Iron Sludge-Derived Photo-Fenton Reaction for Laundry Wastewater Effluent Oxidation and Process Optimization into Industrial Ecology Symbiosis
Abstract
1. Introduction
2. Results and Discussion
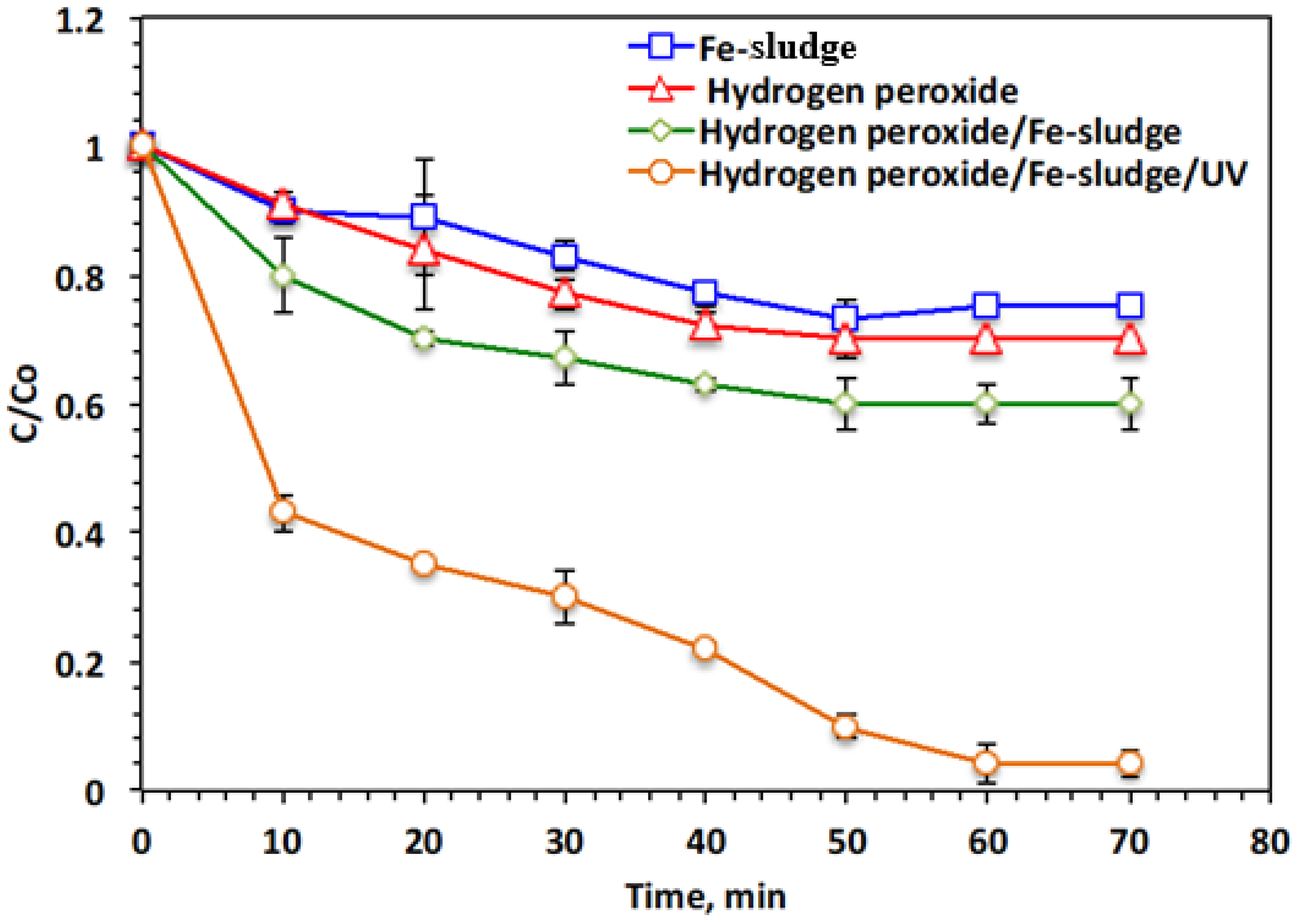
2.1. Comparative Oxidation Systems and Operating Time
2.2. Fenton’s Multiple Parameters (Fe-Sludge, H2O2, and pH Effect)
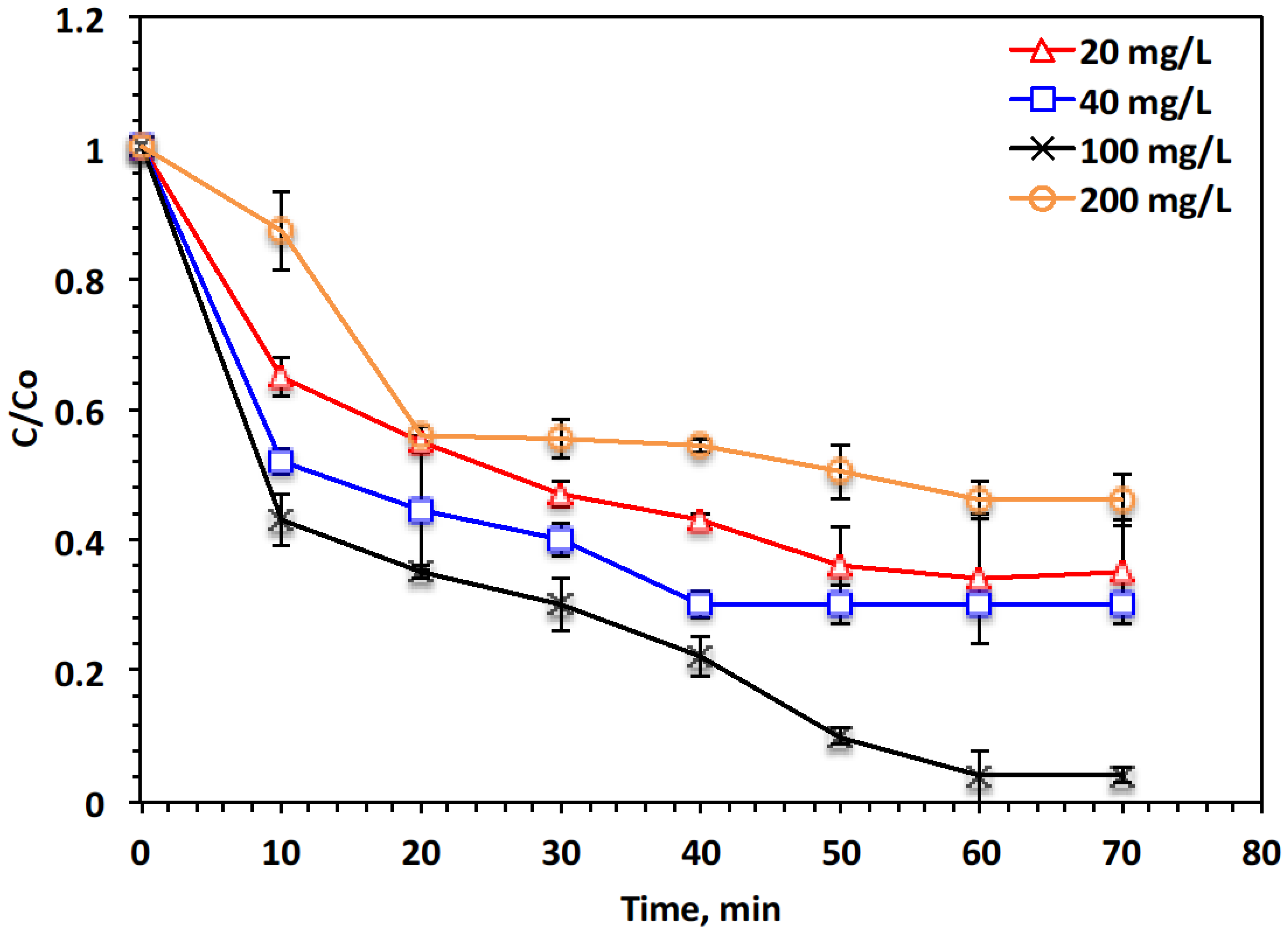
- Fe Sludge
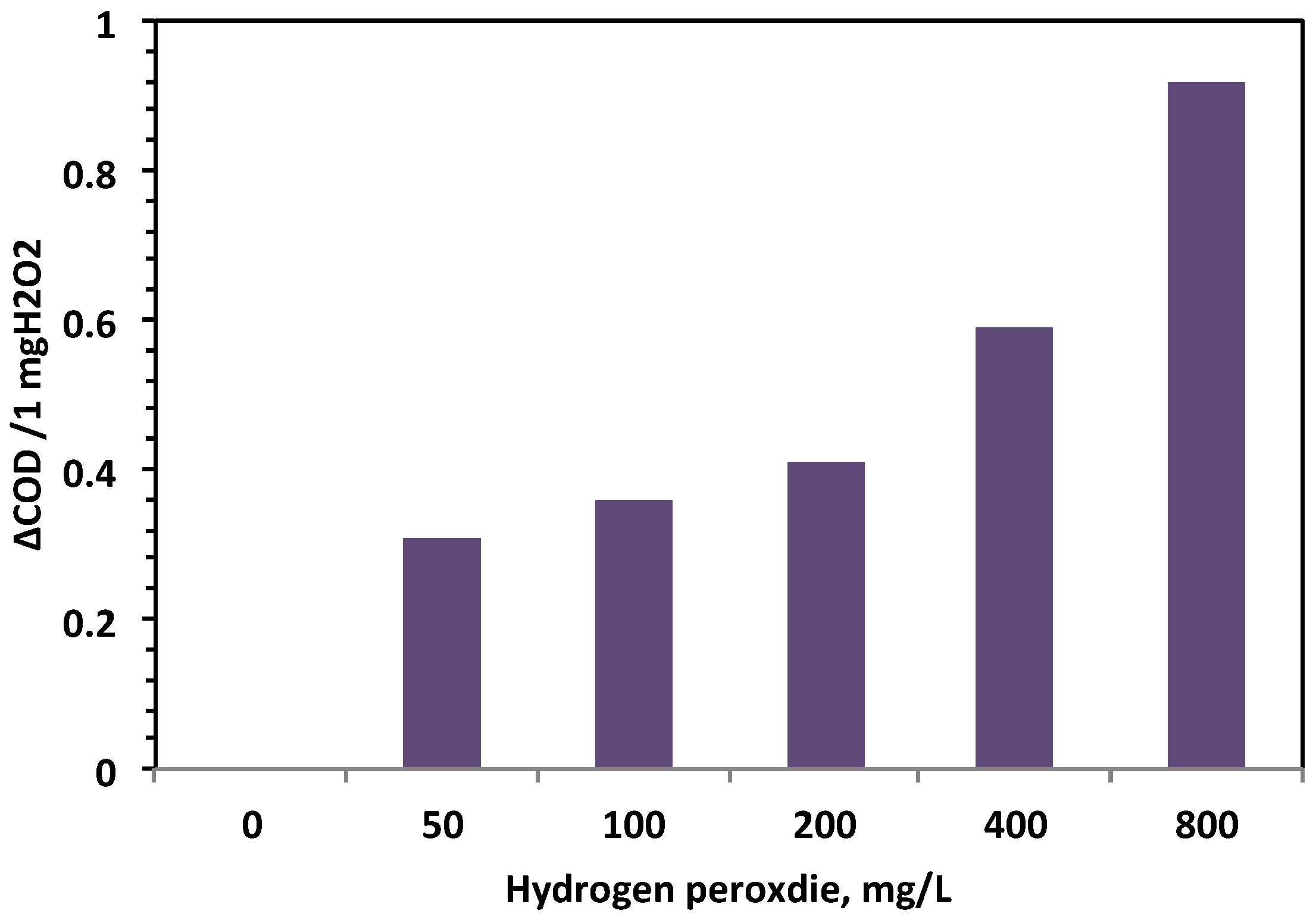
- H2O2 concentration
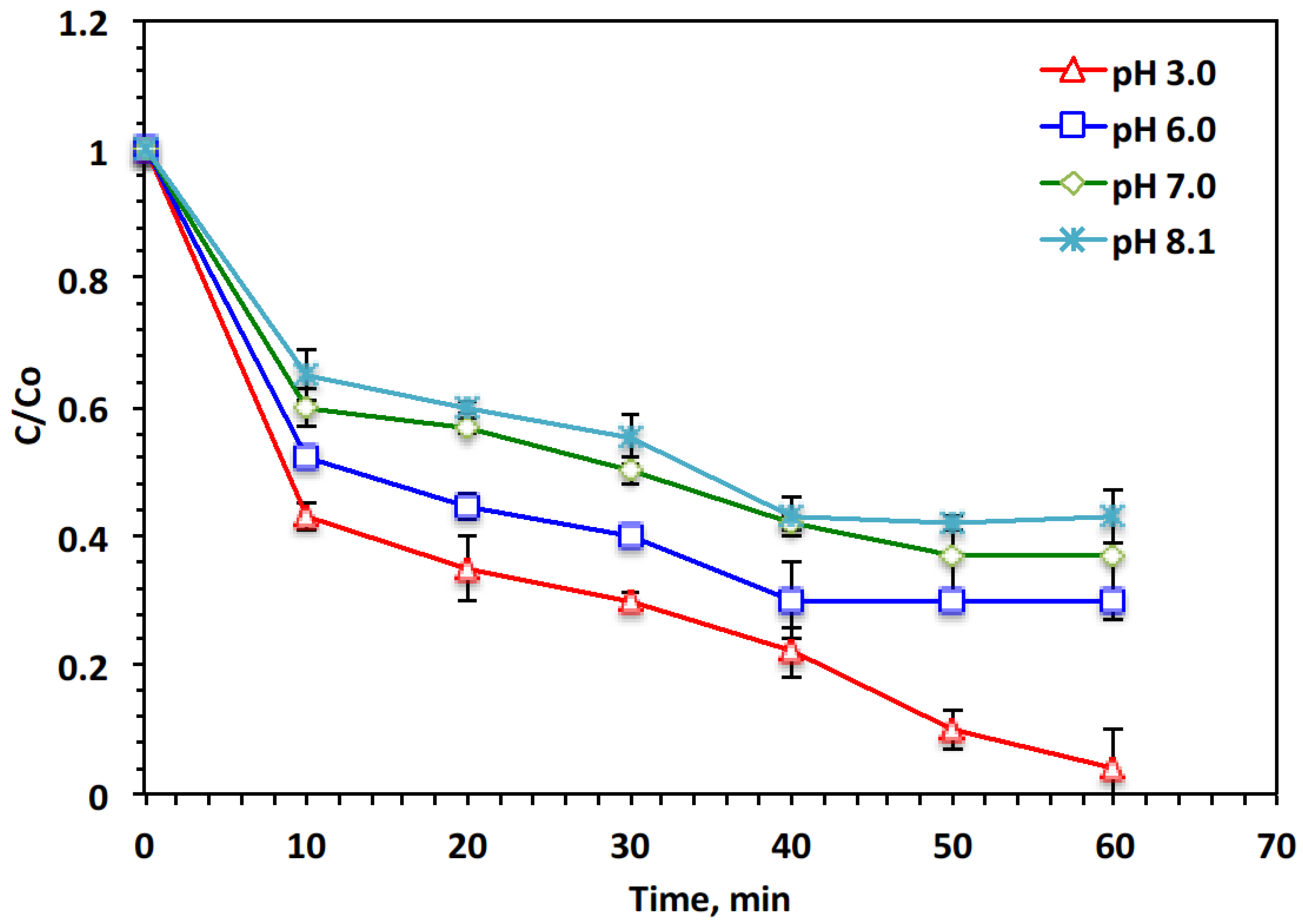
- pH
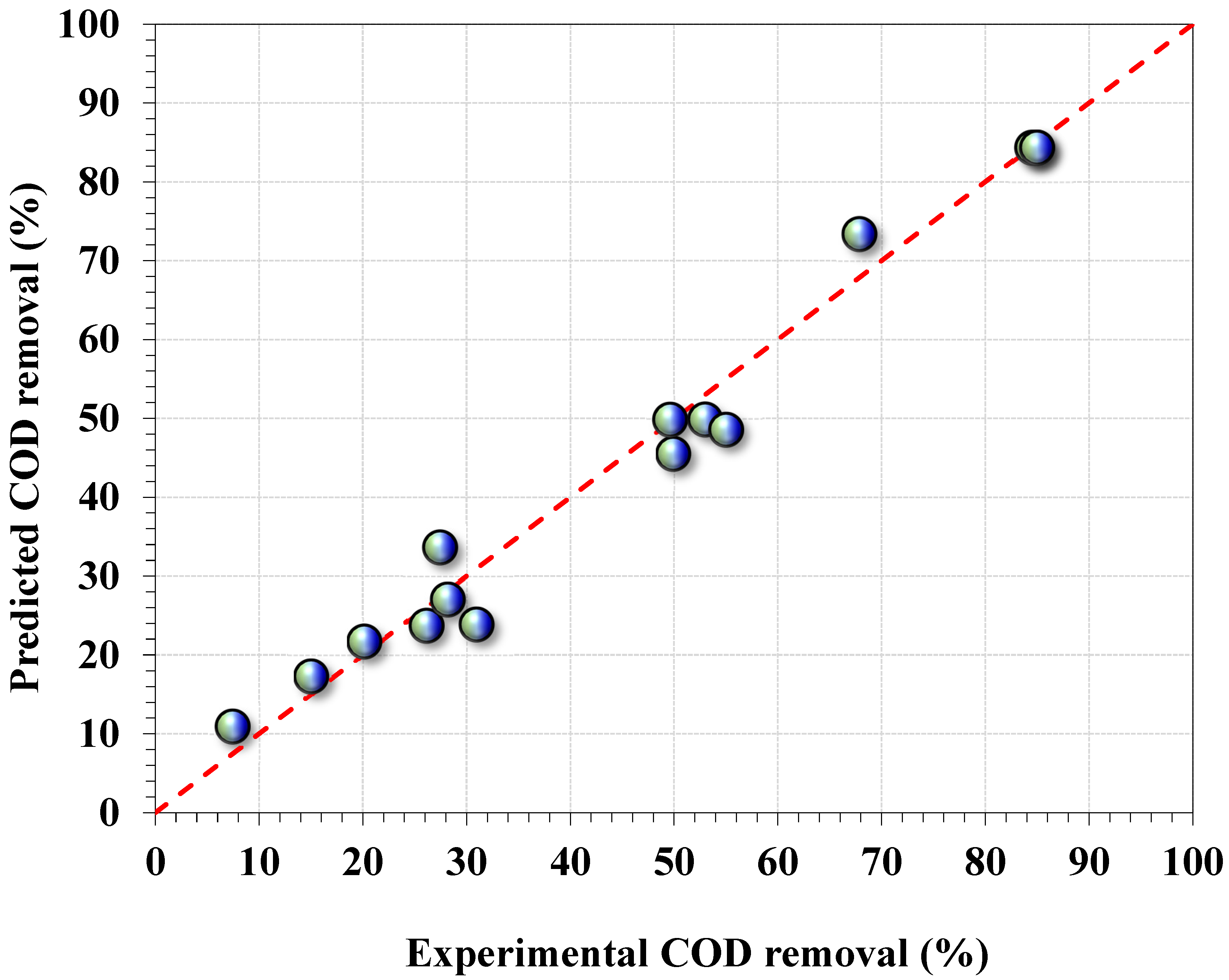
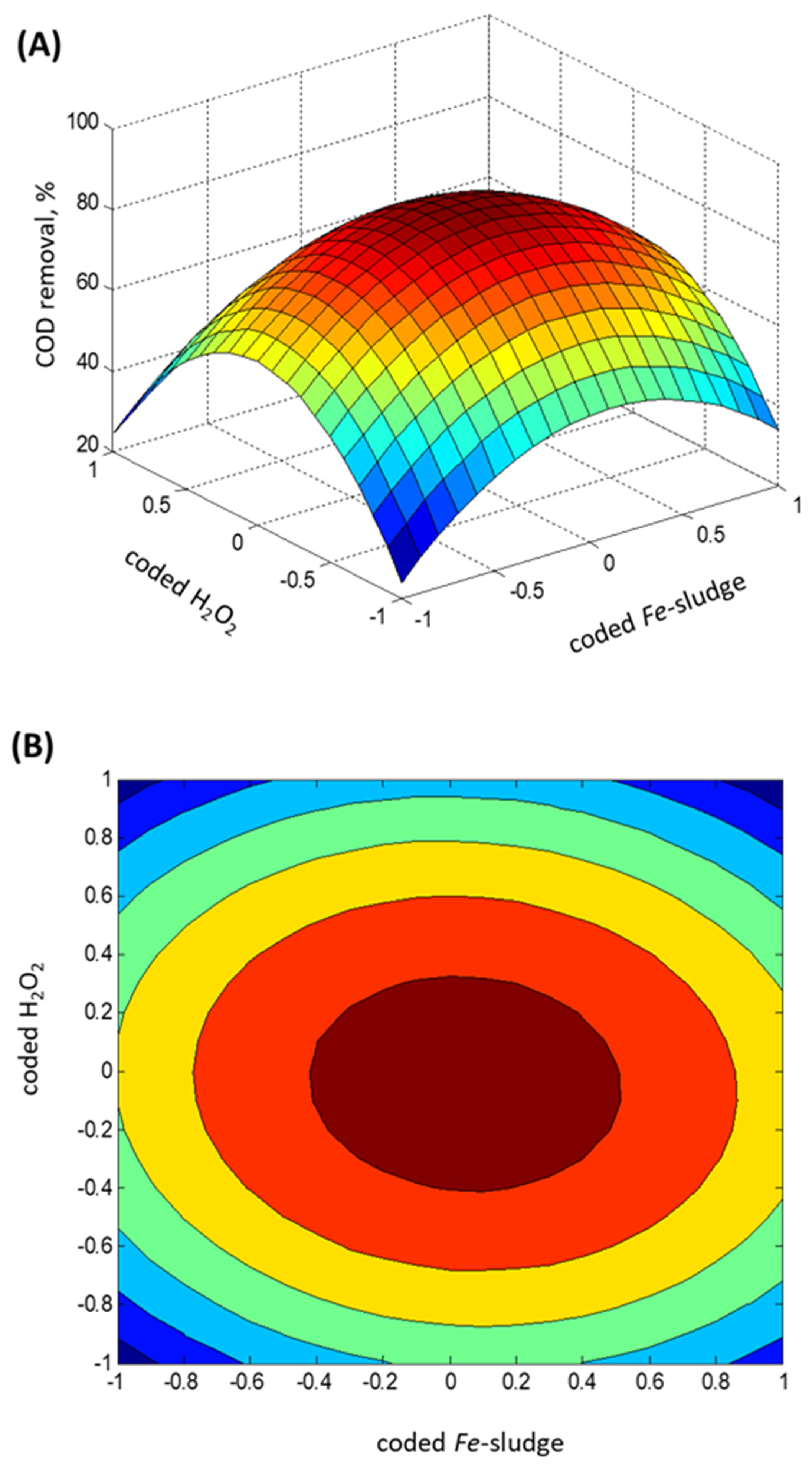
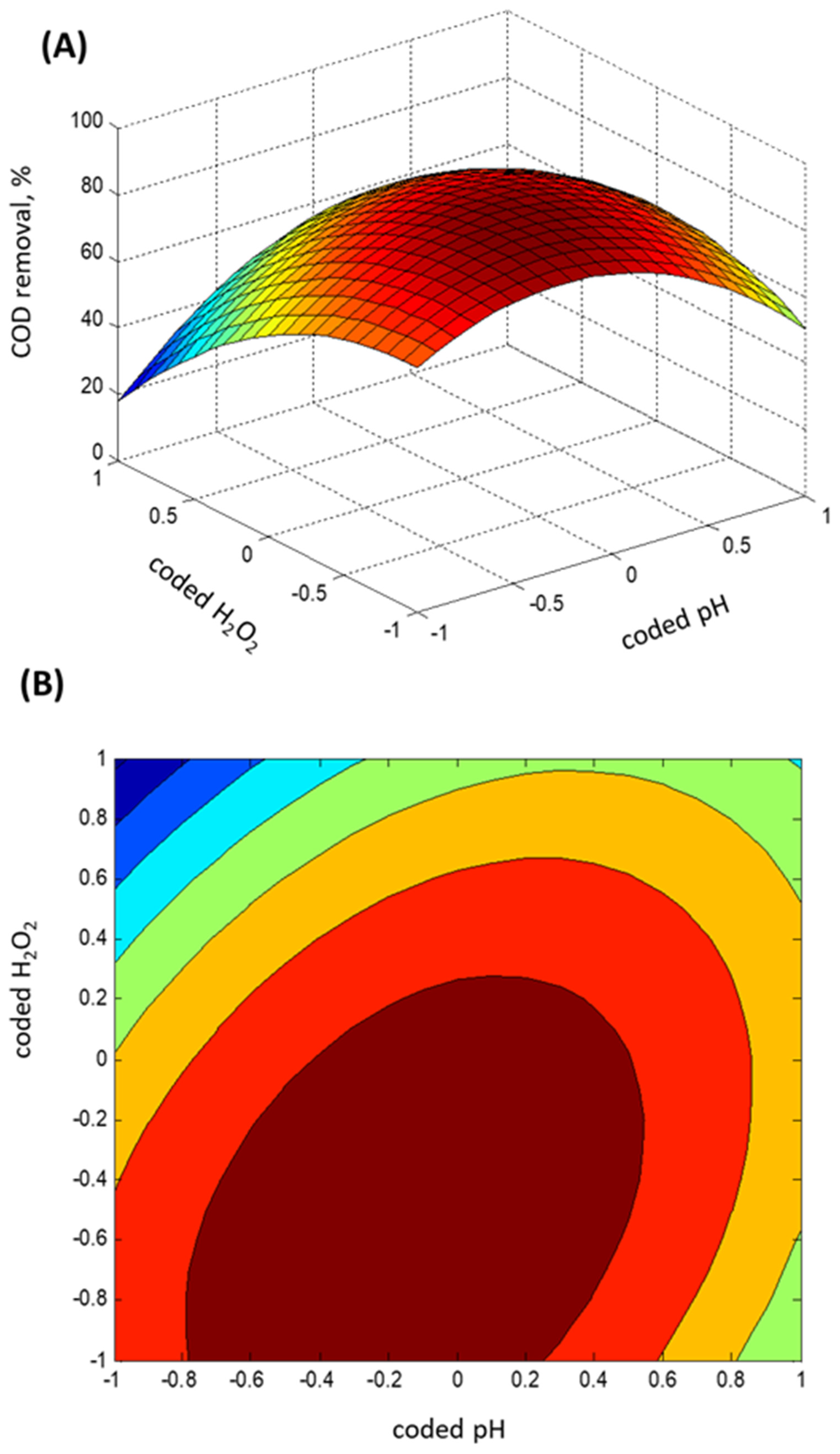
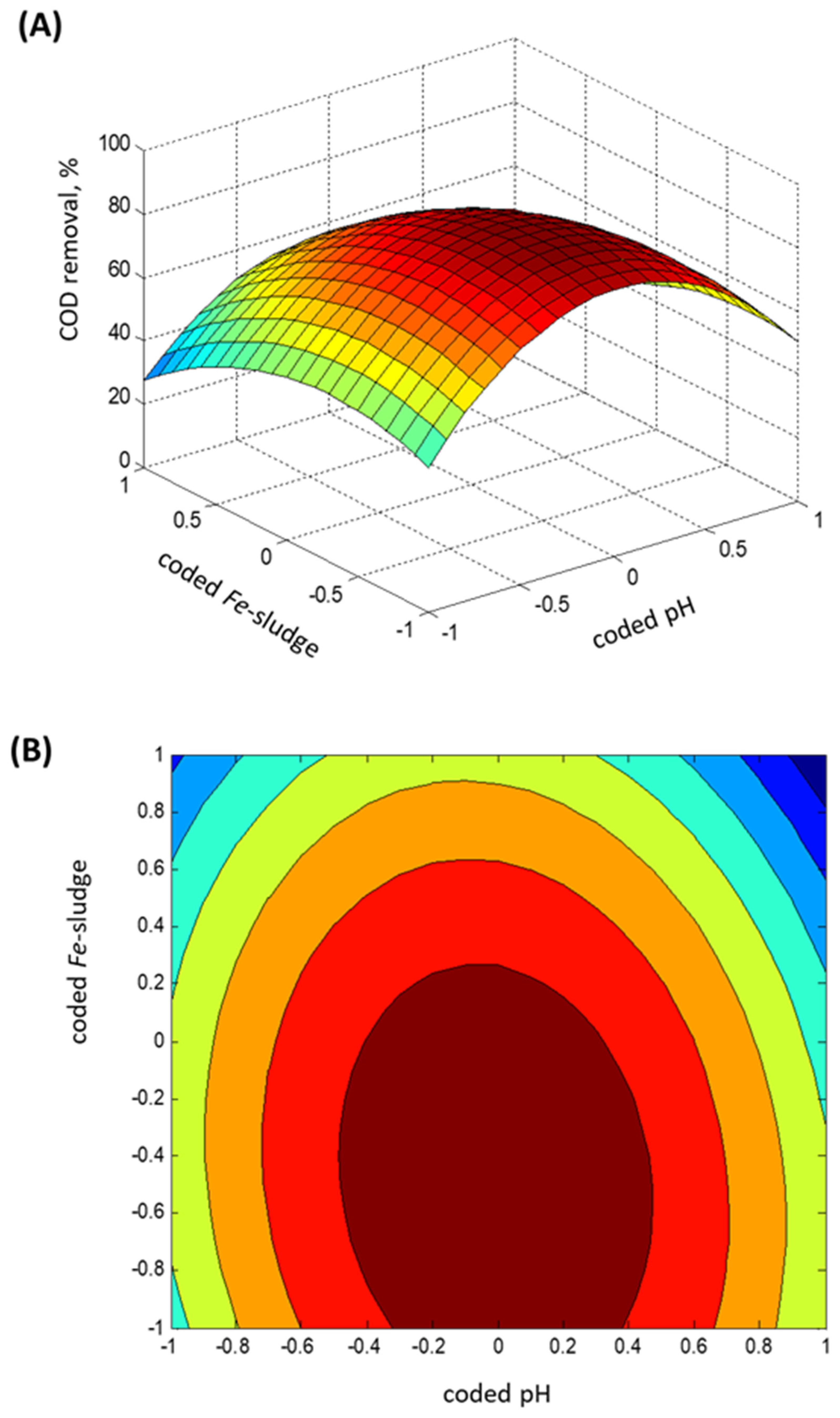
2.3. Experimental Box–Behnken Design
- Regression model fitting
- Statistical ANOVA testing
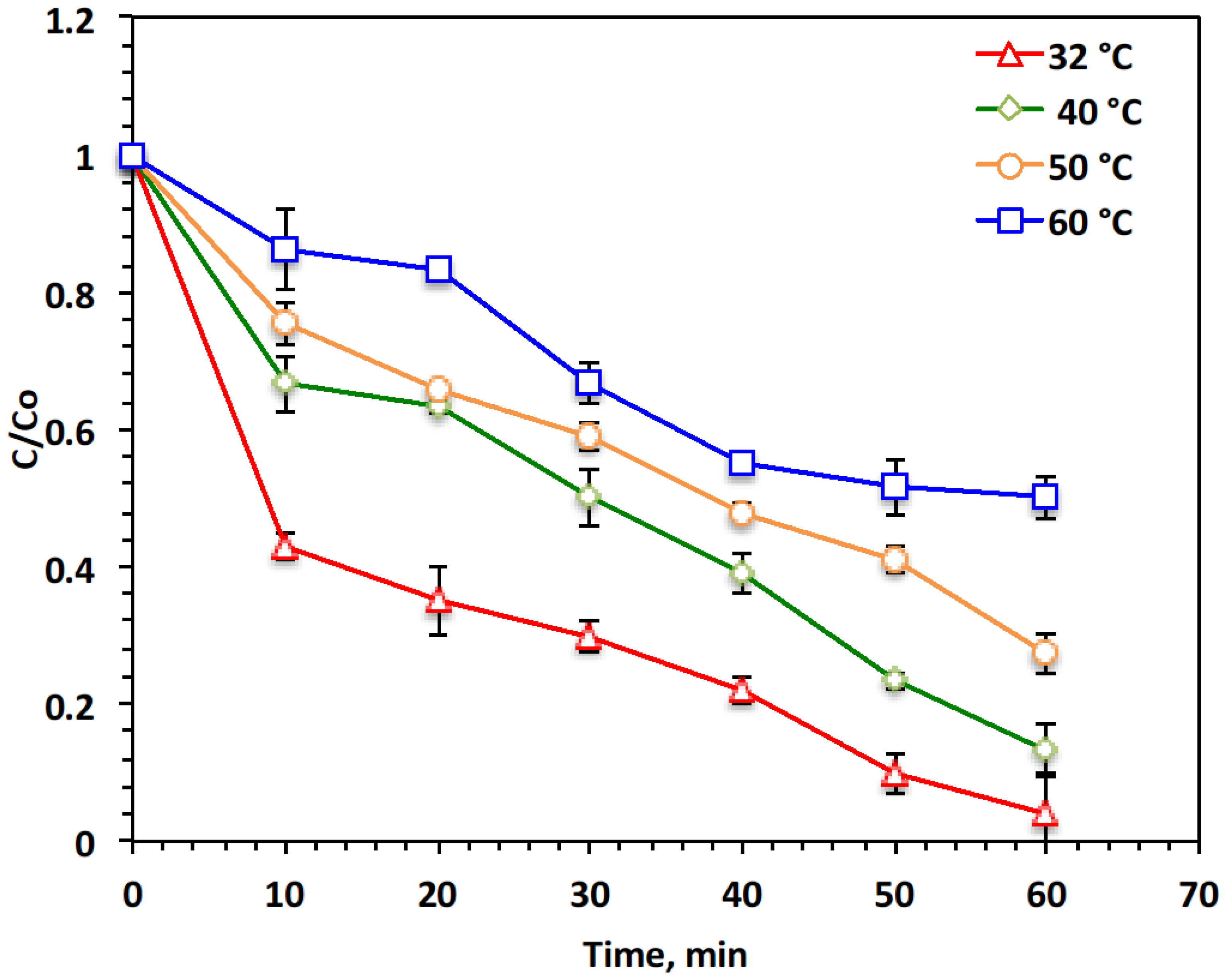
2.4. Temperature Influence, Kinetics, and Thermodynamic Investigations
2.5. Catalyst Stability Investigation
2.6. Error in COD Values of Launderette Wastewater Due to H2O2
3. Experimental Procedure
3.1. Laundry Wastewater and Fe Sludge
3.2. Oxidation System
3.3. Factorial Design and Statistical Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sylwan, I.; Thorin, E.; Zambrano, J. Biochar adsorption for separation of heavy metals during municipal wastewater treatment. Int. J. Environ. Sci. Technol. 2016, 13, 359–386. [Google Scholar]
- Van de Walle, A.; Kim, M.; Alam, K.; Wang, X.; Wu, D.; Dash, S.R.; Rabaey, K.; Kim, J. Greywater reuse as a key enabler for improving urban wastewater management. Environ. Sci. Ecotechnology 2023, 16, 100277. [Google Scholar] [CrossRef]
- Sher, F.; Malik, A.; Liu, H. Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 2013, 1, 684–689. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; El Sherbiny, S.A.; Tony, M.A. Zero-waste approach: Assessment of aluminum-based waste as a photocatalyst for industrial wastewater treatment ecology. Int. J. Environ. Res. 2022, 16, 36. [Google Scholar] [CrossRef]
