Revolutionizing Hydrogen Production: Unveiling the Role of Liquid Metals in Methane Pyrolysis over Iron Catalysts Supported on Titanium Dioxide and Alumina
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Analysis
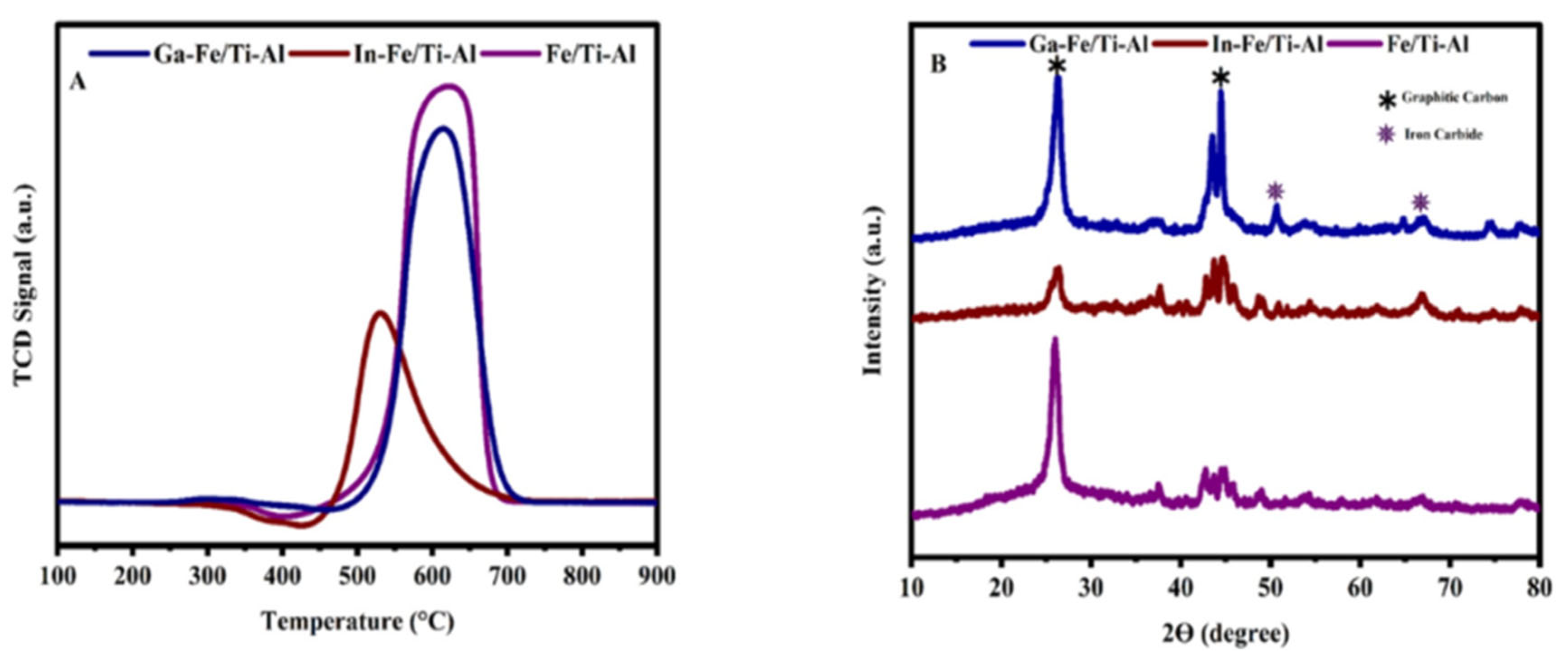
2.2. Reducibility
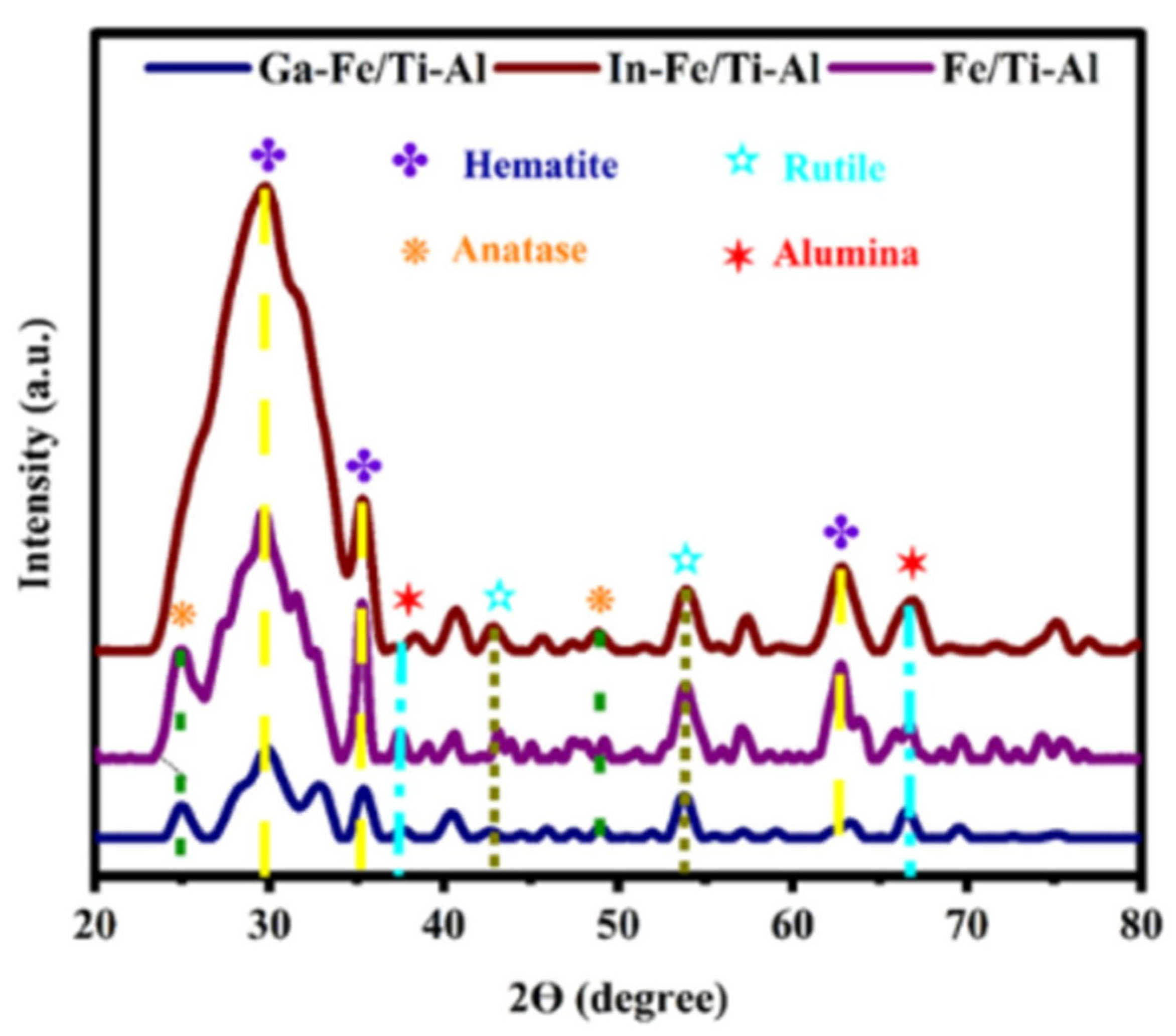
2.3. X-Ray Diffraction
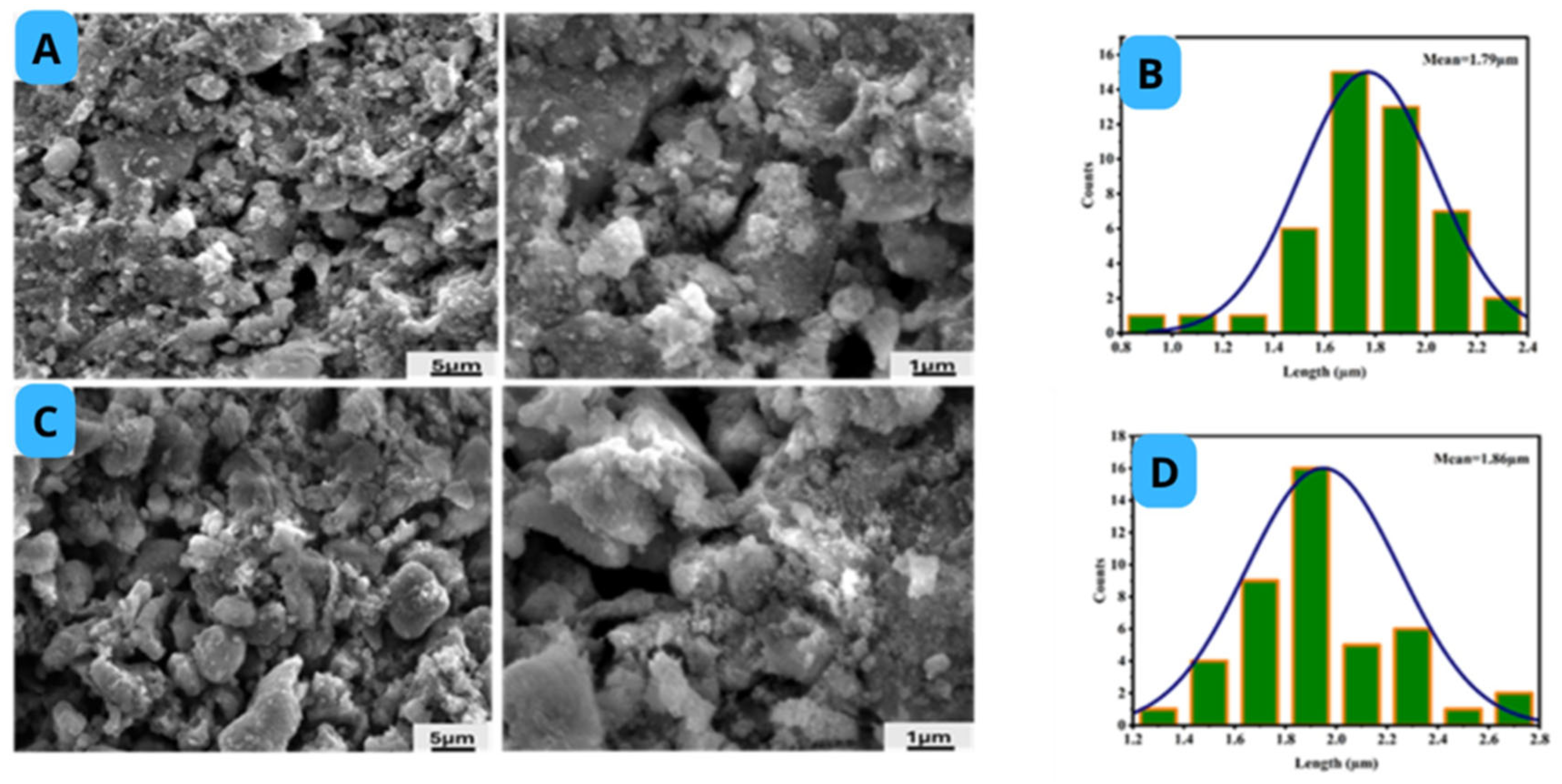
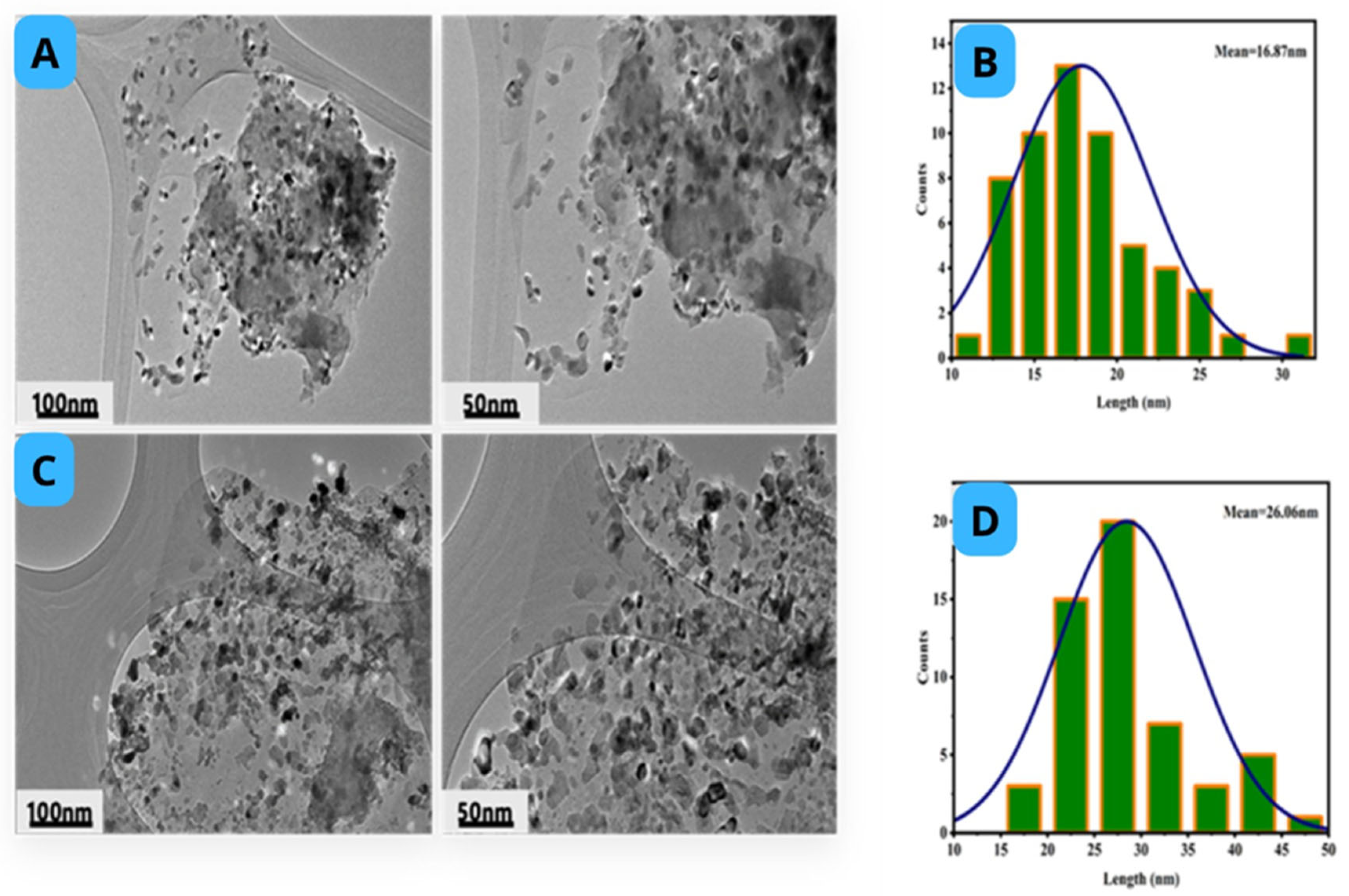
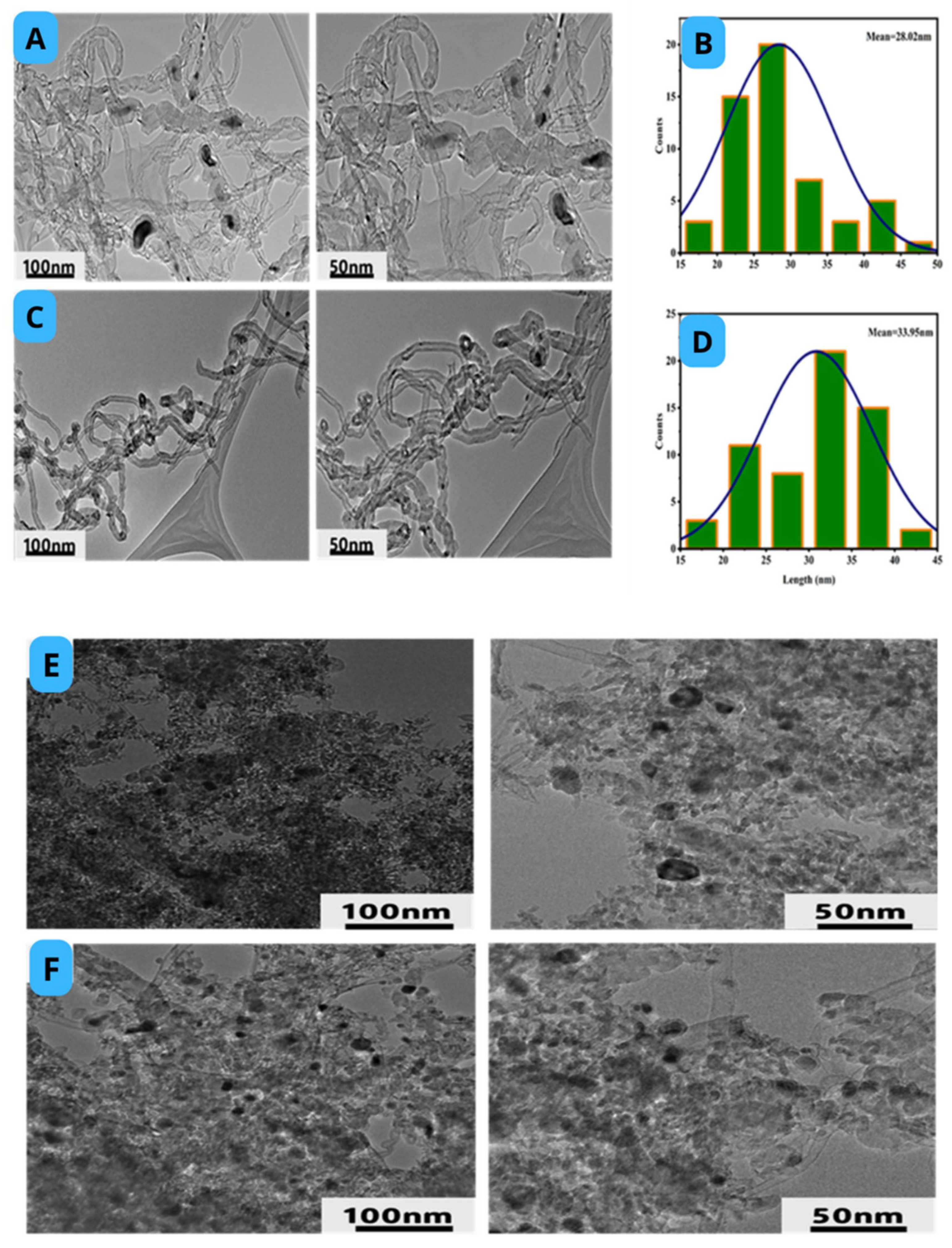
2.4. Morphology of Catalyst
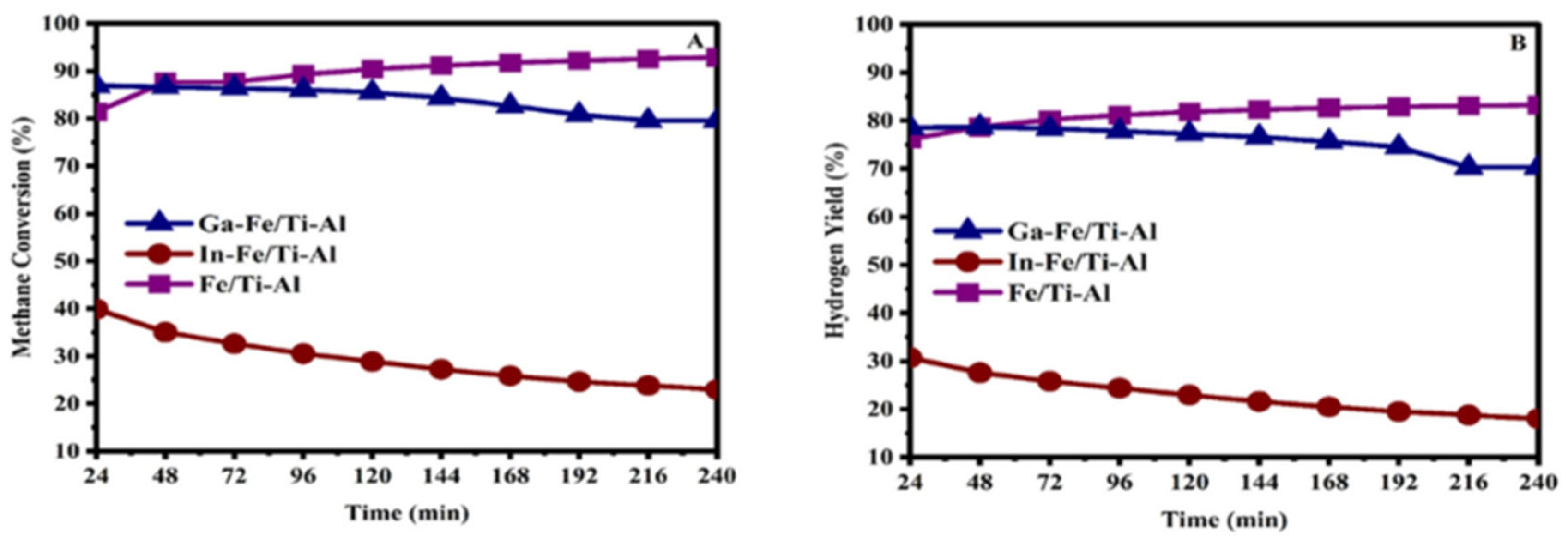
2.5. Catalytic Activity
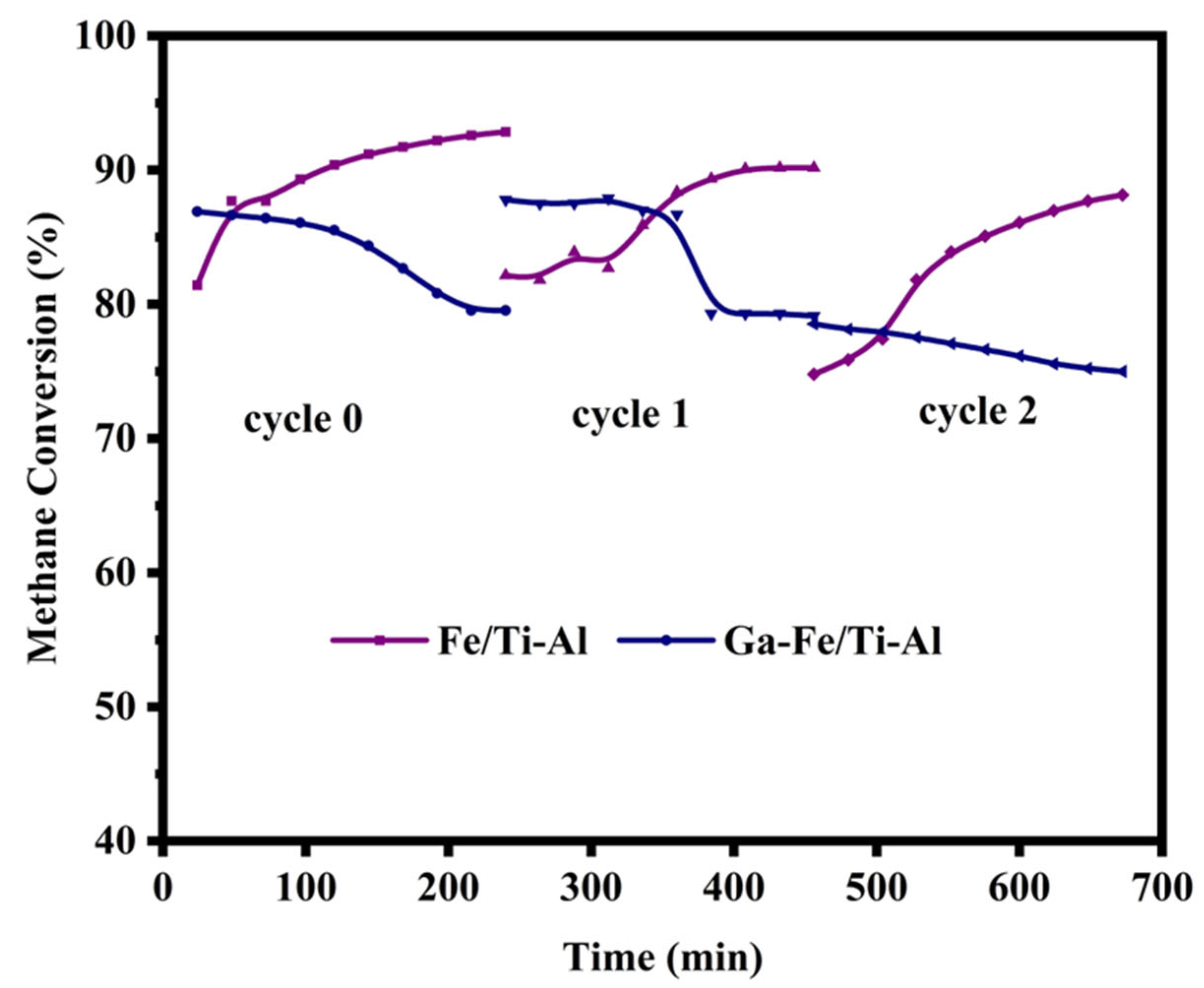
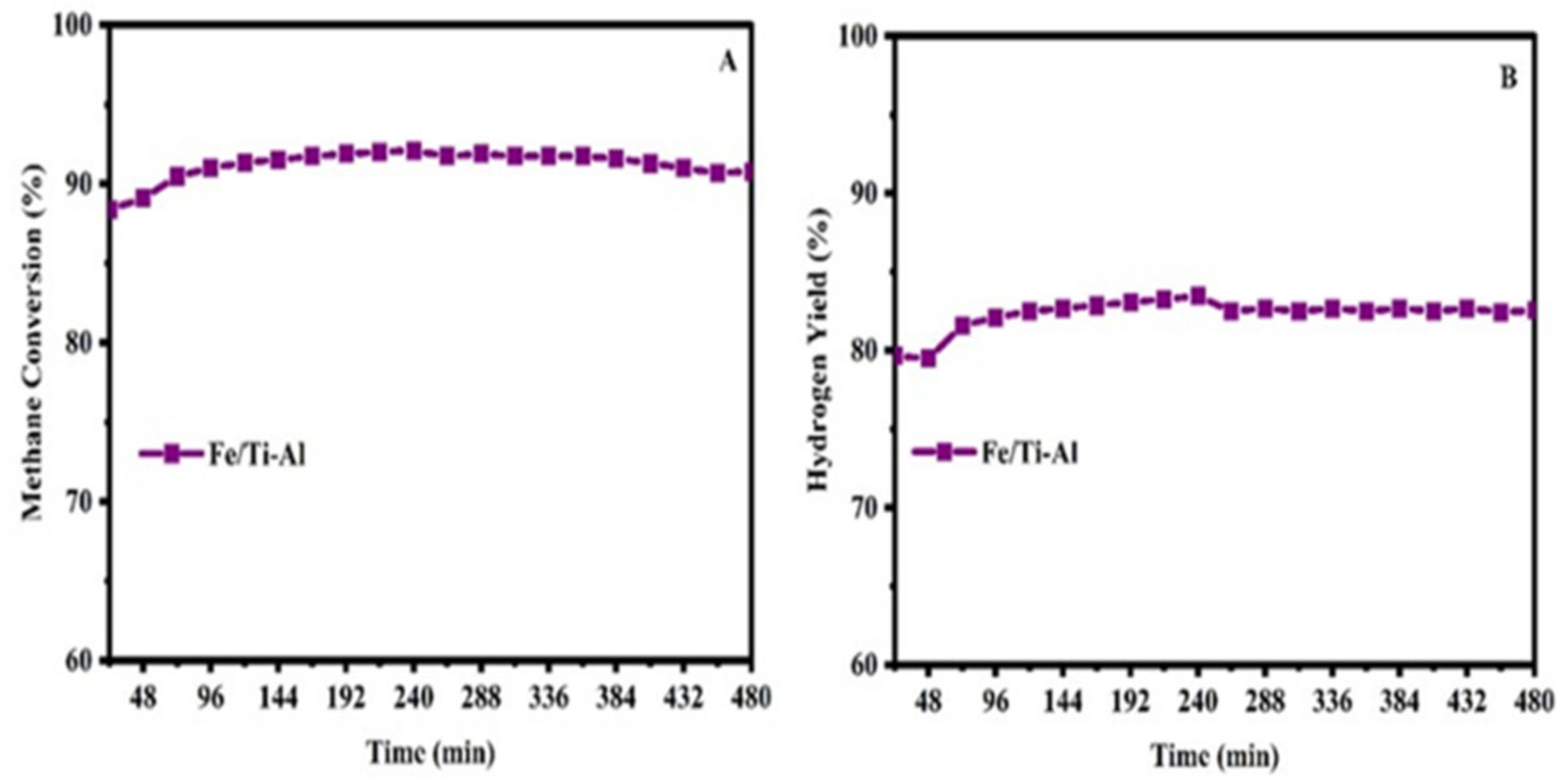
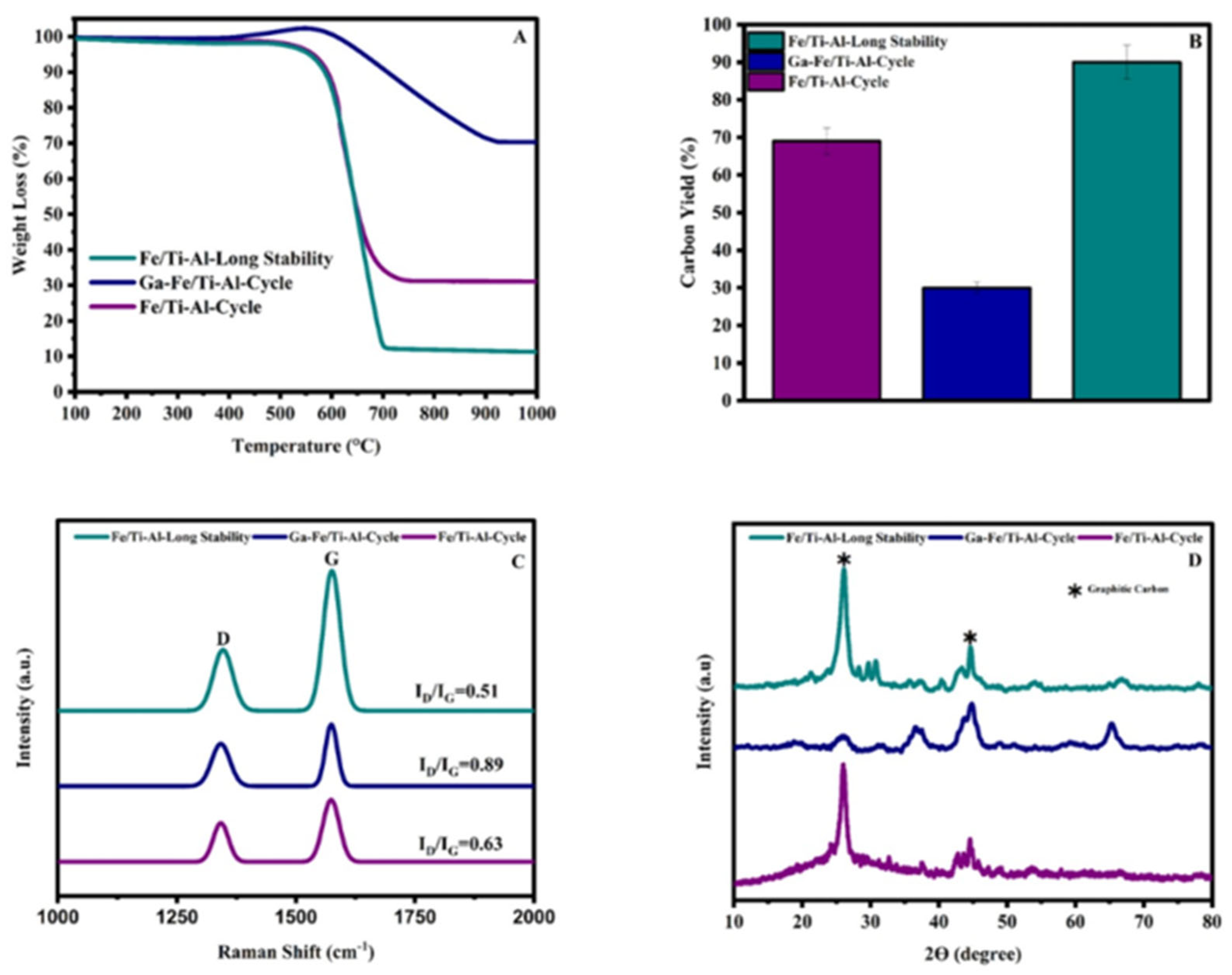
2.6. Regeneration and Long-Term Stability of the Catalysts Test
3. Characterization of Used Catalyst
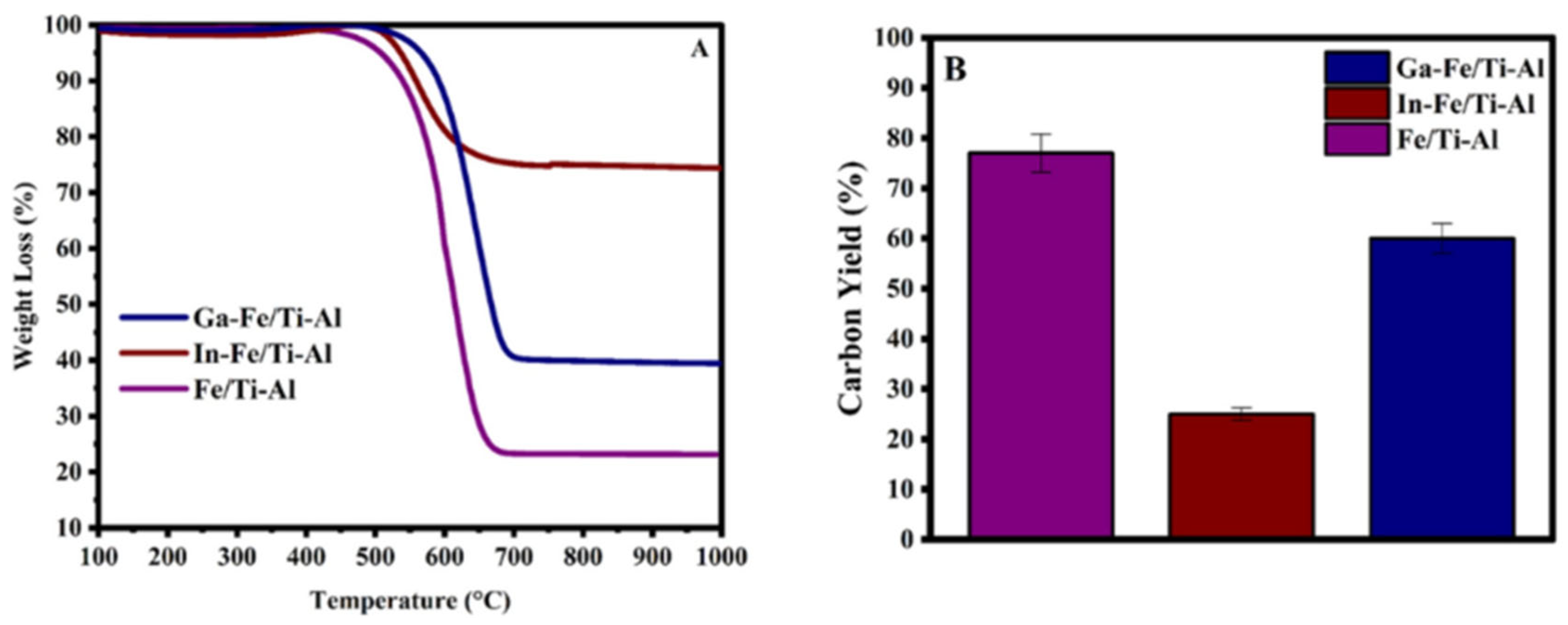
3.1. TGA-Used Catalyst
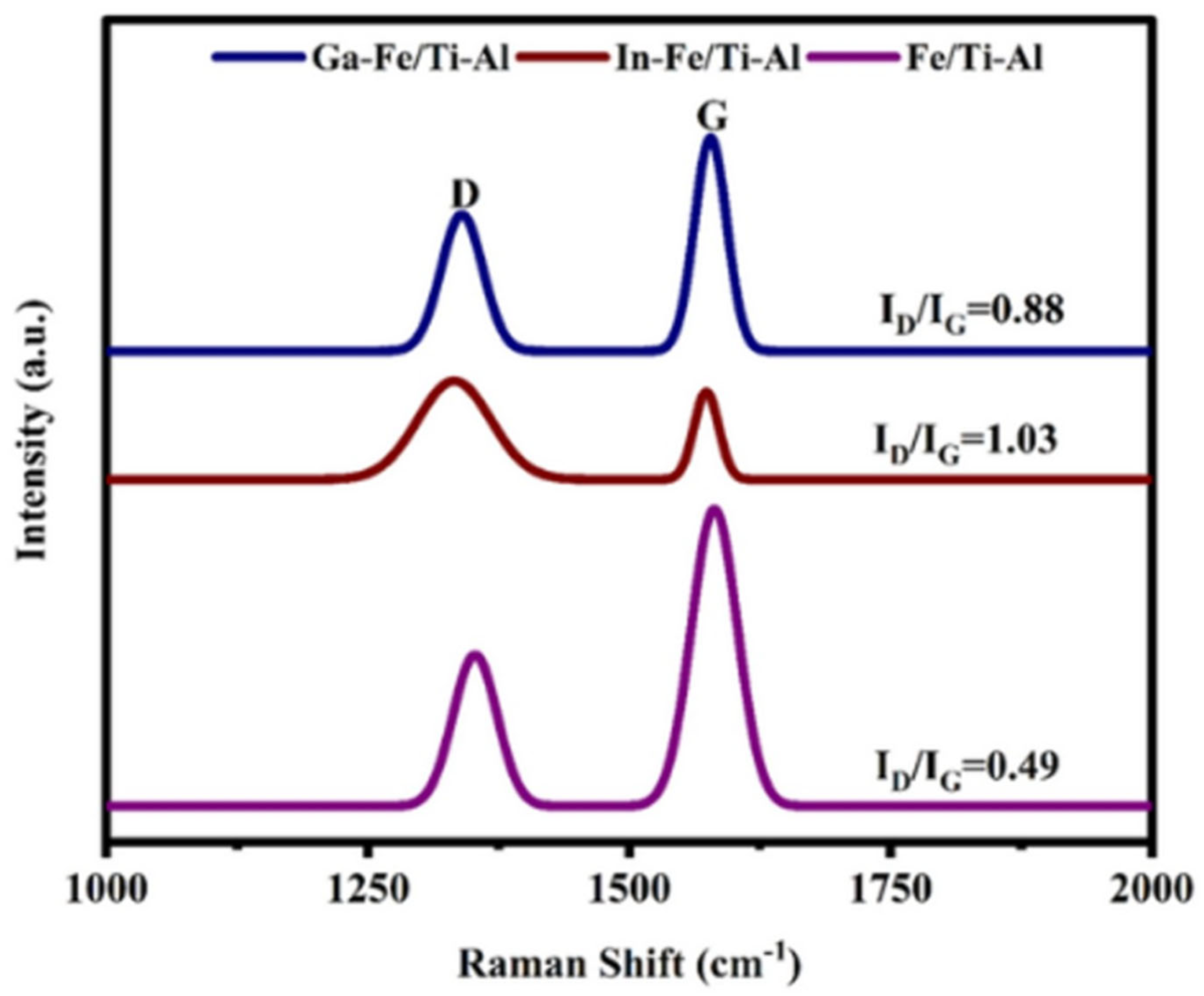
3.2. Raman Spectroscopy Used a Catalyst
3.3. TPO-Used Catalyst
3.4. X-Ray Diffraction Catalyst
3.5. Morphology-Used a Catalyst
4. Supplies and Preparation
4.1. Supplies
4.2. Catalyst Preparation
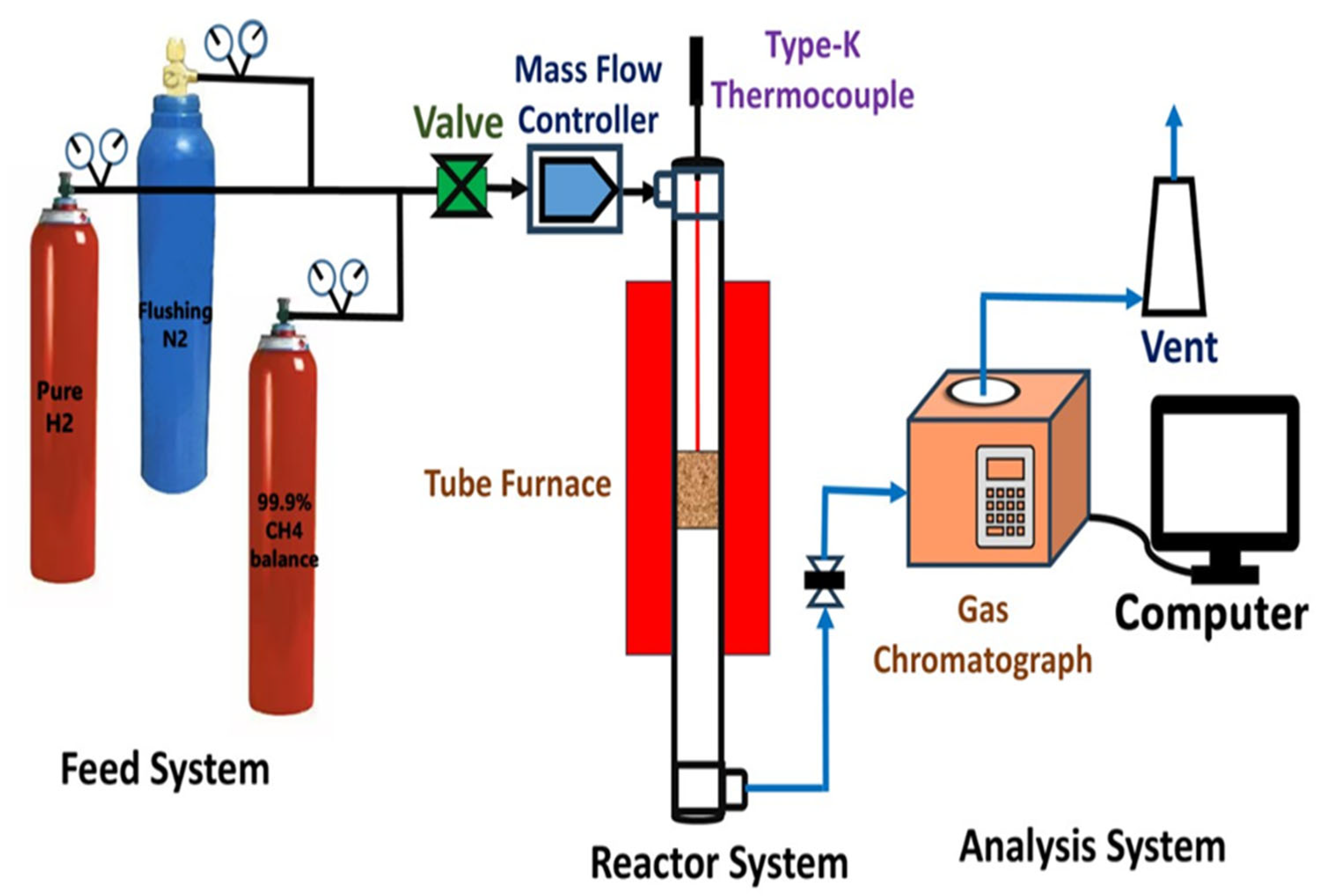
4.3. Catalytic Reactor Set Up
4.4. Fresh Catalyst Characterization
4.5. Post-Catalyst Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xuan, G.; Liu, F.; Zhang, F.; Hu, Y.; Miao, J.; Yang, L. Mechanism of Improving the Stability of Activated Carbon Catalyst by Trace H2S Impurities in Natural Gas for Hydrogen Production from Methane Decomposition. Fuel 2021, 299, 120884. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; El-Naas, M.H.; Kumar, D.; Kumar, A.; Kumar, M.H.; Kumar, D.; Catalytic, A. Catalytic Methane Decomposition to Carbon Nanostructures and CO X-Free Hydrogen: A Mini-Review. Nanomaterials 2021, 11, 1226. [Google Scholar] [CrossRef]
- Barbir, F. Transition to Renewable Energy Systems with Hydrogen as an Energy Carrier. Energy 2009, 34, 308–312. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of Methane Catalytic Cracking for Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Screening of Ni-Cu Bimetallic Catalysts for Hydrogen and Carbon Nanofilaments Production via Catalytic Decomposition of Methane. Appl. Catal. A Gen. 2018, 559, 10–19. [Google Scholar] [CrossRef]
