Direct Conversion of 1,3-Butanediol to 1,3-Butadiene over ZSM-22 Catalysts: Influence of the Si/Al Ratio
Abstract
1. Introduction
2. Results and Discussion
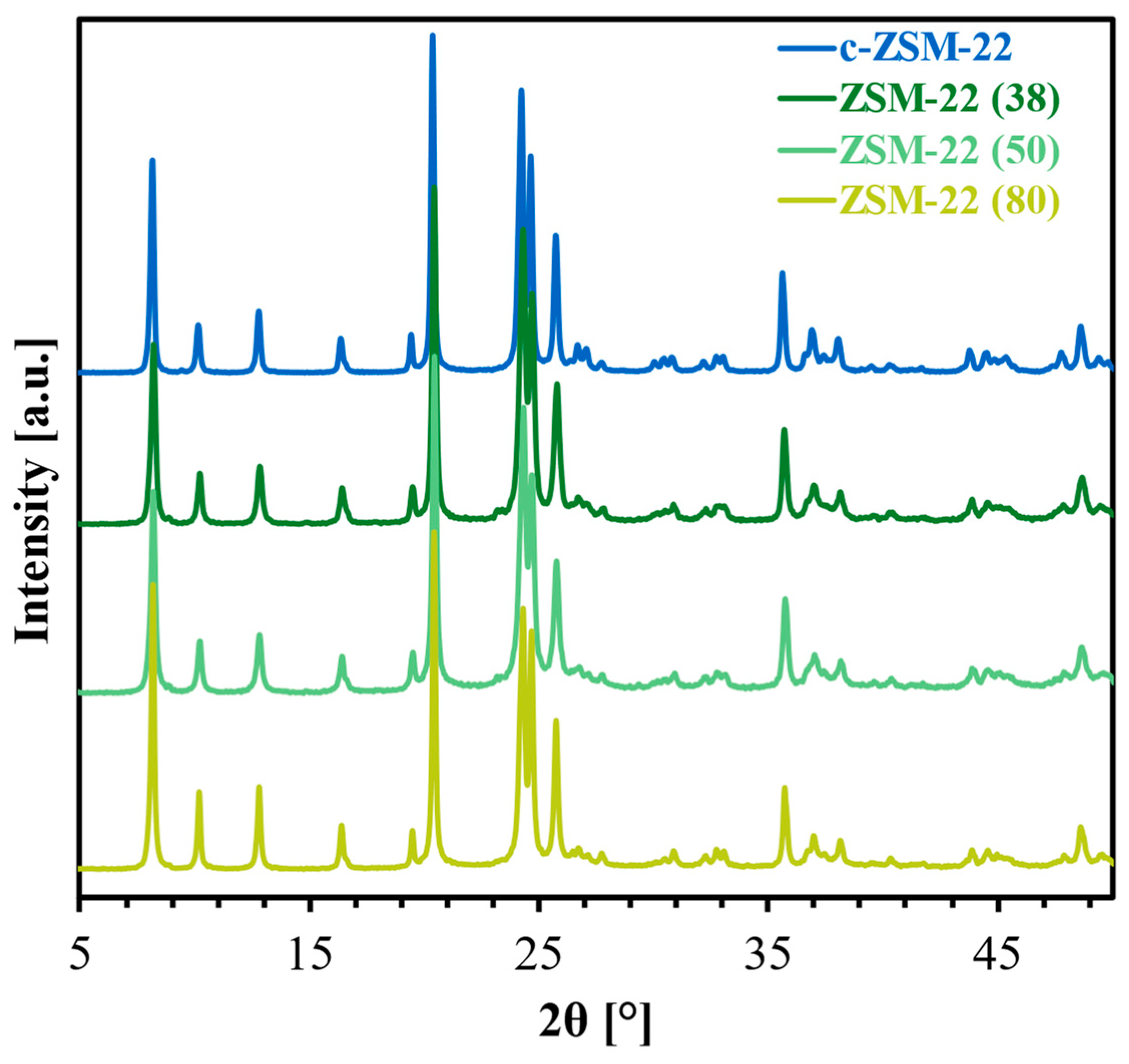
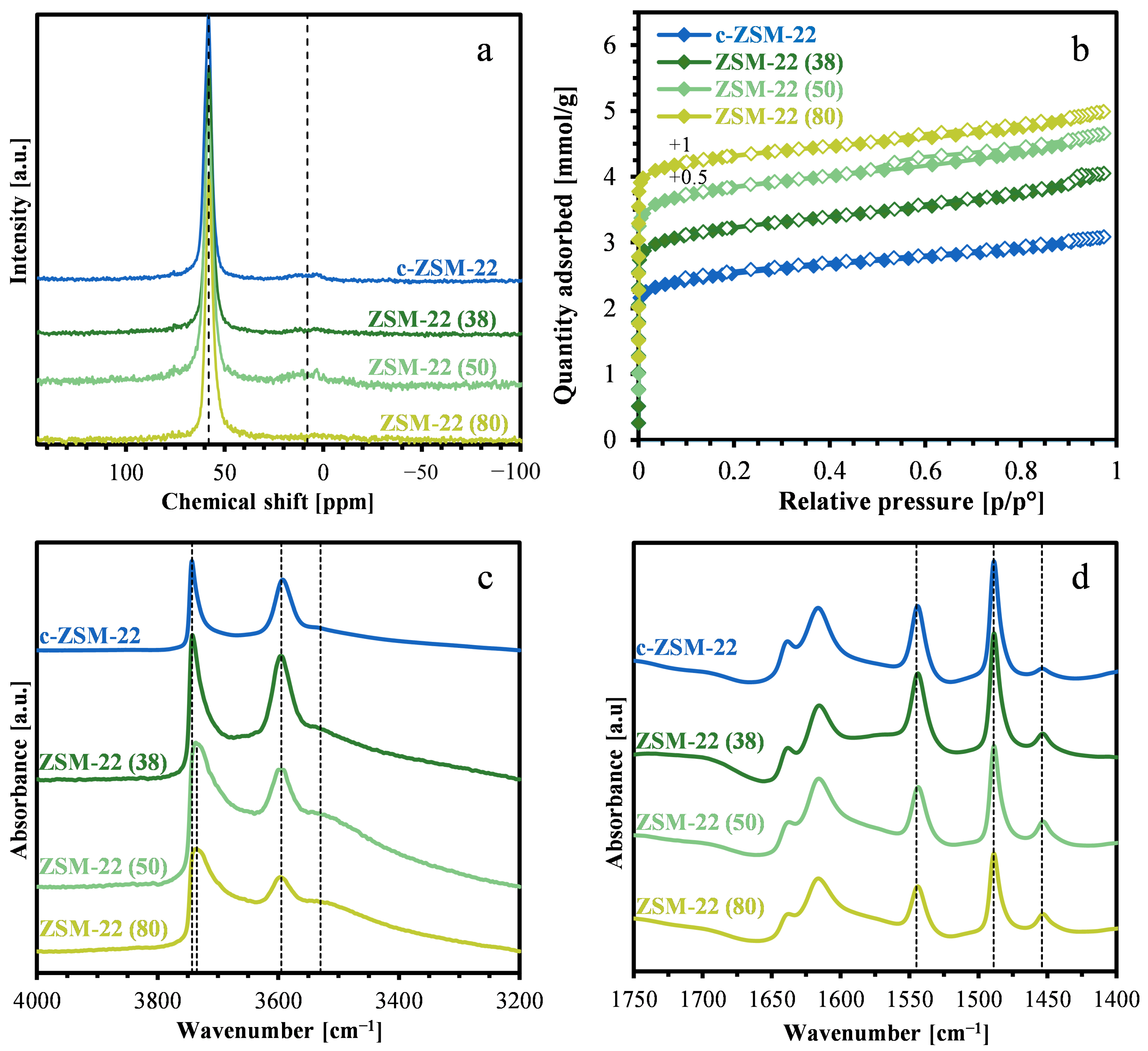
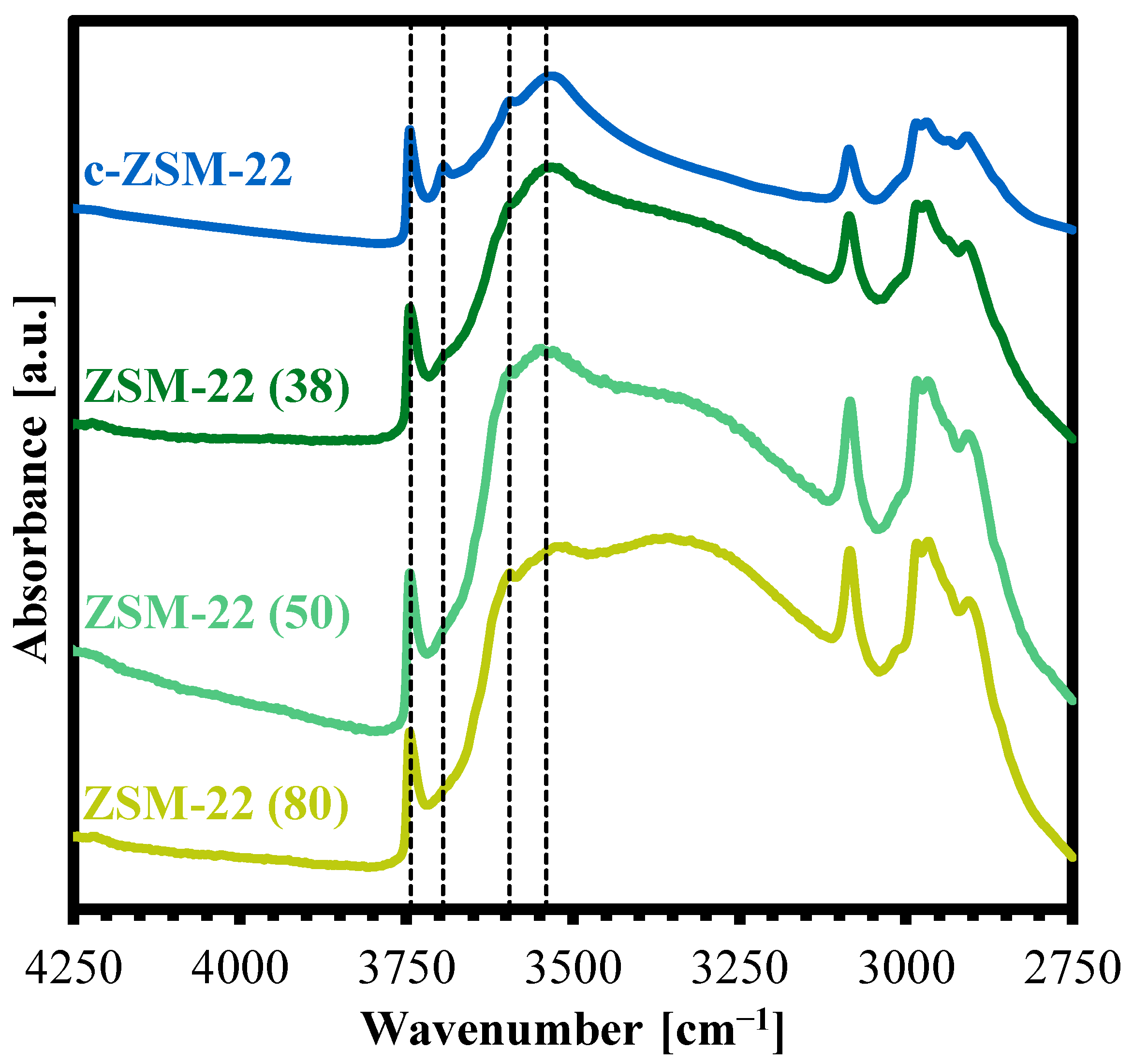
2.1. Characterization of the Zeolite Materials
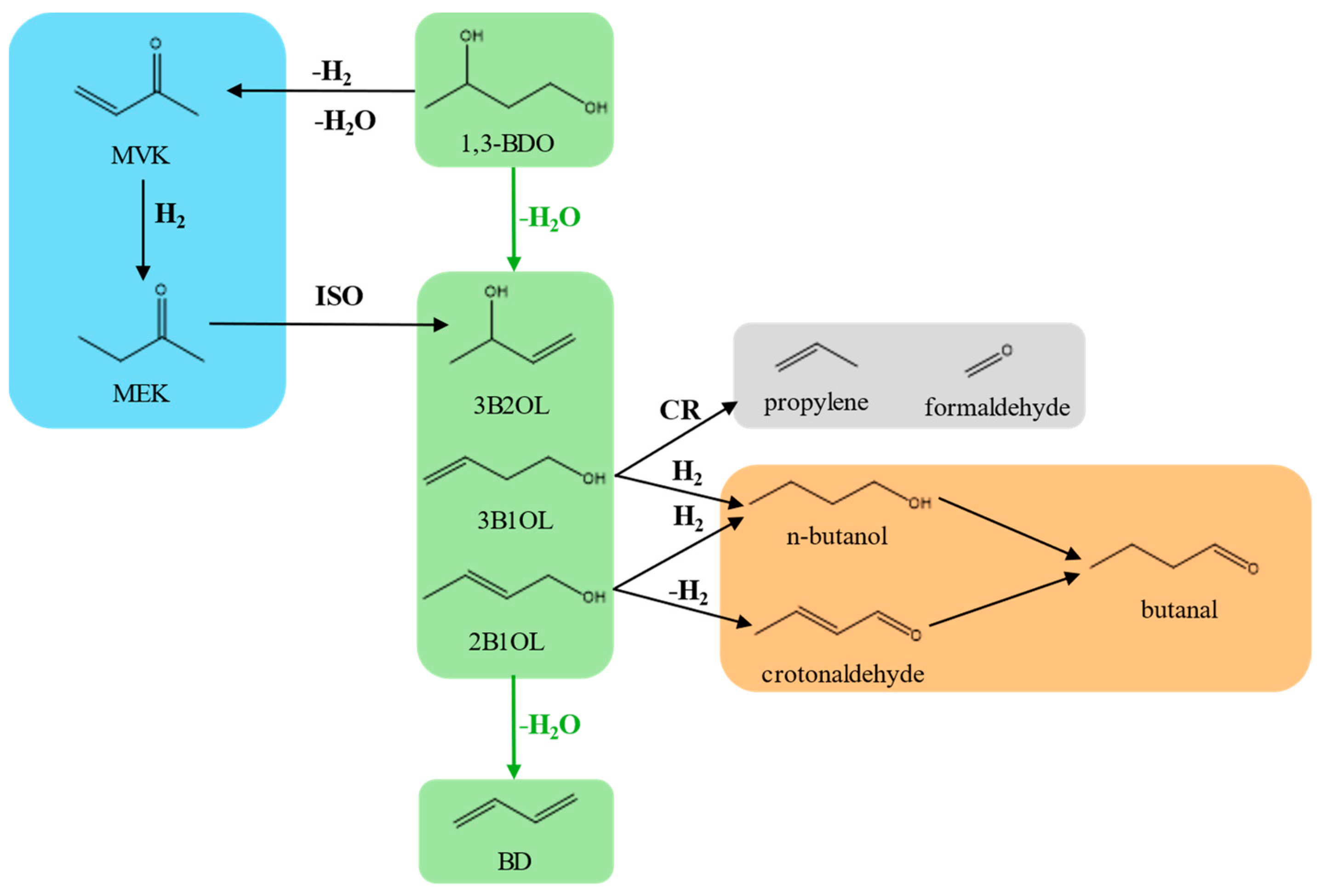
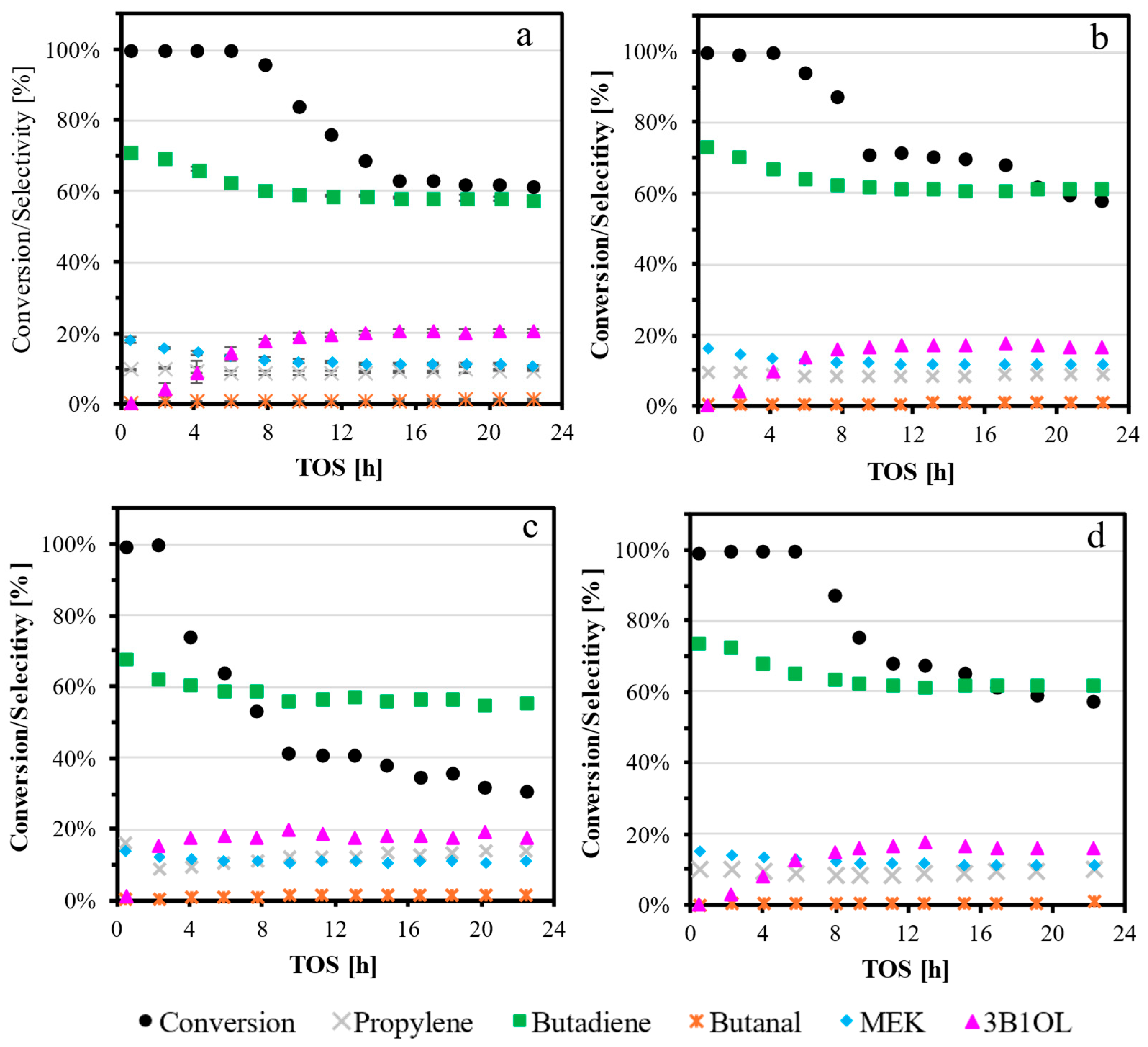
2.2. Catalytic Results: Dehydration of 1,3-Butanediol into Butadiene
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Dehydration Experiments
Definitions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, O.; Khairun, H.S.; Joshi, H.; Sarkar, B.; Gupta, N.K. Advancing light olefin production: Exploring pathways, catalyst development, and future prospects. Fuel 2025, 379, 132992. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [PubMed]
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. (In English) [Google Scholar] [CrossRef]
- Camacho, C.C.; Perales, A.V.; Alonso-Fariñas, B.; Vidal-Barrero, F.; Ollero, P. Assessing the economic and environmental sustainability of bio-olefins: The case of 1,3-butadiene production from bioethanol. J. Clean. Prod. 2022, 374, 133963. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Yang, C.; Su, Y.; Yamada, Y.; Sato, S. Production of 1,3-butadiene from biomass-derived C4 alcohols. Fuel Process. Technol. 2020, 197, 106193. [Google Scholar] [CrossRef]
- Pomalaza, G.; Ponton, P.A.; Capron, M.; Dumeignil, F.Y. Ethanol-to-butadiene: The reaction and its catalysts. Catal. Sci. Technol. 2020, 10, 4860–4911. [Google Scholar] [CrossRef]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic Conversion of Ethanol into Butadiene and Other Bulk Chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef]
- Janssens, W.; Makshina, E.V.; Vanelderen, P.; De Clippel, F.; Houthoofd, K.; Kerkhofs, S.; Martens, J.A.; Jacobs, P.A.; Sels, B.F. Ternary Ag/MgO-SiO2 Catalysts for the Conversion of Ethanol into Butadiene. ChemSusChem 2015, 8, 994–1008. [Google Scholar] [CrossRef]
