Strategies for the Removal of Per- and Polyfluoroalkyl Substances: A Review
Abstract
1. Introduction
2. Physical Treatment
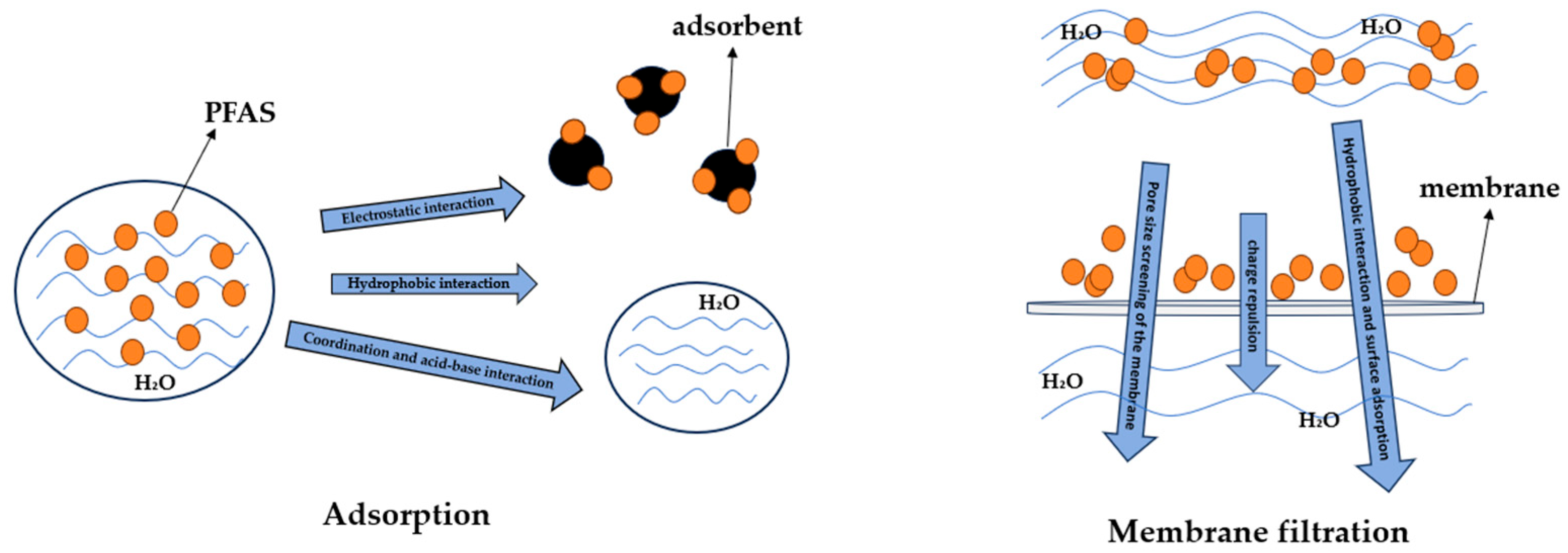
2.1. Adsorption
2.2. Membrane Filtration
2.3. Emerging Technologies
3. Chemical Treatment
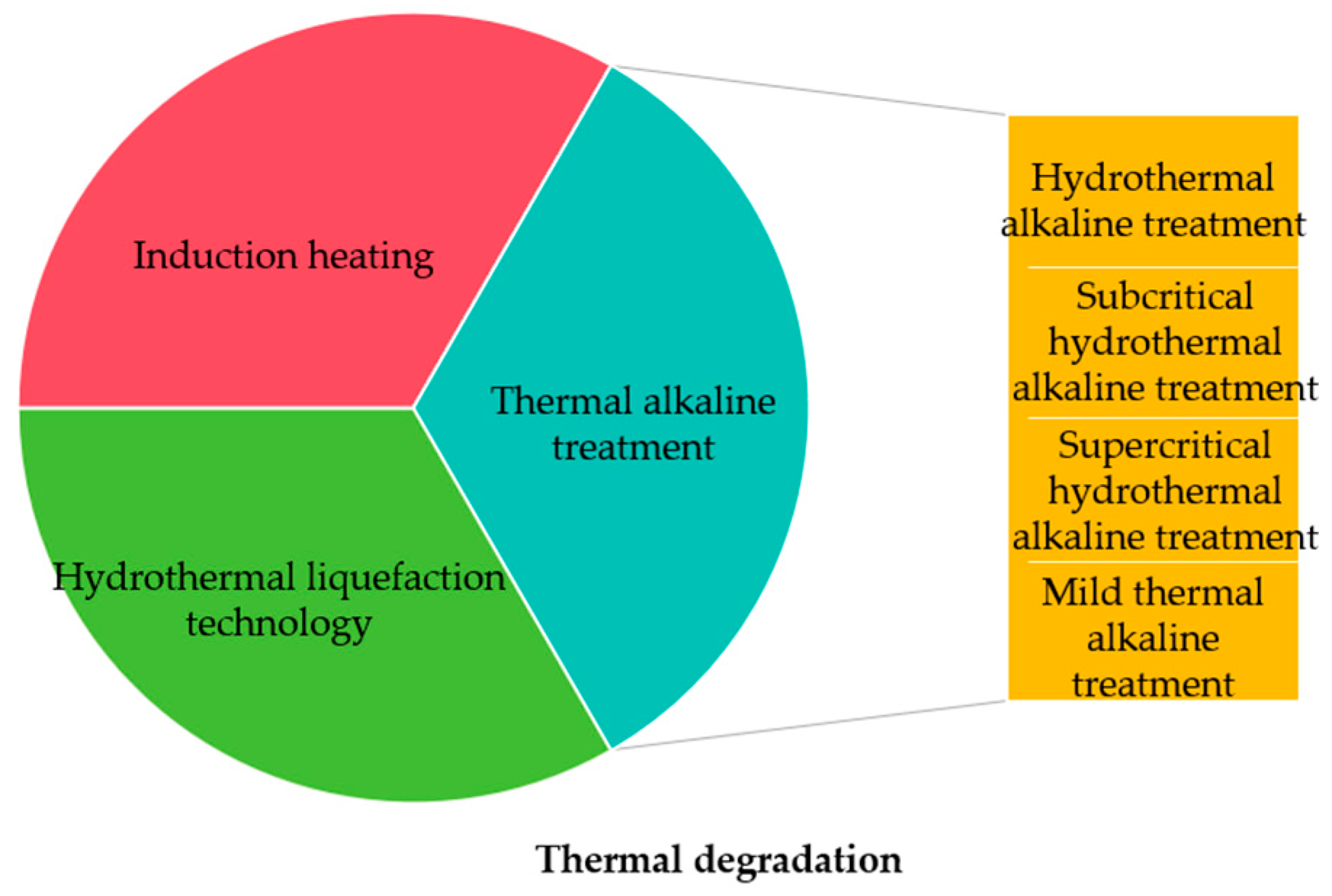
3.1. Thermal Degradation
3.2. Electrochemical Degradation
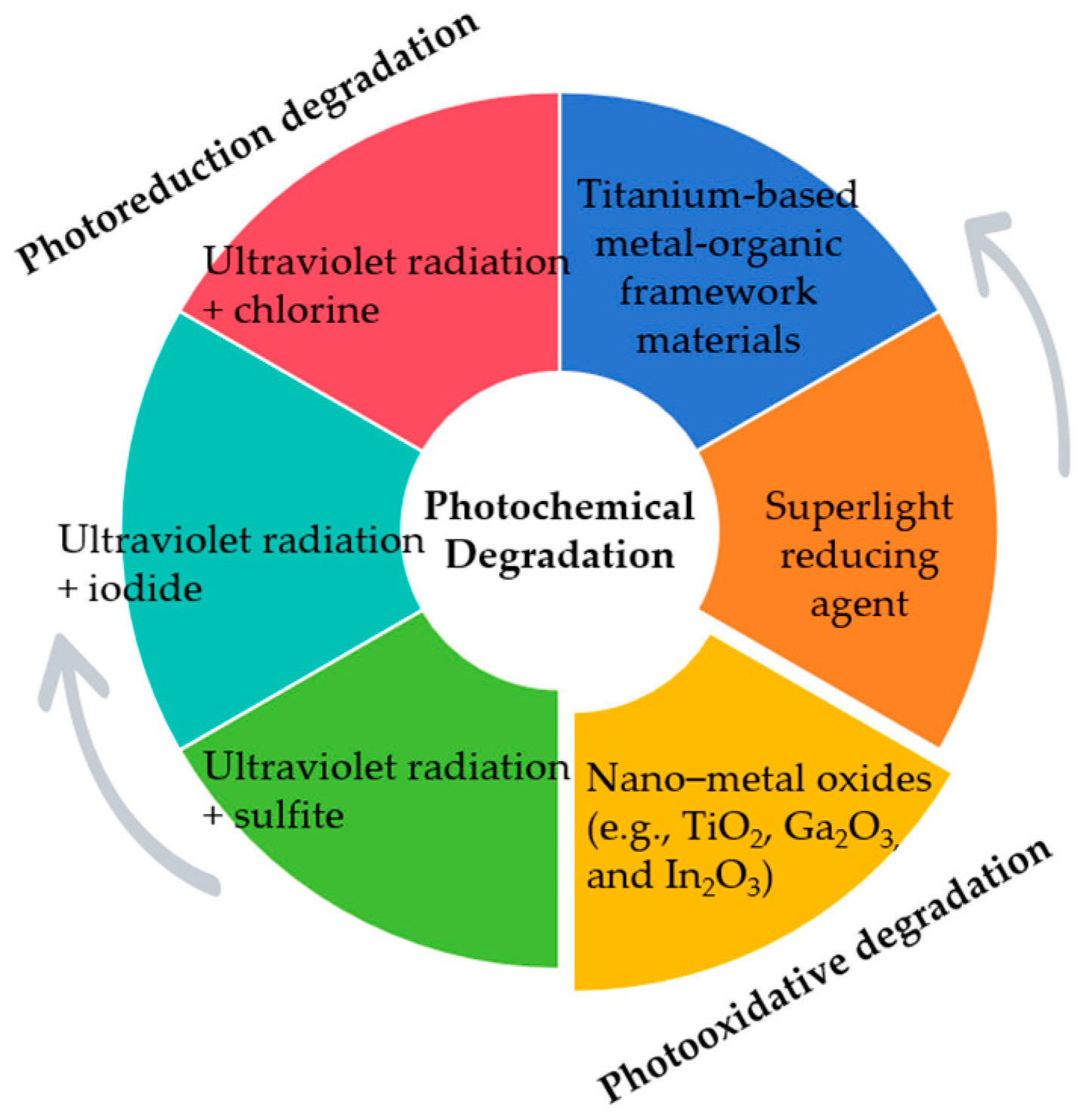
3.3. Photochemical Degradation
3.4. Sonochemical Degradation
3.5. Plasma-Based Technologies
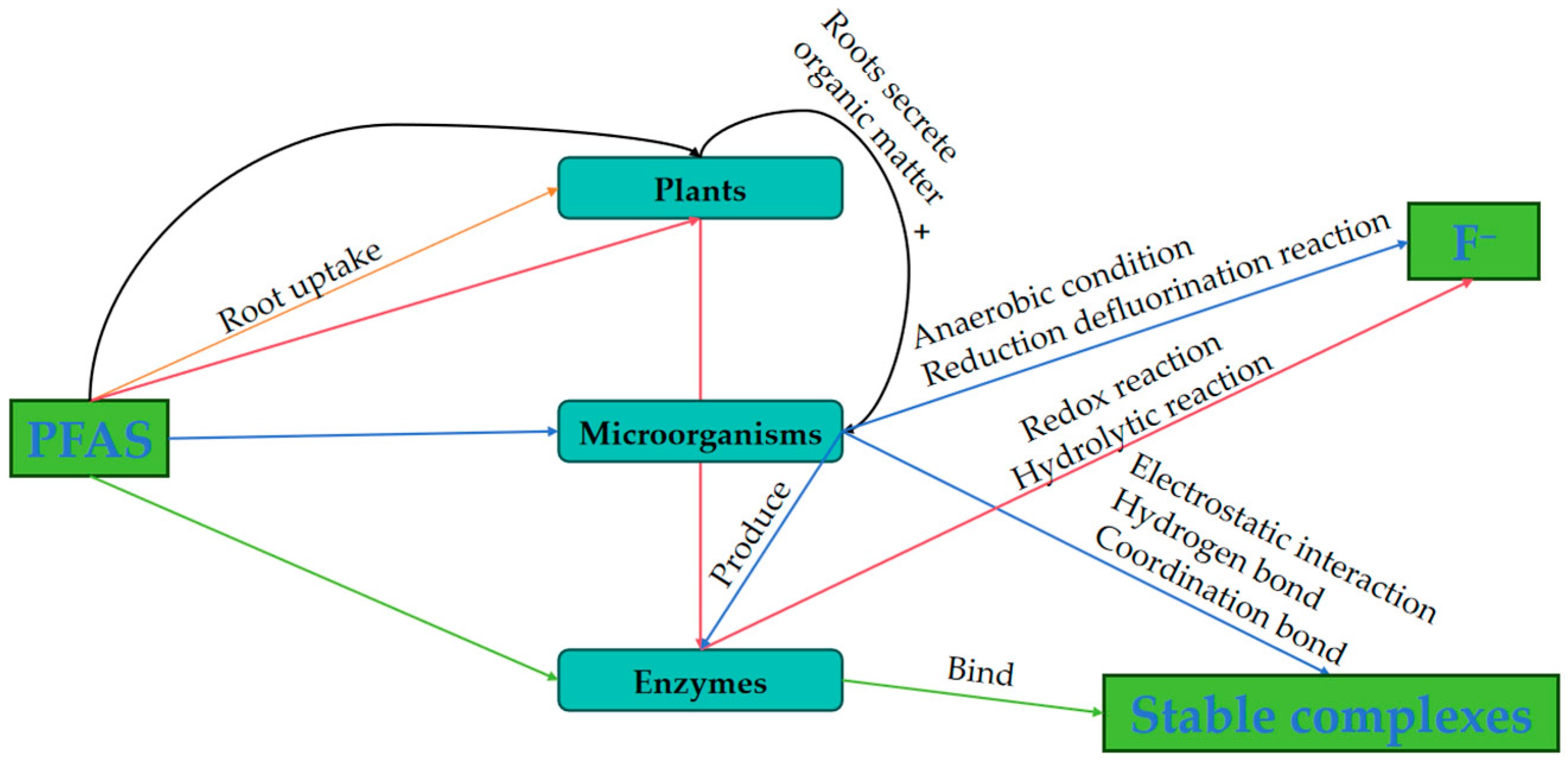
4. Biological Treatment
5. Combined Treatment
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFAS | Perfluoroalkyl and polyfluoroalkyl substance |
| GAC | Granular activated carbon |
| AERs | Anion exchange resins |
| PFOS | Perfluorooctanesulfonic acid |
| PFOA | Perfluorooctanoic acid |
| CERs | Cation exchange resins |
| NIRs | Non-ionic resins |
| COF | Covalent organic framework |
| MOF | Metal–organic framework |
| CNT | Carbon nanotube |
| AFFF | Film-forming foam |
| FBA | 2-fluorobenzoic acid |
| DFBA | 2,6-difluorobenzoic acid |
| TFA | Trifluoroacetic acid |
| PFBA | Heptafluorobutyric acid |
| PFHxA | Perfluorohexanoic acid |
| UF | Ultrafiltration |
| MF | Microfiltration |
| NF | Nanofiltration |
| RO | Reverse osmosis |
| AEC | Aqueous electrostatic concentration |
| PFCA | Perfluoroalkyl carboxylic acid |
| PFSA | Perfluorosulfonic acid |
| FTS | Fluorotelomer sulfonic acid |
| FTCA | Fluorotelomer carboxylic acid |
| PFBA | Perfluorobutanoic acid |
| FTAB | Fluorotelomer sulfonamide alkylbetaine |
| FTB | Fluoroalkyl chain betaine |
| PFAAs | Perfluoroalkyl acids |
| PFCAs | Perfluoroalkyl carboxylic acids |
| CERCLA | Comprehensive Environmental Response Compensation and Liability Act |
| HALT | Hydrothermal alkali treatment |
| DMSO | Dimethyl sulfoxide |
| PFBS | Perfluorobutanesulfonic acid |
| BDD | Boron-doped diamond |
| B-RGO | Boron-doped graphene sponge anode |
| Bph-RGO | Borophene functionalized graphene sponge anode |
| FTSAs | Fluorotelomer sulfonates |
| TOC | Total organic carbon |
| VOF | Volatile organic fluorine |
| PSDR | Plasma rotating disk reactor |
| PFDA | Perfluorodecanoic acid |
| HFPO-DA | Hexafluoropropylene oxide dimer acid |
| PFPeA | Perfluoropentanoic acid |
| HRP | Horseradish peroxidase |
| LiP | Lignin peroxidase |
| MnP | Manganese peroxidase |
| FOSA | Perfluorooctane Sulfonamide |
| RAC | Carbon impregnated with zero-valent nano-iron combined with persulfate |
| GenX | Hexafluoropropylene oxide dimer acid |
References
- Das, S.; Ronen, A. A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes 2022, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—A review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- Barisci, S.; Suri, R. Occurrence and removal of poly/perfluoroalkyl substances (PFAS) in municipal and industrial wastewater treatment plants. Water Sci. Technol. 2021, 84, 3442–3468. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cai, Y.; Zhou, B.; Yuan, R.; Chen, Z.; Chen, H. Removal of PFASs from water by carbon-based composite photocatalysis with adsorption and catalytic properties: A review. Sci. Total Environ. 2022, 836, 155652. [Google Scholar] [CrossRef] [PubMed]
- Appah, S.; Jia, W.D.; Ou, M.X.; Wang, P.; Asante, E.A. Analysis of potential impaction and phytotoxicity of surfactant-plant surface interaction in pesticide application. Crop Prot. 2020, 127, 104961. [Google Scholar] [CrossRef]
- Vakili, M.; Bao, Y.; Gholami, F.; Gholami, Z.; Deng, S.; Wang, W.; Awasthi, A.K.; Rafatullah, M.; Cagnetta, G.; Yu, G. Removal of HFPO-DA (GenX) from aqueous solutions: A mini-review. Chem. Eng. J. 2021, 424, 130266. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Liu, X.Q.; Chen, H.L.; Tian, X. Automatic cruise system for water quality monitoring. Int. J. Agric. Biol. Eng. 2018, 11, 244–250. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, X.H.; Hou, B.X.; Hao, C.; Li, X.; Wu, J.B. Construction of a Lignosulfonate-Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef]
- Verma, S.; Varma, R.S.; Nadagouda, M.N. Remediation and mineralization processes for per- and polyfluoroalkyl substances (PFAS) in water: A review. Sci. Total Environ. 2021, 794, 148987. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.Y.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol. 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.Y.; Aheto, J.H.; Osae, R. Application of Hyperspectral Imaging as a Nondestructive Technique for Foodborne Pathogen Detection and Characterization. Foodborne Pathog. Dis. 2019, 16, 712–722. [Google Scholar] [CrossRef]
- Li, B.Y.; Xu, X.Y.; Gan, R.Y.; Sun, Q.C.; Meng, J.M.; Shang, A.; Mao, Q.Q.; Li, H.B. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods 2019, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Dickman, R.A.; Aga, D.S. A review of recent studies on toxicity, sequestration, and degradation of per- and polyfluoroalkyl substances (PFAS). J. Hazard. Mater. 2022, 436, 129120. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Zhang, J.K.; Liu, E.M.; Yang, M.Q.; Chen, S.; Hu, F.; Ma, H.L.; Liu, Z.; Yu, X.T. Enhancing the taste of raw soy sauce using low intensity ultrasound treatment during moromi fermentation. Food Chem. 2019, 298, 124928. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Vidal, P.; Jiang, L.; Mei, W.; Luo, C.; Lacorte, S.; Rigol, A.; Zhang, G. Plant uptake of perfluoroalkyl substances in freshwater environments (Dongzhulong and Xiaoqing Rivers, China). J. Hazard. Mater. 2022, 421, 126768. [Google Scholar] [CrossRef]
- Huang, S.; Jaffe, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Tseng, N.; Wang, N.; Szostek, B.; Mahendra, S. Biotransformation of 6:2 Fluorotelomer Alcohol (6:2 FTOH) by a Wood-Rotting Fungus. Environ. Sci. Technol. 2014, 48, 4012–4020. [Google Scholar] [CrossRef]
- Grgas, D.; Petrina, A.; Stefanac, T.; Beslo, D.; Dragicevic, T.L. A Review: Per- and Polyfluoroalkyl Substances-Biological Degradation. Toxics 2023, 11, 446. [Google Scholar] [CrossRef]
