Sustainable Treatment of Plastic Wastes with Photocatalytic Technologies: A Review
Abstract
1. Introduction
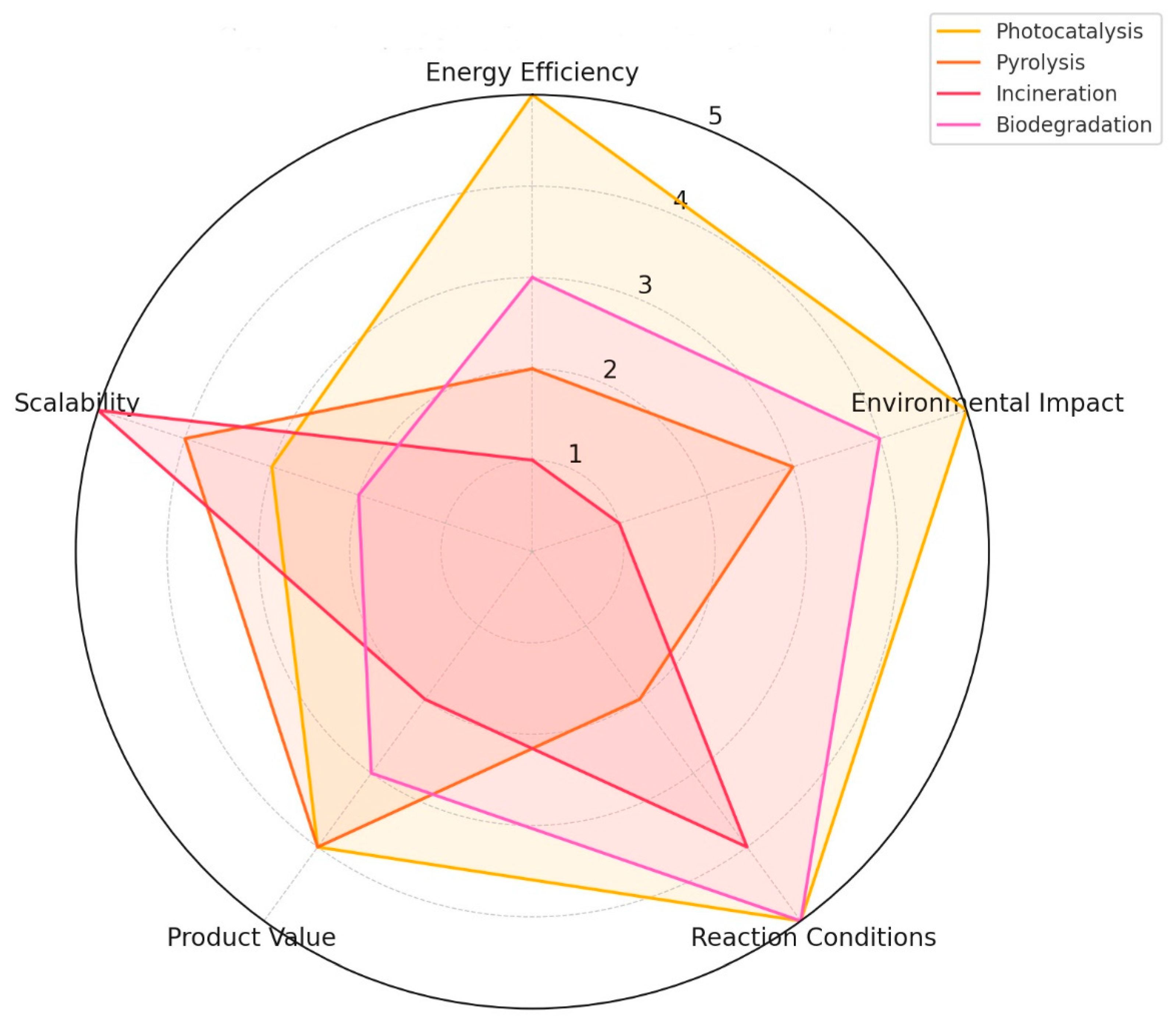
2. Current Status of Plastic Waste Management
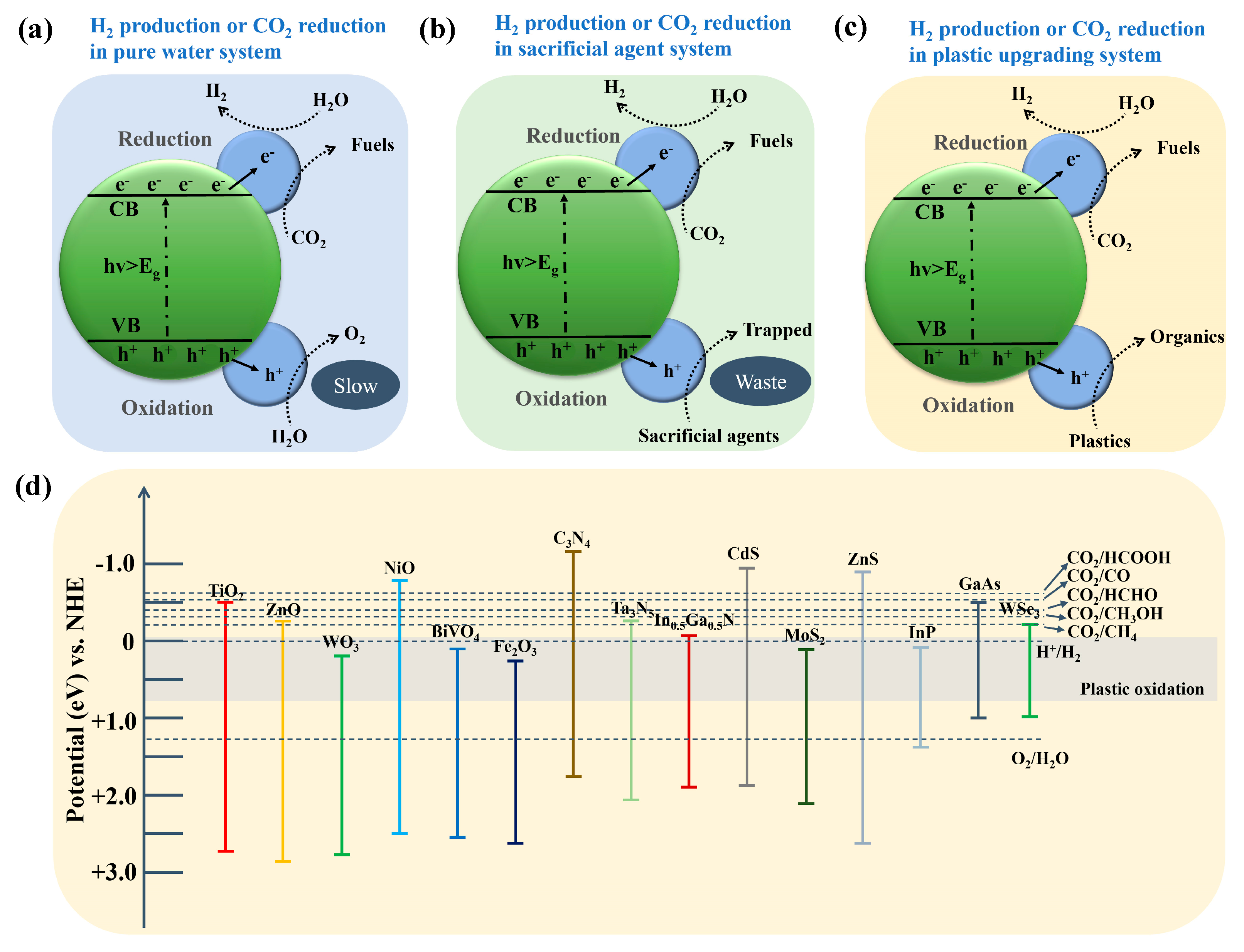
3. Mechanistic Insights and Design Principles of Photo-Reforming
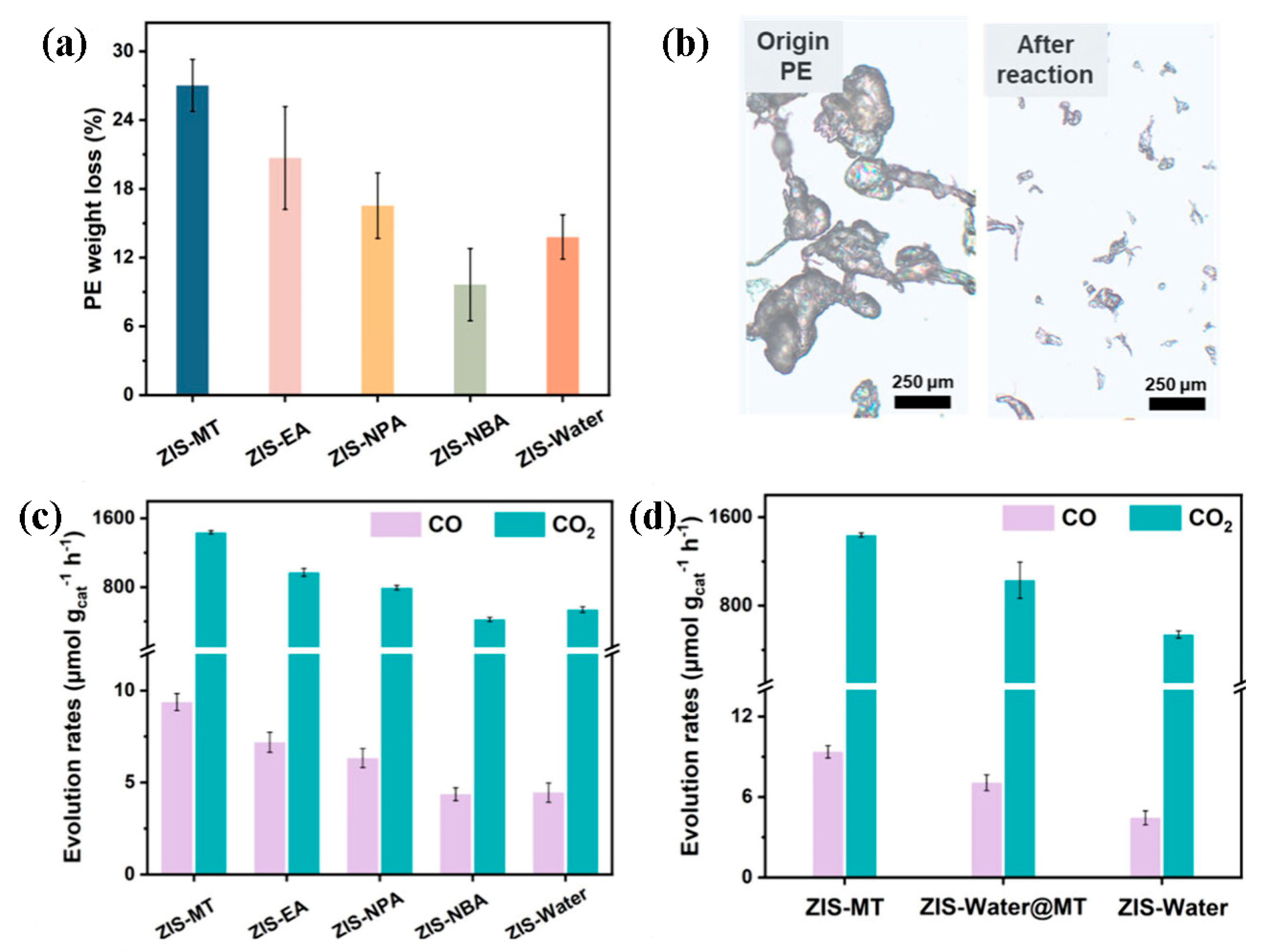
4. Recent Advances in Photocatalytic Conversion of Plastics
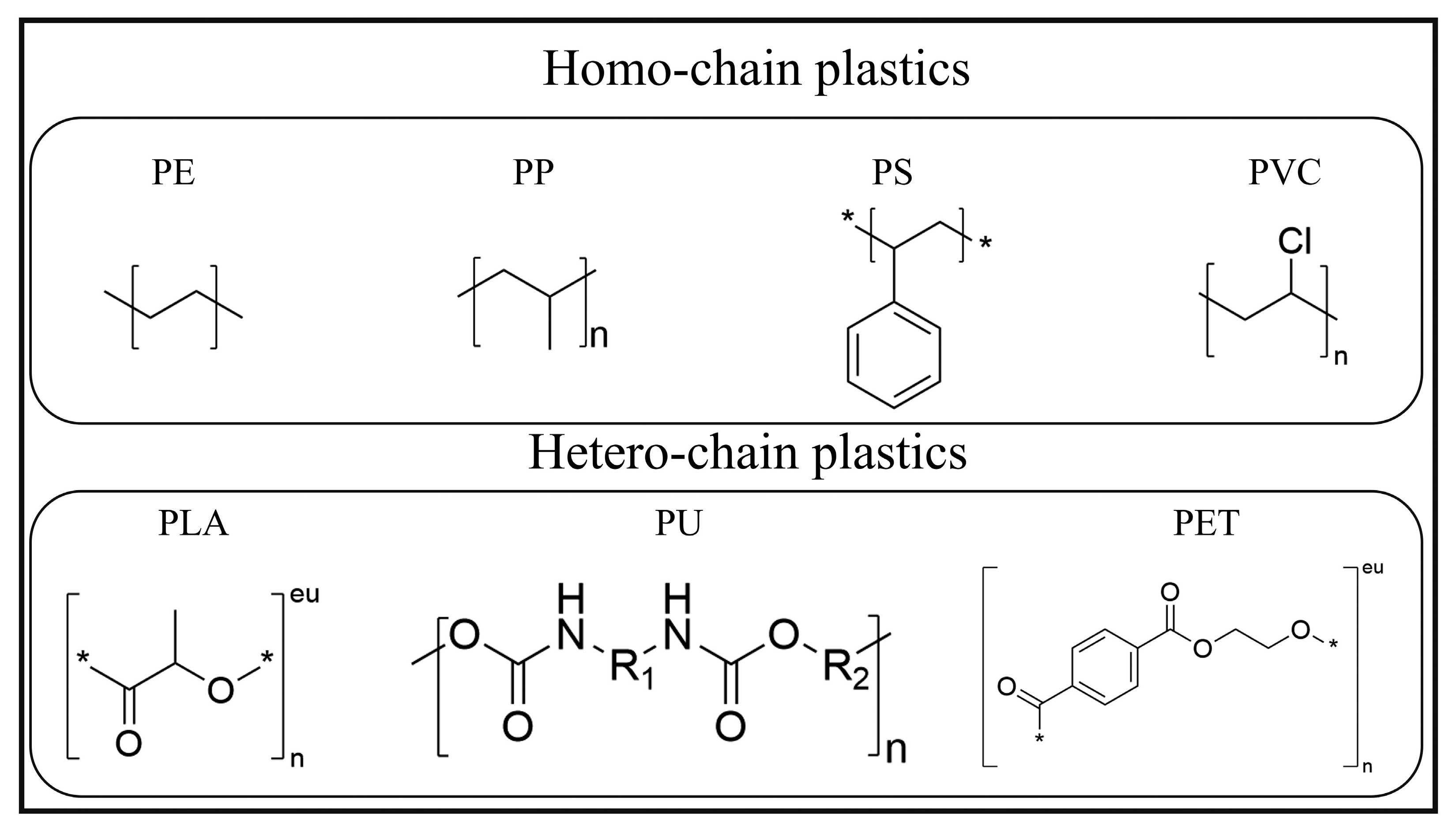
4.1. Overview and Conversion Strategies for Homochain Polymer Waste
| Plastic | Photocatalyst | Light Source | Main Product | Ref. |
|---|---|---|---|---|
| PVC | CDs/Zr-MOF | AM 1.5 | CH3COOH | [56] |
| PVC | Ni-TCPP | AM 1.5 | diesel olefin products | [57] |
| PS | g-C3N4 | 300 W Xe lamp | benzoic acid | [58] |
| PS | Triton X-100 TiO2 | 32 W lamp | CO2, H2O | [59] |
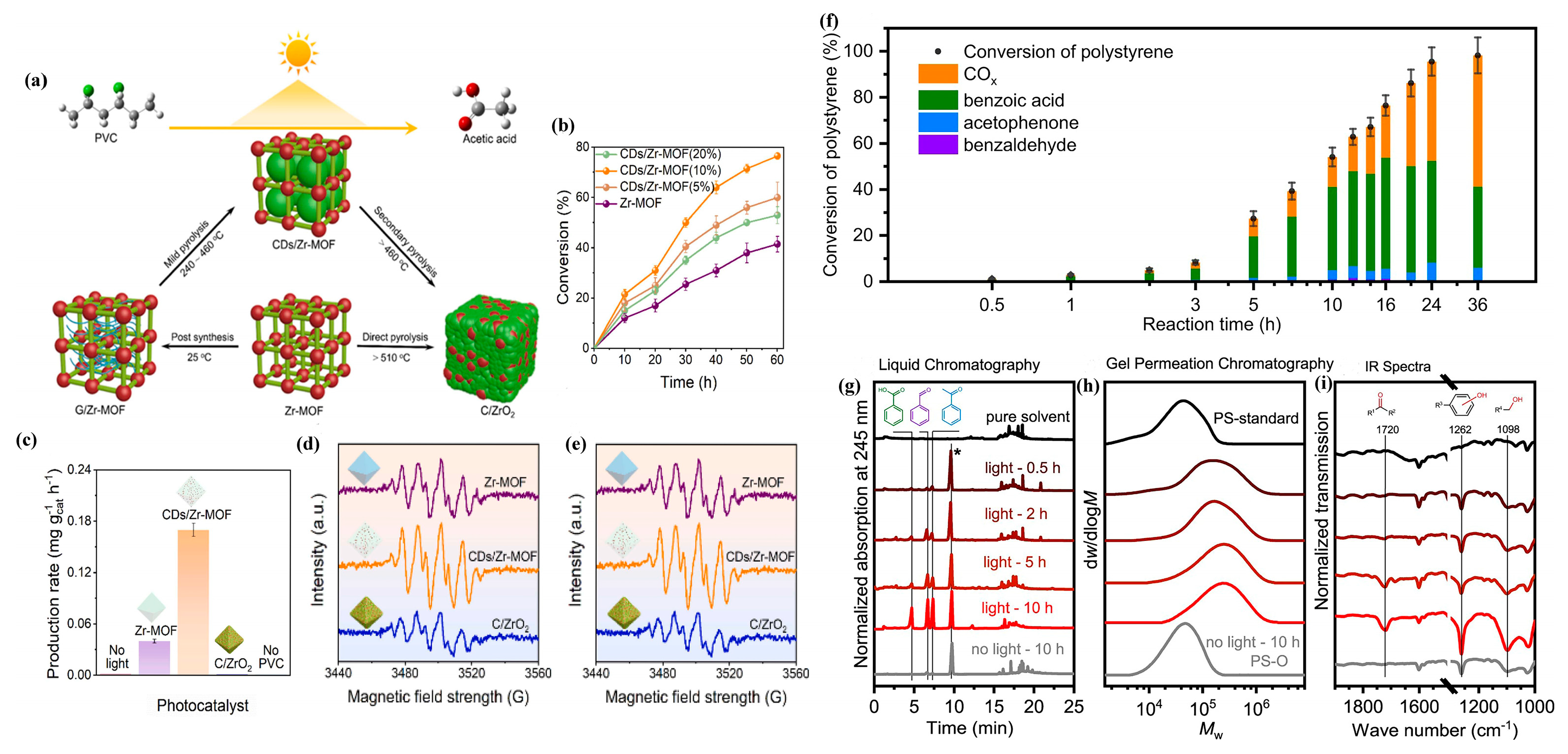
4.2. Photocatalytic Conversion of Heterochain Plastics
5. Photocatalytic Product Selectivity
6. Future Directions in Photocatalytic Plastic Conversion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Hossain, K.B.; Chen, K.; Chen, P.; Wang, C.; Cai, M. Socioeconomic Relation with Plastic Consumption on 61 Countries Classified by Continent, Income Status and Coastal Regions. Bull. Environ. Contam. Toxicol. 2021, 107, 786–792. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Worch, J.C.; Dove, A.P. 100th Anniversary of Macromolecular Science Viewpoint: Toward Catalytic Chemical Recycling of Waste (and Future) Plastics. ACS Macro Lett. 2020, 9, 1494–1506. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhang, L. Recent Progress in the Chemical Upcycling of Plastic Wastes. ChemSusChem 2021, 14, 4137–4151. