Effect of Sulfur Poisoning During Worldwide Harmonized Light Vehicles Test Cycle on NOx Reduction Performance and Active Sites of Selective Catalytic Reduction Filter
Abstract
1. Introduction
2. Results
2.1. Research on Sulfur Poisoning and Regeneration Performance
2.2. Characterization Analysis
2.2.1. Sulfur Content Analysis
2.2.2. BET
2.2.3. XRD
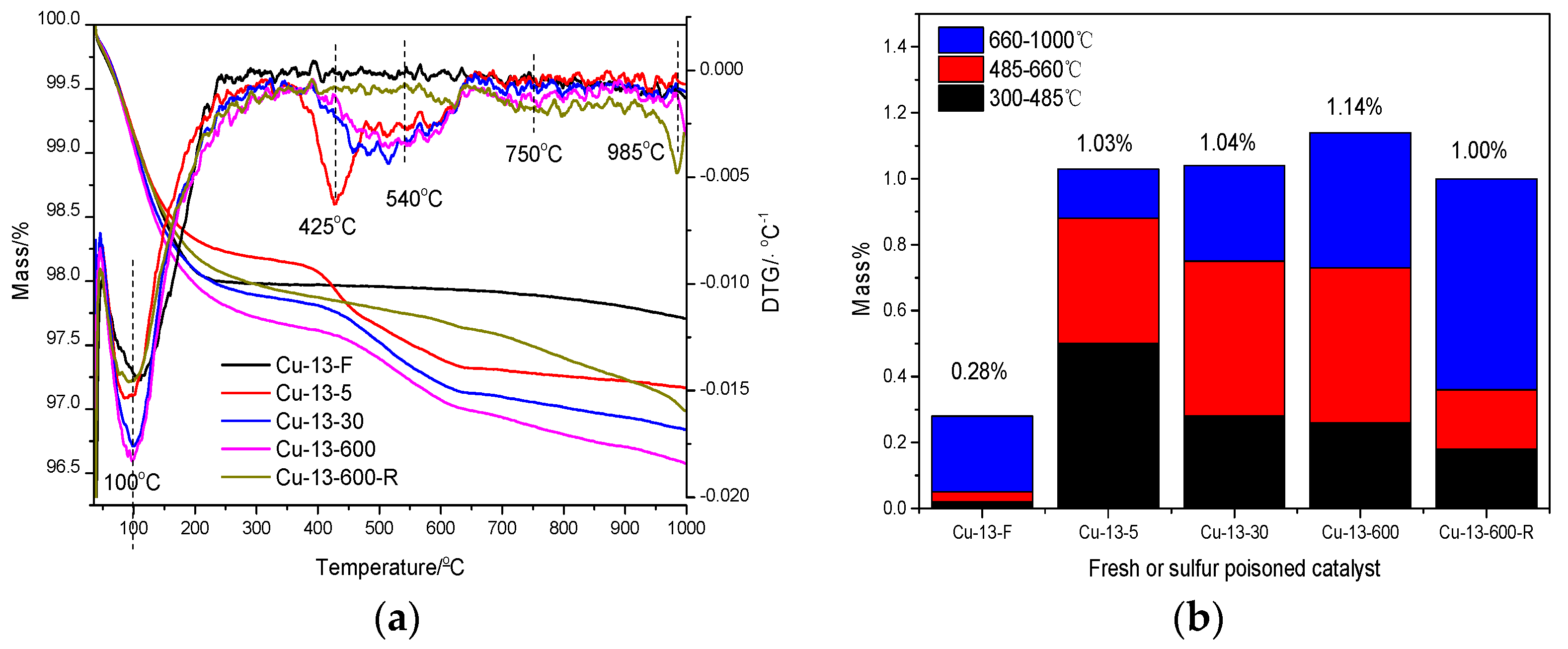
2.2.4. TG
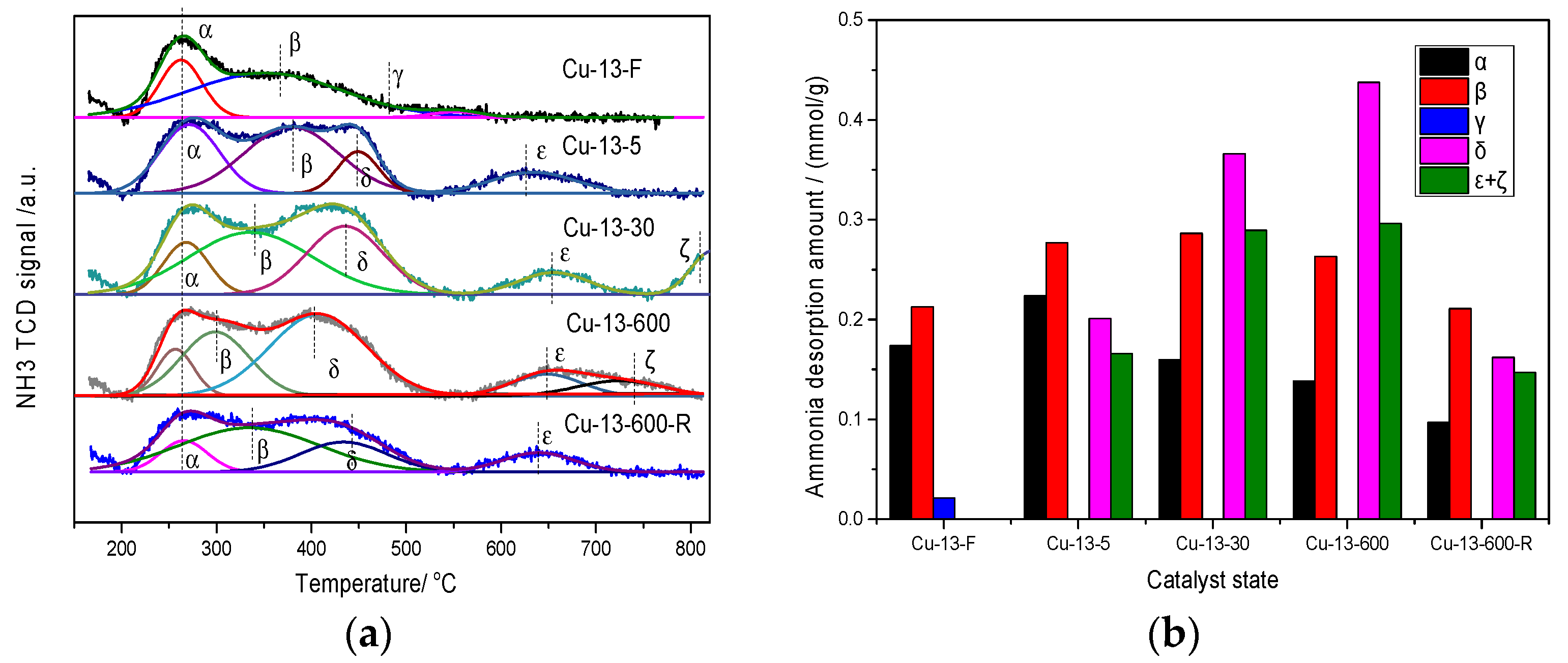
2.2.5. TPD
TPD
NH3-TPD
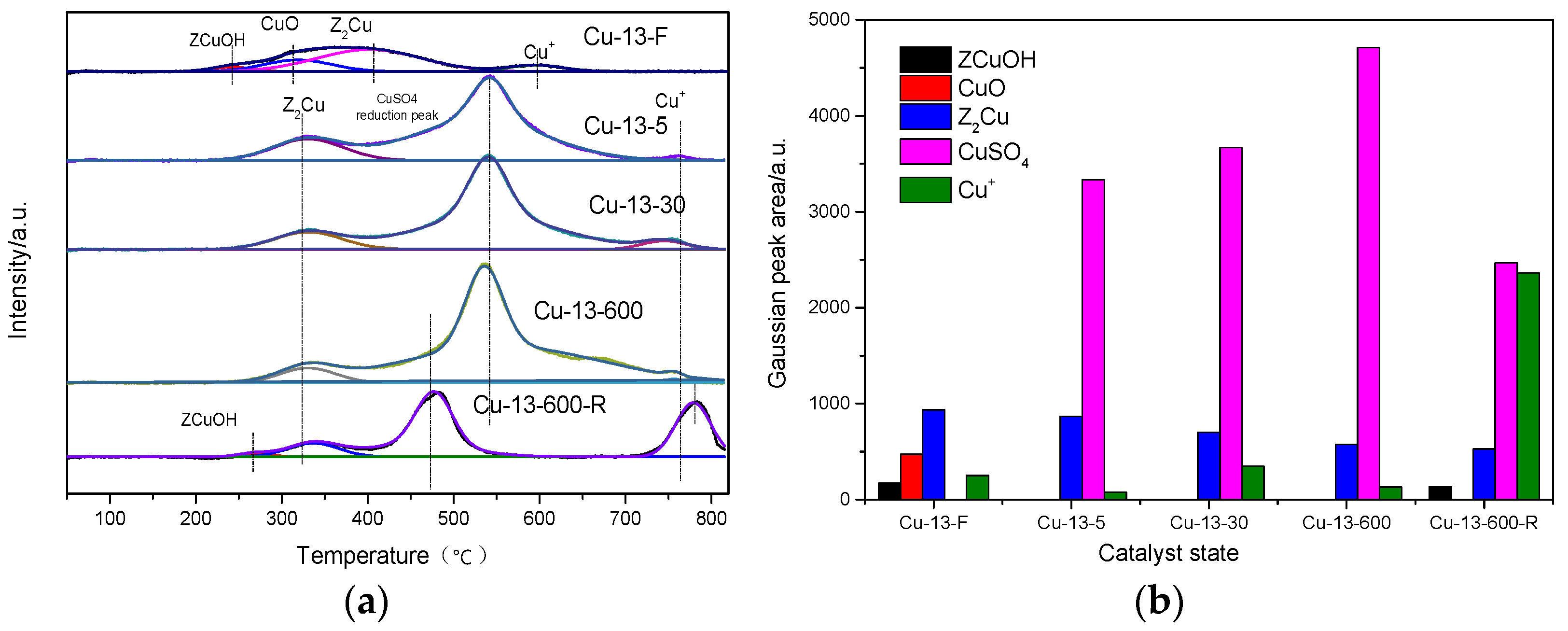
2.2.6. H2-TPR
2.2.7. UV–Vis Spectra
2.2.8. XPS
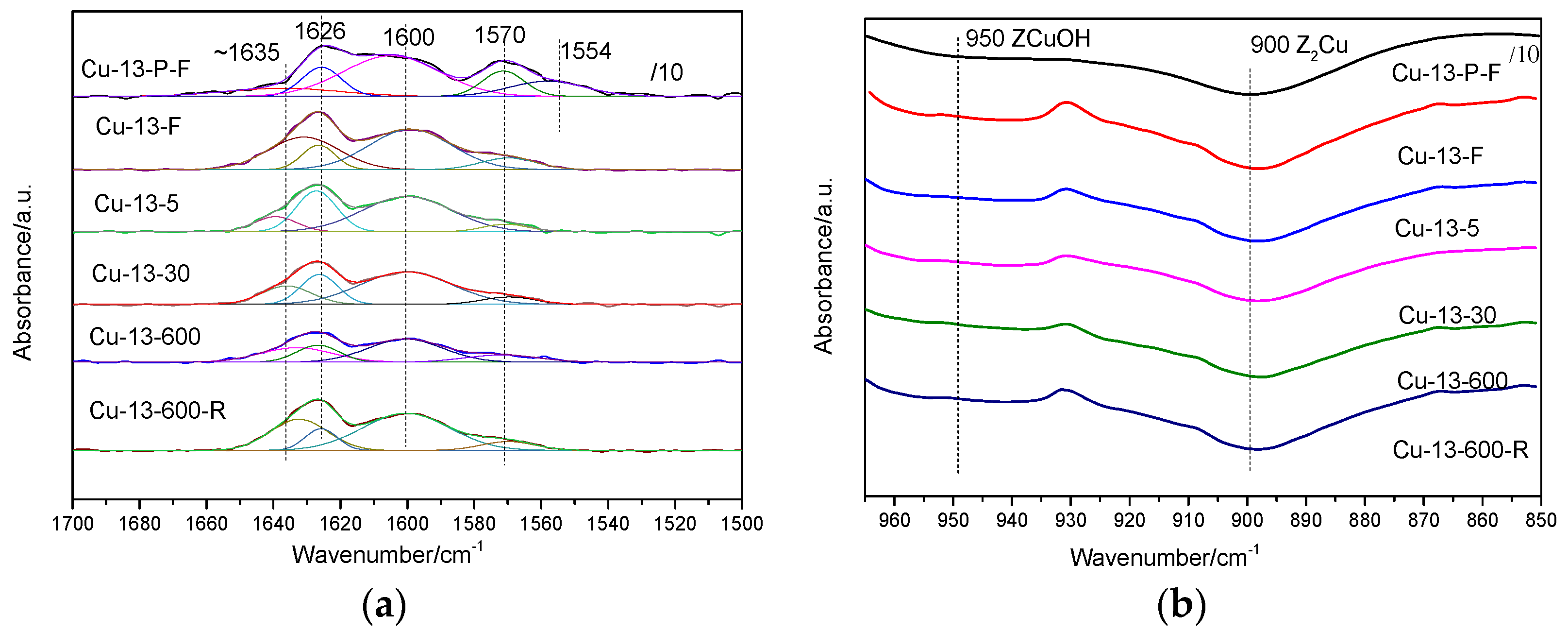
2.2.9. In Situ DRIFTS
NO + O2 Adsorption
NH3 + O2 Adsorption
2.3. Possible Sulfur Poisoning Reaction Pathway
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Sulfur Poisoning During WLTC and Emission Testing
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Ma, J.; Chang, S.; Yu, F.; Lai, H.; Zhao, Y. Research Progress on Sulfur Deactivation and Regeneration over Cu-CHA Zeolite Catalyst. Catalysts 2022, 12, 1499. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.-S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Wang, Q.; Ning, F.; He, Q.; Li, G.; Liu, C.; Li, Z.; Peng, H. Effects of Cu species in Cu-SSZ-13 zeolites on the performance of NOx reduction reactions. Sep. Purif. Technol. 2024, 346, 127544. [Google Scholar] [CrossRef]
- Ding, W.; Sun, Y.; Liu, J.; Xue, S.; Han, X.; Yan, Z.; Yu, Y.; Shan, Y.; He, H. Mechanism of propylene effect on the NH3-SCR performance of Cu-SSZ-13 catalyst. Chem. Eng. J. 2024, 483, 149272. [Google Scholar] [CrossRef]
- Wang, A.; Olsson, L. Insight into the SO2 poisoning mechanism for NOx removal by NH3-SCR over Cu/LTA and Cu/SSZ-13. Chem. Eng. J. 2020, 395, 125048. [Google Scholar] [CrossRef]
