Different Routes for the Hierarchization of *BEA Zeolite, Followed by Impregnation with Niobium and Application in Ethanol and 1-Propanol Dehydration
Abstract
1. Introduction
2. Results and Discussion
2.1. Powder XRD and XRF Characterizations
2.2. FT-IR Spectroscopy
2.3. Textural Properties Using N2 Adsorption/Desorption Isotherms at −196 °C
2.4. SEM Images and Analysis
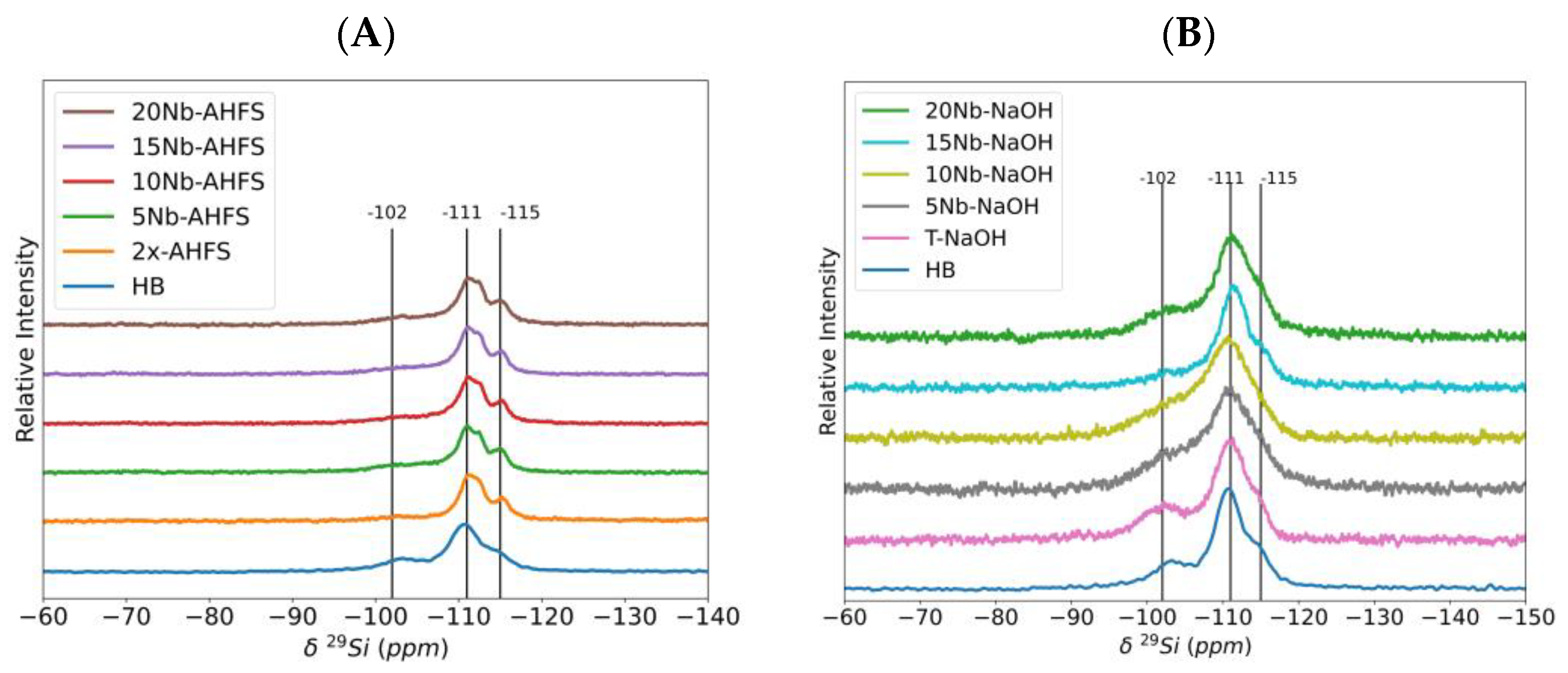
2.5. 27Al and 29Si MAS NMR Spectroscopy
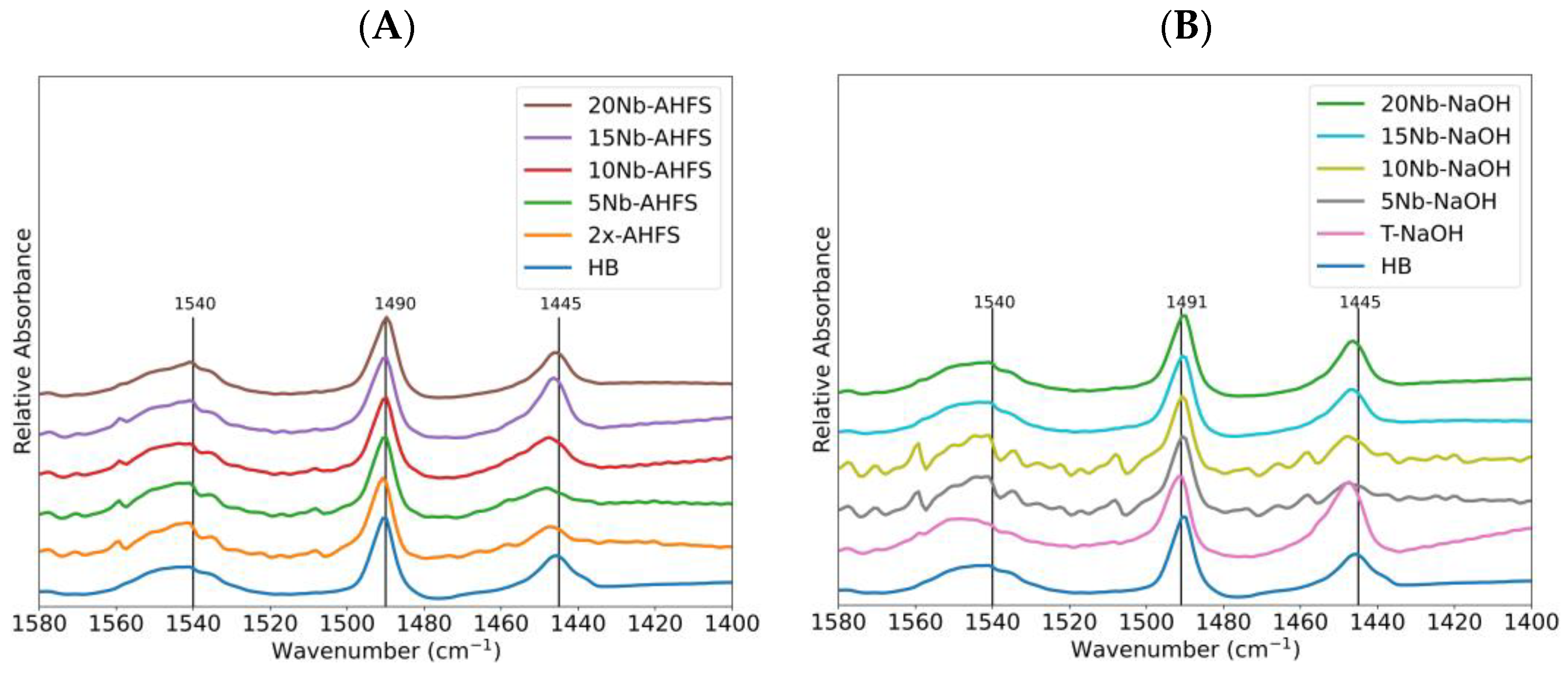
2.6. Acidity of the Catalysts
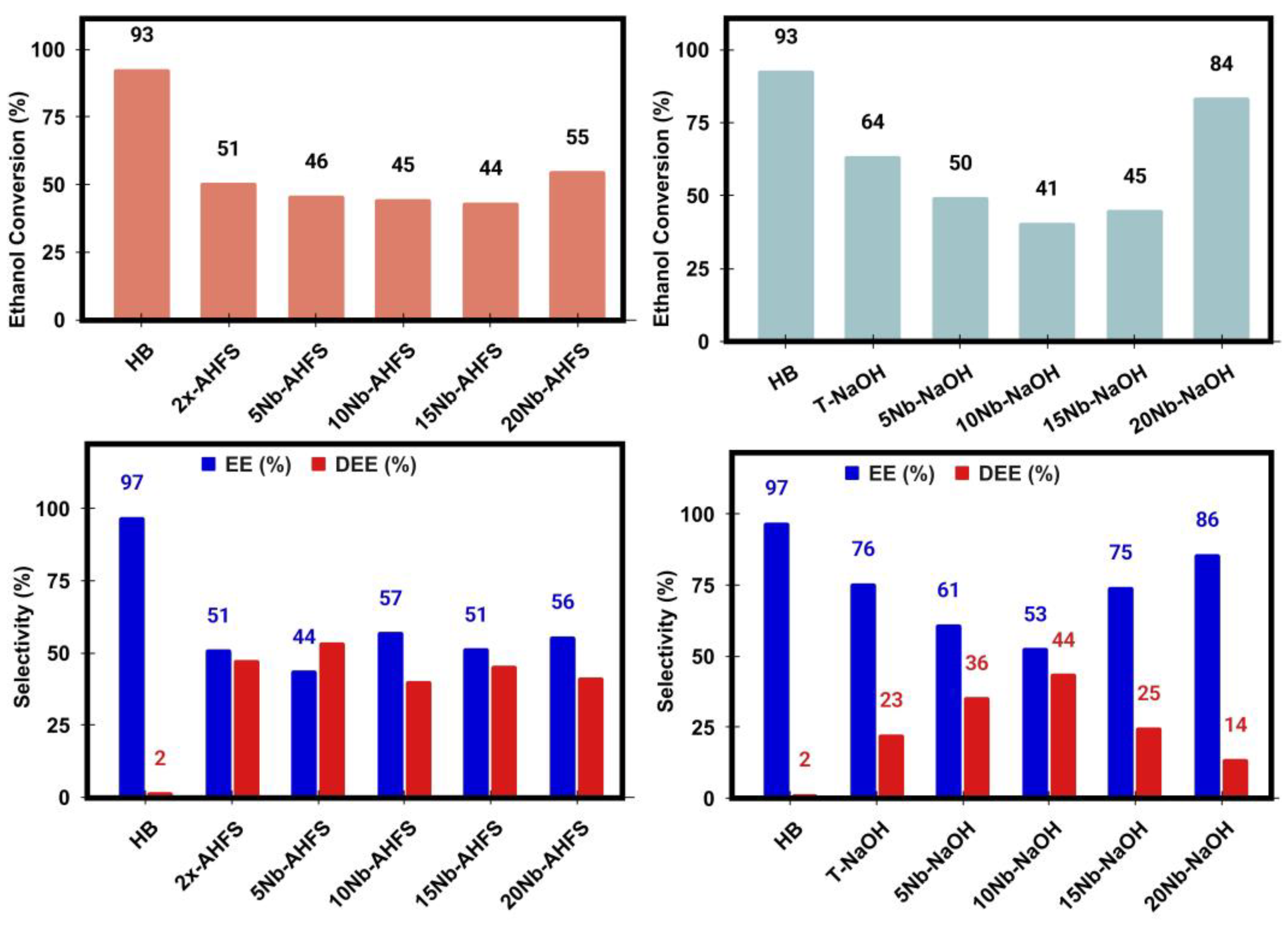
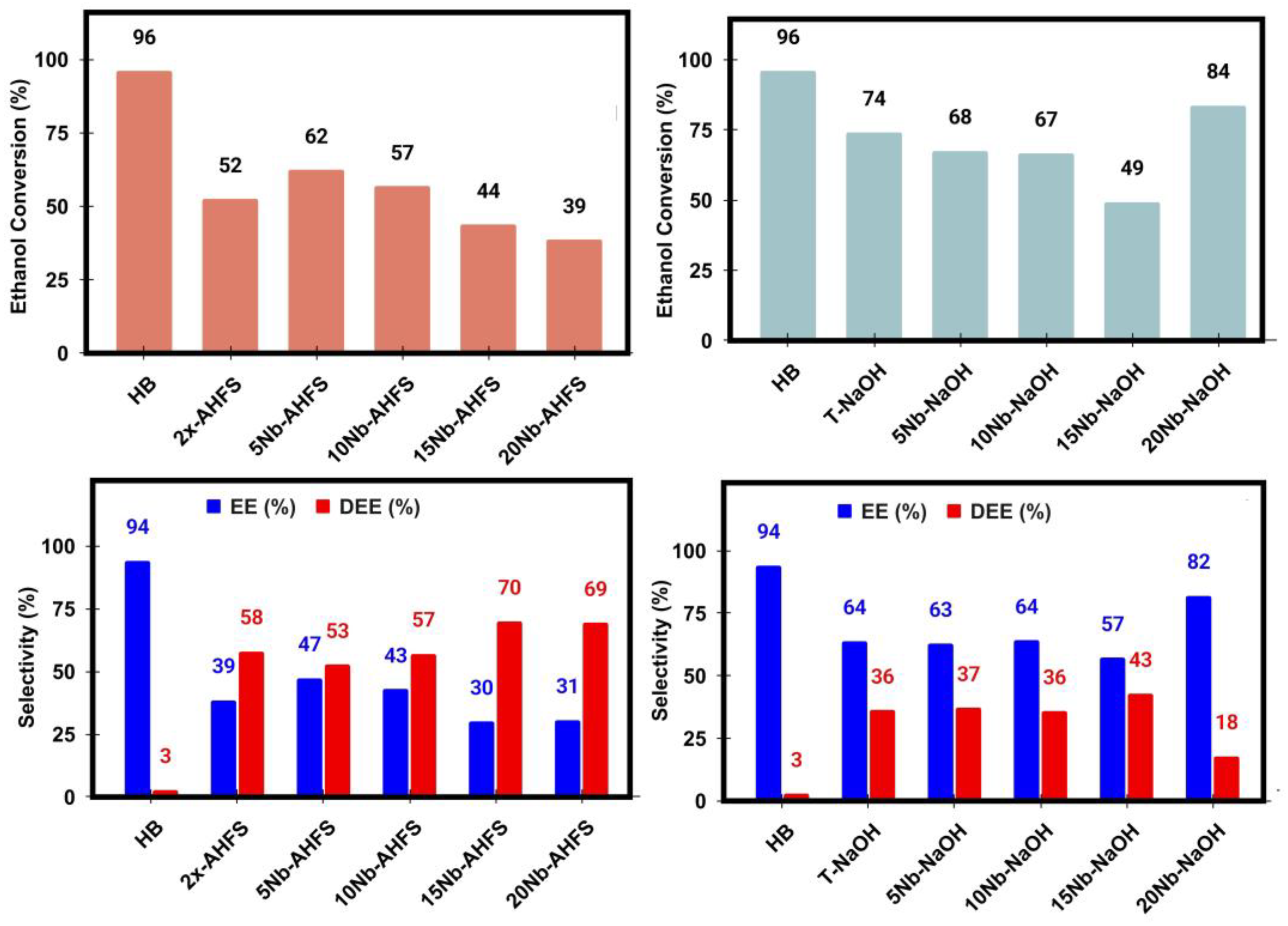
2.7. Ethanol Catalytic Dehydration
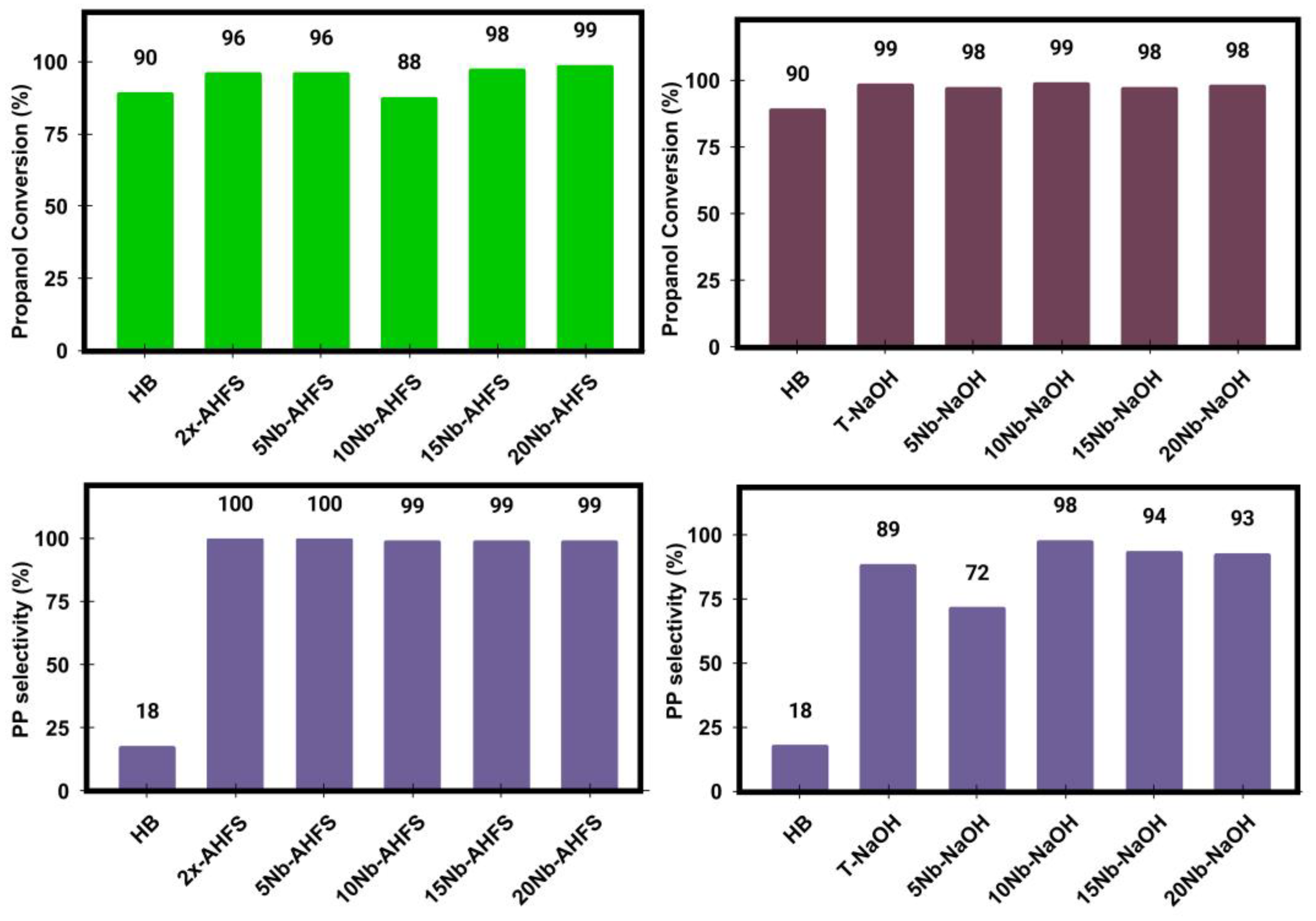
2.8. 1-Propanol Catalytic Dehydration
3. Materials and Methods
3.1. Hierarchization of *BEA Zeolite
- (i)
- Solid state hierarchization: a fraction of HB was subjected to solid-state dealumination using ammonium hexafluorosilicate (AHFS, 98%, Aldrich, Burlington, MA, USA) with the intention of removing a theoretical percentage of 70 mol% of Al from its crystal lattice by successive (2x) for comparison purposes. The solids were placed in an agate mortar and pestle, and mixed for 10 min, followed by placing the mixture in a desiccator containing a saturated ammonium chloride solution (>99.5%, Sigma-Aldrich, Burlington, MA, USA) at atmospheric pressure. After 24 h, the mixture was heated in a muffle furnace (3 h, 190 °C), followed by washing with ammonium acetate solution and deionized water (Milli-Q), both at room temperature. Finally, the mixture was dried in an oven (24 h, 120 °C) and calcined (8 h, 550 °C) [14].