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Saito, K.; Eisenreich, F.; Türel, T.; Tomović, Ž. Closed-Loop Recycling of Poly(Imine-Carbonate) Derived from Plastic Waste and Bio-based Resources. Angew. Chem. Int. Ed. Engl. 2022, 61, e202211806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Z.; Wang, B.; Li, P.; Zhu, J.; Ma, S. Closed-loop chemical recycling of thermosetting polymers and their applications: A review. Green Chem. 2022, 24, 5691–5708. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent Developments in the Chemical Recycling of Postconsumer Poly(ethylene terephthalate) Waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, B.; Zhao, X.; Soo, H.S.; Wang, F.; Xiao, R.; Zhang, H. Photocatalytic Conversion of Plastic Waste: From Photodegradation to Photosynthesis. Adv. Energy Mater. 2022, 12, 2200435. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Photoredox-Catalyzed Plastic Waste Conversion: Nonselective Degradation versus Selective Synthesis. ACS Catal. 2023, 13, 3575–3590. [Google Scholar] [CrossRef]
- Saquing, J.M.; Saquing, C.D.; Knappe, D.R.U.; Barlaz, M.A. Impact of Plastics on Fate and Transport of Organic Contaminants in Landfills. Environ. Sci. Technol. 2010, 44, 6396–6402. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.; Ye, J.; He, P. Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 2021, 401, 123429. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. Engl. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Shi, R.; Liu, K.-S.; Liu, F.; Yang, X.; Hou, C.-C.; Chen, Y. Electrocatalytic reforming of waste plastics into high value-added chemicals and hydrogen fuel. Chem. Commun. 2021, 57, 12595–12598. [Google Scholar] [CrossRef]