- Vatani, P.; Aliannezhadi, M.; Tehrani, F.S. Improvement of optical and structural properties of ZIF-8 by producing multifunctional Zn/Co bimetallic ZIFs for wastewater treatment from copper ions and dye. Sci. Rep. 2024, 14, 15434. [Google Scholar] [CrossRef] [PubMed]
- Karamat, S.; Akhter, T.; Hassan, S.U.; Faheem, M.; Mahmood, A.; Al-Masry, W.; Razzaque, S.; Ashraf, S.; Kim, T.; Han, S.-K.; et al. Recycling of polyethylene terephthalate to bismuth-embedded bimetallic MOFs as photocatalysts toward removal of cationic dye in water. J. Ind. Eng. Chem. 2024, 137, 503–513. [Google Scholar] [CrossRef]
- Qureshi, K.; Ahmad, Z.; Bhatti, I.A.; Iqbal, M.; Khan, A. Cytotoxicity reduction of wastewater treated by advanced oxidation process. Chem. Int. 2015, 1, 153–159. [Google Scholar]
- Abeie, B.; Mahmoodi, N.M. Heterogeneous MIL-88A on MIL-88B hybrid: A promising eco-friendly hybrid from green synthesis to dual application (Adsorption and photocatalysis) in tetracycline and dyes removal. J. Colloid Interface Sci. 2024, 654, 495–522. [Google Scholar]
- Tony, M.A.; Purcell, P.J.; Zhao, Y.Q.; Tayeb, A.M.; El-Sherbiny, M.F. Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process, Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process. Environ. Eng. Manag. J. 2015, 14, 11–16. [Google Scholar] [CrossRef]
- Tony, M.A. An industrial ecology approach: Green cellulose based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int. J. Environ. Anal. Chem. 2019, 101, 167–183. [Google Scholar] [CrossRef]
- Daij, K.B.; Bellebia, S.; Bengharez, Z. Comparative experimental study on the COD removal in aqueous solution of pesticides by the electrocoagulation process using monopolar iron electrodes. Chem. Int. 2017, 3, 420–427. [Google Scholar]
- Minas, F.; Chandravanshi, B.S.; Leta, S. Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem. Int. 2017, 3, 392–405. [Google Scholar]
- Mishra, S.; Maiti, A. Biological Methodologies for Treatment of Textile Wastewater. In Environmental Processes and Management; Singh, R., Shukla, P., Singh, P., Eds.; Water Science and Technology Library: New York, NY, USA, 2020; Volume 9. [Google Scholar]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 1120. [Google Scholar]
