Abstract
This study investigates the dual catalytic inhibition mechanisms of chlorobenzene (CBz) formation during combustion using N- and S-modified layered double hydroxides (LDHs). The metal hydroxide layers in these LDHs primarily suppress lower-chlorinated CBzs (e.g., trichlorobenzene-dichlorobenzene) under inert conditions by inhibiting direct chlorination, achieving inhibition rates above 80%. In contrast, N/S functional groups, particularly thioacetamide, enhance catalytic inhibition efficiency under air, increasing it from 17.8% to 77.3% in the solid phase by controlling catalytic chlorination and limiting highly chlorinated CBzs (e.g., pentachlorobenzene–hexachlorobenzene). These findings highlight the complementary roles of metal hydroxide layers and N/S functional groups in reducing CBz formation, offering insights for developing efficient, multifunctional inhibitors for waste incineration pollution control. While promising, the scaling up of the application of LDH-based inhibitors may face challenges related to synthesis complexity and cost, requiring further research to provide a theoretical foundation for their large-scale application.
1. Introduction
Waste incineration has become a cornerstone of modern waste management, particularly for municipal solid waste (MSW), medical waste, and hazardous waste, owing to its efficiency in reducing waste volume and recovering energy [1,2,3,4]. However, this process presents critical environmental and health challenges, particularly through the emission of hazardous pollutants such as polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) [5,6]. These toxic compounds, commonly referred to as dioxins, are formed through multiple chemical pathways, including catalytically driven de novo synthesis catalyzed by CuCl2 at moderate temperatures (300–500 °C), reactions with chlorinated precursors, and gas-phase reactions at high temperatures (500–800 °C) [7,8,9,10]. Catalytically driven de novo synthesis is widely recognized as the dominant pathway for the formation of PCDD/Fs during MSW incineration [9,11]. Currently, the integration of activated carbon injection and fabric filtration technology is extensively employed for the capture of PCDD/Fs generated via this pathway [12]. However, this approach is limited to end-of-pipe control and fails to address the root cause of PCDD/F formation. The catalytically driven de novo synthesis of PCDD/Fs is highly complex, especially in the presence of metal catalysts and chloride compounds, and is strongly linked to the formation of chlorinated precursors like chlorobenzene (CBz) [13,14]. Consequently, recent research efforts have shifted towards the development of novel inhibitors aimed at regulating the catalytic activity of metal compounds and chloride sources, thereby suppressing the catalytic formation of chlorinated precursors and effectively controlling PCDD/F generation [15,16].
Nitrogen-based, sulfur-based, and alkaline inhibitors effectively suppress PCDD/F formation through distinct mechanisms, each with their own advantages and limitations. Nitrogen-based inhibitors (e.g., NH3, urea) react with Cl2 to form stable NH4Cl and neutralize HCl, disrupting dioxin precursor synthesis [15,16,17]. Sulfur-based inhibitors (e.g., SO2, Na2S) passivate catalytic metal chlorides (CuCl2, FeCl3) by converting them into inert compounds (Cu2S, CuSO4) while sulfonating aromatic precursors to reduce chlorination potential [18,19]. Yet, SO2 emissions necessitate co-injection with alkaline materials [20,21]. Alkaline inhibitors (e.g., KOH, NaOH, CaO) promote CuCl2-to-CuO conversion and reduce Cl2 and HCl concentrations, further limiting dioxin formation [22,23,24]. However, the efficiency of these three types of inhibitors depends on combustion conditions, posing challenges in complex environments [25,26]. Thus, multifunctional inhibitors tailored to dynamic incineration settings are essential for enhanced dioxin control [27,28].
Layered double hydroxides (LDHs), an emerging class of multifunctional materials, offer unique potential for pollution control due to their structural flexibility, functional versatility, and catalytic properties [29,30]. LDHs can adsorb acidic gases (e.g., HCl, CO2, SO2), passivate catalytic sites, stabilize reaction intermediates, and catalytically promote the inhibition of dioxin formation [31,32]. Numerous studies have demonstrated that LDH-based materials are highly effective in removing volatile organic compounds (VOCs), with LDH catalysts exhibiting excellent resistance to complex gas compositions [33,34]. Moreover, LDHs feature a hydroxyl-rich layered structure with tunable interlayer properties, allowing the incorporation of various functional groups (e.g., N and S) to achieve synergistic effects [33,35,36]. However, the application of functionalized LDHs in catalytically suppressing dioxin formation via de novo synthesis remains largely unexplored, and the underlying catalytic mechanisms are yet to be fully understood.
This study investigates the multifunctional properties of functionalized LDHs and their potential to catalytically mitigate key pollutant formation. By synthesizing various N- and S-modified layered double hydroxides, it systematically examines their catalytical inhibitory effects on the de novo synthesis of CBzs under different atmospheric conditions. A combination of gas-phase and solid-phase analyses is employed to elucidate the catalytical inhibition mechanisms, including catalytic site passivation, gas adsorption capacity, and the stabilization of reaction intermediates. This research provides both a solid theoretical foundation and technical support for the development of efficient, environmentally friendly pollution control systems, with promising applications in industrial flue gas treatment and environmental protection.