- Sundararajan, N.K.; Bhagavathi, A.R. Experimental Investigation on Thermocatalytic Pyrolysis of HDPE Plastic Waste and the Effects of Its Liquid Yield over the Performance, Emission, and Combustion Characteristics of CI Engine. Energy Fuels 2016, 30, 5379–5390. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, Y.; Zhang, C.; Zhu, S.; Qin, L.; Wang, W.; He, D.; Zhou, Y.; Luo, H.; Qin, F. Recent advances in photocatalytic degradation of plastics and plastic-derived chemicals. J. Mater. Chem. A 2021, 9, 13402–13441. [Google Scholar] [CrossRef]
- Pan, D.; Su, F.; Liu, C.; Guo, Z. Research progress for plastic waste management and manufacture of value-added products. Adv. Compos. Hybrid Mater. 2020, 3, 443–461. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Additives of plastics: Entry into the environment and potential risks to human and ecological health. J. Environ. Manag. 2023, 348, 119364. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.; Paull, G.C.; Van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Morris, J. Recycling versus incineration: An energy conservation analysis. J. Hazard. Mater. 1996, 47, 277–293. [Google Scholar] [CrossRef]
- Bernardo, C.; Simões, C.L.; Pinto, L.M.C. Environmental and economic life cycle assessment of polymers and polymer matrix composites: A review. Ciência Tecnol. Dos Mater. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef]
- Kakadellis, S.; Rosetto, G. Achieving a circular bioeconomy for plastics. Science 2021, 373, 49–50. [Google Scholar] [CrossRef]
- Plastic Upcycling. Available online: https://www.nature.com/articles/s41929-019-0391-7 (accessed on 7 July 2025).
