Application of Fused Filament Fabrication in Preparation of Ceramic Monolithic Catalysts for Oxidation of Gaseous Mixture of Volatile Aromatic Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Polymer–ZrO2-Based Filament
2.1.1. Moisture Resistance Test
2.1.2. Determination of the Proportion of the Ceramic Phase in the Filament
2.1.3. FTIR Analysis
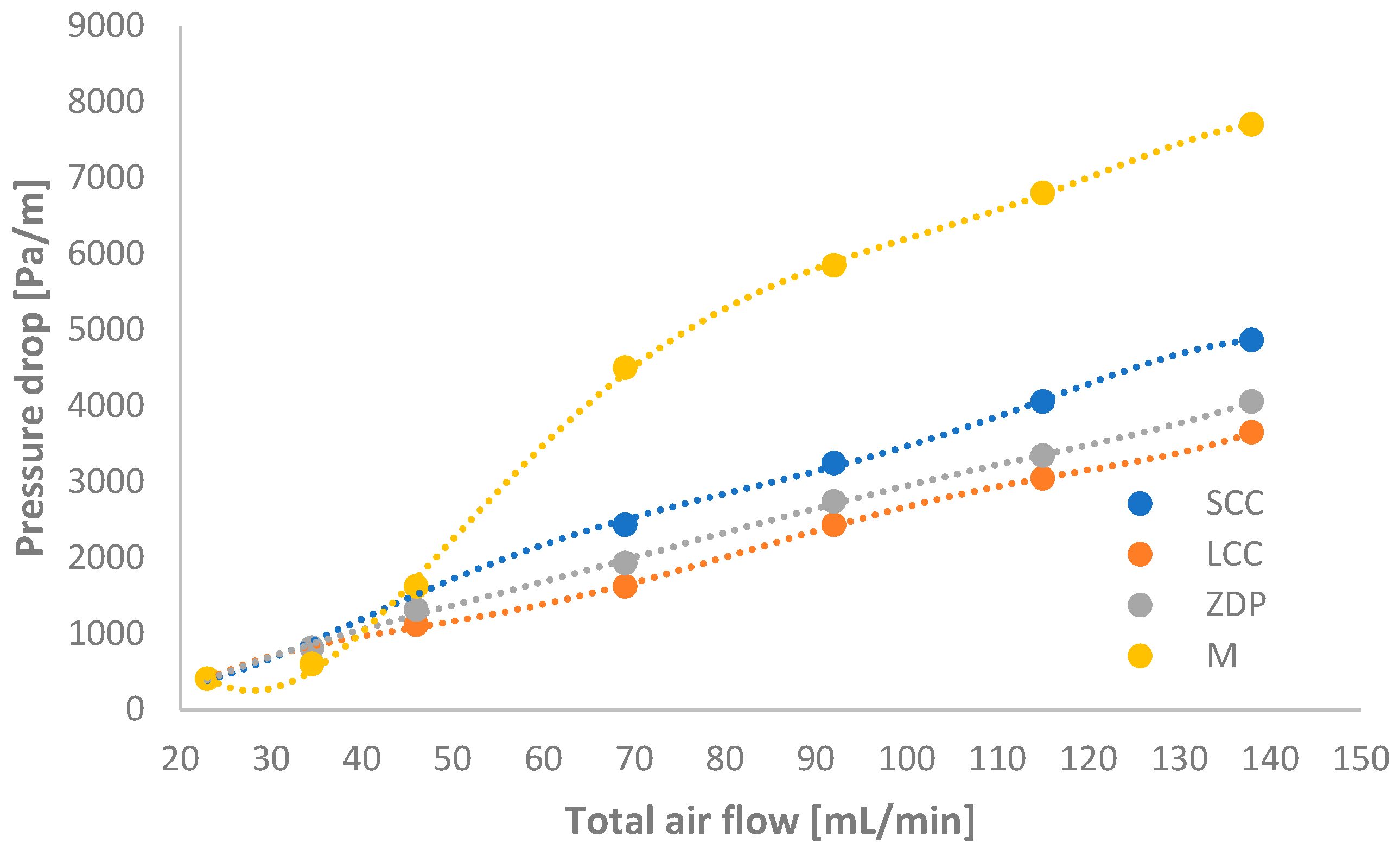
2.1.4. Measurement of Pressure Drop Through Various Monolithic Structures
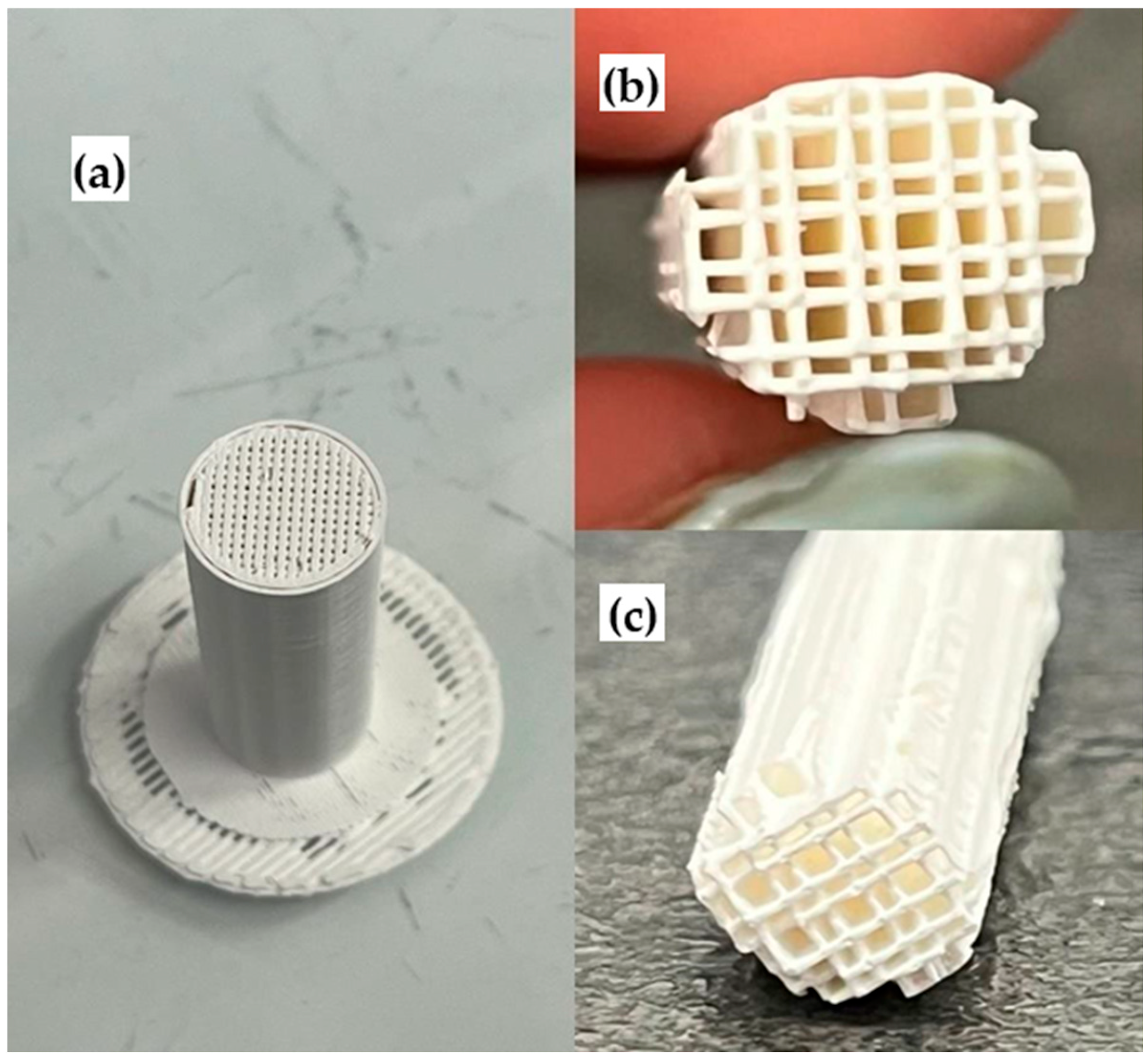
