Abstract
The development of cost-effective and scalable air/oxygen electrode materials is crucial for the advancement of Zn–air batteries (ZABs). Porous carbon materials doped with heteroatoms have attracted considerable attention in energy and environmental fields because of their tunable nanoporosity and high electrical conductivity. In this work, we report the synthesis of a three-dimensional (3D) N and P co-doped porous carbon (PA@pDC-1000), derived from a conjugated polyaniline–phytic acid polymer. The cross-linked, rigid conjugated polymeric framework plays a crucial role in maintaining the integrity of micro- and mesoporous structures and promoting graphitization during carbonization. As a result, the material exhibits a hierarchical pore structure, a high specific surface area (1045 m2 g−1), and a large pore volume (1.02 cm3 g−1). The 3D N, P co-doped PA@pDC-1000 catalyst delivers a half-wave potential of 0.80 V (vs. RHE) and demonstrates a higher current density compared to commercial Pt/C. A primary ZAB utilizing this material achieves an open-circuit voltage of 1.51 V and a peak power density of 217 mW cm−2. This metal-free, self-templating presents a scalable route for the generating and producing of high-performance oxygen reduction reaction catalysts for ZABs.
1. Introduction
Zinc–air batteries (ZABs) have emerged as promising candidates for next-generation energy storage, owing to their high theoretical energy density, inherent safety, and cost- effectiveness [1,2]. However, the sluggish oxygen reduction reaction (ORR) at the air cathode remains a critical bottleneck, severely limiting their practical performance [3,4]. Although commercial Pt/C catalysts exhibit excellent ORR activity, their high cost, scarcity, and limited durability under alkaline conditions hinder large-scale applications [5]. Therefore, developing low-cost, metal-free ORR catalysts is crucial for advancing ZAB technology.
Recently, heteroatom-doped porous carbons have attracted significant interest due to their tunable electronic structure and robust chemical stability [6,7]. In particular, metal-free dual doping with nitrogen (N) and phosphorus (P) has been demonstrated to create synergistic active sites that markedly enhance ORR kinetics. For example, Hu et al. developed a novel N, P co-doped porous carbon catalyst by a two-step thermal treatment process, utilizing ZIF-8 as the N-rich precursor and red phosphorus as the P source. The resulting material exhibited a high surface area and a significantly reduced work function (from 4.32 to 3.86 eV), leading to enhanced ORR activity with a half-wave potential (E1/2) of 0.87 V vs. RHE and excellent durability after 2000 CV cycles [8]. Han et al. employed Fructus azedarach biomass through microwave-assisted hydrothermal treatment, followed by calcination, to obtain hierarchical N, P co-doped carbon nanosheets delivering E1/2 = 0.84 V and durable performance over 84 h in ZABs [9]. Takada et al. reported a straightforward synthesis of N and P co-doped porous carbon by co-pyrolyzing of glycine and phytic acid, followed by CO2 activation to fine-tune the pore structure. The resulting catalyst showed a high onset potential of 0.925 V and a E1/2 of 0.838 V vs. RHE in 0.1 M KOH, demonstrating its potential as a metal-free ORR electrocatalyst [10].
Despite these notable advances, several critical challenges persist in the synthesis of heteroatom-doped porous carbons. First, MOF- and template-derived methods, while delivering well-defined pore structures, rely on expensive organic ligands, metal salts, or surfactants and require harsh chemical etching (e.g., HF or NaOH) to remove the template, which severely undermines cost-efficiency and scalability [11,12,13]. Second, polymer- and biomass-based strategies often rely on energy- and time-intensive preprocessing processes, such as freeze-drying to retain 3D structures, multi-step chemical activation (e.g., KOH and ZnCl2), or two-stage calcination protocols, each of which adds operational complexity and environmental burden [14,15,16]. Finally, while individual methods may achieve high surface area, hierarchical porosity, or improved graphitization, integrating all three attributes, a large specific surface area for abundant active sites, interconnected micro-/meso-/macroporous channels for fast mass transport, and sufficient graphitic domains for excellent electronic conductivity, into a single, streamlined synthesis remains an unmet challenge.
In this context, we propose a scalable, template- and metal-free strategy for synthesizing 3D N, P co-doped porous carbon (PA@PDC-1000) using a cross-linked polyaniline–phytic acid (PANI–PA) polymer as the precursor. Unlike previous reports, our method involves a direct carbonization process without freeze-drying, template removal, or chemical activation, simplifying the synthesis while preserving a hierarchical porous architecture. The resulting PA@PDC-1000 features a high surface area (1045 m2 g−1), large pore volume (1.02 cm3 g−1), and abundant N and P dopants, delivering superior ORR performance with an E1/2 of 0.80 V vs. RHE and surpassing Pt/C in current density. A primary ZAB assembled with this material achieves an open-circuit voltage of 1.51 V and a peak power density of 217 mW cm−2. This work offers a sustainable and scalable pathway for the development of high-performance ORR catalysts for ZABs.