2. Results and Discussion
2.1. Characterization of LDH Inhibitors
XRD patterns (Figure 1) were used to analyze the structural characteristics of the Ca2Al-LDHs, including the unmodified sample (LDH) and those functionalized with different groups, such as sulfonamide (LDH-1), thioacetamide (LDH-2), and aminosulfonic acid (LDH-3). The reference sample (LDH) exhibited a (003) diffraction peak at 2θ ≈ 11.3°, corresponding to an interlayer spacing (d003) of 0.78 nm, indicating a well-defined and highly ordered layered framework, which served as a benchmark for comparison [37]. Upon sulfonamide modification (LDH-1), the (003) peak shifted to 2θ ≈ 10.8°, with the interlayer spacing increasing to 0.82 nm. This shift suggests that sulfonamide functionalization induced structural adjustments within the framework, while the broader diffraction peak reflects reduced crystallinity, likely due to steric effects and local distortions. For LDH-2, modification with thioacetamide further shifted the (003) diffraction peak to 2θ ≈ 10.6°, corresponding to an interlayer spacing of 0.83 nm. The larger molecular size and hydrogen-bonding capability of thioacetamide disrupted the regular stacking of the layers, leading to increased framework flexibility and a decline in long-range order. Additionally, the reduced peak intensity and broadening suggest partial structural disorder, which may be associated with the weakening of interlayer interactions and localized lattice distortions. In contrast, the (003) diffraction peak of LDH-3 transformed from a sharp peak into two weaker peaks, appearing at 2θ ≈ 10.5° and 2θ ≈ 11.0°, corresponding to interlayer spacings of 0.84 nm and 0.80 nm, respectively. This phenomenon suggests that aminosulfonic acid modification led to heterogeneous structural rearrangements within the framework, resulting in multiple stacking configurations. Some regions of the framework retained their original organization, while others underwent modifications that introduced local strain and disrupted the uniformity of the layer alignment, leading to peak splitting. The trend in interlayer spacing across these samples is as follows: LDH-2 (0.83 nm) ≈ LDH-1 (0.82 nm) > LDH-3 (0.84/0.80 nm) > LDH (0.78 nm). The relatively small variation in interlayer spacing after modification suggests that the functional groups were not intercalated into the LDH layers but were instead present in the form of surface adsorption or coordination [31]. The larger interlayer spacing in LDH-2 may facilitate the diffusion and thermal release of modified functional groups [38], while potentially enhancing adsorption capacity by increasing the surface area and accessible sites for pollutant capture [39].

Figure 1.
XRD analysis of various LDHs.
Due to LDH-2’s unique structural advantage, it was selected for detailed investigation. Specifically, Fourier transform infrared spectroscopy (FTIR) and thermogravimetric-differential scanning calorimetry (TGA-DSC) analyses were performed to characterize the N- and S-modified functional groups in LDH-2. The FTIR spectrum of LDH-2 is presented in Figure 2a. The absorption bands at 3640 cm−1 and 3480 cm−1 are assigned to the O–H stretching vibrations of structural water, whereas the band at 1620 cm−1 corresponds to the bending vibration of interlayer water molecules (H2O) [38]. Additionally, the bands observed at 789 cm−1 and 532 cm−1 are attributed to metal–oxygen vibrational modes (Al–O and Al–OH), indicating the characteristic layered structure of the LDH. Notably, the appearance of new absorption bands at 1144 cm−1 and 1299 cm−1, corresponding to S=O stretching vibrations, suggests the successful incorporation of sulfur-containing functional groups [39]. Furthermore, the characteristic band at 1402 cm−1, assigned to N–H bending vibrations, provides unequivocal evidence of nitrogen-group integration. These spectral features collectively confirm the successful chemical modification of the LDH matrix.
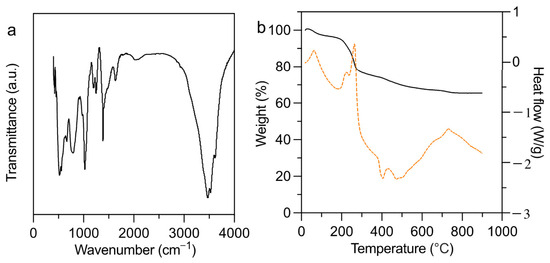
Figure 2.
(a) FT-IR and (b) TG-DSC spectra of LDH-2.
Based on the analysis of the TG-DSC spectra, the results for LDH-2 show distinct stage characteristics in both mass loss and heat flow as the temperature increases (Figure 2b). In the low-temperature region (<150 °C), a slight mass loss of approximately 3% is observed, indicating the evaporation of water. The DSC curve also shows a small endothermic peak, confirming the heat absorption associated with water evaporation. In the mid-temperature range (150–350 °C), a significant mass loss of about 20% is observed, accompanied by a pronounced endothermic peak in the DSC curve, indicating the decomposition of the thioacetamide modification. This process leads to a rapid loss of mass, and the heat flow changes reflect the instability of the modification layer. In the high-temperature region (>350 °C), mass loss stabilizes, indicating that the inorganic layers of the LDH, composed of metal hydroxides, remain thermally stable without significant decomposition or phase transition. The DSC curve shows no significant thermal effects in this region, confirming the resilience of the inorganic components under high thermal stress. These findings suggest that thioacetamide modification not only enhances the functionality of LDHs but also offers a promising strategy for inhibiting chlorobenzene (CBz) formation during its synthesis.
2.2. Formation and Inhibition of CBzs
2.2.1. Formation of CBzs
Previous studies have shown that in the post-combustion stage of the flue gas cooling zone [18,40], CBzs partition between adsorption onto fly ash and volatilization into the gas phase. As shown in Figure 3, this study analyzed the concentration and chlorination degree distributions of CBzs in both phases under different atmospheric conditions.

Figure 3.