- (ii)
- Hierarchization in base and acid solutions: the HB zeolite was treated with a 0.2 M sodium hydroxide solution (97%, Aldrich, Burlington, MA, USA), under magnetic stirring at 75 °C, for 4 h. Subsequently, this mixture was washed with deionized water for 1 h, also at 75 °C. Following this step, the resulting material was treated with a 0.5 M hydrochloric acid solution (37%, Aldrich, Burlington, MA, USA) under the same conditions as the base treatment. Finally, the same washing procedure was conducted, and the resulting zeolite was placed in a crucible, dried in an oven for 12 h at 120 °C, and calcined for 8 h at 550 °C.
3.2. Impregnation of Niobium
3.3. Methods of Characterization
3.4. Catalytic Dehydration Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-López, P.; Puente-Santiago, A.; Castro-Beltrán, A.; Nascimento, L.A.S.; Balu, A.M.; Luque, L.; Alvarado-Beltrán, C.G. Nanomaterials and catalysis for green chemistry. Curr. Opin. Green Sustain. Chem. 2020, 24, 48–55. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Mohammed, B.B.; Yamni, K.; Tijani, N.; Alrashdi, A.A.; Zouihri, H.; Dehmani, Y.; Chung, I.; Kim, S.; Lgaz, H. Adsorptive removal of phenol using faujasite-type Y zeolite: Adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies. J. Mol. Liq. 2019, 296, 111997. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112. [Google Scholar] [CrossRef]
