1. Introduction
Over the last few decades, significant advancements have been made in solar energy conversion, particularly in generating electricity and chemical fuels [
1]. Solar energy has become a key focus for the development of sustainable methods in chemical transformations, aligning with modern trends in green chemistry [
2,
3]. In academic research, scientists have aimed to replicate how plants use sunlight as an energy input to facilitate complex molecular transformations through photosynthesis [
4]. However, despite the progress in solar energy technologies, utilizing direct sunlight as an energy source for chemical reactions remains relatively underexplored [
5]. Advancing this approach could improve the utilization of direct solar energy, paving the way for sustainable environmental solutions and real-world applicability.
Photochemistry has recently gained significant attention, marking a shift from its earliest niche status to a central area of research ending up with studies that primarily focused on transition metal-based semiconductors [
6]. These materials have been extensively studied for their photocatalytic properties; however, they often require a noble metal cocatalyst such as Pt or RuO
2 to enhance charge separation and surface reaction kinetics. Without the metallic cocatalyst, the photo-induced charge carriers, electrons (e
−), and holes (h
+), tend to recombine, reducing catalytic efficiency [
2]. By improving the understanding of photon capture and energy transfer, researchers have designed metal-free photocatalysts such as 2D crystalline graphitic carbon nitrides (g-C
3N
4) [
7], boron nitrides [
8], etc., making them promising candidates for future photocatalytic applications. While significant progress has been made, challenges such as stability, costs, and environmental impact remain [
9]. Addressing these challenges, scientists have increasingly focused on designing novel heterogeneous photocatalysts, which has led to the introduction of the latest addition to the family of metal-free photocatalysts: conjugated microporous polymers (CMPs) [
10,
11].
Conjugated microporous polymers have emerged as a promising class of materials due to their unique combination of π-conjugated systems and a permeable porous structure [
12], possessing high electronic conductivity, chemical tunability, and mechanical robustness, which distinguishes them from unstable porous materials and non-porous conjugated polymers [
13]. The π-conjugated system facilitates electron delocalization across the polymer backbone, resulting in remarkable electronic properties, such as higher conductivity and strong light absorption [
14]. Despite this, CMPs typically suffer from charge recombination, which can hinder the overall catalytic performance. As a solution, previous studies have successfully established Mott–Schottky interfaces by introducing metals into the CMP’s framework, thereby creating delocalized energy states within the CMP’s bandgap [
15]. In solid-state physics, the contact between a metal and a semiconductor forms a Mott–Schottky junction at the composite’s heterojunction interface due to their different work functions [
16,
17]. When this contact occurs, electrons from the semiconductor’s conduction band (CB) migrate to the metal interface until the Fermi levels of both materials equilibrate [
17,
18]. This electron migration results in a positive charge remaining at the semiconductor’s surface, thereby creating a space charge region and a built-in electric field (BEF), which causes band bending within the semiconductor, thereby determining the height of the Mott–Schottky barrier, which is crucial for charge transfer efficiency at the metal/semiconductor interface. The interaction between these materials at the heterojunction significantly alters the electronic structure, impacting charge transfer dynamics and electrocatalytic behavior. Additionally, the surface charge heterogeneity at the interface shifts the flat band potential of the semiconductor without significantly altering the valence band (VB) or the conduction band (CB) positions, causing the semiconductor to retain its fundamental electronic properties while gaining improved catalytic behavior [
19]. Therefore, the Mott–Schottky junction formed between the semiconductor and the noble metal improves the catalytic activity by accelerating interfacial charge transfer and optimizing the light-harvesting in the reaction intermediates [
20]. Nevertheless, many metal-based photocatalysts suffer from poor recyclability due to their degradation over time after prolonged exposure to light or reactive species, which further increases both cost and environmental impact [
21].
Graphene 2D materials are increasingly recognized for their ability to serve as a template for the synthesis of various crystalline materials [
22], including g-C
3N
4, silica, metal oxides, and sulfides. They can serve as excellent electron conductors that facilitate electron–hole (e
−/h
+) separation by forming a Mott–Schottky interface, offering a promising alternative to the traditional metal doping [
23,
24,
25]. For example, the photocatalytic activity of g-C
3N
4 was significantly improved by adjusting the electrical properties through graphene doping. Furthermore, the incorporation of graphene g-C
3N
4 and boron nitride into a sandwich-type nanohybrid structure enables the creation of heterojunctions that promote charge separation, improving light absorption and enhancing stability, making these hybrids more suitable for a wide range of applications [
26]. The crystallization process of these materials on the graphene surface typically begins with nucleation, which is often driven by thermodynamic principles [
27,
28]. On the other hand, CMP synthesis is a kinetically controlled pathway [
29], diverging from the thermodynamic mechanisms, which can result in more disordered final products. During the CMP synthesis, a small, flexible intermediate that is not fully polymerized attaches to the graphene surface through π–π stacking interactions between the aromatic ring and the graphene surface [
30]. This attachment influences the network propagation, resulting in morphological control; however, the intrinsic amorphous nature of the CMP remains unchanged.
The construction of graphene-based CMP heterostructures is still an underexplored research area. In 2018, a study was published by Zhuang and his coworkers where they synthesized several sandwich graphene template CMPs covalently connected to the actual CMP’s skeleton. The work demonstrated a novel strategy for synthesizing a new class of 2D porous polymers by using functionalized 2D materials as a template [
31]. In another study published in 2012, Due et al. revealed that g-C
3N
4/graphene exhibits strong π–π interactions due to the attractive forces between the electron clouds of adjacent aromatic rings or π-bounded systems. These interactions play a significant role in the stabilization of the structure and electronic properties of the composite material. The authors reported that the interplanar distance between the graphene and g-C
3N
4 layers was 3.03 Å, which is consistent with the typical distance observed for π–π stacking interactions. This optimized distance allows for an effective interaction between the layers, maximizing the strength of the π–π interactions while maintaining structural integrity [
32]. As the research in the domain of CMPs continues, we aim to contribute to it by introducing Mott–Schottky heterojunction-promoted advanced photo-responsive properties in the CMP skeleton to generate a novel all-organic, metal-free photocatalytic heterostructure, achieving activity levels similar to noble metal-based catalysts while maintaining lower cost and scalability [
25] by replacing noble metal nanoparticles with graphene as a dopant [
33]. This study introduces a new graphene-based composite material (GCMP) synthesized through a simple and efficient straightforward technique. GCMP, with its Mott–Schottky interactions, exhibited beneficial physical and optoelectronic properties compared to pristine CMP-1. GCMP demonstrated a high degradation efficiency for both cationic and anionic dyes, establishing its promise for water purification and organic pollutant removal from wastewater.