(a) Formation of CBzs and (b) their chlorination degree distribution under different atmospheres and phases.
Under both conditions, CBz concentrations in the gas phase remained consistently below 1.5 μg/g (Figure 3a). In contrast, in the solid phase, they reached more than 20 μg/g. The results indicate that CBzs predominantly accumulate in the solid phase under both inert and air atmospheres, primarily due to the strong adsorption capacity of the fly ash surface for CBzs. Notably, CBz concentrations increased by more than a factor of five, from 25.5 ± 3.7 μg/g under an inert atmosphere to 134.3 ± 13.2 μg/g under an air atmosphere, highlighting the significant effect of oxygen on CBz partitioning. Figure 3b further illustrates the distribution of different CBz species. Under inert conditions, the CBzs in the gas phase were primarily low-chlorinated CBzs, such as dichlorobenzene (DCB) and trichlorobenzene (TCB), collectively accounting for nearly 100% of the total. When the atmosphere shifted to air, the formation pathway shifted towards higher-chlorinated CBzs, leading to an increase in pentachlorobenzene (PeCB) and hexachlorobenzene (HCB), which accounted for approximately 20% of the gas phase. This effect was even more pronounced in the solid phase, where the proportion of PeCB increased markedly to ~50%, and HCB reached approximately 30% under air conditions. These results demonstrate that oxygen significantly influences CBz concentration and chlorination degree distribution. On the one hand, under oxygen-deficient conditions, limited C–C bond cleavage promotes the retention of aromatic structures, consistent with the findings of Yan et al. [41]. On the other hand, the presence of oxygen modifies the electrophilic chlorination mechanism, promoting the formation of higher-chlorinated CBzs and potentially shifting the chlorination pathway from direct chlorination to catalytic chlorination [19,42].
2.2.2. Effects of Inhibitors on CBz Formation and Degree of Chlorination
To systematically evaluate the effects of different inhibitors on CBz formation and chlorination degree, this study analyzed the concentrations, inhibition efficiencies, and species distribution of CBzs in both the solid and gas phases under varying atmospheric conditions. As shown in Figure 4, Figure 5, Figure 6 and Figure 7, the addition of inhibitors not only markedly suppressed CBz formation, confirming their inhibitory effects, but also altered the chlorination pathways, resulting in substantial changes in CBz species distribution across different chlorination degrees. These findings reveal significant variations in inhibition efficiencies among the tested inhibitors, which are closely related to their chemical composition and distinct inhibition mechanisms.
Figure 4.
(a) The concentrations and inhibition efficiencies and (b) the species distribution of CBzs in the solid phase under an inert atmosphere with various LDH inhibitors.

Figure 5.
(a) The concentrations and inhibition efficiencies and (b) the species distribution of CBzs in the solid phase under an air atmosphere with various LDH inhibitors.

Figure 6.
(a) The concentrations and inhibition efficiencies and (b) the species distribution of CBzs in the gas phase under an inert atmosphere with various LDH inhibitors.

Figure 7.
(a) The concentrations and inhibition efficiencies and (b) the species distribution of CBzs in the gas phase under an air atmosphere with various LDH inhibitors.
Under an inert atmosphere, the introduction of various LDH inhibitors significantly reduced CBz formation in the solid phase, maintaining concentrations below 5 μg/g and achieving inhibition efficiencies exceeding 80% (Figure 4a). Among these, LDH-3 exhibited the highest inhibition efficiency (91.3% ± 3.8%), followed by LDH-1, LDH, and LDH-2. These findings suggest that the inhibition efficiencies of unmodified LDH and the three N/S-modified LDHs are comparable, indicating that the primary inhibition effect originates from the intrinsic properties of the metal hydroxide layers, while the contribution of functional groups is relatively minor. Beyond the overall suppression, LDH inhibitors markedly influenced the speciation of CBzs (Figure 4b). The proportion of TCB decreased significantly, whereas MCB and DCB production increased, suggesting that the LDH inhibitors effectively impeded the stepwise chlorination of lower-chlorinated CBzs into TCB and higher-chlorinated species. Under inert conditions, the formation of low-chlorinated CBzs in the solid phase is primarily attributed to the electrophilic substitution mechanism (direct chlorination). Therefore, it can be inferred that the suppression effect of LDHs mainly results from their inhibition of CuCl2-based chlorinating agents, which reduces their reactivity for further chlorination. Additionally, LDHs promote the dechlorination of Cu-based catalysts and the solidification of Cl (e.g., conversion of CuCl2 to CuO within the interlayers of alkali metal hydroxide sheets) [43,44,45].
Upon transitioning from an inert to an air atmosphere, the inhibition efficiency of the unmodified LDH decreased significantly, dropping to only 17.8% ± 6.7% (Figure 5a). This decline is likely due to the interaction between oxygen and the LDH metal hydroxide layers, which alters their surface properties and catalytic activity, thereby reducing their inhibition capacity against CBz formation. In contrast, when N- and S-incorporated LDHs were used as inhibitors, the inhibition efficiency notably increased, with LDH-2 achieving the highest inhibition efficiency (77.3% ± 3.4%). This finding underscores the crucial role of N and S functional groups in the inhibition mechanism [23,34,46]. Furthermore, as shown in Figure 5b, under an air atmosphere, the proportion of highly chlorinated CBzs significantly decreased. The relative abundance of PeCB and HCB dropped from 80% to below 45%, whereas MCB, TCB, and TeCB became more prevalent. Previous studies have reported that N- and S-containing compounds (e.g., nitrilotriacetic acid (NTA), ethylenediaminetetraacetic acid (EDTA), or Na2S) exhibit varying inhibitory effects on chlorinated pollutants, likely due to the transformation of N and S groups into different functional species under high-temperature conditions [47]. Therefore, it can be inferred that N and S functional groups in the presence of oxygen not only capture the chlorinating agent Cl2, but also suppress the catalytic activity of CuCl2, thereby reducing the formation of higher-chlorinated CBzs and promoting the generation of lower-chlorinated CBzs [44].
