Ultraviolet Photocatalytic Performance of ZnO Nanorods Selectively Deposited with Bi2O3 Quantum Dots
Abstract
1. Introduction
2. Results
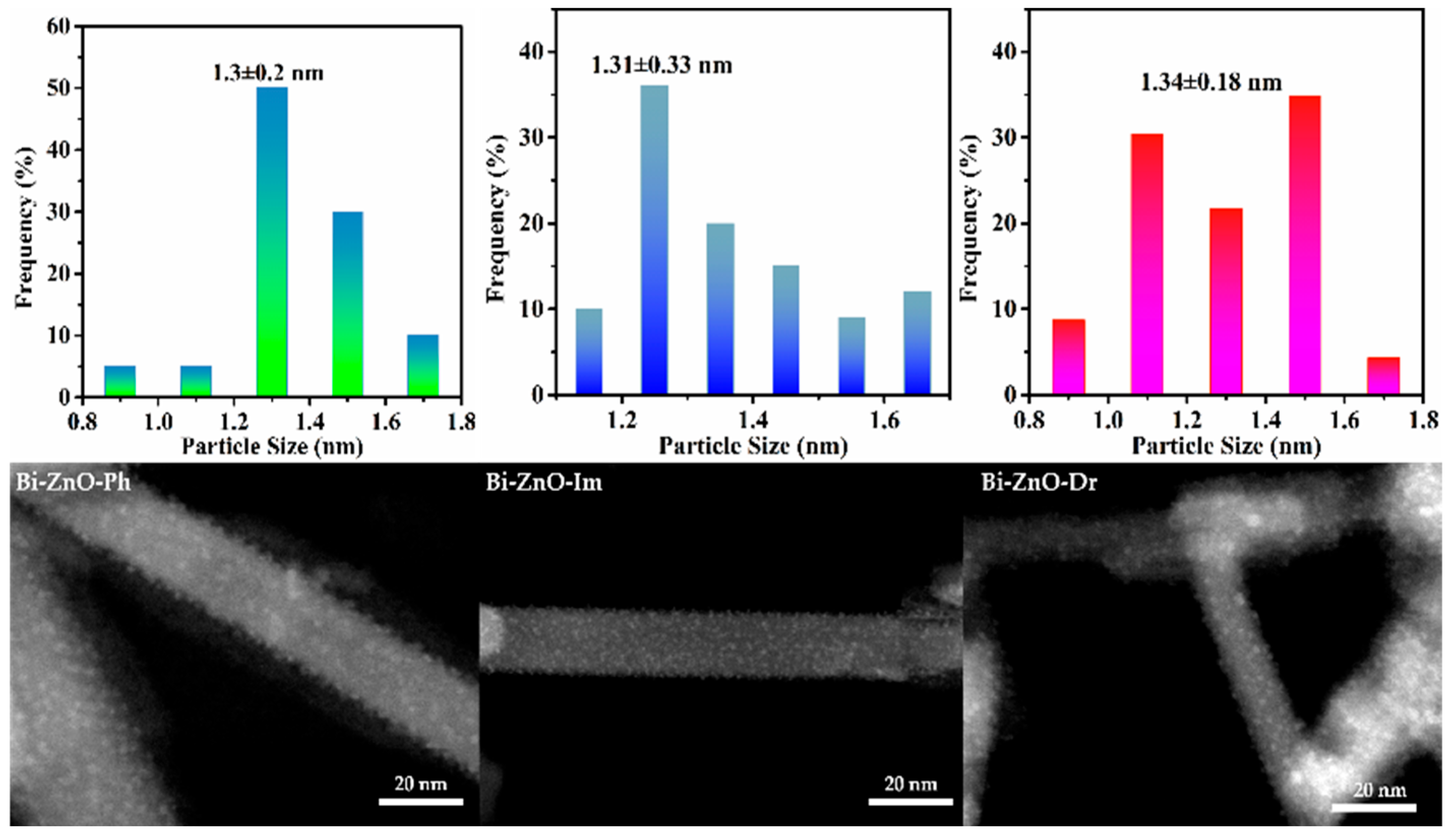
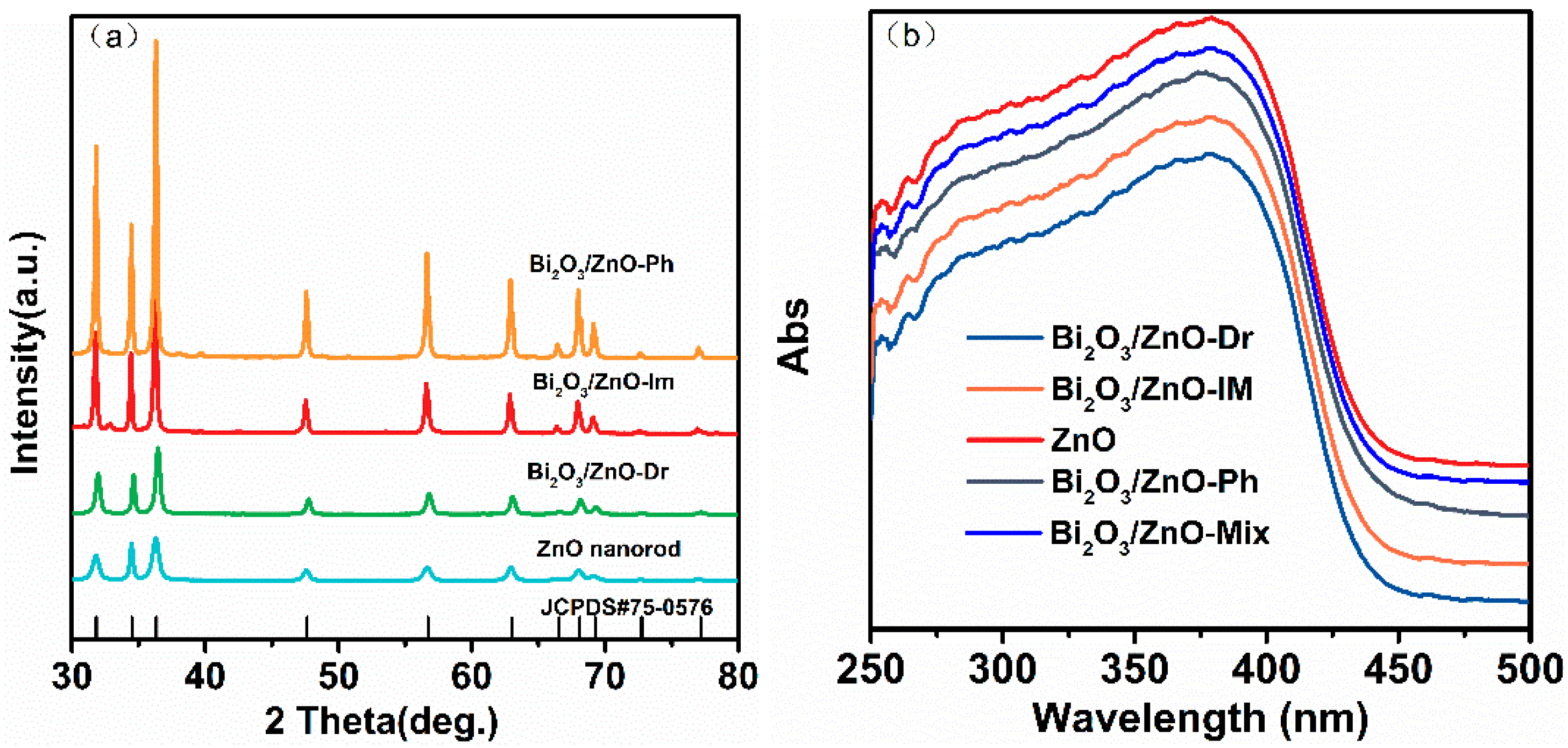
2.1. Structural Characterization
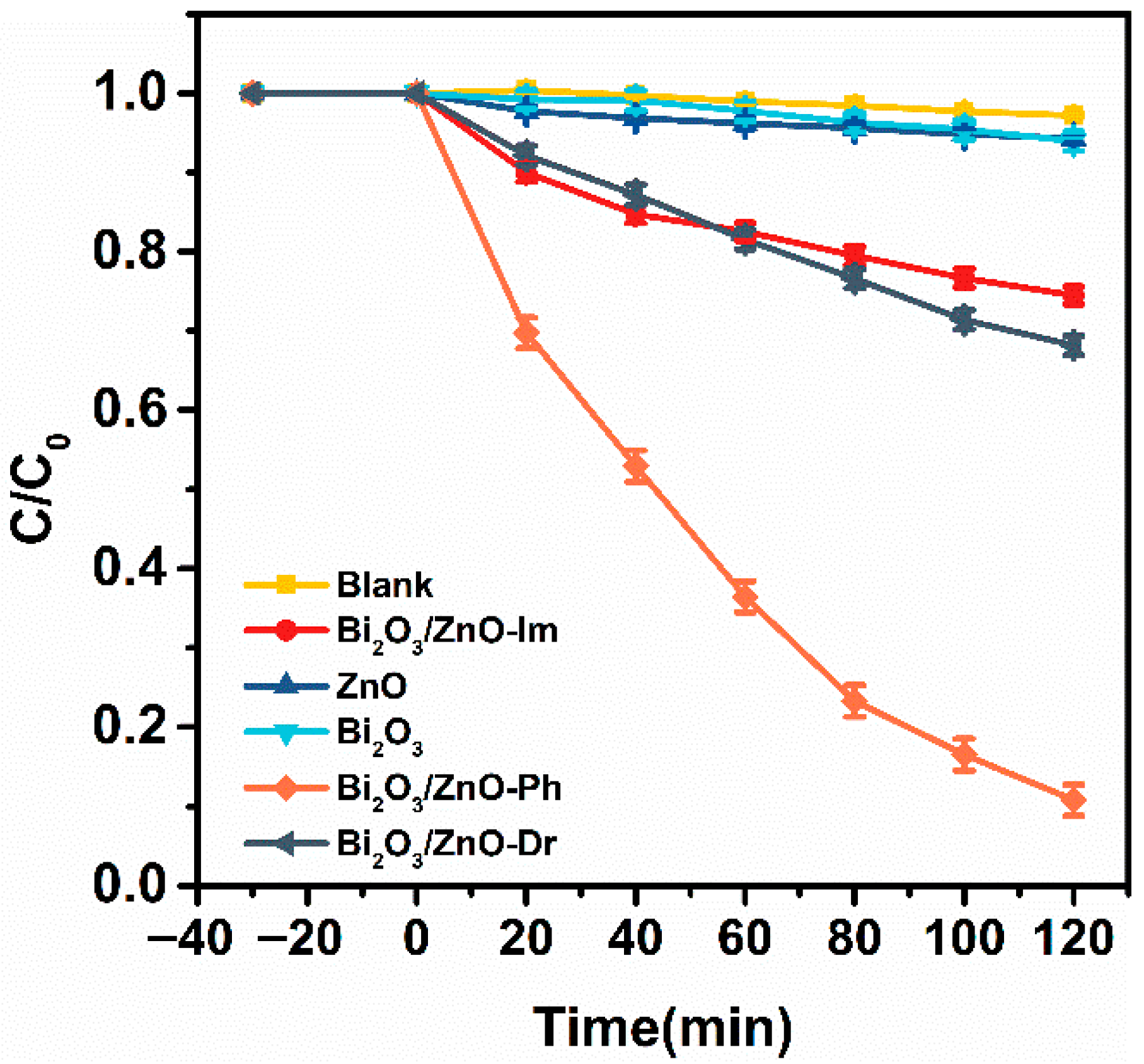
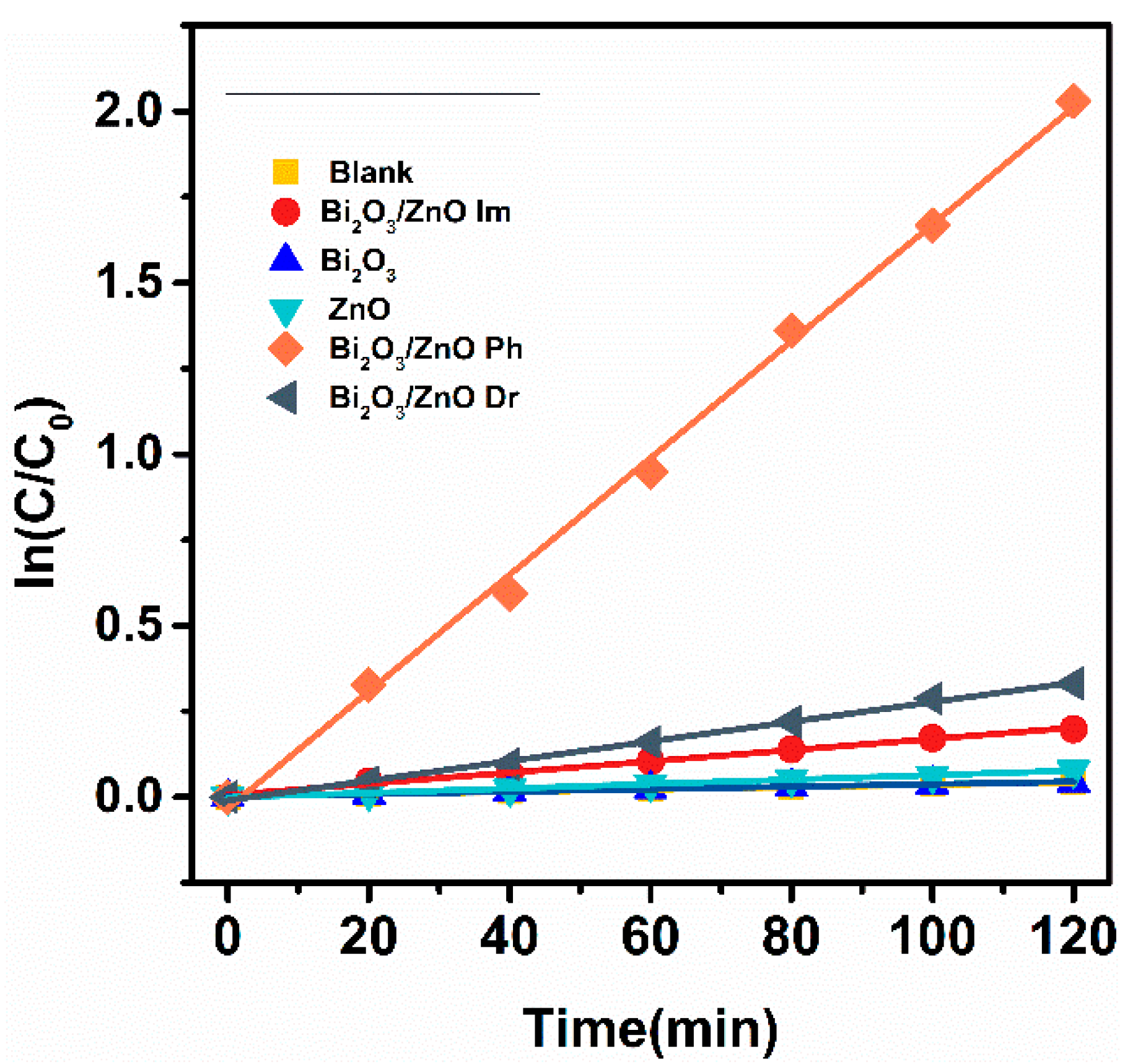
2.2. Photocatalytic Degradation Performance Analysis of RhB
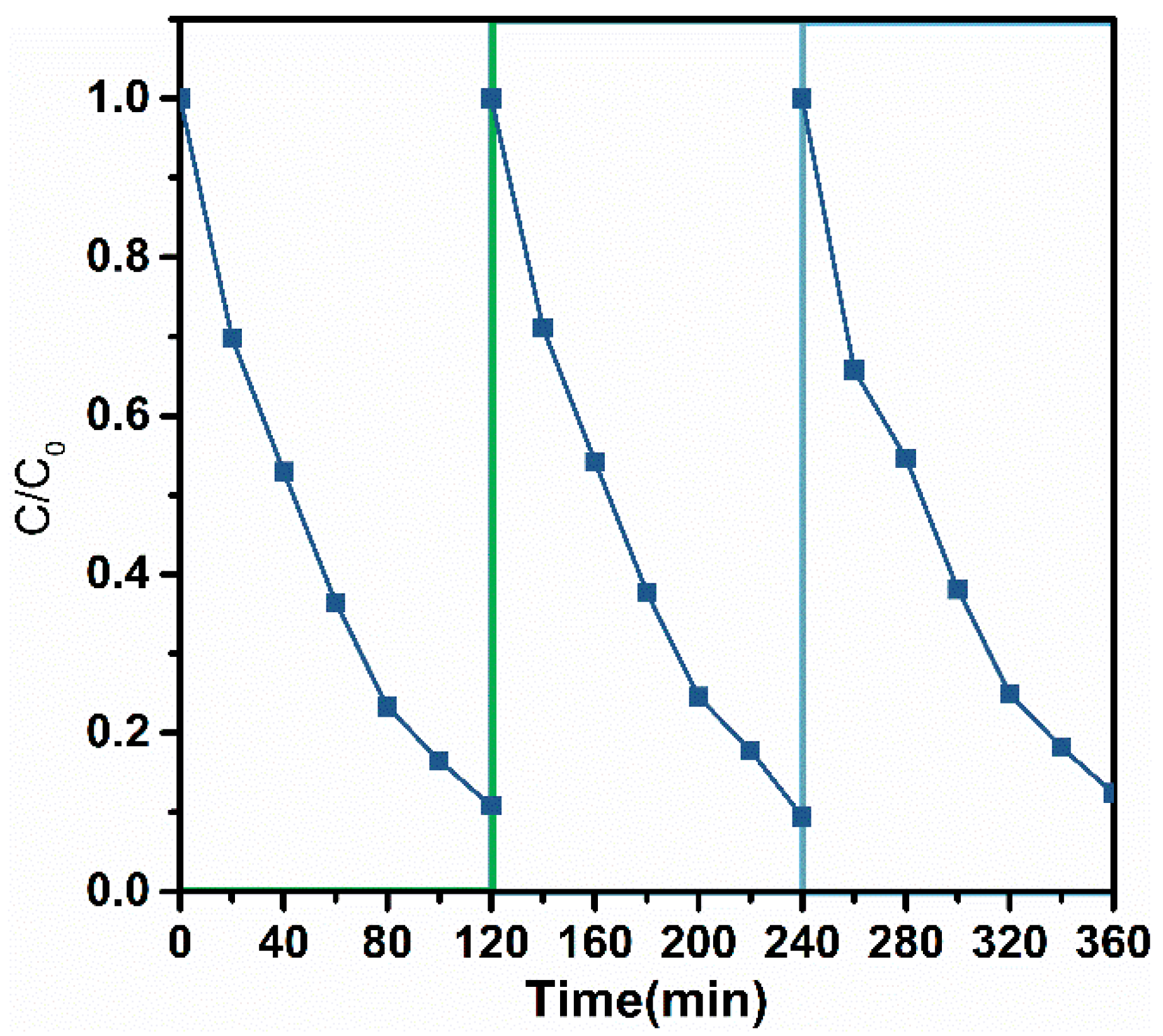
2.3. Photocatalyst Stability Evaluation
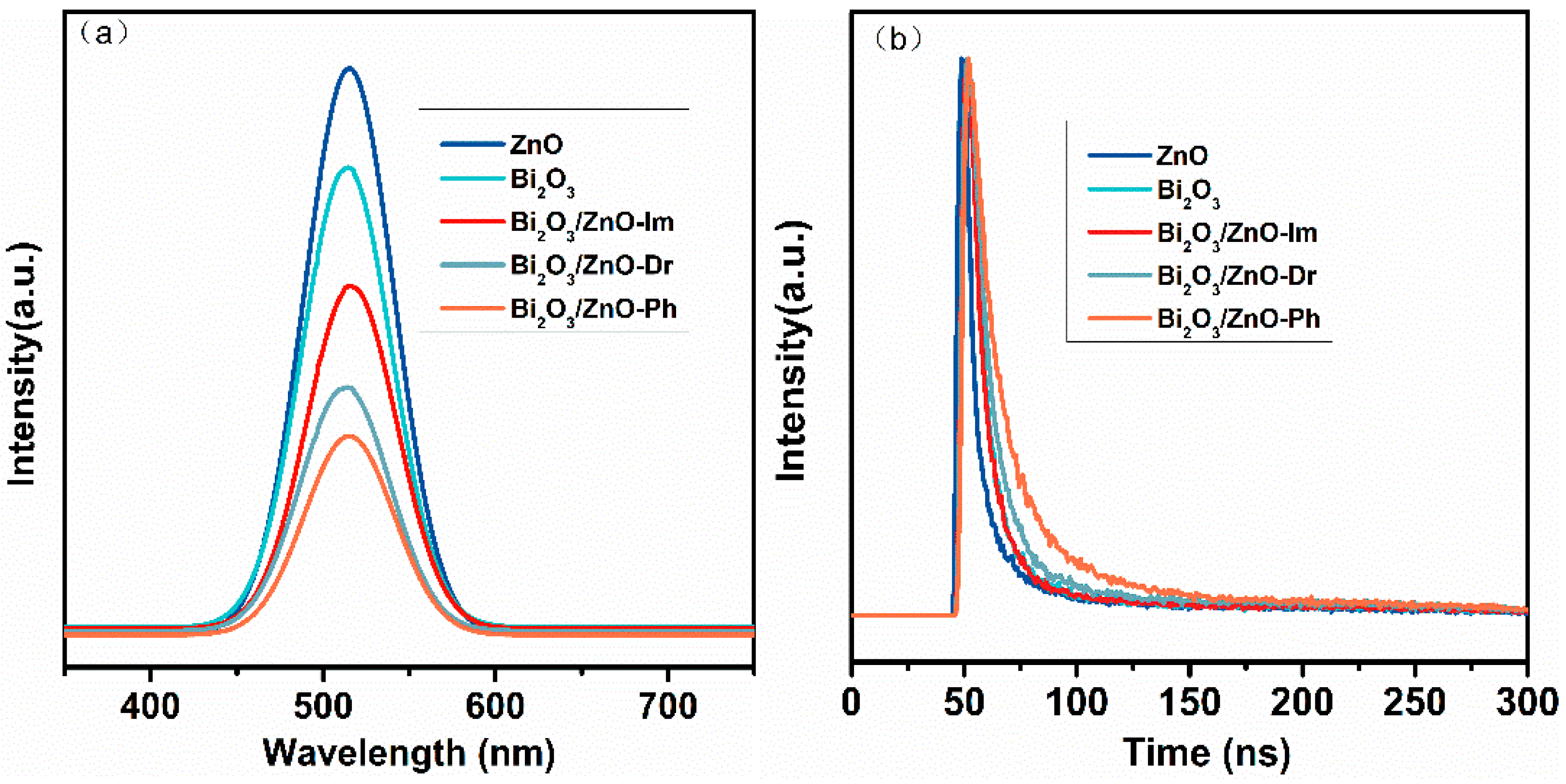
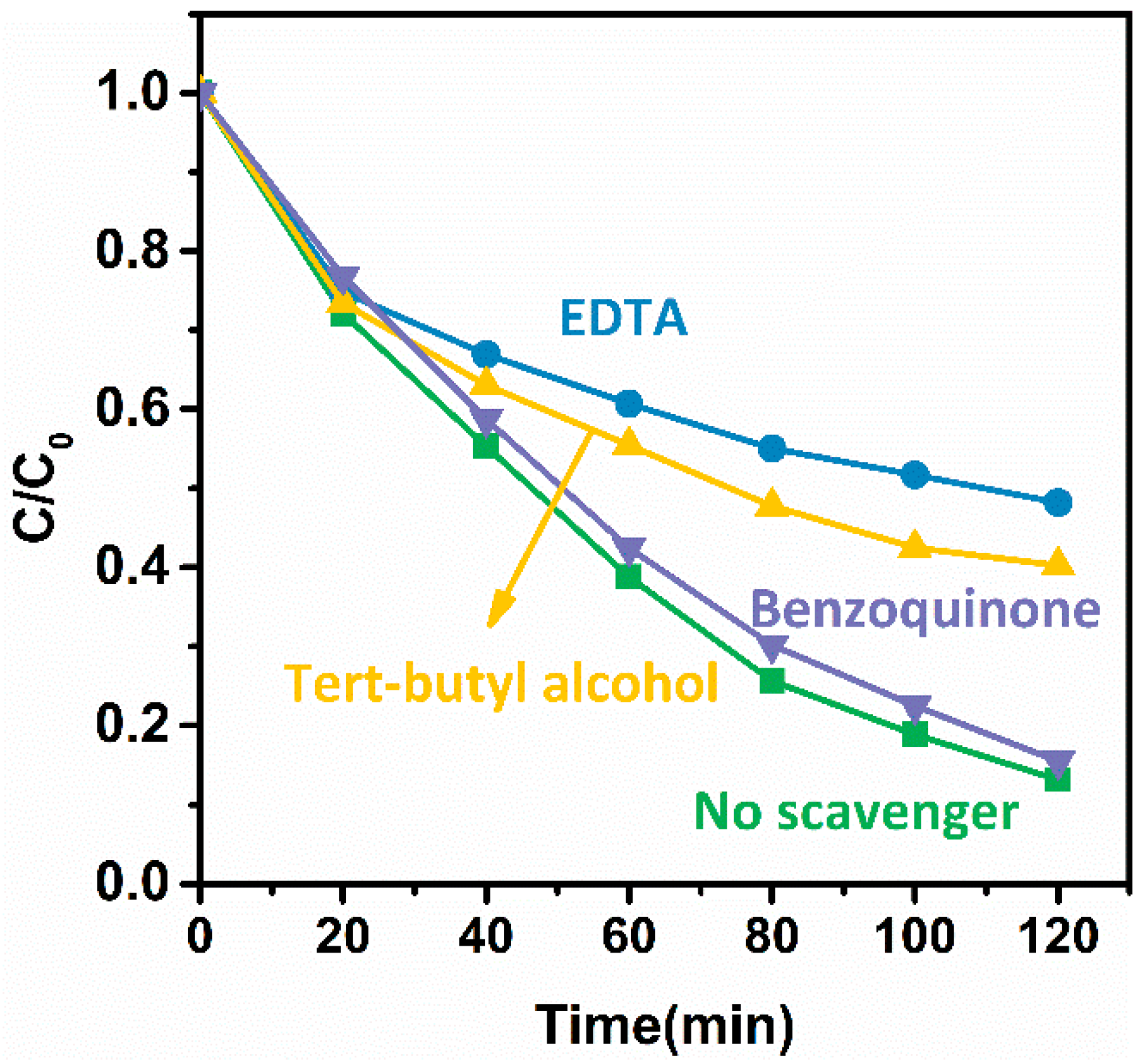
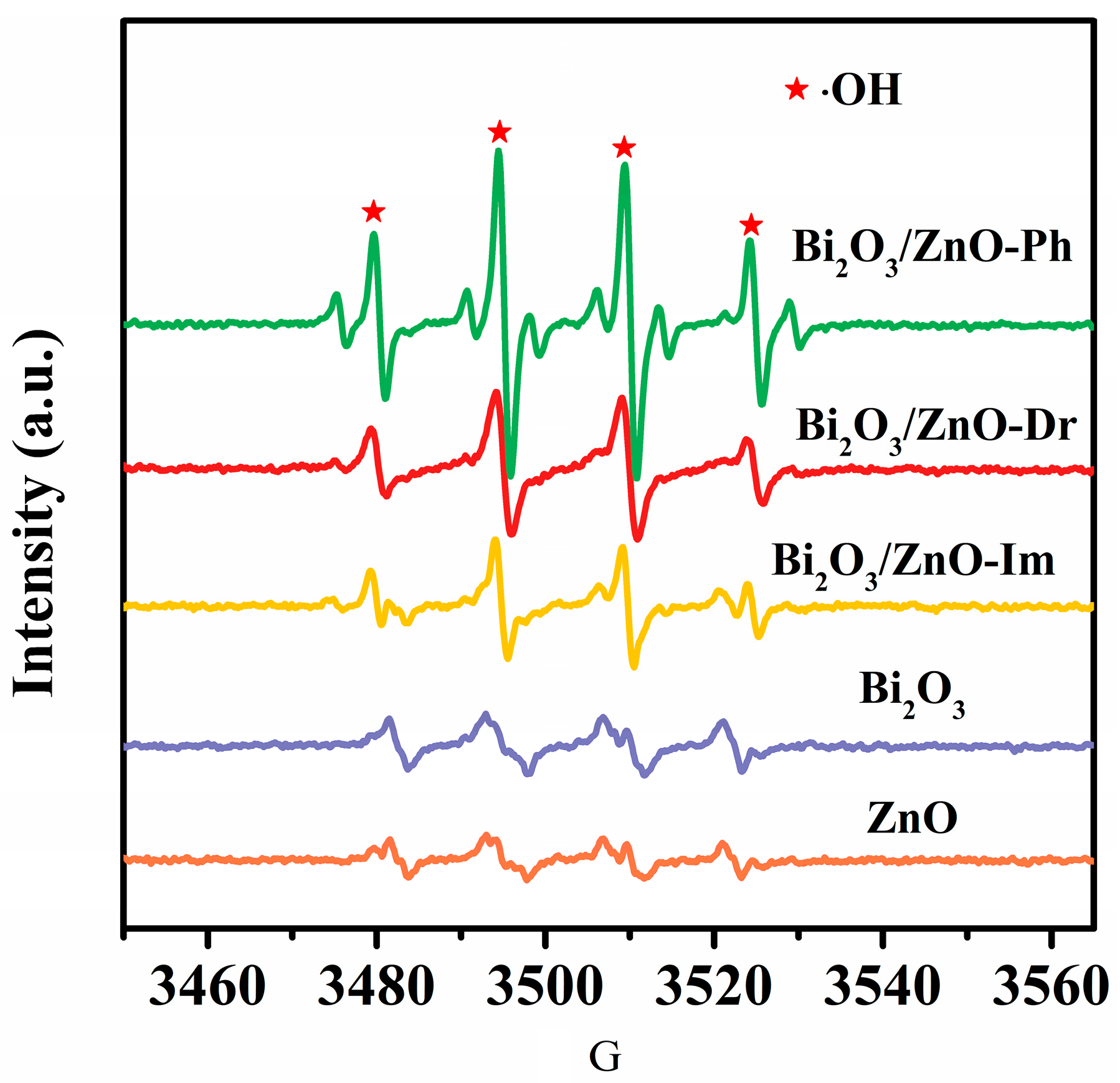
3. Discussion
4. Materials and Methods
4.1. Synthesis of Catalysts
4.1.1. Synthesis of the ZnO Nanorods
4.1.2. Preparation of the 5 wt.% Bi2O3/ZnO-Ph Composite Catalyst
4.1.3. Preparation of the 5 wt.% Bi2O3/ZnO-Dr Composite Catalyst
4.1.4. Preparation of the 5 wt.% Bi2O3/ZnO-Im Composite Catalyst
4.2. Material Characterization Methods
