Enantioselective Iodination and Bromination for the Atroposelective Construction of Axially Chiral Compounds
Abstract
1. Introduction
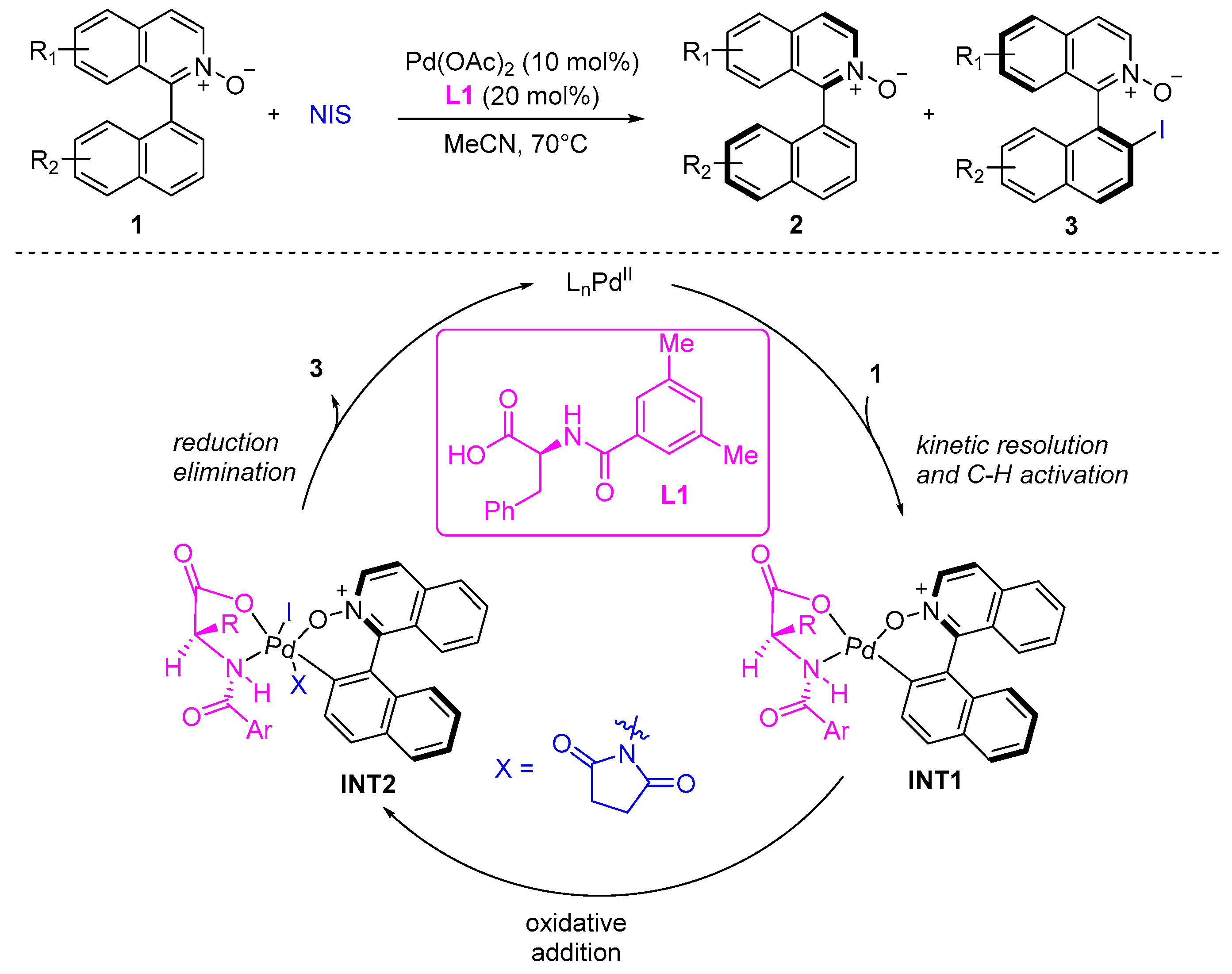
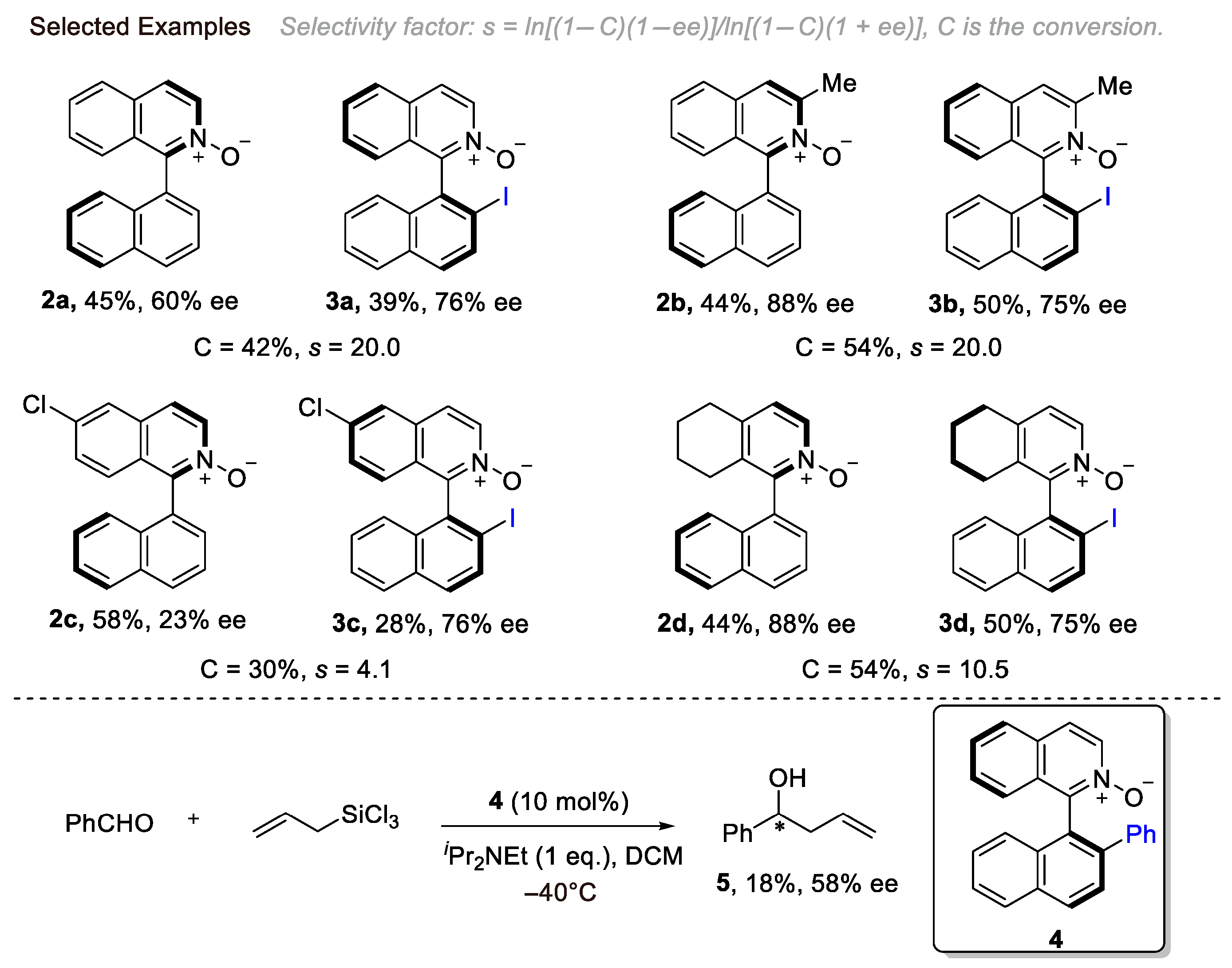
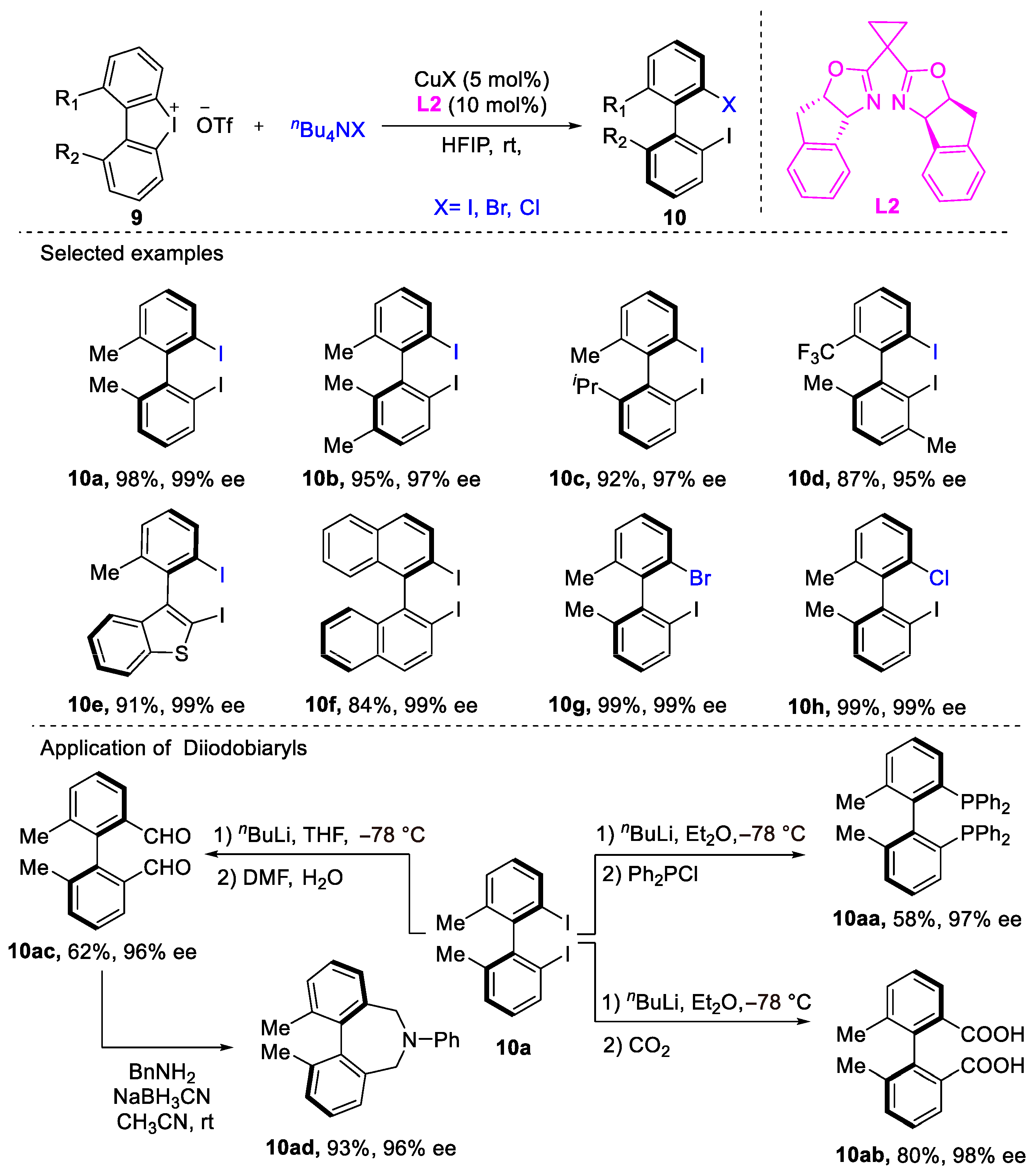
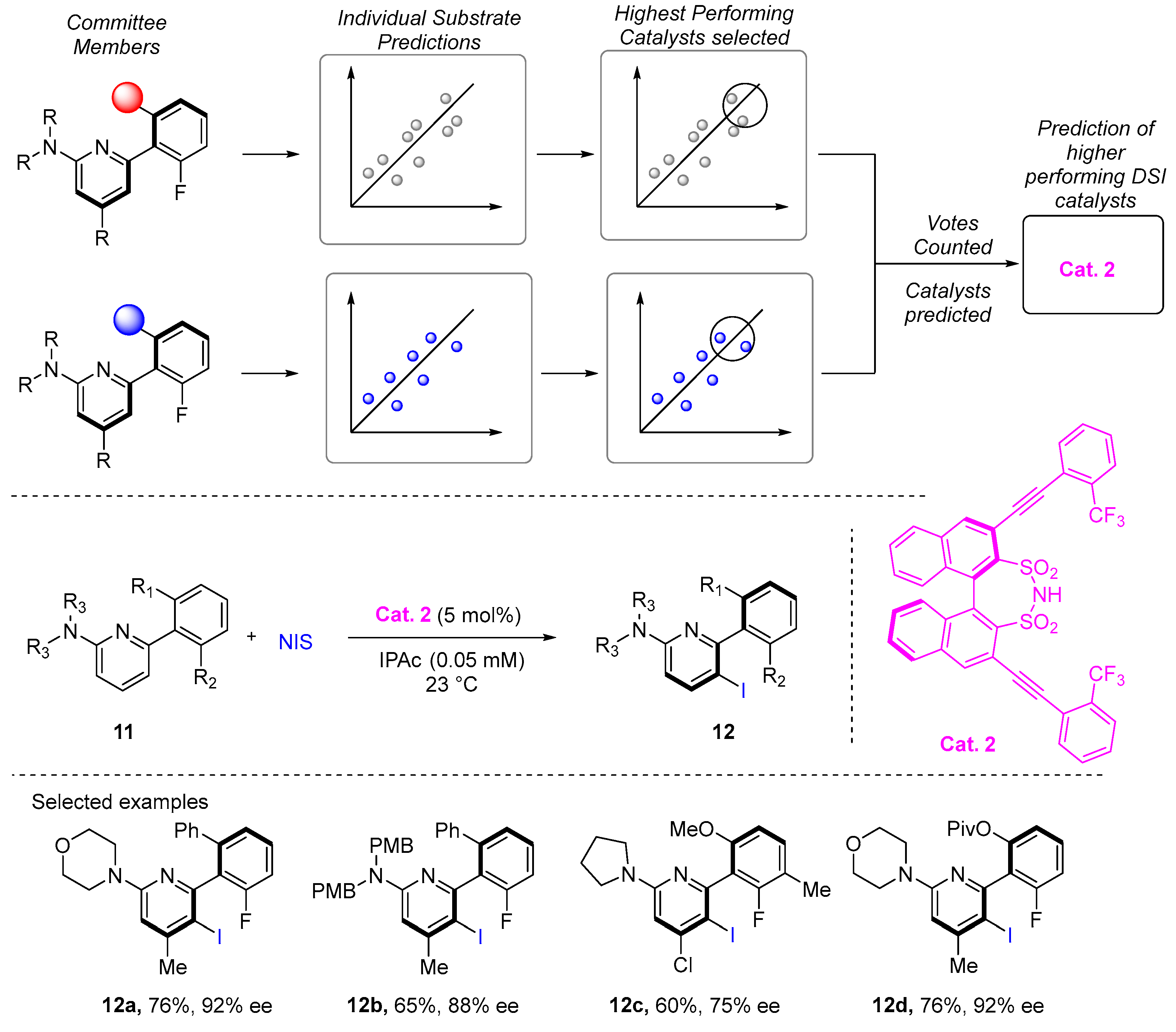
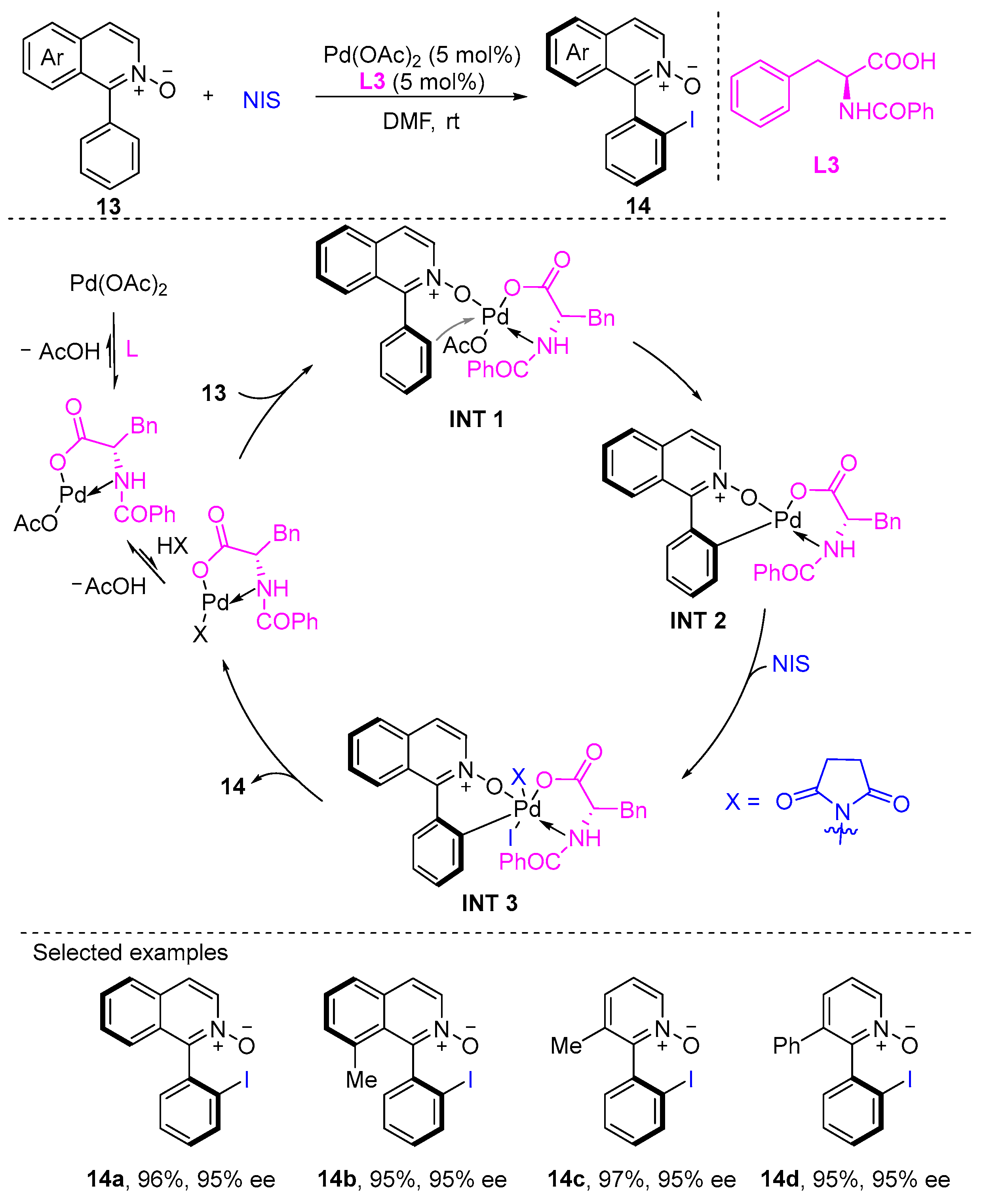
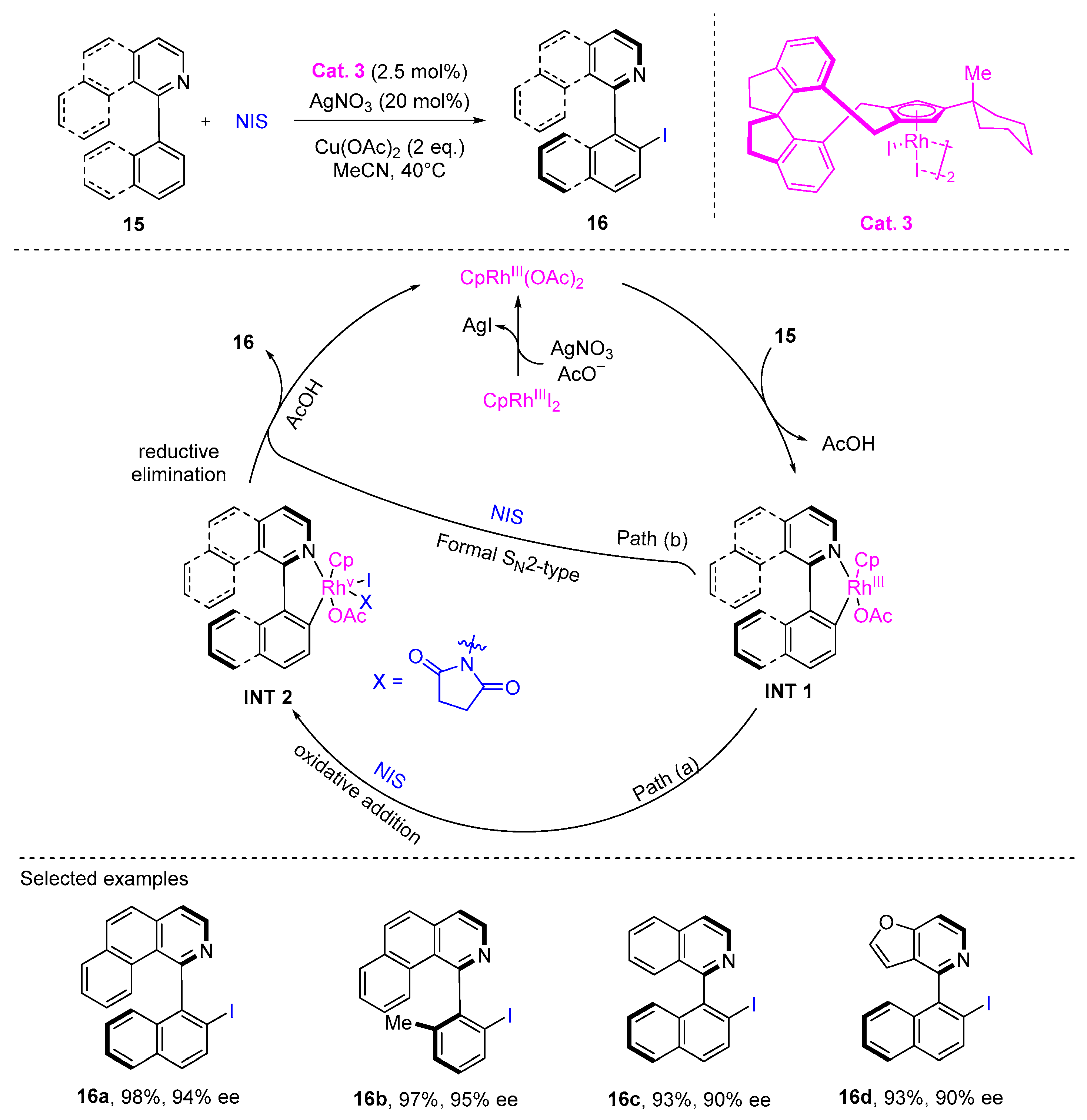
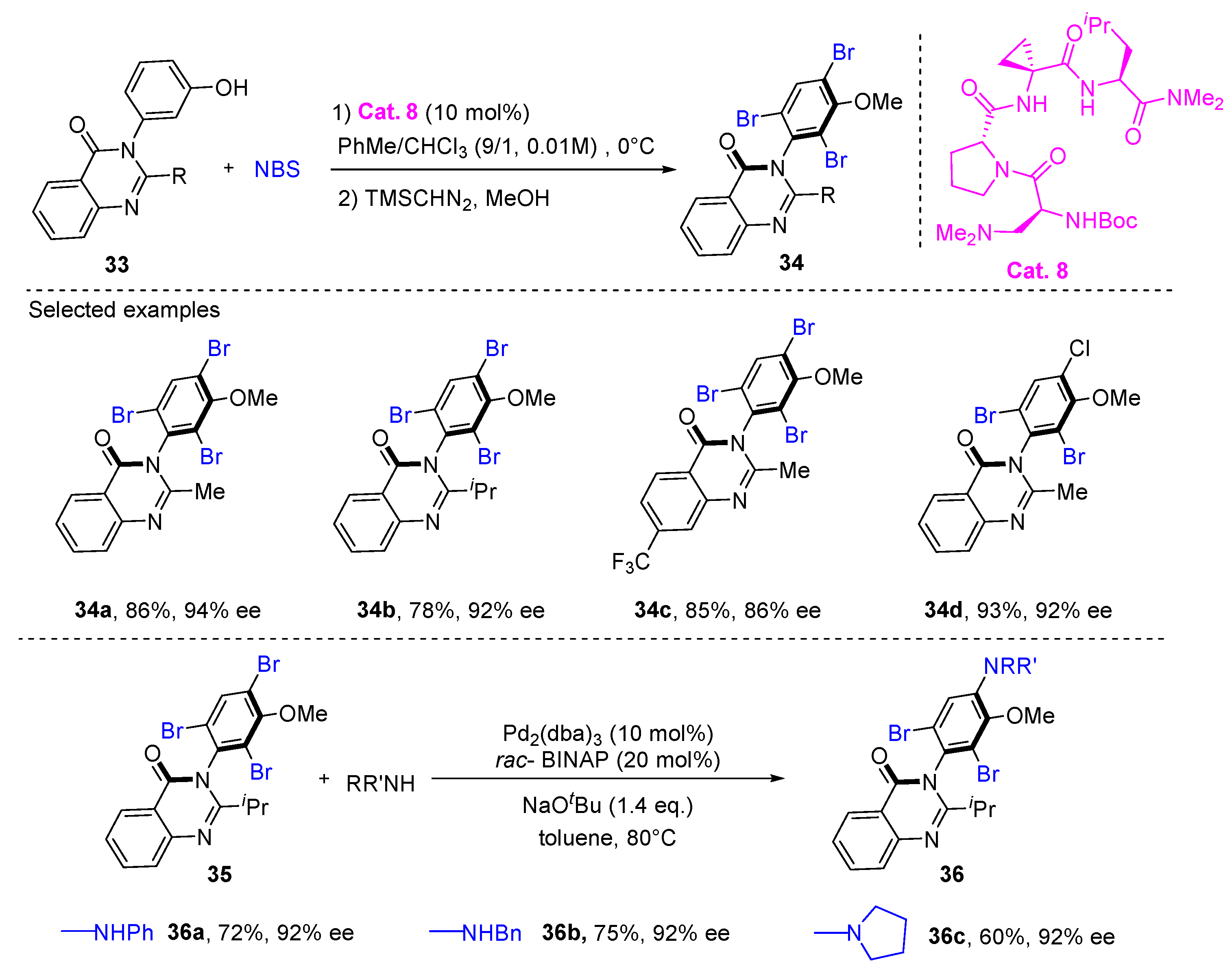
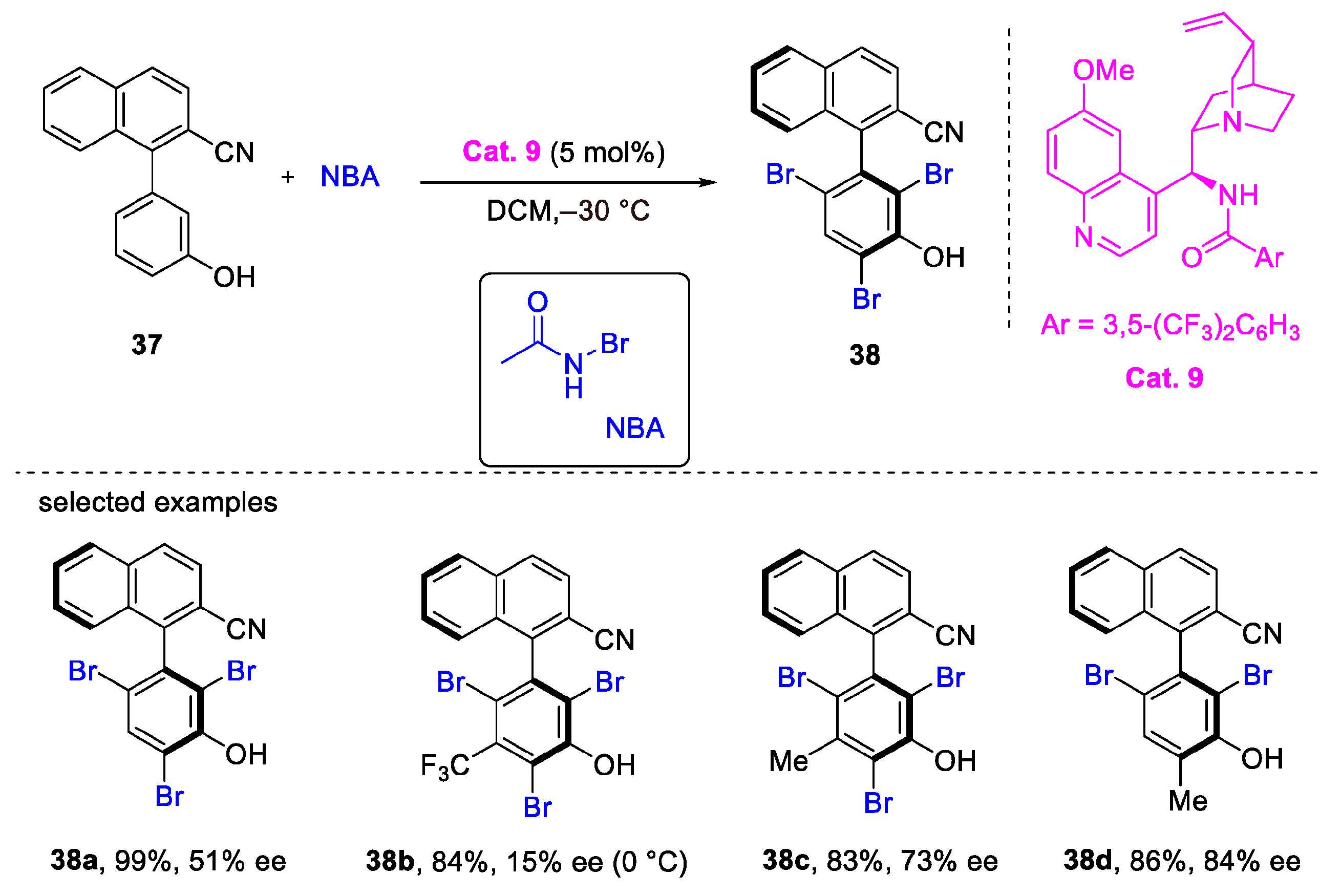
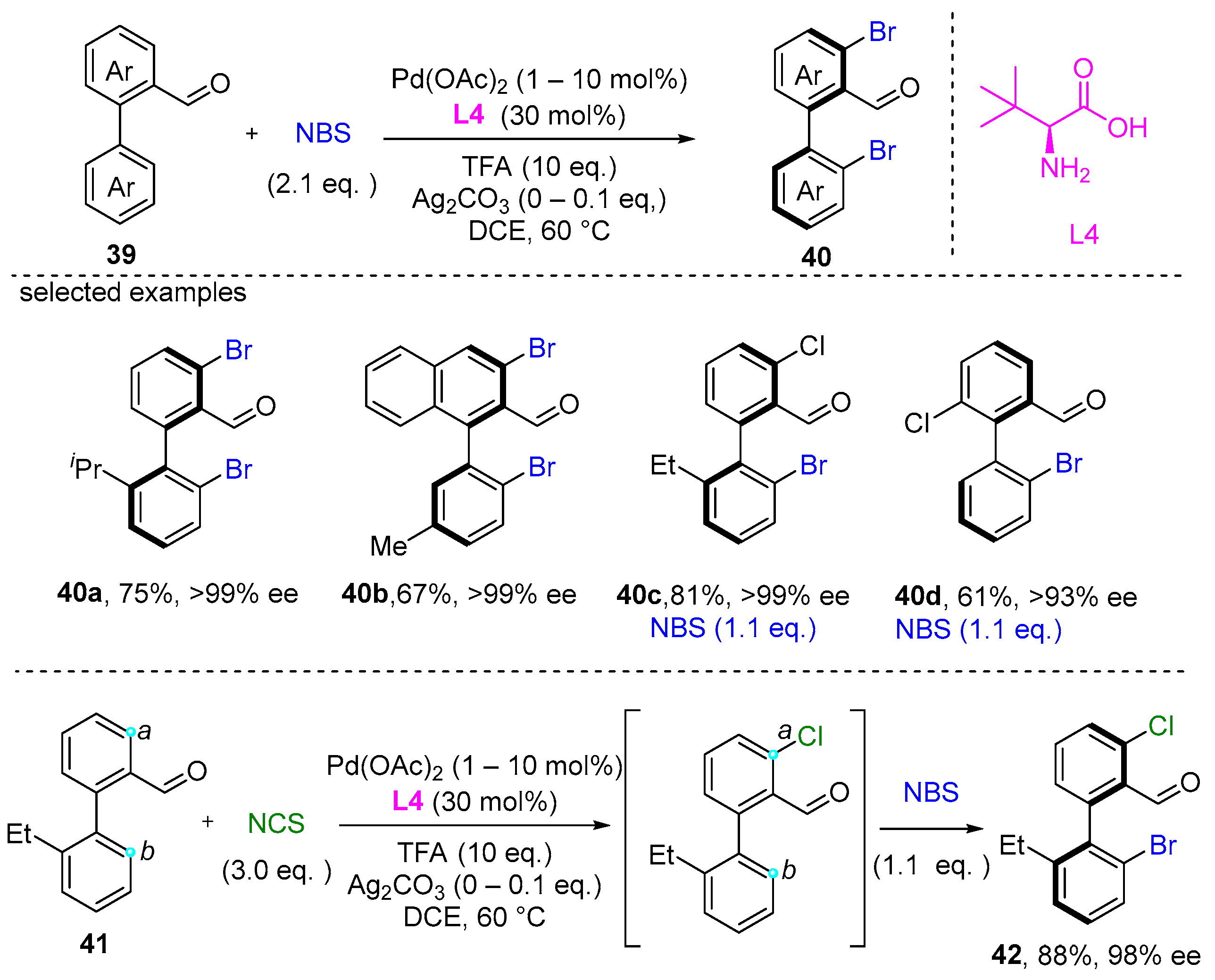
2. Atroposelective Iodination
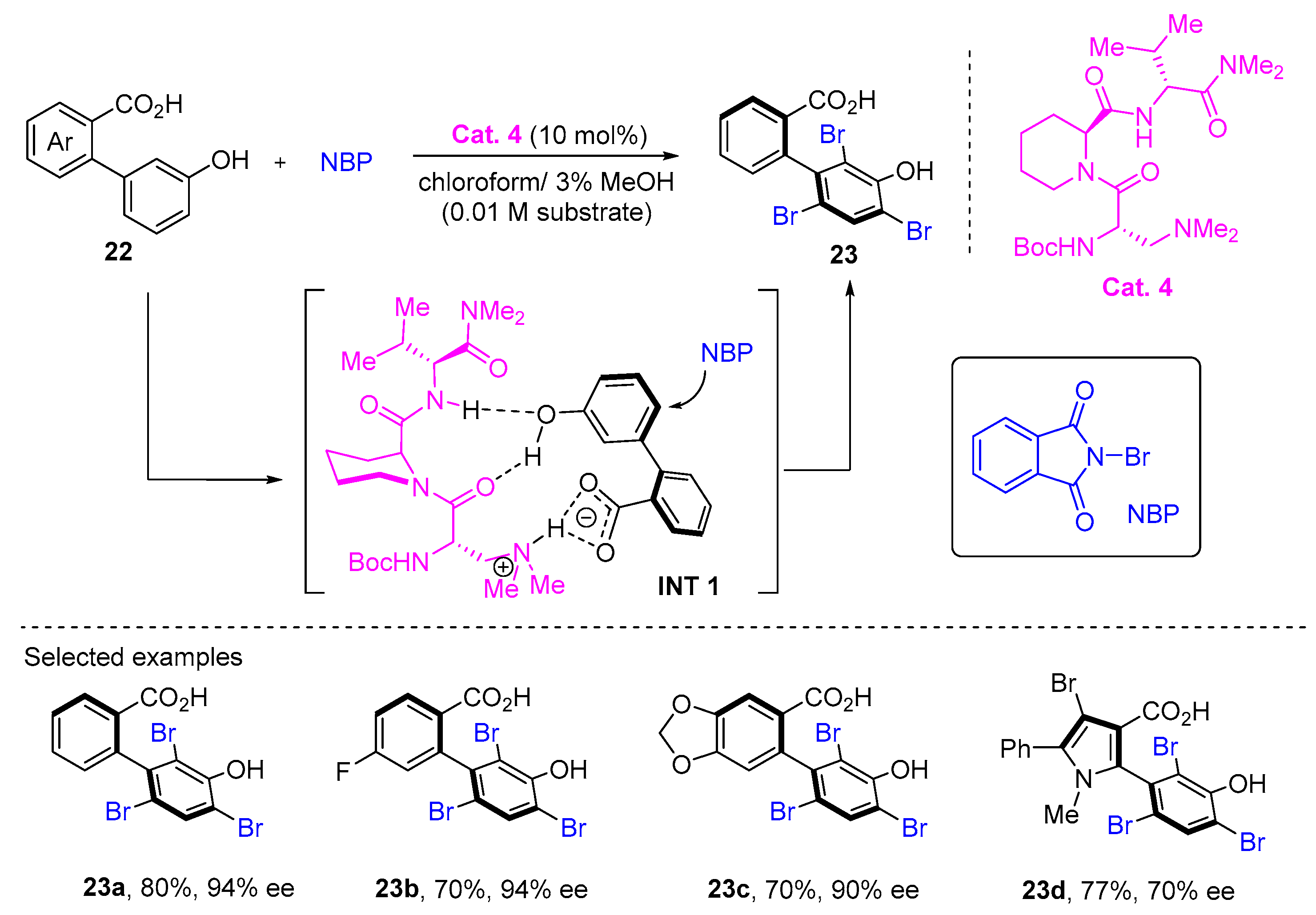
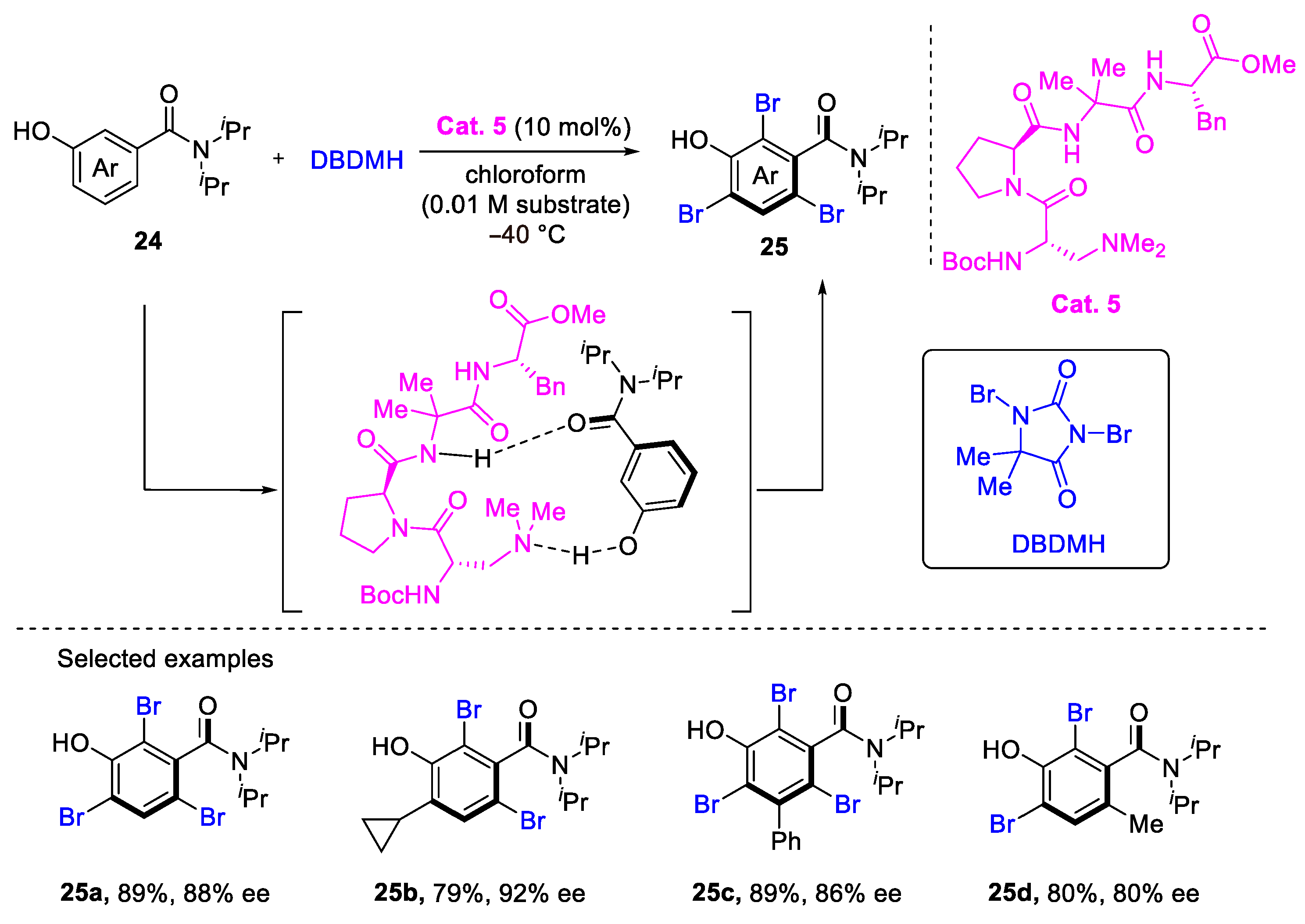
3. Atroposelective Bromination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Yekta, S.; Yudin, A.K. Modified BINOL Ligands in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3155–3212. [Google Scholar] [CrossRef]
- Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Modified BINAP: The How and the Why. Chem. Rev. 2005, 105, 1801–1836. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.; Moran, W.J.; Edwards, P.J.; LaPlante, S.R. The Challenge of Atropisomerism in Drug Discovery. Angew. Chem. Int. Ed. 2009, 48, 6398–6401. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Pu, L. Enantioselective Fluorescent Sensors: A Tale of BINOL. Acc. Chem. Res. 2012, 45, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef]
- Smyth, J.E.; Butler, N.M.; Keller, P.A. A twist of nature—The significance of atropisomers in biological systems. Nat. Prod. Rep. 2015, 32, 1562–1583. [Google Scholar] [CrossRef]
- Kumarasamy, E.; Raghunathan, R.; Sibi, M.P.; Sivaguru, J. Nonbiaryl and Heterobiaryl Atropisomers: Molecular Templates with Promise for Atroposelective Chemical Transformations. Chem. Rev. 2015, 115, 11239–11300. [Google Scholar] [CrossRef]
- Basilaia, M.; Chen, M.H.; Secka, J.; Gustafson, J.L. Atropisomerism in the Pharmaceutically Relevant Realm. Acc. Chem. Res. 2022, 55, 2904–2919. [Google Scholar] [CrossRef]
- Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. Synthesis of 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of.alpha.-(acylamino)acrylic acids. J. Am. Chem. Soc. 1980, 102, 7932–7934. [Google Scholar] [CrossRef]
