Heteropolyacid-Based Poly(Ionic Liquid) Catalyst for Ultra-Deep and Recyclable Oxidative Desulfurization of Fuels
Abstract
1. Introduction
2. Results
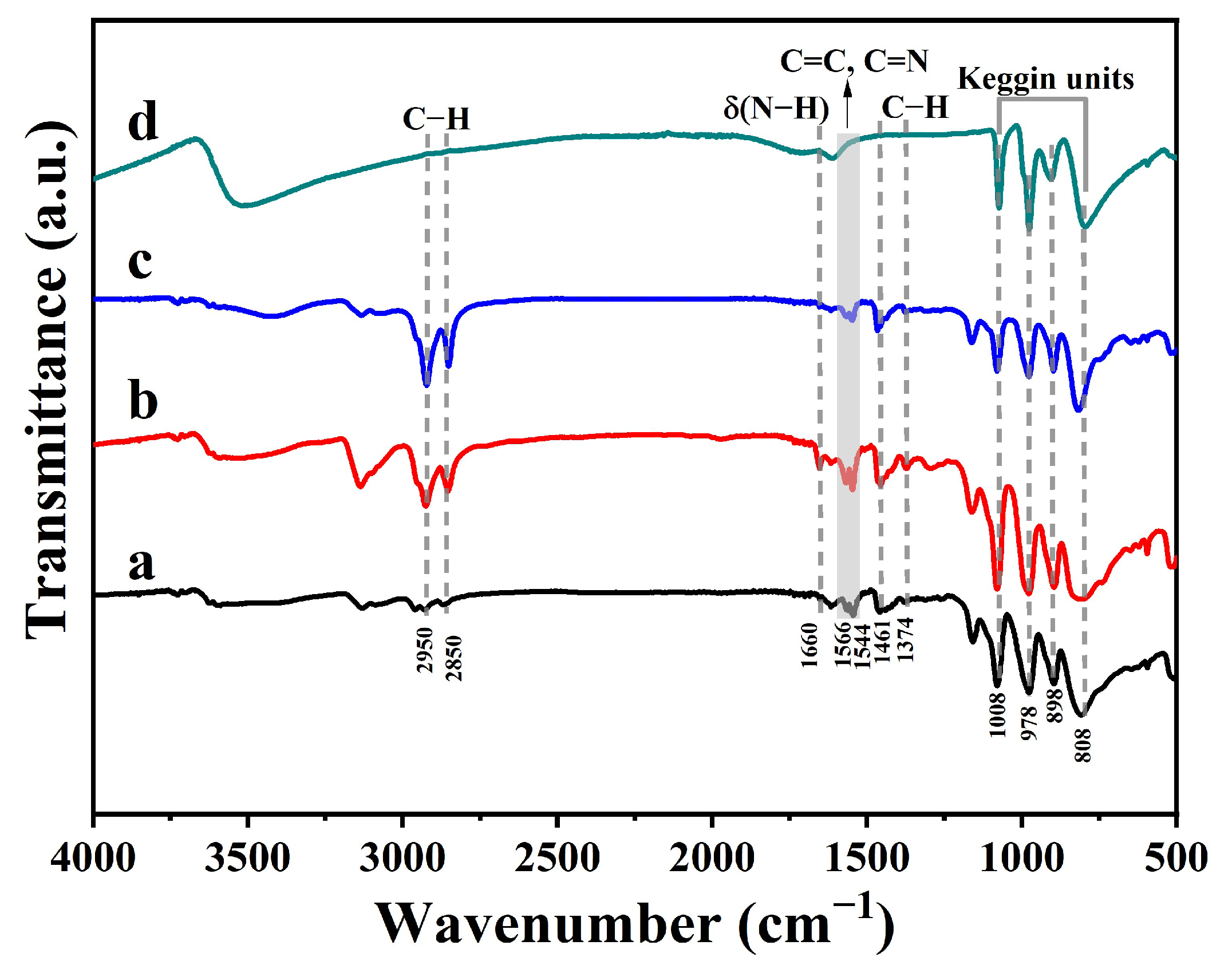
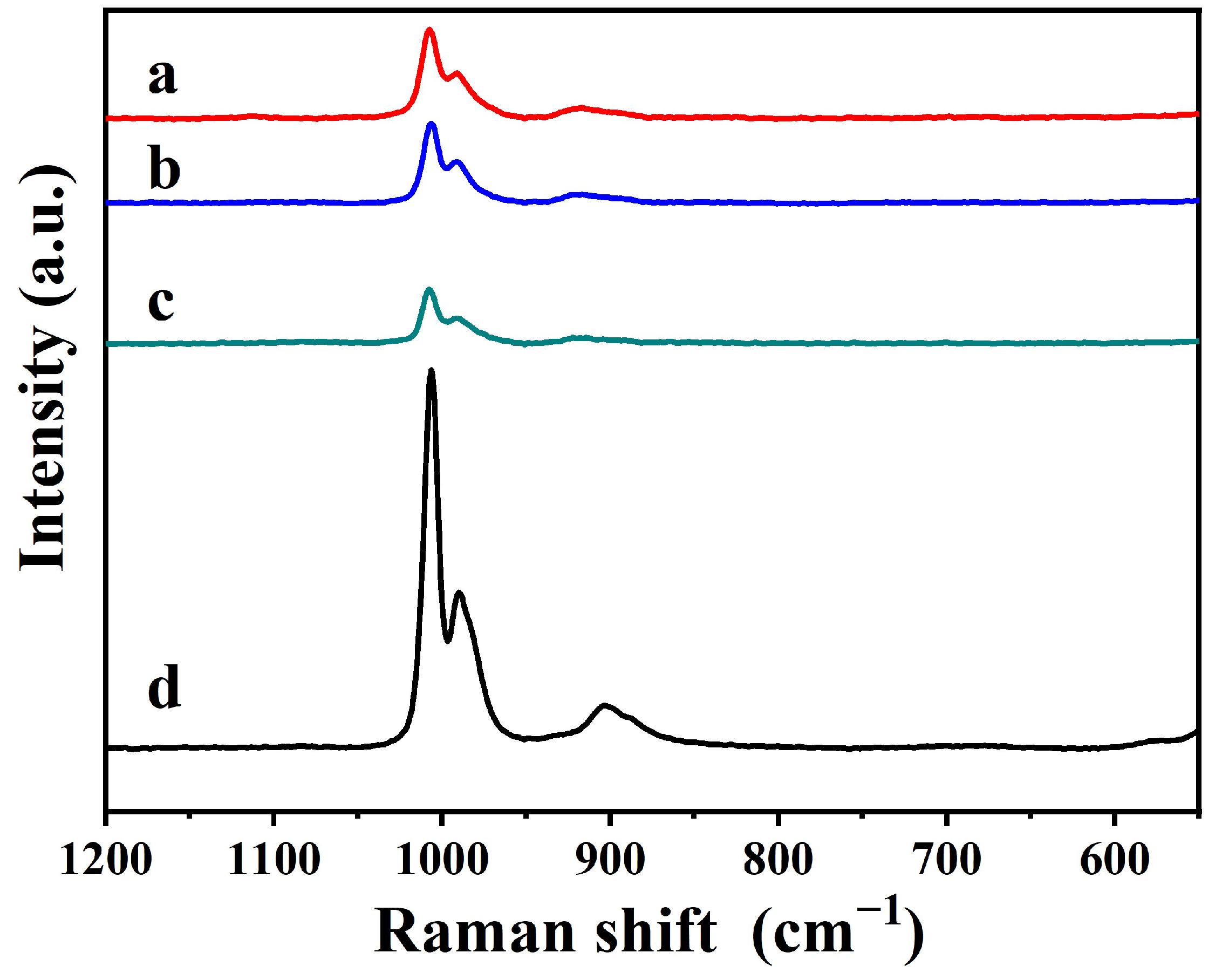
2.1. Characterization of Materials
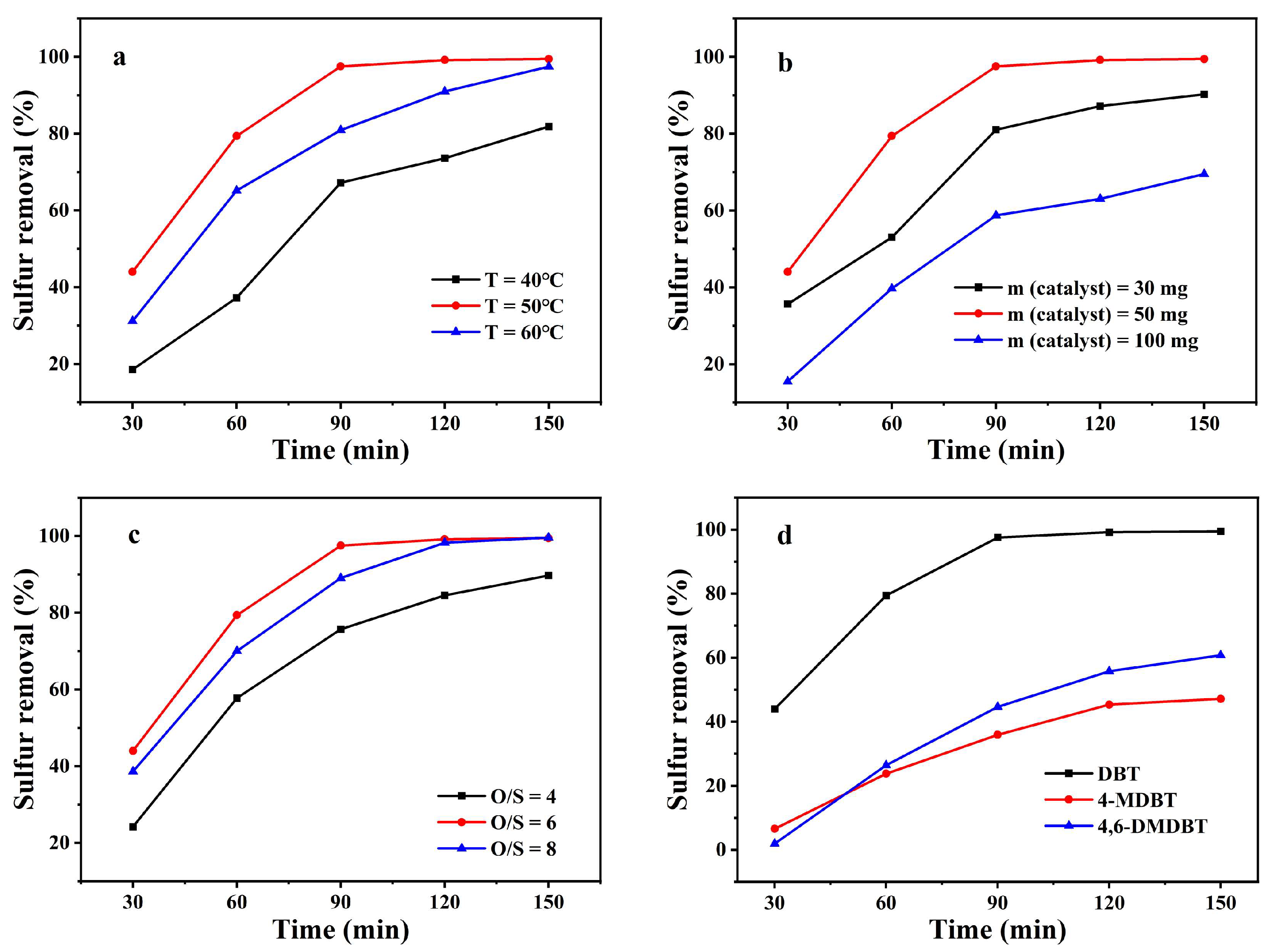
2.2. ODS Performance
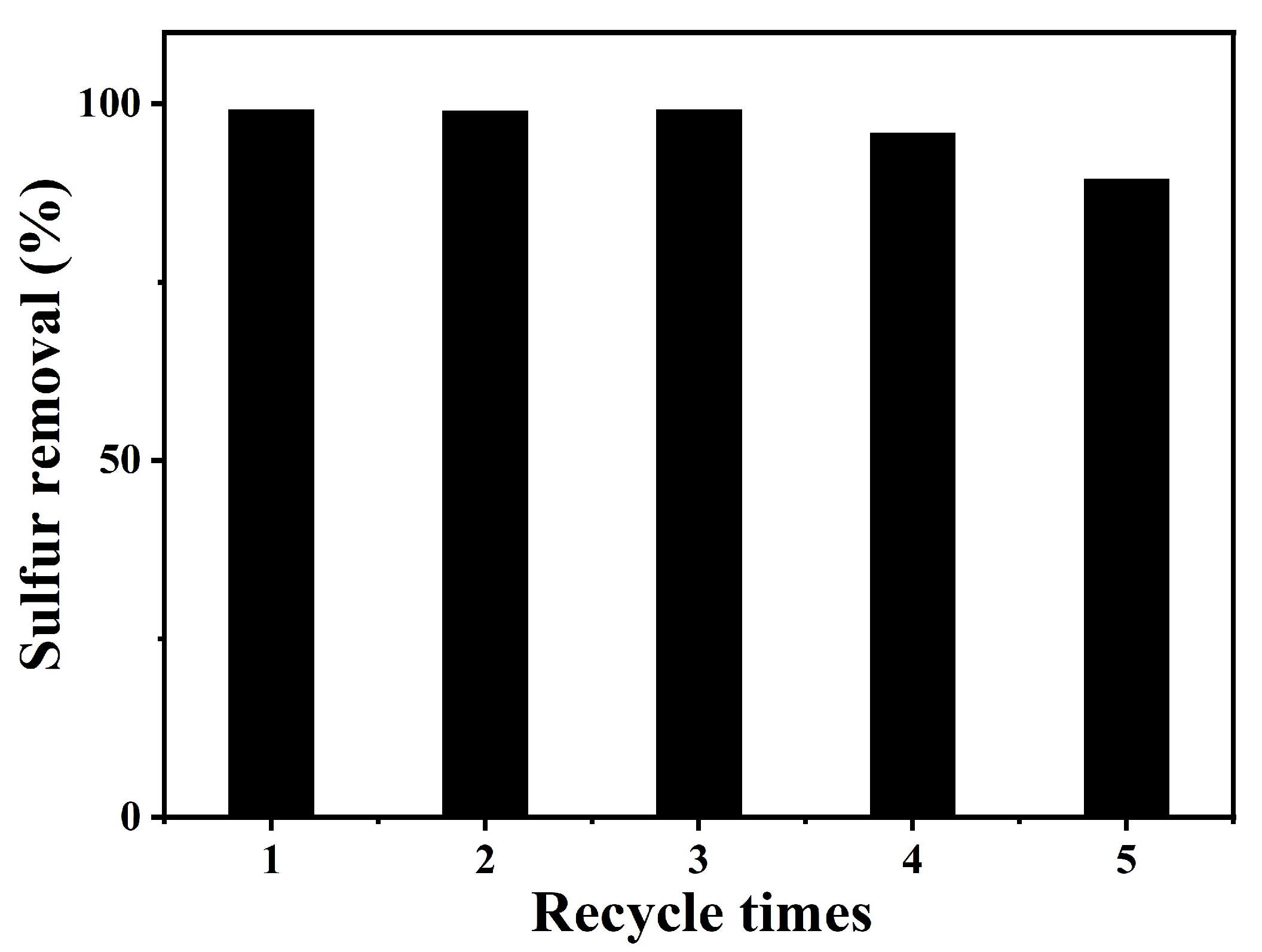
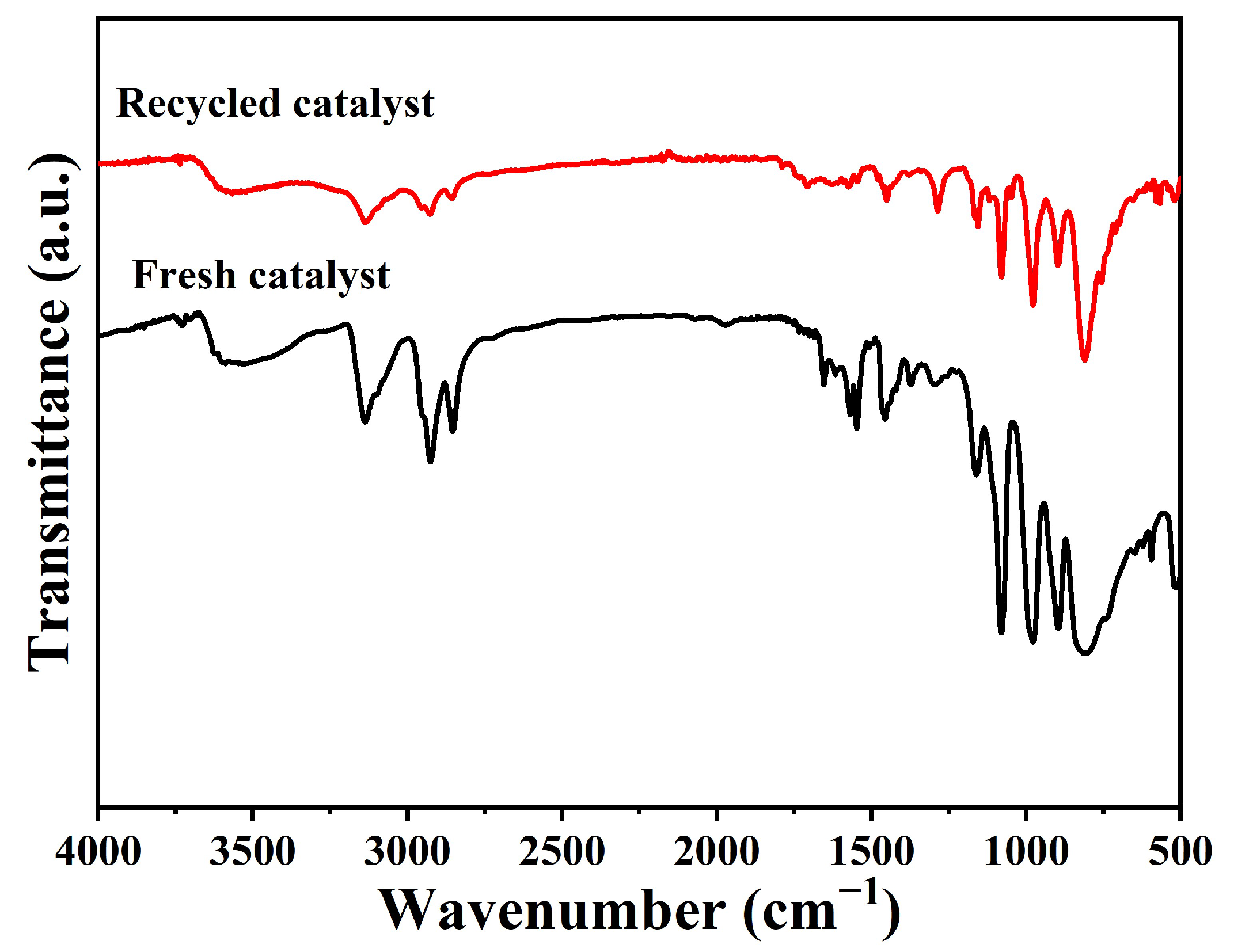
2.3. Catalyst Recycling Capacity
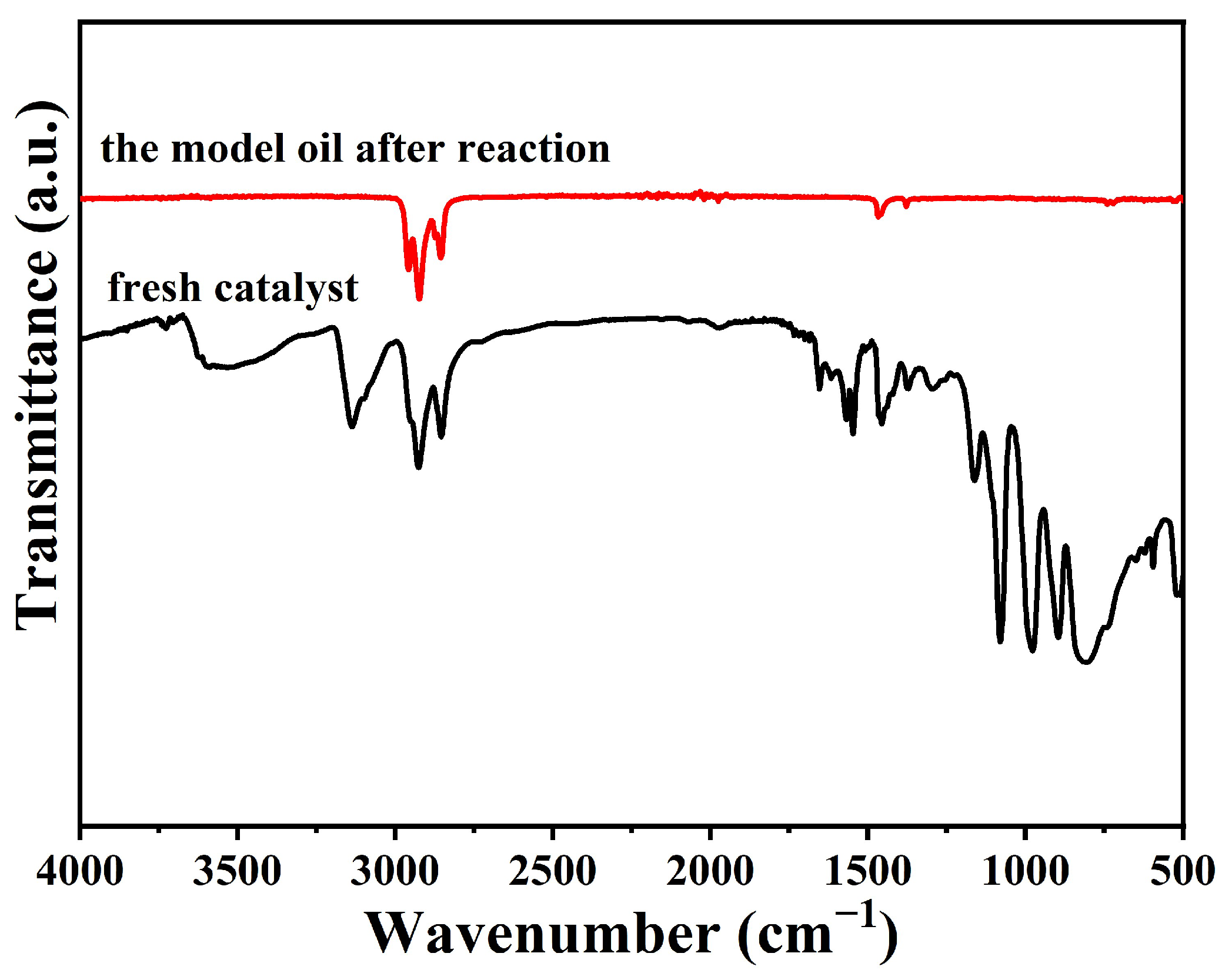
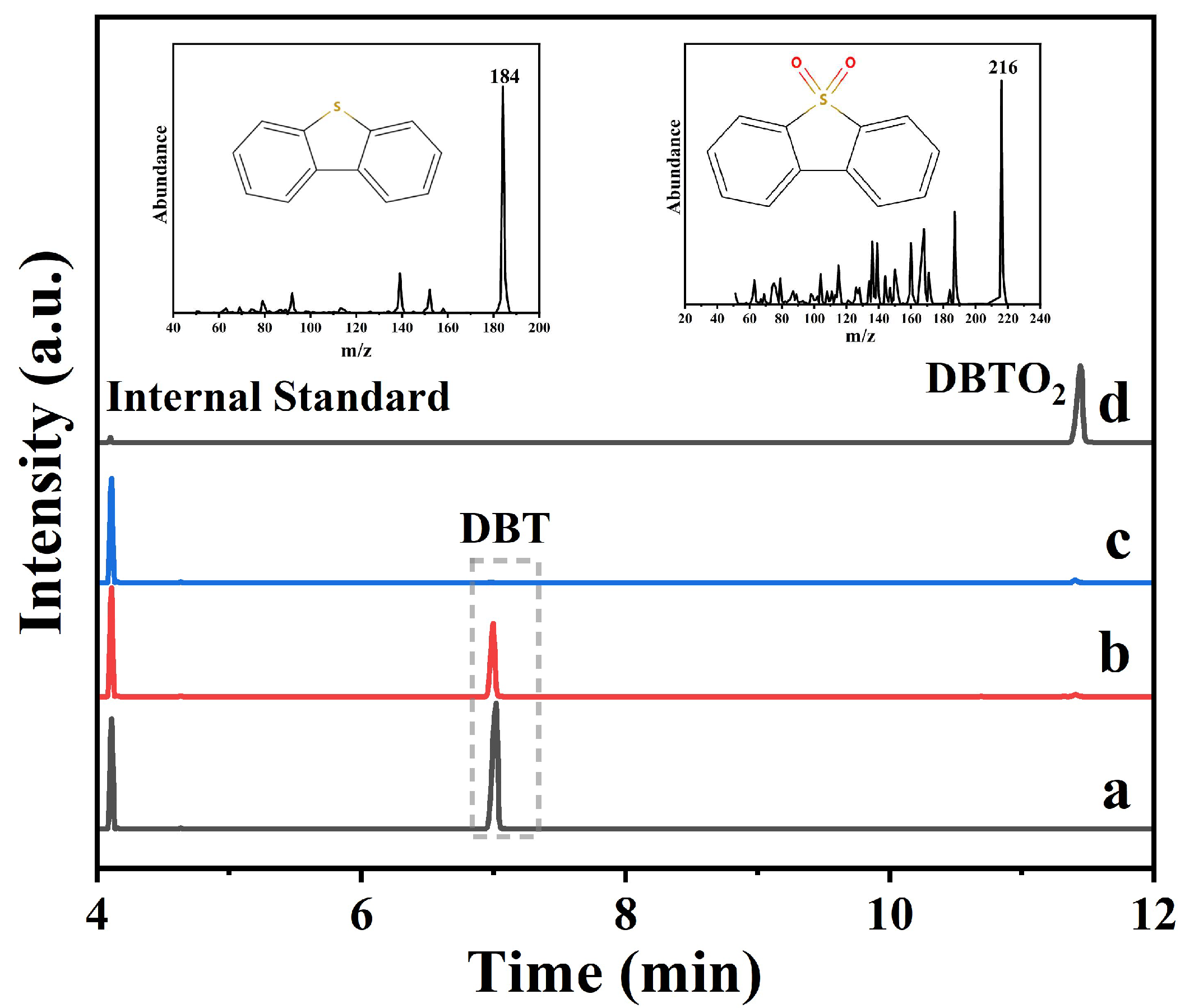
2.4. Catalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
- Polymerization of ionic liquid
- Synthesis of PIL@PW and IL@PW
3.3. Characterization
3.4. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, I.; Kim, C.-U.; Jhung, S.H. Efficient metal-free aerobic oxidative desulfurization with nitrogen and sulfur co-doped covalent organic polymer-derived carbon. Chem. Eng. J. 2023, 474, 145652. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, R.; Feng, Z.; Li, J. Amide-assisted synthesis of TS-1 zeolites with active Ti(OH2)2(OH)2(OSi)2 sites toward efficient oxidative desulfurization. Appl. Catal. B Environ. 2024, 342, 123339. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, J.; Yang, L.; Ma, Z.; Wang, L.; Li, H.; Bai, L.; Wei, D.; Wang, W.; et al. Cellulose nanocrystal shelled with poly(ionic liquid)/polyoxometalate hybrid as efficient catalyst for aerobic oxidative desulfurization. J. Colloid Interface Sci. 2019, 554, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Li, J.; Liu, G.; Qi, X.; Zhang, X.; Ning, Y.; Zhang, B.; Fu, Y.; Liu, S. Adsorption desulfurization performances of Zn/Co porous carbons derived from bimetal-organic frameworks. Chem. Eng. J. 2019, 362, 287–297. [Google Scholar] [CrossRef]
- Tong, S.; Huang, T.; Chen, M.; Wang, C.; Ji, M.; Zhu, L.; Li, H.; Zhang, M. Unraveling the enhanced oxidative desulfurization activity: Synergistically boosting the formation of reactive oxygen species and active centers. J. Colloid Interface Sci. 2025, 689, 137268. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, B.; Sun, Y.; Zhang, L.; Sun, Z.; Wang, J.; Tantai, X. Polymeric cation and isopolyanion ionic self-assembly: Novel thin-layer mesoporous catalyst for oxidative desulfurization. Chem. Eng. J. 2017, 317, 32–41. [Google Scholar] [CrossRef]
