A Scoring Model for Catalyst Informatics Based on Real-Time High-Throughput Fluorogenic Assay for Catalyst Discovery and Kinetic Profiling
Abstract
1. Introduction
2. Methodology
2.1. Assay Preparation for Data Collection
2.2. Well Plate Set-Up
2.3. Initial Processing of Spectroscopic Data
2.4. Parameter Database
- Material abundance was evaluated based on the available supply of the material. The information was adapted from the American Chemical Society—“the periodic table’s endangered elements” [27];
- The affordability of the material was evaluated based on the material price per gram, which was obtained from Sigma Aldrich on 10 September 2021 (St. Louis, MO, USA), or estimated for material prepared in our laboratories. This estimate was usually four times the cost of precursors;
- Recoverability is the first step towards re-useability. Recoverability was ranked based on the material’s solubility in the reaction solvent (H2O), with heterogenous catalysts scoring higher on our scale, and unrecoverable soluble catalysts scoring low;
- Safety was measured as the inverse of each material’s relative hazard score. The National Fire Protection Association (NFPA) evaluates materials’ hazard levels based on health, flammability, and instability. Each hazard factor was ranked from 0 to 4 based on severity, where a higher ranking indicated a greater hazard level. Overall, the hazard levels were added up and the inverse value was used to guide the level of relative safety.