- Alphy, M.P.; Hazeena, S.H.; Binoop, M.; Madhavan, A.; Arun, K.; Vivek, N.; Sindhu, R.; Awasthi, M.K.; Binod, P. Synthesis of C2-C4 diols from bioresources: Pathways and metabolic intervention strategies. Bioresour. Technol. 2022, 346, 126410. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Wei, Z.; Park, S. Recent advances in metabolic engineering of microorganisms for the production of monomeric C3 and C4 chemical compounds. Bioresour. Technol. 2023, 377, 128973. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-G.; Seo, J.-H. Production of 2,3-butanediol from glucose and cassava hydrolysates by metabolically engineered industrial polyploid Saccharomyces cerevisiae. Biotechnol. Biofuels 2019, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, K.; Wang, Y.; Chen, C.; Xu, Y.; Zhang, L.; Han, B.; Gao, C.; Tao, F.; Ma, C.; et al. Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab. Eng. 2015, 28, 19–27. [Google Scholar] [CrossRef]
- Cho, S.; Kim, T.; Woo, H.M.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels 2015, 8, 146. [Google Scholar] [CrossRef]
- Matsuyama, A.; Yamamoto, H.; Kawada, N.; Kobayashi, Y. Industrial production of (R)-1,3-butanediol by new biocatalysts. J. Mol. Catal. B Enzym. 2001, 11, 513–521. [Google Scholar] [CrossRef]
- Kataoka, N.; Vangnai, A.S.; Tajima, T.; Nakashimada, Y.; Kato, J. Improvement of (R)-1,3-butanediol production by engineered Escherichia coli. J. Biosci. Bioeng. 2013, 115, 475–480. [Google Scholar] [CrossRef]
- Islam, T.; Nguyen-Vo, T.P.; Gaur, V.K.; Lee, J.; Park, S. Metabolic engineering of Escherichia coli for biological production of 1, 3-Butanediol. Bioresour. Technol. 2023, 376, 128911. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Nakaji, Y.; Yabushita, M.; Nakagawa, Y.; Tomishige, K. Selective synthesis of 1,3-butadiene by vapor-phase dehydration of 1,4-butanediol over cerium oxide catalyst. Appl. Catal. A Gen. 2023, 663, 119321. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sato, S.; Takahashi, R.; Inui, K. Synthesis of homoallyl alcohol from 1,4-butanediol over ZrO2 catalyst. Catal. Commun. 2005, 6, 480–484. [Google Scholar] [CrossRef]