- Balyan, S.; Jiang, C.; Caiola, A.; Hu, J. Microwave Catalytic Conversion of Acetylene for Co-Production of Hydrogen and Carbon Nanotubes. Chem. Eng. J. 2023, 454, 140115. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic Partial Oxidation of Methane to Syngas: Review of Perovskite Catalysts and Membrane Reactors. Catal. Rev. Sci. Eng. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen Production through Sorption-Enhanced Steam Methane Reforming and Membrane Technology: A Review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen Production by Methane Decomposition Using Bimetallic Ni–Fe Catalysts. Int. J. Hydrogen Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Catalan, L.J.J.; Rezaei, E. Coupled Hydrodynamic and Kinetic Model of Liquid Metal Bubble Reactor for Hydrogen Production by Noncatalytic Thermal Decomposition of Methane. Int. J. Hydrogen Energy 2020, 45, 2486–2503. [Google Scholar] [CrossRef]
- Al -Fatesh, A.S.; Kasim, S.O.; Ibrahim, A.A.; Al-Awadi, A.S.; Abasaeed, A.E.; Fakeeha, A.H.; Awadallah, A.E. Catalytic Methane Decomposition over ZrO2 Supported Iron Catalysts: Effect of WO3 and La2O3 Addition on Catalytic Activity and Stability. Renew. Energy 2020, 155, 969–978. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.M.; Zhou, L. Methane Decomposition to Produce COx-Free Hydrogen and Nano-Carbon over Metal Catalysts: A Review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Khan, U.M.; Sarmad, Q.; Anwar, M.; Khoja, A.H.; Muhammed Ali, S.A.; Khan, Z.S.; Hassan, M.; Shakir, S. Synthesis of Cobalt Loaded Double Perovskite Sr2TiFeO6-δ (STF) as a Stable Catalyst for Enhanced Hydrogen Production via Methane Decomposition. Int. J. Energy Res. 2021, 45, 20073–20088. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Rezaei, M.; Meshkani, F.; Zhang, K.; Zhao, X.; Pei, W.; Liu, Y.; Deng, J.; Arandiyan, H.; Dai, H. Influence of Group VIB Metals on Activity of the Ni/MgO Catalysts for Methane Decomposition. Appl. Catal. B Environ. 2019, 248, 515–525. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E.; Khan, W.U. Methane Decomposition over Iron Catalyst for Hydrogen Production. Int. J. Hydrogen Energy 2015, 40, 7593–7600. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane Decomposition over Pd Promoted Ni/MgAl2O4 Catalysts for the Production of COx Free Hydrogen and Multiwalled Carbon Nanotubes. Appl. Surf. Sci. 2015, 356, 1320–1326. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L.P. Recent Developments in Methane Decomposition over Heterogeneous Catalysts: An Overview. Mater Renew Sustain. Energy 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Yergaziyeva, G.; Makayeva, N.; Abdisattar, A.; Yeleuov, M.; Soloviev, S.; Anissova, M.; Taurbekov, A.; Dossumov, K.; Akkazin, E.; Daulbayev, C. Electrochemical Synthesis of Fe-Containing Composite for Decomposition of Methane into COx-Free Hydrogen and Nano-Carbon. Chem. Pap. 2022, 76, 1–13. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, H.; Huang, M.; Wan, C.; Li, D.; Jiang, L. Catalytic Methane Decomposition to Hydrogen and Carbon over Hydrotalcite-Derivative Composition-Uniform and Sintering-Resistant Ni-Fe/Al2O3 Alloy Catalysts. Int. J. Energy Res. 2022, 46, 16810–16822. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.L.; Li, J.; Wei, N.; Gary, D.; et al. Fe Catalysts for Methane Decomposition to Produce Hydrogen and Carbon Nano Materials. Appl. Catal. B Environ. 2017, 208, 44–59. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Abdel-Fattah, E.; Alotaibi, M.A.; Al-Shalwi, M.N. Facile Modification of Cobalt Ferrite by SiO2 and H-ZSM-5 Support for Hydrogen and Filamentous Carbon Production from Methane Decomposition. Int. J. Energy Res. 2022, 46, 17497–17510. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Al-Awadi, A.S.; Alzahrani, E.; Abasaeed, A.E.; Awadallah, A.E.; Al-Fatesh, A.S. Methane Decomposition Over ZrO2-Supported Fe and Fe–Ni Catalysts—Effects of Doping La2O3 and WO3. Front. Chem. 2020, 8, 527057. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Alotaibi, M.A.; Alharthi, A.I. Thermo-Catalytic Methane Decomposition over Unsupported Fe–Al and Co–Al Catalysts for Hydrogen and Carbon Nanostructures Production. Int. J. Hydrogen Energy 2024, 64, 685–694. [Google Scholar] [CrossRef]