- Cheng, Y.; Lambert, C.; Kim, D.H.; Kwak, J.H.; Cho, S.J.; Peden, C.H.F. The different impacts of SO2 and SO3 on Cu/zeolite SCR catalysts. Catal. Today 2010, 151, 266–270. [Google Scholar] [CrossRef]
- Li, P.; Yu, F.; Zhu, M.; Tang, C.; Dai, B.; Dong, L. Selective catalytic reduction De-NOx catalysts. Prog. Chem. 2016, 28, 1578–1590. [Google Scholar]
- Su, W.; Li, Z.; Zhang, Y.; Meng, C.; Li, J. Identification of sulfate species and their influence on SCR performance of Cu/CHA catalyst. Catal. Sci. Technol. 2017, 7, 1523–1528. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jangjou, Y.; Epling, W.S.; Jensen, A.D.; Janssens, T.V. Reversible and irreversible deactivation of Cu-CHA NH3-SCRcatalysts by SO2 and SO3. Appl. Catal. B Environ. 2018, 226, 38–45. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Wang, J.; Wang, C.; Wang, J. Nature of SO3 poisoning on Cu/SAPO-34 SCR catalysts. J. Catal. 2018, 358, 277–286. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Molokova, A.Y.; Borfecchia, E.; Martini, A.; Pankin, I.A.; Atzori, C.; Mathon, O.; Bordiga, S.; Wen, F.; Vennestrm, P.N.R.; Berlier, G.; et al. SO2 Poisoning of Cu-CHA deNOx Catalyst: The Most Vulnerable Cu Species Identified by X-ray Absorption Spectroscopy. JACS Au 2022, 2, 787–792. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Ando, R.; Hihara, T.; Banno, Y.; Nagata, M.; Ishitsuka, T.; Matsubayashi, N.; Tomie, T. Detailed Mechanism of S Poisoning and De-Sulfation Treatment of Cu-SCR Catalyst. In Proceedings of the WCX TM 17 SAE World Congress Experience, Detroit, MI, USA, 3–5 April 2017. [Google Scholar]