- Duan, H.; Sun, D.; Yamada, Y.; Sato, S. Dehydration of 2,3-butanediol into 3-buten-2-ol catalyzed by ZrO2. Catal. Commun. 2014, 48, 1–4. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Honda, N. Dehydration of diols catalyzed by CeO2. J. Mol. Catal. A Chem. 2004, 221, 177–183. [Google Scholar] [CrossRef]
- Bekele, B.A.; Poissonnier, J.; Thybaut, J.W. The potential of ZrO2 catalysts for the dehydration of 2,3-butanediol into 3-buten-2-ol: Impact of synthesis method and operating conditions. J. Catal. 2022, 411, 200–211. [Google Scholar] [CrossRef]
- Gascoyne, J.L.; Bommareddy, R.R.; Heeb, S.; Malys, N. Engineering Cupriavidus necator H16 for the autotrophic production of (R)-1,3-butanediol. Metab. Eng. 2021, 67, 262–276. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Sad, M.E.; Cruchade, H.; Pinard, L.; Padró, C.L. Study of catalyst deactivation during 1,3-butanediol dehydration to produce butadiene. Microporous Mesoporous Mater. 2021, 320, 111066. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, S.B. Dehydration of 1,3-butanediol to butadiene over medium-pore zeolites: Another example of reaction intermediate shape selectivity. Appl. Catal. B Environ. 2021, 280, 119446. [Google Scholar] [CrossRef]
- Fang, L.; Jing, F.; Lu, J.; Hu, B.; Pera-Titus, M. Nano-flowered Ce@MOR hybrids with modulated acid properties for the vapor-phase dehydration of 1,3-butanediol into butadiene. Green Chem. 2017, 19, 4610–4621. [Google Scholar] [CrossRef]
- Jing, F.; Katryniok, B.; Paul, S.; Fang, L.; Liebens, A.; Shen, M.; Hu, B.; Dumeignil, F.; Pera-Titus, M. Al-doped SBA-15 Catalysts for Low-temperature Dehydration of 1,3-Butanediol into Butadiene. ChemCatChem 2017, 9, 258–262. [Google Scholar] [CrossRef]
- Jing, F.; Katryniok, B.; Araque, M.; Wojcieszak, R.; Capron, M.; Paul, S.; Daturi, M.; Clacens, J.-M.; De Campo, F.; Liebens, A.; et al. Direct dehydration of 1,3-butanediol into butadiene over aluminosilicate catalysts. Catal. Sci. Technol. 2016, 6, 5830–5840. [Google Scholar] [CrossRef]
- Ichikawa, N.; Sato, S.; Takahashi, R.; Sodesawa, T. Catalytic reaction of 1,3-butanediol over solid acids. J. Mol. Catal. A Chem. 2006, 256, 106–112. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Sad, M.E.; Padró, C.L. Acid site requirement and reaction pathway for selective bio-butadiene synthesis by 1,3-butanediol dehydration. Appl. Catal. A Gen. 2023, 664, 119349. [Google Scholar] [CrossRef]