- Tony, M.A. Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 2485–2498. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Tureli, G.; Olmez-Hanci, T. Optimization of the photo-Fenton- like process for real synthetic azo dye production wastewater treatment using response surface methodology. Photochem. Photobiol. 2009, 8, 628–638. [Google Scholar] [CrossRef]
- Tony, M.A. Optimization of Bismarck Brown Dye Removal from Aqueous Solution by Central Composite Design. Appl. Water Sci. 2020, 10, 108. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Q.; Zhang, H.; Wang, N.; Chang, S.; Wang, H.; Pang, H.; Chen, H. Treatment of real benzene dye intermediates wastewater by the Fenton method: Characteristics and multi-response optimization. RSC Adv. 2018, 8, 80–90. [Google Scholar] [CrossRef]
- Deng, D.; Lin, O.; Rubenstein, A.; Weidhaas, J.L.; Lin, L.-S. Elucidating biochemical transformations of Fe and S in an innovative Fe(II)-dosed anaerobic wastewater treatment process using spectroscopic and phylogenetic analyses. Chem. Eng. J. 2019, 358, 1208–1217. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.S. Iron recovery form acid mine drain sludge as a Fenton source for municipal wastewater treatment. Int. J. Environ. Anal. Chem. 2020, 102, 1245–1260. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Jaiyeola, A.T.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Deng, D.; Lin, L.S. Continuous sulfidogenic wastewater treatment with iron sulfide sludge oxidation and recycle. Water Res. 2017, 114, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, G.; Zheng, T.; Wang, P. Degradation of p-nitrophenol using CuO/Al2O3 as a Fenton-like catalyst under microwave irradiation. RSC Adv. 2015, 5, 27043–27051. [Google Scholar] [CrossRef]
- Laureni, M.; Weissbrodt, D.G.; Szivak, I.; Robin, O.; Nielsen, J.; Morgenroth, E.; Joss, A. Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater. Water Res. 2015, 80, 325–336. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water & Wastewater; Port City Press: Baltimore, MD, USA, 2005; American Water Works Association, Water Environment Federation. [Google Scholar]