- Dadashi Firouzjaei, M.; Zolghadr, E.; Ahmadalipour, S.; Taghvaei, N.; Akbari Afkhami, F.; Nejati, S.; Elliott, M.A. Chemistry, abundance, detection and treatment of per- and polyfluoroalkyl substances in water: A review. Environ. Chem. Lett. 2022, 20, 661–679. [Google Scholar] [CrossRef]
- Li, J.; Pinkard, B.R.; Wang, S.; Novosselov, I.V. Review: Hydrothermal treatment of per- and polyfluoroalkyl substances (PFAS). Chemosphere 2022, 307, 135888. [Google Scholar] [CrossRef]
- Liu, F.; Guan, X.; Xiao, F. Photodegradation of per- and polyfluoroalkyl substances in water: A review of fundamentals and applications. J. Hazard. Mater. 2022, 439, 129580. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.-S.; Wang, J.; Yang, L.; Wang, J.; Zhang, Y.; Zhu, L. A review on the advancement in photocatalytic degradation of poly/ perfluoroalkyl substances in water: Insights into the mechanisms and structure-function relationship. Sci. Total Environ. 2024, 946, 174137. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Yamijala, S.S.R.K.C.; Wong, B.M. A review of emerging photoinduced degradation methods for per- and polyfluoroalkyl substances in water. Curr. Opin. Chem. Eng. 2023, 41, 100947. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, T.; Yang, X.; Ding, S.; Ma, M.; Muhmood, T.; Yang, X. Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes. Catalysts 2023, 13, 1308. [Google Scholar] [CrossRef]
- McBeath, S.T.; Mora, A.S.; Zeidabadi, F.A.; Mayer, B.K.; McNamara, P.; Mohseni, M.; Hoffmann, M.R.; Graham, N.J.D. Progress and prospect of anodic oxidation for the remediation of perfluoroalkyl and polyfluoroalkyl substances in water and wastewater using diamond electrodes. Curr. Opin. Electrochem. 2021, 30, 100865. [Google Scholar] [CrossRef]
- Sivagami, K.; Sharma, P.; Karim, A.V.; Mohanakrishna, G.; Karthika, S.; Divyapriya, G.; Saravanathamizhan, R.; Kumar, A.N. Electrochemical-based approaches for the treatment of forever chemicals: Removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) from wastewater. Sci. Total Environ. 2023, 861, 160440. [Google Scholar] [CrossRef]
- Mirabediny, M.; Sun, J.; Yu, T.T.; Akermark, B.; Das, B.; Kumar, N. Effective PFAS degradation by electrochemical oxidation methods-recent progress and requirement. Chemosphere 2023, 321, 138109. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Vo, P.H.N.; Shukla, P.; Ge, L.; Zhao, C.-X. Electrochemical advanced oxidation of per- and polyfluoroalkyl substances (PFASs): Development, challenges and perspectives. Chem. Eng. J. 2024, 500, 157222. [Google Scholar] [CrossRef]
- Zhou, X.; Zhong, Y.; Tian, X.; Zhao, F. Electrochemical oxidation of perfluoroalkyl and polyfluoroalkyl substances: Mechanisms, implications, and challenges. Sci. China-Technol. Sci. 2024, 67, 2972–2990. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, S.; Chen, W.; Lin, J.; Yu, X.; Feng, M.; Wan, K. Removal of Per- and Polyfluoroalkyl Substances by Electron Beam and Plasma Irradiation: A Mini-Review. Water 2022, 14, 1684. [Google Scholar] [CrossRef]
- Mbanugo, V.S.; Ojo, B.S.; Lin, T.C.; Huang, Y.-W.; Locmelis, M.; Han, D. Per- and Polyfluoroalkyl Substance (PFAS) Degradation in Water and Soil Using Cold Atmospheric Plasma (CAP): A Review. ACS Phys. Chem. Au 2025, 5, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Helbling, D.E.; Liu, J.; Olivares, C.I.; Higgins, C.P. Microbial biotransformation of aqueous film-forming foam derived polyfluoroalkyl substances. Sci. Total Environ. 2022, 824, 153711. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344, 126223. [Google Scholar] [CrossRef]
- Berhanu, A.; Mutanda, I.; Ji, T.; Qaria, M.A.; Yang, B.; Zhu, D. A review of microbial degradation of per- and polyfluoroalkyl substances (PFAS): Biotransformation routes and enzymes. Sci. Total Environ. 2023, 859, 160010. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Lin, X.; Zheng, X.; Wu, Y.; Long, M.; Chen, Y. Aerobic or anaerobic? Microbial degradation of per- and polyfluoroalkyl substances: A review. J. Hazard. Mater. 2024, 480, 136173. [Google Scholar] [CrossRef]
- Dey, D.; Shafi, T.; Chowdhury, S.; Dubey, B.K.; Sen, R. Progress and perspectives on carbon-based materials for adsorptive removal and photocatalytic degradation of perfluoroalkyl and polyfluoroalkyl substances (PFAS). Chemosphere 2024, 351, 141164. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Usman, M.; Haderlein, S.; Hanna, K. Carbonaceous materials for adsorptive removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) and regeneration of spent adsorbents. J. Water Process Eng. 2024, 68, 106498. [Google Scholar] [CrossRef]
- Yin, S.; Villagran, D. Design of nanomaterials for the removal of per- and poly-fluoroalkyl substances (PFAS) in water: Strategies, mechanisms, challenges, and opportunities. Sci. Total Environ. 2022, 831, 154939. [Google Scholar] [CrossRef]
- Li, H.; Junker, A.L.; Wen, J.; Ahrens, L.; Sillanpaa, M.; Tian, J.; Cui, F.; Vergeynst, L.; Wei, Z. A recent overview of per- and polyfluoroalkyl substances (PFAS) removal by functional framework materials. Chem. Eng. J. 2023, 452, 139202. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Bandosz, T.J. Activated carbon versus metal-organic frameworks: A review of their PFAS adsorption performance. J. Hazard. Mater. 2022, 425, 127810. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, B.; Liu, H.; Yuan, R.; Wang, X.; Feng, Z.; Chen, Z.; Chen, H. Strategies to improve adsorption and photocatalytic performance of metal-organic frameworks (MOFs) for perfluoroalkyl and polyfluoroalkyl substances (PFASs) removal from water: A review. Environ. Res. 2024, 240, 117483. [Google Scholar] [CrossRef]
- Pervez, M.N.; Jiang, T.; Liang, Y. Structure and mechanism of nanoengineered membranes toward per- and polyfluoroalkyl substances (PFAS) removal from water: A critical review. J. Water Process Eng. 2024, 63, 105471. [Google Scholar] [CrossRef]