2.2. Prepared Monolithic Catalyst Carriers for Study of Catalytic Activity
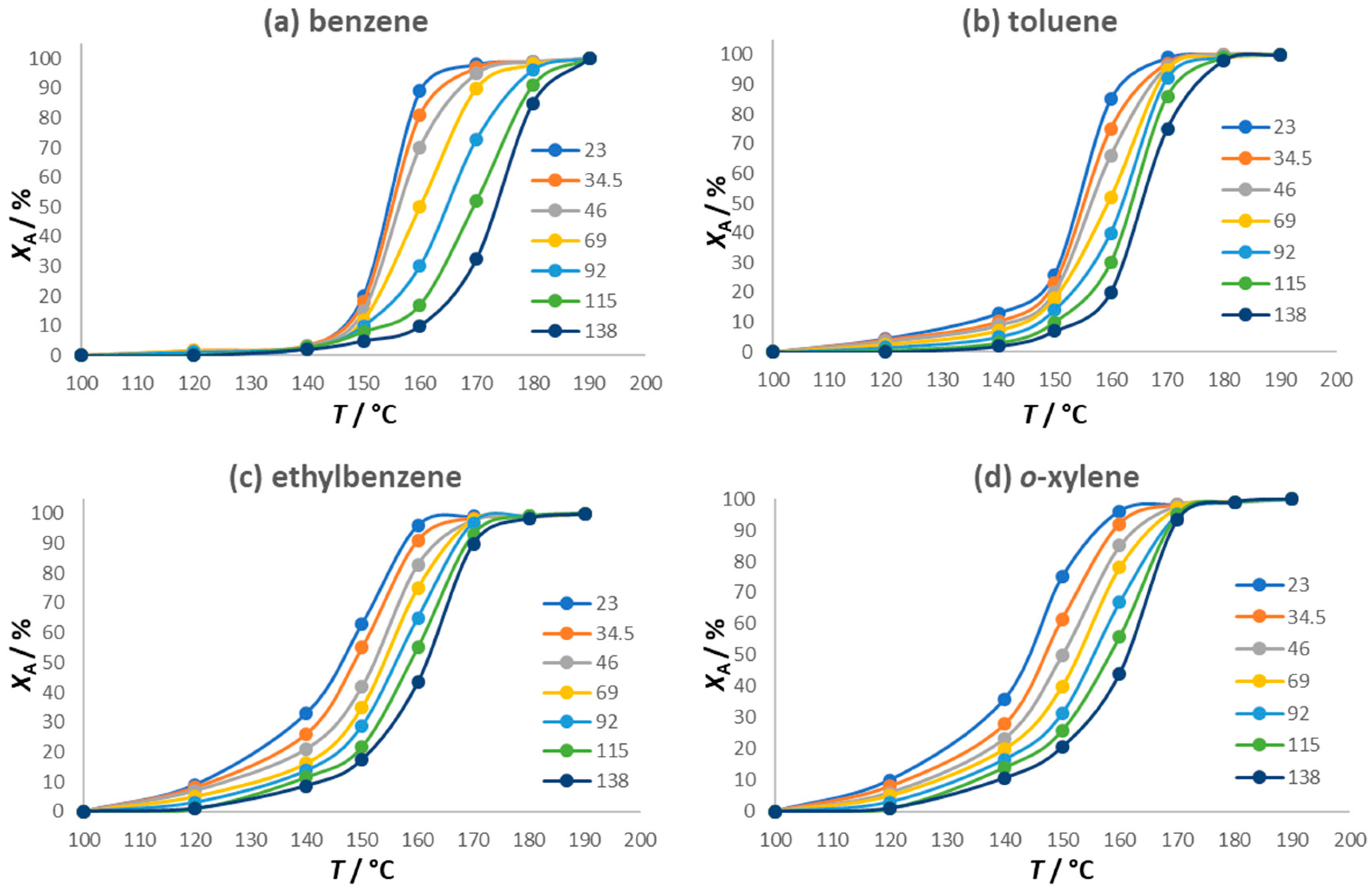
2.3. Catalytic Activity for BTEX Oxidation
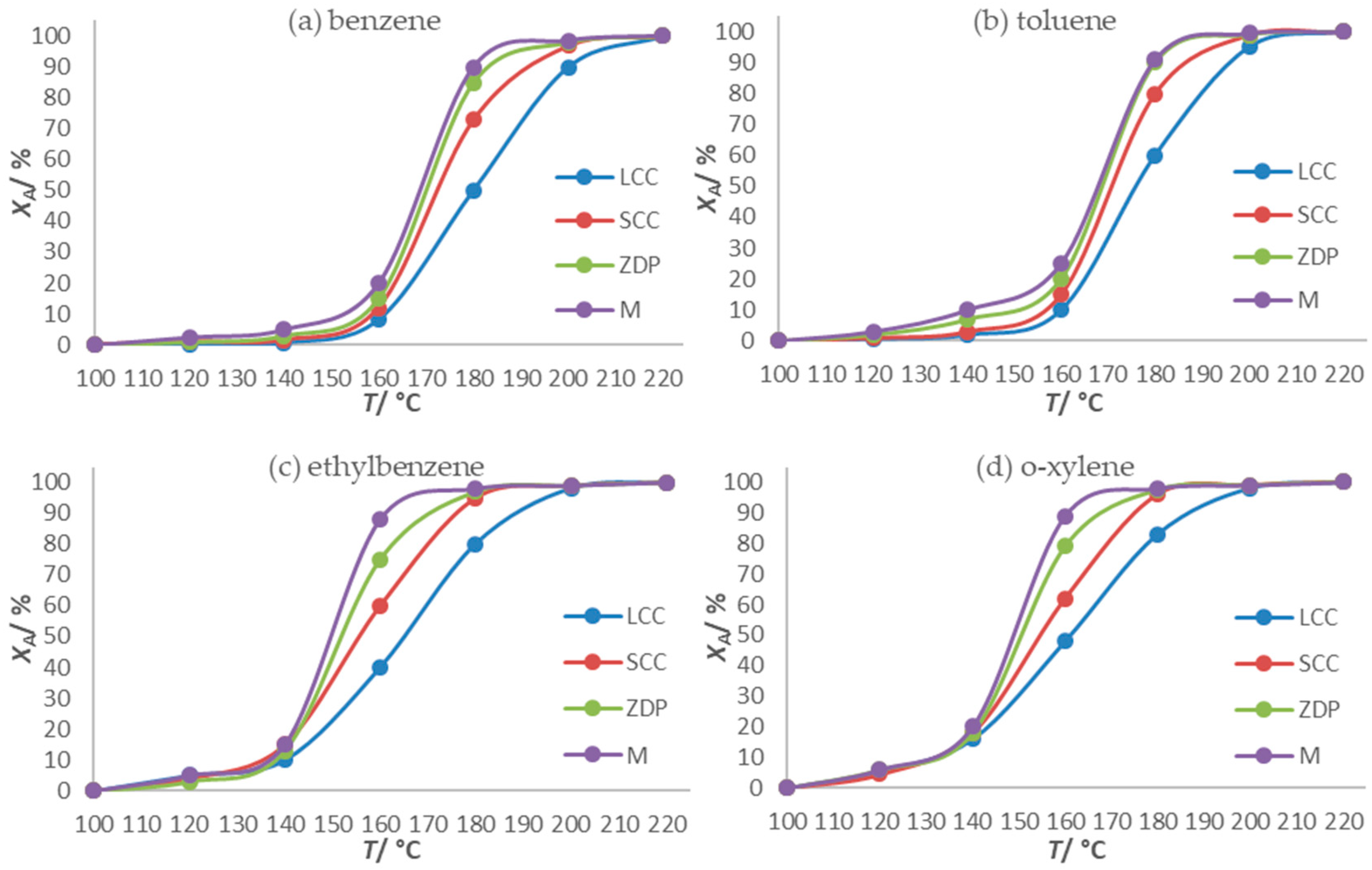
Comparison of Catalytic Activity of Monolithic Catalysts Obtained by 3D-Printing Technology with That of Commercial Cordierite
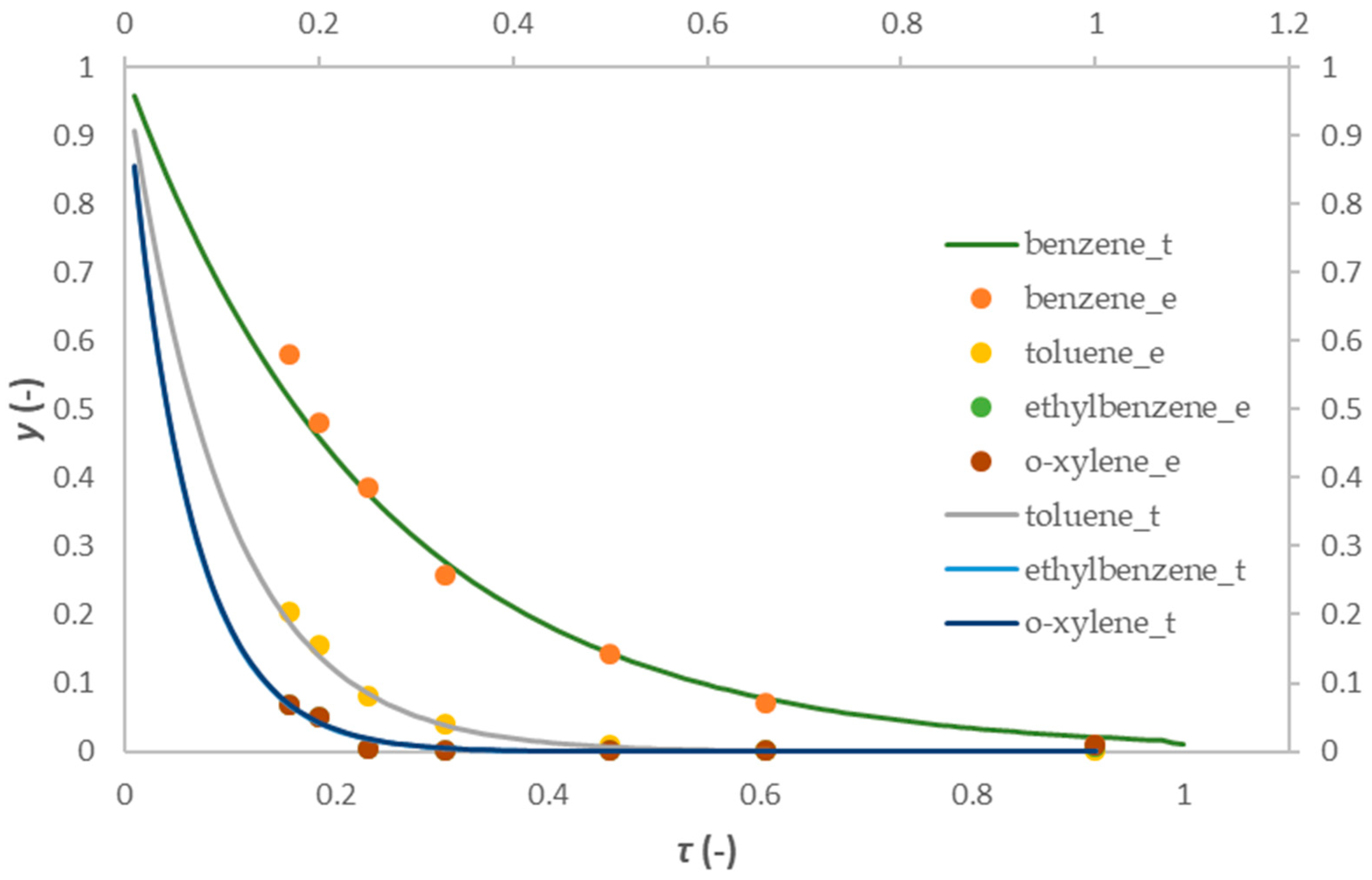
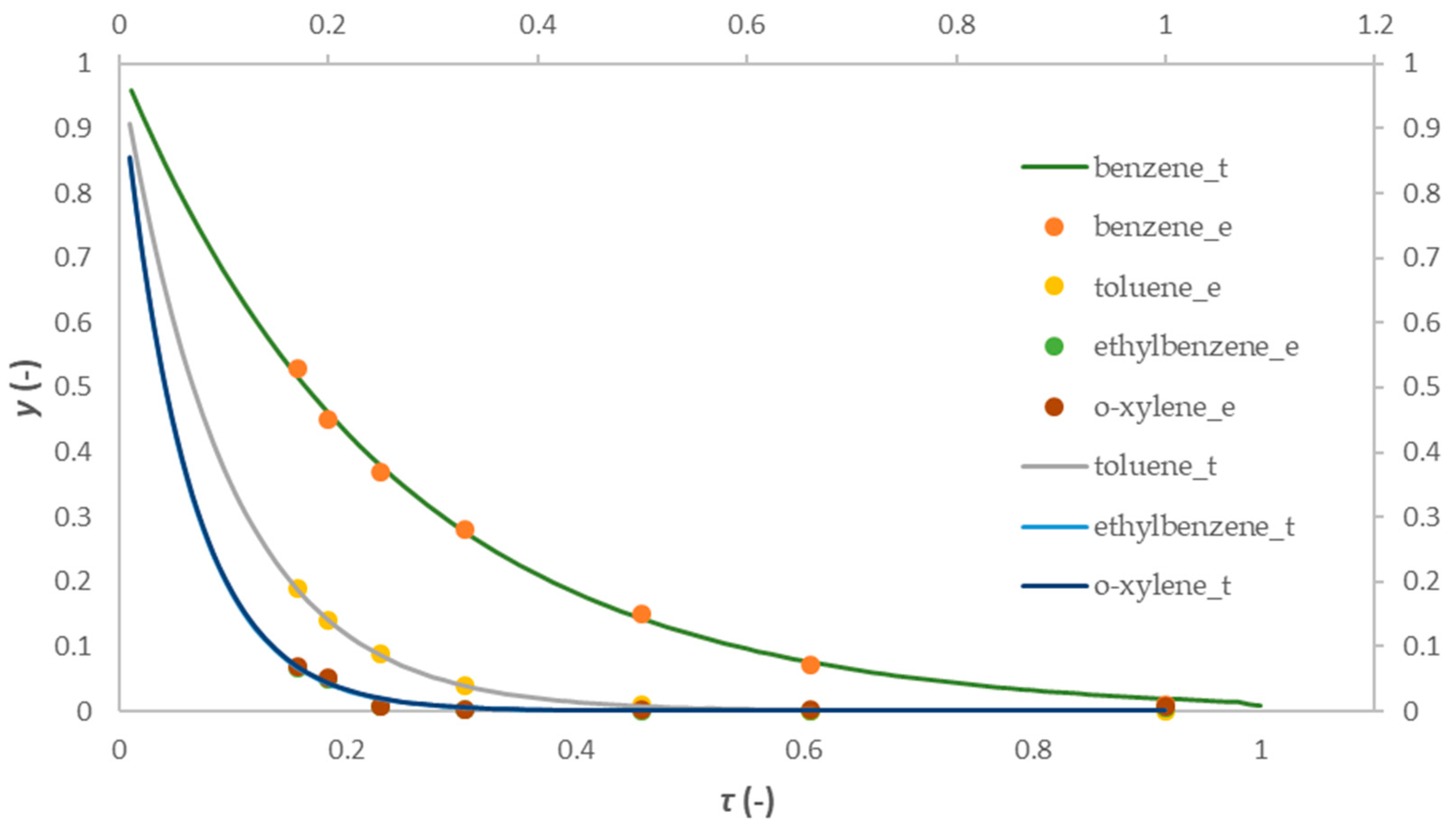
2.4. Mathematical Modeling Results
2.4.1. One-Dimensional Pseudo-Homogeneous Reactor Model
- −
- Steady-state operation (monolithic reactors usually operate at a steady state with a constant inflow of the reaction mixture for a given measurement cycle, while any non-stationarity is present at the beginning of operation or may occur as a result of a malfunction);
- −
- Isothermal operating conditions (due to operation with very low concentrations of the model components and with a low mass of the catalytic layer);
- −
- The ideal flow of the reaction mixture through the reactor (due to operation with relatively high flow rates);
- −
- The negligible influence of intraphase diffusion (due to a very thin catalytic layer);
- −
- A negligible pressure drop along the length of the monolithic catalyst (a common assumption for monolithic structures, experimentally confirmed by independent measurements);
- −
- The absence of catalyst deactivation;
- −
- The presence of interphase diffusion (i.e., the mass transfer of substances between the phases to the outer surface of the catalyst (a key factor when working with monolithic catalysts));
- −
- The chemical reaction takes place on the surface of the catalyst layer.