- Wang, J.; Li, X.; Wang, M.; Zhang, T.; Chai, X.; Lu, J.; Wang, T.; Zhao, Y.; Ma, D. Electrocatalytic Valorization of Poly(ethylene terephthalate) Plastic and CO2 for Simultaneous Production of Formic Acid. ACS Catal. 2022, 12, 6722–6728. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.T.; Oh, L.S.; Kim, H.J.; Lee, J. Marine waste upcycling—Recovery of nylon monomers from fishing net waste using seashell waste-derived catalysts in a CO2-mediated thermocatalytic process. J. Mater. Chem. A 2022, 10, 20024–20034. [Google Scholar] [CrossRef]
- Pham, D.D.; Cho, J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2020, 23, 511–525. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Zuo, Z.; Niu, Z. Recent advances in plastic recycling and upgrading under mild conditions. Green Chem. 2023, 25, 6949–6970. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; McClure, B.A.; Edri, E.; Frei, H. Coupling carbon dioxide reduction with water oxidation in nanoscale photocatalytic assemblies. Chem. Soc. Rev. 2016, 45, 3221–3243. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Liu, M.; Wang, A.; Huang, Q.; Li, C. Water Oxidation Catalysts for Artificial Photosynthesis. Adv. Mater. 2019, 31, e1902069. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Grills, D.C.; Polyansky, D.E.; Szalda, D.J.; Fujita, E. Unexpected Roles of Triethanolamine in the Photochemical Reduction of CO2 to Formate by Ruthenium Complexes. J. Am. Chem. Soc. 2019, 142, 2413–2428. [Google Scholar] [CrossRef]
- Gisbertz, S.; Pieber, B. Heterogeneous Photocatalysis in Organic Synthesis. ChemPhotoChem 2020, 4, 456–475. [Google Scholar] [CrossRef]
- Eisenreich, F. Photocatalysis as an Effective Tool for Upcycling Polymers into Value-Added Molecules. Angew. Chem. Int. Ed. Engl. 2023, 62, e202301303. [Google Scholar] [CrossRef]
- Fan, X.; Lan, L.; Chang, Y.; Yang, L.; Huang, Y.; Dan, Y.; Jiang, L. Construction of Multi-step Charge Transfer Pathways in Bi0@Bi3+-KNbO3 for Significantly Accelerated Photoconversion of Waste Plastics. Angew. Chem. Int. Ed. Engl. 2025, 64, e202502874. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.; Wang, J.; Shakouri, M.; Shi, B.; Liu, X.; Guo, Y.; Hu, Y.; Wu, X.-P.; Wang, Y. Pt enhanced C–H bond activation for efficient and low-methane-selectivity hydrogenolysis of polyethylene over alloyed RuPt/ZrO2. Appl. Catal. B Environ. 2024, 353, 124046. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Tian, J.; Zhao, C. Coupled conversion of polyethylene and carbon dioxide catalyzed by a zeolite–metal oxide system. Sci. Adv. 2024, 10, eadn0252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, B.; Qu, Y.; Jing, L.; Jaroniec, M.; Ran, J.; Qiao, S.-Z. Photocatalytic production of ethylene and propionic acid from plastic waste by titania-supported atomically dispersed Pd species. Sci. Adv. 2023, 9, eadk2407. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Qin, J.; Yin, B.; Zhang, Y.; Li, C.; Dou, Y.; Hélix-Nielsen, C.; Zhang, W. In-and-out of inert sites on high-entropy layered double hydroxide to facilitate peroxymonosulfate-assisted photocatalytic removal of microplastics. Appl. Catal. B Environ. 2024, 365, 124853. [Google Scholar] [CrossRef]