2.5. Materials Tested
3. Results and Discussion
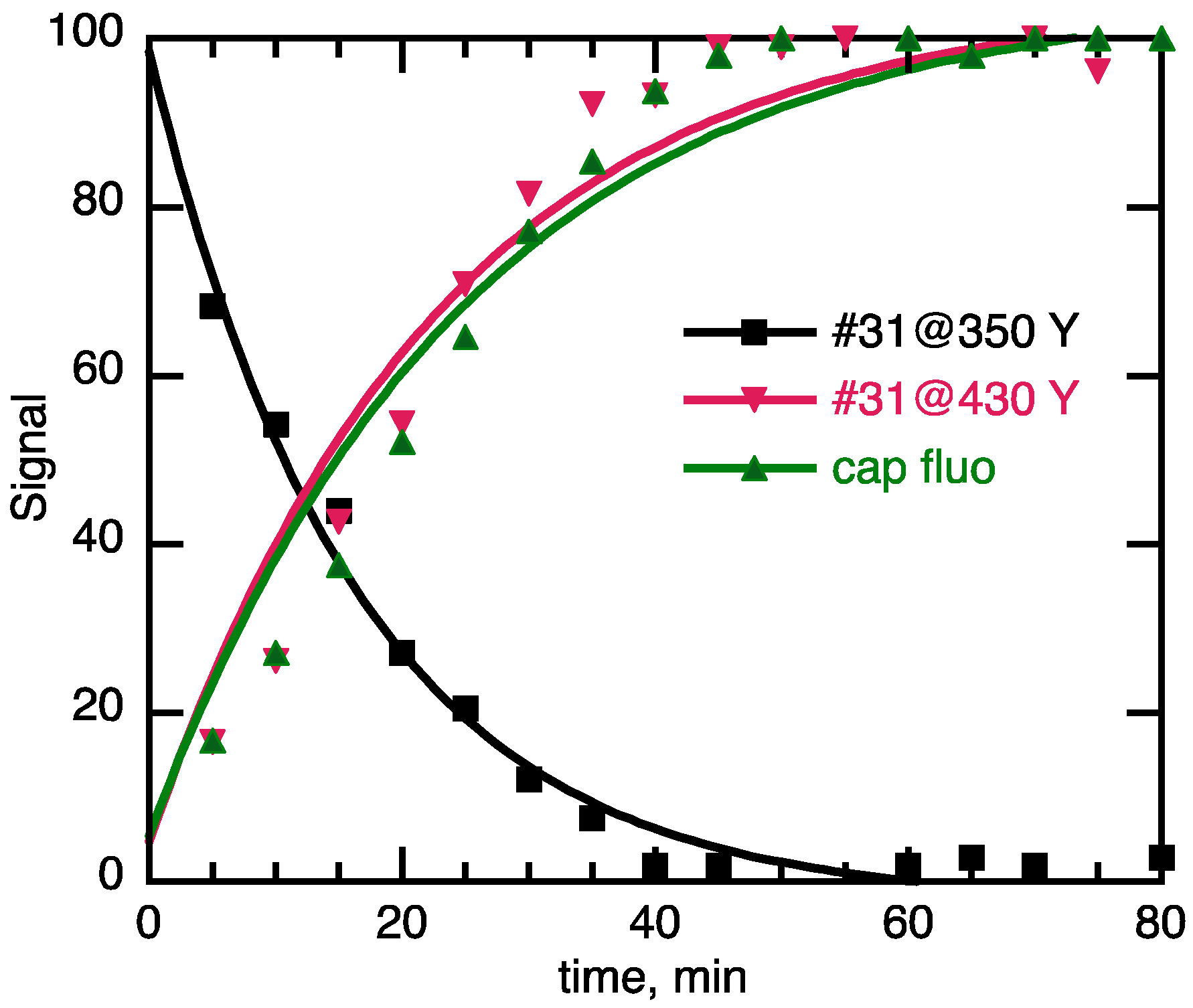
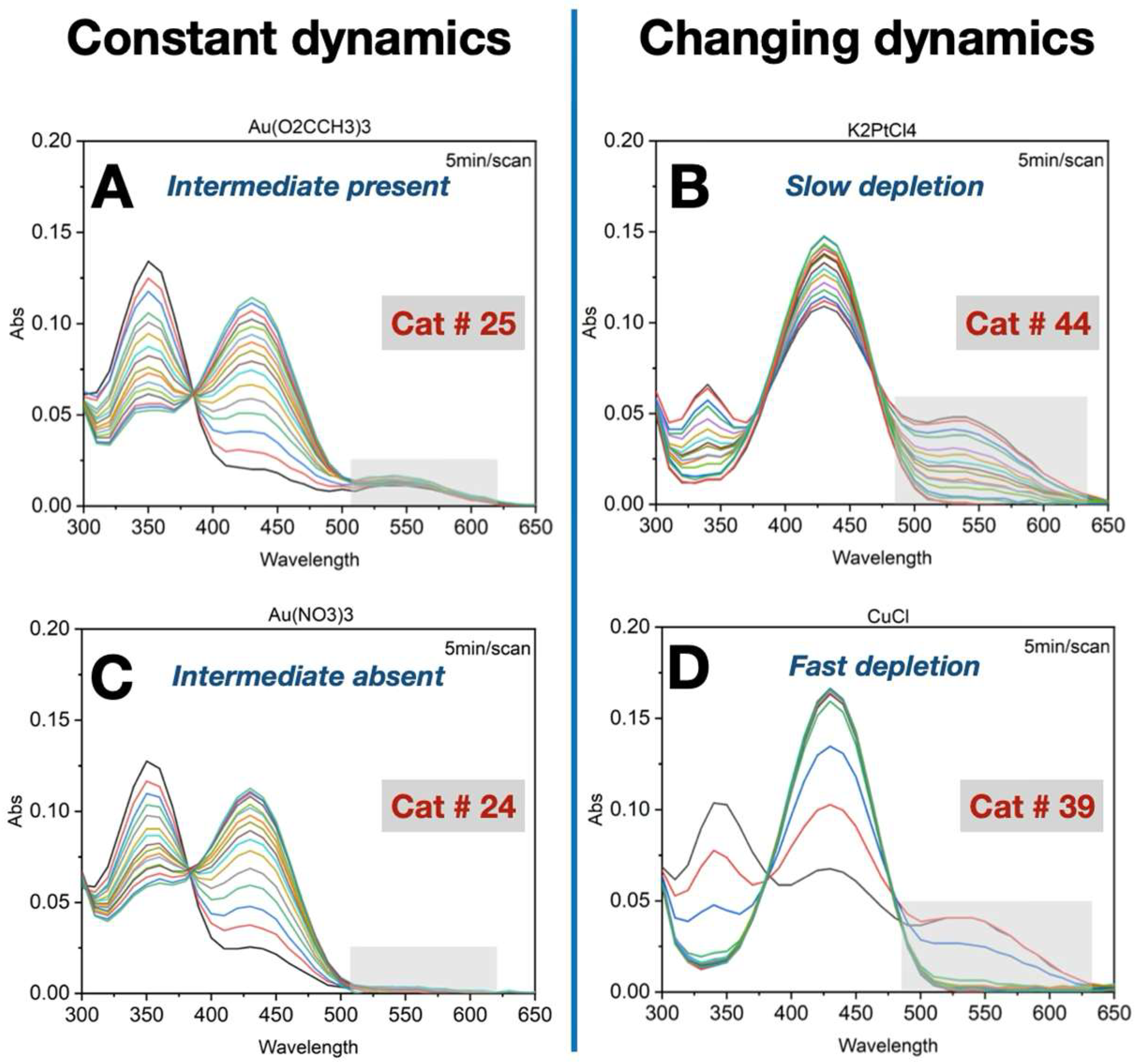
3.1. Importance of Time-Course Analysis in Catalyst Discovery
- A constant amount of intermediate was maintained throughout the reaction.
- In some cases, the intermediate was undetectable.
- A significant accumulation of the intermediate occurred at the beginning of the reaction, followed by a slow depletion rate.
- In other cases, the intermediate also accumulated early, but was followed by a rapid depletion rate.
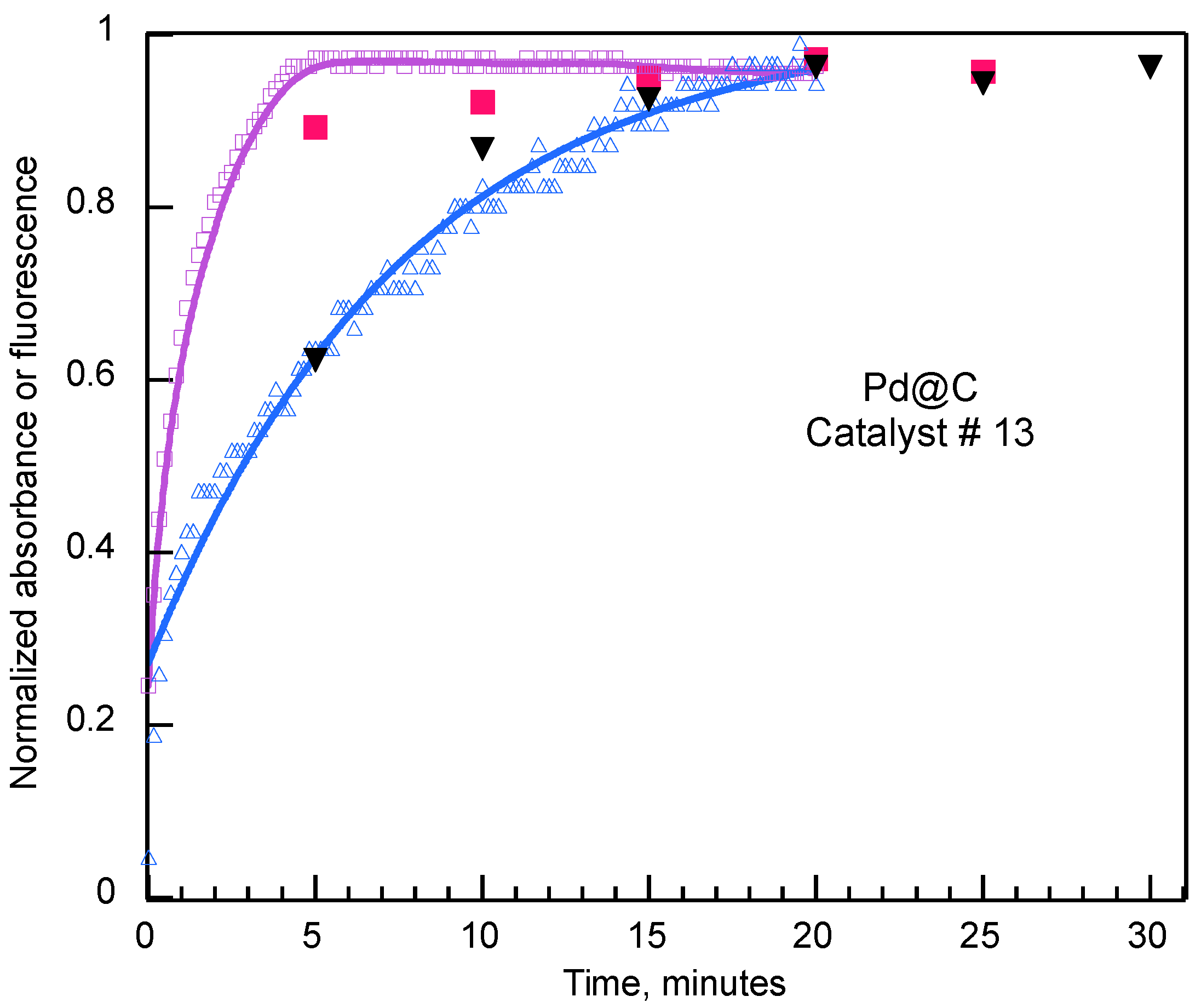
3.2. Fast Kinetics
3.3. Kinetic Models for Heterogeneous Catalysts
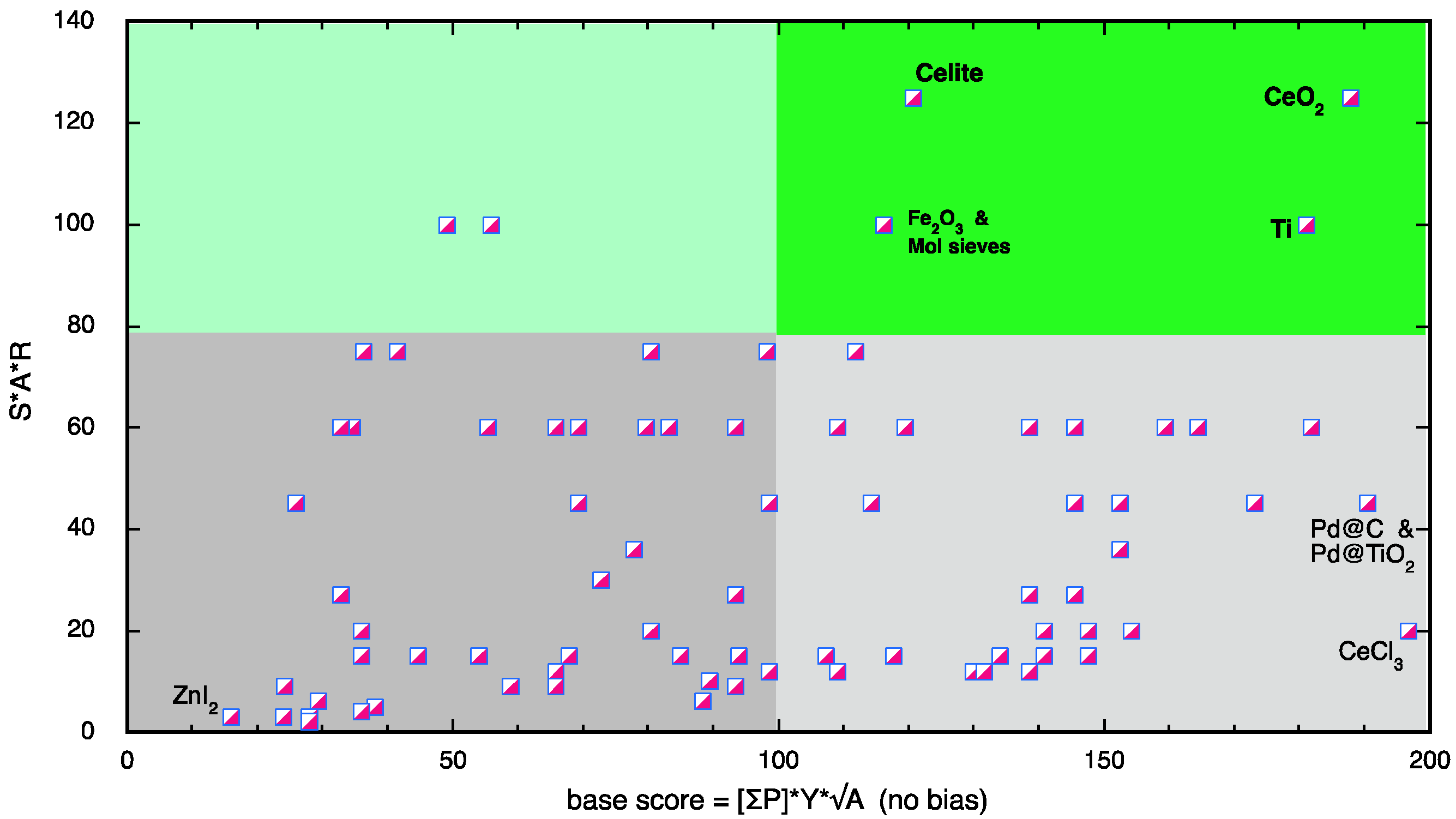
3.4. Scoring System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trunschke, A. Prospects and challenges for autonomous catalyst discovery viewed from an experimental perspective. Catal. Sci. Technol. 2022, 12, 3650–3669. [Google Scholar] [CrossRef]
- Kearnes, S.M.; Maser, M.R.; Wleklinski, M.; Kast, A.; Doyle, A.G.; Dreher, S.D.; Hawkins, J.M.; Jensen, K.F.; Coley, C.W. The Open Reaction Database. J. Am. Chem. Soc. 2021, 143, 18820–18826. [Google Scholar] [CrossRef]