- Kurniawan, E.; Yu, L.; Kobayashi, R.; Hara, T.; Yamada, Y.; Sato, S. Vapor-phase dehydration of 1, 3-butanediol to 1, 3-butadiene over WO3/SiO2 catalyst. Appl. Catal. A Gen. 2023, 666, 119408. [Google Scholar] [CrossRef]
- Li, Y.; Kurniawan, E.; Sato, F.; Hara, T.; Yamada, Y.; Sato, S. Amorphous silica-alumina modified with silver as an efficient catalyst for vapor-phase dehydration of 1,3-butanediol to 1,3-butadiene. Appl. Catal. A Gen. 2024, 669, 119493. [Google Scholar] [CrossRef]
- Matsumura, Y.; Matsuda, A.; Yamada, Y.; Sato, S. Selective Production of 1,3-Butadiene from 1,3-Butanediol over Y2Zr2O7 Catalyst. Bull. Chem. Soc. Jpn. 2021, 94, 1651–1658. [Google Scholar] [CrossRef]
- Zhai, M.; Wu, W.; Xing, E.; Zhang, Y.; Ding, H.; Zhou, J.; Luo, Y.; Liu, J.; Zhu, K.; Zhu, X. Generating TON zeolites with reduced [001] length through combined mechanochemical bead-milling and porogen-directed recrystallization with enhanced catalytic property in hydroisomerization. Chem. Eng. J. 2022, 440, 135874. [Google Scholar] [CrossRef]
- Wang, Q.; Shan, H.; Sim, L.B.; Xie, J.; Ye, S.; Fu, J.; Wang, J.; Zhang, N.; Zheng, J.; Chen, B. ZSM-22 Synthesized Using Structure-Directing Agents of Different Alkyl Chain Lengths for Controlled n-Hexadecane Hydroisomerizations. Ind. Eng. Chem. Res. 2023, 62, 11470–11479. [Google Scholar] [CrossRef]
- Jamil, A.K.; Muraza, O. Facile control of nanosized ZSM-22 crystals using dynamic crystallization technique. Microporous Mesoporous Mater. 2016, 227, 16–22. [Google Scholar] [CrossRef]
- Hong, Z.; Deng, L.; Wang, F.; Zhu, F.; Fang, Y.; Song, L.; Li, L.; Zhu, Z. Intergrowth MFI Zeolite with Inverse Al Zoning and Predominant Sinusoidal Channels for Highly Selective Production of Styrene. Inorg. Chem. 2024, 63, 20888–20899. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q.; Li, S.; Deng, F. (Eds.) Solid-State NMR Characterization of Framework Structure of Zeolites and Zeotype Materials. In Solid-State NMR in Zeolite Catalysis; Singapore Springer: Singapore, 2019; pp. 93–132. [Google Scholar]