2. Results and Discussion
2.1. Physical Characterization of the PA@pDC
The synthesis process of N, P-co-doped porous carbon (PA@pDC-1000) is depicted in Figure 1a. The PANI molecular framework was synthesized with the aid of a cross-linking agent. A hydrogel network was swiftly established upon interaction between the monomer, oxidizing agent, and cross-linker. Upon dehydration via drying, the hydrogel was converted into an aerogel while preserving the original macroscopic structure of the polymer network (Figure 1e). Figure 1b shows the XRD patterns of PA@pDC-1000 and pDC-1000. Both samples show two broad peaks at 2θ = 24.5° and 43.7°, which are related to the (002) and (101) diffraction peaks of graphitic carbon, respectively. With the introduction of phytic acid (PA), the diffraction peak of (002) undergoes a blue shift accompanied with a slightly reduced intensity, indicating an increase in the interplanar space of the (002) plane, which is expected to facilitate mass transfer during the ORR process [17,18]. Moreover, the broad and low-intensity peak observed at 24.5° indicates the presence of an amorphous carbon structure [19]. Such an amorphous structure is beneficial for enhancing the electrical conductivity, providing abundant active sites, and improving structural stability for the electrocatalyst [20]. Raman spectra of the as-prepared samples display two peaks at 1350 and 1600 cm−1, which are assigned to the typical D and G bands, respectively. The D band reflects the existence of disordered and defective carbon structures, while the G band is related to the stretching vibrations of sp2-hybridized C atoms in graphitic domains [21]. The ID/IG ratios of PA@pDC-1000 and pDC-1000 were calculated to be 0.84 and 1.21, respectively (Figure 1c). The higher ID/IG ratio suggests an increased number of surface (edge) defects, which likely contributes to the enhanced electrocatalytic activity [22].
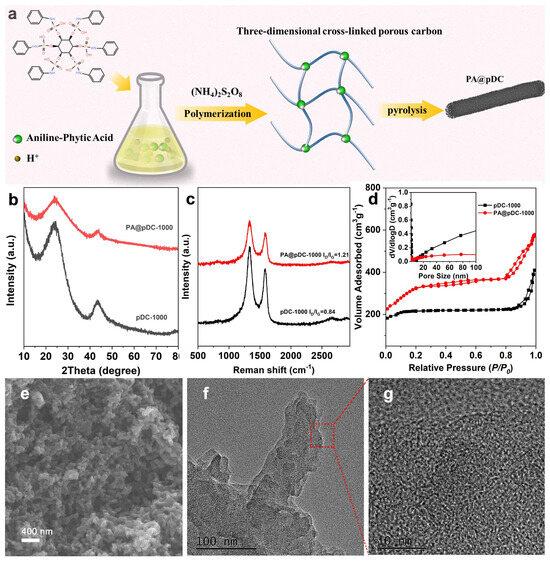
Figure 1.
(a) Schematic illustration of the preparation route for PA@pDC-1000 electrocatalysts. (b) XRD patterns. (c) Raman spectra. (d) N2 adsorption/desorption profiles with inset pore size distribution plots for the pDC-1000 and PA@pDC-1000 catalysts. (e) SEM, (f) TEM, and (g) HR-TEM images of the PA@pDC-1000 catalyst.
The nitrogen adsorption–desorption isotherms were conducted to evaluate the specific surface area, pore size distribution, and overall porosity of the catalysts. As illustrated in Figure 1d, both pDC-1000 and PA@pDC-1000 exhibit Type IV nitrogen adsorption–desorption isotherms with pronounced hysteresis loops, which are characteristic of mesoporous materials. Notably, the PA@pDC-1000 sample demonstrates a substantially higher nitrogen uptake across the entire range of relative pressures, particularly in the high-pressure region (P/P0 > 0.8). This behavior indicates a significantly larger total pore volume and a more developed mesoporous structure compared to pDC-1000. The corresponding pore size distribution curves (inset of Figure 1d) further elucidate the differences in porosity between the two materials. The PA@pDC-1000 sample presents a broader and more flattened distribution profile, suggesting the presence of a wider range of mesopore sizes. However, its contribution from larger pores is somewhat reduced relative to pDC-1000. Conversely, pDC-1000 displays a distinct peak in the 20–100 nm range, indicative of a higher proportion of large mesopores or even macropores. These observations are consistent with the structural parameters summarized in Table S1. The BET-specific surface area of PA@pDC-1000 reaches 1045.8 m2/g, representing an approximately 22.6% increase over that of pDC-1000 (852.8 m2/g). The BJH average pore diameter also shows a slight expansion from 4.0 nm to 4.5 nm, while the total pore volume increases from 0.88 cm3/g to 1.02 cm3/g. These enhancements suggest that PA introduction effectively augments the textural properties of the carbon framework. Overall, the PA@pDC-1000 material exhibits enhanced mesoporosity, a higher specific surface area, and a larger total pore volume relative to pDC-1000. These features are advantageous for applications such as catalysis and adsorption, where efficient mass transport and abundant active surface sites are critical [13].
In the SEM of pDC-1000 (Figure S1a), the carbon appears as a relatively dense, agglomerated sponge with a few well-defined macropores. After PA incorporation, SEM (Figure 1e and Figure S1b) reveals a transition to a “coral-like” assembly of interwoven nanofibers, creating abundant macropores (several hundred nanometers) that were absent in pDC-1000. TEM of PA@pDC-1000 (Figure 1f) further uncovers a continuous, entangled matrix punctuated by secondary mesopores, forming a foam-like structure that greatly enhances mass transport. HR-TEM images display largely amorphous carbon interspersed with short-range, turbostratic, graphene-like fringe, indicative of partial graphitization and abundant edge defects. Finally, SEM-EDS elemental maps (Figure S1) confirm the homogeneous distribution of C, N, O, and P in the structure, with no observable phase segregation. The introduction of PA not only introduces uniformly dispersed N/P dopants but also self-templates a multiscale porous network and promotes localized graphitization. The resulting combination of hierarchical pores, conductive graphitic domains, and homogeneous N/P incorporation synergistically maximizes active site exposure, accelerates O2 diffusion, and enables rapid ion/electron transport, thereby markedly enhancing the ORR activity of PA@pDC-1000.