- Zikrata, O.V.; Larina, O.V.; Balakin, D.Y.; Nychiporuk, Y.M.; Khalakhan, I.; Švegovec, M.; Volavšek, J.; Yaremov, P.S.; Soloviev, S.O.; Orlyk, S.M. Influence of acid-base characteristics of different structural-type zeolites (FER, MFI, FAU, BEA) on their activity and selectivity in isobutanol dehydration. ChemCatChem 2024, 16, e202400068. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Kumar, N.D.; Swaminathan, M.; Swaminathan, M. Review on hierarchically porous BEA and ZSM-5 zeolites and its industrial catalytic applications. ES Mater. Manuf. 2024, 24, 1151. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Hydrocracking catalysts based on hierarchical zeolites: A recent progress. J. Ind. Eng. Chem. 2018, 61, 265–280. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xiao, P.; Bekhti, S.; Kunkes, E.; Iemhoff, A.; Bottke, N.; Vos, D.E.; Meng, X.; Xiao, F.; et al. Needle-like hierarchical Beta zeolite synthesis via postsynthetic morphology control in the presence of cetyltrimethylammonium bromide for catalytic dehydration of sorbitol. ACS Appl. Nano Mater. 2025, 8, 1042–1053. [Google Scholar] [CrossRef]
- Fernandez, S.; Ostraat, M.L.; Zhang, K. Toward rational design of hierarchical beta zeolites: An overview and beyond. AIChE J. 2020, 66, e16943. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Waeyenberg, J.V.; Zhang, Q.; Yu, J.; Sels, B.F. State of the art and perspectives of hierarchical zeolites: Practical overview of synthesis methods and use in catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating hierarchical pores in zeolite catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Valadares, D.S.; Clemente, M.C.H.; Freitas, E.F.; Martins, G.A.V.; Dias, J.A.; Dias, S.C.L. Niobium on BEA dealuminated