- Melián-Cabrera, I. Catalytic Materials: Concepts to Understand the Pathway to Implementation. Ind. Eng. Chem. Res. 2021, 60, 18545–18559. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, W.; Ou, Y.; Li, G.; You, R.; Li, S.; Yang, H.; Zhang, Z.; Wang, Y. Recent Progresses on Structural Reconstruction of Nanosized Metal Catalysts via Controlled-Atmosphere Transmission Electron Microscopy: A Review. ACS Catal. 2020, 10, 14419–14450. [Google Scholar] [CrossRef]
- Costa, P.; Sandrin, D.; Scaiano, J.C. Real-time fluorescence imaging of a heterogeneously catalysed Suzuki–Miyaura reaction. Nat. Catal. 2020, 3, 427–437. [Google Scholar] [CrossRef]
- Ouyang, R.; Liu, J.-X.; Li, W.-X. Atomistic Theory of Ostwald Ripening and Disintegration of Supported Metal Particles under Reaction Conditions. J. Am. Chem. Soc. 2013, 135, 1760–1771. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Pérez-Ramírez, J.; Berger, R.J.; Hamminga, G.; Mul, G.; Kapteijn, F. High-throughput experimentation in catalyst testing and in kinetic studies for heterogeneous catalysis. Catal. Today 2003, 81, 457–471. [Google Scholar] [CrossRef]
- Takahashi, K.; Takahashi, L.; Nishimura, S.; Fujima, J.; Ohyama, J. The Rise of Catalysts Informatics. In Crystalline Metal Oxide Catalysts; Ueda, W., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 349–371. [Google Scholar]
