Investigation of Anti-Corrosion Behavior of Epoxy-Based Tannic Acid/Benzoxazine and Embedded ZnO Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
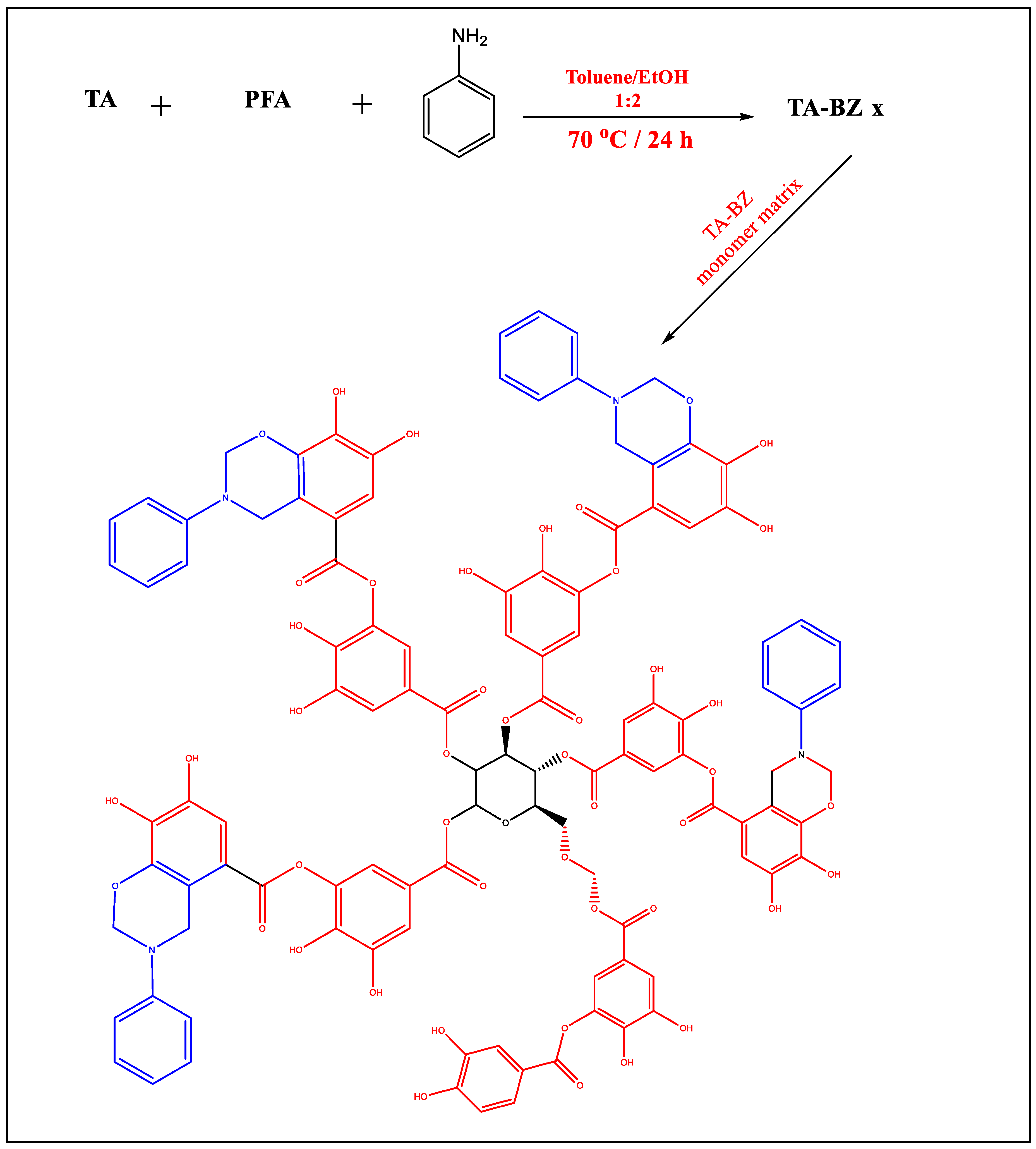
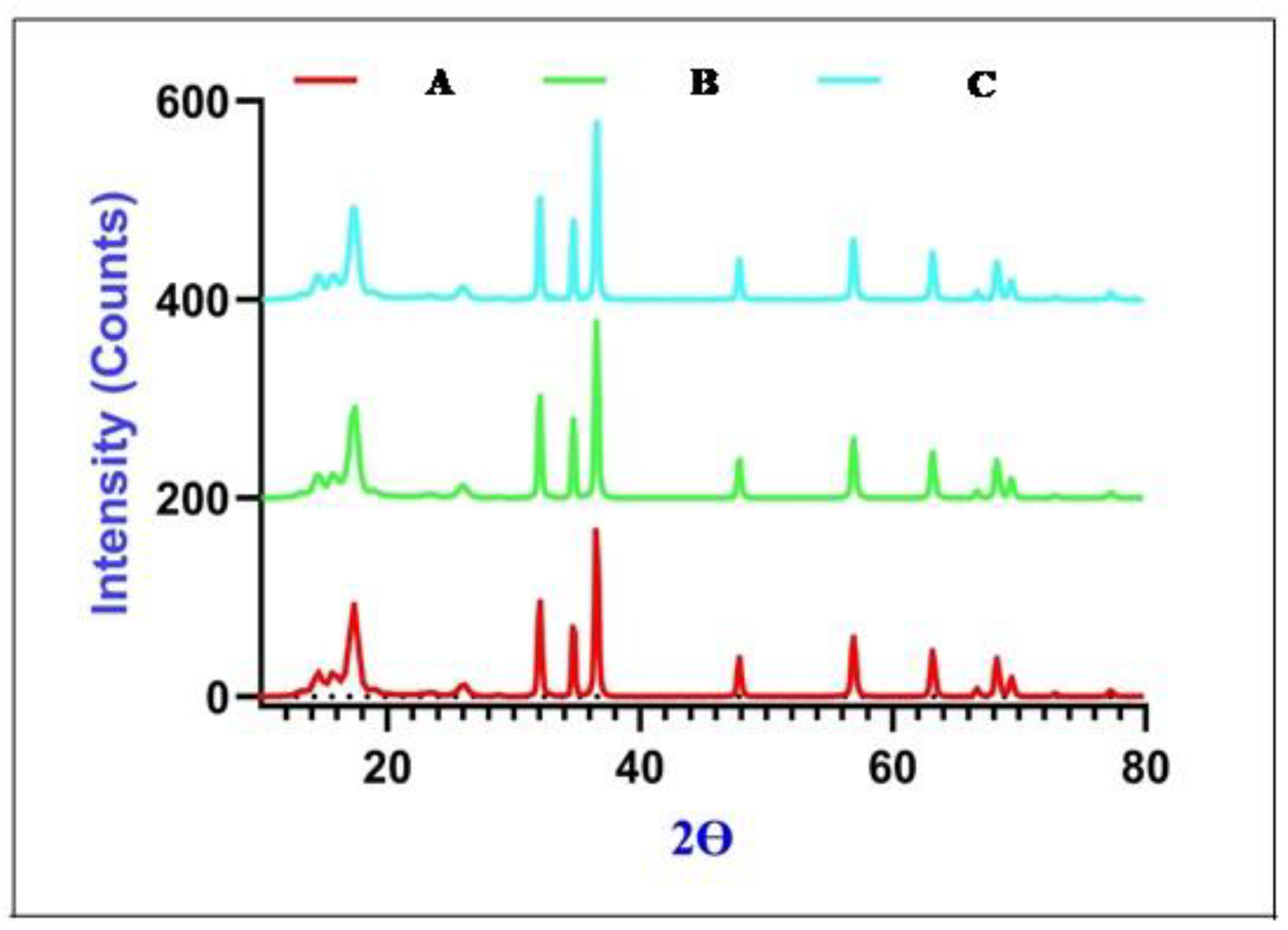
2.1. Chemistry
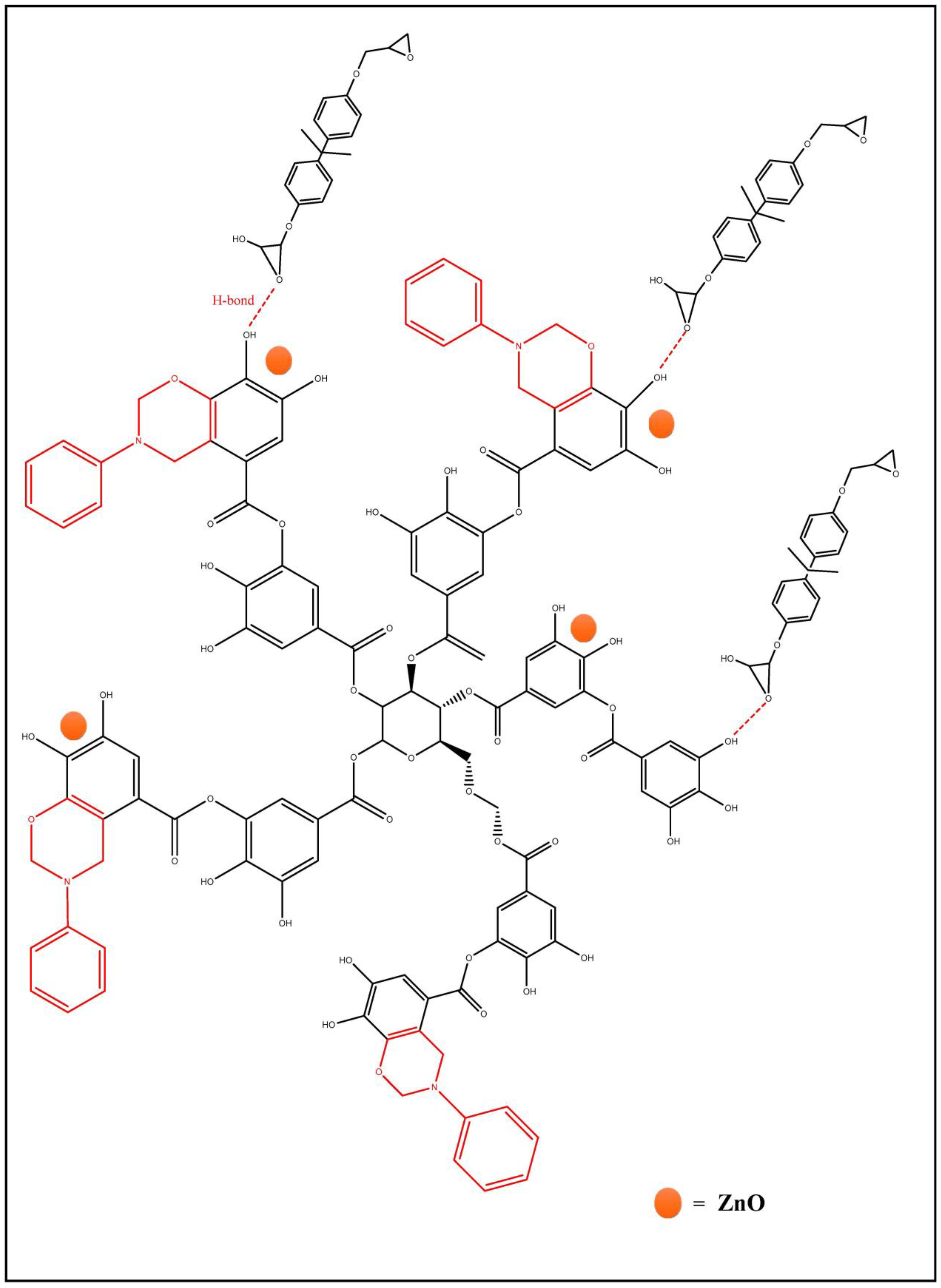
2.2. Long-Term Stability Prediction Based on Polymer Structure
2.3. Anticorrosion Properties of Uncured Epoxy-TA-BZ-ZnO Samples
3. Experimental
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Methods
3.3.1. Synthesis of Tannic Acid Benzoxazine (TA-Bz) Monomer
3.3.2. Preparation of Pure Epoxy Resin
3.3.3. The Preparation of TA-BZ Blend Epoxy-ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, H.; Wang, D.; Fu, S. Tannic acid-assisted green exfoliation and functionalization of MoS2 nanosheets: Significantly improve the mechanical and flame-retardant properties of polyacrylonitrile composite fibers. J. Chem. Eng. 2020, 384, 123288. [Google Scholar] [CrossRef]
- Lin, H.; Pei, L.; Zhang, L. Enhanced thermal conductivity of PLA-based nanocomposites by incorporation of graphite nanoplatelets functionalized by tannic acid. J. Appl. Polym. Sci. 2018, 135, 46397. [Google Scholar] [CrossRef]
- Fei, X.; Zhao, F.; Wei, W.; Luo, J.; Chen, M.; Liu, X. Tannic Acid as a Bio-Based Modifier of Epoxy/Anhydride Thermosets. Polymers 2016, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, S.A.; Najmi, P.; Keshmiri, N.; Tanguy, N.; Van der Kuur, C.; Yan, N.; Mekonnen, T.; Arjmand, M. Cerium-doped tannic acid-reduced graphene oxide nanoplatform/epoxy nanocomposite coatings with enhanced mechanical and Bi-functional corrosion protection properties. Compos. Part B Eng. 2022, 239, 109969. [Google Scholar] [CrossRef]
- Zhou, C.; Fu, M.; Xie, H.; Gong, Y.; Chen, J.; Liu, J.; Xin, Z. Polybenzoxazine/Epoxy Composite Coatings: Effect of Crosslinking on Corrosion Resistance. Ind. Eng. Chem. Res. 2021, 60, 1675–1683. [Google Scholar] [CrossRef]
- Krishnan, S.; Hariharan, A.; Kuppan, C.; Goswami, A.; Chavali, M.; Muthukaruppan, A. Silane-Functionalized Polybenzoxazines: A Superior Corrosion Resistant Coating for Steel Plates. Mater. Corros. 2017, 68, 1343–1354. [Google Scholar] [CrossRef]
- Li, S.; Zhao, C.; Wang, Y.; Li, H.; Li, Y. Synthesis and electrochemical properties of electroactive aniline-dimer-based benzoxazines for advanced corrosion-resistant coatings. J. Mater. Sci. 2018, 53, 7344–7356. [Google Scholar] [CrossRef]
- Lligadas, G.; Tüzün, A.; Ronda, J.C.; Galià, M.; Cádiz, V. Polybenzoxazines: New players in the bio-based polymer arena. Polym. Chem. 2014, 5, 6636–6644. [Google Scholar] [CrossRef]
