Ag/AgCl-Decorated Layered Lanthanum/Niobium Oxide Microparticles as Efficient Photocatalysts for Azo Dye Remediation and Cancer Cell Inactivation
Abstract
1. Introduction
2. Results and Discussion
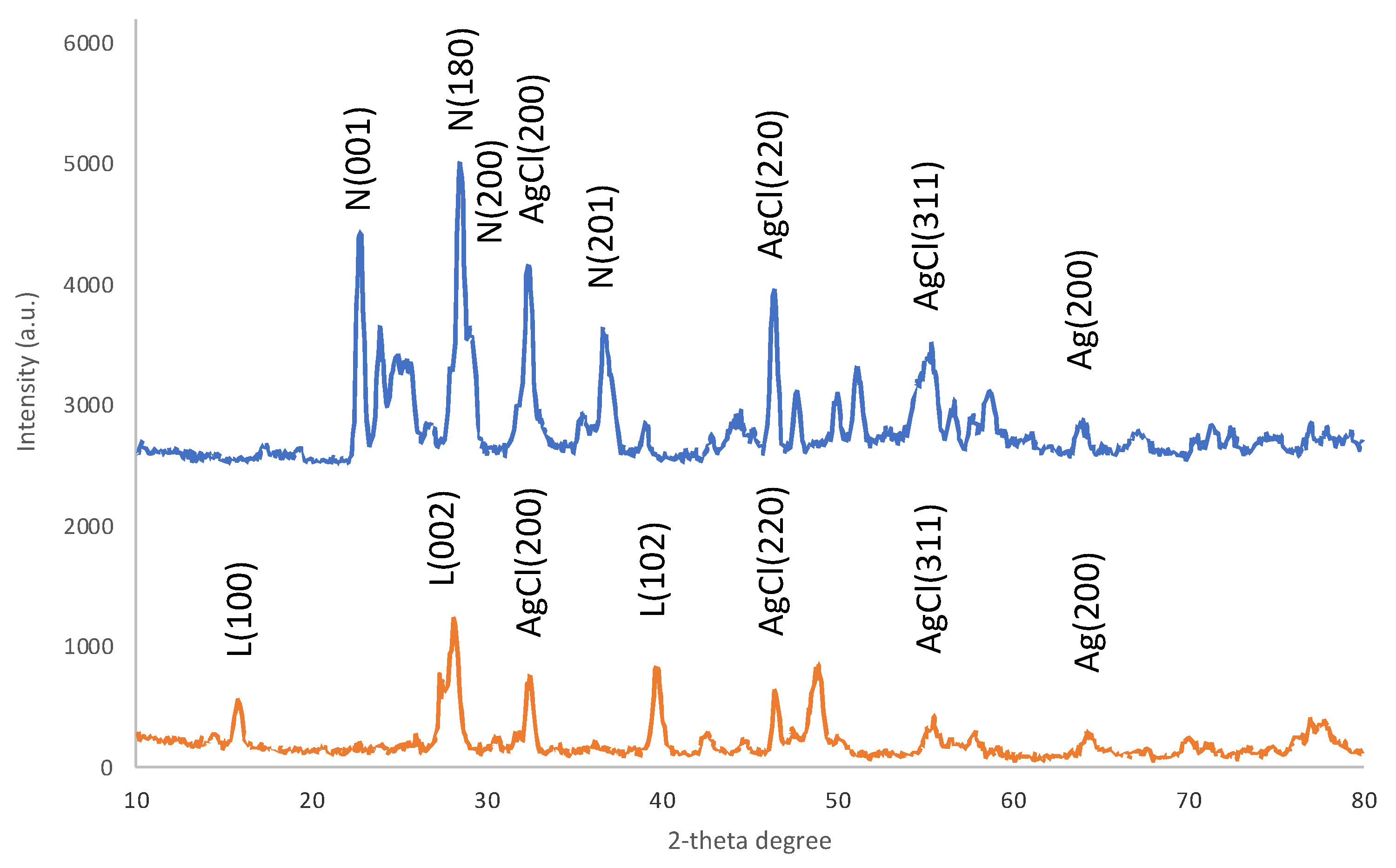
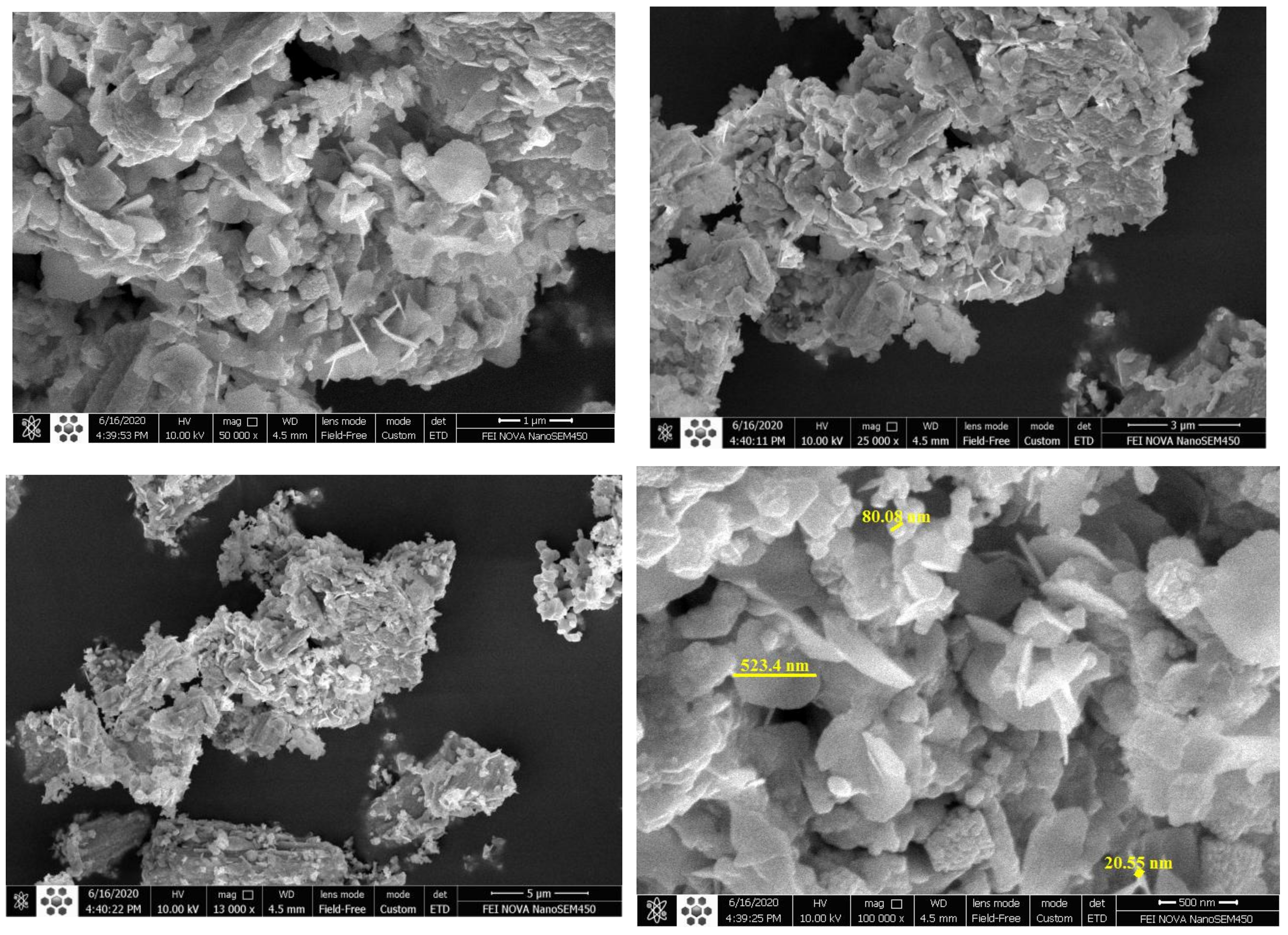
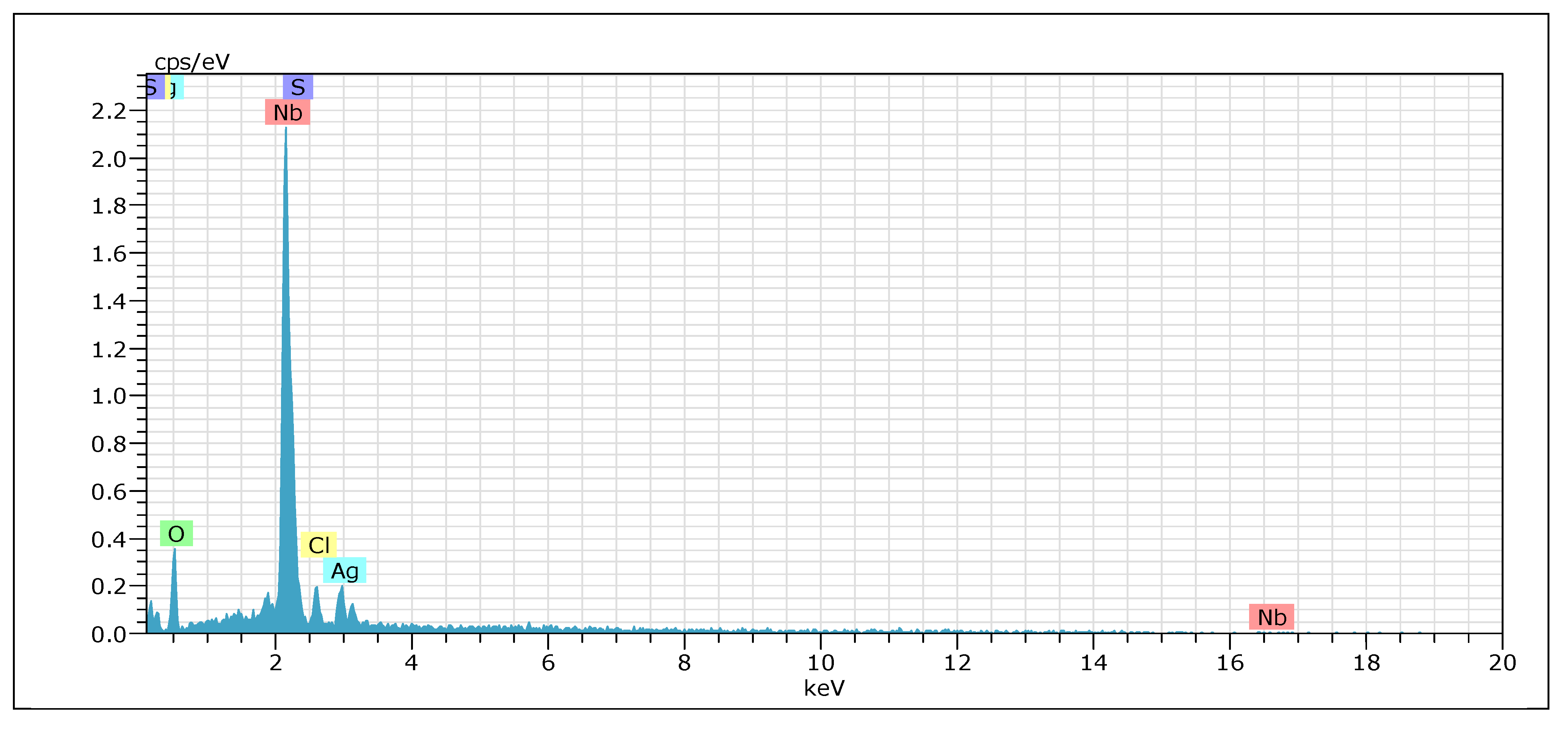
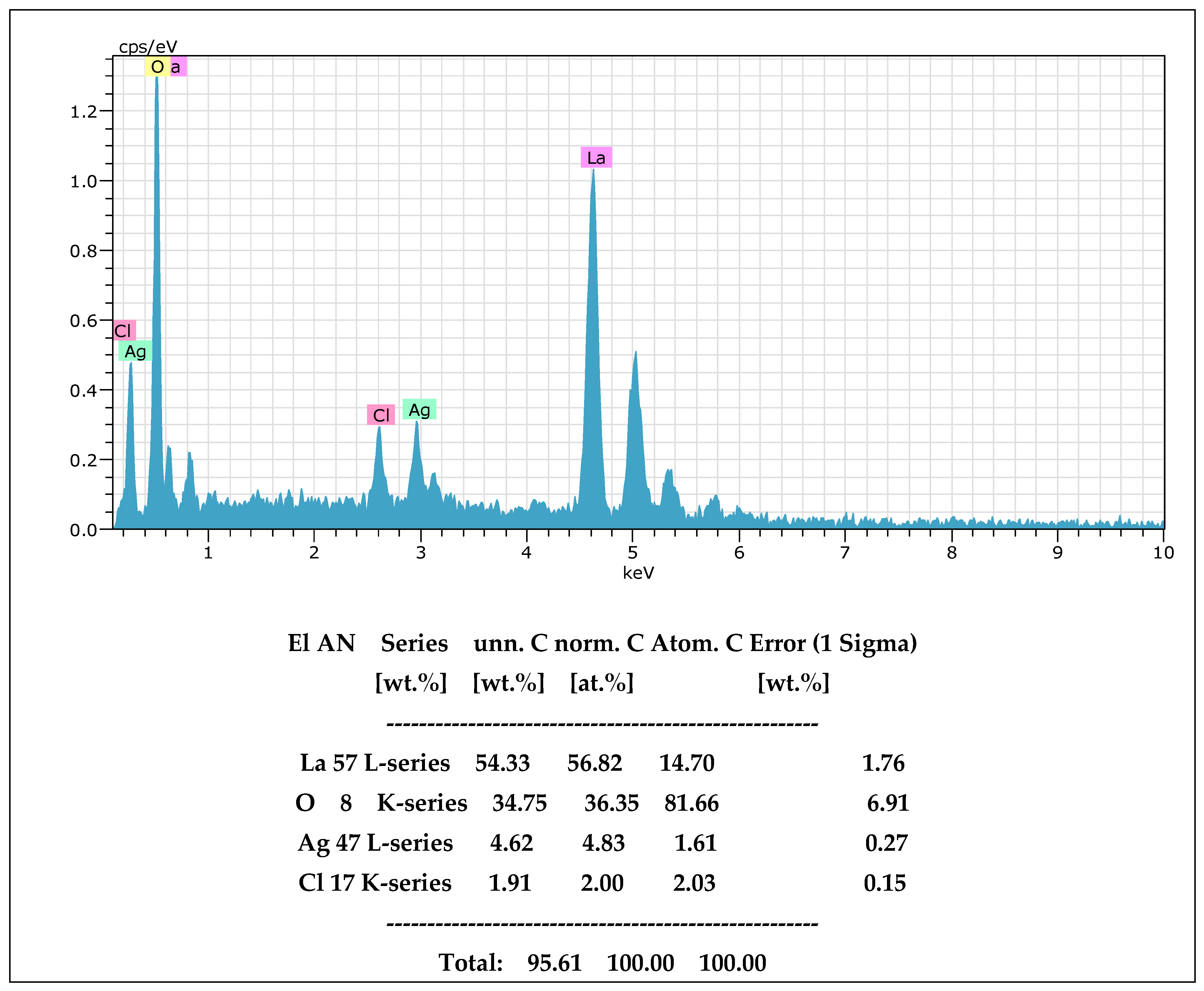
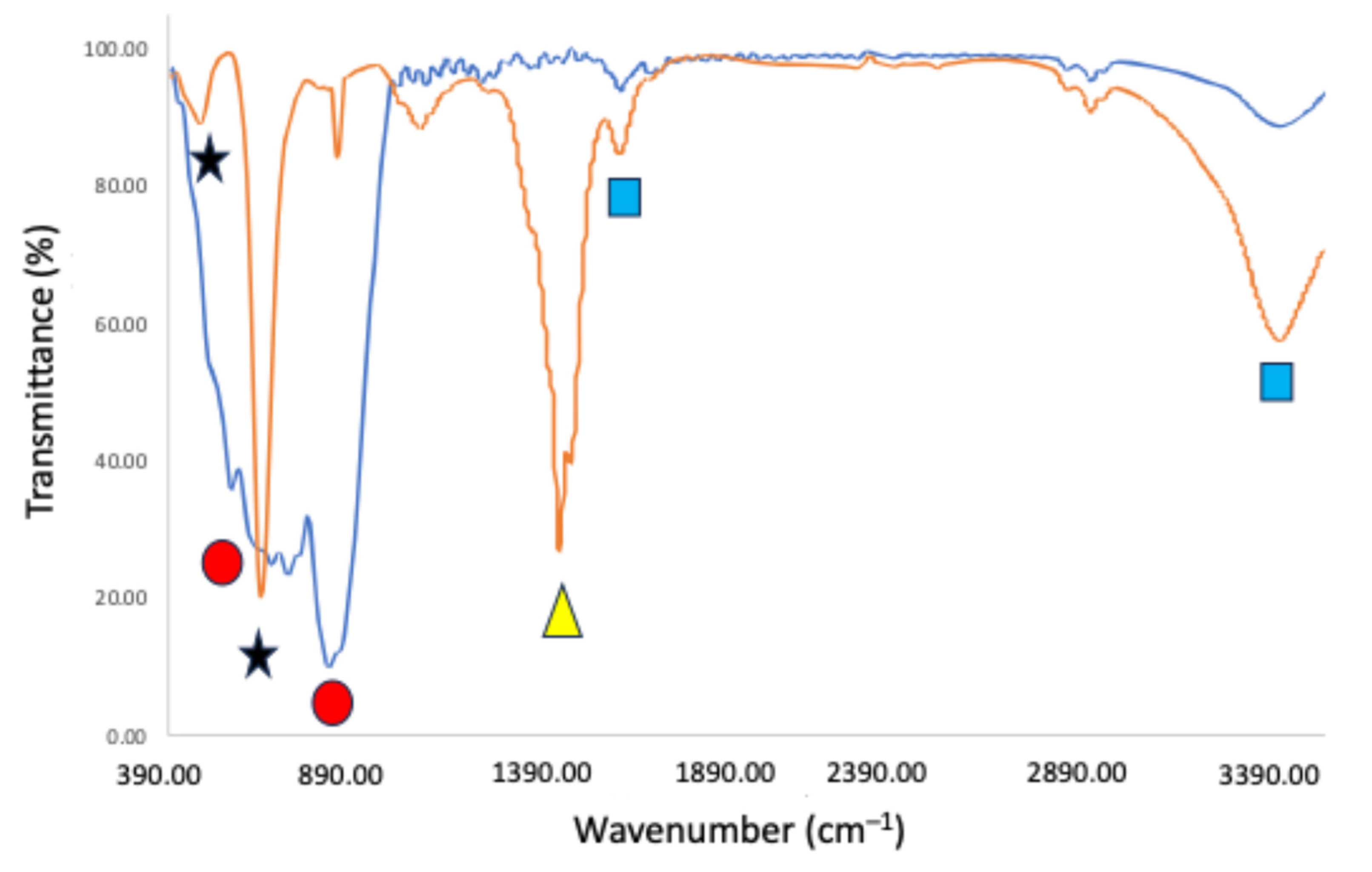
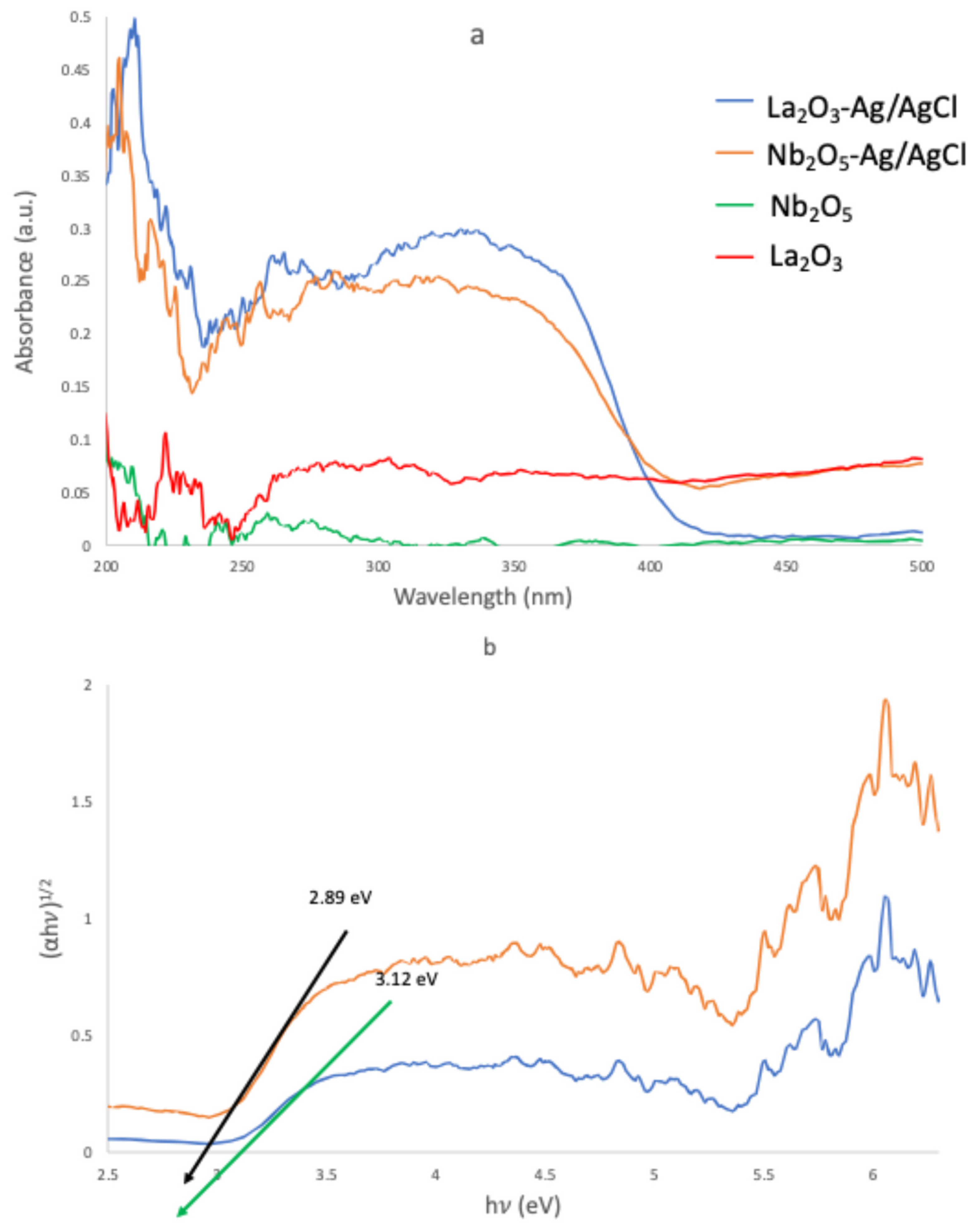
2.1. Characterization of the Materials
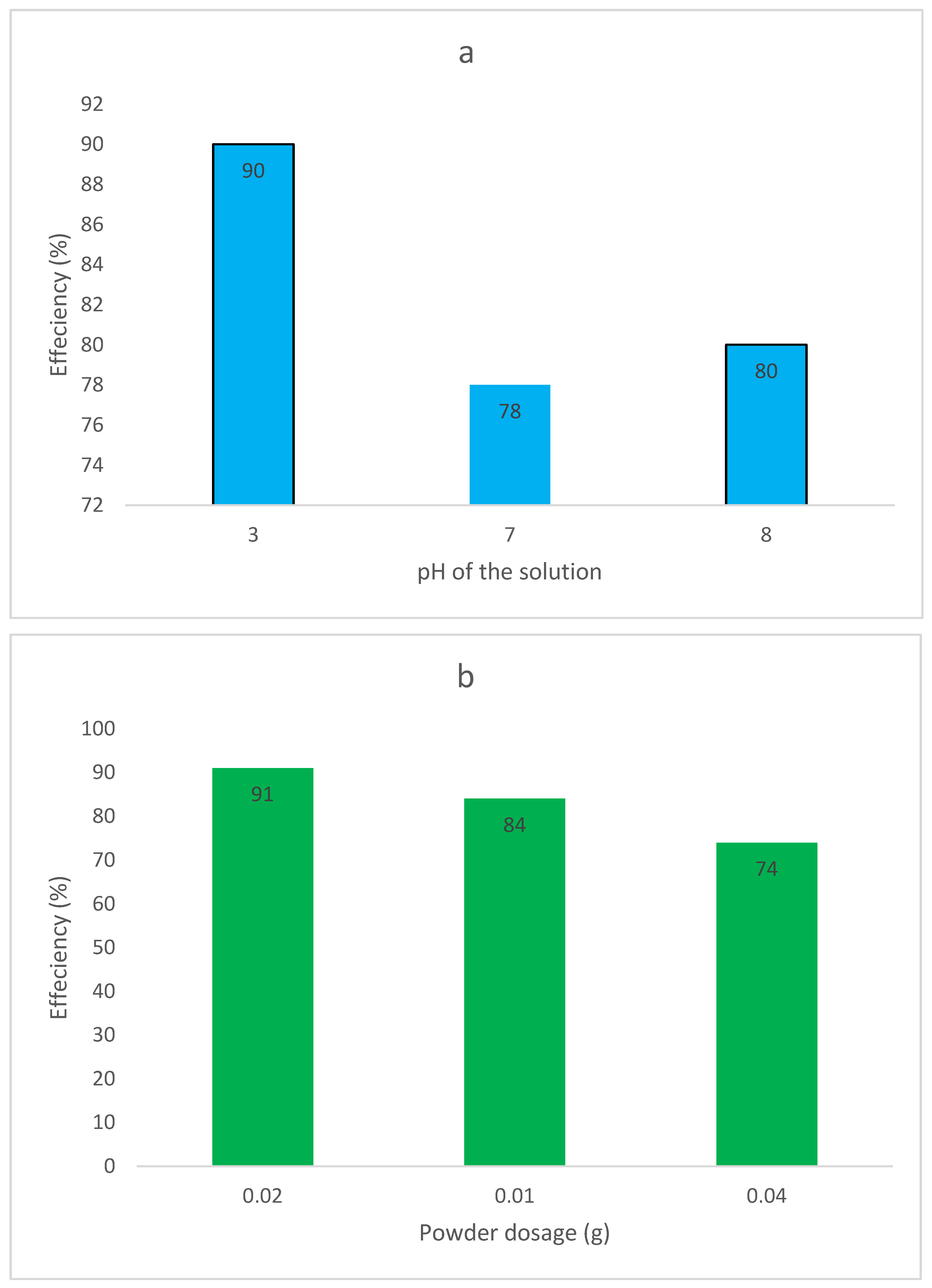
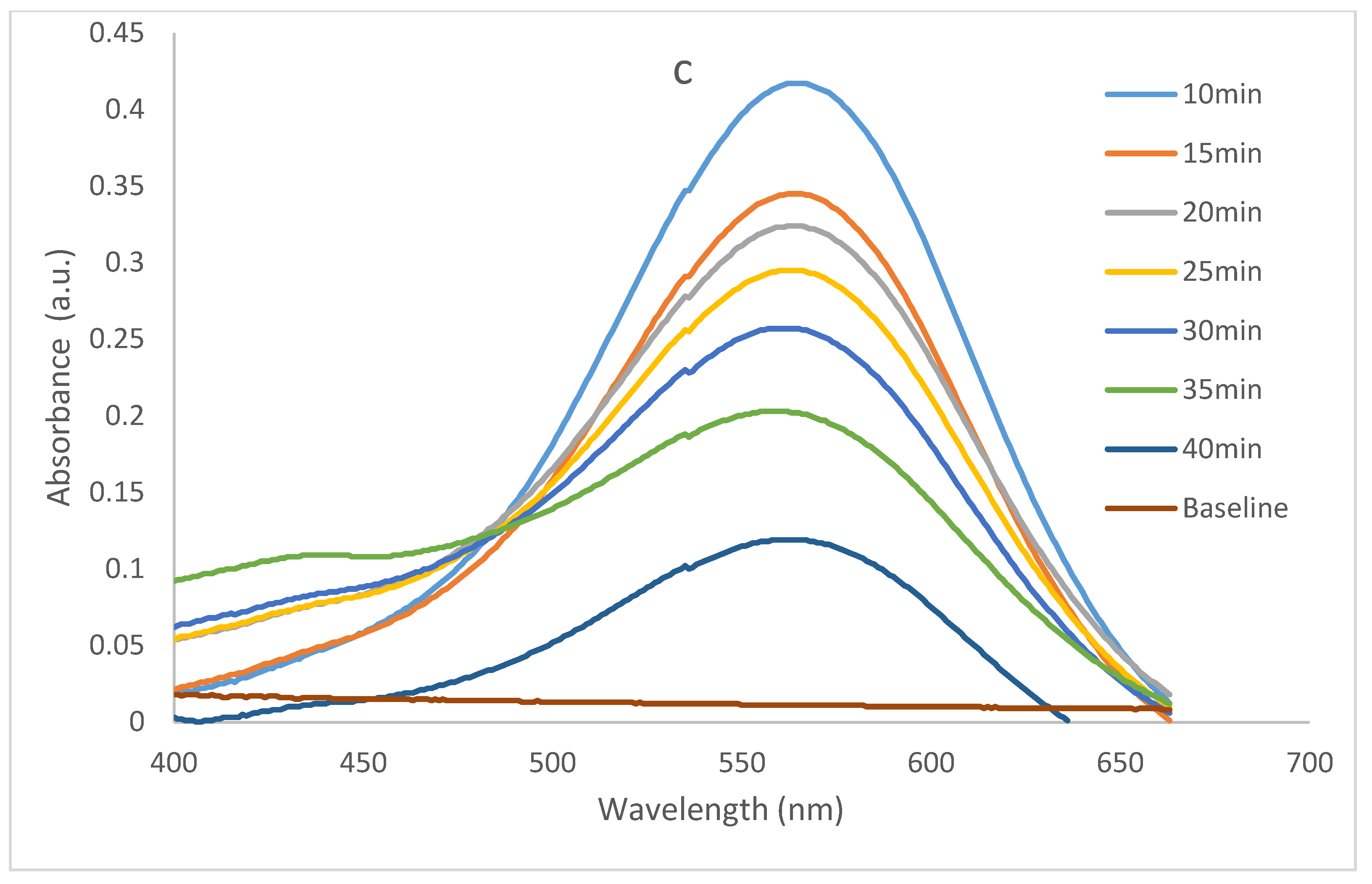
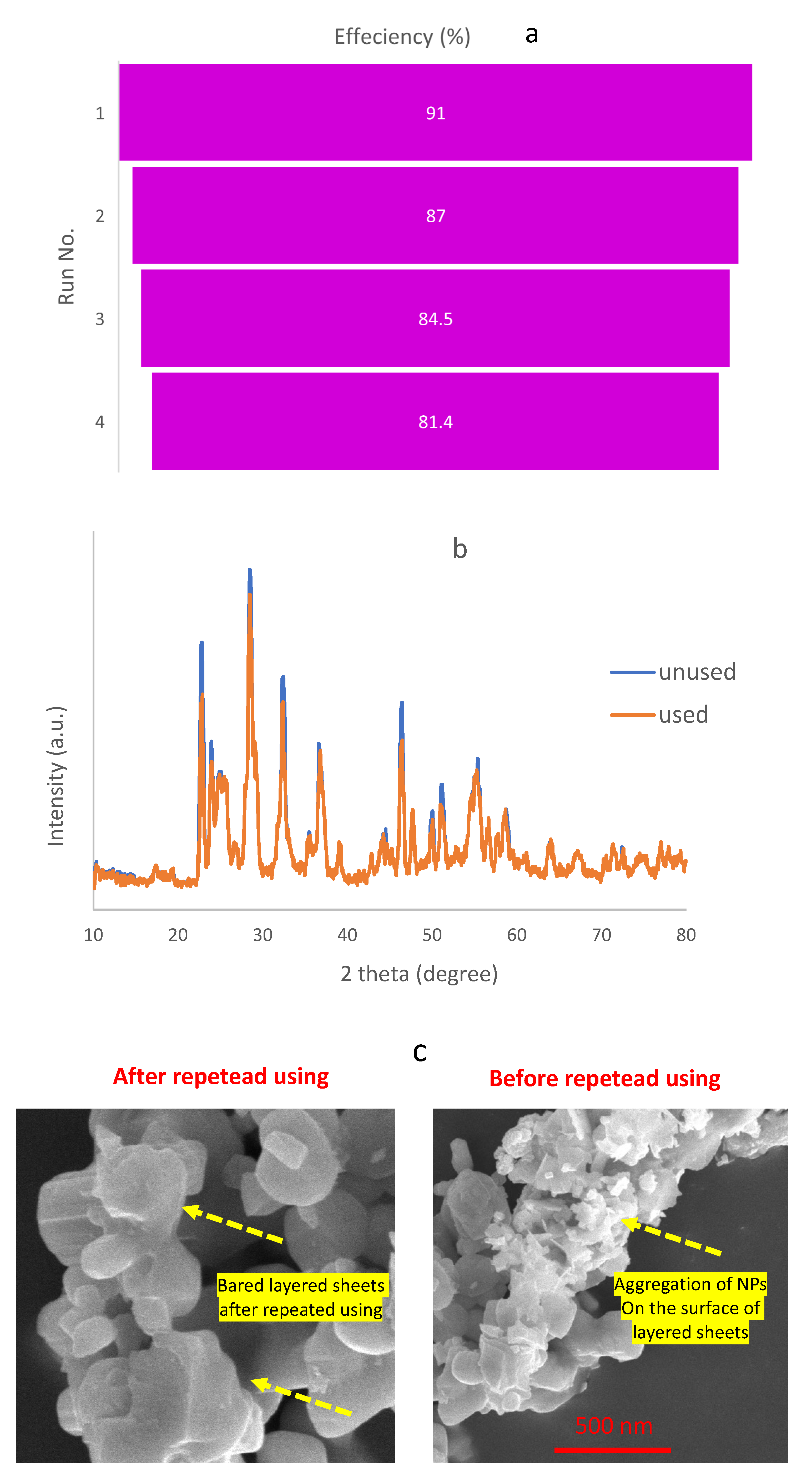
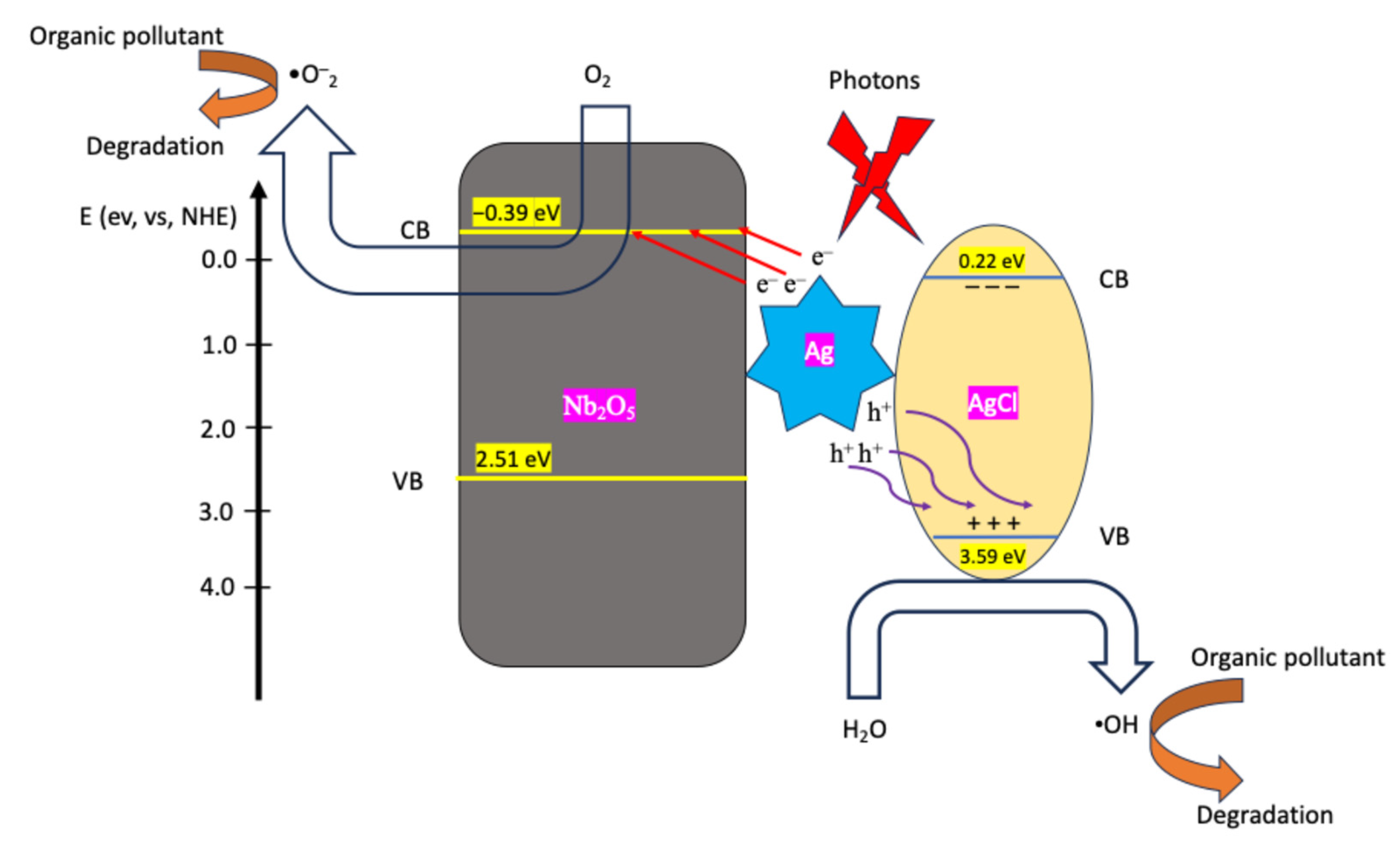
2.2. Photocatalytic Performance of the Constructions
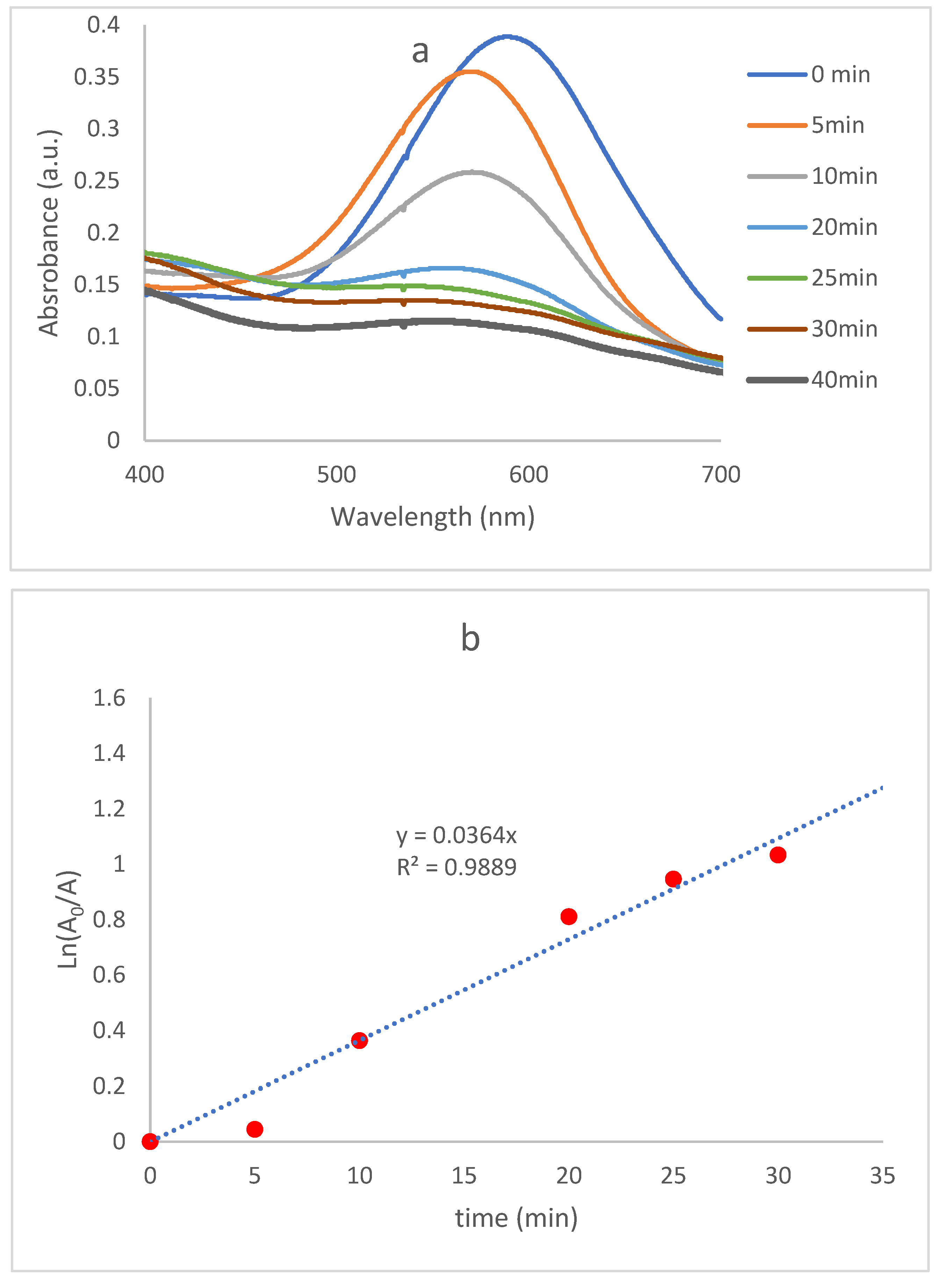
2.2.1. Dye Contaminant Removal
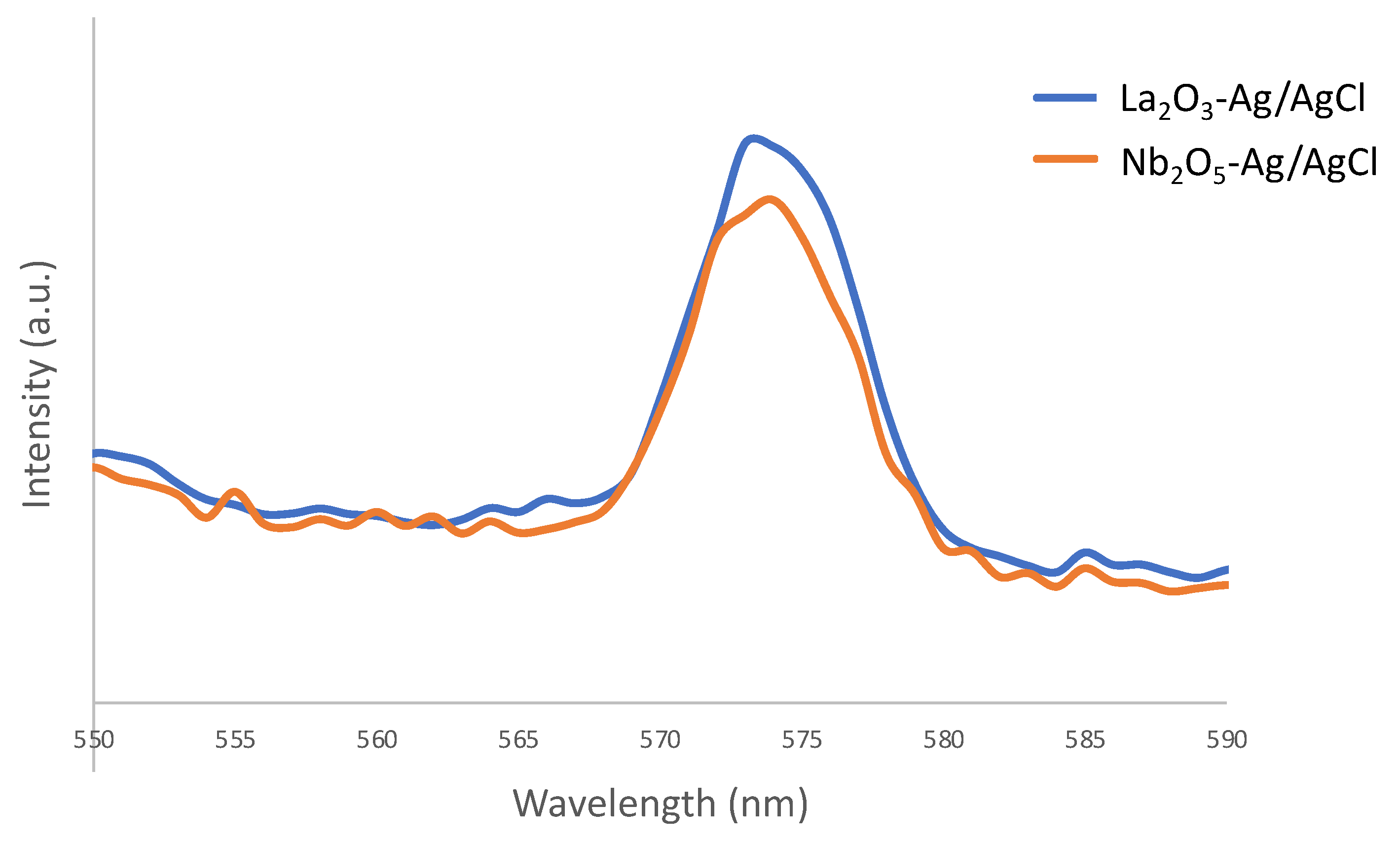
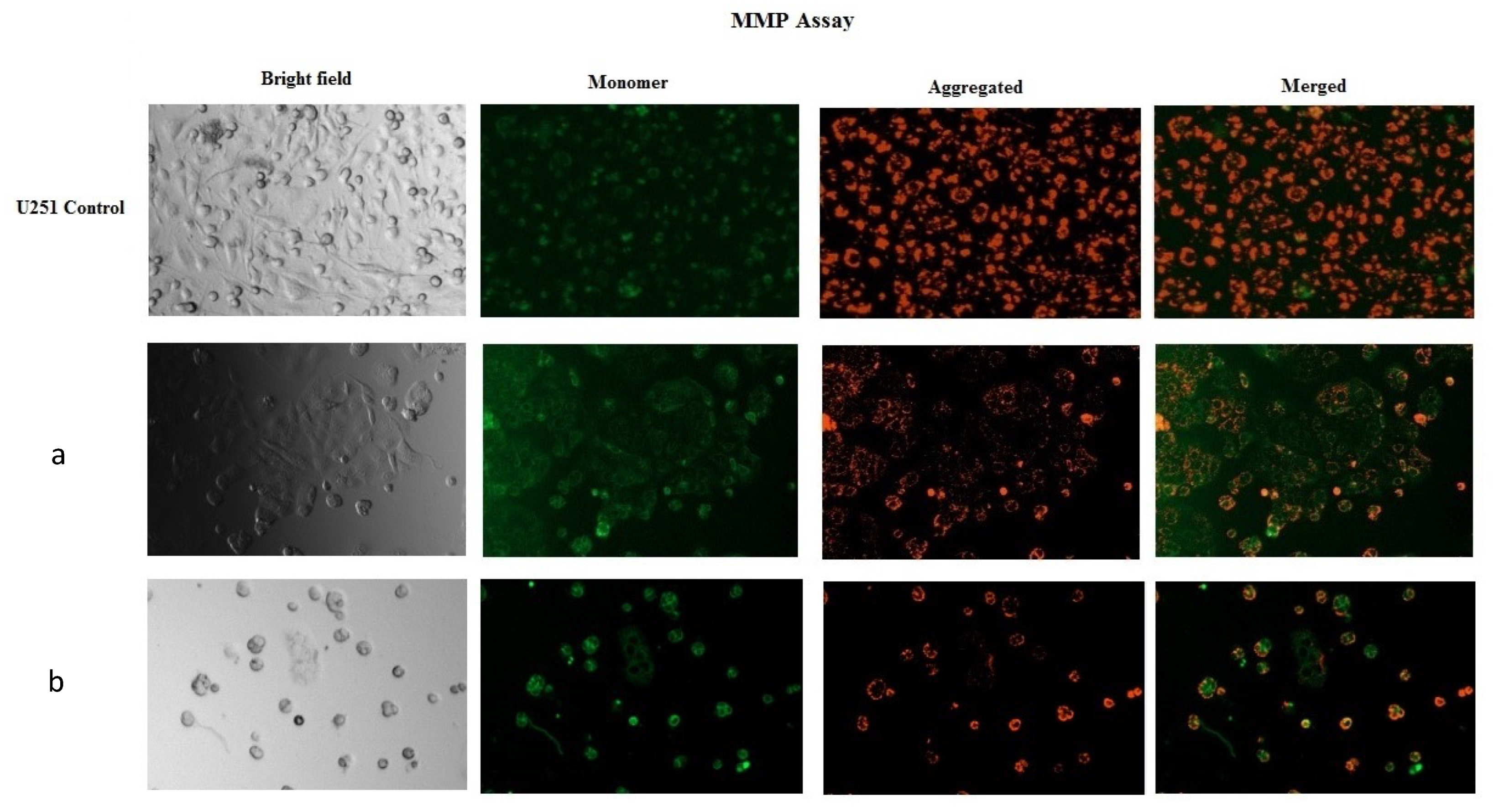
2.2.2. Anticancer Activity of the Materials
3. Materials and Methods
3.1. Chemical Reagents
3.2. Preparation of the Constructions
3.3. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tai, A.P.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Change 2014, 4, 817–821. [Google Scholar] [CrossRef]
- Vats, S.; Srivastava, S.; Maurya, N.; Saxena, S.; Mudgil, B.; Yadav, S.; Chandra, R. Advances in dye contamination: Health hazards, biodegradation, and bioremediation. In Biological Approaches to Controlling Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–162. [Google Scholar]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Ghany, N.A.A. Advanced electrochemical degradation of basic yellow 28 textile dye using IrO2/Ti meshed electrode in different supporting electrolytes. J. Electroanal. Chem. 2021, 882, 114979. [Google Scholar] [CrossRef]
- Perumal, V.; Inmozhi, C.; Uthrakumar, R.; Robert, R.; Chandrasekar, M.; Mohamed, S.B.; Honey, S.; Raja, A.; Al-Mekhlafi, F.A.; Kaviyarasu, K. Enhancing the photocatalytic performance of surface-Treated SnO2 hierarchical nanorods against methylene blue dye under solar irradiation and biological degradation. Environ. Res. 2022, 209, 112821. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W. Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Janik-Karpinska, E.; Brancaleoni, R.; Niemcewicz, M.; Wojtas, W.; Foco, M.; Podogrocki, M.; Bijak, M. Healthcare waste—A serious problem for global health. Healthcare 2023, 11, 242. [Google Scholar] [CrossRef]
- Kenny, C.; Priyadarshini, A. Review of current healthcare waste management methods and their effect on global health. Healthcare 2021, 9, 284. [Google Scholar] [CrossRef]