2. Results and Discussion
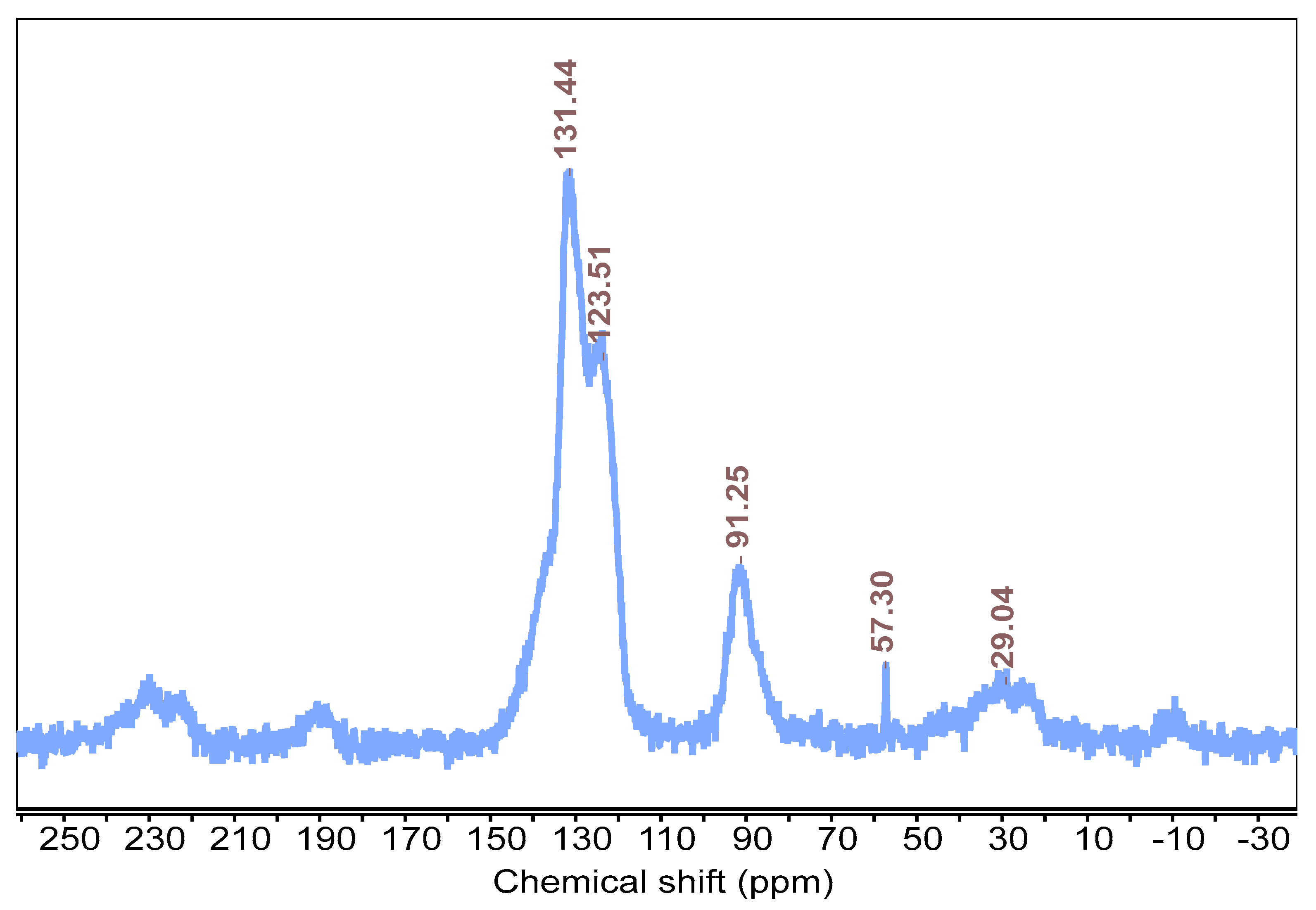
In order to verify the formation of the GCMP network and identify key carbon signals corresponding to its structure, a
13C cross-polarization solid-state NMR analysis was performed. The analysis revealed a peak at 91 ppm, which is generally associated with C≡C acetylenic carbon, along with two additional peaks at 123 ppm and 131 ppm corresponding to aromatic carbon, thereby confirming the aromatic backbone of the polymer and the presence of a triple bond within the polymer skeleton (
Figure 1). The chemical shifts observed were consistent with the
13C cross-polarization solid-state NMR data of CMP-1, thus confirming that the desired polymerization reaction occurred without deviations (
Figure S1) [
34]. Additionally, X-ray photoelectron spectra were conducted to gain additional structural insight and confirm the elemental composition of the material. Based on the survey spectra, the primary detected element was carbon, with the presence of a small oxygen peak that can be attributed to surface-adsorbed oxygen rather than being a part of the network, which is a common occurrence in polymeric and graphene-based materials exposed to air. The C1s’ peak was split into a graphitic C=C peak at 284.8 eV and an acetylenic C≡C peak at 286.0 eV [
35] (
Figures S2 and S3), further confirming the presence of a triple-bonded carbon from the acetylene groups in the CMPs network, thus validating the successful polymerization and formation of the extended GCMP structure. The consistency between the
13C cross-polarization solid-state NMR chemical shifts and XPS binding energy provides robust evidence for the successful synthesis of a clean, well-polymerized GCMP network.