- Mohamed, A.T.; Ali, S.; Kumar, A.; Mondal, K.C.; El-Naas, M.H. Evaluation of Highly Active and Stable SiO2 Supported Fe-Based Catalysts for the Catalytic Methane Decomposition into COx Free Hydrogen and CNTs. Catal. Commun. 2023, 180, 106703. [Google Scholar] [CrossRef]
- Pashchenko, D. Thermodynamic Equilibrium Analysis of Steam Methane Reforming Based on a Conjugate Solution of Material Balance and Law Action Mass Equations with the Detailed Energy Balance. Int. J. Energy Res. 2020, 44, 438–447. [Google Scholar] [CrossRef]
- Rein, D.; Friedel Ortega, K.; Weidenthaler, C.; Bill, E.; Behrens, M. The Roles of Co-Precipitation PH, Phase-Purity and Alloy Formation for the Ammonia Decomposition Activity of Ga-Promoted Fe/MgO Catalysts. Appl. Catal. A Gen. 2017, 548, 52–61. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Ibrahim, A.A.; AlSharekh, A.A.M.; Alqahtani, F.S.; Kasim, S.O.; Al-Zahrani, S.A.; Fakeeha, A.H. Methane Decomposition over Strontium Promoted Iron Catalyst: Effect of Different Ratio of Al/Si Support on Hydrogen Yield. Chem. Eng. Commun. 2020, 207, 1148–1156. [Google Scholar] [CrossRef]
- Bao, A.; Ma, Y.; Guo, X.; Wang, J.; Zhao, Y.; Liu, Z.; Wang, Y.; Liu, X.; Zhang, Y. Tunable Electronic and Optical Properties of a Type-II GaP/SiH van Der Waals Heterostructure as Photocatalyst: A First-Principles Study. Int. J. Hydrogen Energy 2024, 88, 1256–1266. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Jia, Q.; Takriff, M.S. Catalytic Decomposition of Methane over Rare Earth Metal (Ce and La) Oxides Supported Iron Catalysts. Appl. Surf. Sci. 2019, 467–468, 236–248. [Google Scholar] [CrossRef]
- Tang, L.; Yamaguchi, D.; Burke, N.; Trimm, D.; Chiang, K. Methane Decomposition over Ceria Modified Iron Catalysts. Catal. Commun. 2010, 11, 1215–1219. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Khan, W.U.; Ibrahim, A.A.; Al-Otaibi, R.L.; Abasaeed, A.E. Suitability of Titania and Magnesia as Support for Methane Decomposition Catalyst Using Iron as Active Materials. J. Chem. Eng. Jpn. 2016, 49, 552–562. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Echegoyen, Y.; Alegre, C.; Suelves, I.; Moliner, R.; Palacios, J.M. TiO2 as Textural Promoter on High Loaded Ni Catalysts for Methane Decomposition. Int. J. Hydrogen Energy 2008, 33, 3320–3329. [Google Scholar] [CrossRef]