- Ishida, H.; Agag, T. Preface. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, Netherlands, 2011; pp. xix–xx. [Google Scholar] [CrossRef]
- Lyu, Y.; Rachita, E.; Pogharian, N.; Froimowiczc, P.; Ishida, H. Electronic effects of asymmetric and meta-alkoxy substituents on the polymerization behavior of bis-benzoxazines. Polym. Chem. 2020, 11, 800–809. [Google Scholar] [CrossRef]
- Hanif, Z.; Dinh, D.K.; Pornea, A.G.M.; Yanar, N.; Kwak, M.S.; Kim, J. Protruding Boron Nitride Nanotubes on the Al2O3 Surface Enabled by Tannic Acid-Assisted Modification to Fabricate a Thermal Conductive Epoxy/Al2O3 Composite. ACS Omega 2024, 9, 38946–38956. [Google Scholar] [CrossRef]
- Li, X.K.; Zhang, A.P.; Bian, J.; Ni, K.Y.; Zhao, W.; Yang, K.C.; Lin, H.L.; Chen, D.Q. Progress on the simulation, synthesis, processing and application of high performance thermosetting polybenzoxazine resin and its composites: A review. Prog. Org. Coat. 2024, 192, 108506. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Sun, Z. Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review. Polymers 2024, 16, 2752. [Google Scholar] [CrossRef] [PubMed]
- Klfout, H.A.; Asiri, A.M.; Alamry, K.A.; Hussein, M.A. Recent Advances in Bio Based Polybenzoxazines as an Interesting Adhesive Coating. RSC Adv. 2023, 13, 19817–19835. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Wang, Z. Study of nano-ZnO improvement of the mechanical properties and corrosion resistance of modified-SiO2/PTFE superhydrophobic nanocomposite coatings by one-step spraying. New J. Chem. 2023, 47, 6246–6257. [Google Scholar] [CrossRef]
- Bani, H.; Nouri, H.; Dardouri, F. Recent Advances in Polymer Nanocomposites for Corrosion Protection Applications. . Materials 2020, 13, 903. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Li, Z. Nanocomposite coatings for corrosion protection: The role of zinc oxide nanoparticles. Prog. Org. Coat. 2022, 167, 106838. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, J.; Lu, X.; Xin, Z. Enhanced corrosion resistance of polybenzoxazine coatings by epoxy incorporation. RSC Adv. 2016, 6, 28428–28434. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Jungwirth, S.; Seely, N.; Fang, Y.; Shi, X. Review: The Application of Anti-Corrosion Coating for Preserving the Value of Equipment Asset in Chloride-Laden Environments: A Review. Int. J. Electrochem. Sci. 2015, 10, 10756–10780. [Google Scholar] [CrossRef]