- Dawi, E.; Mustafa, E.; Padervand, M.; Ashames, A.; Hajiahmadi, S.; Saleem, L.; Baghernejad, M.; Nur, O.; Willander, M. Ag/AgCl decorated ionic liquid@ tantalum pentoxide nanostructures: Fabrication, photocatalytic activity, and cytotoxicity effects against human brain tumor cells. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2647–2660. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Wu, J. Construction of a Z-scheme heterojunction for high-efficiency visible-light-driven photocatalytic CO2 reduction. Nanoscale 2021, 13, 4359–4389. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, H.; Li, Z.; Liu, A.; Wang, E.; Wu, Y.; Tang, X.; Du, H.; Jin, L.; Zhu, H. Efficient photocatalytic degradation of antibiotics using Z-scheme MIL-88 (Fe)/Ti3C2/MoO3: Mechanistic insights and toxicity assessment. J. Hazard. Mater. 2025, 486, 137051. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, A. Effect of metal and non-metal doping on the photocatalytic performance of titanium dioxide (TiO2): A review. Curr. Nanosci. 2022, 18, 499–519. [Google Scholar] [CrossRef]
- Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Recent development in defects engineered photocatalysts: An overview of the experimental and theoretical strategies. Energy Environ. Mater. 2022, 5, 68–114. [Google Scholar] [CrossRef]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Chen, Y.; Qian, Y.; Xu, B.; Chu, W.; An, D. Recent progress of silver-containing photocatalysts for water disinfection under visible light irradiation: A review. Sci. Total Environ. 2022, 804, 150024. [Google Scholar] [CrossRef]
- Lee, P.-C.; Yang, Z.-R.; Kuo, C.-Y.; Shin, C.-H.; Lin, C.-B. Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 porous structure. J. Mater. Eng. Perform. 2023, 32, 7183–7194.s. [Google Scholar] [CrossRef]
- Han, Z.; Zhong, D.; Xu, Y.; Chang, H.; Dong, L.; Liu, Y. Ag nanofilm enhanced S-type Ag@ AgCl/tubular g-C3N4/Ti photoanode visible light response photocatalytic fuel cell. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133858. [Google Scholar] [CrossRef]
- Lei, D.; Xue, J.; Peng, X.; Li, S.; Bi, Q.; Tang, C.; Zhang, L.; Zhang, J. Effective photocatalytic removal of As (III) by ZnFe2O4/Ag/AgCl coupled peroxymonosulfate: Z-Scheme charge transfer and dual active sites. Appl. Surf. Sci. 2021, 567, 150860. [Google Scholar] [CrossRef]
- Mu, F.; Liu, C.; Xie, Y.; Zhou, S.; Dai, B.; Xia, D.; Huang, H.; Zhao, W.; Sun, C.; Kong, Y. Metal-organic framework-derived rodlike AgCl/Ag/In2O3: A plasmonic Z-scheme visible light photocatalyst. Chem. Eng. J. 2021, 415, 129010. [Google Scholar] [CrossRef]
- Zheng, J.; Tang, X.; Fan, C.; Deng, Y.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Luo, J.; Chen, Z. Facile synthesis of Ag@ AgCl/ZnAl-LDH sesame balls nanocomposites with enhanced photocatalytic performance for the degradation of neonicotinoid pesticides. Chem. Eng. J. 2022, 446, 136485. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Feng, X. Z-scheme flower-like Ag/AgCl/NiZnAl-LDH for high efficiency removal of Methylene Blue: Performance and mechanism insights. Mater. Sci. Semicond. Process. 2025, 185, 108900. [Google Scholar] [CrossRef]