- Dickey, M.D. Emerging Applications of Liquid Metals Featuring Surface Oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef]
- Taccardi, N.; Grabau, M.; Debuschewitz, J.; Distaso, M.; Brandl, M.; Hock, R.; Maier, F.; Papp, C.; Erhard, J.; Neiss, C.; et al. Gallium-Rich Pd–Ga Phases as Supported Liquid Metal Catalysts. Nat. Chem. 2017, 9, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Geißler, T.; Plevan, M.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; et al. Experimental Investigation and Thermo-Chemical Modeling of Methane Pyrolysis in a Liquid Metal Bubble Column Reactor with a Packed Bed. Int. J. Hydrogen Energy 2015, 40, 14134–14146. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic Molten Metals for the Direct Conversion of Methane to Hydrogen and Separable Carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef]
- Rahman, S.; Santra, C.; Kumar, R.; Bahadur, J.; Sultana, A.; Schweins, R.; Sen, D.; Maity, S.; Mazumdar, S.; Chowdhury, B. Highly Active Ga Promoted Co-HMS-X Catalyst towards Styrene Epoxidation Reaction Using Molecular O2. Appl. Catal. A Gen. 2014, 482, 61–68. [Google Scholar] [CrossRef]
- Palmer, C.; Tarazkar, M.; Kristoffersen, H.H.; Gelinas, J.; Gordon, M.J.; McFarland, E.W.; Metiu, H. Methane Pyrolysis with a Molten Cu-Bi Alloy Catalyst. ACS Catal. 2019, 9, 8337–8345. [Google Scholar] [CrossRef]
- Plevan, M.; Geißler, T.; Abánades, A.; Mehravaran, K.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; Wetzel, T. Thermal Cracking of Methane in a Liquid Metal Bubble Column Reactor: Experiments and Kinetic Analysis. Int. J. Hydrogen Energy 2015, 40, 8020–8033. [Google Scholar] [CrossRef]