- Bringmann, G.; Price Mortimer, A.J.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective Synthesis of Axially Chiral Biaryl Compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Panossian, A.; Leroux, F.R.; Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. [Google Scholar] [CrossRef] [PubMed]
- Loxq, P.; Manoury, E.; Poli, R.; Deydier, E.; Labande, A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. Coord. Chem. Rev. 2016, 308, 131–190. [Google Scholar] [CrossRef]
- Newton, C.G.; Wang, S.-G.; Oliveira, C.C.; Cramer, N. Catalytic Enantioselective Transformations Involving C–H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [Google Scholar] [CrossRef]
- Link, A.; Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 2018, 47, 3804–3815. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Tan, B. Construction of Axially Chiral Compounds via Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. [Google Scholar] [CrossRef]
- Metrano, A.J.; Miller, S.J. Peptide-Based Catalysts Reach the Outer Sphere through Remote Desymmetrization and Atroposelectivity. Acc. Chem. Res. 2019, 52, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhou, T.; Yao, Q.-J.; Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun. 2019, 55, 8514–8523. [Google Scholar] [CrossRef]
- Lassaletta, J.M. (Ed.) Atropisomerism and Axial Chirality; World Scientific: London, UK, 2019. [Google Scholar]
- Cheng, J.K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902. [Google Scholar] [CrossRef]
- Liu, C.-X.; Zhang, W.-W.; Yin, S.-Y.; Gu, Q.; You, S.-L. Synthesis of Atropisomers by Transition-Metal-Catalyzed Asymmetric C–H Functionalization Reactions. J. Am. Chem. Soc. 2021, 143, 14025–14040. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Shi, F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562–2580. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.-J.; Koay, W.L.; Guan, C.-Y.; Lu, Y. Atropisomers beyond the C−C axial chirality: Advances in catalytic asymmetric synthesis. Chem 2022, 8, 1855–1893. [Google Scholar] [CrossRef]
- Feng, J.; Lu, C.-J.; Liu, R.-R. Catalytic Asymmetric Synthesis of Atropisomers Featuring an Aza Axis. Acc. Chem. Res. 2023, 56, 2537–2554. [Google Scholar] [CrossRef]