- Bryzhin, A.A.; Gantman, M.G.; Buryak, A.K.; Tarkhanova, I.G. Brønsted acidic SILP-based catalysts with H3PMo12O40 or H3PW12O40 in the oxidative desulfurization of fuels. Appl. Catal. B Environ. 2019, 257, 117938. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Peng, X.L.; Ding, Y.C.; Yu, S.S.; Zhang, F.Q.; Zhang, L.F.; Wu, H.D.; Guo, J. Design and preparation of polyoxometalate-based catalyst MIMPs 3PMo6W6O40 and its application in deep oxidative desulfurization with excellent recycle performance and low molar O/S ratio. Chem. Eng. J. 2022, 429, 132446. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, W.; Li, H.; Zhang, M.; Jiang, W.; Chen, G.; Han, C. Polyoxometalate-based ionic liquids as catalysts for deep desulfurization of fuels. Fuel Process. Technol. 2011, 92, 1842–1848. [Google Scholar] [CrossRef]
- Macaud, M.; Milenkovic, A.; Schulz, E.; Lemaire, M.; Vrinat, M. Hydrodesulfurization of Alkyldibenzothiophenes: Evidence of Highly Unreactive Aromatic Sulfur Compounds. J. Catal. 2000, 193, 255–263. [Google Scholar] [CrossRef]
- Ma, X.; Sakanishi, K.; Mochida, I. Hydrodesulfurization reactivities of various sulfur compounds in diesel fuel. Ind. Eng. Chem. Res. 1994, 33, 218–222. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Wei, A.; Zhu, W.; Xun, S.; Li, Y.; Li, H.; Li, H. Facile synthesis of amphiphilic polyoxometalate-based ionic liquid supported silica induced efficient performance in oxidative desulfurization. J. Mol. Catal. A Chem. 2015, 406, 23–30. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, W.; Dai, B.; Chao, Y.; Li, C.; Li, H.; Zhang, M.; Jiang, W.; Li, H. Copper nanoparticles advance electron mobility of graphene-like boron nitride for enhanced aerobic oxidative desulfurization. Chem. Eng. J. 2016, 301, 123–131. [Google Scholar] [CrossRef]
- Lü, H.; Gao, J.; Jiang, Z.; Yang, Y.; Song, B.; Li, C. Oxidative desulfurization of dibenzothiophene with molecular oxygen using emulsion catalysis. Chem. Commun. 2007, 150–152. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Wang, X.; Jin, K.; Ma, W. Oxidative desulfurization of dibenzothiophene based on molecular oxygen and iron phthalocyanine. Fuel Process. Technol. 2009, 90, 317–323. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Oveisi, M.; Nia Asli, M.A. Phosphotungestovanadate immobilized on PVA as an efficient and reusable nano catalyst for oxidative desulphurization of gasoline. J. Mol. Catal. A Chem. 2015, 410, 121–132. [Google Scholar] [CrossRef]
- García-Gutiérrez, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; Murrieta, F.; Navarrete, J.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3. Appl. Catal. A Gen. 2006, 305, 15–20. [Google Scholar] [CrossRef]