- Khan, G.; Newaz, K.M.S.; Basirun, W.J.; Ali, H.B.M.; Faraj, F.L.; Khan, G.M. Application of Natural Product Extracts as Green Corrosion Inhibitors for Metals and Alloys in Acid Pickling Processes-A review. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhan, G.; Zhuang, Q.; Zhang, R.; Qian, J. Study on the synergistic anticorrosion property of a fully bio-based polybenzoxazine copolymer resin. Eur. Polym. J. 2019, 119, 477–486. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S. Enhancement of anti-corrosive performances of cardanol based amine functional benzoxazine resin by copolymerizing with epoxy resins. Prog. Org. Coat. 2017, 105, 18–28. [Google Scholar] [CrossRef]
- Saleh, B.; Fathi, R.; Shi, H.; Wei, H. Advanced Corrosion Protection through Coatings and Surface Rebuilding. Coatings 2023, 13, 180. [Google Scholar] [CrossRef]
- Saji, V.S. Advanced Corrosion Prevention Approaches: Smart Coating and Photoelectrochemical Cathodic Protection. In Corrosion and Fouling Control in Desalination Industry; Saji, V.S., Ed.; Springer: Cham, Switzerland, 2020; pp. 225–247. [Google Scholar] [CrossRef]
- Hamak, K.F. Synthetic of Phthalimides via the Reaction of Phthalic Anhydride with Amines and Evaluating Its Biological and Anticorrosion Activity. Int. J. Chem. Technol. Res. 2014, 6, 324–333. [Google Scholar]
- Hegazy, M.A. A Novel Schiff Base-Based Cationic Gemini Surfactants: Synthesis and Effect on Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution. Corros. Sci. 2009, 51, 2610–2618. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Crown Ether-Functionalized Polybenzoxazine for Metal Ion Adsorption. Macromolecules 2020, 53, 2420–2429. [Google Scholar] [CrossRef]
- Ma, C.; Han, L.; Ma, Z.; Ishida, H. Preparation and Characterization of Carbon Fiber Reinforced Polybenzoxazine and Polybenzoxazole Composites from the Same Precursor: Use of a Smart, Ortho-Substituted and Amide-co-Imide Functional Matrix. Compos. Sci. Technol. 2020, 195, 108205. [Google Scholar] [CrossRef]
- Sharma, S.; Ganjoo, R.; Kumar, A. Polyphenols for Anticorrosion Application. In Green Corrosion Inhibitors; Verma, C., Ebenso, E.E., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 134–156. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, X.; Lou, C.; Zhou, C.; Xin, Z. Preparation of Diamine-Based Polybenzoxazine Coating for Corrosion Protection on Mild Steel. J. Polym. Res. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Barbouchi, M.; Benzidia, B.; Aouidate, A.; Ghaleb, A.; El Idrissi, M.; Choukrad, M. Theoretical Modeling and Experimental Studies of Terebinth Extracts as Green Corrosion Inhibitor for Iron in 3% NaCl Medium. J. King Saud Univ. Sci. 2020, 32, 2995–3004. [Google Scholar] [CrossRef]