zeolite for high selectivity dehydration reactions of ethanol and xylose into diethyl ether and furfural. Nanomaterials 2020, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez-Ramírez, J. Mechanism of hierarchical porosity development in MFI zeolites by desilication: The role of aluminum as a pore-directing agent. Chem. Eur. J. 2005, 11, 4983–4994. [Google Scholar] [CrossRef]
- Clemente, M.C.H.; Valadares, D.S.; Lacava, A.B.; Barbosa, L.S.; Martins, G.A.V.; Dias, J.A.; Dias Sílvia, C.L. Catalytic transformation conditions of ethanol on dealuminated BEA zeolites. J. Braz. Chem. Soc. 2019, 30, 2182–2190. [Google Scholar] [CrossRef]
- Groen, J.C.; Abelló, S.; Villaescusa, L.A.; Pérez-Ramírez, J. Mesoporous beta zeolite obtained by desilication. Microporous Mesoporous Mater. 2008, 114, 93–102. [Google Scholar] [CrossRef]
- Ouayloul, L.; Agirrezabal-Telleria, I.; Arias, P.L.; Dumeignil, F.; ElDoukkali, M. Tuning the acid nature of the ZSM-5 surface for selective production of ethylene from ethanol at low temperatures. Energy Fuels 2024, 38, 4492–4503. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Vu, K.B.; Busca, G. (Bio)Propylene production processes: A critical review. J. Environ. Chem. Eng. 2021, 9, 105673. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, X.; Liu, T.; Liu, H.; Wang, T.; Yuan, P.; Zhu, H.; Bai, Z.; Bao, X. Template free synthesis of hierarchical porous zeolite beta with natural kaolin clay as alumina source. Microporous Mesoporous Mater. 2020, 293, 109772. [Google Scholar] [CrossRef]