- Miao, G.; Huang, D.; Ren, X.; Li, X.; Li, Z.; Xiao, J. Visible-light induced photocatalytic oxidative desulfurization using BiVO4/C3N4@SiO2 with air/cumene hydroperoxide under ambient conditions. Appl. Catal. B Environ. 2016, 192, 72–79. [Google Scholar] [CrossRef]
- Li, S.-W.; Li, J.-R.; Gao, Y.; Liang, L.-L.; Zhang, R.-L.; Zhao, J.-S. Metal modified heteropolyacid incorporated into porous materials for a highly oxidative desulfurization of DBT under molecular oxygen. Fuel 2017, 197, 551–561. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef]
- Jiang, W.; Zheng, D.; Xun, S.; Qin, Y.; Lu, Q.; Zhu, W.; Li, H. Polyoxometalate-based ionic liquid supported on graphite carbon induced solvent-free ultra-deep oxidative desulfurization of model fuels. Fuel 2017, 190, 1–9. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Haghighat Mamaghani, A.; Beheshtian, J.; Thompson, L.T.; Mortazavi, Y. Microporous titania–silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 65–77. [Google Scholar] [CrossRef]
- Huang, T.; Qiu, X.; Zhu, L.; Wang, C.; Li, H.; Fan, Y.; Zhang, M.; Li, H.; Zhu, W. Engineering high specific surface area over poly(ionic liquids)-derived molybdenum-silica hybrid materials for enhanced oxidative desulfurization. J. Environ. Chem. Eng. 2023, 11, 111487. [Google Scholar] [CrossRef]
- Xun, S.; Hu, C.; Yang, B.; Jiang, W.; He, M.; Zhu, W.; Li, H. Construction of imidazole-based ionic liquid modified MoO3-x for enhancing photocatalytic oxidation desulfurization in diesel. Green. Energy Environ. 2025. [Google Scholar] [CrossRef]
- Hamid, A.; Azam, A.; Mohammad Reza, O. Catalytic oxidative desulfurization performance of immobilized NMP.FeCl3 ionic liquid on γ-Al2O3 support. Chem. Eng. J. 2017, 320, 189–200. [Google Scholar]
- Zhang, J.; Wu, H.; Yesire, Y.; Zhang, Y.; Ding, J.; Fan, Y.; Zhang, M.; Wang, C.; Li, H. Amphiphilic Catalysts Comprising Phosphomolybdic Acid Fastened on MIL-101(Cr): Enabling Efficient Oxidative Desulfurization under Solvent-Free and Moderate Reaction Conditions. Energy Fuels 2024, 38, 8553–8563. [Google Scholar] [CrossRef]