- Wei, X.; Viadero, R.C.; Buzby, K. Recovery of Iron and Aluminium from Acid Mine Drainage by Selective Precipitation. Environ. Eng. Sci. 2005, 22, 745–755. [Google Scholar] [CrossRef]
- Silva, E.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Liu, O.; Qian, K.; Qi, J.; Li, C.; Yao, C.; Song, W.; Wang, Y. Improving the efficiency of Fenton reactions and their application in the degradation of benzimidazole in wastewater. RSC Adv. 2018, 8, 9741. [Google Scholar] [CrossRef]
- Li, F.; Zhu, G.; Jiang, J.; Yang, L.; Deng, F.; Arramel; Li, X. A review of updated S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2024, 177, 142–180. [Google Scholar] [CrossRef]
- Gharsallah, N.; Khannous, L.; Souissi, N.; Nasri, M. Biological treatment of saline wastewaters from marineproducts processing factories by a fixed-bed reactor. J. Chem. Technol. Biotechnol. 2002, 77, 865–870. [Google Scholar] [CrossRef]
- Kubo, M.; Hiroe, J.; Murakami, M.; Fukami, H.; Tachiki, T. Treament of hypersaline-containing wastewater with salt- tolerant microorganisms. J. Biosci. Bioeng. 2001, 91, 222–224. [Google Scholar] [CrossRef]
- Aspé, E.; Marti, M.C.; Roeckel, M. Anaerobic treatment of fishery wastewater using a marine sediment inoculum. Water Res. 1997, 31, 2147–2160. [Google Scholar] [CrossRef]
- Uygur, A.; Kargi, F. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzym. Microb. Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]
- Lefebvre, O.; Habouzit, F.; Bru, V.; Delgenes, J.P.; Godon, J.J.; Moletta, R. Treatment of hypersaline industrial wastewater by a microbial consortium in a sequencing batch reactor. Environ Technol. 2004, 25, 543–553. [Google Scholar] [CrossRef]
- Omil, F.; Méndez, R.J.; Lema, J.M. Characterization of biomass from a pilot plant digester treating saline wastewater. J. Chem. Technol. Biotechnol. 1995, 63, 384–392. [Google Scholar] [CrossRef]