- Li, H.; Jiang, S.; He, S.; Zhang, Y.; Chen, Y.; Wang, L.; Yang, J. Accelerated Solar-Driven Polyolefin Degradation via Self-Activated Hydroxy-Rich ZnIn2S4. Nano Lett. 2024, 24, 11624–11631. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Chu, M.; Zhang, W.; Fu, J.; Li, S.; Wang, L.; Chen, J.; Zhang, Q.; Cao, M. Selective Liquid Chemical Production in Waste Polyolefin Photorefinery by Controlling Reactive Species. J. Am. Chem. Soc. 2025, 147, 5228–5237. [Google Scholar] [CrossRef]
- Jiao, X.; Hu, Z.; Zheng, K.; Zhu, J.; Wu, Y.; Zhang, X.; Hu, J.; Yan, W.; Zhu, J.; Sun, Y.; et al. Direct Polyethylene Photoreforming into Exclusive Liquid Fuel over Charge-Asymmetrical Dual Sites under Mild Conditions. Nano Lett. 2022, 22, 10066–10072. [Google Scholar] [CrossRef]
- Lin, J.; Hu, K.; Wang, Y.; Tian, W.; Hall, T.; Duan, X.; Sun, H.; Zhang, H.; Cortés, E.; Wang, S. Tandem microplastic degradation and hydrogen production by hierarchical carbon nitride-supported single-atom iron catalysts. Nat. Commun. 2024, 15, 8769. [Google Scholar] [CrossRef]
- Xing, C.; Yu, G.; Zhou, J.; Liu, Q.; Chen, T.; Liu, H.; Li, X. Solar energy-driven upcycling of plastic waste on direct Z-scheme heterostructure of V-substituted phosphomolybdic acid/g-C3N4 nanosheets. Appl. Catal. B Environ. 2022, 315, 121496. [Google Scholar] [CrossRef]
- Venkataramana, C.; Botsa, S.M.; Shyamala, P.; Muralikrishna, R. Photocatalytic degradation of polyethylene plastics by NiAl2O4 spinels-synthesis and characterization. Chemosphere 2021, 265, 129021. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, Y.; Waterhouse, G.I.N.; Shi, R.; Wu, L.-Z.; Zhang, T. Photothermal recycling of waste polyolefin plastics into liquid fuels with high selectivity under solvent-free conditions. Nat. Commun. 2023, 14, 4242. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Zhang, Q.; Liu, Z.; Wang, H.; Hao, S.; Zhang, F.; Zhang, L.; Han, Q.; Zheng, G. Brønsted-acid sites induced photocatalytic cracking of low-polarity polyethylene plastics. J. Energy Chem. 2024, 96, 509–515. [Google Scholar] [CrossRef]
- Meng, X.; Peng, X.; Xue, J.; Wei, Y.; Sun, Y.; Dai, Y. A biomass-derived, all-day-round solar evaporation platform for harvesting clean water from microplastic pollution. J. Mater. Chem. A 2021, 9, 11013–11024. [Google Scholar] [CrossRef]
- Selvam, E.; Yu, K.; Ngu, J.; Najmi, S.; Vlachos, D.G. Recycling polyolefin plastic waste at short contact times via rapid joule heating. Nat. Commun. 2024, 15, 5662. [Google Scholar] [CrossRef]
