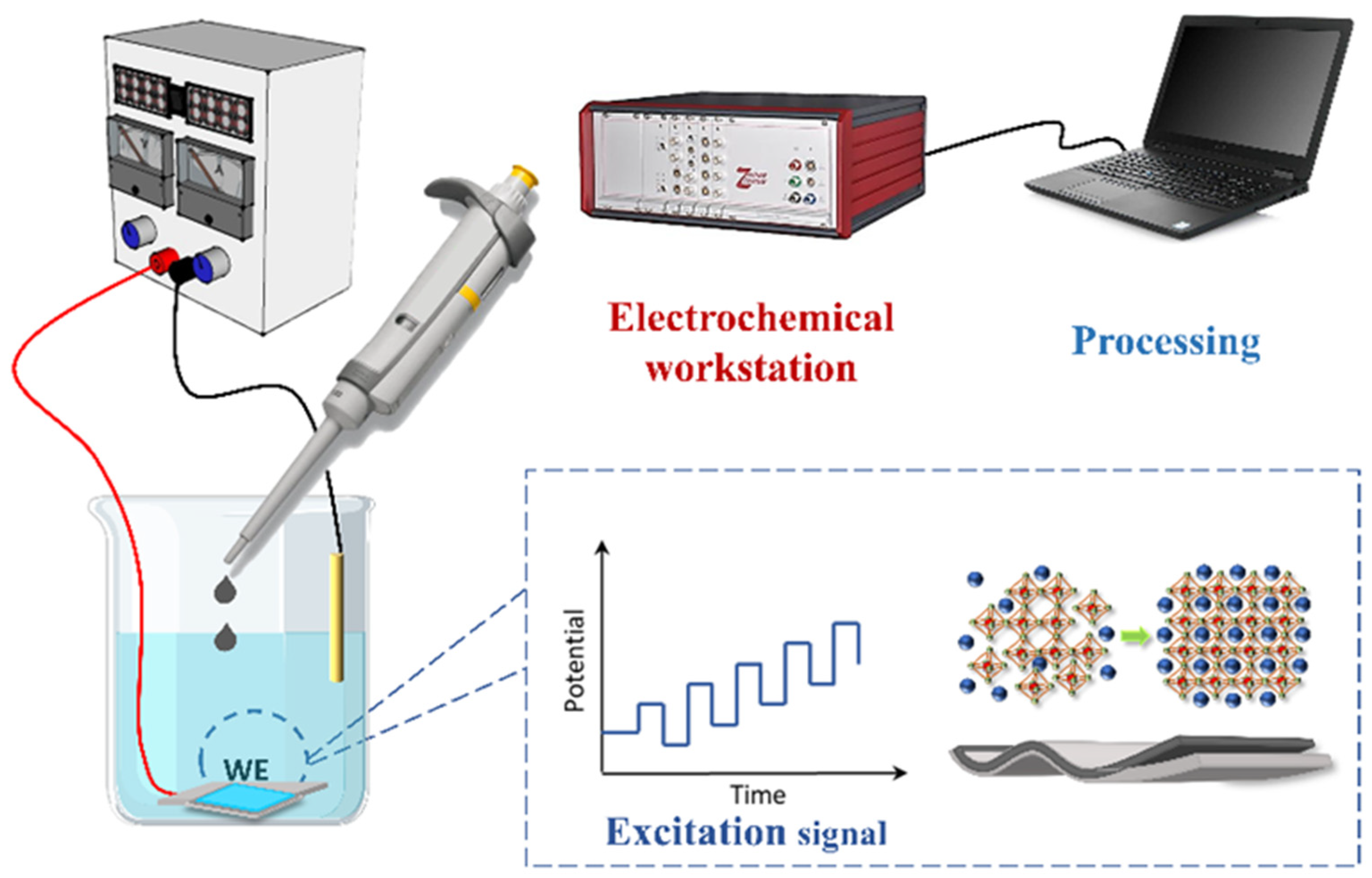
2.1. Material Characterization
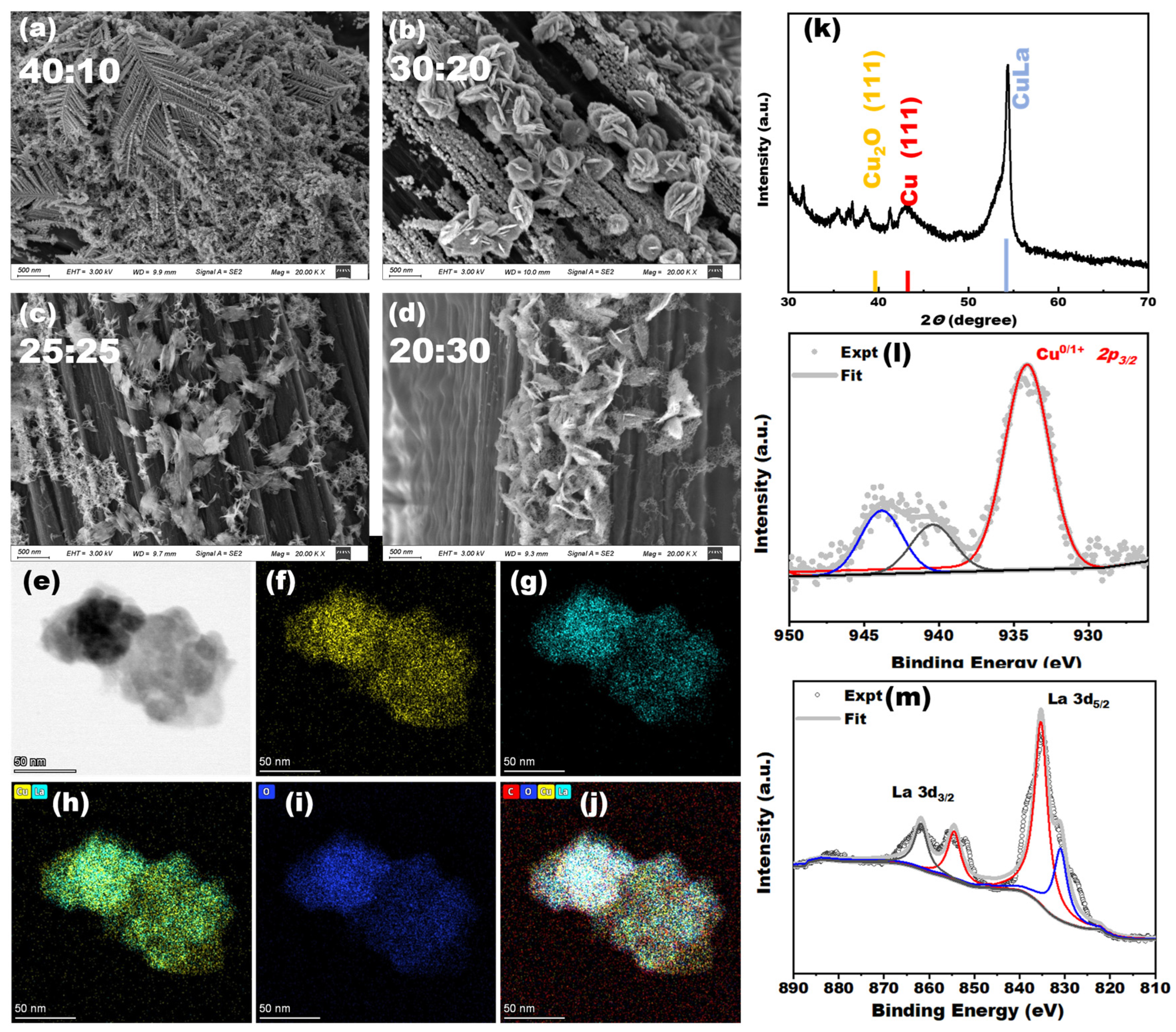
The Cu-La bimetallic electron cathode was subjected to comprehensive characterization and testing utilizing scanning electron microscopy (SEM) and other characterization tools. The original morphology of the carbon paper electrodes, as well as the apparent morphology after deposition, were observed using SEM. The results are presented in
Figure 2a and
Figure S1. The analysis of the morphology of Cu-La bimetallic catalyst with different ratios revealed significant changes in the morphology of the electrode surface after electrodeposition, as observed in the SEM images. These changes were particularly evident when the ratio of copper and lanthanum was 40:10; the electrode surface was primarily a dendritic deposit. During this phase, the electrode surface mainly exhibited the formation of crystalline precipitation of metallic copper. It was observed that copper and lanthanum had not yet formed a heterojunction structure. This may be attributable to the low concentration of metallic lanthanum ions in the deposition solution, which may not have effectively combined with the copper ions. As the ratio of lanthanum metal increased, the morphological characteristics of the electrode underwent a significant transformation. As illustrated in
Figure 2b, with the increase in the ratio of copper to lanthanum to 30:20, the deposits on the surface of the electrode underwent a transformation from leafy copper crystals to Cu-La nanoflowers. This indicates that bonding between copper and lanthanum occurred during the electrodeposition process, thus altering the microscopic morphology. As the ratio of lanthanum to metal in the electrodeposited solution increased, the microscopic morphology of the surface of the electrode further changed, gradually becoming thin clusters of flowers. This was probably due to the low concentration of copper ions in the deposited solution, which prevented the two elements from forming heterogeneous structures. As the proportion of lanthanum metal in the electrodeposition solution increased, the microstructure of the electrode surface underwent further alteration, from nanoflowers to thin clusters. This may be attributed to the low concentration of copper ions in the deposition solution, which prevented the formation of a heterojunction structure between the two elements. Instead, precipitation of lanthanum metal crystals on the electrode surface occurred, rather than the desired copper-lanthanum metal heterojunction. The elemental distribution of the electrode surface in the SEM characterization was further analyzed by the EDS technique, and the results are shown in
Supplementary Figure S2. These results demonstrated that the dendritic crystals formed when Cu
40La
10 was electrodeposited were essentially composed of bluish-green Cu elements. This was due to the low concentration of lanthanum metal, which resulted in the surface of the electrode being predominantly precipitated by the crystallization of Cu ions. As the proportion of lanthanum increased, the proportion of the yellow part of the elemental surface scan (representing the metal La) also increased. This indicates that the proportion of La gradually increased during the deposition process, leading to the crystallization of crystals, which was the main reason for the significant change in the morphology of the electrode surface.
To investigate the crystalline structural features of the catalysts, the HR-TEM characterization technique was applied, as illustrated in
Figure 2e. This revealed that the Cu
30La
20 catalysts were exfoliated after sonication to obtain a thin-layered structure. Furthermore, the high-resolution TEM spectra (