In an inert atmosphere, LDH-2 exhibited the highest inhibition efficiency in the gas phase, with the inhibition order proceeding as follows: LDH-2 (84.4%) > LDH-1 (55.1%) > LDH-3 (48.0%) > LDH (11.8%) (Figure 6a). This suggests that the incorporation of N and S functional groups into LDH-2 enhances its ability to suppress CBz formation. When LDH-2 was used as an inhibitor, the total proportion of highly chlorinated CBzs (TeCB, PeCB, and HCB) slightly decreased, while the addition of other inhibitors led to varying degrees of increase in highly chlorinated CBzs (Figure 6b). This supports the hypothesis that the N and S functional groups in LDH-2 play a key role in inhibiting catalytic chlorination, which is the main mechanism for the formation of highly chlorinated CBzs [42]. Under an air atmosphere, the inhibition efficiency of the various LDH inhibitors was relatively low, with all inhibition rates being below 65% (Figure 7a). This trend was consistent across the inhibitors, primarily manifesting in the suppression of low-chlorinated CBzs (Figure 7b). This can be attributed to the dual effect of oxygen. First, oxygen accelerates chlorobenzene formation by enhancing the reaction between Cl2 and the benzene ring in the gas phase, reducing the inhibitor’s effectiveness in suppressing chlorobenzene formation [48]. Second, oxygen exposure alters the surface properties of LDHs, potentially reducing their ability to capture Cl2 in the gas phase due to oxidation. Meanwhile, the decomposition products of the N- and S-functional groups (e.g., stable oxides or sulfates) are more effective in capturing Cl2 in the solid phase than in the gas phase [49].
2.3. Inhibition Mechanism
The electrophilic chlorination of aromatic hydrocarbons to form CBzs proceeds via two pathways [10,42]. In direct chlorination, CuCl2 serves as the chlorine source, transferring a positively charged chlorine group to achieve chlorination while being reduced to CuCl and releasing HCl (Equation (1)). In catalytic chlorination, CuCl2 acts as a Lewis acid catalyst, promoting the dissociation of Cl2 by decomposing CuCl2 (Equation (2)) to generate Cl⁺ for electrophilic substitution, with CuCl2 being regenerated through redox cycling (Equation (3)). The pathway choice depends on reaction conditions, with direct chlorination favoring low chlorination under an inert atmosphere and catalytic chlorination favoring high chlorination under oxidative conditions.
Based on the previous research findings and the analysis of CBz electrophilic chlorination, it can be concluded that N/S-modified LDHs inhibit CBzs synthesis through two mechanisms: one associated with their metal hydroxide layers and the other with the contributions of the N and S functional groups. Under an inert atmosphere, the metal hydroxide layers dominate the inhibition process, while under an air atmosphere the N and S functional groups play a more critical role in suppressing catalytic chlorination. These mechanisms are described as follows:
- Inhibition effect of metal hydroxide layers.
The metal hydroxide layers in LDHs serve as a key structural component, typically consisting of metal ions (Ca2⁺ and Al3⁺) coordinated with hydroxide ions (OH−) to form a layered framework. In direct chlorination, these layers facilitate the dechlorination of CuCl2 by assisting in the conversion of CuCl2 to CuO within the metal hydroxide layers [44,45]. This process effectively reduces the availability of active chlorine species (Cl2, HCl) released from CuCl2 decomposition (Equations (2) and (3)), thereby inhibiting the further chlorination of lower-chlorinated CBz species, such as MCB and DCB, and preventing the formation of more highly chlorinated products.
- Inhibition effect of N and S functional groups.
The introduction of N and S functional groups provides an additional inhibitory mechanism for LDH inhibitors, distinct from the inhibition of direct chlorination by the metal hydroxide layers. This mechanism targets the catalytic chlorination process: within the temperature range of 200–400 °C, N and S groups (e.g., thioacetamide in LDH-2) decompose to form NH3 and H2S (Equations (4) and (5)). Under an oxygen atmosphere, H2S is oxidized to SO2 (Equation (6)), which reacts with CuCl2 to generate CuSO4 (Equation (7)), deactivating the catalyst [27,41]. Simultaneously, NH3 reacts with HCl to form NH4Cl (Equation (8)) [49], effectively capturing chlorine sources. By deactivating the catalyst and sequestering chlorine, the N and S functional groups inhibit the catalytic chlorination process from multiple angles, ultimately preventing the formation of highly chlorinated CBzs.
3. Materials and Methods
3.1. Materials
The following chemicals and reagents were used in this study: sulfonamide (≥99%, Macklin Biochemical Co., Ltd., Shanghai, China), thioacetamide (≥99%, Macklin Biochemical Co., Ltd., Shanghai, China), aminosulfonic acid (≥99.5%, Macklin Biochemical Co., Ltd., Shanghai, China), calcium chloride dihydrate (CaCl2·2H2O, ≥99%, Macklin Biochemical Co., Ltd., Shanghai, China), copper chloride (CuCl2, ≥98%, Macklin Biochemical Co., Ltd., Shanghai, China), aluminum nitrate nonahydrate (Al(NO3)3·9H2O, analytical grade, Macklin Biochemical Co., Ltd., Shanghai, China), sodium hydroxide (NaOH, analytical grade, Macklin Biochemical Co., Ltd., Shanghai, China), and potassium chloride (KCl, ≥99%, Sigma-Aldrich, St. Louis, MO, USA).