- −
- The mass balance in the fluid:
- −
- The mass balance on the catalyst surface:
2.4.2. 1D Heterogeneous Reactor Model
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Ceramic Monolithic Catalyst Carriers
3.2.1. FFF Technology
3.2.2. SLA Technology
3.2.3. Commercial Cordierite
3.3. Characterization of Polymer–ZrO2 Composite Filament
3.3.1. Moisture Resistance Test
3.3.2. Determination of Content of Ceramic Phase in ZrO2-Based Filament
3.3.3. FTIR Analysis
3.3.4. Pressure Drop Measurements
3.4. Coating of 3D-Printed Carriers with Catalytically Active Components
3.5. Evaluation of Catalytic Performance
3.6. Mathematical Modeling
4. Conclusions
- −
- The results of the FTIR analysis showed that the ZrO2 filament probably contained EVA as the polymer phase. Based on the determination of the proportion of the polymer and ceramic phase, it was found that the material was homogeneous, and that a mass reduction of 14% occurred during the heat treatment of the filament.
- −
- The results of the experimental determination of the pressure drop showed that the pressure drop was relatively low; i.e., it ranged from 400 to 7700 Pa/m.
- −
- The comparison of the results of the catalytic oxidation of a mixture of BTEX compounds on monolithic catalysts produced using the SLA process and FFF process and monolithic cordierite catalysts with different channel dimensions showed that 3D printing can not only compete with commercially available ceramics in terms of the conversions achieved, but also that the results are even better than those achieved with monolithic catalyst carriers made of commercially available cordierite ceramics.
- −
- In the last part of the research, 1D pseudo-homogeneous and 1D heterogeneous reactor models were derived, which included a kinetic model for the first-order reaction, and were used to describe the oxidation of a mixture of BTEX compounds in a monolithic reactor prepared by the FFF technique. An assessment of the acceptability of the proposed models was carried out. The values of the key parameters of the proposed models were estimated based on a comparison of the experimental results with the theoretical values obtained according to the proposed models. Based on the corresponding mean-square deviation (SD) values, it was found that the proposed models described the experimental results well. Compared to the 1D pseudo-homogeneous model, the 1D heterogeneous model provides a more realistic description of the studied system and allows conclusions to be drawn about the influence of interphase diffusion on the overall oxidation rate on a heterogeneous catalyst.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Słomińska, M.; Król, S.; Namieśnik, J. Removal of BTEX Compounds from Waste Gases; Destruction and Recovery Techniques. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1417–1445. [Google Scholar] [CrossRef]
- Shojaei, A.; Ghafourian, H.; Yadegarian, L.; Lari, K.; Sadatipour, M.T. Removal of volatile organic compounds (VOCs) from waste air stream using ozone assisted zinc oxide (ZnO) nanoparticles coated on zeolite. J. Environ. Health Sci. Eng. 2021, 19, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, S.; Zhang, X.; Verma, P.; Wen, M. Advances in Catalytic Oxidation of Volatile Organic Compounds over Pd-Supported Catalysts: Recent Trends and Challenges. Front. Mater. 2020, 7, 595667. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Yao, C.; Ma, Y.; Zhao, J. Recent advances of photocatalytic degradation for BTEX: Materials, operation, and mechanism. Chem. Eng. J. 2023, 455, 140461. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Feng, X. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Berenjian, A.; Chan, N.; Malmiri, H.J. Volatile organic compounds removal methods: A review. Am. J. Biochem. Biotechnol. 2012, 8, 220–229. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Johnston, K.R.; Hu, S. Kinetics, Thermodynamics, and Regeneration of BTEX Adsorption in Aqueous Solutions via NaOCl-Oxidized Carbon Nanotubes. In Environanotechnology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 71–97. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Kim, J.H.; Kim, J.H.; Kang, S.H.; Ryu, J.H.; Park, N.K.; Yun, D.S.; Bae, J.W. Noble-Metal-Based Catalytic Oxidation Technology Trends for Volatile Organic Compound (VOC) Removal. Catalysts 2022, 12, 63. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Shakeri, H.; Arshadi, M.; Salvacion, J.W.L. Removal of BTEX by using a surfactant—Bio originated composite. J. Colloid Interface Sci. 2016, 466, 186–197. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.H.; He, J.; Zhang, X.D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 15, 8324826. [Google Scholar] [CrossRef]
- Lou, B.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.; Zhao, Y.; Zhao, W.; Jiang, Y.; Rui, Y. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- De los Santos, C.; Vidal, H.; Gatica, J.M.; Yeste, M.P.; Cifredo, G.; Castiglioni, J. Optimized preparation of washcoated clay honeycomb monoliths as support of manganese catalysts for acetone total combustion. Microporous Mesoporous Mater. 2021, 310, 110651. [Google Scholar] [CrossRef]