- Xiong, G.; Yang, H.; Liu, L.; Liu, J. Post-synthesis of Sn-beta zeolite by aerosol method. RSC Adv. 2023, 13, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Willhammar, T.; Zou, X. Stacking disorders in zeolites and open-frameworks—Structure elucidation and analysis by electron crystallography and X-ray diffraction. Z. Kristallogr. 2013, 228, 11–27. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Murzin, V.Y.; Monakhova, Y.V.; Zubavichus, Y.V.; Knyazeva, E.E.; Nesterenko, N.S.; Ivanova, I.I. Nature, strength and accessibility of acid sites in micro/mesoporous catalysts obtained by recrystallization of zeolite BEA. Microporous Mesoporous Mater. 2007, 105, 101–110. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Cao, Z.; Gao, W.; Cui, L. Crystallization behavior of zeolite beta from acid-leached metakaolin. Pet. Sci. 2010, 7, 541–546. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- Chalupka, K.; Sadek, R.; Valentin, L.; Millot, Y.; Calers, C.; Nowosielska, M.; Rynkowski, J.; Dzwigaj, S. Dealuminated beta zeolite modified by alkaline earth metals. J. Chem. 2018, 2018, 7071524. [Google Scholar] [CrossRef]
- Ma, Y.; Rigolet, S.; Michelin, L.; Paillaud, J.; Mintova, S.; Khoerunnisa, F.T.; Daou, J.; Ng, E. Facile and fast determination of Si/Al ratio of zeolites using FTIR spectroscopy technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Millot, Y.; Méthivier, C.; Che, M. Incorporation of Nb(V) into BEA zeolite investigated by XRD, NMR, IR, DR UV–vis, and XPS. Microporous Mesoporous Mater. 2010, 130, 162–166. [Google Scholar] [CrossRef]