- Hailili, R.; Li, Z. Synergizing Photocatalysis with Aurivillius-Phase Bi4Ti3O12: Current Insights and Emerging Trends. J. Mater. Chem. A 2025, 13, 16345–16381. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Chang, Z.; Chen, C.; Fan, F.; Lu, C. Synthesis of CaTiO3@ Ag@ ZnO and its photocatalytic degradation of Levofloxacin. Ceram. Int. 2025. [Google Scholar] [CrossRef]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Rhimi, B.; Nejad, Z.G.; Karima, S.; Shahsavari, Z.; Wang, C. Multifunctional Ag/AgCl/ZnTiO3 structures as highly efficient photocatalysts for the removal of nitrophenols, CO2 photoreduction, biomedical waste treatment, and bacteria inactivation. Appl. Catal. A Gen. 2022, 643, 118794. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Yan, Z.; Luo, J.; Du, B.; Zhan, J.; Liu, L.; Zhang, J. Double photoelectron-transfer mechanism in Ag− AgCl/WO3/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for trimethoprim degradation. J. Hazard. Mater. 2021, 403, 123964. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Huang, C.; Doong, R.-a.; Chen, C.-W.; Dong, C.-D. In-situ immobilization of Ag/AgCl on sulfurized g-C3N4 nanosheet for enhancing visible-light driven photocatalysis toward simultaneous oxidation of tetracycline and reduction of Cr (VI) in water. J. Environ. Chem. Eng. 2023, 11, 109453. [Google Scholar] [CrossRef]
- Yu, X.; Huang, J.; Zhao, J.; Liu, S.; Xiang, D.; Tang, Y.; Li, J.; Guo, Q.; Ma, X.; Zhao, J. Efficient visible light photocatalytic antibiotic elimination performance induced by nanostructured Ag/AgCl@ Ti3+-TiO2 mesocrystals. Chem. Eng. J. 2021, 403, 126359. [Google Scholar] [CrossRef]
- Cui, N.; Zada, A.; Song, J.; Yang, Y.; Liu, M.; Wang, Y.; Wu, Y.; Qi, K.; Selvaraj, R.; Liu, S.-y. Plasmon-induced ZnO-Ag/AgCl photocatalyst for degradation of tetracycline hydrochloride. Desalination Water Treat. 2022, 245, 247–254. [Google Scholar] [CrossRef]
- Cao, A.; Bai, X.; Yang, C.; Zhang, M. Sphere-rod-like Ag/AgCl@ Fe2O3 Z-scheme heterojunction as photocatalysts for efficient degradation of tetracycline under visible light irradiation. Chemosphere 2024, 346, 140674. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanamoorthy, K.; Manivannan, S. Ionic liquids assist synthesis of Ag/AgX (X = Cl, Br, & F)-decorated rGO for visible light photocatalytic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 8724–8733. [Google Scholar]
- AbuMousa, R.A.; Baig, U.; Gondal, M.A.; AlSalhi, M.S.; Alqahtani, F.Y.; Akhtar, S.; Aleanizy, F.S.; Dastageer, M.A. Photo-catalytic killing of HeLa cancer cells using facile synthesized pure and Ag loaded WO3 nanoparticles. Sci. Rep. 2018, 8, 15224. [Google Scholar] [CrossRef]