- Murbach, G.; Biyani, S.A.; Thompson, D.H. High Throughput Experimentation as a Tool to Guide the Microwave Assisted Catalytic Amidation of Aryl Amines with Aryl Acids. Eur. J. Org. Chem. 2025, 28, e202400764. [Google Scholar] [CrossRef]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Org. Proc. Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
- Isbrandt, E.S.; Sullivan, R.J.; Newman, S.G. High Throughput Strategies for the Discovery and Optimization of Catalytic Reactions. Angew. Chem. Int. Ed. 2019, 58, 7180–7191. [Google Scholar] [CrossRef]
- Benavides-Hernández, J.; Dumeignil, F. From Characterization to Discovery: Artificial Intelligence, Machine Learning and High-Throughput Experiments for Heterogeneous Catalyst Design. ACS Catal. 2024, 14, 11749–11779. [Google Scholar] [CrossRef]
- Suh, C.; Sieg, S.C.; Heying, M.J.; Oliver, J.H.; Maier, W.F.; Rajan, K. Visualization of High-Dimensional Combinatorial Catalysis Data. J. Comb. Chem. 2009, 11, 385–392. [Google Scholar] [CrossRef]
- Shevlin, M. Practical High-Throughput Experimentation for Chemists. ACS Med. Chem. Lett. 2017, 8, 601–607. [Google Scholar] [CrossRef]
- Christensen, M.; Yunker, L.P.E.; Adedeji, F.; Häse, F.; Roch, L.M.; Gensch, T.; dos Passos Gomes, G.; Zepel, T.; Sigman, M.S.; Aspuru-Guzik, A.; et al. Data-science driven autonomous process optimization. Commun. Chem. 2021, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Shevlin, M.; Friedfeld, M.R.; Sheng, H.; Pierson, N.A.; Hoyt, J.M.; Campeau, L.