Dichloromethane (DCM, HPLC grade, Macklin Biochemical Co., Ltd., Shanghai, China), tetrachloroguaiacol internal standard solution (Sigma-Aldrich, St. Louis, MO, USA), and XAD-II macroreticular resin (20–60 mesh, Sigma-Aldrich, St. Louis, MO, USA) were employed for extraction and analytical processes. Adsorbents included activated carbon (AC, 40–60 mesh, derived from nutshells, Sigma-Aldrich, St. Louis, MO, USA) and silica (SiO2, 200 mesh, special grade, Macklin Biochemical Co., Ltd., Shanghai, China). All other reagents and chemicals were of analytical grade or higher and were procured from Macklin Biochemical Co., Ltd. (Shanghai, China) or Sigma-Aldrich (St. Louis, MO, USA).
3.2. Sample Preparation
3.2.1. Preparation of LDH Inhibitors
A typical LDH was synthesized using calcium-aluminum layered double hydroxide (Ca2Al-LDH) as the base material [50]. CaCl2·2H2O and Al(NO3)3·9H2O were dissolved in deionized water, maintaining a molar ratio of Ca2⁺ to Al3⁺ at 2:1. The metal salt solution was added dropwise at 1 mL/min to the NaOH solution (1 M) under continuous stirring (800 rpm). During the precipitation, the pH was carefully monitored and maintained at 10.0 ± 0.5 by the gradual addition of NaOH to facilitate LDH nucleation and growth while minimizing undesired phases. The reaction proceeded at room temperature (25 ± 2 °C) to maintain controlled precipitation kinetics. The resulting slurry was transferred to a sealed glass reactor and hydrothermally aged at 80 °C for 12 h to enhance its crystallinity and improve its structural homogeneity. After aging, the suspension was filtered under a vacuum and thoroughly washed with deionized water until the filtrate reached a neutral pH. The washed precipitate was subsequently dried in an oven at 80 °C for 12 h to completely remove residual moisture, hereafter referred to as LDH.
The preparation of a series of N- and S-modified layered double hydroxides (N/S-modified LDHs) followed a procedure similar to that of Ca2Al-LDH synthesis, with the key modification of replacing Cl− with specific N- and S-containing compounds, such as sulfonamide, thioacetamide, and aminosulfonic acid. The molar ratio of (Ca2⁺ + Al3⁺) to these N- and S-containing compounds was maintained at 1:1 to maximize the modification efficiency. This approach facilitated the synthesis of a series of N/S-modified LDHs, including sulfonamide-modified Ca2Al-LDH (LDH-1), thioacetamide-modified Ca2Al-LDH (LDH-2), and aminosulfonic acid-modified Ca2Al-LDH (LDH-3).
3.2.2. Preparation of Simulated Fly Ash
To ensure optimal performance, activated carbon, serving as the carbon source, was pretreated prior to use. The pretreatment involved grinding the activated carbon in a mortar for 10 min, followed by heating at 600 °C for 60 min under a nitrogen flow (100 mL/min) to remove residual organic content. CuCl2, recognized as the most active catalyst, played a crucial role in the catalytic activity of the SFA.
A fixed amount of 2 g of SFA was used to maintain consistency across the experiments and to align with typical experimental constraints (Table 1) [40]. In experiments involving inhibitors, the inhibitor dosage was set at 2.5 wt%. This experimental design provides a systematic framework to evaluate the synergistic interactions between SFA and the inhibitors, offering valuable insights into the reaction mechanisms.

Table 1.
Mass fractions of compounds used in experimental procedures.
3.3. De Novo Synthesis Experiments
A customized reactor system was used to perform the de novo synthesis experiments, consisting of two main components: a thermal regulation unit and a gas adsorption module (Figure 8). The thermal regulation unit featured a horizontal cylindrical furnace connected to a quartz tube (800 mm in length, 40 mm internal diameter), designed to maintain a stable thermal environment. The gas adsorption module consisted of an adsorption tube filled with 2 g of XAD-II macroreticular resin, optimized for capturing chlorobenzenes (CBzs) released during the reaction.

Figure 8.
Scheme of de novo synthesis experiments.
The experimental setup involved placing 0.2 g of SFA in a quartz boat positioned within the sample loading zone. The reaction atmosphere was adjusted by varying the oxygen-to-nitrogen ratio to either 0% (inert atmosphere) or 21% (air atmosphere), allowing precise control over the gaseous environment. The furnace temperature was set to 300 °C, and once the desired temperature was reached and stabilized, the quartz boat was transferred into the reaction chamber within the quartz tube. Thermal treatment was conducted for 30 min under constant isothermal conditions to ensure the reaction proceeded to completion.
3.4. Sample Characterization and CBzs Analysis
The crystal phases of the inhibitors were analyzed using X-ray diffraction (XRD) on a Bruker D8 Venture diffractometer (Karlsruhe, Germany). Fourier transform infrared (FT-IR) spectroscopy was conducted on a Thermo Scientific Nicolet iS50 FT-IR spectrometer (Waltham, MA, USA), with spectra recorded in the range of 400–4000 cm−1. Thermogravimetric-differential scanning calorimetry (TG-DSC) analysis was performed using a NETZSCH STA 449F5 thermal analyzer (Selb, Germany), where the sample was heated from 20 °C to 900 °C at a heating rate of 10.0 K/min under a controlled nitrogen atmosphere. X-ray photoelectron spectroscopy (XPS) was used to examine valence-state variations with a Thermo Scientific K-Alpha spectrometer (USA).