- Mohan, H.; Ramalingam, V.; Lim, J.-M.; Lee, S.-W.; Kim, J.; Lee, J.-H.; Park, Y.-J.; Seralathan, K.-K.; Oh, B.-T. E-waste based graphene oxide/V2O5/Pt ternary composite: Enhanced visible light driven photocatalyst for anti-microbial and anti-cancer activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125469. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kargozar, S.; Ghorbani, A.; Darroudi, M.; Keshavarz, M.; Baino, F.; Kim, H.-W. Biomedical waste management by using nanophotocatalysts: The need for new options. Materials 2020, 13, 3511. [Google Scholar] [CrossRef] [PubMed]
- Fooladi, S.; Nematollahi, M.H.; Iravani, S. Nanophotocatalysts in biomedicine: Cancer therapeutic, tissue engineering, biosensing, and drug delivery applications. Environ. Res. 2023, 231, 116287. [Google Scholar] [CrossRef]
- Graça, M.; Meireles, A.; Nico, C.; Valente, M. Nb2O5 nanosize powders prepared by sol–gel–Structure, morphology and dielectric properties. J. Alloys Compd. 2013, 553, 177–182. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef]
- Ha, H.; Payer, J. The effect of silver chloride formation on the kinetics of silver dissolution in chloride solution. Electrochim. Acta 2011, 56, 2781–2791. [Google Scholar] [CrossRef]
- Muhumuza, E.; Wu, P.; Nan, T.; Zhao, L.; Bai, P.; Mintova, S.; Yan, Z. Perovskite-type LaCoO3 as an efficient and green catalyst for sustainable partial oxidation of cyclohexane. Ind. Eng. Chem. Res. 2020, 59, 21322–21332. [Google Scholar] [CrossRef]
- Ismail, W.; Belal, A.; Abdo, W.; El-Shaer, A. Investigating the physical and electrical properties of La2O3 via annealing of La(OH)3. Sci. Rep. 2024, 14, 7716. [Google Scholar] [CrossRef] [PubMed]
- Ouzaouit, K.; Bakiz, B.; Villain, S.; Benlhachemi, A.; Essoumhi, A.; Benyaich, H.; Guinneton, F.; Gavarri, J.-R. Lanthanum hydroxycarbonates and langasite ceramics: Stability, infrared spectroscopy and electrical behaviors. In Proceedings of the Advanced Materials & Technologies, AMT 2007, XVIII Physical Metallurgy and Materials Science Conference, Warsaw, Poland, 18–21 June 2007. [Google Scholar]
- Nagaraju, P.; Vasudevan, R.; Alsalme, A.; Alghamdi, A.; Arivanandhan, M.; Jayavel, R. Surfactant-free synthesis of Nb2O5 nanoparticles anchored graphene nanocomposites with enhanced electrochemical performance for supercapacitor electrodes. Nanomaterials 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Khan, Z.S.; Ali, A.; Iqbal, N. Studies on solution processed Graphene-Nb2O5 nanocomposite based photoanode for dye-sensitized solar cells. J. Alloys Compd. 2017, 694, 401–407. [Google Scholar] [CrossRef]
- Heidarpour, H.; Padervand, M.; Soltanieh, M.; Vossoughi, M. Enhanced decolorization of rhodamine B solution through simultaneous photocatalysis and persulfate activation over Fe/C3N4 photocatalyst. Chem. Eng. Res. Des. 2020, 153, 709–720. [Google Scholar] [CrossRef]