- Guo, C.; Hu, S.; Cheng, P.; Cheng, K.; Yang, Y.; Chen, G.; Wang, Q.; Wang, Y.; Liu, T. Speciation and biogeochemical behavior of perfluoroalkyl acids in soils and their environmental implications: A review. Eco-Environ. Health 2024, 3, 505–515. [Google Scholar] [CrossRef]
- Loukopoulos, E.; Marugan-Benito, S.; Raptis, D.; Tylianakis, E.; Froudakis, G.E.; Mavrandonakis, A.; Platero-Prats, A.E. Chemically Tailored Metal-Organic Frameworks for Enhanced Capture of Short- and Long-Chain Per- and Polyfluoroalkyl Substances from Water. Adv. Funct. Mater. 2024, 34, 2409932. [Google Scholar] [CrossRef]
- Lu, J.; Lu, H.; Liang, D.; Feng, S.; Li, Y.; Li, J. A review of the occurrence, monitoring, and removal technologies for the remediation of per- and polyfluoroalkyl substances (PFAS) from landfill leachate. Chemosphere 2023, 332, 138824. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Li, R.; Alomari, S.; Islamoglu, T.; Farha, O.K.; Fernando, S.; Thagard, S.M.; Holsen, T.M.; Wriedt, M. Systematic Study on the Removal of Per- and Polyfluoroalkyl Substances from Contaminated Groundwater Using Metal-Organic Frameworks. Environ. Sci. Technol. 2021, 55, 15162–15171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ellis, A.; Choi, Y.J.; Boyer, T.H.; Higgins, C.P.; Schaefer, C.E.; Strathmann, T.J. Removal of Per- and Polyfluoroalkyl Substances (PFASs) in Aqueous Film-Forming Foam (AFFF) Using Ion-Exchange and Nonionic Resins. Environ. Sci. Technol. 2021, 55, 5001–5011. [Google Scholar] [CrossRef]
- Dixit, F.; Munoz, G.; Mirzaei, M.; Barbeau, B.; Liu, J.; Duy, S.V.; Sauve, S.; Kandasubramanian, B.; Mohseni, M. Removal of Zwitterionic PFAS by MXenes: Comparisons withAnionic, Nonionic, and PFAS-Specific Resins. Environ. Sci. Technol. 2022, 56, 6212–6222. [Google Scholar] [CrossRef]
- Huang, X.-W.; Li, Z.-H.; Zou, X.-B.; Shi, J.-Y.; Mao, H.-P.; Zhao, J.-W.; Hao, L.-M.; Holmes, M. Detection of meat-borne trimethylamine based on nanoporous colorimetric sensor arrays. Food Chem. 2016, 197, 930–936. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zou, X.B.; Zhai, X.D.; Huang, X.W.; Jiang, C.P.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Wen, C.T.; Zhang, J.X.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.J.; Ahmad, W.; Li, H.H.; Chen, Q.S. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef]
- Liu, C.J.; Strathmann, T.J.; Bellona, C. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Res. 2021, 188, 116546. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Peydayesh, M.; Joerss, H.; Zhou, J.; Bolisetty, S.; Mezzenga, R. Amyloid fibril-based membranes for PFAS removal from water. Environ. Sci.-Water Res. Technol. 2021, 7, 1873–1884. [Google Scholar] [CrossRef]
- Chandler, T.; Liao, S. Treating PFAS-Laden Waste Using Aqueous Electrostatic Concentration. J. Am. Water Work. Assoc. 2025, 117, 71–74. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Y.; Tishchenko, V.; Huang, Q. Removing per- and polyfluoroalkyl substances (PFAS) in water by foam fractionation. Chemosphere 2023, 311, 137004. [Google Scholar] [CrossRef]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef]
- Fennell, B.D.; Chavez, S.; McKay, G. Destruction of Per- and Polyfluoroalkyl Substances in Reverse Osmosis Concentrate Using UV-Advanced Reduction Processes. ACS ES T Water. 2024, 4, 4818–4827. [Google Scholar] [CrossRef]
- Hao, S.; Choi, Y.-J.; Wu, B.; Higgins, C.P.; Deeb, R.; Strathmann, T.J. Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam. Environ. Sci. Technol. 2021, 55, 3283–3295. [Google Scholar] [CrossRef]
- Xiao, F.; Sasi, P.C.; Alinezhad, A.; Golovko, S.A.; Golovko, M.Y.; Spoto, A. Thermal Decomposition of Anionic, Zwitterionic, and Cationic Polyfluoroalkyl Substances in Aqueous Film-Forming Foamse. Environ. Sci. Technol. 2021, 55, 9885–9894. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; de Souza, B.B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.S.; Hu, J.L.; Yu, X.J.; Yagoub, A.E.A.; Zhang, Y.Y.; Ma, H.L.; Gao, X.L.; Otu, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT 2017, 77, 488–496. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Z.; Lu, X.; Chen, D.; Li, Z.; Wang, F. The degradation mechanisms of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) by different chemical methods: A critical review. Chemosphere 2021, 283, 131168. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chaturvedi, A.; Gautam, R.K.; Jiang, J.J. Molecular Cu Electrocatalyst Escalates Ambient Perfluorooctanoic Acid Degradation. J. Am. Chem. Soc. 2023, 145, 27390–27396. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Y. Effects of hydrothermal treatments on destruction of per- and polyfluoroalkyl substances in sewage sludge*. Environ. Pollut. 2021, 285, 117276. [Google Scholar] [CrossRef]
- Hao, S.; Choi, Y.J.; Deeb, R.A.; Strathmann, T.J.; Higgins, C.P. Application of Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Contaminated Groundwaterand Soil. Environ. Sci. Technol. 2022, 56, 6647–6657. [Google Scholar] [CrossRef]
- Kim, J.; Xin, X.; Germolus, N.P.; Huang, C.-H. Thermal destruction of per-and polyfluoroalkyl substances in alkaline aprotic solvent. Chem. Eng. J. 2025, 505, 159296. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Hu, Y.G.; Li, P.P. Consistency of electrical and physiological properties of tea leaves on indicating critical cold temperature. Biosyst. Eng. 2017, 159, 89–96. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wen, C.T.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Luo, X.P.; Cui, J.M.; Zhang, H.H.; Duan, Y.Q. Subcritical water extraction of polyphenolic compounds from sorghum (Sorghum bicolor L.) bran and their biological activities. Food Chem. 2018, 262, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Wen, C.T.; Zhang, H.H.; Zandile, M.; Luo, X.P.; Duan, Y.Q.; Ma, H.L. Structure of the zein protein as treated with subcritical water. Int. J. Food Prop. 2018, 21, 143–153. [Google Scholar] [CrossRef]