XPS was employed to analyze the chemical states and bonding configurations of the dopants. As shown in the survey spectra (Figure 2a), C, O, and N elements are present in both pDC-1000 and PA@pDC-1000, whereas P is exclusively detected in PA@pDC-1000, indicating the successful incorporation of P into the carbon framework via PA treatment. This finding is in good agreement with the earlier SEM-EDS analysis, which revealed a uniform phosphorus distribution across the carbon matrix (Figure S1). High-resolution C 1s spectra of pDC-1000 (Figure 2b) exhibit characteristic peaks, corresponding to C=C/C–C (284.3 eV), C–N (284.9 eV), C=O (286.1 eV) and O–C=O (289.8 eV) [23], indicative of sp2-hybridized graphitic carbon structures with oxygen and nitrogen functionalities. Upon PA introduction, an additional peak emerges at 285.5 eV in PA@pDC-1000, assigned to C–P bonding [24], further confirming the incorporation of P into the carbon matrix. Moreover, the C=O and O=C–O peaks shift to higher binding energies (287.0 eV and 290.3 eV, respectively), which may be attributed to the electron-withdrawing nature of P-containing groups, reflecting electronic modulation of the carbon framework. The N 1s spectra (Figure 2c) reveal a marked difference in N configuration upon P doping. While pDC-1000 primarily contains pyrrolic N (401.0 eV) and graphitic N (403.8 eV), PA@pDC-1000 exhibits all three types of typical N species, namely pyridinic N (398.8 eV), pyrrolic N (401.2 eV), and graphitic N (405.3 eV). Notably, pyridinic nitrogen, which is frequently associated with enhanced ORR activity because of its capability to lower the adsorption energy barrier for oxygen molecules and to facilitate electron transfer at adjacent carbon sites [25,26], is absent in pDC-1000 but appears in PA@pDC-1000. This observation suggests that the introduction of PA not only incorporates P into the carbon matrix but also promotes the formation of catalytically active nitrogen species. Quantitative deconvolution of the N 1s spectrum further confirms this trend. In PA@pDC-1000, pyrrolic N dominates (72.6%), but pyridinic N accounts for a significant 22.0% of the total N, with graphitic N contributing 5.4%. In contrast, pDC-1000 contains 94.4% pyrrolic N and only 5.6% graphitic N, with a negligible pyridinic N content. The generation of pyridinic N upon PA doping suggests a synergistic structure–function effect involving both N and P heteroatoms. The high-resolution P 2p spectrum of PA@pDC-1000 (Figure 2d) displays two peaks at 133.3 eV and 134.1 eV, corresponding to P–C and P–O bonds, respectively [27]. Quantitatively, P–C bonds constitute 58.3% and P–O bonds 41.7% of the total phosphorus species, as summarized in Table S2. The coexistence of covalently bonded P–C and oxidized P–O groups indicates that phosphorus is doped into the carbon lattice both structurally and electronically, further modifying the surface chemistry. Overall, XPS analysis confirms that PA treatment enables the successful incorporation of phosphorus into the carbon framework through the formation of C–P/P–O bonds. Moreover, the PA treatment appears to modulate the nitrogen configuration, facilitating the formation of pyridinic nitrogen species known for their catalytic activity. These changes are expected to modulate the electronic structure, enhance charge delocalization, and ultimately contribute to the improved electrocatalytic activity observed in subsequent electrochemical measurements. Further mechanistic studies using in situ spectroscopy or DFT simulations will be pursued to elucidate the site-specific activity of these heteroatom-doped configurations.
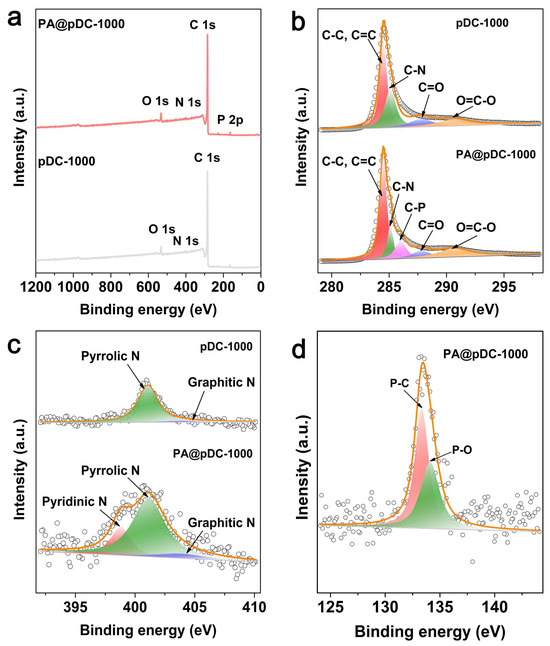
Figure 2.
High-resolution XPS spectra survey with (a) C 1s, (b) N 1s, (c) P 2p, and (d) O 1s of pDC-1000 and PA@pDC-1000.