FT-IR spectroscopy was employed to verify the incorporation of graphene into the CMP-1 polymeric network. The resulting spectrum of GCMP represents a superposition of individual spectra of pure graphene and CMP-1, confirming the incorporation of the graphene into the polymeric network. The fingerprint regions of CMP-1 match the GCMP, confirming that its polymeric structure remains intact. Even after graphene incorporation, several characteristic peaks associated with graphene are observed in the GCMP spectrum at 2900 cm
−1 and 2973 cm
−1 [
36]. The C=C stretching bands that were observed between 1500 cm
−1 and 1670 cm
−1 are indicative of conjugated aromatic structures that are present in both CMP-1 and graphene, reflecting the π–π stacking interactions between the aromatic rings of the graphene and polymeric network. Another peak was observed around 800–1100 cm
−1, representing the aryl C-H bending peak attributed to the same π–π interactions between the CMP-1 and the graphene [
37]. These observations confirm the successful incorporation of the graphene into the CMP-1 polymeric network, with interactions between the two components leading to enhanced π–π stacking interactions (
Figure 2a).
The XRD pattern of GCMP demonstrates a broad hump centered at 2θ = 20.8°, highlighting the amorphous nature. Notably, this hump appears at a lower position compared to CMP-1 (23.3°), the (002) peak of monolayer graphene (24.4°), and graphite (26.5°) [
38]. This shift to lower 2θ values suggests an increased interlayer spacing and a highly disordered 3D network in GCMP [
22] (
Figure S4). The observed disorder reflects a breakdown of the regular π−π interactions that are typically observed in ordered graphitic and CMP materials. This detected structural irregularity likely arises from the incorporation of graphene, which disturbs the uniform stacking pattern of CMP-1. Thereby, the graphene incorporation significantly modifies the π−π stacking interactions between the CMP layers, which suggests a potential benefit for applications where increased surface area and tailored electronic properties are desirable [
27].
This attachment influences the network propagation, resulting in morphological control; however, the intrinsic amorphous nature of the CMP remains unchanged. Transmission electron microscopy (TEM) images provided a visualization, supporting the previously mentioned hypothesis about the graphene’s role in influencing the morphology of CMP-1. Analysis of the TEM data reveals that the CMP-1 matrix exhibits increased density following graphene doping, confirming the successful synthesis of graphene-doped CMP-1 (GCMP). This structural densification likely arises from the integration of graphene into the CMP-1 framework, which enhances interfacial interactions and reinforces the composite architecture [
39] (
Figure 2b and