After the heating process, the concentrations of CBzs in both the solid residues and the XAD-II macroreticular adsorbent were comprehensively analyzed using gas chromatography–mass spectrometry (GC-MS, Agilent 7890B-5977B, Santa Clara, CA, USA). The sample preparation procedure strictly followed the Chinese National Standard GB/T 20384-2024, which provides detailed protocols to ensure the accurate identification and quantification of chlorinated aromatic compounds. Each heating experiment was performed in duplicate (n = 2) to enhance the reliability and statistical robustness of the results. The analysis showed consistently low variability in CBz levels across the different congeners, further confirming the high reproducibility, precision, and reliability of the employed analytical method.
4. Conclusions
This study systematically evaluated the effects of N- and S-modified LDH inhibitors on CBz formation and chlorination pathways under inert and air atmospheres. The results demonstrate that the metal hydroxide layers of LDHs effectively suppress lower-chlorinated CBz formation under inert conditions by capturing Cl2 and promoting CuCl2 dechlorination, with LDH-3 achieving an inhibition efficiency of 91.3% ± 3.8%. Under an air atmosphere, the introduction of N and S functional groups significantly enhances the inhibition efficiency, with LDH-2 reaching 77.3% ± 3.4%, compared to just 17.8% ± 6.7% for the unmodified LDH. This improvement is primarily attributed to the decomposition of N and S groups (e.g., thioacetamide) into NH3 and H2S, which react with CuCl2 to form stable complexes (e.g., CuSO4) and capture chlorine sources (e.g., NH4Cl), thus inhibiting catalytic chlorination and the formation of highly chlorinated CBzs. These findings highlight the dual inhibition mechanisms of N- and S-modified LDHs: metal hydroxide layers suppress direct chlorination under inert conditions, while N and S functional groups target catalytic chlorination under oxidative conditions. This study provides valuable insights for designing multifunctional inhibitors aimed at controlling chlorinated by-products in waste incineration.
Author Contributions
Y.L.: Conceptualization; Investigation; Methodology; Project Administration; Writing—Original Draft. W.L.: Investigation; Methodology; Validation; Writing—Original Draft. J.L.: Methodology; Software; Validation; Formal Analysis. D.P.: Supervision; Investigation; Data Curation. F.O.: Conceptualization; Methodology; Software; Data Curation. D.C.: Supervision: Investigation; Resources. S.F.: Writing—Original Draft; Investigation; Supervision. X.X.: Project Administration; Supervision; Resources. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51808132), the Stable Support Plan Program of the Shenzhen Natural Science Fund (Grant No. 20220820003755001), the 2022 Innovation Team Project of Colleges and Universities in Guangdong Province (Grant No. 2022KCXTD058), and the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2024A1515010469).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Astrup, T.F.; Tonini, D.; Turconi, R.; Boldrin, A. Life cycle assessment of thermal waste-to-energy technologies: Review and recommendations. Waste Manag. 2015, 37, 104–115. [Google Scholar] [PubMed]
- Ramos, A. Sustainability assessment in waste management: An exploratory study of the social perspective in waste-to-energy cases. J. Clean. Prod. 2024, 475, 143693. [Google Scholar]
- Marshall, R.E.; Farahbakhsh, K. Systems approaches to integrated solid waste management in developing countries. Waste Manag. 2013, 33, 988–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Y. Economic analysis of large-scale farm biogas power generation system considering environmental benefits based on LCA: A case study in China. J. Clean. Prod. 2020, 258, 120985. [Google Scholar] [CrossRef]
- Brzuzy, L.P.; Hites, R.A. Global mass balance for polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ. Sci. Technol. 1996, 30, 1797–1804. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Kurup, L.; Singh, A.K. Process development for the removal and recovery of hazardous dye erythrosine from wastewater by waste materials-bottom ash and de-oiled soya as adsorbents. J. Hazard. Mater. 2006, 138, 95–105. [Google Scholar] [CrossRef]
- He, F.; Peng, Y.; Wang, F.; Dong, Y.; Chen, K.; Lu, S. Inhibition of PCDD/Fs in a full-scale hazardous waste incinerator by the quench tower coupled with inhibitors injection. Environ. Pollut. 2022, 314, 120261. [Google Scholar]
- Addink, R.; Olie, K. Mechanisms of formation and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans in heterogeneous systems. Environ. Sci. Technol. 1995, 29, 1425–1435. [Google Scholar] [CrossRef]