Supplementary Figure S3) indicated that several crystal lattices were dispersed on the surface of the material. Lattice fringes were observed with a spacing of 2.08 Å, corresponding to the (111) crystallographic plane of Cu, and a lattice stripe of 1.8 Å, corresponding to the (211) crystallographic plane of (III) (JCPDS#40-1284) of the La element in the material. At the intersection of the two crystalline surfaces, the presence of additional lattice defects was evident, which served to confirm the successful formation of a heterojunction structure between the two metal elements on the electrode surface. Furthermore, elemental face-scanning analysis was conducted to ascertain the surface elemental composition of the catalyst. The results of this analysis are presented in
Figure 2f–j. The elemental scans demonstrated the uniform distribution of copper and lanthanum within the nanostructures.
The crystal structure, elemental composition, and valence state of the electrode surface were also investigated by XRD and XPS, and the results are presented in
Figure 2k–m. As can be observed in
Figure 2k, the XRD results indicated that the diffraction peaks in the material were primarily situated at the 38.8°, 43.3°, and 54.6° positions. These peaks can be attributed to the structural characteristics of the primary Cu2O (111) and Cu (111) crystal surfaces observed at the 38.8° and 43.3° positions [
24], while the 54.6° peak correlates with the structural peaks of the Cu-La heterojunction, as evidenced by the TEM characterization. The lattices of Cu and La were relatively fragmented, which may be the reason why the characteristic peaks in the XRD patterns were not particularly prominent. The elemental composition and valence states of the electrode surface were also investigated by XPS, and the results are shown in
Figure 2l,m; Cu and La elements were detected in the Cu-La catalysts. The positions of their spectral peaks were observed at 932.1 eV (Cu 2p3/2) and 952.0 eV (Cu 2p1/2), respectively, corresponding to the characteristics of the Cu physical phase. The Cu phases were primarily present as Cu
0 and Cu
+, with Cu
0 being the dominant form. Furthermore, the XPS spectra exhibited characteristic peaks of La
3+ corresponding to the peaks at 835.6 eV (La 3d5/2) and 852.5 eV (La 3d3/2) [
11,
15], respectively. These findings once again substantiated the simultaneous existence of Cu and La in the Cu30-La20 catalyst. In order to determine the valence state of copper, as shown in
Supplementary Figure S4, LMM analysis of the element Cu was carried out, which revealed a certain amount of Cu
+ on the surface of the electrode, determined using least squares fitting in XPS; part of this belonged to Cu+. The same Cu0 also made a certain contribution. The presence of Cu
2+ may have been due to exposure to oxygen in the air and formation of copper oxides.
The micro-morphological characteristics, elemental composition, and valence features of the fabricated Cu30La20 catalysts were subjected to analysis. The micro-morphological analysis revealed that the morphology of the electrodes underwent significant alterations upon adjusting the Cu–La ratio. When the proportion of Cu was more than 40%, the electrode surface predominantly deposited Cu crystals. However, upon doping with metallic lanthanum, the morphology of the electrode surface transformed, adopting a nanoflower-like structure. The lattice of the Cu and La was be observed via the TEM characterization; there were increased numbers of lattice defects at the junctions of the crystal surfaces, indicating the synthesis of Cu-La heterojunctions. Subsequently, the successful doping of Cu and La elements was confirmed by XPS analysis. The Cu element was predominantly present in monovalent and zero-valent forms on the electrode surface, while the La element existed in an ortho-trivalent valence state.