- Do, Q.C.; Kim, D.-G.; Ko, S.-O. Catalytic activity enhancement of a Fe3O4@ SiO2 yolk-shell structure for oxidative degradation of acetaminophen by decoration with copper. J. Clean. Prod. 2018, 172, 1243–1253. [Google Scholar] [CrossRef]
- Arimi, M.M. Modified natural zeolite as heterogeneous Fenton catalyst in treatment of recalcitrants in industrial effluent. Prog. Nat. Sci. Mater. Int. 2017, 27, 275–282. [Google Scholar] [CrossRef]
- Schrank, S.G.; Santos, J.R.; Souza, D.S.; Souza, E.S. Decolourisation effects of Vat Green 01 textile dye and textile wastewater using H2O2/UV process. J. Photochem. Photobiol. A Chem. 2007, 186, 125–129. [Google Scholar] [CrossRef]
- Tony, M.A.; Mansour, S. A commercial reactive dye Procion Blue MX-7RX removal from real textile wastewater using different particle sizes of synthesized Fe2O3 nanoparticles as a source of Fenton’s reagent. Nanoscale Adv. 2019, 1, 1362–1371. [Google Scholar] [CrossRef]
- Cetinkaya, S.C.; Hakan, M.; Akarsu, S.A.; Ziba, C.Z.; Dolaz, M. Comparison of classic Fenton with ultrasound Fenton processes on industrial textile wastewater. Sustain. Environ. Res. 2018, 28, 165–170. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Ban, Y.; Ren, B. A comparative study of UV–Fenton, UV–H2O2 and Fenton reaction treatment of landfill leachate. Environ. Technol. 2011, 32, 945. [Google Scholar] [CrossRef]
- Sun, H.; Xu, R.; Jia, X.; Liu, Z.; Chen, H.; Lu, T. Recent advances in the photocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Biomass Convers. Biorefinery 2023, 14, 26707–26724. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh, K.A.; Padungthon, S.; Pranudta, A.; Amonpattaratkit, P.; Tran, L.B.; Phan, P.T.; Nguyen, N.H. Synthesis of natural flowerlike iron-alum oxide with special interaction of Fe-Si-Al oxides as an effective catalyst for heterogeneous Fenton process. J. Environ. Chem. Eng. 2021, 9, 105732. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Clark, L.A.; Zeltner, W.A.; Anderson, M.A. Effects of reaction temperature and water vapor content on the heterogeneous photocatalytic oxidation of ethylene. J. Photochem. Photobiol. A Chem. 1996, 97, 181–186. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Li, Y.; Cheng, F.; Liu, Y.; Gao, M.; Liu, G.; Hu, L.; Liang, Y. Effectiveness of discarded cigarette butts derived carbonaceous adsorbent for heavy metals removal from water. Microchem. J. 2021, 168, 106474. [Google Scholar] [CrossRef]