- Cheng, Y.; Montreuil, C.; Cavataio, G.; Lambert, C. The effects of SO2 and SO3 poisoning on Cu/zeolite SCR catalysts. In Proceedings of the SAE World Congress & Exhibition, Detroit, MI, USA, 20–23 April 2009. [Google Scholar]
- Mandal, K.; Rani, P.; Chen, Y.-R.; Wijerathne, A.; Nam, K.; Meena, K.; Kiani, D.A.; Daya, R.; Epling, W.S.; Paolucci, C. Impact of sulfur exposure on high-temperature Cu speciation in SSZ-13 Zeolites. Appl. Catal. B Environ. Energy 2024, 358, 124361. [Google Scholar] [CrossRef]
- Shan, Y.; Shi, X.; Yan, Z.; Liu, J.; Yu, Y.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2019, 320, 84–90. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, B.; Peng, X.; Wei, Y.; Zhan, R.; Lin, H.; Huang, Z. Effect of sulfur poisoning on the performance and active sites of Cu/SSZ-13 catalyst. Chem. Eng. Sci. 2020, 226, 115855. [Google Scholar] [CrossRef]
- Abasabadi, R.K.; Janssens, T.V.W.; Bordiga, S.; Berlier, G. Probing the effect of the Si/Al ratio in Cu-CHA zeolite catalysts on SO2 exposure: In situ DR UV-vis spectroscopy and deactivation measurements. Catal. Sci. Technol. 2024, 14, 3076–3085. [Google Scholar] [CrossRef]
- Shen, M.; Li, X.; Wang, J.; Wang, C.; Wang, J. Nature Identification of Cu Active Sites in Sulfur-Fouled Cu/SAPO-34 Regeneration. Ind. Eng. Chem. Res. 2018, 57, 3501–3509. [Google Scholar] [CrossRef]
- Raja, S.; Alphin, M.; Sivachandiran, L.; Singh, P.; Damma, D.; Smirniotis, P.G. TiO2-carbon nanotubes composite supported MnOx-CuO catalyst for low-temperature NH3-SCR of NO: Investigation of SO2 and H2O tolerance. Fuel 2022, 307, 121886. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Brookshear, D.W.; Nam, J.