-C.; Chirik, P.J. Nickel-Catalyzed Asymmetric Alkene Hydrogenation of α,β-Unsaturated Esters: High-Throughput Experimentation-Enabled Reaction Discovery, Optimization, and Mechanistic Elucidation. J. Am. Chem. Soc. 2016, 138, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.T.; Pilicer, S.L.; Anslyn, E.V.; Joyce, L.A.; Wolf, C. Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc. 2018, 140, 10385–10401. [Google Scholar] [CrossRef]
- Wang, B.; Bourgonje, C.R.; Scaiano, J.C. Fiber-glass supported catalysis: Real-time, high-resolution visualization of active palladium catalytic centers during the reduction of nitro compounds. Catal. Sci. Technol. 2023, 13, 1021–1031. [Google Scholar] [CrossRef]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S.; Pandey, B.N.; Kulkarni, S.D.; Varadavenkatesan, T.; Venkatachalam, G.; V, A.K.N. A Nitronaphthalimide Probe for Fluorescence Imaging of Hypoxia in Cancer Cells. J. Fluores. 2021, 31, 1665–1673. [Google Scholar] [CrossRef]
- Carrillo, A.I.; Stamplecoskie, K.G.; Marin, M.L.; Scaiano, J.C. ‘From the mole to the molecule’: Ruthenium catalyzed nitroarene reduction studied with ‘bench’, high-throughput and single molecule fluorescence techniques. Catal. Sci. Technol. 2014, 4, 1989–1996. [Google Scholar] [CrossRef]