- Stepanov, A.G. Chapter 4—Basics of Solid-State NMR for Application in Zeolite Science: Material and Reaction Characterization. In Zeolites and Zeolite-Like Materials; Sels, B.F., Kustov, L.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 137–188. [Google Scholar]
- Qin, Z.; Lakiss, L.; Tosheva, L.; Gilson, J.; Vicente, A.; Fernandez, C.; Valtchev, V. Comparative Study of Nano-ZSM-5 Catalysts Synthesized in OH− and F− Media. Adv. Funct. Mater. 2014, 24, 257–264. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Yan, W.; Yu, J. Regulation of the Si/Al ratios and Al distributions of zeolites and their impact on properties. Chem. Sci. 2023, 14, 1935–1959. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, S.; Wang, X.; Lu, G. Facile synthesis of HZSM-5 with controlled crystal morphology and size as efficient catalysts for chlorinated hydrocarbons oxidation and xylene isomerization. J. Porous Mater. 2014, 21, 1041–1049. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Wang, H.; Ning, Q.; Zhang, H.; Yun, Y.; Ren, J.; Li, Y.-W. Synthesis and characterization of bundle-shaped ZSM-22 zeolite via the oriented fusion of nanorods and its enhanced isomerization performance. J. Catal. 2018, 361, 177–185. [Google Scholar] [CrossRef]
- Ge, S.; Hu, Z.; Xie, H.; Gao, S.; Zhang, Z.; Wu, Z. Shape selection of alkane hydroisomerization over one-dimensional zeolite supported Pt catalyst: Pt/ZSM-48 versus Pt/ZSM-22. Microporous Mesoporous Mater. 2024, 376, 113179. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Zhang, H.; Lv, E.; Yang, Y.; Li, Y.-W. Synthesis, characterization and isomerization performance of micro/mesoporous materials based on H-ZSM-22 zeolite. J. Catal. 2016, 335, 11–23. [Google Scholar] [CrossRef]
- Dalena, F.; Dib, E.; Onida, B.; Ferrarelli, G.; Daturi, M.; Giordano, G.; Migliori, M.; Mintova, S. Evaluation of Zeolite Composites by IR and NMR Spectroscopy. Molecules 2024, 29, 4450. [Google Scholar] [CrossRef] [PubMed]