2.2. CO2RR Electrocatalytic Performance
To investigate the selectivity of the Cu-La bimetallic catalyst electron cathode with regard to electrocatalytic reduction products and the reaction pathways during the CO
2 reduction reaction (CO
2RR), the fabricated catalytic electrodes were employed in electrocatalytic carbon dioxide reduction experiments conducted in a conventional H electrolytic cell. The experimental conditions were as follows. The electrolyte was 0.5 M potassium bicarbonate saturated with CO
2; the area of the electrocatalytic cathode was 1 × 1 cm
2; and the CO
2RR experiments were conducted at a constant voltage. The liquid-phase products of CO
2 reduction were detected via NMR, and the gas-phase products were detected using gas chromatography. No liquid-phase products were detected during the experiments (
Supplementary Figure S5). The gas-phase products were identified as CO, CH
4, C
2H
4, and H
2, and calculations indicated that the Faraday sum of the four products was approximately 100%. This further corroborates the conclusion that no liquid-phase products were generated in the CO
2RR experiments. First, different catalytically active metals were screened, and the amount of copper nitrate and urea in the electrodeposition solution was kept constant. Then, the deposited metal of the other active component was replaced, to study the electrocatalytic CO
2RR performance and product distribution of the different active metals. Four different active metals, namely lanthanum, zinc, palladium, and cesium, were selected, and the electrodeposition was performed with a constant current of 1 A/cm
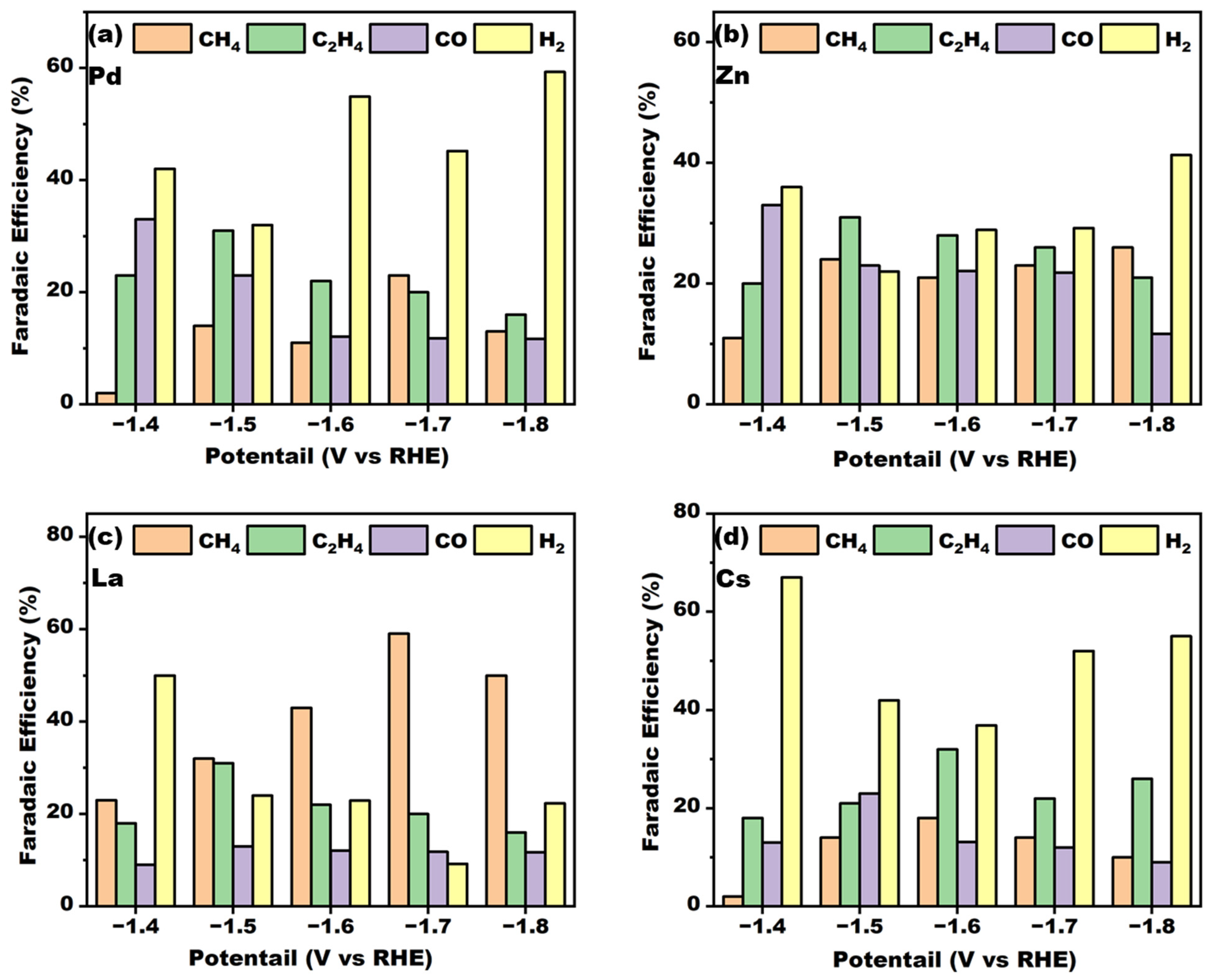
2 for 30 s. The results obtained are shown in
Figure 3. The electrodeposition was carried out for 30 s, after which the obtained electrodes were dried and tested; the results obtained are shown in
Figure 3.
As illustrated in the figure, all four metals demonstrated some CO2RR activity following electrodeposition with elemental copper. However, the resulting reduction products and Faraday efficiencies exhibited notable variation. The doping with Pd metal was observed to enhance selectivity for ethylene at low potentials. With the increase in voltage, selectivity for methane also improved, although the overall level remained relatively low. The doping with Zn metal was observed to exhibit a similar trend; with the emergence of some ethylene selectivity at −1.6 V vs. RHE, the overall reaction was still dominated by the hydrogen precipitation reaction. The doping with metal Cs showed some ethylene selectivity at all potentials, which may have the potential to be an efficient catalyst for electrocatalytic ethylene production. The doping with metal La showed the best methane selectivity, and initially showed 56.9% Faraday efficiency for methane production at −1.7 V vs. RHE, already at a high level. The incorporation of La markedly enhanced methane selectivity and effectively suppress the occurrence of hydrogen precipitation side reactions in comparison with doping with other metals. This suggests that the bimetallic heterojunction formed following the co-deposition of La and Cu can effectively regulate the adsorption of reaction intermediates in the CO2RR process, thereby improving the selectivity of the electrocatalytic methane product.