4.3. Catalytic Performance Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Riente, P.; Adams, A.M.; Albero, J.; Palomares, E.; Pericàs, M.A. Light-driven organocatalysis using inexpensive, nontoxic Bi2O3 as the photocatalyst. Angew. Chem. Int. Ed. 2014, 53, 9613–9616. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.H.; Han, Q. A review on the preparation, microstructure, and photocatalytic performance of Bi2O3 in polymorphs. Nanoscale 2021, 13, 17687–17724. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, X. A review on quantum dots modified g-C3N4-based photocatalysts with improved photocatalytic activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Xi, Q.; Zhou, H.; Zhao, Y.; Wu, C.; Wang, L.; Guo, P.; Xu, J. Selectivity of quantum dot sensitized ZnO nanotube arrays for improved photocatalytic activity. Phys. Chem. Chem. Phys. 2017, 19, 11366–11372. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.R.; Pujar, G.; Sannaikar, M. FRET from ZnSe/ZnS QDs to coumarin dyes: Role of acceptor dipole moment and QD surface states on FRET efficiency. J. Lumin. 2018, 203, 67–73. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nomngongo, P.N. Review on metal chalcogenides and metal chalcogenide-based nanocomposites in photocatalytic applications. Chem. Afr. 2023, 6, 1127–1143. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Hartmann, F.; Etter, M.; Cibin, G.; Groß, H.; Kienle, L.; Bensch, W. Understanding sodium storage properties of ultra-small Fe 3 S 4 nanoparticles–a combined XRD, PDF, XAS and electrokinetic study. Nanoscale 2022, 14, 2696–2710. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.J.; Andreko, S.K.; Ernst, L.A.; Waggoner, A.S.; Ballou, B.; Bruchez, M.P. Long-term persistence and spectral blue shifting of quantum dots in vivo. Nano Lett. 2009, 9, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Singla, M.L.; Kumar, M. Optical characterization of ZnO nanoparticles capped with various surfactants. J. Lumin. 2009, 129, 434–438. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Zhang, J.; Peng, Y.; Xie, E. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Shi, C.; Zhang, C.; Yi, G.; Chen, L.; Guo, H.; Huang, G.; Cao, J. Preparation of TiO2/activated carbon composites for photocatalytic degradation of RhB under UV light irradiation. J. Nanomater. 2016, 2016, 8393648. [Google Scholar] [CrossRef]