- Lu, T.; Su, F.; Zhao, Q.; Li, J.; Zhang, C.; Zhang, R.; Liu, P. Catalytic oxidation of volatile organic compounds over manganese-based oxide catalysts: Performance, deactivation and future opportunities. Sep. Purif. Technol. 2022, 296, 121436. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Kreutzer, M.T.; Nijhuis, T.A.; Kapteijn, F. Monolithic Catalysts and Reactors: High Precision with Low Energy Consumption. Chem. React. Eng. 2011, 54, 249–327. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Hosseini, S.; Moghaddas, H.; Soltani, S.M.; Kheawhom, S. Technological Applications of Honeycomb Monoliths Prepared via Extrusion and Coating of Structured Supports in Environmental Processes: A Review. Process Saf. Environ. Prot. 2019, 133, 286–300. [Google Scholar] [CrossRef]
- Alimi, O.A.; Akinnawo, C.A.; Meijboom, R. Monolith catalyst design by 3D printing: A reusable support for modern palladium-catalyzed cross-coupling reactions. New J. Chem. 2020, 44, 18867–18878. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Li, K.; Yu, X.; Wei, Y.; Liu, J.; Peng, Y.; Li, Y.; Chen, Y. Highly Active Monolith Catalysts of LaKCoO3 Perovskite-type Complex Oxide on Alumina-washcoated Diesel Particulate Filter and the Catalytic Performances for the Combustion of Soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Figueiredo, J.L.; Sanz, O.; Montes, M.; Pereira, M.F.R. Preparation of ceramic and metallic monoliths coated with cryptomelane as catalysts for VOC abatement. Chem. Eng. J. 2019, 382, 122923. [Google Scholar] [CrossRef]
- Aguero, F.N.; Morales, M.R.; Duran, F.G.; Barbero, B.P.; Cadus, L.E. MnCu/Cordierite Monolith used for Catalytic Combustion of Volatile Organic Compounds. Chem. Eng. Technol. 2013, 36, 1749–1754. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Li, M.; Mo, F.; Yu, S.; Wei, J. Catalytic Materials by 3D Printing: A Mini Review. Catalysts 2022, 12, 1081. [Google Scholar] [CrossRef]
- Lawson, S.; Li, X.; Thakkar, H.; Rownaghi, A.A.; Rezai, F. Recent Advances in 3D Printing of Structured Materials for Adsorption and Catalysis Applications. Chem. Rev. 2021, 121, 6246–6291. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3D printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef]
- Guan, Z.; Yang, X.; Liu, P.; Xu, X.; Li, Y.; Yang, X. Additive manufacturing of zirconia ceramic by fused filament fabrication. Ceram. Int. 2023, 49, 27742–27749. [Google Scholar] [CrossRef]
- Nötzel, D.; Eickhoff, R.; Pfeifer, C.; Hanemann, T. Printing of Zirconia Parts via Fused Filament Fabrication. Materials 2021, 14, 5467. [Google Scholar] [CrossRef]
- Bogdan, E.; Michorczyk, P. 3D Printing in Heterogeneous Catalysis—The State of the Art. Materials 2020, 13, 4534. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, P.; Chao, Y.; Yu, J.; Zhu, W.; Liu, Z.; Xu, C. Recent advances in 3D printing for catalytic applications. Chem. Eng. J. 2022, 433, 134341. [Google Scholar] [CrossRef]
- He, Q.; Jiang, J.; Yang, X.; Zhang, L.; Zhou, Z.; Zhong, Y.; Shen, Z. Additive manufacturing of dense zirconia ceramics by fused deposition modeling via screw extrusion. J. Eur. Ceram. Soc. 2021, 41, 1033–1040. [Google Scholar] [CrossRef]
- Cano, S.; Gonzalez-Gutierrez, J.; Sapkota, J.; Spoerk, M.; Arbeiter, F.; Schuschnigg, S.; Holzer, C.; Kukla, C. Additive manufacturing of zirconia parts by fused filament fabrication and solvent debinding: Selection of binder formulation. Addit. Manuf. 2019, 26, 117–128. [Google Scholar] [CrossRef]
- Cano, S.; Lube, T.; Huber, P.; Gallego, A.; Naranjo, J.A.; Berges, C.; Schuschnigg, S.; Herranz, G.; Kukla, C.; Holzer, C.; et al. Influence of the Infill Orientation on the Properties of Zirconia Parts Produced by Fused Filament Fabrication. Materials 2020, 13, 3158. [Google Scholar] [CrossRef]
- Hadian, A.; Koch, L.; Koberg, P.; Sarraf, F.; Liersch, A.; Sebastian, T.; Clemens, F. Material extrusion based additive manufacturing of large zirconia structures using filaments with ethylene vinyl acetate based binder composition. Addit. Manuf. 2021, 47, 102227. [Google Scholar] [CrossRef]
- Car, F.; Brnadić, G.; Vrsaljko, D.; Tomašić, V. Advanced preparation method of monolithic catalyst carriers using 3D-printing technology. Prog. Addit. Manuf. 2022, 7, 797–808. [Google Scholar] [CrossRef]
- Car, F.; Sušec, I.; Tomašić, V. Preparation and testing of cordierite monolithic catalysts for oxidation of aromatic volatile organic compounds. Chem. Eng. Trans. 2021, 86, 673–678. [Google Scholar] [CrossRef]
- Car, F.; Zekić, N.; Vrsaljko, D.; Tomašić, V. Innovative Production of 3D-Printed Ceramic Monolithic Catalysts for Oxidation of VOCs by Using Fused Filament Fabrication. Catalysts 2025, 15, 125. [Google Scholar] [CrossRef]
- D’Amelia, R.P.; Gentile, S.; Nirode, W.F.; Huang, L. Quantitative Analysis of Copolymers and Blends of Polyvinyl Acetate (PVAc) Using Fourier Transform Infrared Spectroscopy (FTIR) and Elemental Analysis (EA). World J. Chem. Educ. 2016, 4, 25–31. [Google Scholar]
- Jiang, Z.; Hu, C.; Easa, S.M.; Zheng, X.; Zhang, Y. Evaluation of physical, rheological, and structural properties of vulcanized EVA/SBS modified bitumen. J. Appl. Polym. Sci. 2017, 134, 44850. [Google Scholar] [CrossRef]
- Barretta, C.; Oreski, G.; Kyranaki, N.; Mansour, D.E.; Betts, T.R.; Bauermann, L.P.; Resch-Fauster, K. Effects of artificial ageing tests on EVA degradation: Influence of microclimate and methodology approach. In Proceedings of the IEEE 48th Photovoltaic Specialists Conference (PVSC), Fort Lauderdale, FL, USA, 20–25 June 2021. [Google Scholar] [CrossRef]
- Available online: https://zetamix.fr/en/produit/white-zirconia-filament/ (accessed on 4 June 2023).