In the above experiments to determine the metal La and metal Cu as the main active metal in the two-electrode system for electrodeposition experiments for co-deposition, urea was added to adjust the deposition valence of Cu. Constant current was used for electrodeposition, with a current density of 1 A/cm
2 and electrodeposition time of 30 s. The Cu-La electrodes were prepared by adjusting the ratio of Cu to La in the deposition solution, and the prepared electrodes were dried and subjected to CO
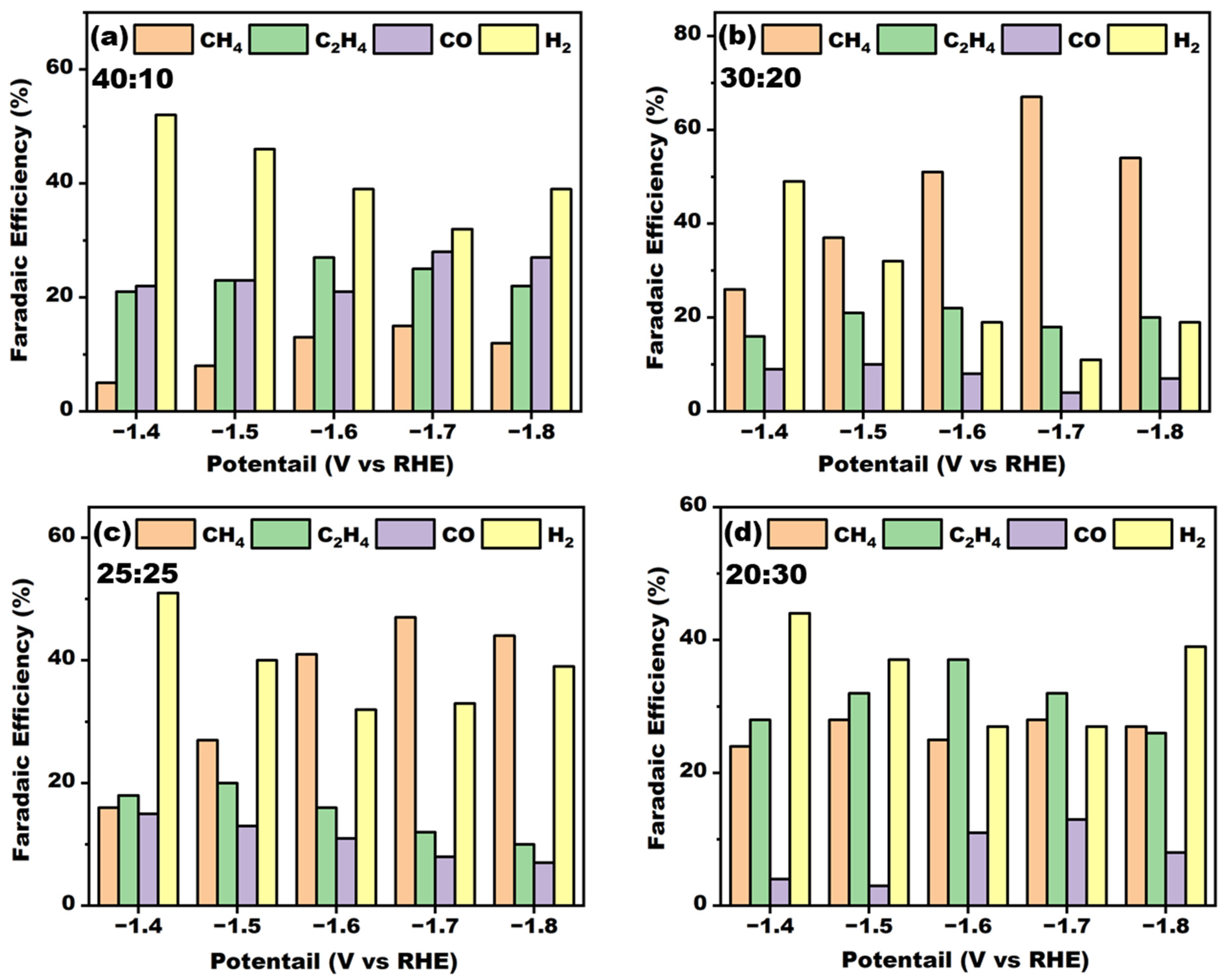
2RR testing; the results are shown in
Figure 4. From the figure, it can be seen that the ratio of metal Cu and metal La had a great influence on the selectivity of the products. When the electrode is not doped with La, the electrocatalytic reduction products were mainly dominated by ethylene (
Supplementary Figure S6). It was also observed from the previous XRD as well as SEM characterization that when the percentage of Cu in the electrolyte was high, the electrode surface was mainly crystallized with copper and copper oxide. The selectivity for ethylene was still maintained in the Cu
40-La
10 electrode, but with the continuous enhancement of the proportion of metal La, the electrode surface generated more Cu-La heterojunction structures. This phenomenon was also observed in the TEM characterization (
Supplementary Figure S3). At this ratio, the Cu
30-La
20 electrode achieved high Faraday efficiency of methane under conditions of −1.7 V vs RHE potential, reaching 66.9%, indicating that the bimetallic sites on the surface of the electrode had a good synergistic catalytic effect. With the gradual enhancement of the proportion of La in the electrode, the methane’s Faraday efficiency gradually reduced, and that of the ethylene gradually increased. Finally, the Faraday efficiencies of both were close to each other, but both of them were maintained at a relatively low level. This may have been due to the large difference in the ratio of the two metals in the deposition solution, leading to the fact that Cu and La did not form more catalytically active heterojunction structures during the deposition process and thus, a reduction in the catalytic performance. From the microscopic morphology of the electrodes, it was also be seen that the morphology of the surface of the electrodes changed significantly with the increase in the ratio of La, which also indicates that the electrodes prepared by electrodeposition with different Cu-La ratios had obvious differences in the constitutive relationships of their catalytic sites.
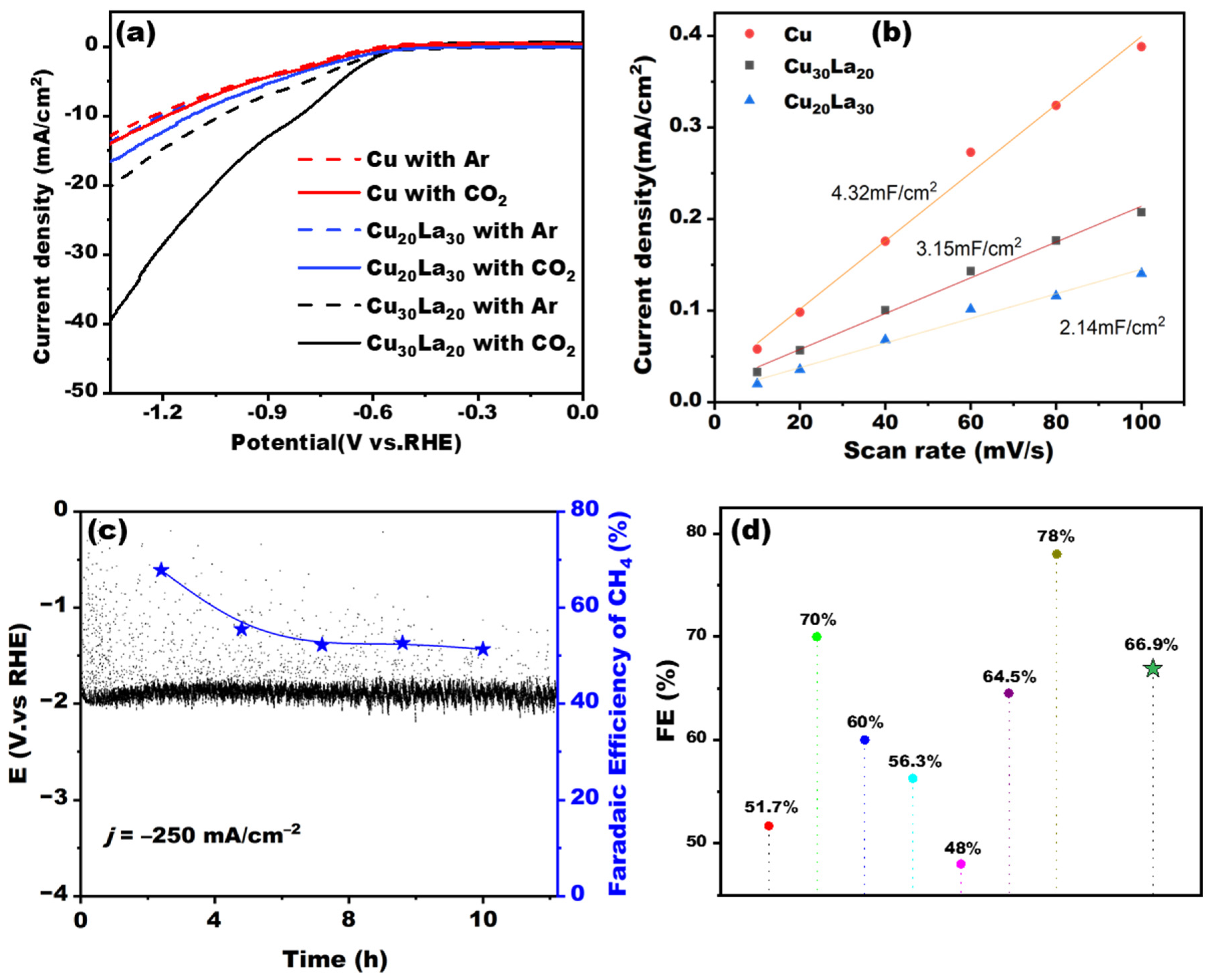
After exploring the deposition ratio of Cu and La, the linear voltammetric curves of pure Cu, bimetallic Cu
30La
20, and Cu
20La
30 under Ar and CO
2 atmospheres were determined to further investigate their performance in CO
2 electrocatalytic reduction. As shown in
Figure 5, the preliminary performance testing of the electrodes was carried out using linear voltammetry; the results, shown in
Figure 5a, show that the current density of the Cu
30La
20 electrode under CO
2 atmosphere was significantly higher than that under Ar atmosphere, and this electrode had the highest current in response to CO
2 and the first current inflection point among the Cu
30La
20 electrodes, followed by the Cu
20La
30 and the pure Cu electrodes, in that order. This indicates that the presence of La induced the Cu
30La
20 electrode’s significant catalytic activity towards CO
2. Subsequently, the current densities in the Ar atmosphere all showed a significant decrease, which indicated that in the CO
2 atmosphere, the CO
2RR reaction occurred on the electrode surface, and the current of the Cu
30La
20 electrode was more negative compared with the Cu
20La
30 electrode; this is consistent with the results of the study of the Faraday efficiency of methane.