- McKay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar]
- Altarawneh, M.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs). Prog. Energy Combust. Sci. 2009, 35, 245–274. [Google Scholar]
- Dong, M. Investigation on the PCDD/Fs distribution of quenched off-gas from electric arc furnace. Chemosphere 2021, 272, 129932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, H.; Lu, S.; Qiu, J.; Ning, P.; Hou, X.; Zhu, L.; Peng, Y. Removal characteristics of PCDD/Fs by an adsorbent injection coupled with a baghouse filter system. Atmos. Pollut. Res. 2024, 15, 102243. [Google Scholar] [CrossRef]
- Blumenstock, M.; Zimmermann, R.; Schramm, K.W.; Kettrup, A. Identification of surrogate compounds for the emission of PCDD/F (I-TEQ value) and evaluation of their on-line real-time detectability in flue gases of waste incineration plants by REMPI-TOFMS mass spectrometry. Chemosphere 2001, 42, 507–518. [Google Scholar] [CrossRef]
- Chen, T.; Xiang, W.; Wu, A.; Lin, X.; Chen, Z.; Li, X.; Yan, J. Suppression on PCDD/Fs formation by a novel inhibition system consisting of phosphorous-based compounds coupled with a chlorine-deactivation material. Waste Manag. 2023, 156, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, S.; Sato, H.; Tsubouchi, N.; Ohtsuka, Y.; Kasai, E. Effect of nitrogen-containing compounds on polychlorinated dibenzo-p-dioxin/dibenzofuran formation through de novo synthesis. Environ. Sci. Technol. 2005, 39, 795–799. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, L.; Yu, Z.; Chun, T.; Long, H.; Wu, X.; Li, J. Inhibition behavior of PCDD/Fs congeners by addition of N-containing compound in the iron ore sintering. Aerosol Air Qual. Res. 2020, 20, 2568–2579. [Google Scholar] [CrossRef]
- Hajizadeh, Y.; Onwudili, J.A.; Williams, P.T. Effects of gaseous NH3 and SO2 on the concentration profiles of PCDD/F in fly ash under post-combustion zone conditions. Waste Manag. 2012, 32, 1378–1386. [Google Scholar] [CrossRef]
- Fujimori, T.; Nishimoto, Y.; Shiota, K.; Takaoka, M. Contrasting effects of sulfur dioxide on cupric oxide and chloride during thermochemical formation of chlorinated aromatics. Environ. Sci. Technol. 2014, 48, 13644–13651. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, H.; Fan, Y.; Wang, D.; Cao, R.; Gao, Y.; Chen, J. Inhibition effect and Mmechanism of thiourea on electrophilic chlorination of aromatics in combustion flue gas. Environ. Sci. Technol. 2021, 55, 700–708. [Google Scholar] [CrossRef]
- Lin, X.; Zhan, M.; Yan, M.; Dai, A.; Wu, H.; Li, X.; Chen, T.; Lu, S.; Yan, J. Suppression of dioxins in waste incinerator emissions by recirculating SO2. Chemosphere 2015, 133, 75–81. [Google Scholar] [CrossRef]
- Chang, M.B.; Cheng, Y.C.; Chi, K.H. Reducing PCDD/F formation by adding sulfur as inhibitor in waste incineration processes. Sci. Total Environ. 2006, 366, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, M.; Zhang, B.; Qian, Y.; Ma, X.; Liu, W. Inhibition of PCDD/Fs formation from dioxin precursors by calcium oxide. Chemosphere 2005, 60, 785–790. [Google Scholar] [PubMed]
- Xia, D.; Xu, J.; Li, W.; Sun, Y. Exploring N/S-based polymers for synergistic inhibition of multiple unintentional persistent organic pollutants during iron ore sintering. ACS ES T Eng. 2022, 2, 2095–2103. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Lin, X.; Chen, T.; Li, X. Formation and inhibition of polychlorinated-ρ-dibenzodioxins and dibenzofurans from mechanical grate municipal solid waste incineration systems. J. Hazard. Mater. 2021, 403, 123812. [Google Scholar] [CrossRef]
- Ruokojärvi, P.H.; Asikainen, A.H.; Tuppurainen, K.A.; Ruuskanen, J. Chemical inhibition of PCDD/F formation in incineration processes. Sci. Total Environ. 2004, 325, 83–94. [Google Scholar] [CrossRef]
- Pekárek, V.; Punčochář, M.; Bureš, M.; Grabic, R.; Fišerová, E. Effects of sulfur dioxide, hydrogen peroxide and sulfuric acid on the de novo synthesis of PCDD/F and PCB under model laboratory conditions. Chemosphere 2007, 66, 1947–1954. [Google Scholar] [CrossRef]
- Wang, P.; Xie, F.; Yan, F.; Shen, X.; Wei, X.; Qu, F.; Su, Y.; Zhong, R.; Li, Z.; Zhang, Z. Inhibitory effect and mechanism of an N-S-based inhibitor (CH4N2S) on PCDD/Fs in flue gas and fly ash in a full-scale municipal solid waste incinerator. ACS ES T Eng. 2023, 3, 1557–1567. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, X.; Lu, S.; Li, X.; Yan, J. Suppressing formation pathway of PCDD/Fs by S-N-containing compound in full-scale municipal solid waste incinerators. Chem. Eng. J. 2019, 359, 1391–1399. [Google Scholar] [CrossRef]
- Jamil, S.; Khan, S.R.; Alvi, A.R.; Kausar, F.; Ali, S.; Khan, S.A.; Naim, M.; Malik, A.; Janjua, M.R.S.A. Morphologically controlled synthesis, characterization and application of zinc-aluminum layered double hydroxide nano needles. Chem. Phys. 2020, 528, 110530. [Google Scholar] [CrossRef]