- Li, L.; Jia, C.; Zhu, X.; Zhang, S. Utilization of cigarette butt waste as functional carbon precursor for supercapacitors and adsorbents. J. Clean. Prod. 2020, 256, 120326. [Google Scholar] [CrossRef]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Ghasemian, M.B. A zinc oxide-coated nanoporous carbon adsorbent for lead removal from water: Optimization, equilibrium modeling, and kinetics studies. Int. J. Environ. Sci. Technol. 2013, 10, 325–340. [Google Scholar] [CrossRef][Green Version]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Argun, M.E.; Karatas, M. Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: Kinetics and thermodynamics. Environ. Prog. Sustain. Energy 2011, 30, 540–548. [Google Scholar] [CrossRef]
- Ahmadi, M.; Behin, J.; Mahnam, A.R. Kinetics and thermodynamics of peroxydisulfate oxidation of Reactive Yellow 84. J. Saudi Chem. Soc. 2016, 20, 644–650. [Google Scholar] [CrossRef]
- Shende, T.P.; Bhanvase, B.A.; Rathod, A.P.; Pinjari, D.V.; Sonawane, S.H. Sonochemical synthesis of Graphene-Ce-TiO2 and Graphene-Fe-TiO2 ternary hybrid photocatalyst nanocomposite and its application in degradation of crystal violet dye. Ultrason. Sonochemistry 2018, 41, 582–589. [Google Scholar] [CrossRef]
- SAS/STAT. User’s Guide; SAS Institute, Inc.: Cary, NC, USA, 1990. [Google Scholar]
- Lefebvre, O.; Vasudevan, N.; Torrijos, M.; Thanasekaran, K.; Moletta, R. Halophilic biological treatment of tannery soak liquor in a sequencing batch reactor. Water Res. 2005, 39, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health 2010, A45, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.A.; Tebbutt, T.Y. Significance of COD, BOD and TOC correlations in kinetic–models of biological oxication. Water Res. 1980, 14, 319–324. [Google Scholar] [CrossRef]