The electrochemically active areas of the three electrodes, including Cu
30La
20 and Cu
20La
30, were further measured using cyclic voltammetry (
Supplementary Figure S7), and the electrochemically active areas (ECSAs) of the electrode materials were obtained by calculation. As shown in
Figure 5b, the double-layer capacitance value of the pure Cu electrode was 4.32 mF-cm
−2, the electrochemically active area of the Cu
30La
20 electrode was 3.15 mF-cm
−2, and the electrochemically active area of the Cu
20La
30 electrode was 2.14 mF-cm
−2. For electrocatalysts, enhancement of catalytic effect is generally due to an increase in electrochemically active sites or the reaction caused by a decrease in thermodynamics. The above electrochemical activity measurements revealed that the increase in La doping concentration decreased the electrochemically active area, similar to the experimental findings indicating that overdoped La led to a decrease in the Faraday efficiency of methane. We also tested the electrochemical impedance of the electrodes, and the results are shown in
Supplementary Figure S8. The results showed that the Cu
30La
20 catalytic electrode had the lowest resistance and therefore, the fastest electron transfer rate during this reaction.
Meanwhile, a long-time stability experiment was carried out in a flow cell reaction cell, using the Cu
30La
20 electron cathode as the reducing electrode. The electrolysis conditions were a current density of −250 mA/cm
2 during the 12 h long-time stability test. The obtained curves of voltage versus time as well as the Faraday efficiency of the methane product are shown in
Figure 5c. The results demonstrate that the Faradaic efficiency (FE) of methane production over the Cu
30La
20 electrode stabilized at approximately 50% after 7 h of electrolysis and maintained 51.3% after 12 h, confirming the long-term operational stability of our material. The methane Faraday efficiency reached 66.9% and maintains a high conversion efficiency across the 12 h, which is a notable achievement among recently reported copper-based catalysts for methane production via CO2RR [
10,
15,
17,
18,
21,
34,
42].
The microscopic morphology of the electrode was observed after the persistence experiment, as shown in
Supplementary Figure S9, in which it can be seen that after a long period of electrolysis, the morphology of the electrode greatly changed from its initial nanosized shape to granular clusters, which may occur under conditions of high current density when the microstructure undergoes reconstruction. The elemental surface scanning revealed that the surface of the electrode was dominated by the elements of Cu and La. There were some potassium elements, which may have been caused by the crystallization of the electrolyte on the electrode surface during the electrolysis process. The reconstruction of the microstructure during the catalytic process led to a change in the micro-morphology, which may have been the main reason for the degradation of the electrode’s performance.