Figure S6). Notably, the TEM data showed 1 μm-sized CMP-1 layers warping around graphene sheets, contrasting significantly with the morphology of pure CMP-1, which appeared as a chunk of smaller particles lacking structural uniformity. Scanning electron microscopy (SEM) further validated these observations, confirming the warping of CMP-1 layers around graphene sheets, reinforcing the role of graphene as a templating scaffold (
Figure S7).
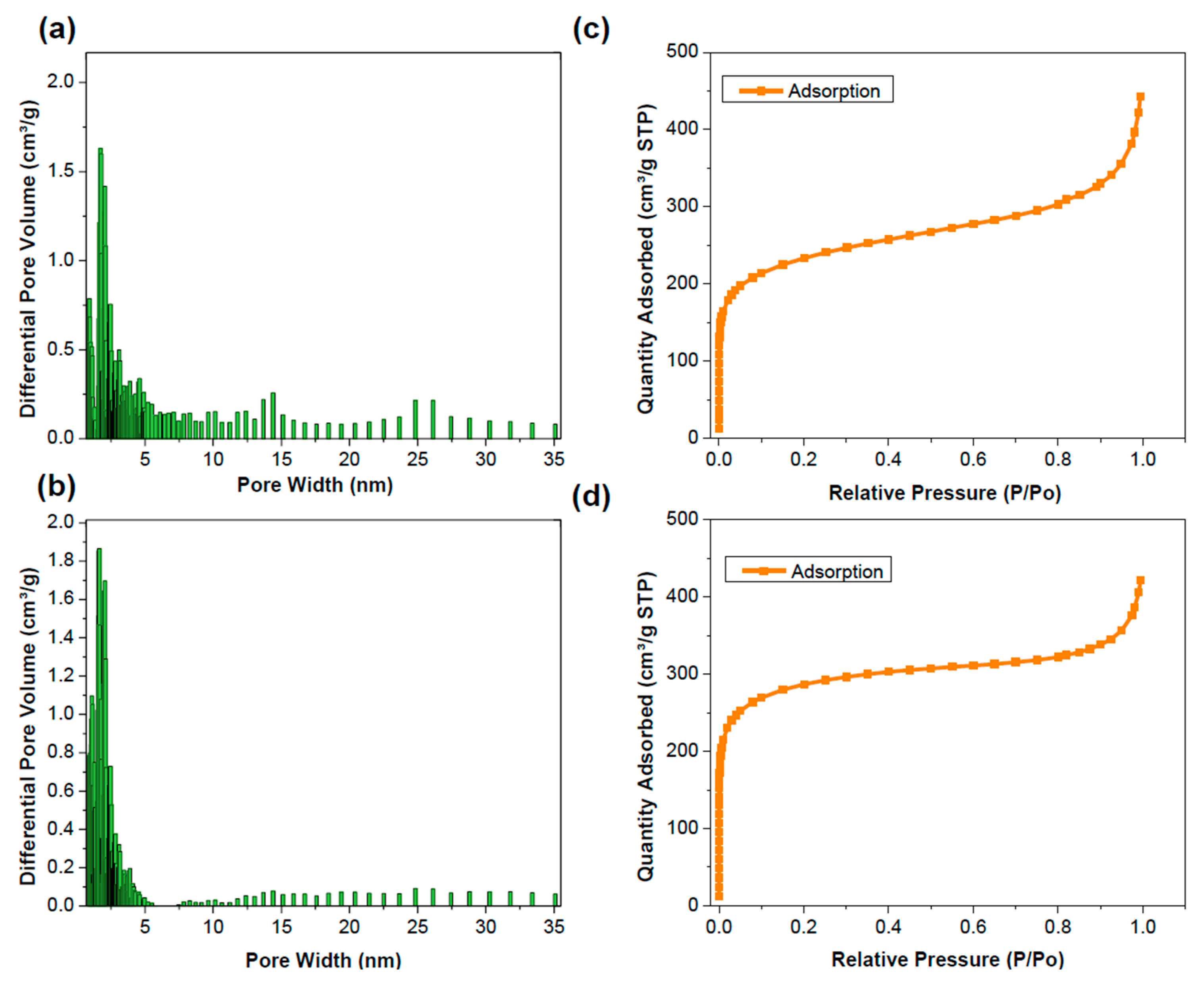
2.1. Surface and Porosity
In order to evaluate the impact of graphene intercalation on the surface area and porosity of CMP-1, we performed the Brunauer–Emmett–Teller (BET) surface area analysis. GCMP presented a BET surface area of 1035 m
2/g, significantly surpassing the 639 m
2/g surface area observed for pure CMP-1. Therefore, the incorporation of the graphene resulted in an impressive 62% increase in surface area, emphasizing the critical role of the graphene intercalation (
Figure 3c,d) [
40]. Notably, GCMP showed a higher microporosity and reduced mesoporosity compared to CMP-1, as indicated by the local density functional theory (DFT) pore size distribution. Graphene intercalation promotes a uniform microporous network while simultaneously limiting the formation of mesopores, contributing to enhanced material properties.
2.2. Photophysical and Electrochemical Properties
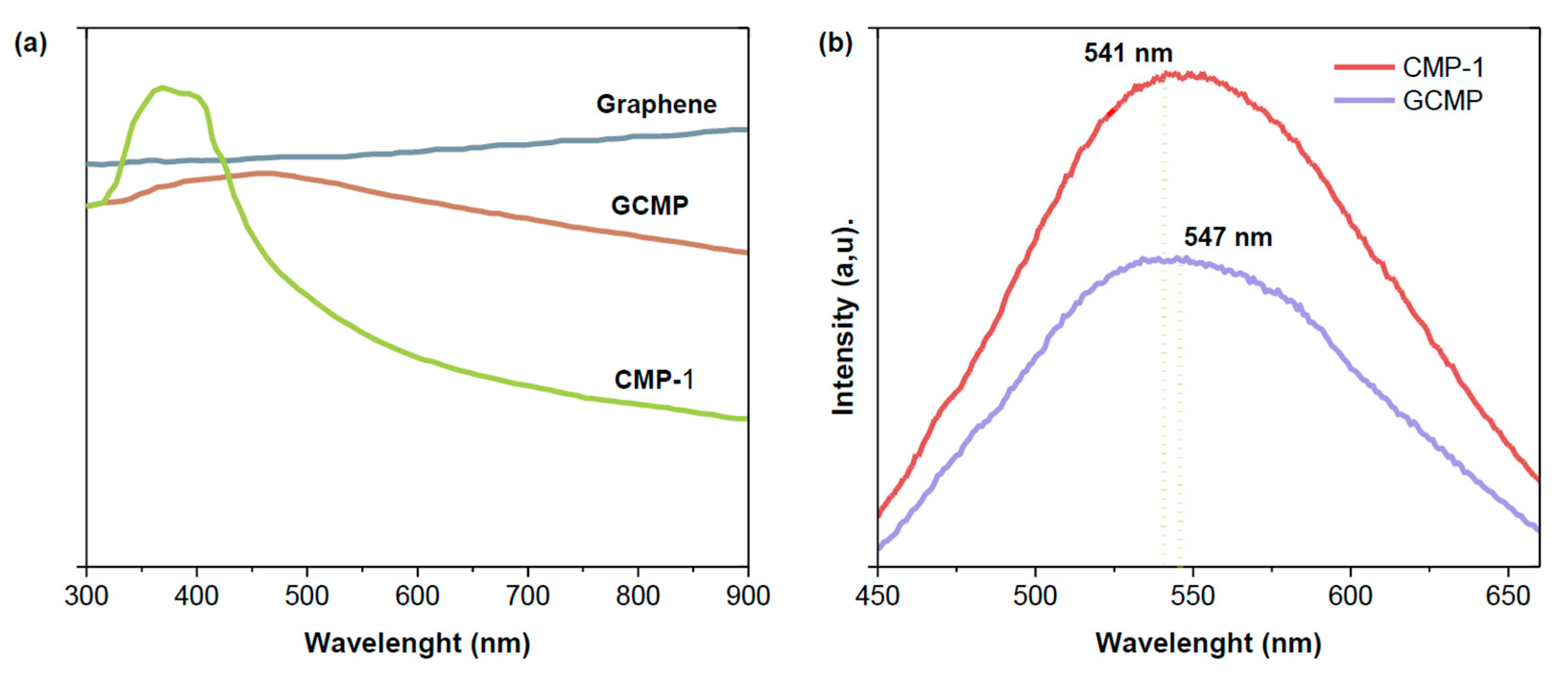
Graphene’s zero bandgap [
26] presents a promising opportunity to enhance the photo-responsive properties of CMP-1. As presented in
Figure 4, after graphene intercalation, GCMP presented a 90 nm redshift (λ
max) and a significant increase in the absorption tail, which significantly influenced the fluorescence spectra by resulting in a 6 nm shift of λ
max. Beyond the shifts, the fluorescence spectrum of GCMP presented a 42% reduction in emission intensity compared to CMP-1, implying an extended exciton lifetime and reduced radiative recombination rates, endorsing the formation of Mott–Schottky junctions. Upon light excitation, electrons from the CB of CMP-1 migrate to the CB of graphene. This charge separation leaves behind holes accumulating on the CMP-1 surface, creating an electrostatic potential difference that surpasses radiative recombination, making it thermodynamically unfavorable. This enhances the charge carriers’ mobility and contributes to the material’s photocatalytic efficiency [
16]. The photocurrent response of CMP-1 and GCMP was evaluated under intermittent illumination using an Xe-lamp in a light-sealed environment. Upon illumination, the photocurrent increases immediately to a stable value of 1.4 × 10
−4 A. The process was repeated over three on-off light cycles to confirm the reproducibility and stability of the response. As illustrated in
Figure S8, GCMP demonstrated higher charge efficiencies consistent with the role of the graphene in reducing charge recombination, thereby enhancing the overall photoelectrochemical performance.