2.2. Electrochemical Performance of PA@pDC
The high surface area and 3D porous framework of PA@pDC-1000 render it highly suitable for various electrode applications. Additionally, the comparatively small size of the carbon particles contributes to the material’s scalability and enhances its processing flexibility. The interconnected coral-like nanofibers comprising the carbon particles endow the electrodes with excellent mechanical flexibility, positioning PA@pDC-1000 as a promising candidate for applications in supercapacitors, batteries, electrocatalysts, and other related fields.
The electrocatalytic performance of PA@pDC-1000 toward the ORR was systematically evaluated and compared with that of pDC-1000 and commercial Pt/C catalysts. As shown in the cyclic voltammetry (CV) curves in Figure 3a, PA@pDC-1000 displays a distinct ORR peak at 0.71 V vs. RHE in O2-saturated 0.1 M KOH, which is significantly more pronounced than that of the undoped counterpart pDC-1000, indicating superior ORR activity. In addition to the cathodic ORR peak, PA@pDC-1000 also displays a distinct anodic feature in the potential range of 0.2–0.4 V vs. RHE, which is absent in pDC-1000. This anodic peak is attributed to reversible redox processes involving phosphorus-containing surface groups, particularly C–P moieties introduced via P doping. These groups likely engage in Faradaic-type interactions with OH− in the alkaline electrolyte, resulting in pseudocapacitive behavior. Although no directly comparable anodic peak has been reported for P- or N, P co-doped carbon materials within this potential window, the reproducibility of this feature underscores the unique surface chemistry and electronic structure of the PA@pDC-1000 catalyst. To quantitatively evaluate the electrocatalytic activity of the materials toward the ORR, linear sweep voltammetry (LSV) measurements were performed at a rotation speed of 1600 rpm in 0.1 M KOH electrolyte (Figure 3b). Among all the tested catalysts, PA@pDC-1000 exhibited the most promising performance, delivering a high limiting diffusion current density of 7.19 mA cm−2, outperforming commercial Pt/C (5.20 mA cm−2). In addition, PA@pDC-1000 presented a more positive onset potential of 1.04 V and a higher half-wave potential of 0.80 V, closely matching that of Pt/C (E1/2 = 0.78 V). These results collectively indicate that PA@pDC-1000 possesses superior ORR activity, both in terms of onset potential and diffusion-limited current density. Furthermore, the Tafel slope provides insight into the kinetics of the ORR. PA@pDC-1000 exhibits a Tafel slope of 96.6 mV dec−1, which is lower than that of pDC-1000 (124.4 mV dec−1) and comparable to Pt/C (96.9 mV dec−1), suggesting more favorable reaction kinetics (Figure 3c). Moreover, PA@pDC-1000 exhibits a superior mass activity of 29.01 mA mg−1, significantly outperforming Pt/C, which shows 14.11 mA mg−1 (Figure 3d). This indicates a superior catalytic efficiency of PA@pDC-1000. Figure S2 presents the LSV curves of PA@pDC-1000 recorded in an O2-saturated 0.1 M KOH electrolyte at various rotation speeds (400, 625, 900, 1225, and 1600 rpm). These measurements were employed to conduct the Koutecky–Levich (K–L) analysis presented in Figure 3e. As illustrated in Figure 3e, the current density increases with the increasing rotation speed from 400 to 1600 rpm. This trend indicates improved mass transport at higher rotation speeds. The corresponding Koutecky–Levich (K–L) plots, constructed from these LSV curves, exhibit excellent linearity. The calculated electron transfer number (n) is approximately 4, indicating that the ORR on PA@pDC-1000 proceeds predominantly via an efficient four-electron pathway. In addition, a fast cyclic aging experiment was performed to assess the catalytic durability of PA@pDC-1000. After 5000 cycles, only a slight shift in the negative potential was observed, confirming its excellent long-term stability (Figure 3f). These findings suggest that the enhanced electrocatalytic performance of PA@pDC-1000 is likely attributed to the synergistic effect of nitrogen and phosphorus co-doping and the presence of a porous architecture that exposes abundant active sites [28].
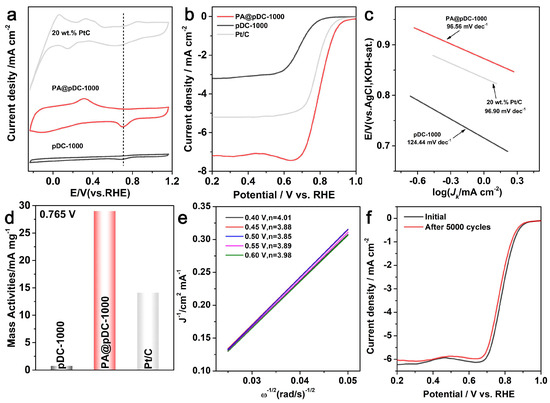
Figure 3.
(a) CV curves, (b) LSV curves recorded at 1600 rpm, (c) Tafel slopes, and (d) mass activities of pDC-1000, PA@pDC-1000, and the 20 wt% Pt/C benchmark catalyst evaluated in O2-saturated 0.1 M KOH. (e) Koutecky–Levich plots of PA@pDC-1000 at different potentials, based on LSV measurements at various rotation speeds (400, 625, 900, 1225, and 1600 rpm). (f) ORR activity curves of PA@pDC-1000 recorded before and after 5000 cycles.