- Ghubayra, R.; Nuttall, C.; Hodgkiss, S.; Craven, M.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids. Appl. Catal. B Environ. 2019, 253, 309–316. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Wang, R. Homogeneous oxidative desulfurization catalyzed by a recoverable reaction-controlled phase transfer catalyst based on trilacunary Keggin polyoxometalate. New J. Chem. 2019, 43, 7363–7370. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.; Ding, W.; Yang, L.; Chao, Y.; Li, H.; Zhu, F.; Li, H. Phosphotungstic Acid Immobilized on Ionic Liquid-Modified SBA-15: Efficient Hydrophobic Heterogeneous Catalyst for Oxidative Desulfurization in Fuel. Ind. Eng. Chem. Res. 2014, 53, 19895–19904. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, G.; Zhang, Y.; Zeng, D.; Chen, Y. Direct synthesis of mesoporous H3PMo12O40/SiO2 and its catalytic performance in oxidative desulfurization of fuel oil. Fuel 2015, 147, 195–202. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, L.; Wu, Y.; Liu, B.; Dai, L.; Li, Z.; Xia, Q.; Xi, H. Effect of gasoline composition on oxidative desulfurization using a phosphotungstic acid/activated carbon catalyst with hydrogen peroxide. Appl. Energy 2014, 113, 78–85. [Google Scholar] [CrossRef]
- Poli, E.; De Sousa, R.; Jerome, F.; Pouilloux, Y.; Clacens, J.-M. Catalytic epoxidation of styrene and methyl oleate over peroxophosphotungstate entrapped in mesoporous SBA-15. Catal. Sci. Technol. 2012, 2, 910–914. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yoshida, C.; Uchida, S.; Mizuno, N. Peroxotungstate immobilized on ionic liquid-modified silica as a heterogeneous epoxidation catalyst with hydrogen peroxide. J. Am. Chem. Soc. 2005, 127, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, Y.; Li, R.; Zhu, W.; Li, H.; Zhang, Q.; Wang, M.; Chen, X.; Li, H. Magnetic POM-based mesoporous silica for fast oxidation of aromatic sulfur compounds. Fuel 2017, 209, 545–551. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, H.; Wei, Y.; Fu, Y.; Liao, W.; Zhu, L.; Chen, G.; Zhu, W.; Li, H. Tuning the electrophilicity of vanadium-substituted polyoxometalate based ionic liquids for high-efficiency aerobic oxidative desulfurization. Appl. Catal. B Environ. 2020, 271, 118936. [Google Scholar] [CrossRef]
- Huang, W.; He, X.; Wu, J.; Ma, X.; Han, J.; Wang, L.; Wang, Y. The evaluation of deep eutectic solvents and ionic liquids as cosolvents system for improving cellulase properties. Ind. Crops Prod. 2023, 197, 116555. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, J.; Liang, J.; Li, M.; Fu, Y.; Wang, H.; Tu, S.; Li, J. Hypercrosslinked mesoporous poly(ionic liquid)s with high density of ion pairs: Efficient adsorbents for Cr(VI) removal via ion-exchange. Chem. Eng. J. 2019, 378, 122107. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Liu, Y.; Wang, L.; Bai, L.; Yang, L.; Wei, D.; Wang, W.; Niu, Y.; Chen, H. Rapid removal of anionic dye from water by poly(ionic liquid)-modified magnetic nanoparticles. J. Mol. Liq. 2019, 284, 383–392. [Google Scholar] [CrossRef]