- Fu, Y.; Chi, J.; Wu, Y.; Li, J.; Tan, M.; Li, C.; Du, H.; Hao, D.; Zhu, H.; Wang, Q. Synergistic electric fields induced by unilateral doping modulation for enhanced organic pollutant degradation and sterilization. Appl. Surf. Sci. 2025, 692, 162711. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Wu, Y.; Shi, Y.; Liu, Y.; Deng, H.; Chen, S.; Li, Z.; Wang, E.; Zhu, H. Dual S-scheme heterojunction via MOF-on-MOF strategy for efficient photoelectrocatalytic removal of organic contaminants: Detoxification and mechanism. J. Environ. Sci. 2025, 155, 111–126. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. In situ deposition of Ag/AgCl on the surface of magnetic metal-organic framework nanocomposite and its application for the visible-light photocatalytic degradation of Rhodamine dye. J. Hazard. Mater. 2019, 378, 120741. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Photocatalytic degradation and chlorination of azo dye in saline wastewater: Kinetics and AOX formation. Chem. Eng. J. 2012, 192, 171–178. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Han, Q.; Guo, P.; Zhu, J.; Yin, R. Photo-assisted Ag/AgCl nanoparticle formation process can be used in the degradation of fluorescent dyes. Inorg. Chem. Commun. 2020, 112, 107716. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Y.; Dai, M.; Gong, Q.; Dang, Z. Ag/AgCl/MIL-101 (Fe) catalyzed degradation of methylene blue under visible light irradation. Materials 2019, 12, 1453. [Google Scholar] [CrossRef]
- Heidarpour, H.; Golizadeh, M.; Padervand, M.; Karimi, A.; Vossoughi, M.; Tavakoli, M.H. In-situ formation and entrapment of Ag/AgCl photocatalyst inside cross-linked carboxymethyl cellulose beads: A novel photoactive hydrogel for visible-light-induced photocatalysis. J. Photochem. Photobiol. A Chem. 2020, 398, 112559. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, X.; Cheng, Z.; Liao, Y.; Wang, Z.; Pu, Q.; Duan, M. Synthesis of Pp-16@ Ag/AgCl of high performance photocatalyst particles for decomposition of Rhodamine B and fast green dyes. Mater. Chem. Phys. 2018, 218, 98–107. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Abdi, V.; Sourinejad, I. Green fabrication of Ag/AgCl@ TiO2 superior plasmonic nanocomposite: Biosynthesis, characterization and photocatalytic activity under sunlight. J. Alloys Compd. 2020, 841, 155593. [Google Scholar] [CrossRef]
- Ahmad, A.; e Noor, A.; Anwar, A.; Majeed, S.; Khan, S.; Nisa, Z.U.; Ali, S.; Gnanasekaran, L.; Rajendran, S.; Li, H. Support based metal incorporated layered nanomaterials for photocatalytic degradation of organic pollutants. Environ. Res. 2024, 260, 119481. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, P.; Lei, Y.; Chen, F.; Yu, Z.; Fang, P.; Liu, Y. Sun-light-driven plasmonic Ag/AgCl@ TNT photocatalysts for high-efficient absorption-regeneration and photocatalytic degradation. Appl. Surf. Sci. 2020, 529, 147010. [Google Scholar] [CrossRef]