Compared with recently reported metal-free ORR catalysts derived from MOFs, PA@pDC-1000 demonstrates a balanced combination of electrocatalytic performance and practical advantages. Although its ORR activity may not exceed that of the highest-performing MOF-derived materials in all aspects, PA@pDC-1000 still delivers a competitive performance while being synthesized through a significantly more straightforward process. More importantly, our material was prepared via a template-free, one-pot strategy using low-cost and readily available precursors, eliminating the need for complex synthesis procedures, expensive metal centers, or harsh reaction conditions often required in MOF-based systems. This accessible and scalable synthesis approach, coupled with the favorable electrochemical properties, positions PA@pDC-1000 as a promising and practically viable alternative to conventional MOF-derived catalysts, particularly for applications where simplicity, sustainability, and cost-effectiveness are essential.
Electrochemical impedance spectroscopy (EIS) further supports the role of the hierarchical pore structure in facilitating ORR activity. The Nyquist plot was fitted using a multilayered equivalent circuit model [R(C(R(C(R(CR))))], capturing the multiscale transport processes within the porous framework. The fitted resistances (Figure S3 and Table S3) increase progressively from Rs to R3, corresponding to transport through macropores, mesopores, and micropores, respectively [29]. The absence of a Warburg tail and the depressed semicircle shape indicate efficient mass transport and distributed reaction kinetics, which are consistent with the interconnected, hierarchical pore network confirmed by BET analysis.
A primary ZAB was first assembled using PA@pDC-1000 combined with RuO2 as the electrocatalyst (Figure 4b). The two-electrode ZAB exhibited an open-circuit potential of about 1.51 V (Figure 4a), confirming the outstanding catalytic performance of PA@pDC-1000+RuO2 configuration (1.42 V). Figure 4c displays the polarization and power density curves of ZABs equipped with PA@pDC-1000+RuO2 air cathodes. The PA@pDC-1000+RuO2 catalysts achieved a peak power density of 217 mW cm−2, which is comparable to that of Pt/C+RuO2 (195 mW cm−2). The excellent performance of PA@pDC-1000 can be attributed to its porous structure, which promotes the efficient diffusion of O2 and electrolytes to the active sites. A specific capacity of 698 mA h gZn−1 was achieved at 5 mA cm−2, based on the amount of zinc consumed. This value exceeds that of ZABs employing Pt/C and RuO2 catalysts, which delivered 635 mA h gZn−1 under the same conditions (Figure 4d). Although Zn is gradually depleted during discharge, the battery can be reactivated by mechanical replacement of the Zn plate and replenishment of the KOH electrolyte. To further evaluate the long-term cycling stability and reversibility, batteries based on PA@pDC-1000+RuO2 and Pt/C+RuO2 were tested at 5 mA cm−2, with a 10-min charge/discharge interval. As shown in Figure 4e, the PA@pDC-1000+RuO2-based battery displayed excellent cycling stability and rechargeability, even after 400 h, significantly outperforming the Pt/C+RuO2-based battery. Finally, a blue LED (OCP, 1.51 V) was successfully powered by the PA@pDC-1000+RuO2-based ZAB (Figure 4a), further demonstrating the potential of PA@pDC-1000 as an efficient metal-free catalyst for ORR and ZAB applications. In addition, the low-cost, template-free synthesis route and the use of abundant precursors suggest that this method holds promise for the scalable production of carbon-based electrocatalysts with minimal cost barriers.
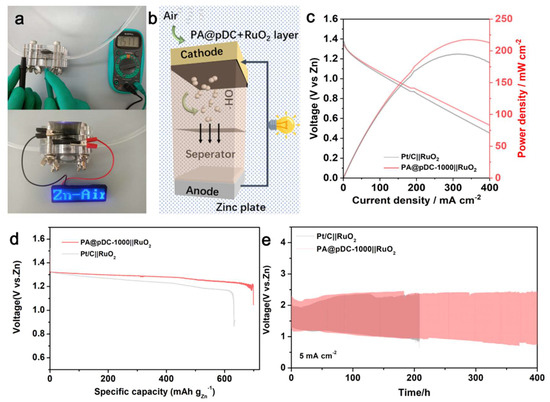
Figure 4.
(a) Photograph of the OCV test of ZABs and powered LED lights by aqueous rechargeable ZAB assembled from PA@pDC-1000||RuO2. (b) The schematic design of PA@pDC-1000||RuO2 ZABs. (c) Polarization and power density curves of the ZABs with PA@pDC-1000||RuO2 and Pt/C||RuO2. (d) Specific discharge capacity of PA@pDC-1000||RuO2 and Pt/C||RuO2 electrodes at 5 mA/cm2. (e) Galvanostatic discharge and charge cycling curves with 10-min discharge and 10-min charge at 5 mA/cm2.
3. Materials and Methods
3.1. Catalysts Preparation
Nanorod composites of polyaniline (PANI) and PA were prepared using a chemical oxidative polymerization technique. PA was chosen as the cross-linking agent due to its ability to form stable electrostatic and phosphate bonds that facilitate 3D framework assembly and enhance carbon structure stability during high-temperature pyrolysis. Initially, 1 g of aniline monomer, 4 mL of PA, and 16 mL of hydrochloric acid (37 wt.%) were dissolved in 100 mL of deionized water to prepare a homogeneous solution at 10 °C. Simultaneously, 2.45 g of ammonium persulfate (APS) was dissolved in 50 mL of deionized water to prepare the initiator solution. This APS solution was subsequently introduced dropwise into the aniline–hydrochloric acid–phytic acid mixture over a 50-min period. The reaction system was maintained under continuous stirring for a further 6 h at 10 °C. The obtained dark green powder was harvested, rinsed with ethanol, and dried in an oven at 60 °C for 12 h. The polyaniline–phytic acid nanorod composite was first pre-heated at 400 °C in a N2 flow for 2 h, followed by annealing at 1000 °C for another 5 h. The resulting catalyst was denoted as PA@pDC-1000. In addition, the hydrochloric acid and aniline monomer mixture processed by the same procedure without PA was also calcinated at 1000 °C for 5 h under N2 flow, and the as-obtained carbon was termed as pDC-1000.