The electrochemical potential of CMP-1 and GCMP was assessed using cyclovoltammetry (CV) with the values determined by applying a Standard Calomel Electrode (SCE) as a reference [
40]. The conduction band potentials of CMP-1 were measured as −0.71 V, while GCMP was slightly more positive by giving a potential of −0.68 V. Similarly, the valence band potentials were determined to be 1.94 V and 1.98 V for CMP-1 and GCMP (
Figures S9 and S10). These values correspond to a band gap of 2.65 eV for CMP and 2.66 eV for GCMP. Despite the graphene intercalation in GCMP, the electrochemical measurements indicate that there is no significant change in the bandgap. Graphene does not significantly alter the electronic band gap of CMP-1, yet it helps in separating the photoexcited electron–hole pairs more efficiently.
3. Photocatalytic Application
To evaluate the adsorptive capacity of the photocatalyst (a critical precursor to its photocatalytic activity), a 10 ppm RhB dye solution was treated with 0.1 mg/mL of GCMP and stirred in the dark to establish adsorption equilibrium. Remarkably, GCMP achieved complete dye adsorption within 15 min, whereas pristine CMP-1 adsorbed only 28% of the dye after 2 h under identical conditions (
Figure S11). This dramatic enhancement in adsorption efficiency attributed to the incorporation of graphene into the CMP-1 framework-arises from two synergistic effects:
Increased Surface Area: Graphene doping expanded the surface area by 62%, providing abundant active sites for RhB adsorption.
These structural and electronic modifications not only enhance adsorption but also create a foundation for accelerated photocatalytic degradation by ensuring efficient utilization of photogenerated carriers. Within light exposure, the conductive graphene network shortens the exciton migration pathway, suppressing electron–hole recombination and facilitating faster interfacial charge transfer to adsorbed dye molecules.