- Qin, J.; Dou, Y.; Zhou, J.; Zhao, D.; Orlander, T.; Andersen, H.R.; Hélix-Nielsen, C.; Zhang, W. Encapsulation of carbon-nanodots into metal-organic frameworks for boosting photocatalytic upcycling of polyvinyl chloride plastic. Appl. Catal. B Environ. 2023, 341, 123355. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, Z.; Zhang, T.; Li, F.; Liu, K.; Zhan, S. Selective Photoreforming of Waste Plastics into Diesel Olefins via Single Reactive Oxygen Species. Angew. Chem. Int. Ed. Engl. 2024, 63, e202406795. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, M.-Q.; Hu, C.; Xiao, D.; Wang, M.; Ma, D. Catalytic oxidation of polystyrene to aromatic oxygenates over a graphitic carbon nitride catalyst. Nat. Commun. 2022, 13, 4809. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Li, H. Photocatalytic Degradation of Plastic Waste: A Mini Review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Uekert, T.; Kuehnel, M.F.; Wakerley, D.W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857. [Google Scholar] [CrossRef]
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of Nonrecyclable Plastic Waste over a Carbon Nitride/Nickel Phosphide Catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, Y.; Kang, S.; Guo, X.; Ma, Y.; Xing, M.; Zhu, Y.; Chai, Y.; Qiu, B. Trash to Treasure: Photoreforming of Plastic Waste into Commodity Chemicals and Hydrogen over MoS2-Tipped CdS Nanorods. ACS Catal. 2022, 12, 12823–12832. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, H.; Zhang, F.; Wu, J.; Yang, J.; Wang, H. Efficient Photocatalytic Degradation of the Persistent PET Fiber-Based Microplastics over Pt Nanoparticles Decorated N-Doped TiO2 Nanoflowers. Adv. Fiber Mater. 2022, 4, 1094–1107. [Google Scholar] [CrossRef]
- Li, Y.; Wan, S.; Lin, C.; Gao, Y.; Lu, Y.; Wang, L.; Zhang, K. Engineering of 2D/2D MoS2/CdxZn1−xS Photocatalyst for Solar H2 Evolution Coupled with Degradation of Plastic in Alkaline Solution. Sol. RRL 2020, 5, 2000427. [Google Scholar] [CrossRef]
- Kang, H.; Washington, A.; Capobianco, M.D.; Yan, X.; Cruz, V.V.; Weed, M.; Johnson, J.; Johns, G.; Brudvig, G.W.; Pan, X.; et al. Concentration-Dependent Photocatalytic Upcycling of Poly(ethylene terephthalate) Plastic Waste. ACS Mater. Lett. 2023, 5, 3032–3041. [Google Scholar] [CrossRef]
- Du, M.; Xing, M.; Yuan, W.; Zhang, L.; Sun, T.; Sheng, T.; Zhou, C.; Qiu, B. Upgrading polyethylene terephthalate plastic into commodity chemicals paired with hydrogen evolution over a partially oxidized CuIn5S8 nanosheet photocatalyst. Green Chem. 2023, 25, 9818–9825. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S. Tandem Chemical Depolymerization and Photoreforming of Waste PET Plastic to High-Value-Added Chemicals. ACS Catal. 2024, 14, 2949–2958. [Google Scholar] [CrossRef]
- Ya, Z.; Tang, L.; Xu, D.; Wang, H.; Zhang, S. Photoreforming of waste plastic by B-doped carbon nitride nanotube: Atomic-level modulation and mechanism insights. AIChE J. 2025, 71, e18740. [Google Scholar] [CrossRef]