- Dai, J.; Yang, S.; Teng, N.; Liu, Y.; Liu, X.; Zhu, J.; Zhao, J. Synthesis of Eugenol-Based Silicon-Containing Benzoxazines and Their Applications as Bio-Based Organic Coatings. Coatings 2018, 8, 88. [Google Scholar] [CrossRef]
- Xu, J.; Gao, F.; Wang, H.; Dai, R.; Dong, S.; Wang, H. Organic/Inorganic Hybrid Waterborne Polyurethane Coatings with Self-Healing Properties for Anticorrosion Application. Prog. Org. Coat. 2023, 174, 107244. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Okbi, A.K.; Jamil, D.M.; Qussay, A.; Al-Amiery, A.A.; Gaaz, T.S.; Kadhum, A.A.H.; Mohamad, A.B.; Nassir, M.H. Experimental and Theoretical Studies of Benzoxazines Corrosion Inhibitors. Results Phys. 2017, 7, 4013–4019. [Google Scholar] [CrossRef]
- Kusmierek, E.; Chrzescijanska, E. Tannic Acid as Corrosion Inhibitor for Metals and Alloys. Mater. Corros. 2015, 66, 169–174. [Google Scholar] [CrossRef]
- Abdus Samad, U.; Alam, M.A.; Anis, A.; Sherif, E.M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of Incorporated ZnO Nanoparticles on the Corrosion Performance of SiO2 Nanoparticle-Based Mechanically Robust Epoxy Coatings. Materials 2020, 13, 3767. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Zhang, J.; Habib, S.; Lu, G.; Dai, J.; Liu, X. Bio-Based Polybenzoxazines Coatings for Efficient Marine Antifouling. Prog. Org. Coat. 2023, 174, 107298. [Google Scholar] [CrossRef]
- Both, J.; Szabó, G.; Katona, G.; Muresan, L.M. Tannic Acid Reinforced Sol-Gel Silica Coatings for Corrosion Protection of Zinc Substrates. Mater. Chem. Phys. 2022, 282, 125912. [Google Scholar] [CrossRef]
- Sun, J.; Duan, J.; Liu, X.; Dong, X.; Zhang, Y.; Liu, C.; Hou, B. Environmentally Benign Smart Self-Healing Silicone-Based Coating with Dual Antifouling and Anti-Corrosion Properties. Appl. Mater. Today 2022, 28, 101551. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Xiang, F.; Liu, P.; Wang, H.; Wei, W.; Dong, S. Enhanced Corrosion Resistance of Self-Healing Waterborne Polyurethane Coating Based on Tannic Acid Modified Cerium-Montmorillonites Composite Fillers. Prog. Org. Coat. 2023, 178, 107454. [Google Scholar] [CrossRef]
- Aly, K.I.; Mahdy, A.; Hegazy, M.A.S.; Kuo, S.; Gamal Mohamed, M. Corrosion Resistance of Mild Steel Coated with Phthalimide-Functionalized Polybenzoxazines. Coatings 2020, 10, 1114. [Google Scholar] [CrossRef]