- Ahmed, H.; Fakeeha, A.H.; Al-Alweet, F.M.; Abasaeed, A.E.; Ibrahim, A.A.; Kumar, R.; Saeed, A.M.M.; Al-Fatesh, A.S. Methane Decomposition over a Titanium-Alumina and Iron Catalyst Assisted by Lanthanides to Produce High-Performance COx-Free H2 and Carbon Nanotubes. Catalysts 2025, 15, 77. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Vadodariya, D.M.; Bayazed, M.O.; Osman, A.I.; Ibrahim, A.A.; Fakeeha, A.H.; Alanazi, Y.M.; Abasaeed, A.E.; Kumar, R. Optimizing Hydrogen Production: Influence of Promoters in Methane Decomposition on Titania-Modified-Zirconia Supported Fe Catalyst. ACS Omega 2024, 9, 20322–20330. [Google Scholar] [CrossRef] [PubMed]
- Beasley, C.; Gnanamani, M.K.; Hamdeh, H.H.; Martinelli, M.; Davis, B.H. Effect of Gallium Additions on Reduction, Carburization and Fischer–Tropsch Activity of Iron Catalysts. Catal. Lett. 2018, 148, 1920–1928. [Google Scholar] [CrossRef]
- Rahman, S.; Farooqui, S.A.; Rai, A.; Kumar, R.; Santra, C.; Prabhakaran, V.C.; Bhadu, G.R.; Sen, D.; Mazumder, S.; Maity, S.; et al. Mesoporous TUD-1 Supported Indium Oxide Nanoparticles for Epoxidation of Styrene Using Molecular O2. RSC Adv. 2015, 5, 46850–46860. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amorós-Pérez, A.; Ouzzine, M.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. One-Step Hydrothermal Synthesis of TiO2 with Variable HCl Concentration: Detailed Characterization and Photocatalytic Activity in Propene Oxidation. Appl. Catal. B Environ. 2018, 220, 645–653. [Google Scholar] [CrossRef]
- Rameshan, C.; Lorenz, H.; Armbrüster, M.; Kasatkin, I.; Klötzer, B.; Götsch, T.; Ploner, K.; Penner, S. Impregnated and Co-Precipitated Pd-Ga2O3, Pd-In2O3 and Pd-Ga2O3-In2O3 Catalysts: Influence of the Microstructure on the CO2 Selectivity in Methanol Steam Reformingprecipitated Pd-Ga. Catal. Lett. 1234, 148, 3062–3071. [Google Scholar] [CrossRef]
- Hexana, W.M.; Coville, N.J. Indium as a Chemical Promoter in Fe-Based Fischer-Tropsch Synthesis. Appl. Catal. A Gen. 2010, 377, 150–157. [Google Scholar] [CrossRef]
- Karaismailoğlu, M.; Figen, H.E.; Baykara, S.Z. Methane Decomposition over Fe-Based Catalysts. Int. J. Hydrogen Energy 2020, 45, 34773–34782. [Google Scholar] [CrossRef]