- Wang, B.; Lanterna, A.E.; Scaiano, J.C. Mechanistic Insights on the Semihydrogenation of Alkynes over Different Nanostructured Photocatalysts. ACS Catal. 2021, 11, 4230–4238. [Google Scholar] [CrossRef]
- Gawargy, T.A.; Costa, P.; Lanterna, A.E.; Scaiano, J.C. Photochemical benzylic radical arylation promoted by supported Pd nanostructures. Org. Biomol. Chem. 2020, 18, 6047–6052. [Google Scholar] [CrossRef] [PubMed]
- Elhage, A.; Lanterna, A.E.; Scaiano, J.C. Catalytic farming: Reaction rotation extends catalyst performance. Chem. Sci. 2019, 10, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors vs. True Water Splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef]
- Wang, B.; Durantini, J.; Nie, J.; Lanterna, A.E.; Scaiano, J.C. Heterogeneous Photocatalytic Click Chemistry. J. Am. Chem. Soc. 2016, 138, 13127–13130. [Google Scholar] [CrossRef]
- Cely-Pinto, M.; Wang, B.; Scaiano, J.C. Photocatalytic Semi-Hydrogenation of Alkynes: A Game of Kinetics, Selectivity and Critical Timing. Nanomaterials 2023, 13, 2390. [Google Scholar] [CrossRef]
- American Chemical Society. Green Chemistry: Endangered Elements. Available online: https://www.acs.org/green-chemistry-sustainability/research-innovation/endangered-elements.html (accessed on 28 June 2025).