- Yuan, J.; Prescher, S.; Sakaushi, K.; Antonietti, M. Novel polyvinylimidazolium nanoparticles as high-performance binders for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 7229–7234. [Google Scholar] [CrossRef]
- Safa, M.; Mokhtarani, B.; Mortaheb, H.R. Deep extractive desulfurization of dibenzothiophene with imidazolium or pyridinium-based ionic liquids. Chem. Eng. Res. Des. 2016, 111, 323–331. [Google Scholar] [CrossRef]
- Al Kaisy, G.M.J.; Abdul Mutalib, M.I.; Bustam, M.A.; Leveque, J.-M.; Muhammad, N. Liquid-Liquid extraction of aromatics and sulfur compounds from base oil using ionic liquids. J. Environ. Chem. Eng. 2016, 4, 4786–4793. [Google Scholar] [CrossRef]
- Gao, S.; Li, J.; Chen, X.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. A combination desulfurization method for diesel fuel: Oxidation by ionic liquid with extraction by solvent. Fuel 2018, 224, 545–551. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Mao, D.; Chen, G.; Wang, X.; Yang, X.; Wang, M.; Peng, L.; Wang, J. Heteropolyanion-based ionic liquid-functionalized mesoporous copolymer catalyst for Friedel–Crafts benzylation of arenes with benzyl alcohol. Chem. Eng. J. 2014, 254, 54–62. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Sun, Y.; Jiang, B.; Chen, Y.; Gao, X.; Yang, H. Deep catalytic oxidative desulfurization of fuels by novel Lewis acidic ionic liquids. Fuel Process. Technol. 2018, 177, 81–88. [Google Scholar] [CrossRef]
- Mayuri, P.; Nellepalli, P.; Vijayakrishna, K.; Senthil Kumar, A. Tuning Poly(ionic liquid) as a Facile Anion (Hexacyanoferrate(III) ion) Exchanger after Being Adsorbed on Graphitic Nanomaterial and Its Versatile Electrocatalytic Oxidation of Ascorbic Acid. J. Phys. Chem. C 2019, 123, 19637–19648. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Meng, H.; Lu, Y.; Li, C. Poly(ionic liquid)s based nano core-shell catalyst SiO2@V-PIL for efficient oxidative desulfurization of diesel. Appl. Catal. A Gen. 2021, 616, 118096. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Li, D.; Wu, J.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Sulfadiazine detection in aquatic products using upconversion nanosensor based on photo-induced electron transfer with imidazole ligands and copper ions. Food Chem. 2024, 456, 139992. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, M.; Gao, Y.; Tan, H.; Li, H.; Wang, C.; Zhu, W.; Li, H. Facile Construction of Magnetic Ionic Liquid Supported Silica for Aerobic Oxidative Desulfurization in Fuel. Catalysts 2021, 11, 1496. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, W.; Xun, S.; Li, H.; Gu, Q.; Zhao, Z.; Wang, Q. Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids. Chem. Eng. J. 2013, 220, 328–336. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, D.; Xun, S.; He, M.; Ma, R.; Jiang, W.; Li, H.; Zhu, W.; Li, H. Amorphous TiO2-supported Keggin-type ionic liquid catalyst catalytic oxidation of dibenzothiophene in diesel. Pet. Sci. 2018, 15, 870–881. [Google Scholar] [CrossRef]
- Liu, F.; Huang, T.; Chen, M.; He, J.; Li, H.; Wang, C.; Li, H.; Zhang, M. Facile preparation of decavanadate-based poly (ionic liquids) for efficient aerobic oxidative desulfurization. J. Mol. Struct. 2025, 1324, 140768. [Google Scholar] [CrossRef]
- Kumar, A.; Omar, R.A.; Verma, N. Efficient electro-oxidation of diclofenac persistent organic pollutant in wastewater using carbon film-supported Cu-rGO electrode. Chemosphere 2020, 248, 126030. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the Development of Advanced Catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhu, W.; Xiong, J.; Yang, L.; Wei, A.; Zhang, M.; Li, H. Novel heterogeneous iron-based redox ionic liquid supported on SBA-15 for deep oxidative desulfurization of fuels. Chem. Eng. J. 2015, 266, 213–221. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Lv, Y.; Ren, J.; Wang, R.; Jiao, W. Oxidative desulfurization of liquid fuels catalyzed by W2C@C derived from metallophthalocyanine/phosphotungstic acid composites. Sep. Purif. Technol. 2022, 281, 119953. [Google Scholar] [CrossRef]
- García-Gutiérrez, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; García, P.; Murrieta-Guevara, F.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel by the Mo/Al2O3-H2O2 system: The effect of system parameters on catalytic activity. Appl. Catal. A Gen. 2008, 334, 366–373. [Google Scholar] [CrossRef]