- Alves Silva, J.; Oliveira Santos, J.B.; Torres, D.; Pinilla, J.L.; Suelves, I.; Batista, O. Natural Fe-Based Catalysts for the Production of Hydrogen and Carbon Nanomaterials via Methane Decomposition. Int. J. Hydrogen Energy 2021, 46, 35137–35148. [Google Scholar] [CrossRef]
- Bayat, N.; Meshkani, F.; Rezaei, M. Thermocatalytic Decomposition of Methane to COx-Free Hydrogen and Carbon over Ni–Fe–Cu/Al2O3 Catalysts. Int. J. Hydrogen Energy 2016, 41, 13039–13049. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Atia, H.; Eckelt, R.; Seshan, K.; Chowdhury, B. Decomposition of Methane over Alumina Supported Fe and Ni–Fe Bimetallic Catalyst: Effect of Preparation Procedure and Calcination Temperature. J. Saudi Chem. Soc. 2018, 22, 239–247. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Gryglewicz, G. Growth of Carbon Nanofibers from Methane on a Hydroxyapatite-Supported Nickel Catalyst. J. Mater. Sci. 2016, 51, 5367–5376. [Google Scholar] [CrossRef]
- Kameya, Y.; Hanamura, K. Kinetic and Raman Spectroscopic Study on Catalytic Characteristics of Carbon Blacks in Methane Decomposition. Chem. Eng. J. 2011, 173, 627–635. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Messerer, A.; Yang, X.; Niessner, R.; Pöschl, U. Raman Microspectroscopic Analysis of Changes in the Chemical Structure and Reactivity of Soot in a Diesel Exhaust Aftertreatment Model System. Environ. Sci. Technol. 2007, 41, 3702–3707. [Google Scholar] [CrossRef]
- Fujimoto, H. Theoretical X-Ray Scattering Intensity of Carbons with Turbostratic Stacking and AB Stacking Structures. Carbon 2003, 41, 1585–1592. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-Ray Diffraction Patterns of Graphite and Turbostratic Carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
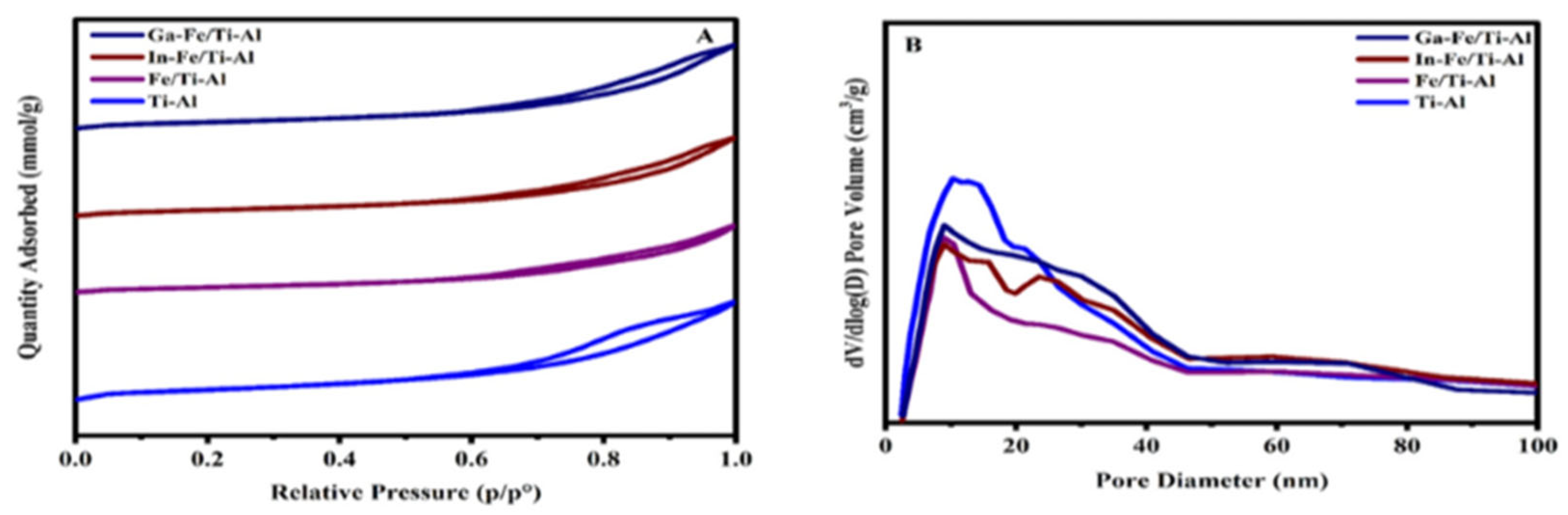


| Catalyst’s Name | BET(m2/g) Surface Area | Pore Volume (cm3/g) | Average Pore Size (nm) | Hydrogen Consumption (cm3/g STP) | (DR)a (%) |
|---|---|---|---|---|---|
| 20Ti-Al | 239 | 0.68 | 10.04 | -------- | ----------- |
| Fe/Ti-Al | 143 | 0.46 | 10.10 | 25.50 | 19.23 |
| In-Fe/Ti-Al | 164 | 0.54 | 10.56 | 75.65 | 86.99 |
| Ga-Fe/Ti-Al | 174 | 0.58 | 10.47 | 71.26 | 79.93 |