3.2. Catalysts Characterization
The surface structure of the materials was characterized using scanning electron microscopy (SEM; Zeiss Gemini SEM300, Carl Zeiss AG, Oberkochen, Germany). The internal ultrastructural characterization was performed on both HRTEM and HAADF imaging in a scanning–transmission configuration (Thermo Fisher Talos F200S, Thermo Fisher Scientific, Waltham, MA, USA). The crystalline structure was analyzed by powder X-ray diffraction (XRD; Hitachi SmartLab SE, Hitachi High-Tech, Tokyo, Japan). The surface area, pore volume, and size distribution were determined by the N2 adsorption–desorption method (Micromeritics Tristar II 3020 chemisorption–physisorption analyzer, Micromeritics Instrument Corp., Norcross, GA, USA). The element chemical environment of the catalyst was characterized by X-ray photoelectron spectra (XPS; Thermo VG MultiLab 2000, Thermo Fisher Scientific, Waltham, MA, USA).
3.3. Electrochemical Measurements
Electrochemical tests were performed with a rotating disk electrode instrument (Pine Instruments Co. Ltd., Grove City, PA, USA) and a Princeton electrochemical workstation, employing a three-electrode configuration in the 0.1 M KOH electrolyte. The cell configuration employed a platinum sheet as the counter electrode, while an Ag/AgCl (3 M KCl) electrode and a glassy carbon disk operated as the reference and working electrodes, respectively. A uniform catalyst dispersion was obtained by sonicating 4 mg of catalyst in 300 µL of isopropanol containing 15 µL of 5 wt.% Nafion solution for 1 h. Subsequently, 10 μL of the catalyst dispersion was pipetted on the polished RDE glassy carbon (5 mm in diameter). The catalyst loading was estimated to be 0.45 mg cm−2. The ink composition was chosen according to established procedures for carbon-based ORR catalysts to ensure optimal loading and uniform film formation. CV was recorded over the −1.2 V to 0.2 V (vs. Ag/AgCl) potential window at a scan rate of 50 mV s–1. ORR polarization curves were recorded by linear sweep voltammetry in O2-saturated 0.1 M KOH at 5 mV s−1 over the 1.2–1.8 V (vs. RHE) range, with electrode rotation rates that varied from 400 to 1600 rpm. Measured potentials were referenced to the RHE scale using the Nernst equation: ERHE = EAg/AgCl + 0.197 + 0.0591pH. The number of electrons transferred (n) during the ORR was determined using the Koutecky–Levich equation:
where J represents the experimentally measured current density (mA cm−2), Jk denotes the kinetic current density (mA cm−2), and JL signifies the diffusion-limiting current density (mA cm−2). The variable ω corresponds to the rotating speed (rpm). The viscosity of the electrolyte (ν) is 1.0 × 10−2 cm2 s−1, and Do is the diffusion coefficient of oxygen, with D = 1.9 × 10−5 cm2 s−1. Co is the saturated concentration of oxygen, with Co = 1.2 × 10−6 mol cm−3. F is the Faraday constant, with F = 96,500 C mol−1.
Liquid ZAB was assembled with a polished zinc plate (purity > 99.99%) serving as the anode, while the cathode comprised a hydrophobic carbon cloth equipped with a gas diffusion layer and loaded with an electrocatalyst at a mass loading of 1 mg cm−2. A 6 M KOH aqueous solution was employed as the electrolyte. In the case of the rechargeable ZAB, the air cathode consisted of a composite containing equal weight proportions (50 wt.%) of the synthesized catalyst and RuO2. The assembled ZAB was evaluated by recording its charge–discharge polarization curves (each cycle lasting 20 min) and galvanostatic discharge profiles at a constant current density of 5 mA cm−2. Additionally, polarization data were collected using a linear sweep rate of 5 mV s−1 within the voltage window of 1.6–0 V. All electrochemical measurements were conducted at ambient temperature using a LAND CT2001A battery testing system. For comparison purposes, the primary ZAB was assembled with a Pt/C-based air cathode.
4. Conclusions
In conclusion, we developed a scalable, template-free route to fabricate 3D N, P co-doped porous carbon (PA@pDC-1000) via in situ interfacial polymerization of aniline with phytic acid, followed by direct carbonization. Our approach leverages phytic acid as both a P source and internal cross-linker, acting as a self-templating agent that generates hierarchical micro-/meso-/macropores without the need for external templates or activation chemicals. The resulting PA@pDC-1000 catalyst displayed excellent electrocatalytic performance for ORR, comparable to commercial Pt/C. Our experimental results suggest that the observed enhanced catalytic performance is attributed to the formation of a highly porous graphitic framework structure enhanced by edge site doping with N and P atoms. Notably, ZABs assembled with these PA@pDC-1000 electrocatalysts in aqueous electrolytes exhibited excellent battery performance and long-term cycling stability. This study not only experimentally demonstrates the influence of heteroatom doping on catalytic activity but also introduces a novel strategy for developing high-performance, affordable metal-free PA@pDC-1000 catalysts for ZABs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15070683/s1: Figure S1: (a) SEM image of pDC-1000; (b) SEM image of PA@pDC-1000 and corresponding EDS mapping. Table S1: The structure parameters of the catalysts. Figure S2: LSV curves of PA@pDC-1000 in O2 -saturated 0.1 M KOH solution at various rotation speeds (400, 625, 900, 1225, and 1600 rpm). These measurements were used to perform the Koutecky–Levich analysis shown in Figure 3e of the main text. Table S2: Relative atomic percentages of N and P species in pDC-1000 and PA@pDC-1000 based on XPS deconvolution. Figure S3: Nyquist plot of PA@pDC-1000 performed in the frequency range of 0.01 Hz to 100 kHz with AC amplitude of 10 mV and its equivalent circuit fitting using a multilayered R(CR) model. Table S3: Fitted impedance parameters and relative errors extracted from the EIS equivalent circuit model.