The incorporation of graphene significantly boosts light absorption and photocatalytic efficiency in GCMP, enabling effective degradation of diverse dye pollutants. This broad-spectrum activity, a critical advantage for real-world wastewater treatment, demonstrates GCMP’s non-selectivity—an essential feature for treating complex industrial effluents. By utilizing visible light, an abundant and sustainable energy source, GCMP overcomes the limitations of conventional catalysts, which often suffer from narrow spectral sensitivity or reliance on UV irradiation. Consistent with previous literature reports, the degradation rates were fitted against a pseudo-first-order reaction pathway given as
where
C0 is the initial concentration of the dye solution,
C is the concentration at time
t, and
k is the rate constant. As depicted in
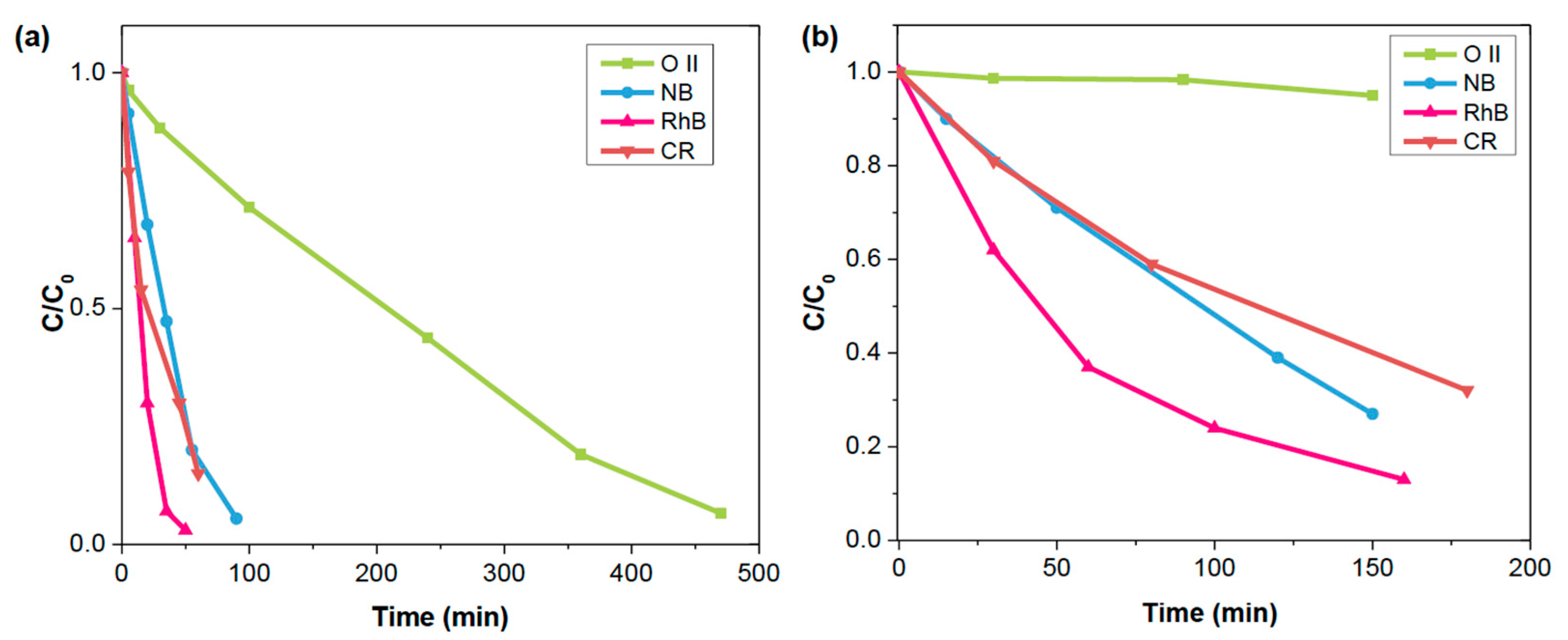
Table 1 and
Figure 5, GCMP demonstrated excellent degradation efficiency for all dyes with high-rate constants and impressive t
1/2. The many-fold catalytic enhancement of GCMP over CMP-1 can be prominently portrayed by rationally comparing their individual activities recorded under identical conditions (
Figures S12–S15). As illustrated in
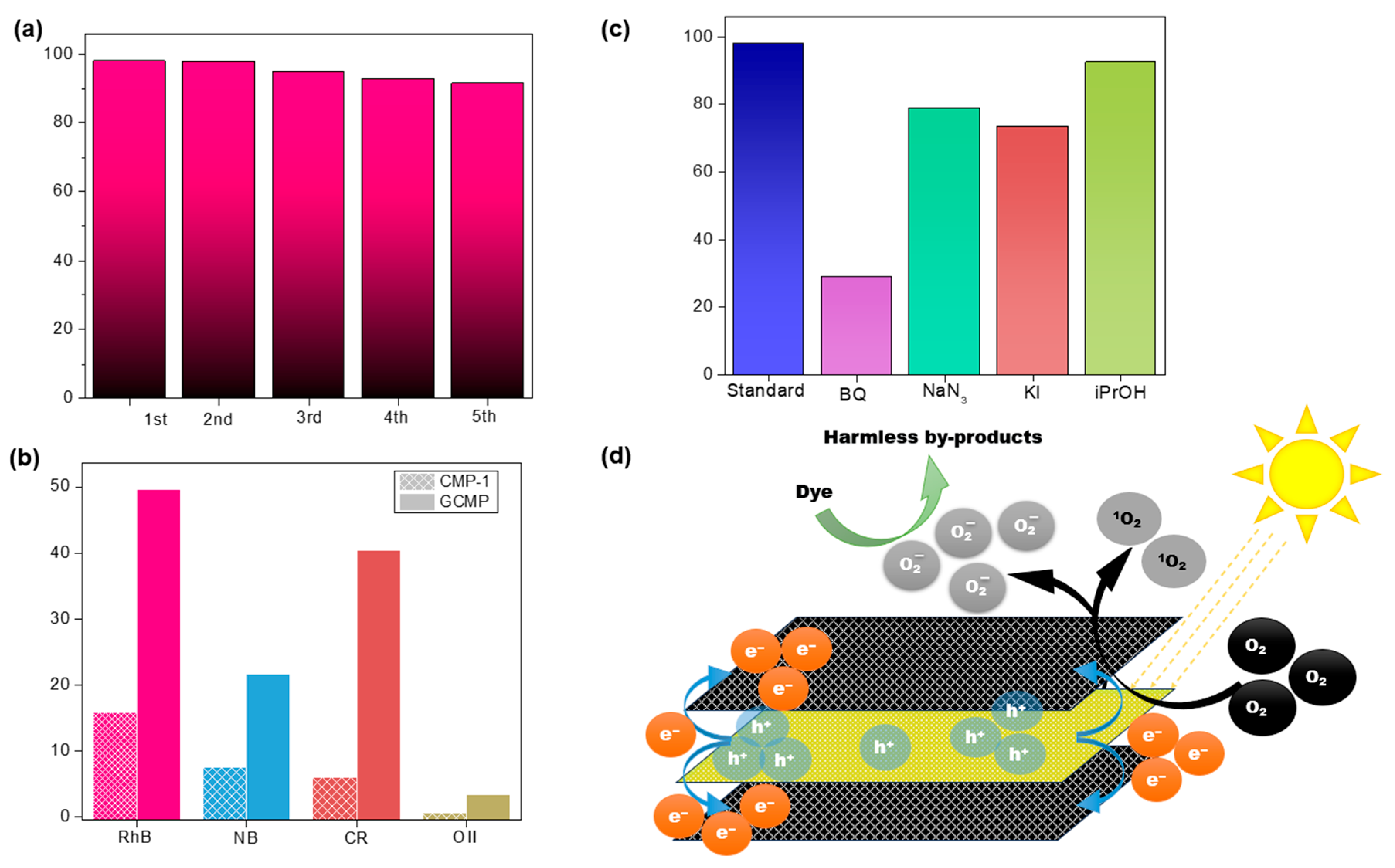
Table 1, CMP-1 degraded RhB significantly slower compared to GCMP, with a degradation rate three times lower than that of GCMP. A similar trend was observed for other dyes, including Nile Blue (NB), Congo Red (CR), and Orange II (O II). However, GCMP not only outperformed CMP-1 in terms of degradation rate but also demonstrated superior overall dye degradation efficiency. Specifically, the dye degradation rate of GCMP was three times higher than CMP-1 for NB, seven times higher for CR, and an impressive eleven times higher for O II. On the other hand, CMP-1 struggled to degrade more challenging dyes like O II, which underscored its limited catalytic activity. Thus, the incorporation of graphene dramatically enhanced its photocatalytic performance, making it far more effective in degrading a broader range of dyes.