- Tong, S.; Gao, Y.; Huang, T.; Chen, M.; Zhu, Z.; Li, H.; Wang, C.; Li, H.; Zhang, M. Engineering porous poly-ionic liquids via a soft template approach: Novel carrier for robust incorporation of phosphomolybdic acid for oxidative desulfurization. New J. Chem. 2025, 49, 3002–3005. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Yang, H.; Dong, Y.; Liu, Y.; Yang, L.; Wei, D.; Wang, W.; Bai, L.; Chen, H. Efficient aerobic oxidative desulfurization over Co–Mo–O bimetallic oxide catalysts. Catal. Sci. Technol. 2019, 9, 2915–2922. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Tan, J.; Hu, B. Polyoxometalate Dicationic Ionic Liquids as Catalyst for Extractive Coupled Catalytic Oxidative Desulfurization. Catalysts 2021, 11, 356. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Hadi, M.; Rezvani, H. Synthesis of new nanocomposite based on ceramic and heteropolymolybdate using leaf extract of Aloe vera as a high-performance nanocatalyst to desulfurization of real fuel. Appl. Organomet. Chem. 2021, 35, e6176. [Google Scholar] [CrossRef]
- Banisharif, F.; Dehghani, M.R.; Capel-Sánchez, M.; Campos-Martin, J.M. Desulfurization of Fuel by Extraction and Catalytic Oxidation Using a Vanadium Substituted Dawson-Type Emulsion Catalyst. Ind. Eng. Chem. Res. 2017, 56, 3839–3852. [Google Scholar] [CrossRef]
- Komal, D.; Swapnil, D.; Jalaja, P.; Satyam, S. Ultrasound-assisted extractive/oxidative desulfurization of oil using environmentally benign trihexyl tetradecyl phosphonium chloride. Environ. Technol. Innov. 2021, 24, 101965. [Google Scholar]
- Ribeiro, S.O.; Duarte, B.; De Castro, B.; Granadeiro, C.M.; Balula, S.S. Improving the Catalytic Performance of Keggin [PW12O40]3− for Oxidative Desulfurization: Ionic Liquids versus SBA-15 Composite. Materials 2018, 11, 1196. [Google Scholar] [CrossRef]
- Lingwan, H.; Lulu, S.; Ting, S.; Dongmei, H.; Weiping, L.; Changliang, D.; Wanzhong, R.; Yanmei, Z.; Hongying, L. Polyoxometalate-based ionic liquid catalyst with unprecedented activity and selectivity for oxidative desulfurization of diesel in Omim BF4. Chem. Eng. J. 2019, 358, 419–426. [Google Scholar]
- He, L.; Li, H.; Zhu, W.; Guo, J.; Jiang, X.; Lu, J.; Yan, Y. Deep Oxidative Desulfurization of Fuels Using Peroxophosphomolybdate Catalysts in Ionic Liquids. Ind. Eng. Chem. Res. 2008, 47, 6890. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, X.; Bai, J.; Yang, Z.; Chen, H.; Yang, L.; Liang, Y.; Bai, L.; Yang, H. Co,N-codoped MoOx nanoclusters on graphene derived from polyoxometalate for highly efficient aerobic oxidation desulfurization of diesel. J. Catal. 2023, 428, 115186. [Google Scholar] [CrossRef]