- Mahdy, A.; Aly, K.I.; Mohamed, M.G. Construction Novel Polybenzoxazine Coatings Exhibiting Corrosion Protection of Mild Steel at Different Concentrations in a Seawater Solution. Heliyon 2023, 9, e17977. [Google Scholar] [CrossRef]
- Li, S.; Cui, B.; Jia, X.; Wang, W.; Cui, Y.; Ding, J.; Yang, C.; Fang, Y.; Song, Y.; Zhang, X. A Cellulose-Based Light-Management Film Incorporated with Benzoxazine Resin/Tannic Acid Exhibiting UV/Blue Light Double Blocking and Enhanced Mechanical Property. Int. J. Biol. Macromol. 2024, 278, 134461. [Google Scholar] [CrossRef]
- Lin, L.; Wu, C.; Su, W.; Liu, Y. Diethylphosphonate-Containing Benzoxazine Compound as a Thermally Latent Catalyst and a Reactive Property Modifier for Polybenzoxazine-Based Resins. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3523–3530. [Google Scholar] [CrossRef]
- Liu, J.; Wuliu, Y.; Dai, J.; Hu, J.; Liu, X. Synthesis and Properties of the Bio-Based Isomeric Benzoxazine Resins: Revealing the Effect of the Neglected Short Alkyl Substituents. Eur. Polym. J. 2021, 157, 110671. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, K. Synthesis and Properties of Biobased Mono-Benzoxazine Resins from Natural Renewable Pterostilbene. Eur. Polym. J. 2021, 156, 110607. [Google Scholar] [CrossRef]
- Marmiroli, M. Review for Review of the Corrosion Behaviour in Tannic-Acid Coated Magnesium Implants. Mater. Res. Express 2023. [Google Scholar] [CrossRef]
- Rao, B.; Palanisamy, A. Synthesis of Bio-Based Low Temperature Curable Liquid Epoxy, Benzoxazine Monomer System from Cardanol: Thermal and Viscoelastic Properties. Eur. Polym. J. 2013, 49, 2365–2376. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Zhu, L.; Meng, J.; He, F. Tannic Acid-Based Functional Coating: Surface Engineering of Membranes for Oil-in-Water Emulsion Separation. Chem. Commun. 2022, 58, 12629–12641. [Google Scholar] [CrossRef]
- Kannaiyan, J.; Mani, S. Study of Corrosion Resistant Property of Benzoxazine Synthesized from Eugenol with N-Butylamine and Copolymerized with Polyurethane on Mild Steel. Orient. J. Chem. 2023, 39, 1194–1204. [Google Scholar] [CrossRef]
- Caldona, E.B.; De Leon, A.C.C.; Pajarito, B.B.; Advincula, R.C. Novel Anti-Corrosion Coatings from Rubber-Modified Polybenzoxazine-Based Polyaniline Composites. Appl. Surf. Sci. 2017, 422, 162–171. [Google Scholar] [CrossRef]
- Laoutid, F.; Karaseva, V.; Costes, L.; Brohez, S.; Mincheva, R.; Dubois, P. Novel Bio-Based Flame Retardant Systems Derived from Tannic Acid. J. Renew. Mater. 2018, 6, 559–572. [Google Scholar] [CrossRef]