- Available online: https://formlabs.com/materials/ceramics/ (accessed on 18 March 2023).


| Sample | Mass Before Heat Treatment (g) | Mass After Heat Treatment (g) | Mass Loss (%) |
|---|---|---|---|
| A | 5.0034 | 4.2994 | 14.07 |
| B | 4.9941 | 4.2879 | 14.14 |
| C | 4.9989 | 4.2931 | 14.12 |
| Monolithic Catalyst Carrier/Fabrication Method | Geometric Surface Area (cm2) | Mass of the Catalytic Layer (mg) | Loading of Catalyst Per Gram of Carrier (mg/g) |
|---|---|---|---|
| M/FFF | 53.4 | 50.3 | 22.2 |
| ZDP/SLA | 28.0 | 33.4 | 7.1 |
| SCC/Extrusion | 29.0 | 6.9 | 9.0 |
| LCC/Extrusion | 21.0 | 6.4 | 11.2 |
| Monolithic Catalyst Carrier | Benzene | Toluene | Ethylbenzene | o-xylene | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T10 | T50 | T90 | T10 | T50 | T90 | T10 | T50 | T90 | T10 | T50 | T90 | |
| SCC | 162 | 171 | 184 | 157 | 169 | 179 | 124 | 157 | 172 | 122 | 154 | 172 |
| LCC | 163 | 179 | 200 | 161 | 170 | 184 | 126 | 165 | 180 | 135 | 164 | 176 |
| ZDP | 161 | 170 | 179 | 151 | 168 | 177 | 122 | 155 | 170 | 121 | 150 | 170 |
| M | 156 | 166 | 176 | 149 | 159 | 168 | 120 | 154 | 165 | 120 | 148 | 162 |
| T/°C | Benzene | Toluene | Ethylbenzene | o-Xylene | ||||
|---|---|---|---|---|---|---|---|---|
| k (min−1) | SD∙103 | k (min−1) | SD∙103 | k (min−1) | SD∙103 | k (min−1) | SD∙103 | |
| 130 | 0.02 | 1.16 | 0.05 | 2.96 | 0.12 | 4.72 | 0.13 | 6.68 |
| 140 | 0.05 | 4.26 | 0.16 | 7.73 | 0.46 | 6.56 | 0.50 | 12.13 |
| 150 | 0.06 | 6.00 | 0.30 | 11.08 | 1.26 | 11.98 | 1.26 | 16.62 |
| 160 | 1.95 | 63.38 | 3.35 | 67.46 | 5.70 | 34.77 | 5.71 | 33.78 |
| 170 | 4.23 | 48.60 | 9.83 | 11.68 | 15.86 | 2.30 | 15.71 | 2.45 |
| 180 | 13.73 | 4.14 | 29.72 | 0.06 | 38.39 | 0.19 | 71.64 | 0.39 |
| 190 | 23.57 | 0.61 | 193.70 | 2.17 × 10−5 | 165.16 | 1.69 × 10−4 | 97.82 | 1.57 × 10−5 |
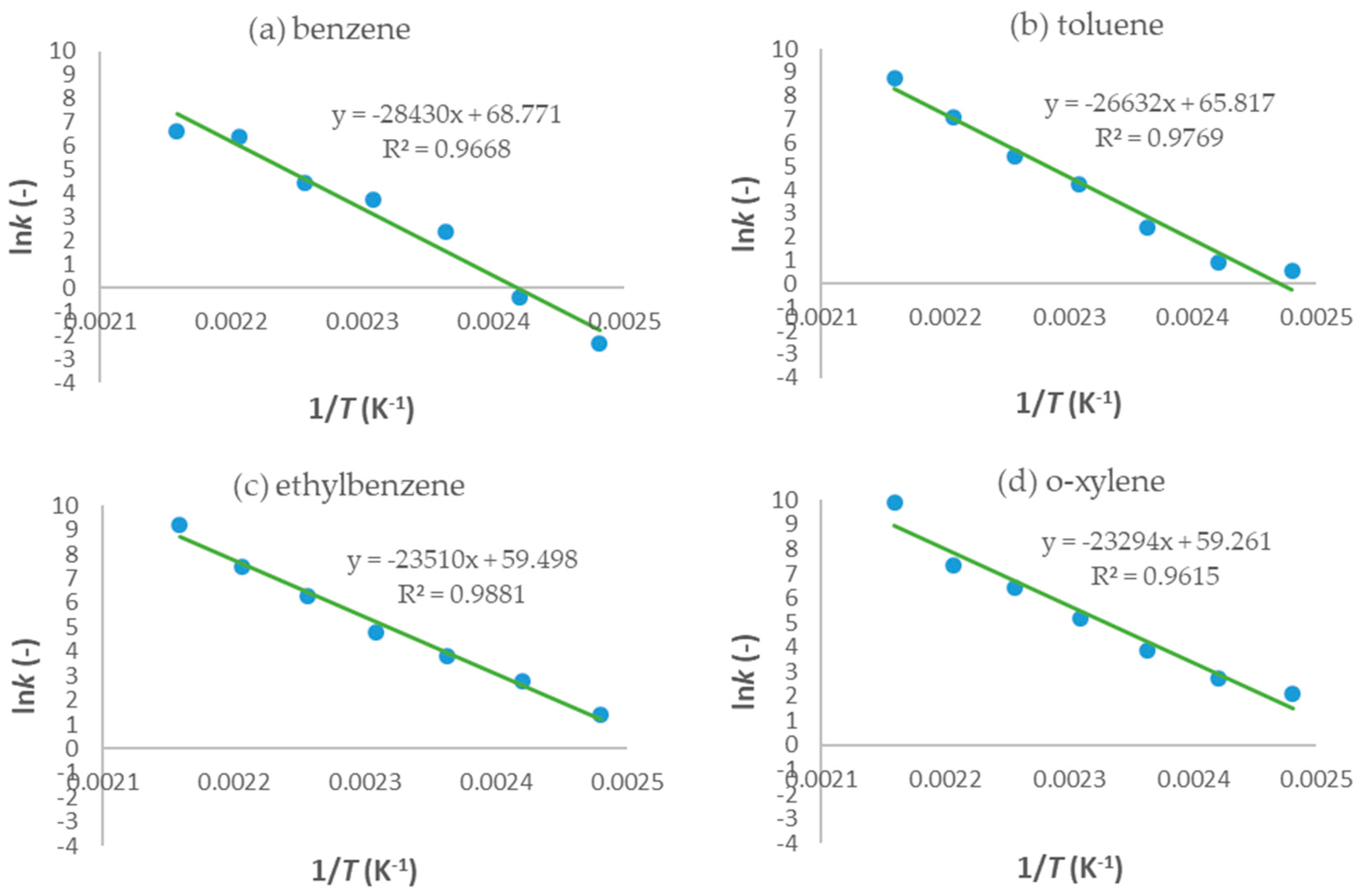
| BTEX Component | Ea (kJ/mol) | A (min−1) |
|---|---|---|
| Benzene | 229.96 | 7.24 × 1027 |
| Toluene | 205.08 | 1.33 × 1025 |
| Ethylbenzene | 182.59 | 5.34 × 1022 |
| o-xylene | 179.36 | 2.24 × 1022 |
| T/°C | Benzene | Toluene | Ethylbenzene | o-Xylene | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k (min−1) | kg (cm/min) | SD∙103 | k (min−1) | kg (cm/min) | SD∙103 | k (min−1) | kg (cm/min) | SD∙103 | k (min−1) | kg (cm/min) | SD∙103 | |