- Yarangsee, C.; Narakaew, S.; Utara, S.; Thungprasert, S.; Promanan, T.; Chaisena, A. Ag/AgCl-NW/rGO composite for high-efficiency visible-light-driven photocatalytic activity of rhodamine B. Environ. Sci. Pollut. Res. 2025, 32, 6658–6677. [Google Scholar] [CrossRef]
- Al-Marri, A.H.; Moulahi, A.; Mogharbel, A.T.; Al-Mohaimeed, A.M.; Janene, F.; Ouda, A.S.; Al-Farraj, E.S.; Almaslamani, M.N.; Almaslamani, M.A.; Mjejri, I. Photocatalytic performance of Nb2O5-graphene heterojunction for the degradation of methylene blue. Polyhedron 2024, 260, 117080. [Google Scholar] [CrossRef]
- Osman, N.S.; Sulaiman, S.N.; Muhamad, E.N.; Mukhair, H.; Tan, S.T.; Abdullah, A.H. Synthesis of an Ag3PO4/Nb2O5 photocatalyst for the degradation of dye. Catalysts 2021, 11, 458. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Almeida, S.R.; Moreira, E.C.; Ferrer, M.M.; Jardim, P.L.; Moreira, M.L.; Raubach, C.W.; Cava, S. Photocatalytic degradation of rhodamine B using Nb2O5 synthesized with different niobium precursors: Factorial design of experiments. Ceram. Int. 2021, 47, 20570–20578. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Han, P. Electrospinning preparation of g-C3N4/Nb2O5 nanofibers heterojunction for enhanced photocatalytic degradation of organic pollutants in water. Sci. Rep. 2021, 11, 22950. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.; Ren, E.; Xiao, H.; Lai, X.; Qin, Q.; Jiang, S.; Shen, H.; Zhou, M.; Qin, W. Facile hydrothermal synthesis of rod-like Nb2O5/Nb2CTx composites for visible-light driven photocatalytic degradation of organic pollutants. Environ. Res. 2021, 193, 110587. [Google Scholar] [CrossRef]
- Zulkiflee, A.; Khan, M.M.; Khan, M.Y.; Khan, A.; Harunsani, M.H. Nb2O5/BiOCl composite as a visible-light-active photocatalyst for the removal of RhB dye and photoelectrochemical studies. J. Photochem. Photobiol. A Chem. 2024, 446, 115177. [Google Scholar] [CrossRef]
- Ganesh, V.; AlAbdulaal, T.H.; AlShadidi, M.; Hussien, M.S.; Bouzidi, A.; Algarni, H.; Zahran, H.Y.; Abdel-Wahab, M.S.; Mohammed, M.I.; Yahia, I.S. Enhancement in the structural, electrical, optical, and photocatalytic properties of La2O3-doped ZnO nanostructures. Materials 2022, 15, 6866. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Ambikapathi, N.; Inbaraj, P.; Jing, Q.; Liu, B. Harvesting high-performance electro-water oxidation and selective MB degradation through dual functional Gd2O3–La2O3 photo-electrocatalysts. Mater. Today Sustain. 2024, 27, 100947. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Dhanakodi, K.; Surendhiran, S.; Vanasundari, K.; Arunraja, L.; Rajamanickam, A. Unveiling the photocatalytic property of La2O3–CuO nanocomposites for organic pollutants in wastewater treatment. J. Indian Chem. Soc. 2023, 100, 101104. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Al-Saeedi, S.I.; Al-Senani, G.M.; Nafady, A.; Ahamad, T.; Naushad, M.; Stadler, F.J. Fabrication of oxidized graphite supported La2O3/ZrO2 nanocomposite for the photoremediation of toxic fast green dye. J. Mol. Liq. 2019, 277, 738–748. [Google Scholar] [CrossRef]
- Kumar, M.; Rahman, A. Facile synthesis, characterization, and photocatalytic study of La2O3/SnO2 nanocomposites. J. Inst. Eng. Ser. E 2023, 104, 95–108. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.; Sun, C.; Ling, Y.; Li, S.; Li, X.; Zhao, J.; Yang, K. La2O3-modified graphite carbon nitride achieving the enhanced photocatalytic degradation of different organic pollutants under visible light irradiation. Mater. Chem. Phys. 2020, 246, 122846. [Google Scholar] [CrossRef]
- Padervand, M.; Nasiri, F.; Hajiahmadi, S.; Bargahi, A.; Esmaeili, S.; Amini, M.; Nami, R.K.; Shahsavari, Z.; Karima, S. Ag@ Ag2MoO4 decorated polyoxomolybdate/C3N4 nanostructures as highly efficient photocatalysts for the wastewater treatment and cancer cells killing under visible light. Inorg. Chem. Commun. 2022, 141, 109500. [Google Scholar] [CrossRef]
- Dawi, E.; Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Kakaei, K.; Shahsavari, Z.; Karima, S.; Baghernejad, M.; Signoretto, M.; Ibupoto, Z. Multi-functional fluorinated NiTiO3 perovskites for CO2 photocatalytic reduction, electrocatalytic water splitting, and biomedical waste management. J. Water Process Eng. 2023, 54, 103979. [Google Scholar] [CrossRef]
- Li, X.-f.; Zhou, Y.-t.; Feng, X.-q. Superior photocatalytic activity and antibacterial behavior of chitosan-anchored Ag/AgCl composites. Water Air Soil Pollut. 2024, 235, 103. [Google Scholar] [CrossRef]
- Ferreira, V.; Eugenio, M.; Del Nery, E.; De Souza, W.; Sant’Anna, C. Quantitative Characterization of the Effect of Biogenic silver-based Nanoparticles on Breast Cancer Cells by High Content Analysis. Curr. Nanomater. 2024, 9, 355–366. [Google Scholar] [CrossRef]
- Chankaew, C.; Somsri, S.; Tapala, W.; Mahatheeranont, S.; Saenjum, C.; Rujiwatra, A. Kaffir lime leaf extract mediated synthesis, anticancer activities and antibacterial kinetics of Ag and Ag/AgCl nanoparticles. Particuology 2018, 40, 160–168. [Google Scholar] [CrossRef]
- Alishah, H.; Pourseyedi, S.; Mahani, S.E.; Ebrahimipour, S.Y. Extract-mediated synthesis of Ag@ AgCl nanoparticles using Conium maculatum seeds: Characterization, antibacterial activity and cytotoxicity effect against MCF-7 cell line. RSC Adv. 2016, 6, 73197–73202. [Google Scholar] [CrossRef]