- Wang, X.; Ren, W.; Zhang, J.; Zhao, S.; Zhou, D.; Chen, H.; Liu, T. Recent Advances in Palladium-Catalyzed Enantioselective Cyclization for the Construction of Atropisomers. Catalysts 2025, 15, 320. [Google Scholar] [CrossRef]
- Shirakawa, S.; Liu, K.; Maruoka, K. Catalytic Asymmetric Synthesis of Axially Chiral o-Iodoanilides by Phase-Transfer Catalyzed Alkylations. J. Am. Chem. Soc. 2012, 134, 916–919. [Google Scholar] [CrossRef]
- Li, S.-L.; Yang, C.; Wu, Q.; Zheng, H.-L.; Li, X.; Cheng, J.-P. Atroposelective Catalytic Asymmetric Allylic Alkylation Reaction for Axially Chiral Anilides with Achiral Morita–Baylis–Hillman Carbonates. J. Am. Chem. Soc. 2018, 140, 12836–12843. [Google Scholar] [CrossRef]
- Lu, S.; Ng, S.V.H.; Lovato, K.; Ong, J.-Y.; Poh, S.B.; Ng, X.Q.; Kürti, L.; Zhao, Y. Practical access to axially chiral sulfonamides and biaryl amino phenols via organocatalytic atroposelective N-alkylation. Nat. Commun. 2019, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Torabi Kohlbouni, S.; Borhan, B. Cu-Catalyzed Oxidation of C2 and C3 Alkyl-Substituted Indole via Acyl Nitroso Reagents. Org. Lett. 2019, 21, 14–17. [Google Scholar] [CrossRef]
- Yang, G.-H.; Zheng, H.; Li, X.; Cheng, J.-P. Asymmetric Synthesis of Axially Chiral Phosphamides via Atroposelective N-Allylic Alkylation. ACS Catal. 2020, 10, 2324–2333. [Google Scholar] [CrossRef]
- Zheng, G.; Li, X.; Cheng, J.-P. Access to Axially and Centrally Chiral Sulfinamides via Asymmetric Allylic Alkylation. Org. Lett. 2021, 23, 3997–4001. [Google Scholar] [CrossRef]
- Pan, M.; Shao, Y.-B.; Zhao, Q.; Li, X. Asymmetric Synthesis of N–N Axially Chiral Compounds by Phase-Transfer-Catalyzed Alkylations. Org. Lett. 2022, 24, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liou, Y.-C.; Oliveira, J.C.A.; Ackermann, L. Ruthenium(II)/Imidazolidine Carboxylic Acid-Catalyzed C−H Alkylation for Central and Axial Double Enantio-Induction. Angew. Chem. Int. Ed. 2022, 61, e202212595. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Qi, L.-W.; Fang, F.; Tan, B. Organocatalytic Atroposelective Arylation of 2-Naphthylamines as a Practical Approach to Axially Chiral Biaryl Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 16308–16312. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.G.; Braconi, E.; Kuziola, J.; Wodrich, M.D.; Cramer, N. Axially Chiral Dibenzazepinones by a Palladium(0)-Catalyzed Atropo-enantioselective C−H Arylation. Angew. Chem. Int. Ed. 2018, 57, 11040–11044. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cai, Z.-J.; Liu, C.-X.; Gu, Q.; You, S.-L. Rhodium-Catalyzed Atroposelective C–H Arylation: Efficient Synthesis of Axially Chiral Heterobiaryls. J. Am. Chem. Soc. 2019, 141, 9504–9510. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Deng, S.; Gao, Q.; Hua, Y.; Cheng, H.-G.; Qi, X.; Zhou, Q. Construction of Axially Chiral Biaryls via Atroposelective ortho-C–H Arylation of Aryl Iodides. ACS Catal. 2023, 13, 2968–2980. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Wang, Z.-K.; Jia, Z.-S.; Chen, J.-H.; Huang, F.-R.; Zhan, B.-B.; Yao, Q.-J.; Shi, B.-F. Synthesis of Axially Chiral Biaryls through Cobalt(II)-Catalyzed Atroposelective C−H Arylation. Angew. Chem. Int. Ed. 2023, 62, e202310004. [Google Scholar] [CrossRef]
- Yan, S.-B.; Wang, R.; Li, Z.-G.; Li, A.-N.; Wang, C.; Duan, W.-L. Copper-catalyzed asymmetric C(sp2)–H arylation for the synthesis of P- and axially chiral phosphorus compounds. Nat. Commun. 2023, 14, 2264. [Google Scholar] [CrossRef]
- Parmar, D.; Kumar, R.; Sarthi; Sharma, A.K.; Sharma, U. Synthesis of axially chiral biaryls via Pd(ii)-catalysed direct C(sp2)–H arylation. Org. Chem. Front. 2024, 11, 4986–4991. [Google Scholar] [CrossRef]
- Ma, Y.-N.; Zhang, H.-Y.; Yang, S.-D. Pd(II)-Catalyzed P(O)R1R2-Directed Asymmetric C–H Activation and Dynamic Kinetic Resolution for the Synthesis of Chiral Biaryl Phosphates. Org. Lett. 2015, 17, 2034–2037. [Google Scholar] [CrossRef]
- Li, S.-X.; Ma, Y.-N.; Yang, S.-D. P(O)R2-Directed Enantioselective C–H Olefination toward Chiral Atropoisomeric Phosphine–Olefin Compounds. Org. Lett. 2017, 19, 1842–1845. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Y.; Ma, W.-Y.; Yang, K.-F.; Cao, J.; Zheng, Z.-J.; Xu, Z.; Cui, Y.-M.; Xu, L.-W. Enantioselective synthesis of axially chiral vinyl arenes through palladium-catalyzed C–H olefination. Chem. Commun. 2018, 54, 10706–10709. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, T.; Wang, L.; Liao, G.; Yao, Q.-J.; Wu, Y.-J.; Zhan, B.-B.; Lan, Y.; Lin, X.-F.; Shi, B.-F. Enantioselective Synthesis of Biaryl Atropisomers by Pd-Catalyzed C−H Olefination using Chiral Spiro Phosphoric Acid Ligands. Angew. Chem. Int. Ed. 2019, 58, 6708–6712. [Google Scholar] [CrossRef]