- Talinli, I.; Anderson, G.K. Interference of hydrogen peroxide on the standard COD test. Water Res. 1992, 26, 107–110. [Google Scholar] [CrossRef]
- Zak, S. Problem of correction of the chemical oxygen demand values determined in wastewaters treated by methods with hydrogen peroxide. Proc. ECOpole 2008, 2, 409–414. [Google Scholar]
- Lee, E.; Lee, H.; Kim, Y.K.; Sohn, K.; Lee, K. Hydrogen peroxide interference in chemical oxygen demand during ozone based oxidation of anaerobically digested livestock wastewater. Int. J. Environ. Sci. Technol. 2011, 8, 381–388. [Google Scholar] [CrossRef]




| Source of Variant | Degrees of Freedom | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 9 | 9335.072 | 1037.23 | 24.18108 | 0.001331 |
| Linear | 3 | 1745.85387 | 1745.85387 | 40.701311 | 0.700913 |
| Quadratic | 3 | 2631.5661 | 2631.5661 | 61.350039 | 0.407857 |
| Cross Product | 3 | 5767.4384 | 5767.4384 | 134.457 | 0.187558 |
| Error | 5 | 214.4714 | 42.89429 | ||
| Total | 14 | 9549.544 |
| T (°C) | 0-Order Reaction * | 1st-Order Reaction ** | 2nd-Order Reaction *** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ko (min−1) | t1/2 (min) | R2 | k1 (min−1) | t1/2 (min) | R2 | k2, (L·mg−1min−1) | t1/2 (min) | R2 | |
| 32 | 11.58 | 57.91 | 0.77 | 0.039 | 40.76 | 0.94 | 0.0002 | 3.73 | 0.66 |
| 40 | 11.68 | 57.41 | 0.97 | 0.014 | 26.65 | 0.97 | 0.00007 | 10.65 | 0.78 |
| 50 | 17.78 | 37.72 | 0.92 | 0.026 | 49.57 | 0.96 | 0.00001 | 74.57 | 0.95 |
| 60 | 14.55 | 46.08 | 0.93 | 0.017 | 17.76 | 0.98 | 0.00003 | 24.85 | 0.87 |
| Thermodynamic Parameters | Temperature (°C) | |||
|---|---|---|---|---|
| 32 | 40 | 50 | 60 | |
| ∆G′ (kJmol−1) | 82.92 | 86.96 | 89.82 | 94.61 |
| ∆H′ (kJ mol−1) | 34.46 | 34.39 | 34.31 | 34.22 |
| ∆S′ (J mol−1) | −158.87 | −167.95 | −171.87 | −181.32 |
| Ea (kJ mol−1) | 36.99 | |||
| Experimental Factor | Symbol | Coded Levels | |||
|---|---|---|---|---|---|
| Low | Middle | High | |||
| Natural | Coded | −1 | 0 | 1 | |
| H2O2 concentration (mg/L) | 350 | 400 | 450 | ||
| Fe sludge concentration (mg/L) | 80 | 100 | 120 | ||