2.3. Electrodeposition Impact Parameters
After determining the optimal Cu-La deposition ratio, two key parameters of electrode electrodeposition were experimentally investigated, namely the electrodeposition time as well as the current density of electrodeposition. Unlike the electrodeposition in the three-electrode system in the previous chapter, the rapid electrodeposition method used in this study had a high deposition current density, leading to a faster bonding rate of the components in the deposition solution. This method is not suitable for the preparation of monoatomic structures in a refined way, but it can create more heterojunctions and enhance the catalytic effect. Therefore, the deposition time as well as the current density of deposition plays an important role in this process. The lowest deposition current density in this study was 100 mA/cm
2; the density was gradually increased to 1 A/cm
2. The electrochemical performance of electrodes prepared with different deposition current densities in CO
2RR is shown in
Figure 6.
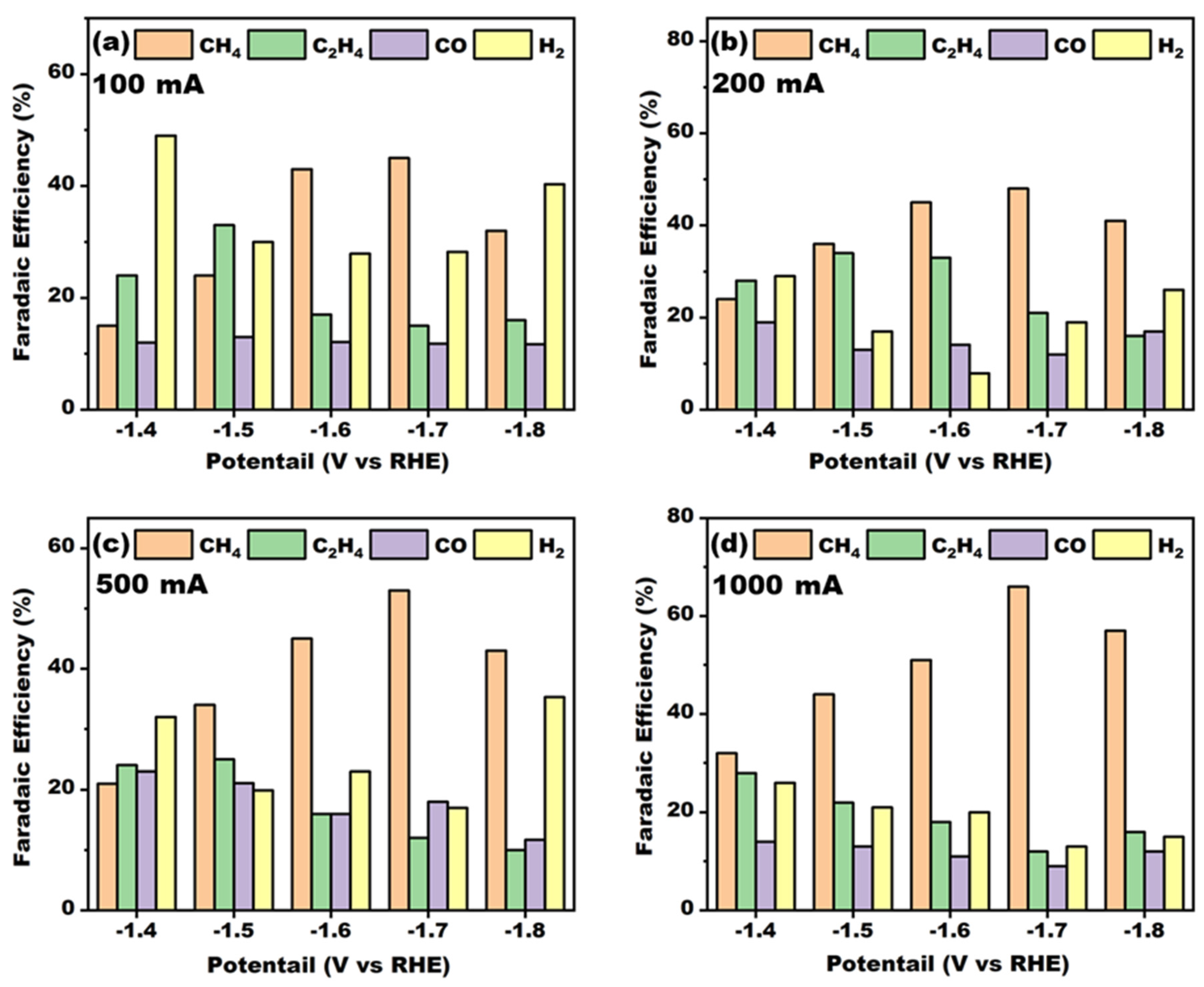
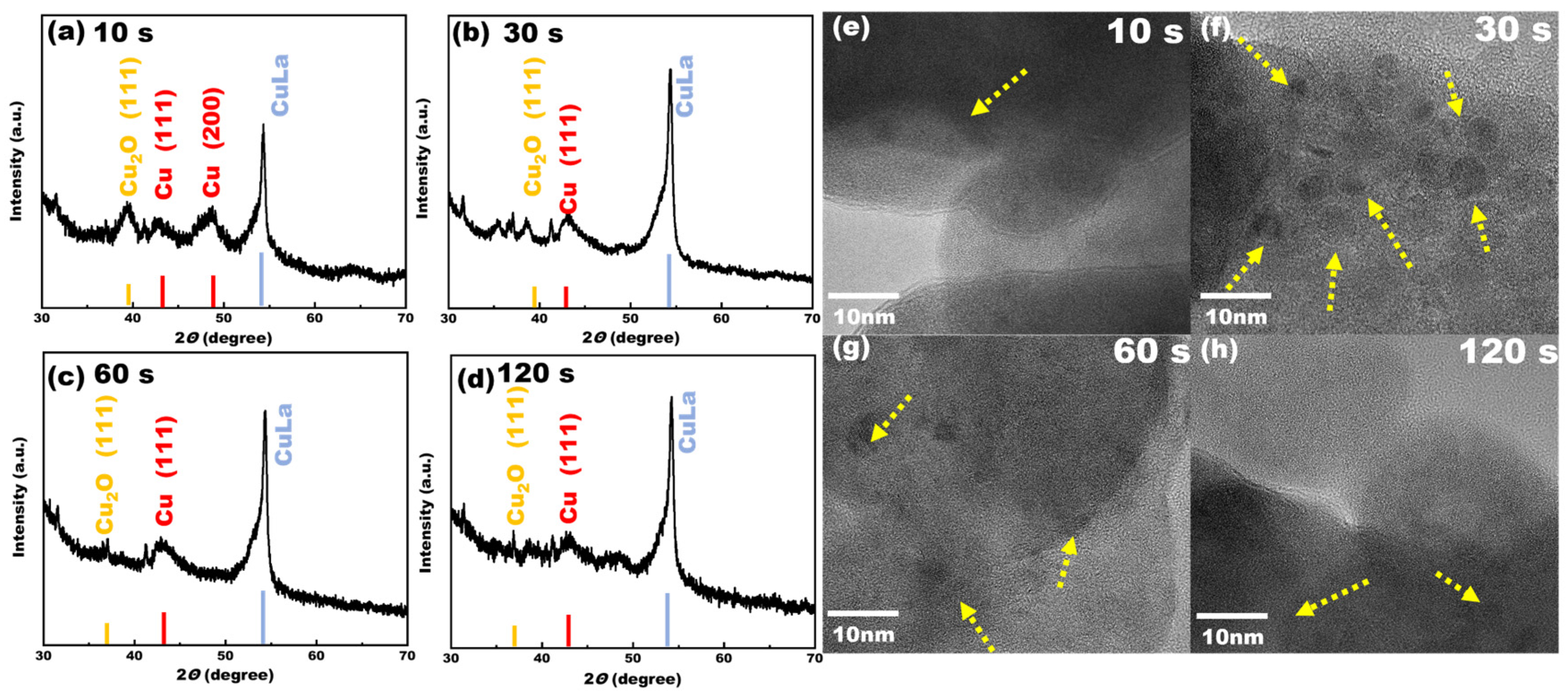
From
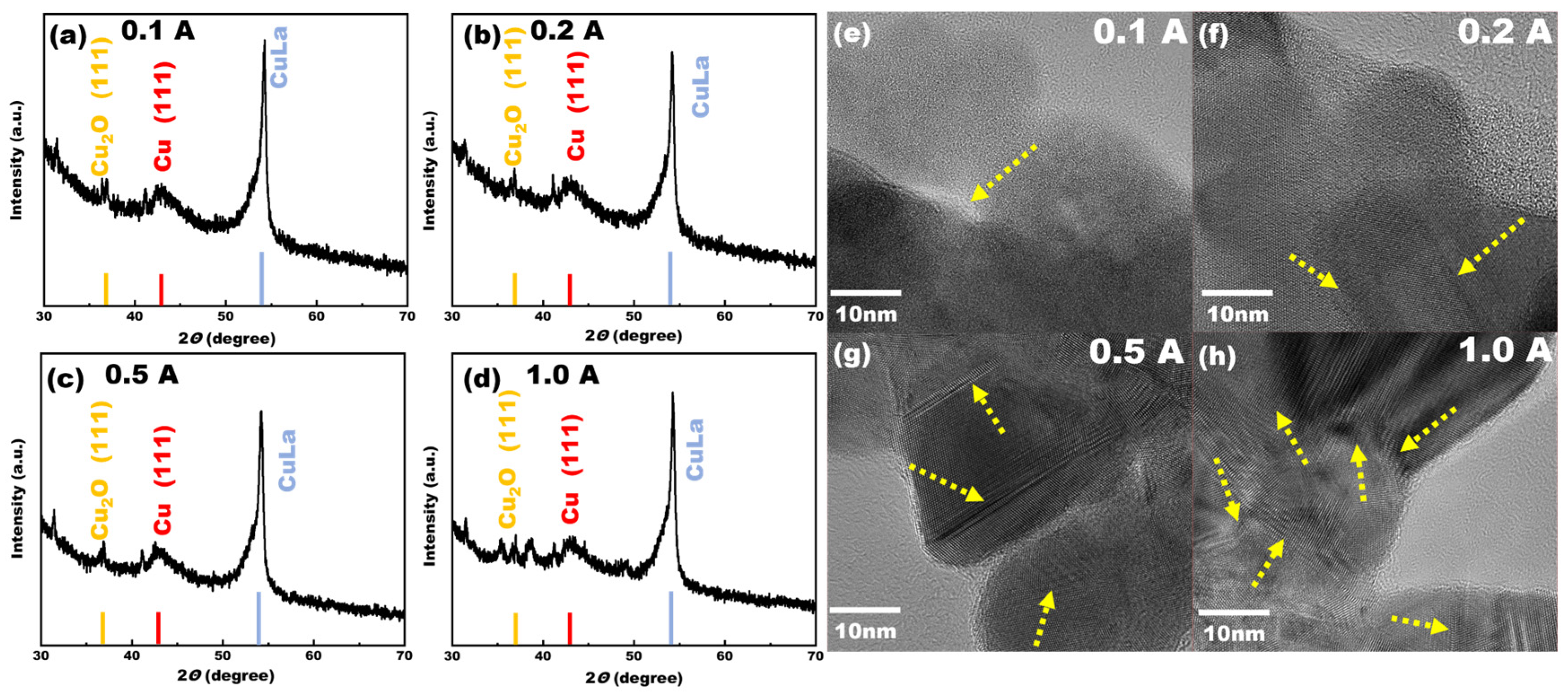
Figure 6, it can be seen that the Faraday efficiency of methane increased with the increase in the deposition current, indicating that there were differences in the structure of the Cu-La complex on the electrode surfaces at this time. For this reason, the electrodes prepared at four different deposition current densities were subjected to XRD characterization to explore their crystal structures; the XRD patterns are shown in
Figure 7. When the deposition current density was lower, the electrodeposition process was more gentle; Cu and La metal formed a part of the heterojunction and the slow deposition of Cu elements on the electrode surface formed Cu (111) crystals. There was some generation of cuprous oxide, but this was not obvious. With the increase in the deposition current density, the characteristic peaks of the cuprous oxide crystals became more and more obvious, indicating that with the increase in the deposition current, the co-deposition of Cu and La was more intense. It was observed from the XRD patterns that when the deposition current reached 1A, more characteristic peaks of cuprous oxide appeared in the prepared electrode and the selectivity of methane also reached a high level. The Cu (111) crystalline surface alone did not demonstrate good catalytic activity, and when the current density increased, the surface of the electrode generated more active substances such as cuprous oxide. A Cu-La A heterojunction structure of Cu-La was also present, and the synergistic effect of many active species was the main reason for the enhancement of the electrocatalytic performance of the electrode.