-G.; Nguyen, K.; Toops, T.J.; Binder, A. Impact of sulfation and desulfation on NOx reduction using Cu-chabazite SCR catalysts. Catal. Today 2015, 258, 359–366. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Zhang, X.; Wu, M.; Qu, H. Construction of multifunctional interface engineering on Cu-SSZ-13@Ce-MnOx/Mesoporous-silica catalyst for boosting activity, SO2 tolerance and hydrothermal stability. J. Hazard. Mater. 2024, 477, 135268. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Chen, H.; Zhao, H.; Wei, M.; Zhao, Y.; Li, Y. Insight into SO2 poisoning and regeneration of one-pot synthesized Cu-SSZ-13 catalyst for selective reduction of NO by NH3. Chin. J. Chem. Eng. 2022, 46, 184–193. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, Z.; Huang, S.; Zhang, W. Cu-SSZ-13 zeolite–metal oxide hybrid catalysts with enhanced SO2-tolerance in the NH3-SCR of NOx. Appl. Catal. B Environ. 2020, 269, 118825. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Z.; Li, X.; Wang, J.; Wang, J.; Wang, C.; Wang, J. Effects of regeneration conditions on sulfated CuSSZ-13 catalyst for NH3-SCR. Korean J. Chem. Eng. 2019, 36, 1249–1257. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, J.; Liu, Z.; Wu, X.; Wei, Y.; Guo, J.; Jiang, H.; Lin, H.; Huang, Z. Degenerating effect of transformation and loss of active sites on NH3-SCR activity during the hydrothermal aging process for Cu-SSZ-13 molecular sieve catalyst. Chem. Eng. Sci. 2023, 269. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Y.; Zhang, Z.; Zhang, C.; Fu, G.; Yi, X.; Huang, Q.; Yang, F.; Liang, W.; Zheng, A.; et al. Remarkable performance of selective catalytic reduction of NOx by ammonia over copper-exchanged SSZ-52 catalysts. Appl. Catal. B Environ. 2021, 283. [Google Scholar] [CrossRef]