- Liang, X.; Gao, T.; Cui, Y.; Dong, Q.; Li, X.; Labidi, A.; Lichtfouse, E.; Li, F.; Yu, F.; Wang, C. Photoreforming of poly(ethylene-terephthalate) plastic into valuable chemicals and hydrogen over BiVO4/MoOx: Synergistic promotion of oxidation and reduction processes. Appl. Catal. B Environ. 2024, 357, 124326. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef]
- Uekert, T.; Pichler, C.M.; Schubert, T.; Reisner, E. Solar-driven reforming of solid waste for a sustainable future. Nat. Sustain. 2020, 4, 383–391. [Google Scholar] [CrossRef]
- Ashraf, M.; Ullah, N.; Khan, I.; Tremel, W.; Ahmad, S.; Tahir, M.N. Photoreforming of Waste Polymers for Sustainable Hydrogen Fuel and Chemicals Feedstock: Waste to Energy. Chem. Rev. 2023, 123, 4443–4509. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Guo, C.; Lam, E.; Holstein, J.M.; Pereira, M.R.; Pichler, C.M.; Pornrungroj, C.; Rahaman, M.; Uekert, T.; Hollfelder, F.; et al. Chemoenzymatic Photoreforming: A Sustainable Approach for Solar Fuel Generation from Plastic Feedstocks. J. Am. Chem. Soc. 2023, 145, 20355–20364. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity Enhancement in Heterogeneous Photocatalytic Transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Chen, J.; Li, J.; Huang, P.; Nie, X.; Yang, J. Persulfate-Based Advanced Oxidation Reforming of Polyethylene Terephthalate Fiber into Formate via Singlet Oxygen Activation. Adv. Fiber Mater. 2025, 7, 664–677. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Wang, N.; Ye, L.; Tang, H. Molecular imprinting for removing highly toxic organic pollutants. Chem. Commun. 2011, 48, 788–798. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Wang, N.; Zhang, T.; Tang, H. Selective photocatalytic degradation of nitrobenzene facilitated by molecular imprinting with a transition state analog. Catal. Today 2013, 225, 164–170. [Google Scholar] [CrossRef]
- Yuan, L.; Qi, M.; Tang, Z.; Xu, Y. Coupling Strategy for CO2 Valorization Integrated with Organic Synthesis by Heterogeneous Photocatalysis. Angew. Chem. Int. Ed. Engl. 2021, 60, 21150–21172. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, M.; Zhang, F.; Wang, L.; Yang, J. Interstitial Boron Atoms in Pd Aerogel Selectively Switch the Pathway for Glycolic Acid Synthesis from Waste Plastics. Adv. Mater. 2024, 36, e2401867. [Google Scholar] [CrossRef]
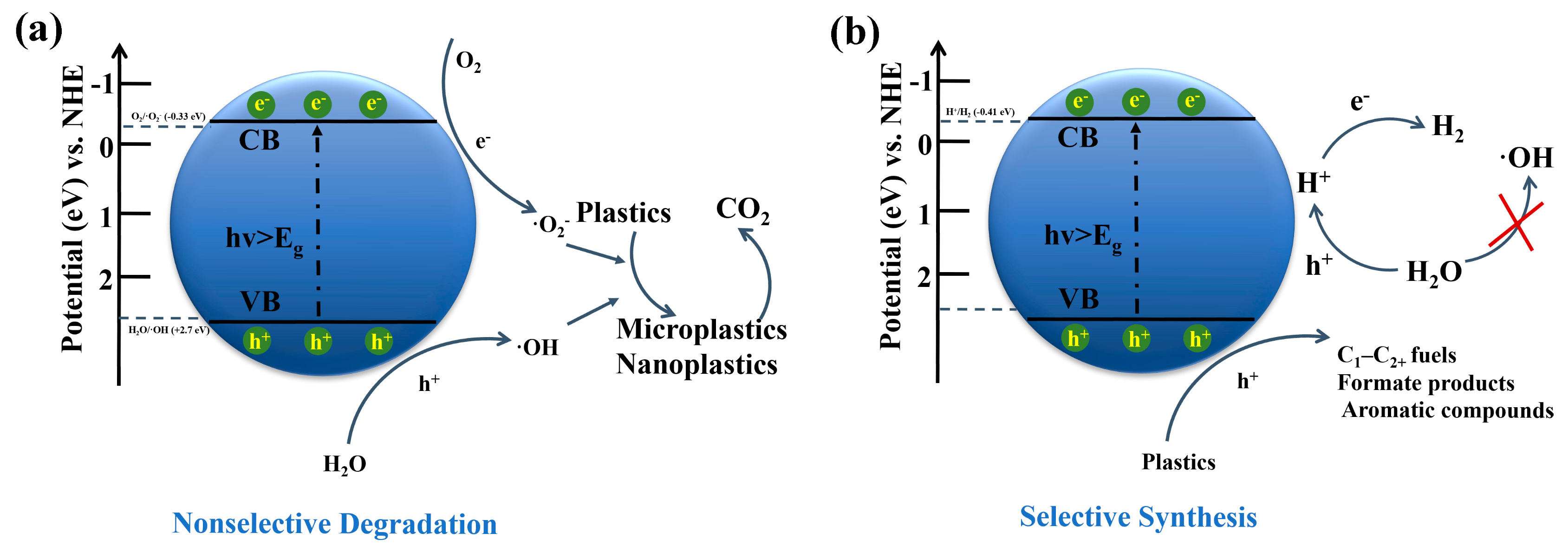
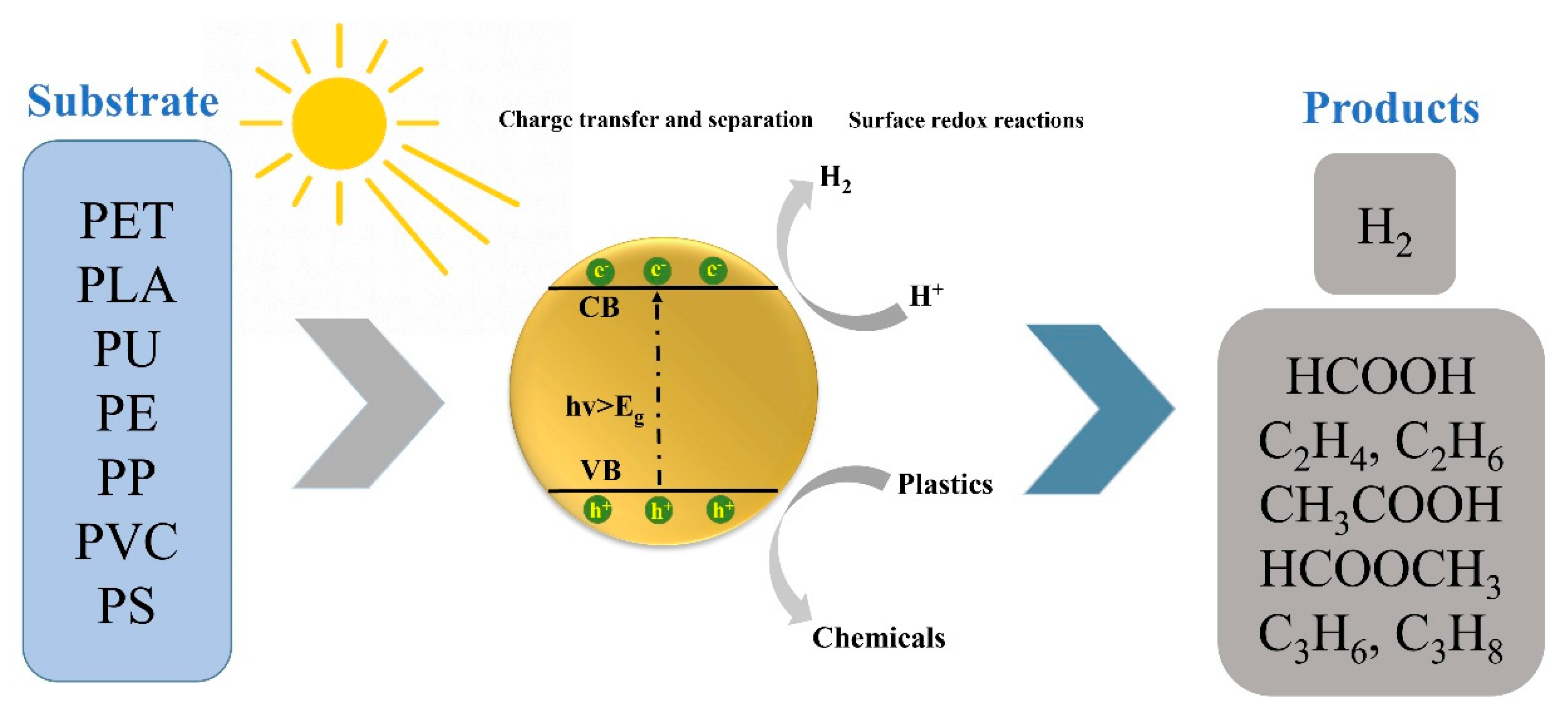
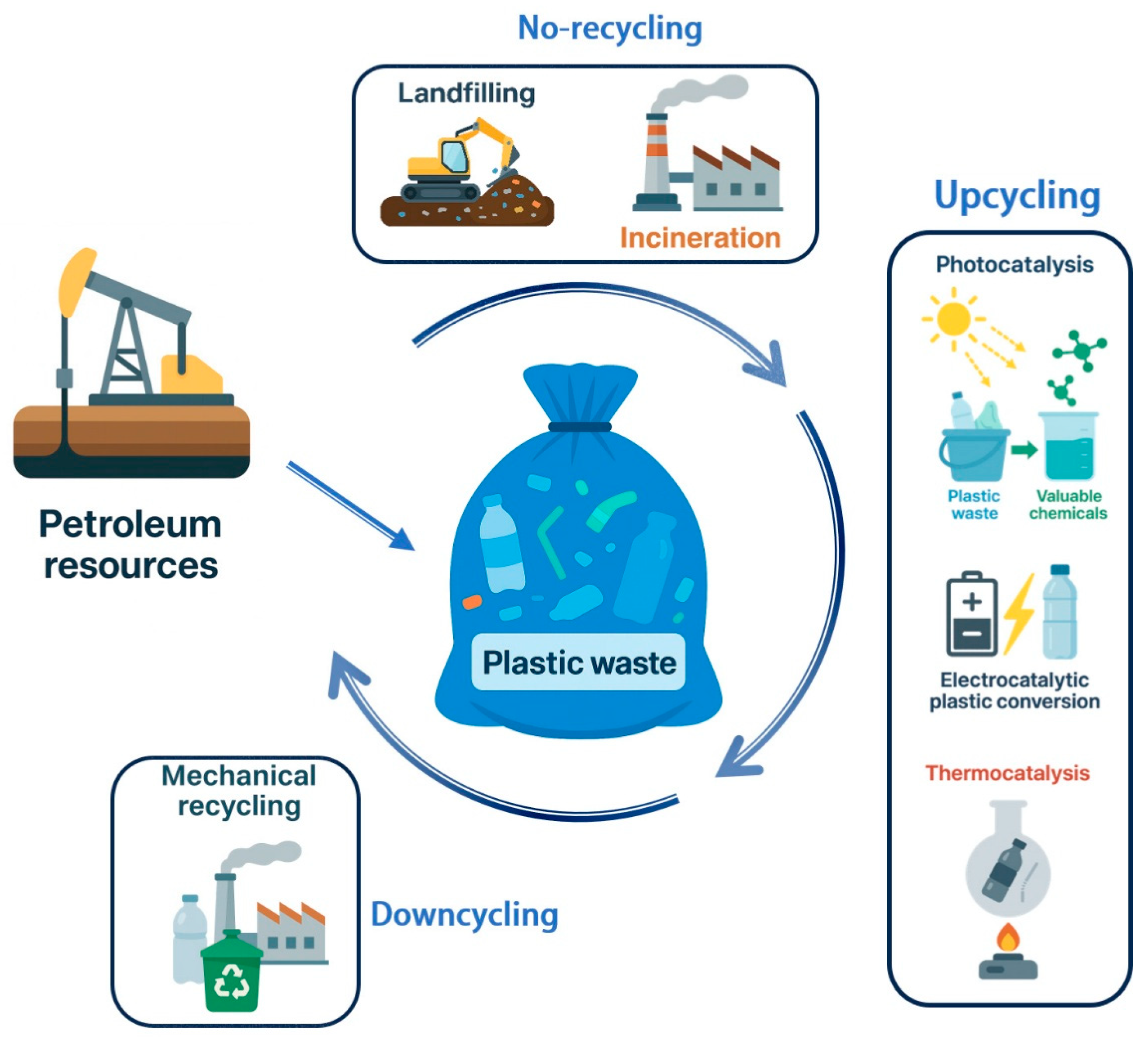

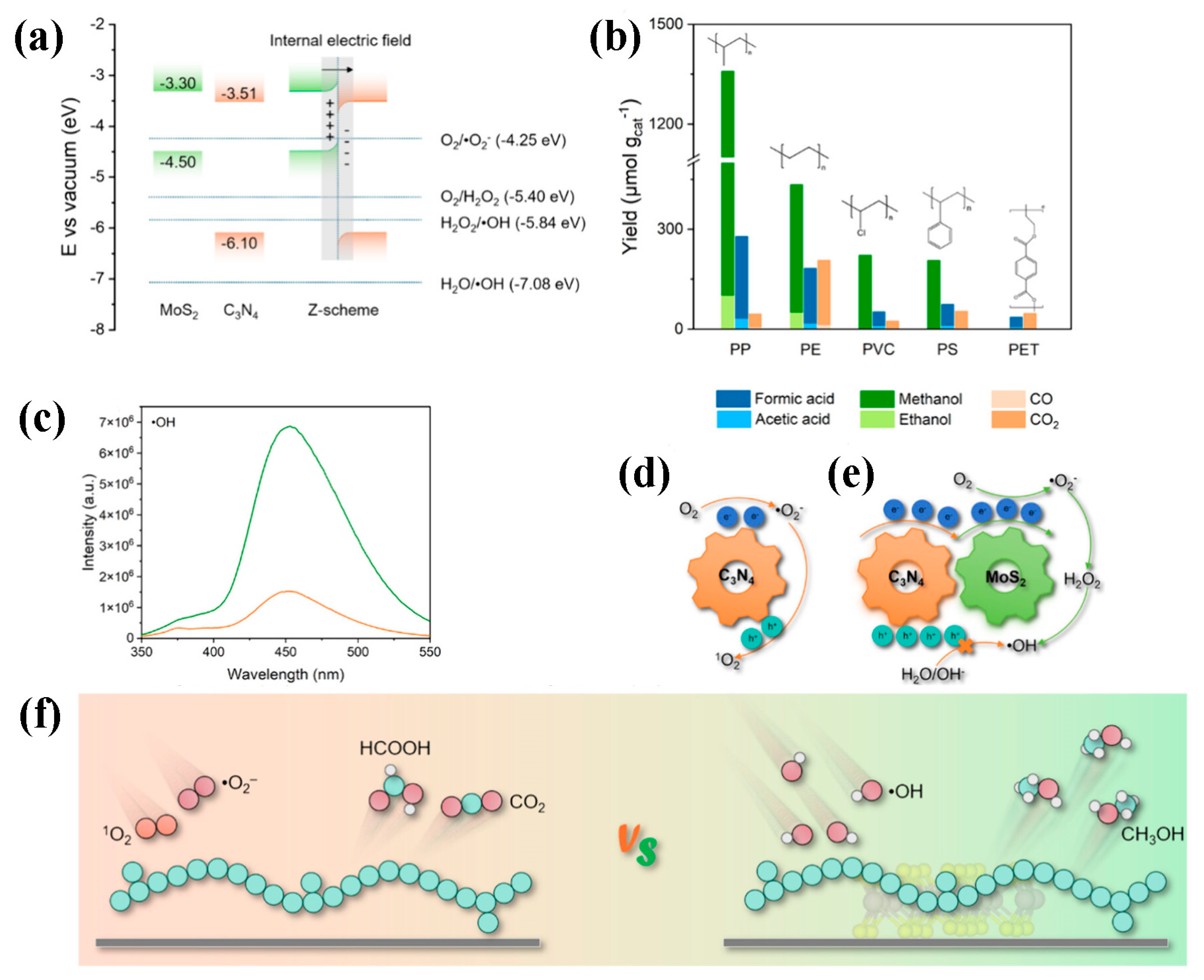
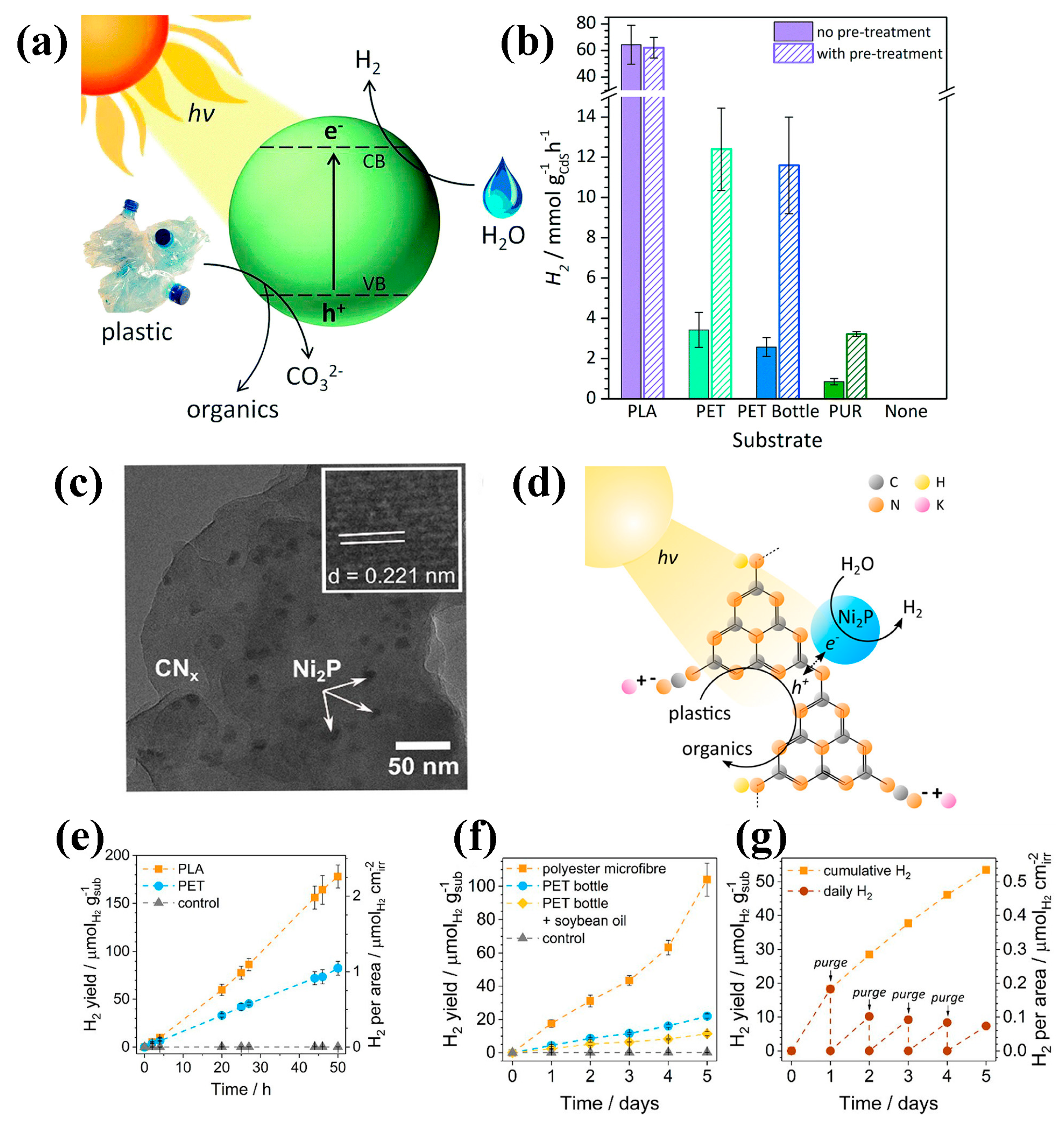
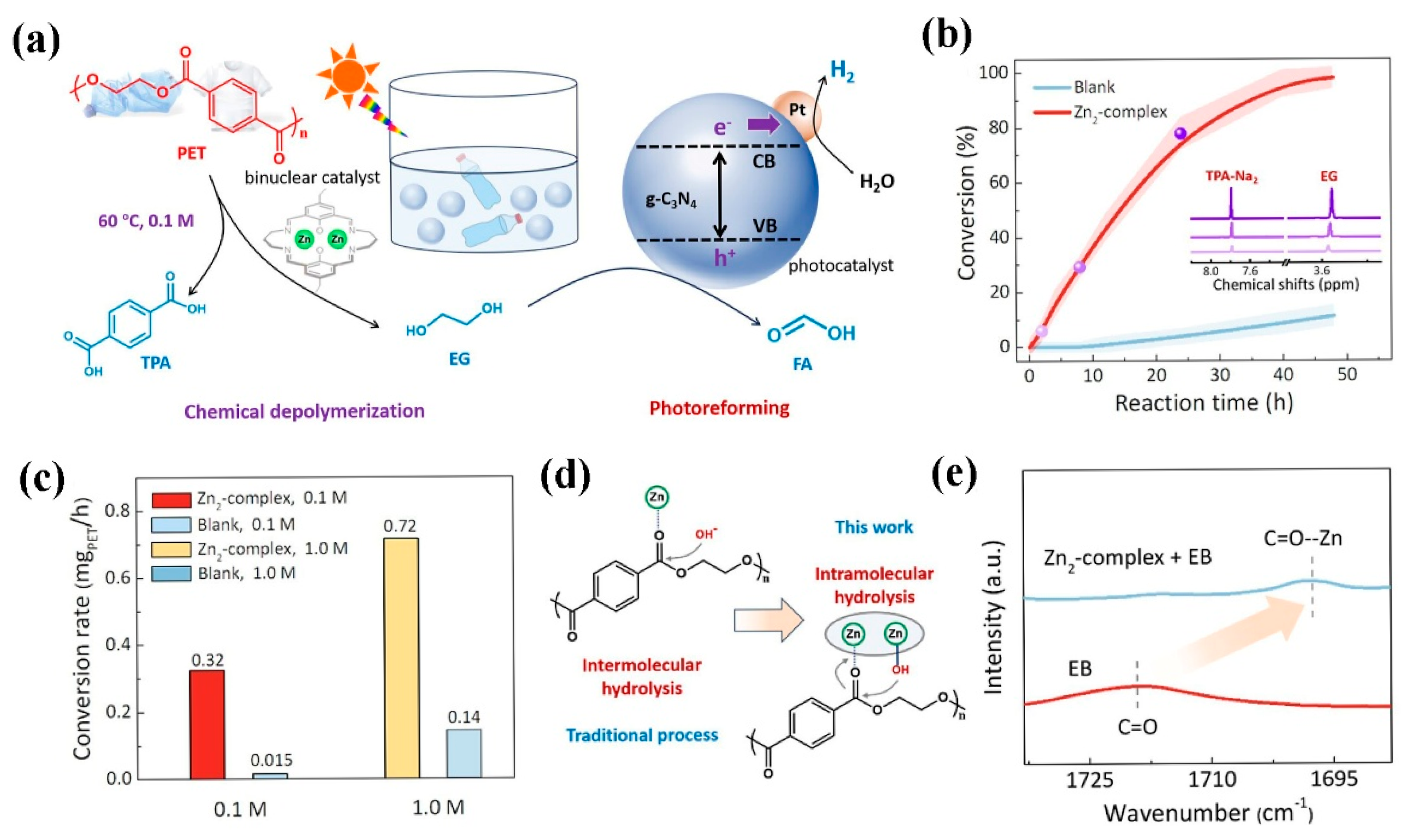
| Particle Size | Typical Source | Risk Level | Can Photocatalysis Address? | Notes |
|---|---|---|---|---|
| Macroplastic (>5 mm) | Packaging, bottles | Medium | Yes | Physical degradation starts the process |
| Microplastic (1 µm–5 mm) | Microplastic (1 µm–5 mm) | High | Yes (slow mineralization) | Needs prolonged irradiation |
| Nanoplastic (<1 µm) | Weathering, fragmentation | Very High | Partially (advanced systems) | Harder to capture and identify |