- Jamil, S.; Khan, S.R.; Ali, S.; Bibi, S.; Khan, R.A.; Gill, W.A.; Janjua, M.R.S.A. Synthesis of calcium-bismuth layered double hydroxide (LADH) nanoparticles: Applications as photo-catalyst and fuel additive. Inorg. Chem. Commun. 2023, 157, 111331. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [PubMed]
- Zhang, R.; Ai, Y.; Lu, Z. Application of multifunctional layered double hydroxides for removing environmental pollutants: Recent experimental and theoretical progress. J. Environ. Chem. Eng. 2020, 8, 103908. [Google Scholar]
- Wu, W.; Wu, Y.; Jin, B.; Gu, Q. Synthesis, Characterization, and high-temperature HCl capture capacity of different proportions of potassium fluoride-doped CaMgAl layered double hydroxides. ACS Omega 2019, 4, 18159–18166. [Google Scholar]
- Xu, S.; Zhang, M.; Li, S.Y.; Zeng, H.Y.; Tian, X.Y.; Wu, K.; Hu, J.; Chen, C.R.; Pan, Y. Intercalation of a novel containing nitrogen and sulfur anion into hydrotalcite and its highly efficient flame retardant performance for polypropylene. Appl. Clay Sci. 2020, 191, 105600. [Google Scholar]
- Yu, Q.; Li, C.; Ma, D.; Zhao, J.; Liu, X.; Liang, C.; Zhu, Y.; Zhang, Z.; Yang, K. Layered double hydroxides-based materials as novel catalysts for gaseous VOCs abatement: Recent advances and mechanisms. Coord. Chem. Rev. 2022, 471, 214738. [Google Scholar]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar]
- Sun, Z.; Park, J.S.; Kim, D.; Shin, C.H.; Zhang, W.; Wang, R.; Rao, P. Synthesis and adsorption properties of Ca-Al layered double hydroxides for the removal of aqueous fluoride. Water Air Soil Pollut. 2016, 228, 23. [Google Scholar]
- Wang, X.; Zhao, H.; Chang, L.; Yu, Z.; Xiao, Z.; Tang, S.; Huang, C.; Fan, J.; Yang, S. First-principles study on interlayer spacing and structure stability of NiAl-layered double hydroxides. ACS Omega 2022, 7, 39169–39180. [Google Scholar]
- Tang, N.; He, T.; Liu, J.; Li, L.; Shi, H.; Cen, W.; Ye, Z. New Insights into CO2 adsorption on layered double hydroxide (LDH)-based nanomaterials. Nanoscale Res. Lett. 2018, 13, 55. [Google Scholar]
- Fujimori, T.; Tanino, Y.; Takaoka, M. Coexistence of Cu, Fe, Pb, and Zn oxides and chlorides as a determinant of chlorinated aromatics generation in municipal solid waste incinerator fly ash. Environ. Sci. Technol. 2014, 48, 85–92. [Google Scholar]
- Yan, M.; Qi, Z.; Yang, J.; Li, X.; Ren, J.; Xu, Z. Effect of ammonium sulfate and urea on PCDD/F formation from active carbon and possible mechanism of inhibition. J. Environ. Sci. 2014, 26, 2277–2282. [Google Scholar]
- Wang, D.; Zhang, H.; Fan, Y.; Ren, M.; Cao, R.; Chen, J. Electrophilic chlorination of naphthalene in combustion flue gas. Environ. Sci. Technol. 2019, 53, 5741–5749. [Google Scholar] [PubMed]
- Wang, X.; Lv, J.; Ying, Y.; Ma, Y.; Wu, A.; Lin, X.; Cao, A.; Li, X.; Yan, J. A new insight into the CaO-induced inhibition pathways on PCDD/F formation: Metal passivation, dechlorination and hydroxide substitution. Sci. Total Environ. 2023, 885, 163782. [Google Scholar] [PubMed]
- Zhang, H.; Lan, D.Y.; Lü, F.; Shao, L.M.; He, P.J. Inhibition of chlorobenzenes formation by calcium oxide during solid waste incineration. J. Hazard. Mater. 2020, 400, 123321. [Google Scholar]
- Wang, X.; Lin, B.; Wang, J.; Ma, Y.; Jin, R.; Liu, G.; Zheng, M. Insight into the inhibition mechanisms of Ca-, N-, S-containing compounds on persistent organic pollutant formation from the thermal reaction of anthracene on copper chloride. Chem. Eng. J. 2024, 497, 154824. [Google Scholar]
- Fu, J.Y.; Li, X.D.; Chen, T.; Lin, X.Q.; Buekens, A.; Lu, S.Y.; Yan, J.H.; Cen, K.F. PCDD/Fs’ suppression by sulfur–amine/ammonium compounds. Chemosphere 2015, 123, 9–16. [Google Scholar]
- Addink, R.; Paulus, R.H.W.L.; Olie, K. Prevention of polychlorinated dibenzo-p-dioxins/dibenzofurans formation on municipal waste incinerator fly ash using nitrogen and sulfur compounds. Environ. Sci. Technol. 1996, 30, 2350–2354. [Google Scholar]
- Lin, F.; Zhang, Z.; Li, N.; Yan, B.; He, C.; Hao, Z.; Chen, G. How to achieve complete elimination of Cl-VOCs: A critical review on byproducts formation and inhibition strategies during catalytic oxidation. Chem. Eng. J. 2021, 404, 126534. [Google Scholar]
- Wang, S.J.; He, P.J.; Lu, W.T.; Shao, L.M.; Zhang, H. Amino compounds as inhibitors of de novo synthesis of chlorobenzenes. Sci. Rep. 2016, 6, 23197. [Google Scholar]
- Razzaq, A.; Ali, S.; Asif, M.; In, S.I. Layered double hydroxide (LDH) based photocatalysts: An outstanding strategy for efficient photocatalytic CO2 conversion. Catalysts 2020, 10, 1185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).