Figure 5b provides an insight into the catalytic performance of GCMP across multiple dye pollutants. As observed, RhB, CR, and NB were rapidly degraded compared to O II, as indicated by lower constant rate
k and higher half-lifetime values t
1/2. These differences in degradation rates can be attributed to the energy required to break specific molecular bonds. For instance, RhB and NB contain iminium chromophores that are easy to cleave, facilitating quicker degradation. However, CR and O II possess highly stable N=N azo linkages, which are more energy-intensive to break, slowing down their degradation rates, reflected in their
k values. However, despite the slower degradation rate, GCMP is still effectively degrading the dye, even at a low catalyst concentration of 0.2 mg/mL for 25 ppm dye under visible light, which highlights GCMP’s excellent performance. The reusability of GCMP was tested over five consecutive degradation cycles using RhB as a model dye (
Figure 6a). The catalyst preserved its catalytic activity with only negligible reductions in efficiency, likely due to pore clogging caused by residual dye, which is a common challenge in reuse scenarios. To evaluate structural integrity, the reused GCMP was characterized by TEM (
Figure S16). The images confirmed that the graphene/CMP-1 sandwich architecture remained intact, demonstrating robust structural stability even after multiple degradation cycles.
This improvement in the photocatalytic performance of GCMP compared to CMP-1, despite the identical bandgap, can be attributed to the formation of a π-stacked intercalated heterostructure. This structure not only increases the surface area, providing more active sites for catalytic reactions but also facilitates the formation of a Mott–Schottky interface at the GCMP surface, leading to a surface charge heterogeneity altering the flat band potential as demonstrated in the study of Zhang et al. on g-C
3N
4/graphene Mott–Schottky contact, which demonstrated a similar mechanism [
32,
41]. Upon exposure to light, electrons from the CB of the CMP-1 travel to the CB of the graphene due to the Schottky effect, resulting in a more stable separation of electron–hole pairs. This allows them to interact more efficiently with the surface-absorbed molecules, such as water, oxygen, and dyes, explaining the superior photocatalytic activity of GCMP (
Figure 6d). Allowing for faster and more efficient degradation of organic dyes, GCMP paves the way for practical and scalable implementations in wastewater treatment and other environmental remediation technologies.
The photocatalytic performance of GCMP was compared to a conventional photocatalyst under identical experimental conditions to evaluate dye degradation efficiency. As summarized in
Table S1, GCMP showed remarkably greater photocatalytic performance, degrading four different dyes with both cationic and anionic nature, with reduced catalyst loading. Furthermore, GCMP surpassed traditional catalysts in the degradation of dyes with complicated aromatic structures, such as Congo Red (CR), which are hardly difficult to break. Importantly, GCMP exhibited a superior catalytic performance at lower catalyst–dye ratios, highlighting its practical benefit in reducing material use while enhancing efficiency.
Aiming to clarify the role of intermediate and reactive species in the degradation of dye, we conducted controlled experiments using RhB as the model dye. Various scavengers were introduced to the reaction system to neutralize the distinct reactive species. The reactions were conducted for 50 min under visible light while monitoring the dye degradation efficiency to help understand how different species contribute to the overall process (
Figure 6c). Our findings indicate that scavenging superoxide (O
2−) contributes to the substantial decrease in the degradation efficiency, resulting in a 70% decrease in the overall dye degradation. Singlet oxygen (
1O
2−) and oxidized holes (h
+) also contributed to the process but to a lesser extent than super-oxides; on the other hand, hydroxyl radicals (
•OH), had the minimal impact on the degradation process even with its great reactivity. Therefore, superoxide radicals are the most influential reactive species in the degradation pathway. The Mott–Schottky junction facilitates efficient electron transfer from the conduction band (CB) of CMP-1 to graphene’s CB which prolongs the lifetime of photoexcited electrons by reducing recombination, thereby enhancing their availability for redox reactions. These long-lived, highly reactive electrons play a pivotal role in driving the degradation of RhB through sustained radical generation (e.g.,
•OH or O
2•−). Despite this, singlet oxygen
1O
2− can be produced through the reaction of excited electrons with oxygen that does participate in the oxidative reactions with the O
2− and h
+, ensuring a comprehensive degradation pathway targeting the dye molecules through the degradation process and clarifying the minor role of
•OH, which suggests that the degradation predominantly relies on species generated on the graphene/CMP interface rather than on hydroxyl radicals.
Notably, singlet oxygen (1O2−) is generated through electron-mediated reduction in molecular oxygen (O2• → O2•−) followed by hole-driven oxidation of superoxide radicals (O2•− + h+ → 1O2−). This diverse oxidative pathway ensures far-reaching dyes degradation, with mechanistic analysis revealing a minor contribution from hydroxyl radicals (•OH). The degradation is primarily driven by reactive species (e.g., 1O2−, O2•−) generated at the graphene/CMP-1 interface, rather than conventional •OH-mediated pathways, highlighting the unique interfacial chemistry enabled by the composite’s architecture.
4. Materials and Methods
4.1. Materials
Monolayer graphene was purchased from XF Nano (XF001H, Shanghai, China). All other chemicals and solvents were purchased from Aladdin (Shanghai, China) and used without further purification.
4.2. GCMP Synthesis
The generated GCMP photocatalyst was synthesized through a straightforward kinetically controlled path following the Sonogashira–Hagihara reaction. In this process, we selected CMP-1 [
34] as the foundation polymeric structure to generate the CMP-1/graphene composite by introducing 126 mg of 1,3,5-tribromobenzene, 82 mg of 1,4-diethynyl benzene, and 2 mg of commercially available monolayer graphene, which were added to a 3:1 solution of DMF and triethylamine in the presence of 0.05 eq. tetrakis (triphenylphosphine) palladium and 0.08 eq. cuprous iodide at 80 °C for 72 h. The resulting material was then washed with methanol and acetone to remove residual impurities and then dried at 104 °C for 24 h to remove any remaining moisture. Consequently, GCMP was obtained as a brownish-yellow solid powder, exhibiting insolubility in common solvents.