- Zhan, B.-B.; Wang, L.; Luo, J.; Lin, X.-F.; Shi, B.-F. Synthesis of Axially Chiral Biaryl-2-amines by PdII-Catalyzed Free-Amine-Directed Atroposelective C−H Olefination. Angew. Chem. Int. Ed. 2020, 59, 3568–3572. [Google Scholar] [CrossRef]
- Yao, Q.-J.; Xie, P.-P.; Wu, Y.-J.; Feng, Y.-L.; Teng, M.-Y.; Hong, X.; Shi, B.-F. Enantioselective Synthesis of Atropisomeric Anilides via Pd(II)-Catalyzed Asymmetric C–H Olefination. J. Am. Chem. Soc. 2020, 142, 18266–18276. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yao, Q.-J.; Xie, P.-P.; Li, Y.; Zhan, B.-B.; Han, Y.-Q.; Hong, X.; Shi, B.-F. Atroposelective Synthesis of Axially Chiral Styrenes via an Asymmetric C−H Functionalization Strategy. Chem 2020, 6, 497–511. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, Y.; Shen, W.; Jin, S.; Zhong, G.; Luo, S.; Xu, L.; Zhong, L.; Zhang, J. Access to axially chiral aryl 1,3-dienes by transient group directed asymmetric C–H alkenylations. Org. Chem. Front. 2022, 9, 2109–2115. [Google Scholar] [CrossRef]
- Li, Y.; Liou, Y.-C.; Chen, X.; Ackermann, L. Thioether-enabled palladium-catalyzed atroposelective C–H olefination for N–C and C–C axial chirality. Chem. Sci. 2022, 13, 4088–4094. [Google Scholar] [CrossRef]
- Bobek, K.B.; Ezzat, N.S.; Jones, B.S.; Bian, Y.; Shaw, T.E.; Jurca, T.; Li, H.; Yuan, Y. Total Synthesis of Polysubstituted γ-Butyrolactone Lignans (−)-Hinokinin, (−)-Bicubebin B, and (−)-Isodeoxypodophyllotoxin via Oxime Carbonate Formation. Org. Lett. 2023, 25, 31–36. [Google Scholar] [CrossRef]
- Liao, G.; Yao, Q.-J.; Zhang, Z.-Z.; Wu, Y.-J.; Huang, D.-Y.; Shi, B.-F. Scalable, Stereocontrolled Formal Syntheses of (+)-Isoschizandrin and (+)-Steganone: Development and Applications of Palladium(II)-Catalyzed Atroposelective C−H Alkynylation. Angew. Chem. Int. Ed. 2018, 57, 3661–3665. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, Q.-J.; Liao, G.; Li, X.; Li, H.; Chen, H.-M.; Hong, X.; Shi, B.-F. Enantioselective Synthesis of Atropisomers Featuring Pentatomic Heteroaromatics by Pd-Catalyzed C–H Alkynylation. ACS Catal. 2019, 9, 1956–1961. [Google Scholar] [CrossRef]