- Kiricsi, I.; Flego, C.; Pazzuconi, G.; Parker, W.O.; Millini, R.; Perego, C.; Bellussi, G. Progress toward understanding zeolite ß acidity: An IR and 27Al NMR spectroscopic study. J. Phys. Chem. 1994, 98, 4627–4634. [Google Scholar] [CrossRef]
- Sazama, P.; Wichterlová, B.; Sklenák, S.; Parvulescu, V.I.; Candu, N.; Sádovská, G.; Dedeck, J.; Klein, P.; Pashkova, V.; Štastny, P. Acid and redox activity of template-free Al-rich H-BEA* and Fe-BEA* zeolites. J. Catal. 2014, 318, 22–33. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium compounds: Preparation, characterization, and application in heterogeneous catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Wachs, I.E.; Jehng, J.M.; Deo, G.; Hu, H.; Arora, N. Redox properties of niobium oxide catalysts. Catal. Today 1996, 28, 199. [Google Scholar] [CrossRef]
- Braga, V.S.; Dias, J.A.; Dias, S.C.L.; Macedo, J.L. Catalyst materials based on Nb2O5 supported on silica-alumina: Preparation and structural characterization. Chem. Mater. 2005, 17, 690–695. [Google Scholar] [CrossRef]
- Candu, N.; Fergani, M.E.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Santos, L.R.M.; Silva, M.A.P.; Menezes, S.C.; Chinelatto, L.S.; Lam, Y.L. Creation of mesopores and structural re-organization in beta zeolite during alkaline treatment. Microporous Mesoporous Mater. 2016, 226, 260–266. [Google Scholar] [CrossRef]
- Hould, N.D.; Kumar, S.; Tsapatsis, M.; Nikolakis, V.; Lobo, R.F. Structure and colloidal stability of nanosized zeolite beta precursors. Langmuir 2010, 26, 1260–1270. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sugiyama, S.; Matsuoka, O.; Kohmura, K.; Honda, T.; Banno, Y.; Nozoye, H. Dissolution of zeolite in acidic and alkaline aqueous solutions as revealed by afm imaging. J. Phys. Chem. 1996, 100, 18474–18482. [Google Scholar] [CrossRef]