- Cai, P.; Chen, T.; Zhan, M.; Ma, X.; Takaoka, M.; Sun, C.; Li, X. Theoretical and experimental insights into the degradation mechanism of PFBS under subcritical hydrothermal conditions. J. Hazard. Mater. 2025, 491, 137935. [Google Scholar] [CrossRef]
- Cai, P.; Chen, T.; Zhan, M.; Ma, X.; Takaoka, M.; Sun, C.; Li, X. Synergetic degradation of PFOS by HALT conditions enhanced by Fe-based amorphous alloys. J. Hazard. Mater. 2025, 486, 137015. [Google Scholar] [CrossRef] [PubMed]
- Pinkard, B.R.; Shetty, S.; Stritzinger, D.; Bellona, C.; Novosselov, I.V. Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor. Chemosphere 2021, 279, 130834. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.J.; Thoma, E.; Sahle-Damesessie, E.; Crone, B.; Whitehill, A.; Shields, E.; Gullett, B. Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction. J. Environ. Eng. 2022, 148, 05021006. [Google Scholar] [CrossRef]
- Xiao, F.; Challa Sasi, P.; Alinezhad, A.; Sun, R.; Abdulmalik Ali, M. Thermal Phase Transition and Rapid Degradation of Forever Chemicals (PFAS) in Spent Media Using Induction Heating. ACS ES T Eng. 2023, 3, 1370–1380. [Google Scholar] [CrossRef]
- Barisci, S.; Suri, R. Degradation of emerging per- and polyfluoroalkyl substances (PFAS) using an electrochemical plug flow reactor. J. Hazard. Mater. 2023, 460, 132419. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.H.; Zou, X.B.; Xu, Y.W.; Xu, X.C. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef]
- Xu, Y.W.; Zhang, W.; Shi, J.Y.; Zou, X.B.; Li, Z.H.; Zhu, Y.D. Microfabricated interdigitated Au electrode for voltammetric determination of lead and cadmium in Chinese mitten crab (Eriocheir sinensis). Food Chem. 2016, 201, 190–196. [Google Scholar] [CrossRef]
- Zhou, W.S.; Li, C.H.; Sun, C.; Yang, X.D. Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem. 2016, 192, 351–357. [Google Scholar] [CrossRef]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wu, J.Y. Biocompatible Polyelectrolyte Complex Nanoparticles from Lactoferrin and Pectin as Potential Vehicles for Antioxidative Curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Duan, N.; Qiu, Y.T.; Li, J.H.; Wang, Z.P. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Lin, Z.; Ersan, M.S.; Garcia-Segura, S.; Perreault, F.; Westerhoff, P. Electrocatalytic water treatment of per- and polyfluoroalkyl substances reduces adsorbable organofluorine and bioaccumulation potential. RSC Adv. 2024, 14, 15627–15636. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vazquez, J.; Santos, C.S.; Montes, R.; Rodil, R.; Quintana, J.B.; Gaebler, J.; Schaefer, L.; Moreira, F.C.; Vilar, V.J.P. Insights into the application of the anodic oxidation process for the removal of per- and polyfluoroalkyl substances (PFAS) in water matrices. Chem. Eng. J. 2024, 482, 148925. [Google Scholar] [CrossRef]
- Samuel, M.S.; Kadarkarai, G.; Ryan, D.R.; McBeath, S.T.; Mayer, B.K.; McNamara, P.J. Enhanced perfluorooctanoic acid (PFOA) degradation by electrochemical activation of peroxydisulfate (PDS) during electrooxidation for water treatment. Sci. Total Environ. 2024, 942, 173736. [Google Scholar] [CrossRef]
- Duinslaeger, N.; Radjenovic, J. Electrochemical degradation of per- and polyfluoroalkyl substances (PFAS) using low-cost graphene sponge electrodes. Water Res. 2022, 213, 118148. [Google Scholar] [CrossRef] [PubMed]
- Duinslaeger, N.; Doni, A.; Radjenovic, J. Impact of supporting electrolyte on electrochemical performance of borophene-functionalized graphene sponge anode and degradation of per-and polyfluoroalkyl substances (PFAS). Water Res. 2023, 242, 120232. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Dong, S.; Huang, Q. The impact of anions on electrooxidation of perfluoroalkyl acids by porous Magnéli phase titanium suboxide anodes. PLoS ONE 2025, 20, e0317696. [Google Scholar] [CrossRef]
- Gomri, C.; Makhoul, E.; Koundia, F.N.; Petit, E.; Raffy, S.; Bechelany, M.; Semsarilar, M.; Cretin, M. Electrochemical advanced oxidation combined to electro-Fenton for effective treatment of perfluoroalkyl substances “PFAS” in water using a Magnéli phase-based anode. Nanoscale Adv. 2024, 7, 261–268. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Shao, T.; Li, X. In2O3 nanoporous nanosphere: A highly efficient photocatalyst for decomposition of perfluorooctanoic acid. Appl. Catal. B Environ. 2012, 125, 350–357. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, P.; Jin, L.; Li, Z. Photocatalytic decomposition of perfluorooctanoic acid in pure water and sewage water by nanostructured gallium oxide. Appl. Catal. B Environ. 2013, 142, 654–661. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Chen, Z.; Chen, H.; Wang, J.; Zhang, Y.; Xu, Y.; Kong, D.; Zhang, M.; Gu, C. New Insights into the Reductive Destruction of Per- and Polyfluoroalkyl Substances in Hydrated Electron-Based Systems. Environ. Sci. Technol. 2025, 59, 5786–5795. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Gao, J.; Yu, Y.; Men, Y.; Gu, C.; Liu, J. Accelerated Degradation of Perfluorosulfonates and Perfluorocarboxylates by UV/Sulfite plus Iodide: Reaction Mechanisms and System Efficiencies. Environ. Sci. Technol. 2022, 56, 3699–3709. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.; Zuo, P.; Wang, B.; Wong, M.S.; Alvarez, P.J.J. Perfluorooctanoic acid Degradation by UV/Chlorine. Environ. Sci. Technol. Lett. 2022, 9, 673–679. [Google Scholar] [CrossRef]
- Wen, Y.; Renteria-Gomez, A.; Day, G.S.; Smith, M.F.; Yan, T.-H.; Ozdemir, R.O.K.; Gutierrez, O.; Sharma, V.K.; Ma, X.; Zhou, H.-C. Integrated Photocatalytic Reduction and Oxidation of Perfluorooctanoic Acid by Metal-Organic Frameworks: Key Insights into the Degradation Mechanisms. J. Am. Chem. Soc. 2022, 144, 11840–11850. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.-X.; Qu, J.-P.; Kang, Y.-B. Photocatalytic low-temperature defluorination of PFASs. Nature 2024, 635, 610–617. [Google Scholar] [CrossRef]