- Treps, L.; Demaret, C.; Wisser, D.; Harbuzaru, B.; Méthivier, A.; Guillon, E.; Benedis, D.V.; Gomez, A.; de Bruin, T.; Rivallan, M.; et al. Spectroscopic Expression of the External Surface Sites of H-ZSM-5. J. Phys. Chem. C 2021, 125, 2163–2181. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Danilova, I.G.; Arzumanov, S.S.; Pirutko, L.V.; Freude, D.; Stepanov, A.G. Direct Measurement of Zeolite Brønsted Acidity by FTIR Spectroscopy: Solid-State 1H MAS NMR Approach for Reliable Determination of the Integrated Molar Absorption Coefficients. J. Phys. Chem. C 2018, 122, 25386–25395. [Google Scholar] [CrossRef]
- Holm, M.S.; Svelle, S.; Joensen, F.; Beato, P.; Christensen, C.H.; Bordiga, S.; Bjørgen, M. Assessing the acid properties of desilicated ZSM-5 by FTIR using CO and 2,4,6-trimethylpyridine (collidine) as molecular probes. Appl. Catal. A Gen. 2009, 356, 23–30. [Google Scholar] [CrossRef]
- Hoffmann, P.; Lobo, J.A. Identification of diverse silanols on protonated ZSM-5 zeolites by means of FTIR spectroscopy. Microporous Mesoporous Mater. 2007, 106, 122–128. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Montanari, T.; Finocchio, E.; Busca, G. Are the active sites of protonic zeolites generated by the cavities? Catal. Today 2006, 116, 132–142. [Google Scholar] [CrossRef]
- Losch, P.; Boltz, M.; Bernardon, C.; Louis, B.; Palčić, A.; Valtchev, V. Impact of external surface passivation of nano-ZSM-5 zeolites in the methanol-to-olefins reaction. Appl. Catal. A Gen. 2016, 509, 30–37. [Google Scholar] [CrossRef]
- Zholobenko, V.; Freitas, C.; Jendrlin, M.; Bazin, P.; Travert, A.; Thibault-Starzyk, F. Probing the acid sites of zeolites with pyridine: Quantitative AGIR measurements of the molar absorption coefficients. J. Catal. 2020, 385, 52–60. [Google Scholar] [CrossRef]
- Trombettaa, M.; Buscaa, G.; Rossinib, S.; Piccolib, V.; Cornarob, U. FT-IR Studies on Light Olefin Skeletal Isomerization Catalysis: II. The Interaction of C4 Olefins and Alcohols with HZSM5 Zeolite. J. Catal. 1997, 168, 349–363. [Google Scholar] [CrossRef]