- Hartman, L.; Fogler, H.S. Reaction kinetics and mechanisms of zeolite dissolution in hydrochloric acid. Ind. Eng. Chem. Res. 2005, 44, 7738–7745. [Google Scholar] [CrossRef]
- Shestakova, P.; Martineau, C.; Mavrodinova, V.; Popova, M. Solid state NMR characterization of zeolite beta based drug formulations containing Ag and sulfadiazine. RSC Adv. 2015, 5, 81957–81964. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, S.; Hu, M.Y.; Bao, X.; Peden, C.H.F.; Hu, J. Investigation of aluminum site changes of dehydrated zeolite H-beta during a rehydration process by high-field solid-state NMR. J. Phys. Chem. C 2015, 119, 1410–1417. [Google Scholar] [CrossRef]
- Hartanto, D.; Yuan, L.S.; Sari, S.M.; Sugiarso, D.; Murwarni, I.K.; Ersam, T.; Prasetyoko, D.; Nur, H. The use of the combination of FTIR, pyridine adsorption, 27Al and 29Si MAS NMR to determine the Brønsted and Lewis acidic sites. J. Teknol. 2016, 78, 2016. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Rijo, B.; Pereira, M.F.C.; Zăvoianu, R.; Pavel, O.D. Valorization of (bio)ethanol over MoO3/(WO3-ZrO2) sol-gel-like catalysts. Reactions 2024, 5, 260–273. [Google Scholar] [CrossRef]
- Thangaraj, B.; Monama, W.; Mohiuddin, E.; Mdleleni, M.M. Recent developments in (bio)ethanol conversion to fuels and chemicals over heterogeneous catalysts. Bioresour. Technol. 2024, 409, 131230. [Google Scholar] [CrossRef]