- Kewalramani, J.A.; Marsh, R.W.; Prajapati, D.; Meegoda, J.N. Kinetics effects of the power density and initial concentration on the sonochemical degradation of PFOS and PFOA in concentrated waste. J. Water Process Eng. 2023, 53, 103752. [Google Scholar] [CrossRef]
- Huang, L.R.; Ding, X.N.; Dai, C.H.; Ma, H.L. Changes in the structure and dissociation of soybean protein isolate induced by ultrasound-assisted acid pretreatment. Food Chem. 2017, 232, 727–732. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.K.; Kwaw, E.; Zhang, H.N.; Li, X.; Afoakwah, N.A. Effects of Ultrasound, High Pressure, and Manosonication Processes on Phenolic Profile and Antioxidant Properties of a Sulfur Dioxide-Free Mulberry (Morus nigra) Wine. Food Bioprocess Technol. 2017, 10, 1210–1223. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C.S.; Sun, Y.H.; Wahia, H.; Sarpong, F.; Owusu-Ansah, P.; Osae, R.; Otu, P.; Ma, H.L. Simultaneous multifrequency: A possible alternative to improve the efficacy of ultrasound treatment on cherry tomato during storage. J. Food Process. Preserv. 2019, 43, e14083. [Google Scholar] [CrossRef]
- Ma, X.K.; Li, T.T.; He, Y.Q.; Chen, M.; Zhou, J.; Yin, L.J.; Ma, H.L. Preliminary Study on Ultrasonic Ageing Zhenjiang Vinegar Mechanism Based on Maillard Simulation System. J. Food Qual. 2020, 2020, 1087863. [Google Scholar] [CrossRef]
- Wali, A.; Ma, H.L.; Aadil, R.M.; Zhou, C.S.; Rashid, M.T.; Liu, X. Effects of multifrequency ultrasound pretreatment on the enzymolysis, ACE inhibitory activity, and the structure characterization of rapeseed protein. J. Food Process. Preserv. 2017, 41, e13413. [Google Scholar] [CrossRef]
- Yagoub, A.A.; Ma, H.; Zhou, C. Ultrasonic-assisted extraction of protein from rapeseed (Brassica napus L.) meal: Optimization of extraction conditions and structural characteristics of the protein. Int. Food Res. J. 2017, 24, 621–629. [Google Scholar]
- Abdualrahman, M.A.Y.; Zhou, C.S.; Zhang, Y.Y.; Yagoub, A.A.; Ma, H.L.; Mao, L.; Wang, K. Effects of ultrasound pretreatment on enzymolysis of sodium caseinate protein: Kinetic study, angiotensin-converting enzyme inhibitory activity, and the structural characteristics of the hydrolysates. J. Food Process. Preserv. 2017, 41, e13276. [Google Scholar] [CrossRef]
- Rashid, M.T.; Ma, H.; Jatoi, M.A.; Safdar, B.; El-Mesery, H.S.; Sarpong, F.; Ali, Z.; Wali, A. Multi-frequency ultrasound and sequential infrared drying on drying kinetics, thermodynamic properties, and quality assessment of sweet potatoes. J. Food Process Eng. 2019, 42, e13127. [Google Scholar] [CrossRef]
- Rashid, M.T.; Ma, H.; Safdar, B.; Jatoi, M.A.; Wali, A.; Sarpong, F.; Zhou, C.S. Synergy of ultrasound and osmotic dehydration in improving drying kinetics and quality of dried sweet potato (Ipomea batatas L.). J. Food Saf. Food Qual.-Arch. Fur Leb. 2019, 70, 72–81. [Google Scholar] [CrossRef]
- Shende, T.; Andaluri, G.; Suri, R. Power density modulated ultrasonic degradation of perfluoroalkyl substances with and without sparging Argon. Ultrason. Sonochem. 2021, 76, 105639. [Google Scholar] [CrossRef]
- Fagan, W.P.; Thayer, S.R.; Weavers, L.K. Kinetics and Mechanism of Ultrasonic Defluorination of Fluorotelomer Sulfonates. J. Phys. Chem. A 2023, 127, 6309–6319. [Google Scholar] [CrossRef]
- Awoyemi, O.S.; Luo, Y.; Niu, J.; Naidu, R.; Fang, C. Ultrasonic degradation of per-and polyfluoroalkyl substances (PFAS), aqueous film-forming foam (AFFF) and foam fractionate (FF). Chemosphere 2024, 360, 142420. [Google Scholar] [CrossRef]
- Fuller, M.E.; Zhao, Y.; Hedman, P.C.; van Groos, P.G.K.; Soto, A.; Boodoo, F.; Yniguez, J.; McKenzie, E.R. Sonochemical degradation of PFAS in ion exchange regeneration wastes. J. Hazard. Mater. 2024, 471, 134291. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Ma, C.X.; Li, C.Z.; Lin, L. Enhancing the antibacterial activity of thyme oil against Salmonella on eggshell by plasma-assisted process. Food Control 2016, 70, 183–190. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Promoting anti-listeria activity of lemongrass oil on pork loin by cold nitrogen plasma assist. J. Food Saf. 2017, 37, e12316. [Google Scholar] [CrossRef]
- Johnson, M.J.; Maza, W.A.; Breslin, V.M.; Boris, D.R.; Petrova, T.B.; Walton, S.G. Low power degradation of perfluorooctane sulfonate (PFOS) in water using a nanosecond pulsed atmospheric pressure plasma. Plasma Sources Sci. Technol. 2022, 31, 085001. [Google Scholar] [CrossRef]
- Cui, H.Y.; Ma, C.X.; Lin, L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Zhang, Y.; Heroux, P.; Cai, L.; Liu, Y. Removal of per- and polyfluoroalkyl substances from water by plasma treatment: Insights into structural effects and underlying mechanisms. Water Res. 2024, 253, 121316. [Google Scholar] [CrossRef]
- Topolovec, B.; Jovanovic, O.; Puac, N.; Skoro, N.; Lumbaque, E.C.; Petrovic, M. Plasma water treatment for PFAS: Study of degradation of perfluorinated substances and their byproducts by using cold atmospheric pressure plasma jet. J. Environ. Chem. Eng. 2024, 12, 112979. [Google Scholar] [CrossRef]
- Eeso, K.; Gallan, R.; Goukeh, M.N.; Tate, K.; Raja, R.K.B.; Popovic, Z.; Abichou, T.; Chen, H.; Locke, B.R.; Tang, Y. Degradation of per- and polyfluoroalkyl substances in landfill leachate by a thin-water-film nonthermal plasma reactor. Waste Manag. 2023, 161, 104–115. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Li, C.Z.; Cui, H.Y.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Chen, C.; Ma, C.; Yang, X.; Gromov, M.; Tian, Y.; Demeestere, K.; Nikiforov, A.; Van Hulle, S.W.H. Degradation of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in water by use of a nonthermal plasma-ozonation cascade reactor: Role of different processes and reactive species. Chem. Eng. J. 2024, 486, 150218. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Wang, Q.; Tian, Y.; Demeestere, K.; Nikiforov, A.; Van Hulle, S.W. Combining nonthermal N2 plasma with a denitrifying biofilm reactor for PFAS-contaminated wastewater remediation. Chem. Eng. J. 2024, 500, 157334. [Google Scholar] [CrossRef]