| pH | 2 | 3 | 4 | ||
| Run No. | Codified Variables | Natural Variables | ||||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | ||||
| 1 | −1 | −1 | 0 | 350 | 80 | 3 |
| 2 | −1 | 1 | 0 | 350 | 120 | 3 |
| 3 | 1 | −1 | 0 | 450 | 80 | 3 |
| 4 | 1 | 1 | 0 | 450 | 120 | 3 |
| 5 | 0 | −1 | −1 | 400 | 80 | 2 |
| 6 | 0 | −1 | 1 | 400 | 80 | 4 |
| 7 | 0 | 1 | −1 | 400 | 120 | 2 |
| 8 | 0 | 1 | 1 | 400 | 120 | 4 |
| 9 | −1 | 0 | −1 | 350 | 100 | 2 |
| 10 | 1 | 0 | −1 | 450 | 100 | 2 |
| 11 | −1 | 0 | 1 | 350 | 100 | 4 |
| 12 | 1 | 0 | 1 | 450 | 100 | 4 |
| 13 | 0 | 0 | 0 | 400 | 100 | 3 |
| 14 | 0 | 0 | 0 | 400 | 100 | 3 |
| 15 | 0 | 0 | 0 | 400 | 100 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trabelsi, A.B.G.; Alkallas, F.H.; Mansour, S.A.; Al Naim, A.F.; Alshoaibi, A.; Rekik, N.; Nour, M.M.; Tony, M.A. Iron Sludge-Derived Photo-Fenton Reaction for Laundry Wastewater Effluent Oxidation and Process Optimization into Industrial Ecology Symbiosis. Catalysts 2025, 15, 669. https://doi.org/10.3390/catal15070669
Trabelsi ABG, Alkallas FH, Mansour SA, Al Naim AF, Alshoaibi A, Rekik N, Nour MM, Tony MA. Iron Sludge-Derived Photo-Fenton Reaction for Laundry Wastewater Effluent Oxidation and Process Optimization into Industrial Ecology Symbiosis. Catalysts. 2025; 15(7):669. https://doi.org/10.3390/catal15070669
Chicago/Turabian StyleTrabelsi, Amira Ben Gouider, Fatemah H. Alkallas, Shehab A. Mansour, Abdullah F. Al Naim, Adil Alshoaibi, Najeh Rekik, Manasik M. Nour, and Maha A. Tony. 2025. "Iron Sludge-Derived Photo-Fenton Reaction for Laundry Wastewater Effluent Oxidation and Process Optimization into Industrial Ecology Symbiosis" Catalysts 15, no. 7: 669. https://doi.org/10.3390/catal15070669
APA StyleTrabelsi, A. B. G., Alkallas, F. H., Mansour, S. A., Al Naim, A. F., Alshoaibi, A., Rekik, N., Nour, M. M., & Tony, M. A. (2025). Iron Sludge-Derived Photo-Fenton Reaction for Laundry Wastewater Effluent Oxidation and Process Optimization into Industrial Ecology Symbiosis. Catalysts, 15(7), 669. https://doi.org/10.3390/catal15070669