- Musa, A.; Alamry, K.A.; Hussein, M.A. Effect of Curing Temperatures on the Thermal Behaviour of New Polybenzoxazine-Modified Epoxy Resin. Polym. Bull. 2020, 77, 5439–5449. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Zhou, Y.; Pervin, F.; Lewis, L.; Jeelani, S. Experimental Study on the Thermal and Mechanical Properties of Epoxy Nanocomposites with Yttria-Stabilized Zirconia Nanoparticles. Mater. Sci. Eng. A 2008, 475, 157–161. [Google Scholar] [CrossRef]
- Shundo, A.; Yamamoto, S.; Tanaka, K. Network Formation and Physical Properties of Epoxy Resins for Future Practical Applications. JACS Au 2022, 2, 1522–1542. [Google Scholar] [CrossRef] [PubMed]
- Balguri, P.K.; Samuel, D.H.; Thumu, U. A Review on Mechanical Properties of Epoxy Nanocomposites. Mater. Today Proc. 2020, 44, 346–355. [Google Scholar] [CrossRef]
- Feng, X.; Fan, J.; Li, A.; Li, G. Biobased Tannic Acid Cross-Linked Epoxy Thermosets with Hierarchical Molecular Structure and Tunable Properties: Damping, Shape Memory, and Recyclability. ACS Sustain. Chem. Eng. 2020, 8, 874–883. [Google Scholar] [CrossRef]
- Kahns, A.H.; Bundgaard, H. Hydrolysis kinetics of 1,3-benzoxazine-2,4-dione (a potential salicylamide prodrug) and various N-substituted derivatives. Acta Pharm. Nord. 1991, 3, 45–50. [Google Scholar]
- Kim, Y.-O.; Cho, J.; Yeo, H.; Lee, B.W.; Moon, B.J.; Ha, Y.-M.; Jo, Y.R.; Jung, Y.C. Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener. ACS Sustain. Chem. Eng. 2019, 7, 3858–3865. [Google Scholar] [CrossRef]
- Zhao, S.; Pei, L.; Li, H.; Zhang, X.; Hu, W.; Zhao, G.; Wang, Z. Enhanced Comprehensive Properties of Polybenzoxazine via Tailored Hydrogen-Bonds. Polymer 2020, 201, 122647. [Google Scholar] [CrossRef]
- Selvi, M.; Devaraju, S.; Vengatesan, M.R.; Go, J.S.; Kumar, M.; Alagar, M. The effect of UV radiation on polybenzoxazine/epoxy/OG-POSS nanocomposites. RSC Adv. 2014, 4, 8238. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M.; Farzam, M. Effect of ZnO Nanoparticles on the Thermal and Mechanical Properties of Epoxy-Based Nanocomposite. J. Therm. Anal. Calorim. 2010, 103, 731–739. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Dhanasekaran, J.; Krishna Mohan, S. Epoxy Benzoxazine Based Ternary Systems of Improved Thermo-Mechanical Behavior for Structural Composite Applications. RSC Adv. 2015, 5, 3709–3719. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Liu, Y.; Guo, Z.; Ge, Q.; Sun, Z. The Curing Characteristics and Properties of Bisphenol A Epoxy Resin/Maleopimaric Acid Curing System. J. Mater. Res. Technol. 2022, 21, 1655–1665. [Google Scholar] [CrossRef]