| Plastic | Photocatalyst | Light Source | Main Product | Conversion | Time | Ref. |
|---|---|---|---|---|---|---|
| PE | Bi0@Bi3+-KNbO3 | 300 W Xe lamp | CO, CO2 | 100% | 12 h | [41] |
| PE | CeO2-nPOx | 300 W Xe lamp | CO2 | 94% | 24 h | [42] |
| PE | BiOl/BiVO4 | 300 W LED | C4-C30 | 100% | 6 h | [43] |
| PE | Pd1-TiO2 | 365 nm LED | CH3CH2COOH, C2H4 | 100% | 3 h | [44] |
| PE | NF@CoNiFe(VZn-Al)-LDHs | 300 W Xe lamp | CH3COOH, CO2 | 100% | 48 h | [45] |
| PE | ZnInS2S4 | 300 W Xe lamp | CO, CO2 | 84.5% | 60 h | [46] |
| PP | MoS2/g-C3N4 | 300 W Xe lamp | ethanolic | 100% | 24 h | [47] |
| PE | ZrCoFe2O4 QDs | 300 W Xe lamp | CH3COOH | 85.4% | 2 h | [48] |
| UHMWPE | FeSA-hCN | 300 W Xe lamp | carboxylic acids, ethers, alkanes, furanone, carboxylic acids | 100% | 12 h | [49] |
| PE | VPOM/CNNS | Visible light (λ > 420 nm) | HCOOH | 100% | 36 h | [50] |
| PP | NaAlO4 | 350 W metal halide lamp | CO2, H2O | 12.5% | 5 h | [51] |
| Plastic | Photocatalyst | Pretreatment Conditions | Light Source | H2 Yield ) | Main Product | Ref. |
|---|---|---|---|---|---|---|
| PET PLA | CdS/CdOX | NaOH 10 M | AM 1.5 G | 12.4 ± 2.0 64.3 ± 14.7 | glycolate, ethanol, acetate, lactate pyruvate | [62] |
| PET PLA | CNx|Ni2P | NaOH 2 M | AM 1.5 | 0.14 0.13 | acetate, formate, glycolate formate, acetate | [63] |
| PET PLA | MoS2/CdS | NaOH 10 M | AM 1.5 G | 3.90 ± 0.07 6.68 ± 0.10 | formate, acetate, glycolate formate | [64] |
| PET | Pt@N-TiO2-x | hydrothermal pretreatment at 180 °C for 12 h. | AM 1.5 G | [65] | ||
| PET | MoS2/CdxZn1-xS | NaOH 10 M | AM 1.5 G | 15.90 | formate, methanol, acetate, ethanol | [66] |
| PET | MoS2/g-C3N4 | NaOH 2 M | AM 1.5 G | 0.0498 | formate, CH4 | [67] |
| PET | CuIn5S8 | KOH 1.5 M | AM 1.5 G | 2.57 ± 0.02 | formate, glycolate, acetate | [68] |
| PET | Pt/g-C3N4 | NaOH 0.1M | AM 1.5 G | 2.00 | formate, methanol, acetate | [69] |
| PET | B-doped g-C3N4 | NaOH 0.1M | Xe lamp λ > 420 nm | 3.24 | formylic acid, acetic acid, glycolic acid | [70] |
| PET | BiVO4/MoOx | KOH 1 M | Xe lamp | 1.60 ± 0.09 | formylic acid, acetic acid | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ye, X.; Zhang, D.; Zhang, B.; Liu, H.; Qu, W. Sustainable Treatment of Plastic Wastes with Photocatalytic Technologies: A Review. Catalysts 2025, 15, 670. https://doi.org/10.3390/catal15070670
Wang X, Ye X, Zhang D, Zhang B, Liu H, Qu W. Sustainable Treatment of Plastic Wastes with Photocatalytic Technologies: A Review. Catalysts. 2025; 15(7):670. https://doi.org/10.3390/catal15070670
Chicago/Turabian StyleWang, Xin, Xiaoling Ye, Duqiang Zhang, Bingxu Zhang, Huimei Liu, and Wenbin Qu. 2025. "Sustainable Treatment of Plastic Wastes with Photocatalytic Technologies: A Review" Catalysts 15, no. 7: 670. https://doi.org/10.3390/catal15070670
APA StyleWang, X., Ye, X., Zhang, D., Zhang, B., Liu, H., & Qu, W. (2025). Sustainable Treatment of Plastic Wastes with Photocatalytic Technologies: A Review. Catalysts, 15(7), 670. https://doi.org/10.3390/catal15070670