- Baloch, A.A.B.; Alharbi, F.H.; Grancini, G.; Hossain, M.I.; Nazeeruddin, K.; Tabet, N. Analysis of photocarrier dynamics at interfaces in perovskite solar cells by time-resolved photoluminescence. J. Phys. Chem. C 2018, 122, 26805–26815. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Li, Z.; Li, H.; Ye, T.; Wetzel, C.; Li, H.; Shi, S.-F. Communicating two states in perovskite revealed by time-resolved photoluminescence spectroscopy. Sci. Rep. 2018, 8, 16482. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Karmakar, K.; Khan, G.G. Designing Co-Pi modified one-dimensional n–p TiO2/ZnCo2O4 nanoheterostructure photoanode with reduced electron–hole pair recombination and excellent photoconversion efficiency (>3%). J. Phys. Chem. C 2017, 121, 25705–25717. [Google Scholar] [CrossRef]
- Ma, J.; Miao, T.J.; Tang, J. Charge carrier dynamics and reaction intermediates in heterogeneous photocatalysis by time-resolved spectroscopies. Chem. Soc. Rev. 2022, 51, 5777–5794. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Gao, X.; Zhang, L.; Wang, D.; Zou, X.; Xie, T. Enhanced photocatalytic hydrogen evolution of NiCoP/g-C3N4 with improved separation efficiency and charge transfer efficiency. ChemSusChem 2018, 11, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Mehata, M.S. Temperature-dependent photoluminescence and decay times of different phases of grown TiO2 nanoparticles: Carrier dynamics and trap states. Ceram. Int. 2021, 47, 32534–32544. [Google Scholar] [CrossRef]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Junkar, I.; Kovač, J.; Zavašnik, J.; Pintar, A. Improved electron–hole separation and migration in anatase TiO2 nanorod/reduced graphene oxide composites and their influence on photocatalytic performance. Nanoscale 2017, 9, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, B.; Wang, C. Rational construction and efficient regulation of stable and long-lived charge-separation state in fullerene materials. Acc. Mater. Res. 2024, 5, 426–437. [Google Scholar] [CrossRef]