The TEM and XRD characterization results are consistent with each other. When the deposition current was low, as illustrated in
Figure 7e,f, the deposition process was relatively gentle. When the current was lower than 0.2 A, the metal ions were arranged in an orderly manner, resulting in only a small part of the lattice interface being visible in the TEM characterization. However, when the deposition current gradually increased to 1 A, as observed in
Figure 7g,h, a significant enhancement in the lattice interface of Cu-La became evident, resulting in a clear and stable interface. It has been demonstrated that the lattice interface serves as a crucial reference point [
43]. Consequently, the catalytic performance of the electrode was progressively enhanced with the increasing deposition current, reaching its optimal level at 1 A. At this current density, the XRD characterization revealed the most prominent characteristic peaks of the catalyst’s active species, such as Cu
2O.
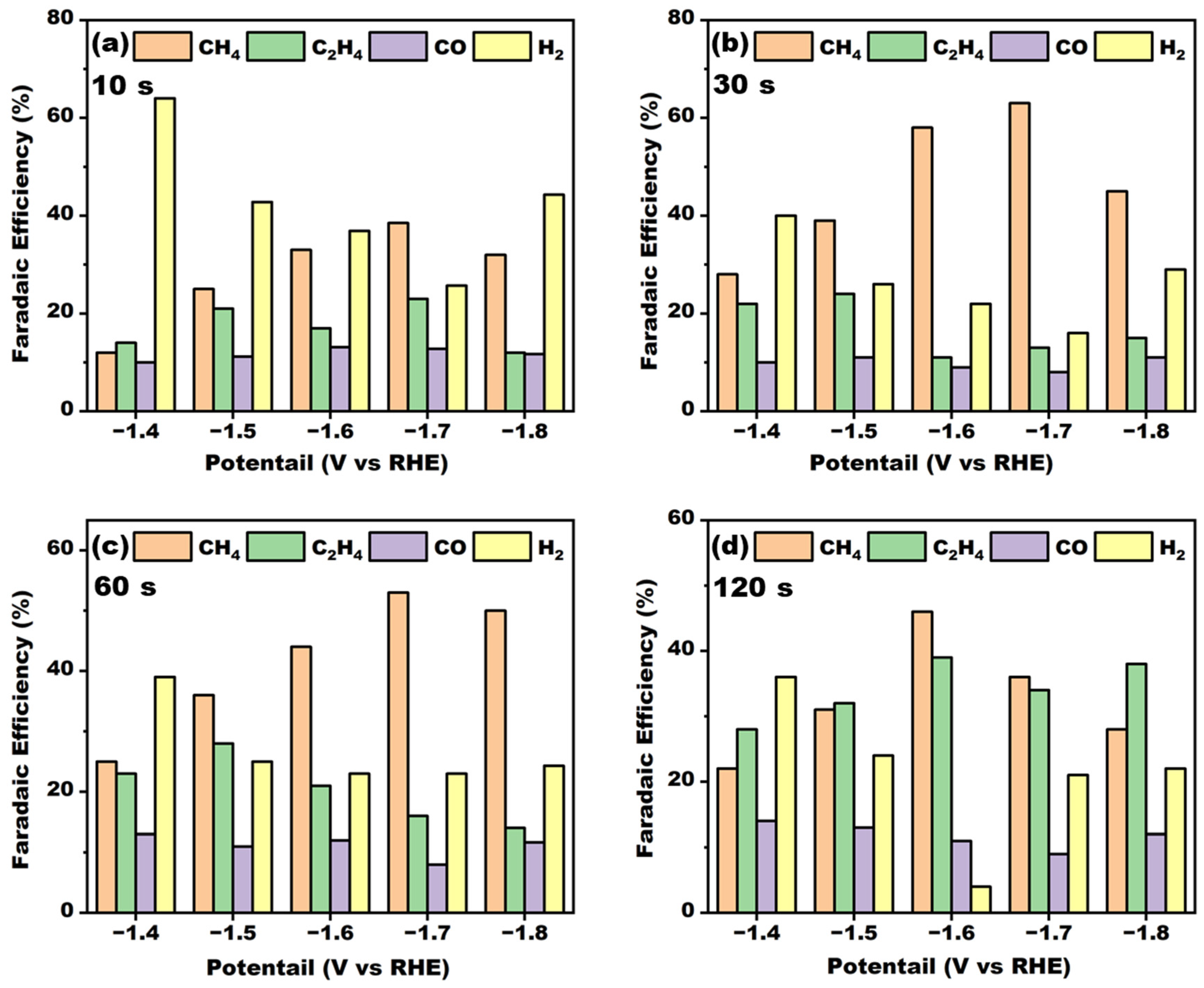
The duration of electrodeposition was further investigated. The length of the deposition time had an important effect on the composition of the active components on the electrode surface. The electrocatalytic performance of the electrodes prepared with different deposition times is shown in
Figure 8. When the deposition time was less than 10 s, the Faraday efficiency of methane was low at 38%, and with the increase of electrolysis time to 30 s, the Faraday efficiency of the electrode was improved and the highest Faraday efficiency reached 63%, indicating that the synergistic effect of the active components on the electrode surface was significant with this deposition time. However, the Faraday efficiency of methane decreased with the increase in deposition time, and the thickness of the electrodeposition layer became thicker and thicker. An overly thick electrode would lead to the weak mass transfer in the electrocatalytic process, which might be the reason for the continuous decrease in the Faraday efficiency of the methane. Furthermore, the crystal structures of the electrodes with different deposition times were analyzed by XRD; the results are shown in
Figure 9. from the figure, it can be seen that when the deposition time was less than 30 s, the crystal structure of the electrode surface was more complex, with the existence of cuprous oxide crystal surfaces, Cu crystal surfaces, and Cu-La heterojunctions, and the electrodes were more electrocatalytic active. With the increase in the deposition time, the copper oxides on the surface of the electrodes and high-valent copper ions on the electrode surface were further reduced to Cu (111) crystals under the action of the electric field. It can also be seen from the figure that the characteristic peaks of cuprous oxide gradually decreased or even disappeared with the increase in the electrodeposition time, but the structure of the Cu-La heterojunction had little effect. There were still more obvious diffraction peaks, and the reduction of copper crystalline phase species may have been the main reason for the decrease in methane Faraday efficiency.