- da Silva, D.R.C.; Mapukata, S.; Currie, S.; Kitos, A.A.; Lanterna, A.E.; Nyokong, T.; Scaiano, J.C. Fibrous TiO2 Alternatives for Semiconductor-Based Catalysts for Photocatalytic Water Remediation Involving Organic Contaminants. ACS Omega 2023, 8, 21585–21593. [Google Scholar] [CrossRef]
- Vivier, L.; Duprez, D. Ceria-Based Solid Catalysts for Organic Chemistry. ChemSusChem 2010, 3, 654–678. [Google Scholar] [CrossRef]
- Yan, B.; Cho, K. CatScore: A High-Efficiency Evaluation Metric for Asymmetric Catalyst Design. ChemRxiv 2023. [Google Scholar] [CrossRef]


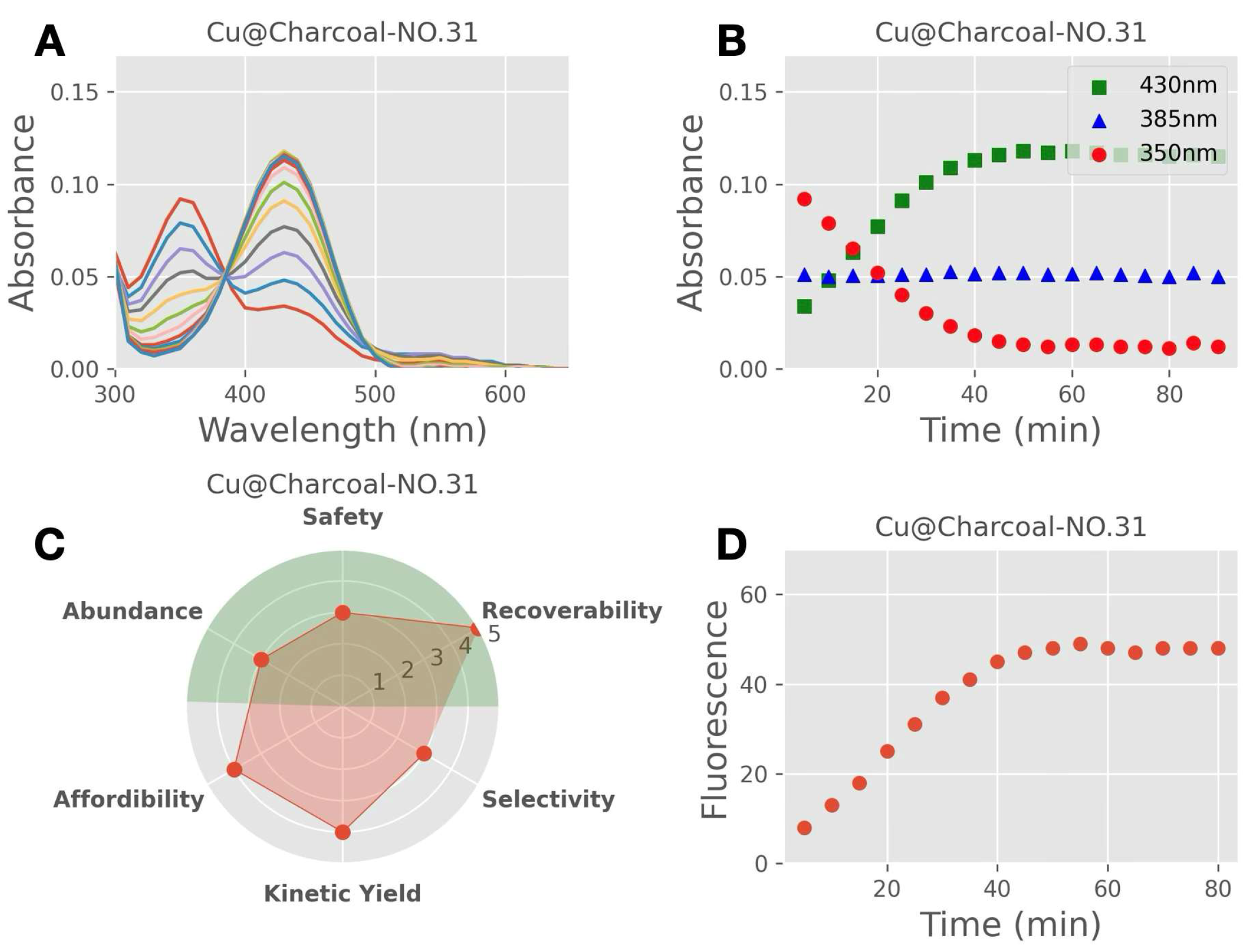



| Score † ➔ Parameter ↓ | 1 (Least Favourable) | 2 | 3 | 4 | 5 (Most Favourable) |
|---|---|---|---|---|---|
| (S) Safety (NFPA score) | 8–7 | 6–5 | 4–3 | 2–1 | 0 |
| Selectivity | Intermediate persists at reaction endpoint (550 nm band remain at 80 min) | NA | Intermediate formed then disappeared by reaction end point; 550 nm band gone at 80 min | NA | Intermediate does not form across the reaction profile; no 550 nm signal |
| Reaction rate | Less than 25% yield in 80 min | 25 ≤ % yield ≤ 60 in 80 min | 60 ≤ % yield ≤ 100 in 80 min | Plateaus to completion in 80 min | Plateaus to completion in <20 min |
| Affordability | USD > 100 | 60 ≤ USD ≤ 100 | 30 ≤ USD ≤ 60 | 30 ≤ USD ≤ 10 | USD < 10 |