Author Contributions
Conceptualization, W.X., Y.K. and X.C.; methodology, W.X. and X.C.; formal analysis, W.X. and Y.K.; investigation, J.X., T.W. and X.C.; writing—original draft preparation, W.X. and X.C.; review and editing, X.C.; supervision, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Start-Up Research Funding of Wuhan Polytechnic University (53210052213) and the Research Project of Wuhan Polytechnic University (2023Y23).
Data Availability Statement
Data are contained within this article.
Acknowledgments
We acknowledge the School of Materials Science and Engineering at Hubei Normal University, for providing instrumental service.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barman, J.; Deka, N.; Rudra, S.; Dutta, G.K. Promising N, P Co-doped Porous Carbon Materials as Metal-Free Electrocatalyst for Oxygen Reduction Reaction in Alkaline Medium. ChemistrySelect 2022, 7, e202200570. [Google Scholar] [CrossRef]
- Al Bostami, R.D.; Al Othman, A.; Tawalbeh, M.; Olabi, A.G. Advancements in zinc-air battery technology and water-splitting. Energy Nexus 2025, 17, 10038. [Google Scholar] [CrossRef]
- Yan, W.; Tang, Q.; Liu, L.; Zhang, Y.; Shi, L.; Chen, W.; Chen, Y. Intramolecular electron transfer for optimized coordination environment in bimetallic molecular electrocatalysts for flexible zinc-air battery applications. J. Energy Storage 2025, 118, 116335. [Google Scholar] [CrossRef]
- Ali, Y.; Dwivedi, S.; Alivand, M.S.; Sanjid, A.; Tanksale, A.; Chakraborty Banerjee, P. Highly stable and porous triple perovskite oxide as a bifunctional electrocatalyst for rechargeable Zn-air batteries. Chem. Eng. J. 2025, 516, 163928. [Google Scholar] [CrossRef]
- Shu, Y.; Takada, R.; Taniguchi, Y.; Yang, X.; Miyake, K.; Uchida, Y.; Nishiyama, N. Facile Synthesis of N-Doped Metal-Free Catalysts for Oxygen Reduction Reaction via a Self-Sacrificed Template Method Using Zinc Amino-Acid Complex. ACS Omega 2023, 8, 46276–46283. [Google Scholar] [CrossRef]
- Liu, F.; Niu, J.; Chuan, X.; Zhao, Y. Nitrogen and phosphorus Co-doped porous carbon: Dopant, synthesis, performance enhancement mechanism and versatile applications. J. Power Sources 2024, 601, 234308. [Google Scholar] [CrossRef]
- Sun, T.; Li, T.; Han, D.; Liu, L.; Wang, H.-G. N, P-doped carbon nanotubes encapsulated with Co2P nanoparticles as efficient bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. J. Electroanal. Chem. 2023, 928, 117016. [Google Scholar] [CrossRef]
- Hu, C.; Liang, Q.; Yang, Y.; Peng, Q.; Luo, Z.; Dong, J.; Isimjan, T.T.; Yang, X. Conductivity-enhanced porous N/P co-doped metal-free carbon significantly enhances oxygen reduction kinetics for aqueous/flexible zinc-air batteries. J. Colloid Interface Sci. 2023, 633, 500–510. [Google Scholar] [CrossRef]
- Han, L.; Cui, X.; Liu, Y.; Han, G.; Wu, X.; Xu, C.; Li, B. Nitrogen and phosphorus modification to enhance the catalytic activity of biomass-derived carbon toward the oxygen reduction reaction. Sustain. Energy Fuels 2020, 4, 2707–2717. [Google Scholar] [CrossRef]
- Takada, R.; Shu, Y.; Taniguchi, Y.; Yang, X.; Miyake, K.; Uchida, Y.; Nishiyama, N. Facile synthesis of carbon co-doped with nitrogen and phosphorus as metal-free electrocatalyst with precisely controlled pore structure and dual heteroatoms for oxygen reduction reaction. Carbon 2024, 218, 118719. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, K.; Ji, X.; Chen, D.; Han, C.; Li, T.-T.; Hu, Y.; Huang, S.; Qian, J. MOF-derived three-dimensional ordered porous carbon nanomaterial for efficient alkaline zinc-air batteries. Sci. China Mater. 2022, 65, 1453–1462. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous Carbons: Structure-Oriented Design and Versatile Applications. Adv. Funct. Mater. 2020, 30, 1909625. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, L.; Ma, J.; Wang, Y.; Dai, L.; Zhang, J. Edge-doping modulation of N, P-codoped porous carbon spheres for high-performance rechargeable Zn-air batteries. Nano Energy 2019, 60, 536–544. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, X.; Liu, S.; Wang, A.; Liu, Y.; Zhou, W.; Sun, K.; Li, S.; Zhou, J.; Li, B.; et al. Biomass-Derived Catalytically Active Carbon Materials for the Air Electrode of Zn-Air Batteries. ChemSusChem 2024, 17, e202301779. [Google Scholar] [CrossRef]