- Das, A.; Adak, M.K.; Mahata, N.; Biswas, B. Wastewater treatment with the advent of TiO2 endowed photocatalysts and their reaction kinetics with scavenger effect. J. Mol. Liq. 2021, 338, 116479. [Google Scholar] [CrossRef]
- Hossain, S.; Mollah, M.Y.A.; Susan, A.B.H.; Islam, M. Role of in situ electrogenerated reactive oxygen species towards degradation of organic dye in aqueous solution. Electrochim. Acta 2020, 344, 136146. [Google Scholar] [CrossRef]
- Xie, Z.H.; He, C.S.; Zhou, H.Y.; Li, L.L.; Liu, Y.; Du, Y.; Liu, W.; Mu, Y.; Lai, B. Effects of molecular structure on organic contaminants’ degradation efficiency and dominant ROS in the advanced oxidation process with multiple ROS. Environ. Sci. Technol. 2022, 56, 8784–8795. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Yin, Y.; Jiang, L.; Xia, Z.; Tan, C.; Li, J.; Zuo, J.; Wang, Y. Synthesis of Ag/ZnO/BiOCl composite material and its photodegradation performance on ciprofloxacin. Coatings 2024, 14, 192. [Google Scholar] [CrossRef]
- Dubey, S.; Thakur, A.S.; Vaish, R.; Algarni, Z.; Ismail, M.A.; Amari, A. Impact of rotational speed and pH on tribocatalytic dye degradation activity of 0.5 Ba (Zr0.2Ti0.8) O3–0.5 (Ba0.7Sr0.3) TiO3. Int. J. Appl. Ceram. Technol. 2025, 22, e15015. [Google Scholar] [CrossRef]
- Shi, W.; Fang, W.-X.; Wang, J.-C.; Qiao, X.; Wang, B.; Guo, X. pH-controlled mechanism of photocatalytic RhB degradation over gC3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Hwang, T.M.; Yoon, Y.; Kang, J.W. Evidence of singlet oxygen and hydroxyl radical formation in aqueous goethite suspension using spin-trapping electron paramagnetic resonance (EPR). Chemosphere 2011, 84, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive oxygen species and catalytic active sites in heterogeneous catalytic ozonation for water purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Skillen, N.C.; Fina, F.; Zhang, G.; Randorn, C.; Lawton, L.A.; Irvine, J.T.; Robertson, P.K. Comparative assessment of visible light and UV active photocatalysts by hydroxyl radical quantification. J. Photochem. Photobiol. A Chem. 2017, 334, 