| Sample | Iron Loading (%) | Reaction Temperature (°C) | Methane Conversion (%) | Hydrogen Yield (%) | GHSV (L g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| Fe/ZrO2 | 20 | 800 | 60 | 58 | 4 | [11] |
| Fe2O3/Y2O3 | 50 | 800 | 34 | 29 | 150 | [49] |
| Tierga Fe-based ores | --- | 800 | 33 | 47 | 2 | [50] |
| Fe/CeO2 | 27 | 800 | 49 | 66 | 4.5 | [30] |
| Fe/La2O3 | 27 | 800 | 33 | 67 | 4.5 | [30] |
| Ni-Fe-Cu/Al2O3 | 10 | 650 | 88 | 60 | 30 | [51] |
| Fe/Al | 20 | 700 | 60 | 55 | 5 | [52] |
| Ga-Fe/Ti-Al | 20 | 800 | 87 | 79 | 6 | This work |
| In-Fe/Ti-Al | 20 | 800 | 40 | 31 | 6 | This work |
| Fe/Ti-Al | 30 | 800 | 89 | 80 | 6 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; BaQais, A.; Ali, F.A.A.; Osman, A.I.; Fakeeha, A.H.; Abasaeed, A.E.; Ibrahim, A.A.; Adil, S.F.; Algarni, T.S.; Al-Fatesh, A.S. Revolutionizing Hydrogen Production: Unveiling the Role of Liquid Metals in Methane Pyrolysis over Iron Catalysts Supported on Titanium Dioxide and Alumina. Catalysts 2025, 15, 631. https://doi.org/10.3390/catal15070631
Ahmed H, BaQais A, Ali FAA, Osman AI, Fakeeha AH, Abasaeed AE, Ibrahim AA, Adil SF, Algarni TS, Al-Fatesh AS. Revolutionizing Hydrogen Production: Unveiling the Role of Liquid Metals in Methane Pyrolysis over Iron Catalysts Supported on Titanium Dioxide and Alumina. Catalysts. 2025; 15(7):631. https://doi.org/10.3390/catal15070631
Chicago/Turabian StyleAhmed, Hamid, Amal BaQais, Fekri Abdulraqeb Ahmed Ali, Ahmed I. Osman, Anis H. Fakeeha, Ahmed E. Abasaeed, Ahmed A. Ibrahim, Syed Farooq Adil, Tahani Saad Algarni, and Ahmed S. Al-Fatesh. 2025. "Revolutionizing Hydrogen Production: Unveiling the Role of Liquid Metals in Methane Pyrolysis over Iron Catalysts Supported on Titanium Dioxide and Alumina" Catalysts 15, no. 7: 631. https://doi.org/10.3390/catal15070631
APA StyleAhmed, H., BaQais, A., Ali, F. A. A., Osman, A. I., Fakeeha, A. H., Abasaeed, A. E., Ibrahim, A. A., Adil, S. F., Algarni, T. S., & Al-Fatesh, A. S. (2025). Revolutionizing Hydrogen Production: Unveiling the Role of Liquid Metals in Methane Pyrolysis over Iron Catalysts Supported on Titanium Dioxide and Alumina. Catalysts, 15(7), 631. https://doi.org/10.3390/catal15070631