- Şomoghi, R.; Semenescu, A.; Pasăre, V.; Chivu, O.R.; Nițoi, D.F.; Marcu, D.F.; Florea, B. The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites. Polymers 2024, 16, 2054. [Google Scholar] [CrossRef] [PubMed]
- ASTM G31; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. TFT-PNEUMATIC: Houston, TX, USA, 2017.
- ISO 17475; Corrosion of Metals and Alloys—Determination of Dezincification Resistance of Copper Alloys with Zinc. TFT-PNEUMATIC: Houston, TX, USA, 2017.
- Soto, M.; Hiller, M.; Oschkinat, H.; Koschek, K. Multifunctional Benzoxazines Feature Low Polymerization Temperature and Diverse Polymer Structures. Polymers 2016, 8, 278. [Google Scholar] [CrossRef]
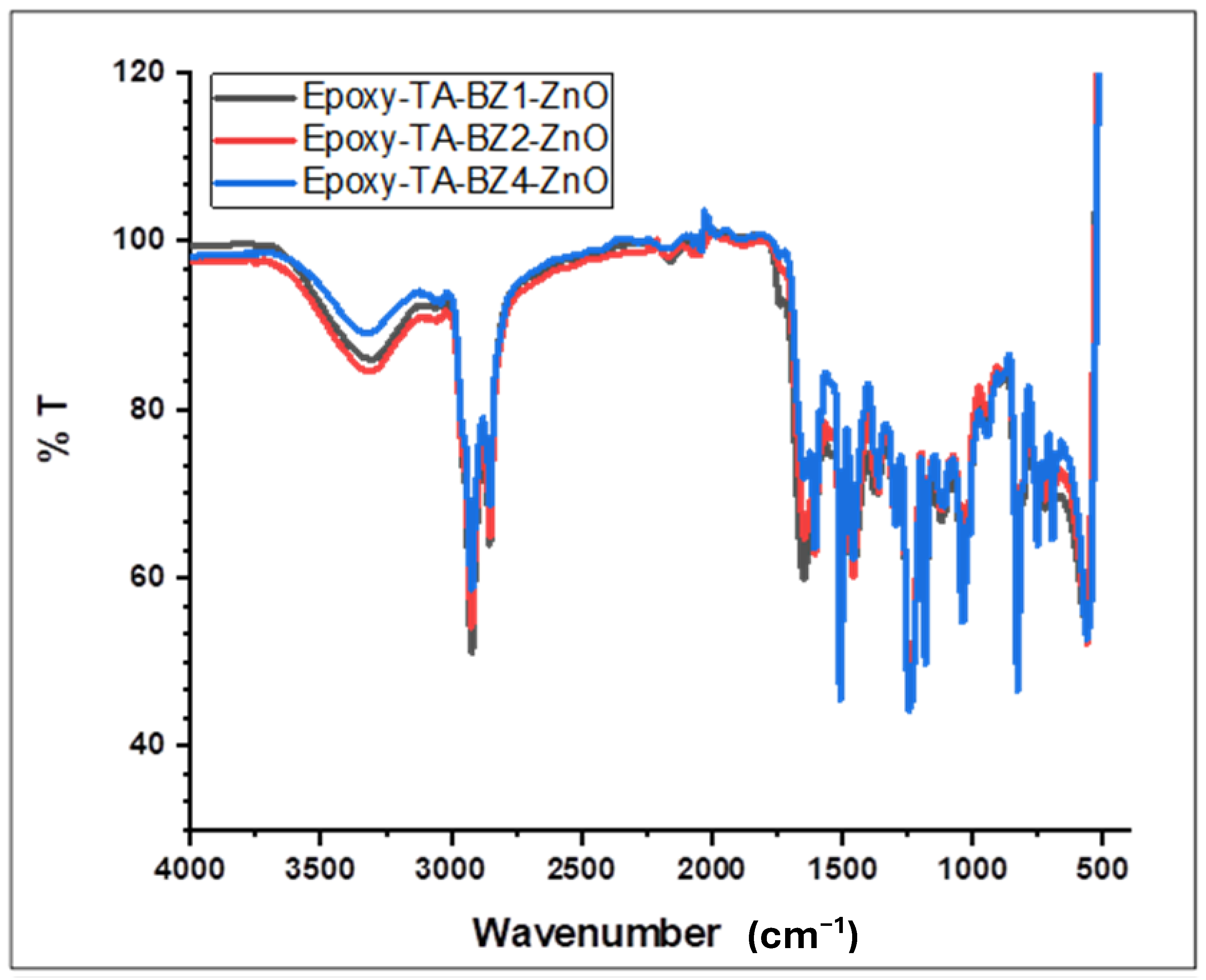

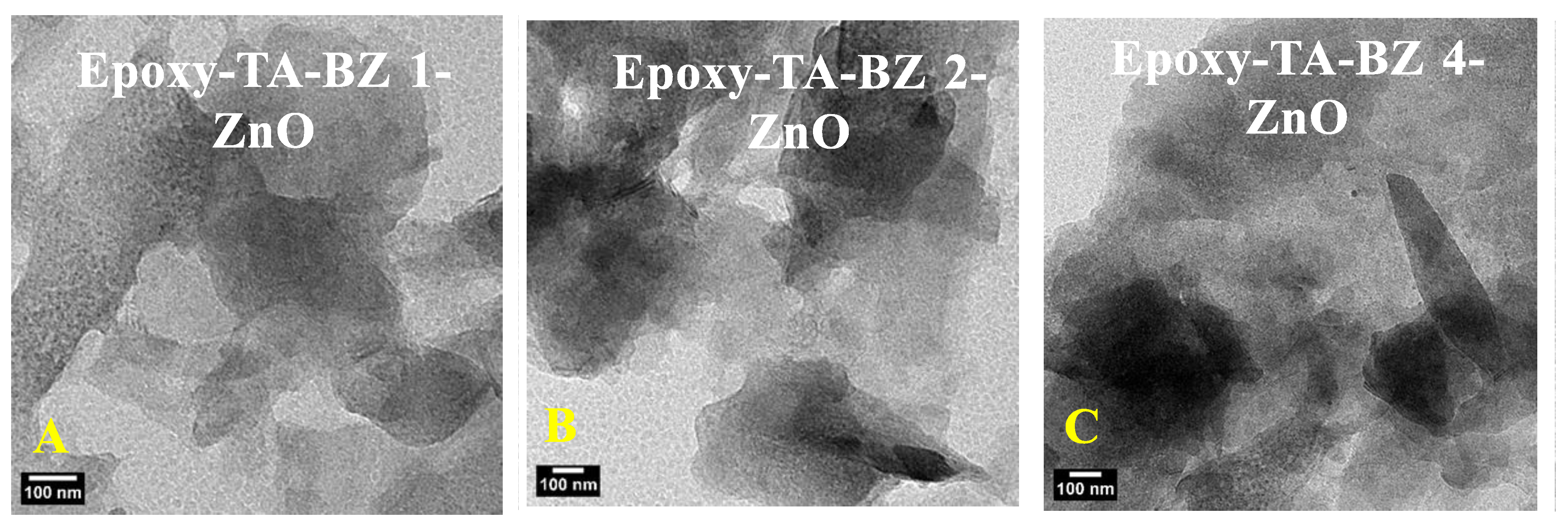


| Wavenumber (cm−1) | Assignment | Molecular Origin | Significance for TA-BZ Formation |
|---|---|---|---|
| 3200–3500 | O-H stretching (phenolic) | Residual tannic acid hydroxyl groups | Confirms retention of polyphenolic character |
| 2850–2950 | C-H stretching (aliphatic) | Methylene bridges in benzoxazine rings | Indicates successful Mannich condensation |
| 1580–1600 | Aromatic C=C stretching | Benzene rings from TA and aniline | Confirms aromatic structure preservation |
| 1500–1550 | Aromatic C=C stretching | Substituted benzene rings | Secondary aromatic vibrations |
| 1220–1260 | C-O-C asymmetric stretch | Oxazine ring formation | Primary confirmation of benzoxazine synthesis |
| 1150–1180 | C-N stretching | Tertiary amine in oxazine ring | Supporting evidence for ring closure |
| 950–970 | C-H out-of-plane deformation | Trisubstituted benzene adjacent to oxazine | Characteristic benzoxazine fingerprint |
| 800–850 | C-H out-of-plane bending | Aromatic substitution patterns | Confirms aromatic substitution |
| <600 | Zn-O stretching | ZnO nanoparticle incorporation | Validates ZnO presence in composite |
| Sample | T10 (°C) | T20 (°C) | T30 (°C) | T50 (°C) | Residue at 800 °C (%) |
|---|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | 398 | 425 | 442 | 465 | 1.45 |
| Epoxy-TA-BZ2-ZnO (B) | 403 | 429 | 448 | 467 | 1.67 |
| Epoxy-TA-BZ4-ZnO (C) | 408 | 435 | 452 | 469 | 1.89 |
| Sample | Stage I (°C) | Stage II (°C) | Stage III (°C) | Residual Mass Transition (°C) |
|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | 300–385 | 385–465 | 465–550 | 550–650 |
| Epoxy-TA-BZ2-ZnO (B) | 305–390 | 390–470 | 470–555 | 555–660 |
| Epoxy-TA-BZ4-ZnO (C) | 310–395 | 395–475 | 475–560 | 560–670 |
| Sample | Char at 400 °C (%) | Char at 500 °C (%) | Char at 600 °C (%) | Char at 800 °C (%) |