- Lakkasandrum, C.; Vasilev, M.; Holsen, T.M.; Thagard, S.M. Assessing the efficacy of the Plasma Spinning Disc Reactor (PSDR) in treating undiluted Aqueous Film Forming Foams (AFFFs). J. Hazard. Mater. 2025, 485, 136805. [Google Scholar] [CrossRef]
- de Souza, N.G.; Parenky, A.C.; Nguyen, H.H.; Jeon, J.; Choi, H. Removal of perfluoroalkyl and polyfluoroalkyl substances in water and water/soil slurry using Fe0-modified reactive activated carbon conjugated with persulfate. Water Environ. Res. 2022, 94, e1671. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, Z.; Qian, X.; Kai, O.; Wu, D.; Tang, F.; Hrynsphan, D.; Savitskaya, T.; Chen, J. Biodegradation of per- and polyfluoroalkyl substances: Microbes, enzymes and their interactions. Rev. Environ. Sci. Bio/Technol. 2025, 24, 43–62. [Google Scholar] [CrossRef]
- Teshita, A.; Khan, W.; Ullah, A.; Iqbal, B.; Ahmad, N. Soil Nematodes in Agroecosystems: Linking Cropping System’s Rhizosphere Ecology to Nematode Structure and Function. J. Soil Sci. Plant Nutr. 2024, 24, 6467–6482. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Olatunji, O.J.; Chen, H.X. Ameliorative effects of Lycium chinensis on male sexual dysfunction and testicular oxidative damage in streptozotocin-induced diabetic rats. Food Sci. Biotechnol. 2019, 28, 1217–1223. [Google Scholar] [CrossRef]
- Wang, C.P.; He, G.; Meng, J.; Wang, S.M.; Kong, Y.Z.; Jiang, J.X.; Hu, R.B.; Zhou, G.K. Improved lignocellulose saccharification of aMiscanthusreddish stem mutant induced by heavy-ion irradiation. Glob. Change Biol. Bioenergy 2020, 12, 1066–1077. [Google Scholar] [CrossRef]
- Osae, R.; Alolga, R.N.; Essilfie, G.; Osei-Adjei, G.; Baduweh, C.A.; Yarley, O.P.N.; Zhou, C.S. Variation in bioactive phytochemicals and sensory attributes of osmosonic convective dried ginger from four African countries. J. Sci. Food Agric. 2020, 100, 3164–3172. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.L.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Ayers, C.; Zhang, J. Defluorination of per- and polyfluoroalkyl carboxylic acids (PFCAs) by wood decomposer fungi. Mycologia 2025, 117, 576–588. [Google Scholar] [CrossRef]
- Merino, N.; Wang, N.; Gao, Y.; Wang, M.; Mahendra, S. Roles of various enzymes in the biotransformation of 6:2 fluorotelomer alcohol (6:2 FTOH) by a white-rot fungus. J. Hazard. Mater. 2023, 450, 131007. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, F.; Holsen, T.M.; Fernando, S.; Wang, S. Biotransformation of 6:2 fluorotelomer sulfonate (6:2 FTS) in sulfur-rich media by Trametopsis cervina. Front. Environ. Sci. Eng. 2024, 18, 107. [Google Scholar] [CrossRef]
- Smorada, C.M.; Sima, M.; Jaffe, P. Bacterial degradation of perfluoroalkyl acids. Curr. Opin. Biotechnol. 2024, 88, 103170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.H.; Sun, H.; Wang, M.X.; Chen, Q.S.; Guo, Z.M.; Wu, J.Z. Rapid Pseudomonas Species Identification from Chicken by Integrating Colorimetric Sensors with Near-Infrared Spectroscopy. Food Anal. Methods 2018, 11, 1199–1208. [Google Scholar] [CrossRef]
- Huang, S.; Pilloni, G.; Key, T.A.; Jaffe, P.R. Defluorination of various perfluoro alkyl acids and selected PFOA and PFOS monomers by Acidimicrobium sp. Strain A6 enrichment cultures. J. Hazard. Mater. 2024, 480, 136426. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, H.; Cheng, D.; Wang, Y.; Hu, Z.; Wu, H.; Xie, H.; Zhang, J. Insights into poly-and perfluoroalkyl substances (PFAS) removal in treatment wetlands: Emphasizing the roles of wetland plants and microorganisms. Water Res. 2025, 268, 122702. [Google Scholar] [CrossRef]
- Mao, H.P.; Kumi, F.; Li, Q.L.; Han, L.H. Combining X-ray computed tomography with relevant techniques for analyzing soil-root dynamics—An overview. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2016, 66, 1–19. [Google Scholar] [CrossRef]
- Shabbir, A.; Mao, H.P.; Ullah, I.; Buttar, N.A.; Ajmal, M.; Lakhiar, I.A. Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato. Agronomy 2020, 10, 1685. [Google Scholar] [CrossRef]
- Chen, A.H.; Liang, H.X.; Chen, T.M.; Yang, W.J.; Ding, C. Influence of long-term irrigation with treated papermaking wastewater on soil ecosystem of a full-scale managed reed wetland. J. Soils Sediments 2016, 16, 1352–1359. [Google Scholar] [CrossRef]
- Ferrario, C.; Peruzzi, C.; Cislaghi, A.; Polesello, S.; Valsecchi, S.; Lava, R.; Zanon, F.; Santovito, G.; Barausse, A.; Bonato, M. Assessment of Reed Grasses (Phragmites australis) Performance in PFAS Removal from Water: A Phytoremediation Pilot Plant Study. Water 2022, 14, 946. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.N.; Chen, H.J.; Sun, D.W.; Deng, B.X.; Li, J.W.; Liu, Y.F.; Wang, F. Inactivation of Lipase and Lipoxygenase of Wheat Germ with Temperature-Controlled Short Wave Infrared Radiation and Its Effect on Storage Stability and Quality of Wheat Germ Oil. PLoS ONE 2016, 11, e0167330. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, L.K.; Miao, W.J.; Wu, Q.F.; Liu, Y.X.; Sun, Y.L.; Gao, C. Thermal versus Microwave Inactivation Kinetics of Lipase and Lipoxygenase from Wheat Germ. J. Food Process Eng. 2016, 39, 247–255. [Google Scholar] [CrossRef]
- Harris, B.A.; Zhou, J.; Clarke, B.O.; Leung, I.K.H. Enzymatic Degradation of PFAS: Current Status and Ongoing Challenges. ChemSusChem 2025, 18, e202401122. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liang, S.; Huang, Q. Laccase induced degradation of perfluorooctanoic acid in a soil slurry. J. Hazard. Mater. 2018, 359, 241–247. [Google Scholar] [CrossRef]
- Steffens, S.D.; Antell, E.H.; Cook, E.K.; Rao, G.; Britt, R.D.; Sedlak, D.L.; Alvarez-Cohen, L. An Artifact of Perfluoroalkyl Acid (PFAA) Removal Attributed to Sorption Processes in a Laccase Mediator System. Environ. Sci. Technol. Lett. 2023, 10, 337–342. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.Y.; Venkitasamy, C.; Wu, B.G.; Pan, Z.L.; Ma, H.L. Effect of pulsed light on activity and structural changes of horseradish peroxidase. Food Chem. 2017, 234, 20–25. [Google Scholar] [CrossRef]