| (A) Supply and Abundance | 0 score | NA | 1, 2 score | NA | 3 score |
| (R) Recoverability | 0 score | NA | 1 score | NA | 2 and 3 scores |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-khawaldeh, R.; Bourgonje, C.R.; Wang, B.; Scaiano, J.C. A Scoring Model for Catalyst Informatics Based on Real-Time High-Throughput Fluorogenic Assay for Catalyst Discovery and Kinetic Profiling. Catalysts 2025, 15, 636. https://doi.org/10.3390/catal15070636
El-khawaldeh R, Bourgonje CR, Wang B, Scaiano JC. A Scoring Model for Catalyst Informatics Based on Real-Time High-Throughput Fluorogenic Assay for Catalyst Discovery and Kinetic Profiling. Catalysts. 2025; 15(7):636. https://doi.org/10.3390/catal15070636
Chicago/Turabian StyleEl-khawaldeh, Rama, Connor R. Bourgonje, Bowen Wang, and Juan C. Scaiano. 2025. "A Scoring Model for Catalyst Informatics Based on Real-Time High-Throughput Fluorogenic Assay for Catalyst Discovery and Kinetic Profiling" Catalysts 15, no. 7: 636. https://doi.org/10.3390/catal15070636
APA StyleEl-khawaldeh, R., Bourgonje, C. R., Wang, B., & Scaiano, J. C. (2025). A Scoring Model for Catalyst Informatics Based on Real-Time High-Throughput Fluorogenic Assay for Catalyst Discovery and Kinetic Profiling. Catalysts, 15(7), 636. https://doi.org/10.3390/catal15070636