- Guo, B.; Ma, R.; Li, Z.; Guo, S.; Luo, J.; Yang, M.; Liu, Q.; Thomas, T.; Wang, J. Hierarchical N-Doped Porous Carbons for Zn–Air Batteries and Supercapacitors. Nano-Micro Lett. 2020, 12, 20. [Google Scholar] [CrossRef]
- Guo, Z.; Han, X.; Zhang, C.; He, S.; Liu, K.; Hu, J.; Yang, W.; Jian, S.; Jiang, S.; Duan, G. Activation of biomass-derived porous carbon for supercapacitors: A review. Chin. Chem. Lett. 2024, 35, 109007. [Google Scholar] [CrossRef]
- Liu, H.; Wang, B.; Bian, Y.; Wang, Y.; Huang, X.; Hu, Z.; Zhang, Z. Single-atom Fe catalysts with pyrrolic-type FeN4 sites for efficient oxygen reduction reaction: Identifying the roles of different N species. J. Power Sources 2024, 608, 234659. [Google Scholar] [CrossRef]
- Hu, X.; Zhong, G.; Li, J.; Liu, Y.; Yuan, J.; Chen, J.; Zhan, H.; Wen, Z. Hierarchical porous carbon nanofibers for compatible anode and cathode of potassium-ion hybrid capacitor. Energy Environ. Sci. 2020, 13, 2431–2440. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Zhu, Z.; Li, X.; Zhu, Z.; Yuan, T.; Yang, J.; Pang, Y.; Zheng, S. Vacancy-defective nano-carbon matrix enables highly efficient Fe single atom catalyst for aqueous and flexible Zn-Air batteries. Chem. Eng. J. 2024, 496, 153669. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, Q.; Zhang, L.; Yang, H.; Chen, Y.; Guo, J.; Zhang, M.; Zhang, Z.; Jiang, Y. Research progress of amorphous catalysts in the field of electrocatalysis. Microstructures 2024, 4, 2024022. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Zhang, S.; Jian, H.; Wang, M.; Lu, T. Stabilization of MOF-derived Co3S4 nanoparticles via graphdiyne coating for efficient oxygen evolution. Sci. China Mater. 2024, 67, 1882–1890. [Google Scholar] [CrossRef]
- Wang, Q.; Lyu, L.; Hu, X.; Fan, W.; Shang, C.; Huang, Q.; Li, Z.; Zhou, Z.; Kang, Y.M. Tailoring the Surface Curvature of the Supporting Carbon to Tune the d-Band Center of Fe−N−C Single-Atom Catalysts for Zinc-Urea-Air Batteries. Angew. Chem. Int. Ed. 2025, 64, e202422920. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, F.; Long, X.; Yu, X.; Qu, K.; Yang, Z. N, P doped carbon nanotubes confined WN-Ni Mott-Schottky heterogeneous electrocatalyst for water splitting and rechargeable zinc-air batteries. Appl. Catal. B Environ. 2021, 298, 120511. [Google Scholar] [CrossRef]
- Rawal, S.; Kumar, Y.; Mandal, U.K.; Kumar, A.; Tanwar, R.; Joshi, B. Synthesis and electrochemical study of phosphorus-doped porous carbon for supercapacitor applications. SN Appl. Sci. 2021, 3, 141. [Google Scholar] [CrossRef]
- Gao, S.; Li, M.; Li, N.; Zhang, L.; Liu, Q.; Wang, X.; Hu, G. Porous carbon-nanostructured electrocatalysts for zinc–air batteries: From materials design to applications. Nanoscale Adv. 2025, 7, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wan, K.; Ming, P.W.; Li, B.; Zhang, C. Advanced and Stable Metal-Free Electrocatalyst for Energy Storage and Conversion: The Structure–Effect Relationship of Heteroatoms in Carbon. ACS Omega 2023, 8, 16364–16372. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Mehdi, S.; Wu, X.; Liu, T.; Zhou, B.; Zhang, P.; Jiang, J.; Li, B. Surface Phosphorus-Induced CoO Coupling to Monolithic Carbon for Efficient Air Electrode of Quasi-Solid-State Zn–Air Batteries. Adv. Sci. 2021, 8, 2101314. [Google Scholar] [CrossRef]
- Ding, M.; Shi, W.; Guo, L.; Leong, Z.Y.; Baji, A.; Yang, H.Y. Bimetallic metal–organic framework derived porous carbon nanostructures for high performance membrane capacitive desalination. J. Mater. Chem. A 2017, 5, 6113–6121. [Google Scholar] [CrossRef]
- Abouelamaiem, D.I.; He, G.; Neville, T.P.; Patel, D.; Ji, S.; Wang, R.; Parkin, I.P.; Jorge, A.B.; Titirici, M.-M.; Shearing, P.R.; et al. Correlating electrochemical impedance with hierarchical structure for porous carbon-based supercapacitors using a truncated transmission line model. Electrochim. Acta 2018, 284, 597–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).