- Shen, Y.; Li, T.; Yang, J.; Wang, A.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. Enhanced low-temperature performance of Al-rich Cu-SSZ-13 by Ce modification upon hydrothermal aging for NH3-SCR. Chem. Eng. J. 2023, 473, 145275. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Kartheuser, B.; Hodnett, B. Overview of the reactivity of copper-on-alumina for the oxidation and sorption of SO2 with simultaneous reduction of NO by NH3 and effect of the modification with a V/TiO2 component. Catal. Today 1993, 17, 103–110. [Google Scholar] [CrossRef]
- Shin, C.S.; Niiyama, H. Oxidative sorption of SO2 by Cu/zeolite. J. Jpn. Petrol. Inst. 1988, 31, 147–153. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Wey, M.-Y. Study of SO2 adsorption and thermal regeneration over activated carbon-supported copper oxide catalysts. Carbon 2004, 42, 2269–2278. [Google Scholar] [CrossRef]
- Jaeckel, B.; Hunger, R.; Webb, L.J.; Jaegermann, W.; Lewis, N.S. High-Resolution Synchrotron Photoemission Studies of the Electronic Structure and Thermal Stability of CH3- and C2H5-Functionalized Si(111) Surfaces. J. Phys. Chem. C 2007, 49, 18204–18213. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, J.; Liang, Y.; Zhang, H.; Zhou, Y.; Shan, R.; Liu, Q.; Liu, W.; Yao, L. In situ DRIFTS and DFT studies of SO2 poisoning over Cu-exchanged X zeolite catalyst for NH3-SCR. J. Environ. Chem. Eng. 2023, 11, 109822. [Google Scholar] [CrossRef]