- Yang, C.; Li, F.; Wu, T.-R.; Cui, R.; Wu, B.-B.; Jin, R.-X.; Li, Y.; Wang, X.-S. Development of Axially Chiral Styrene-Type Carboxylic Acid Ligands via Palladium-Catalyzed Asymmetric C–H Alkynylation. Org. Lett. 2021, 23, 8132–8137. [Google Scholar] [CrossRef]
- Zheng, D.-S.; Zhao, F.; Gu, Q.; You, S.-L. Rh(iii)-catalyzed atroposelective C–H alkynylation of 1-aryl isoquinolines with hypervalent iodine–alkyne reagents. Chem. Commun. 2024, 60, 6753–6756. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kapur, M. Transition-Metal-Catalyzed Site-Selective C−H Halogenation Reactions. Asian J. Org. Chem. 2018, 7, 1524–1541. [Google Scholar] [CrossRef]
- Voskressensky, L.G.; Golantsov, N.E.; Maharramov, A.M. Recent Advances in Bromination of Aromatic and Heteroaromatic Compounds. Synthesis 2016, 48, 615–643. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L.-S.; Shi, B.-F. Forging C–heteroatom bonds by transition-metal-catalyzed enantioselective C−H functionalization. Chem 2022, 8, 384–413. [Google Scholar] [CrossRef]
- Lee, S.; Chung, W.-j. Enantioselective halogenation via asymmetric phase-transfer catalysis. Bull. Korean Chem. Soc. 2022, 43, 896–911. [Google Scholar] [CrossRef]
- Cao, T.; Wei, L.; Wang, L. Recent Progress in N-Iodosuccinimide (NIS)-Mediated Iodination Reactions. Chin. J. Org. Chem. 2024, 44, 508–524. [Google Scholar]
- Jiang, Y.; Lewis, J.C. Asymmetric catalysis by flavin-dependent halogenases. Chirality 2023, 35, 452–460. [Google Scholar] [CrossRef]
- Chu, L.; Xiao, K.-J.; Yu, J.-Q. Room-temperature enantioselective C–H iodination via kinetic resolution. Science 2014, 346, 451–455. [Google Scholar] [CrossRef]
- Gao, D.-W.; Gu, Q.; You, S.-L. Pd(II)-Catalyzed Intermolecular Direct C–H Bond Iodination: An Efficient Approach toward the Synthesis of Axially Chiral Compounds via Kinetic Resolution. ACS Catal. 2014, 4, 2741–2745. [Google Scholar] [CrossRef]