- Kumar, D.; Sauer, J.; Airi, A.; Bordiga, S.; Galimberti, D.R. Assignment of IR spectra of ethanol at Brønsted sites of H-ZSM-5 to monomer adsorption using a Fermi resonance model. Phys. Chem. Chem. Phys. 2025, 27, 550–563. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Johnson, B.A.; Román-Leshkov, Y. Ordered Hydrogen-Bonded Alcohol Networks Confined in Lewis Acid Zeolites Accelerate Transfer Hydrogenation Turnover Rates. J. Am. Chem. Soc. 2020, 142, 19379–19392. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kurniawan, E.; Ozawa, T.; Kobayashi, H.; Yamada, Y.; Sato, S. Catalytic dehydration of crotyl alcohol into 1,3-butadiene over silica-supported metal oxides: Mechanistic features. Mol. Catal. 2023, 537, 112939. [Google Scholar] [CrossRef]
- Lakiss, L.; Ngoye, F.; Canaff, C.; Laforge, S.; Pouilloux, Y.; Qin, Z.; Tarighi, M.; Thomas, K.; Valtchev, V.; Vicente, A.; et al. On the remarkable resistance to coke formation of nanometer-sized and hierarchical MFI zeolites during ethanol to hydrocarbons transformation. J. Catal. 2015, 328, 165–172. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.; Beato, P. Structure–deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196–205. [Google Scholar] [CrossRef]
- Thibault-Starzyk, F.; Vimont, A.; Gilson, J.-P. 2D-COS IR study of coking in xylene isomerisation on H-MFI zeolite. Catal. Today 2001, 70, 227–241. [Google Scholar] [CrossRef]
- Travert, A.; Vimont, A.; Sahibed-Dine, A.; Daturi, M.; Lavalley, J.-C. Use of pyridine CH(D) vibrations for the study of Lewis acidity of metal oxides. Appl. Catal. A Gen. 2006, 307, 98–107. [Google Scholar] [CrossRef]
- Mears, D.E. Tests for Transport Limitations in Experimental Catalytic Reactors. Ind. Eng. Chem. Process Des. Dev. 1971, 10, 541–547. [Google Scholar] [CrossRef]
- Berger, R.J.; Stitt, E.H.; Marin, G.B.; Kapteijn, F.; Moulijn, J.A. Eurokin. Chemical Reaction Kinetics in Practice. CATTECH 2001, 5, 36–60. [Google Scholar] [CrossRef]

| Sample | Si/AlICP−MS | Ar-Sorption | Brønsted Acid Sites [µmol/g] | Lewis Acid Sites [µmol/g] | ||||
|---|---|---|---|---|---|---|---|---|
| SBET [m2/g] | Vtot [cm3/g] | Vmicro [cm3/g] | Tevac = 150 °C | Tevac = 300 °C | Tevac = 150 °C | Tevac = 300 °C | ||
| c-ZSM-22 | 30 | 216 | 0.09 | 0.07 | 128 | 110 | 14 | 9 |