As can be seen from the TEM characterization diagrams, the difference in deposition time greatly affected the density of Cu-La lattice interface formation on the catalyst surface. When the deposition time was shorter than 10 s, a small amount of lattice interface generation could be seen (
Figure 9e). When the deposition time increased to 30 s, the lattice interfaces of Cu-La were observed to increase significantly (
Figure 9f). The lattice junction is an important catalytic active site. Thus, the electrode’s electrocatalytic performance reached its optimum under these deposition time conditions. When the deposition time was increased further, the fragmented lattice on the electrode surface started to reorganize after a long period of reduction, gradually reducing to a large Cu (111) crystal surface, at which time, the catalytic activity gradually decreased, also due to the gradual reduction in the catalytically active center within the electrodeposition process.
2.4. Catalytic Mechanism Study
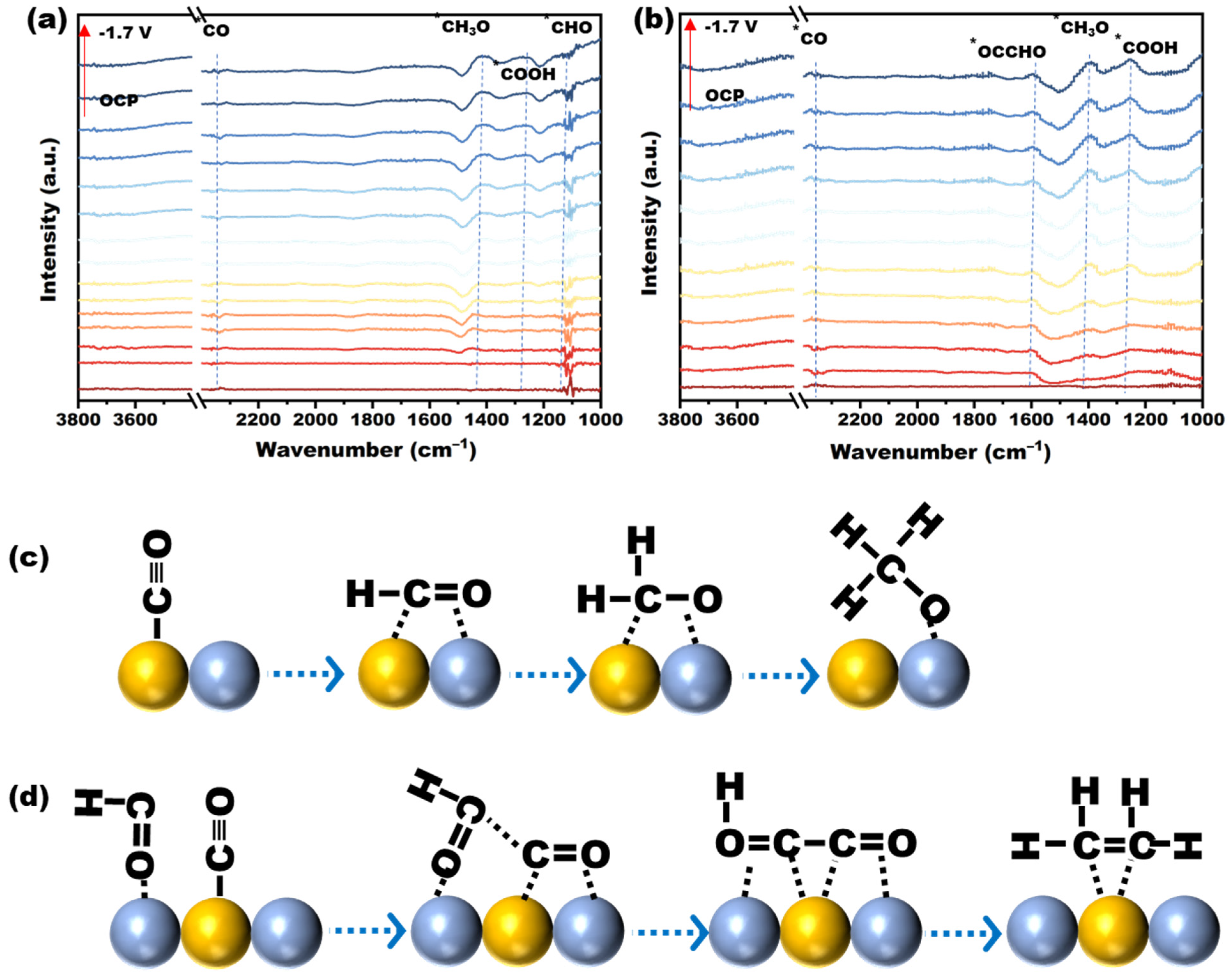
To further reveal the reaction process pathway, the CO
2RR reaction processes of two different catalytic electrodes, Cu
30La
20 as well as Cu
20La
30, were analyzed in situ in an H-reaction cell using enhanced Fourier in situ infrared (FTIR) to observe the generation of the reaction process intermediates, and the results obtained are shown in
Figure 10a,b. The two curves in the diagram exhibit some differences in the peak positions, indicating that the two reaction paths differed during the reaction processes. The following peak positions are worthy of note. In the experimental setup, when the applied voltage exceeded −0.9 V, a minor peak was observed at approximately 2240 cm
−1 in the in situ IR profiles of both catalysts. This peak was attributed to the linear bonding of *CO intermediates, revealing the presence of *CO intermediates on both electrode surfaces, indicating that the CO
2RR reaction occurred on the surface of both electrodes [
44]. Furthermore, a spike was observed at ~1460 cm
−1 for both electrodes, which can be attributed to the carbon dioxide reduction intermediate *CH3O on the surface of the catalysts. The characteristic peaks at 1280 cm
−1 and ~1060 cm
−1 are attributable to the *COOH and *CHO reaction intermediates, which were not detected on the Cu
20La
30 electrode. Notably, the C-C-coupled *OCCHO intermediate included a characteristic peak at 1610 cm
−1 [
33], suggesting that C-C bonds had formed on the Cu
20La
30 electrode surface, facilitating further ethylene production.
As demonstrated in
Figure 10c,d, a synergistic effect was observed between the Cu site and La site on the Cu
30La
20 electrode, which had a reasonable density of metal Cu and La. La (which has low electronegativity) tends to supply electrons to Cu. This results in Cu being electron-rich (Cu becomes more reductive) and La (or its neighbor O) being relatively electron-poor. In the Cu-La catalyst, the Cu 2p3/2 binding energy shifted by ~0.9 eV towards a lower binding energy compared with the pure Cu catalyst, which directly confirmed that the Cu site was electron-acquiring and in an electron-rich state. This is consistent with our proposed La → Cu charge transfer mechanism. In the Cu-La bimetallic/interfacial system, there were significant electronic interactions, mainly manifested by the supply of electrons from La species (e.g., La
2O
3 or doped La atoms) to neighboring Cu atoms/nanoparticles, resulting in the enrichment of the Cu sites with electron density (Cu δ−), whereas the La sites showed a relatively electron-deficient state (La δ+). This electron transfer significantly altered the adsorption behavior of the key intermediates, e.g., the electron-rich state of the Cu weakened the adsorption strength (lower adsorption energy) for the CO intermediates. Weaker CO adsorption favors its detachment from the dissociation or hydrogenation pathway, thus facilitating its further hydrogenation to the *CHxO species, a key precursor for CH
4 generation. In addition, the electron-deficient sites provided by the La component (especially La
2O
3 or interfacial oxygen species) may favor the stabilization of specific oxygen-containing intermediates (e.g., HCOO or H
2CO) and facilitate their further hydrodeoxygenation. At the same time, the unique electronic environment formed at the Cu-La interface may optimize the stability of *CHxO intermediates, making them more inclined to hydrogenate to CH
4.