|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | 85.2 | 28.7 | 1.98 | 1.45 |
| Epoxy-TA-BZ2-ZnO (B) | 87.5 | 30.4 | 2.05 | 1.67 |
| Epoxy-TA-BZ4-ZnO (C) | 89.8 | 32.1 | 2.13 | 1.89 |
| Sample | Peak 1 Temp (°C) | Peak 1 Area (%) | Peak 2 Temp (°C) | Peak 2 Area (%) | Peak 3 Temp (°C) | Peak 3 Area (%) |
|---|---|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | 345 | 18.5 | 432 | 65.7 | 510 | 15.8 |
| Epoxy-TA-BZ2-ZnO (B) | 348 | 17.2 | 438 | 67.9 | 515 | 14.9 |
| Epoxy-TA-BZ4-ZnO (C) | 352 | 16.1 | 445 | 70.2 | 520 | 13.7 |
| Sample | Glass Transition Temperature (°C) | Exothermic Peak Temperature (°C) | Exothermic Peak Height (mW) | Enthalpy of Curing (J/g) |
|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | 152 | 348 | 3.0 | 310 |
| Epoxy-TA-BZ2-ZnO (B) | 155 | 349 | 3.2 | 290 |
| Epoxy-TA-BZ4-ZnO (C) | 158 | 352 | 3.4 | 270 |
| Sample | Onset Temperature (°C) | Peak Temperature (°C) | Endset Temperature (°C) | Peak Width (°C) | Cure Index |
|---|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | 325 | 348 | 365 | 40 | 0.78 |
| Epoxy-TA-BZ2-ZnO (B) | 328 | 349 | 368 | 40 | 0.81 |
| Epoxy-TA-BZ4-ZnO (C) | 332 | 352 | 372 | 40 | 0.85 |
| Sample | Ecorr (mV vs. SCE) | Icorr (µA/cm2) | βa (mV/Decade) | βc (mV/Decade) | Corrosion Rate (mm/Year) |
|---|---|---|---|---|---|
| Epoxy-TA-BZ1-ZnO (A) | −412 ± 8 | 5.8 ± 0.3 | 95 ± 4 | 88 ± 3 | 0.067 ± 0.004 |
| Epoxy-TA-BZ2-ZnO (B) | −408 ± 6 | 5.5 ± 0.2 | 97 ± 3 | 90 ± 4 | 0.064 ± 0.003 |
| Epoxy-TA-BZ4-ZnO (C) | −405 ± 5 | 5.2 ± 0.3 | 98 ± 5 | 91 ± 3 | 0.060 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamry, K.A.; Klfout, H.; Hussein, M.A. Investigation of Anti-Corrosion Behavior of Epoxy-Based Tannic Acid/Benzoxazine and Embedded ZnO Nanocomposites. Catalysts 2025, 15, 644. https://doi.org/10.3390/catal15070644
Alamry KA, Klfout H, Hussein MA. Investigation of Anti-Corrosion Behavior of Epoxy-Based Tannic Acid/Benzoxazine and Embedded ZnO Nanocomposites. Catalysts. 2025; 15(7):644. https://doi.org/10.3390/catal15070644
Chicago/Turabian StyleAlamry, Khalid A., Hafsah Klfout, and Mahmoud A. Hussein. 2025. "Investigation of Anti-Corrosion Behavior of Epoxy-Based Tannic Acid/Benzoxazine and Embedded ZnO Nanocomposites" Catalysts 15, no. 7: 644. https://doi.org/10.3390/catal15070644
APA StyleAlamry, K. A., Klfout, H., & Hussein, M. A. (2025). Investigation of Anti-Corrosion Behavior of Epoxy-Based Tannic Acid/Benzoxazine and Embedded ZnO Nanocomposites. Catalysts, 15(7), 644. https://doi.org/10.3390/catal15070644