- Castoldi, L.; Bonzi, R.; Lietti, L.; Forzatti, P.; Morandi, S.; Ghiotti, G.; Dzwigaj, S. Catalytic behaviour of hybrid LNT/SCR systems: Reactivity and in situ FTIR study. J. Catal. 2011, 282, 128–144. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Nova, I.; Tronconi, E.; Pihl, J.A.; Toops, T.J.; Partridge, W.P. In-situ DRIFTS measurements for the mechanistic study of NO oxidation over a commercial Cu-CHA catalyst. Appl. Catal. B Environ. 2015, 166–167, 181–192. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Yu, F.; Yang, D.; Chang, S.; He, X.; Zhao, Y.; Ma, J.; Chen, T.; Lai, H.; Lin, H. Effect of Sulfur Poisoning During Worldwide Harmonized Light Vehicles Test Cycle on NOx Reduction Performance and Active Sites of Selective Catalytic Reduction Filter. Catalysts 2025, 15, 682. https://doi.org/10.3390/catal15070682
Zhou Z, Yu F, Yang D, Chang S, He X, Zhao Y, Ma J, Chen T, Lai H, Lin H. Effect of Sulfur Poisoning During Worldwide Harmonized Light Vehicles Test Cycle on NOx Reduction Performance and Active Sites of Selective Catalytic Reduction Filter. Catalysts. 2025; 15(7):682. https://doi.org/10.3390/catal15070682
Chicago/Turabian StyleZhou, Zhou, Fei Yu, Dongxia Yang, Shiying Chang, Xiaokun He, Yunkun Zhao, Jiangli Ma, Ting Chen, Huilong Lai, and He Lin. 2025. "Effect of Sulfur Poisoning During Worldwide Harmonized Light Vehicles Test Cycle on NOx Reduction Performance and Active Sites of Selective Catalytic Reduction Filter" Catalysts 15, no. 7: 682. https://doi.org/10.3390/catal15070682
APA StyleZhou, Z., Yu, F., Yang, D., Chang, S., He, X., Zhao, Y., Ma, J., Chen, T., Lai, H., & Lin, H. (2025). Effect of Sulfur Poisoning During Worldwide Harmonized Light Vehicles Test Cycle on NOx Reduction Performance and Active Sites of Selective Catalytic Reduction Filter. Catalysts, 15(7), 682. https://doi.org/10.3390/catal15070682