| ZSM-22 (38) | 38 | 280 | 0.12 | 0.09 | 136 | 110 | 27 | 15 |
| ZSM-22 (50) | 50 | 289 | 0.12 | 0.09 | 105 | 86 | 29 | 24 |
| ZSM-22 (80) | 80 | 291 | 0.11 | 0.09 | 87 | 71 | 26 | 13 |
| Zeolite | TOS [h] | Conversion [%] | Selectivity [%] | Productivity [gBD·g−1cata·h−1] | ||||
|---|---|---|---|---|---|---|---|---|
| PE | BD | BuAL | MEK | 3B1OL | ||||
| c-ZSM-22 | 1 | 100 | 10 | 71 | 0 | 18 | 0 | 2.5 |
| 22 | 61 | 10 | 58 | 1 | 11 | 21 | 1.3 | |
| ZSM-22 (38) | 1 | 100 | 10 | 73 | 0 | 16 | 0 | 2.7 |
| 22 | 58 | 9 | 61 | 1 | 12 | 17 | 1.2 | |
| ZSM-22 (50) | 1 | 99 | 16 | 68 | 0 | 14 | 1 | 2.0 |
| 22 | 31 | 14 | 55 | 2 | 11 | 18 | 0.5 | |
| ZSM-22 (80) | 1 | 99 | 10 | 74 | 0 | 15 | 0 | 1.4 |
| 22 | 57 | 10 | 62 | 1 | 11 | 16 | 0.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eloi, L.; Poissonnier, J.; De Landsheere, A.; Sharma, D.; Al Atrach, J.; Ruaux, V.; Valtchev, V.; Sabbe, M.K.; Thybaut, J.W.; Verberckmoes, A. Direct Conversion of 1,3-Butanediol to 1,3-Butadiene over ZSM-22 Catalysts: Influence of the Si/Al Ratio. Catalysts 2025, 15, 655. https://doi.org/10.3390/catal15070655
Eloi L, Poissonnier J, De Landsheere A, Sharma D, Al Atrach J, Ruaux V, Valtchev V, Sabbe MK, Thybaut JW, Verberckmoes A. Direct Conversion of 1,3-Butanediol to 1,3-Butadiene over ZSM-22 Catalysts: Influence of the Si/Al Ratio. Catalysts. 2025; 15(7):655. https://doi.org/10.3390/catal15070655
Chicago/Turabian StyleEloi, Loïc, Jeroen Poissonnier, Arne De Landsheere, Dhanjay Sharma, Jaouad Al Atrach, Valérie Ruaux, Valentin Valtchev, Maarten K. Sabbe, Joris W. Thybaut, and An Verberckmoes. 2025. "Direct Conversion of 1,3-Butanediol to 1,3-Butadiene over ZSM-22 Catalysts: Influence of the Si/Al Ratio" Catalysts 15, no. 7: 655. https://doi.org/10.3390/catal15070655
APA StyleEloi, L., Poissonnier, J., De Landsheere, A., Sharma, D., Al Atrach, J., Ruaux, V., Valtchev, V., Sabbe, M. K., Thybaut, J. W., & Verberckmoes, A. (2025). Direct Conversion of 1,3-Butanediol to 1,3-Butadiene over ZSM-22 Catalysts: Influence of the Si/Al Ratio. Catalysts, 15(7), 655. https://doi.org/10.3390/catal15070655