- Colosi, L.M.; Pinto, R.A.; Huang, Q.; Weber, W.J., Jr. Peroxidase-mediated degradation of perfluorooctanoic acid. Environ. Toxicol. Chem. 2009, 28, 264–271. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Stoica, C.; Iftode, C.; Pirvu, F.; Petre, V.A.; Paun, I.; Pascu, L.F.; Vasile, G.G.; Nita-Lazar, M. Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability 2023, 15, 14000. [Google Scholar] [CrossRef]
- Bizkarguenaga, E.; Zabaleta, I.; Mijangos, L.; Iparraguirre, A.; Fernandez, L.A.; Prieto, A.; Zuloaga, O. Uptake of perfluorooctanoic acid, perfluorooctane sulfonate and perfluorooctane sulfonamide by carrot and lettuce from compost amended soil. Sci. Total Environ. 2016, 571, 444–451. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q.Y. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.L.; Ayim, I.; Aheto, J.H.; Hong, C.; Zhou, C.S. Reduction of Listeria innocua in fresh-cut Chinese cabbage by a combined washing treatment of sweeping frequency ultrasound and sodium hypochlorite. LWT 2019, 101, 410–418. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Z.; Hong, R.; Chen, Z.; Wu, B.; Ding, S.; Zhu, W.; Lin, Y.; Gu, C. Supramolecular assemblies of a newly developed indole derivative for selective adsorption and photo-destruction of perfluoroalkyl substances. Water Res. 2022, 225, 119147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, X.; Hong, R.; Wang, X.; Chen, Z.; Gao, G.; He, H.; Liu, J.; Gu, C. New Indole Derivative Heterogeneous System for the Synergistic Reduction and Oxidation of Various Per-/Polyfluoroalkyl Substances: Insights into the Degradation/Defluorination Mechanism. Environ. Sci. Technol. 2023, 57, 21459–21469. [Google Scholar] [CrossRef]
- Jiang, T.; Pervez, N.; Ilango, A.K.; Liang, Y. Enhanced removal and destruction of per- and polyfluoroalkyl substances (PFAS) mixtures by coupling magnetic modified clay and photoreductive degradation. J. Water Process Eng. 2025, 69, 106733. [Google Scholar] [CrossRef]
- Tian, S.; Xu, T.; Fang, L.; Zhu, Y.; Li, F.; Leary, R.N., III; Zhang, M.; Zhao, D.; Soong, T.-Y.; Shi, H. A ’Concentrate-&-Destroy’ technology for enhanced removal and destruction of per- and polyfluoroalkyl substances in municipal landfill leachate. Sci. Total Environ. 2021, 791, 148124. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.; Minda, V.; Hawley, E.; Deeb, R.; Hart, M. Development and Laboratory Scalability of Ultraviolet-Activated Silica-Based Granular Media as an Engineered System for the Degradation of Per- and Polyfluoroalkyl Substances in Concentrated Liquid Waste. J. Environ. Eng. 2023, 149, 04023049. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Li, X.; Luo, Y.; Zheng, H.; Li, X.; Chen, L.; Li, F. Adsorption-photocatalysis synergistic removal of perfluoroalkyl/ polyfluoroalkyl substances by reusable chitin/polyethylene imine/O-g-C3N4 sponges in water environments. Chem. Eng. J. 2025, 511, 162006. [Google Scholar] [CrossRef]
- Smith, S.J.; Lauria, M.; Ahrens, L.; McCleaf, P.; Hollman, P.; Bjalkefur Seroka, S.; Hamers, T.; Arp, H.P.H.; Wiberg, K. Electrochemical Oxidation for Treatment of PFAS in Contaminated Water and Fractionated Foam—A Pilot-Scale Study. ACS ES T Water 2023, 3, 1201–1211. [Google Scholar] [CrossRef]
- Kim, N.; Elbert, J.; Shchukina, E.; Su, X. Integrating redox-electrodialysis and electrosorption for the removal of ultra-short- to long-chain PFAS. Nat. Commun. 2024, 15, 8321. [Google Scholar] [CrossRef]
- Broman, J.; Ceja, A.; Godoy, T.; Rivera, D.R.; Dionne, P.; Schipper, J.; Henkemeyer, S.; Cegielski, S.; Wong, G.; Kaur, A. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) via Lacasse Enzymatic Degradation and Electrochemical Advanced Oxidation. In Proceedings of the 31st Waste-Management Education Research Conference (WERC), Las Cruces, NM, USA, 11–14 April 2021. [Google Scholar] [CrossRef]

| Treatment Technology | Source of PFAS | Target PFAS | Removal Efficiency or Adsorption Capacity | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|---|---|
| Adsorption | Granular activated carbon | Contaminated groundwater | PFOA PFBS | 0.86 μg/g 0.015 μg/g | The removal effect of long-chain PFAS is better. | The removal effect of short-chain PFAS is poor. Low adsorption capacity. The mass transfer rate is slow. | [58] |
| Macroporous AERs | AFFF diluent | PFOA PFOS | 0.26–0.88 μmol/mg 0.66–1.36 μmol/mg | Targeted selection. Renewable potential. | The removal effect of short-chain PFAS is poor. Special resins (e.g., PAER) are more expensive. Regeneration processes (e.g., salt solution elution) may lead to secondary pollution. | [48] | |
| MOF NU-1000 | AFFF contaminated groundwater | Anionic PFAS (PFCA, PFSA, FTS, FTCA) Non-ionic PFAS (FASA) | 58% 99% | High structural stability. Fast adsorption speed. | The ions (Cl−, NO3−, and CO32−) in the water matrix compete with PFAS for adsorption sites, resulting in a decrease in adsorption efficiency. | [47] | |
| TFA-MOF-808 | Simulated water samples prepared in the laboratory | PFOA PFBA PFHxA | 2496 mg/g 311 mg/g 436 mg/g | High adsorption capacity. Recycle regeneration. | The synthesis cost of functionalized MOF is high. Ligands such as trifluoroacetic acid released during the adsorption process may cause secondary pollution and require additional treatment. | [44] | |
| Ti3C2 MXenes | AFFF diluent | 6:2 FTAB, 5:1:2 FTB | >80% | Low environmental pollution. | Ti3C2 MXenes need to be prepared by hydrofluoric acid (HF), and the synthesis process is dangerous and costly, which limits large-scale production. | [49] | |
| Membrane Filtration | NF | Laboratory-simulated wastewater | PFAAs | >98% | Low energy consumption. | The removal effect of short-chain PFAS is poor. | [54] |