- Tan, Y.; Jia, S.; Hu, F.; Liu, Y.; Peng, L.; Li, D.; Yan, H. Enantioselective Construction of Vicinal Diaxial Styrenes and Multiaxis System via Organocatalysis. J. Am. Chem. Soc. 2018, 140, 16893–16898. [Google Scholar] [CrossRef]
- Ke, J.; Zu, B.; Guo, Y.; Li, Y.; He, C. Hexafluoroisopropanol-Enabled Copper-Catalyzed Asymmetric Halogenation of Cyclic Diaryliodoniums for the Synthesis of Axially Chiral 2,2′-Dihalobiaryls. Org. Lett. 2021, 23, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.T.; Timmerman, J.C.; Bawel, S.A.; Chin, S.; Zhang, H.; Denmark, S.E. High-Level Data Fusion Enables the Chemoinformatically Guided Discovery of Chiral Disulfonimide Catalysts for Atroposelective Iodination of 2-Amino-6-arylpyridines. J. Am. Chem. Soc. 2022, 144, 22950–22964. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Cho, H.-A.; Oh, B.; Song, J.; Kwon, Y. Chiral phosphoric acid-catalyzed atroposelective iodination of N-arylindoles. Commun. Chem. 2025, 8, 190. [Google Scholar] [CrossRef]
- Yao, L.; Gashaw Woldegiorgis, A.; Huang, S.; Wang, Y.; Lin, X. Palladium-Catalyzed Directed Atroposelective C−H Iodination to Synthesize Axial Chiral Biaryl N-Oxides via Enantioselective Desymmetrization Strategy. Chem. Eur. J. 2023, 29, e202203051. [Google Scholar] [CrossRef]
- Zheng, D.-S.; Zhang, W.-W.; Gu, Q.; You, S.-L. Rh(III)-Catalyzed Atroposelective C–H Iodination of 1-Aryl Isoquinolines. ACS Catal. 2023, 13, 5127–5134. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally produced organohalogens. Chemosphere 2003, 52, 289–297. [Google Scholar] [CrossRef]
- Golantsov, N.E.; Festa, A.A.; Karchava, A.V.; Yurovskaya, M.A. Marine indole alkaloids containing an 1-(indol-3-yl)ethane-1,2-diamine fragment (Review). Chem. Heterocycl. Com. 2013, 49, 203–225. [Google Scholar] [CrossRef]
- Gustafson, J.L.; Lim, D.; Miller, S.J. Dynamic Kinetic Resolution of Biaryl Atropisomers via Peptide-Catalyzed Asymmetric Bromination. Science 2010, 328, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.T.; Miller, S.J. Enantioselective Synthesis of Atropisomeric Benzamides through Peptide-Catalyzed Bromination. J. Am. Chem. Soc. 2013, 135, 2963–2966. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ichikawa, Y.; Kobayashi, M.; Shibata, Y.; Yamanaka, M.; Akiyama, T. Enantioselective Synthesis of Multisubstituted Biaryl Skeleton by Chiral Phosphoric Acid Catalyzed Desymmetrization/Kinetic Resolution Sequence. J. Am. Chem. Soc. 2013, 135, 3964–3970. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, R.; Asano, K.; Matsubara, S. Bifunctional Organocatalysts for the Enantioselective Synthesis of Axially Chiral Isoquinoline N-Oxides. J. Am. Chem. Soc. 2015, 137, 6766–6769. [Google Scholar] [CrossRef]
- Diener, M.E.; Metrano, A.J.; Kusano, S.; Miller, S.J. Enantioselective Synthesis of 3-Arylquinazolin-4(3H)-ones via Peptide-Catalyzed Atroposelective Bromination. J. Am. Chem. Soc. 2015, 137, 12369–12377. [Google Scholar] [CrossRef]
- Wada, Y.; Matsumoto, A.; Asano, K.; Matsubara, S. Enantioselective bromination of axially chiral cyanoarenes in the presence of bifunctional organocatalysts. RSC Adv. 2019, 9, 31654–31658. [Google Scholar] [CrossRef]
- Linde, S.T.; Corti, V.; Lauridsen, V.H.; Lamhauge, J.N.; Jørgensen, K.A.; Rezayee, N.M. Atroposelective brominations to access chiral biaryl scaffolds using high-valent Pd-catalysis. Chem. Sci. 2023, 14, 3676–3681. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, S.; Zhang, Y.; Bai, D.; Qu, F.; Song, Z.; Chen, H.; Liu, T. Enantioselective Iodination and Bromination for the Atroposelective Construction of Axially Chiral Compounds. Catalysts 2025, 15, 679. https://doi.org/10.3390/catal15070679
Wang X, Zhao S, Zhang Y, Bai D, Qu F, Song Z, Chen H, Liu T. Enantioselective Iodination and Bromination for the Atroposelective Construction of Axially Chiral Compounds. Catalysts. 2025; 15(7):679. https://doi.org/10.3390/catal15070679
Chicago/Turabian StyleWang, Xilong, Shunwei Zhao, Yao Zhang, Dongya Bai, Fengbo Qu, Zhiyi Song, Hui Chen, and Tingting Liu. 2025. "Enantioselective Iodination and Bromination for the Atroposelective Construction of Axially Chiral Compounds" Catalysts 15, no. 7: 679. https://doi.org/10.3390/catal15070679
APA StyleWang, X., Zhao, S., Zhang, Y., Bai, D., Qu, F., Song, Z., Chen, H., & Liu, T. (2025). Enantioselective Iodination and Bromination for the Atroposelective Construction of Axially Chiral Compounds. Catalysts, 15(7), 679. https://doi.org/10.3390/catal15070679