4.3. Photocatalytic Dye Degradation
To determine whether incorporating graphene into the CMP-1 framework enhances the photocatalytic degradation of dye molecules in wastewater, we conducted an experiment using GCMP and CMP-1 under visible light irradiation. In this study, we tested both anionic (Nile blue, rhodamine B) and cationic (Congo red, orange II) dyes to assess the photocatalyst’s versatility. These dyes, commonly found in industrial wastewater, are notorious pollutants due to their extensive use in textile printing and related industries [
42]. The degradation process involves two stages: first, the equilibrium for physical adsorption is reached, followed by the onset of the photochemical degradation. The photocatalytic performances were carried out using a 25 ppm concentration of aqueous dye solutions and a 0.2 mg/mL concentration of GCMP. A mixture of dye solution and GCMP was stirred in the dark for 1 h to attain adsorption–desorption equilibrium prior to being subjected to visible light at a distance of 10 cm while carried out in a water bath maintained at room temperature. The experimental data were collected using a UV-Vis spectrometer in a quartz cuvette at room temperature focusing on the wavelength λ
max at which each dye absorbs the most UV-Vis radiation and the way it shifts in the wavelength provides insights into the degradation mechanism of the dye.
4.4. Physical Measurements
Solid state NMR. 13C-CP/MAS NMR spectra were measured on a Bruker Advance 400 DSX spectrometer (Ettlingen, Germany). Cross Polarization Magic Angle Spinning (CP/MAS) was performed at MAS of 10 kHz in a 4 mm zirconia rotor. TPPM decoupling was used for acquisition.
Infrared Spectroscopy. ATR-FTIR spectra were recorded on a 6700Nicolet (Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA).
Gas sorption analysis. The adsorption–desorption isotherms were analyzed by a Micrometrics instrument (ASAP 2020, Micromeritics Instrument Cooperation, Norcross, GA, USA) using ultrapure N2 (99.999%) at 77.3 K. The samples were degassed at 150 °C for 6 h at vacuum prior analysis. Surface area values were calculated using the Bernauer–Emmett––Teller (BET) method at 0.003 < P/P0 < 0.05 range. The pore size distributions were calculated from N2 sorption isotherms using the nonlocal density functional theory (NLDFT).
Morphology study. The morphology study was conducted by a S4800 field emission scanning electron microscope (FESEM, Hitachi, Japan). TEM analysis was carried out using a Philips CM20 microscope at 200 kV. Imaging and diffraction of the structure was performed at low electron dose for minimizing beam damage to the sample.
X-ray photoelectron spectroscopy (XPS). The binding energies of the elements present in the polymer were measured by XPS on a Thermo Fisher ESCALAB 250Xi (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
X-ray Powder Diffraction (XRD). Powder XRD analysis was performed on a Bruker D8 Advance diffractometer with a copper Kα radiation source (λ = 1.54056 Å) at 40 kV and 45 Ma with 5 °/s scanning speed (Ettlingen, Germany).
UV-vis. UV-vis absorption spectra were recorded in solution phase on Shimadzu UV-1800 UV-visible spectrophotometer using a 10 mm path length quartz cuvette (Kyoto, Japan). The materials in respective solvents were sonicated for 10 min to ensure complete dissolution prior to measurement.
Fluorescence Emission spectra were recorded in solid state on a Hitachi F-7000 Fluorescence Spectrophotometer (Hitachi, Tokyo, Japan).
Cyclic voltammetry. The electrochemical study was conducted on a CHI-660E workstation (CH Instruments, Bee Cave, TX, USA).
5. Conclusions
In summary, this research reports a novel design strategy to create Mott–Schottky heterojunction within sandwich-like graphene-based CMP-1 composites (GCMP) specifically engineered for the photocatalytic degradation of organic dyes from aquatic environment. GCMP was obtained through a straightforward, facile protocol involving the integration of graphene sheets into CMP-1 farmwork. The physicochemical properties and morphology of the material was confirmed through serval characterization techniques such as Brunauer–Emmett–Teller (BET) surface area analysis, X-ray diffraction (XRD), transmission and scanning electron microscopy (TEM/SEM), Fourier-transform infrared spectroscopy (FT-IR), solid-state 13C nuclear magnetic resonance (NMR), and UV-Vis spectroscopy, among others. The proposed photocatalytical pathway is primarily driven by efficient separation of photogenerated charge carriers. The generated Mott–Schottky heterojunction between the graphene and CMP-1 facilitates rapid electron transfer from the CMP-1 conduction band to that of graphene. This extends the exciton lifetime by reducing the electron-hole recombination and enhances the generation of reactive oxygen species (ROS) radicals that attack the dye molecules and degrade it into harmless by-products, thereby enhancing the overall catalytic efficiency. GCMP exhibited a significantly enhanced dye adsorption capacity compared to pristine CMP-1, with improvements ranging from 3- to 11-fold. This enhancement is attributed to the increased surface area following graphene intercalation, which enlarged the CMP-1 structure by approximately 62%. Under visible light illumination, GCMP effectively degraded a variety of dyes with different ionic characteristics, including both cationic and anionic species (NB, RhB, CR, OII). Moreover, the catalyst demonstrated excellent reusability, retaining its photocatalytic performance over five consecutive cycles, thus indicating its long-term stability and practical applicability in wastewater treatment.
Mechanistic investigations identified superoxide radicals (O2−) as the dominant reactive species, with singlet oxygen (1O2−) playing a secondary role in the photocatalytic degradation process. This work advances the development and the establishment of GCMP as a sustainable, metal-free platform aligned with green chemistry principles. Nevertheless, the insights gained from this work provide a foundation for the rational design of advanced photocatalytic systems. The potential of GCMP in environmental remediation such as wastewater treatment and renewable energy applications, including solar-driven hydrogen generation, suggests a promising pathway toward scalable and ecologically responsible solutions that bridge material innovation with sustainable development.