- Xun, S.; Jiang, W.; Guo, T.; He, M.; Ma, R.; Zhang, M.; Zhu, W.; Li, H. Magnetic mesoporous nanospheres supported phosphomolybdate-based ionic liquid for aerobic oxidative desulfurization of fuel. J. Colloid Interface Sci. 2019, 534, 239–247. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Zhang, L.; Pei, T.; Dong, L.; Zhou, P.; Li, C.; Xia, L. Acidic polymeric ionic liquids based reduced graphene oxide: An efficient and rewriteable catalyst for oxidative desulfurization. Chem. Eng. J. 2018, 334, 285–295. [Google Scholar] [CrossRef]


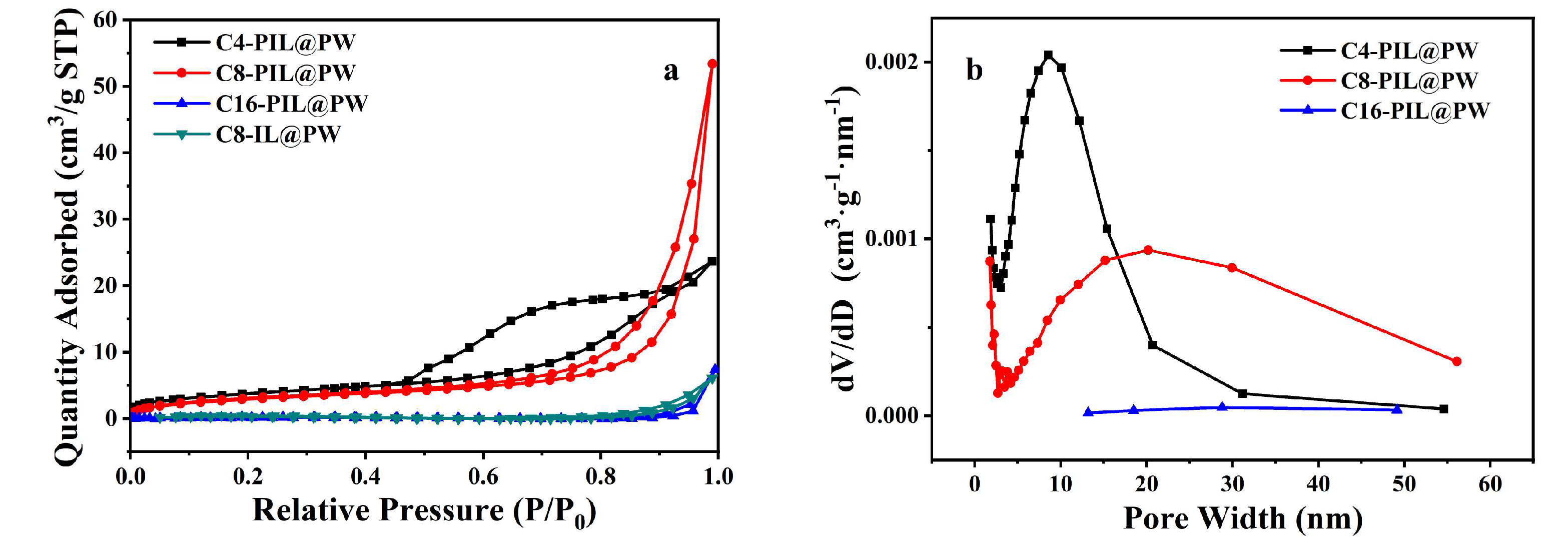


| Sample | SBET (m2g−1) | Pore Volume (cm3g−1) | Average Pore Size (nm) |
|---|---|---|---|
| C4-PIL@PW | 8.2420 | 0.0208 | 4.1 |
| C8-PIL@PW | 10.8744 | 0.0819 | 22.5 |
| C16-PIL@PW | 0.6891 | 0.0114 | 35.8 |
| C8-IL@PW | 1.1102 | 0.0019 | 9.8 |
| Entry | Samples | Sulfur Removal (%) |
|---|---|---|
| 1 | C4-PIL@PW | 41.0 |
| 2 | C8-PIL@PW | 99.2 |
| 3 | C16-PIL@PW | 9.4 |
| 4 | C8-IL@PW | 89.3 |
| 5 | C4-IL@PW | 24.9 |
| 6 | C16-IL@PW | 69.2 |
| Entry | Catalyst | Solvents | T (°C) | O/S | Sulfur Removal (%) | Literature |
|---|---|---|---|---|---|---|
| 1 | [C2(MIM)2]PW12O40 | acetonitrile | 50 | 6 | 98.35 | [61] |
| 2 | PMo12O40@MnFe2O4 | acetonitrile | 35 | 3-mL oxidant (H2O2/CH3COOH) | 98 | [62] |
| 3 | [CTA]11P2W13V5O64 | - | 70 | 6 | 90 | [63] |
| 4 | [THTDP]Cl-MoO | - | 30 | 6 | 98.37 | [64] |
| 5 | PW12@TM–SBA-15 | [BMIM] PF6, acetonitrile | 70 | 8 | 100 | [65] |
| 6 | [PyPS]3(NH4)3Mo7O24 | [Omim]BF4 | 70 | 5 | 99 | [66] |
| 7 | [(C4H9)4N]3{PO4[MoO(O2)2]4 | [Bmim]BF4 | 70 | 6 | 97.3 | [67] |
| 8 | C8-PIL@PW | - | 50 | 6 | 99.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Huang, T.; Tong, S.; Wang, C.; Zhang, M. Heteropolyacid-Based Poly(Ionic Liquid) Catalyst for Ultra-Deep and Recyclable Oxidative Desulfurization of Fuels. Catalysts 2025, 15, 622. https://doi.org/10.3390/catal15070622
Chen M, Huang T, Tong S, Wang C, Zhang M. Heteropolyacid-Based Poly(Ionic Liquid) Catalyst for Ultra-Deep and Recyclable Oxidative Desulfurization of Fuels. Catalysts. 2025; 15(7):622. https://doi.org/10.3390/catal15070622
Chicago/Turabian StyleChen, Mengyue, Tianqi Huang, Shuang Tong, Chao Wang, and Ming Zhang. 2025. "Heteropolyacid-Based Poly(Ionic Liquid) Catalyst for Ultra-Deep and Recyclable Oxidative Desulfurization of Fuels" Catalysts 15, no. 7: 622. https://doi.org/10.3390/catal15070622
APA StyleChen, M., Huang, T., Tong, S., Wang, C., & Zhang, M. (2025). Heteropolyacid-Based Poly(Ionic Liquid) Catalyst for Ultra-Deep and Recyclable Oxidative Desulfurization of Fuels. Catalysts, 15(7), 622. https://doi.org/10.3390/catal15070622