13–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, B.; Zhang, C.; Wu, X.; Liu, Y.; Feng, X.; Huang, F.; Zhao, B.; Zhu, Z. Ultraviolet Photocatalytic Performance of ZnO Nanorods Selectively Deposited with Bi2O3 Quantum Dots. Catalysts 2025, 15, 695. https://doi.org/10.3390/catal15070695
Lou B, Zhang C, Wu X, Liu Y, Feng X, Huang F, Zhao B, Zhu Z. Ultraviolet Photocatalytic Performance of ZnO Nanorods Selectively Deposited with Bi2O3 Quantum Dots. Catalysts. 2025; 15(7):695. https://doi.org/10.3390/catal15070695
Chicago/Turabian StyleLou, Baohui, Chi Zhang, Xianhao Wu, Ying Liu, Xiangdong Feng, Feipeng Huang, Bowen Zhao, and Zhengwang Zhu. 2025. "Ultraviolet Photocatalytic Performance of ZnO Nanorods Selectively Deposited with Bi2O3 Quantum Dots" Catalysts 15, no. 7: 695. https://doi.org/10.3390/catal15070695
APA StyleLou, B., Zhang, C., Wu, X., Liu, Y., Feng, X., Huang, F., Zhao, B., & Zhu, Z. (2025). Ultraviolet Photocatalytic Performance of ZnO Nanorods Selectively Deposited with Bi2O3 Quantum Dots. Catalysts, 15(7), 695. https://doi.org/10.3390/catal15070695