| AFFF-contaminated groundwater | PFCAs Short-chain PFSAs Long-chain PFSAs | 92–98% 92–95% >98% | |||||
| RO | Laboratory-simulated wastewater | PFAAs | >99% | Tolerance to high salt and organic matter. | High cost and energy consumption. | ||
| Amyloid–carbon hybrid membrane | Water samples from Xiaoqing River Basin, China | PFAS (C ≥ 4), PFBA | >96% | Low pollution. Low energy consumption. | The preparation of the membrane requires a high temperature (90 °C) and acidic conditions (pH = 2), and industrial production may face energy consumption and cost challenges. | [55] | |
| Aqueous electrostatic concentration | Actual polluted water samples | PFOA, PFOS | >99% | Low energy consumption. Less waste. | Long-term treatment of high suspended solids or organic wastewater may lead to membrane fouling. | [56] | |
| Foam fractionation | Sewage treatment plant wastewater | PFOS PFOA | 99% 94% | Adapting to complex matrices. Low energy consumption. Environmentally friendly. | The removal effect of short-chain PFAS is poor. A too high air flow rate can easily lead to liquid entrainment and reduce removal efficiency. | [57] | |
| Treatment Technology | Target PFAS | Treatment Conditions | Degradation Efficiency | References | |
|---|---|---|---|---|---|
| Thermal Degradation | Hydrothermal treatment | PFCAs | 300 °C, 0.5 h | 100% | [66] |
| Hydrothermal alkaline treatment | PFOS | 350 °C, 0.5 h, 5 M NaOH | 100% | [60] | |
| Subcritical hydrothermal treatment of Fe-based amorphous alloys | PFOS | 325 °C, 1 h, 1 M NaHCO3 | 85% | [74] | |
| Induction heating | PFCAs, HFPO-DA | 40 s (the temperature rose from 22 °C to 500 °C in the first 30 s and continued to rise to 845 °C in the last 30 s) | >99.5% | [77] | |
| Electrochemical Degradation | Molecular copper electrocatalysts | PFOA | −5 mA, 4 h | 93% | [65] |
| Bph-RGO | PFOS PFOA | Landfill leachate, pH 5.6 | 95% 75% | [88] | |
| BDD | PFOA | 0.5 Mm persulfate, 16.9 A/cm2, 2 h | >99% | [86] | |
| Photochemical Degradation | UV/sulfite + iodide (UV/S + I) system | PFBS | 24 h | >99.7% | [94] |
| UV/chlorine system | PFOA | UV irradiation, 1.4 Mm NaOCl (106 mg/L), 0.5 h | 12% | [95] | |
| Titanium-based MOF material MIL-125-NH2 | PFOA | UV irradiation, 24 h | 98.9% | [96] | |
| Sonochemical Degradation | PFOA PFOS | 30–262 W/L, 2 h | 43–98% 34–97% | [108] | |
| 4:2 FTS 6:2 FTS 8:2 FTS PFOS | 354 kHz, 4 h | >99% >99% 86% 89% | [109] | ||
| Plasma-based Technologies | Falling film dielectric barrier discharge plasma technology | PFOA PFPeA | 1 h 40 min | >95% 42.5% | [116] |
| Non-thermal nitrogen (N2) plasma combined with a denitrifying biofilm reactor | PFOA PFOS | 1 h | 45% 60% | [121] | |
| Category | Target PFAS | Treatment Conditions | Removal Efficiency | References | |
|---|---|---|---|---|---|
| Bacteria | Acidimicrobium sp. Strain A6 | PFOA PFOS | 120 days | 59.1% 39.9% | [135] |
| Pseudomonas aeruginosa | PFOA PFOS | 96 h | 27.9% 47.3% | [148] | |
| Pseudomonas putida | PFOA PFOS | 96 h | 19% 46.9% | [148] | |
| Fungus | Trametopsis cervina | 6:2 FTS | 30 days | 50% | [132] |
| Plants | Reed | PFOS PFBA PFBS PFOA | 5 days | 83.7% 71% 64.9% 61% | [140] |
| Carrot | FOSA | 98 days | 100% | [149] | |
| Lettuce | FOSA | TOC 2.3%, 35 days TOC 53%, 35 days | 50% 80% | [149] | |
| Enzymes | Laccase | PFOA | Aqueous phase, 36 days Soil slurry, 140 days | 24% 40% | [144] |
| PFOA PFOS | Laccase and 1-hydroxybenzotriazole were added twice, 24 h | 64% 67% | [145] | ||
| Horseradish peroxidase | PFOA | Hydrogen peroxide and 4-methoxyphenol as co-substrates, 6 h | 68% | [147] | |
| Combination Mode | Target PFAS | Processing Times | Removal Efficiency | References |
|---|---|---|---|---|
| Physical treatment (di-indole hexadecyl ammonium) + chemical treatment (UV photocatalysis) | PFOA PFOS | 10 s (physical treatment) 2 h (chemical treatment) | >99% (physical treatment) 95% (chemical treatment) >99% (physical treatment) 92% (chemical treatment) | [153] |
| Physical treatment (magnetic modified clay) + chemical treatment (UV photocatalysis) | PFBS GenX PFOA PFOS | 48 h | 27% (defluorination rate) 66% (defluorination rate) 42% (defluorination rate) 27% (defluorination rate) The removal efficiencies of the four substances with physical treatment were all 99% | [154] |
| Physical treatment (foam separation) + chemical treatment (electrochemical oxidation) | PFOA PFOS | 9 h | 92% (physical treatment) 68% (chemical treatment) 91% (physical treatment) 36% (chemical treatment) | [158] |
| Chitin/polyethylene imine/oxygen-doped graphitic carbon nitride sponges | PFOA PFOS | 2 h (adsorption stage) 3 h (photocatalytic stage) | 97.9% 99.7% | [157] |
| Physical treatment (reverse osmosis, foam fractionation) + biological treatment (laccase enzymatic hydrolysis) + chemical treatment (electrochemical oxidation) | PFOA PFOS | Biological treatment time was not mentioned 14 h (chemical treatment) | 35% (biological treatment) >99% (chemical treatment) 35% (biological treatment) 97% (chemical treatment) | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Wang, M.; Xu, L.; Qian, J.; Zou, B.; Huo, S.; Guan, G.; Cui, K. Strategies for the Removal of Per- and Polyfluoroalkyl Substances: A Review. Catalysts 2025, 15, 678. https://doi.org/10.3390/catal15070678
Wang F, Wang M, Xu L, Qian J, Zou B, Huo S, Guan G, Cui K. Strategies for the Removal of Per- and Polyfluoroalkyl Substances: A Review. Catalysts. 2025; 15(7):678. https://doi.org/10.3390/catal15070678
Chicago/Turabian StyleWang, Feng, Mingtong Wang, Ling Xu, Jingya Qian, Bin Zou, Shuhao Huo, Guoqiang Guan, and Kai Cui. 2025. "Strategies for the Removal of Per- and Polyfluoroalkyl Substances: A Review" Catalysts 15, no. 7: 678. https://doi.org/10.3390/catal15070678
APA StyleWang, F., Wang, M., Xu, L., Qian, J., Zou, B., Huo, S., Guan, G., & Cui, K. (2025). Strategies for the Removal of Per- and Polyfluoroalkyl Substances: A Review. Catalysts, 15(7), 678. https://doi.org/10.3390/catal15070678