| 130 | 0.30 | 2.73 | 1.16 | 0.72 | 4.41 | 2.96 | 1.99 | 2.49 | 4.72 | 1.97 | 2.27 | 6.68 |
| 140 | 0.70 | 2.28 | 4.25 | 2.56 | 2.66 | 7.72 | 7.94 | 2.14 | 6.55 | 9.73 | 1.29 | 12.19 |
| 150 | 0.96 | 2.04 | 6.00 | 4.82 | 2.11 | 11.09 | 23.28 | 3.83 | 11.97 | 23.74 | 3.71 | 16.62 |
| 160 | 42.33 | 38.37 | 63.38 | 67.86 | 22.95 | 67.48 | 121.01 | 30.16 | 34.78 | 118.88 | 27.87 | 33.78 |
| 170 | 87.27 | 29.53 | 48.61 | 232.05 | 22.62 | 11.67 | 550.31 | 77.81 | 2.30 | 631.40 | 88.92 | 2.52 |
| 180 | 611.28 | 99.48 | 4.14 | 1218.80 | 30.54 | 0.06 | 1739.32 | 30.44 | 0.07 | 1520.44 | 33.49 | 0.08 |
| 190 | 736.60 | 33.27 | 0.59 | 8493.36 | 78.11 | 5.02 × 10−3 | 11,759.65 | 89.63 | 3.24 × 10−5 | 19,643.23 | 87.28 | 1.26 × 10−5 |
| BTEX Component | Ea (kJ/mol) | A (min−1) |
|---|---|---|
| Benzene | 236.37 | 7.36 × 1029 |
| Toluene | 221.42 | 3.84 × 1028 |
| Ethylbenzene | 195.46 | 6.91 × 1025 |
| o-Xylene | 193.67 | 5.45 × 1025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Car, F.; Horvatić, D.; Tomašić, V.; Vrsaljko, D.; Gomzi, Z. Application of Fused Filament Fabrication in Preparation of Ceramic Monolithic Catalysts for Oxidation of Gaseous Mixture of Volatile Aromatic Compounds. Catalysts 2025, 15, 677. https://doi.org/10.3390/catal15070677
Car F, Horvatić D, Tomašić V, Vrsaljko D, Gomzi Z. Application of Fused Filament Fabrication in Preparation of Ceramic Monolithic Catalysts for Oxidation of Gaseous Mixture of Volatile Aromatic Compounds. Catalysts. 2025; 15(7):677. https://doi.org/10.3390/catal15070677
Chicago/Turabian StyleCar, Filip, Dominik Horvatić, Vesna Tomašić, Domagoj Vrsaljko, and Zoran Gomzi. 2025. "Application of Fused Filament Fabrication in Preparation of Ceramic Monolithic Catalysts for Oxidation of Gaseous Mixture of Volatile Aromatic Compounds" Catalysts 15, no. 7: 677. https://doi.org/10.3390/catal15070677
APA StyleCar, F., Horvatić, D., Tomašić, V., Vrsaljko, D., & Gomzi, Z. (2025). Application of Fused Filament Fabrication in Preparation of Ceramic Monolithic Catalysts for Oxidation of Gaseous Mixture of Volatile Aromatic Compounds. Catalysts, 15(7), 677. https://doi.org/10.3390/catal15070677