- Selvamani, M.; Krishnamoorthy, G.; Ramadoss, M.; Sivakumar, P.K.; Settu, M.; Ranganathan, S.; Vengidusamy, N. Ag@ Ag8W4O16 nanoroasted rice beads with photocatalytic, antibacterial and anticancer activity. Mater. Sci. Eng. C 2016, 60, 109–118. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Sadras, S.R. Green synthesized nano silver: Synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J. Colloid Interface Sci. 2017, 499, 33–45. [Google Scholar] [CrossRef]
- Padervand, M.; Lammel, G.; Bargahi, A.; Mohammad-Shiri, H. Photochemical degradation of the environmental pollutants over the worm-like Nd2CuO4-Nd2O3 nanostructures. Nano-Struct. Nano-Objects 2019, 18, 100258. [Google Scholar] [CrossRef]




| Structure | Pollutant | Irradiation Source | Efficiency (%)/Time (Min) | Ref. |
|---|---|---|---|---|
| Nb2O5/rGO | Methylene blue | Solar irradiation | 99.5/60 | [60] |
| Ag3PO4/Nb2O5 | Methyl orange | Fluorescence lamp (23 W) | 96/60 | [61] |
| Nb2O5 | Rhodamine B | UVC illumination | 98.99/60 | [62] |
| g-C3N4/Nb2O5 | Rhodamine B | 300 W xenon lamp | 98.1/120 | [63] |
| Nb2O5/Nb2CTx | Rhodamine B | Visible light | 91.2/120 | [64] |
| Nb2O5/BiOCl | Rhodamine B | Visible light | 96.7/120 | [65] |
| La2O3–ZnO | Methylene blue | Visible light | 99/120 | [66] |
| Gd2O3–La2O3 | Methylene blue | UV light | 84.8/120 | [67] |
| La2O3–CuO | Crystal violet | Sunlight irradiation | 97.05/135 | [68] |
| OG/La2O3/ZrO2 | Fast green dye | Visible light irradiation | 89/90 | [69] |
| La2O3/SnO2 | Methylene blue | UV light | 83/70 | [70] |
| La2O3/C3N4 | Methyl orange | Visible light (λ > 420 nm) | 100/120 | [71] |
| Nb2O5–Ag/AgCl | Acid blue 92 | 125 W mercury visible lamp | >99/35 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawi, E.; Padervand, M. Ag/AgCl-Decorated Layered Lanthanum/Niobium Oxide Microparticles as Efficient Photocatalysts for Azo Dye Remediation and Cancer Cell Inactivation. Catalysts 2025, 15, 638. https://doi.org/10.3390/catal15070638
Dawi E, Padervand M. Ag/AgCl-Decorated Layered Lanthanum/Niobium Oxide Microparticles as Efficient Photocatalysts for Azo Dye Remediation and Cancer Cell Inactivation. Catalysts. 2025; 15(7):638. https://doi.org/10.3390/catal15070638
Chicago/Turabian StyleDawi, Elmuez, and Mohsen Padervand. 2025. "Ag/AgCl-Decorated Layered Lanthanum/Niobium Oxide Microparticles as Efficient Photocatalysts for Azo Dye Remediation and Cancer Cell Inactivation" Catalysts 15, no. 7: 638. https://doi.org/10.3390/catal15070638
APA StyleDawi, E., & Padervand, M. (2025). Ag/AgCl-Decorated Layered Lanthanum/Niobium Oxide Microparticles as Efficient Photocatalysts for Azo Dye Remediation and Cancer Cell Inactivation. Catalysts, 15(7), 638. https://doi.org/10.3390/catal15070638