- Zhukova, A.; Chuklina, S.; Fionov, Y.; Vakhrushev, N.; Sazonova, A.; Mikhalenko, I.; Zhukov, D.; Isaikina, O.; Fionov, A.; Il’icheva, A. Enhanced ethanol dehydrogenation over Ni-containing zirconia-alumina catalysts with microwave-assisted synthesis. Res. Chem. Intermed. 2024, 50, 1331. [Google Scholar] [CrossRef]
- Ngcobo, M.; Makgwane, P.R.; Mathe, M.K. A minireview on solid acid catalysts for dehydration of bioethanol to renewable ethylene: An update on catalysts development progress. Appl. Catal. O 2024, 193, 206976. [Google Scholar] [CrossRef]
- Garbarino, G.; Pampararo, G.; Phung, T.K.; Riani, P.; Busca, G. Heterogeneous catalysis in (bio)ethanol conversion to chemicals and fuels: Thermodynamics, catalysis, reaction paths, mechanisms and product selectivities. Energies 2020, 13, 3587. [Google Scholar] [CrossRef]
- Nanda, A.S.F.; Kadja, G.T.M. Bio-based templates for generation hierarchical zeolites: An overview for greener synthesis pathway. J. Porous Mater. 2024, 31, 1155–1173. [Google Scholar] [CrossRef]
- Motte, J.; Nachtergaele, P.; Mahmoud, M.; Vleeming, H.; Thybaut, J.W.; Poissonnier, J.; Dewulf, J. Developing circularity, renewability and efficiency indicators for sustainable resource management: Propanol production as a showcase. J. Clean. Prod. 2022, 379, 134843. [Google Scholar] [CrossRef]
- Zhi, Y.; Shi, H.; Mu, L.; Liu, Y.; Mei, D.; Camaioni, D.M.; Lercher, J.A. Dehydration pathways of 1-propanol on HZSM-5 in the presence and absence of water. J. Am. Chem. Soc. 2015, 137, 15781–15794. [Google Scholar] [CrossRef]
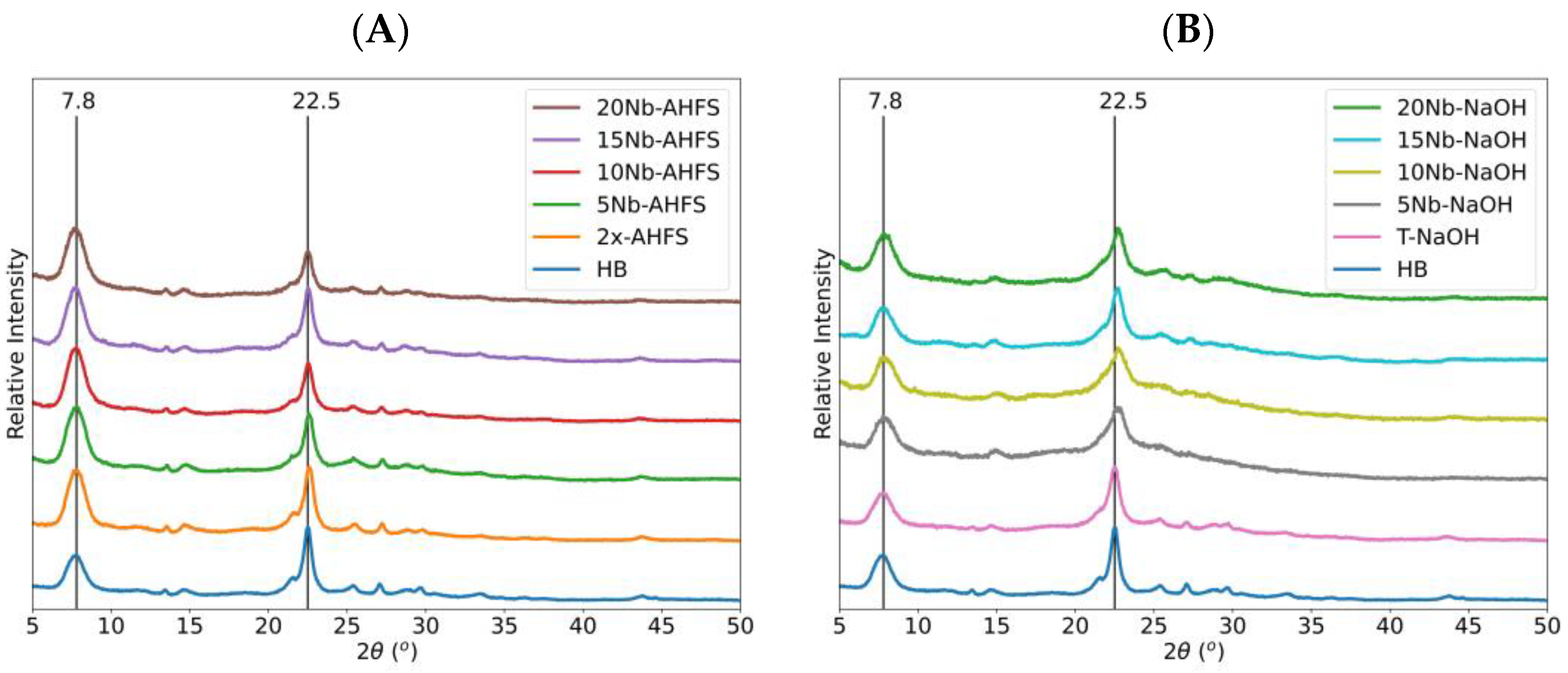
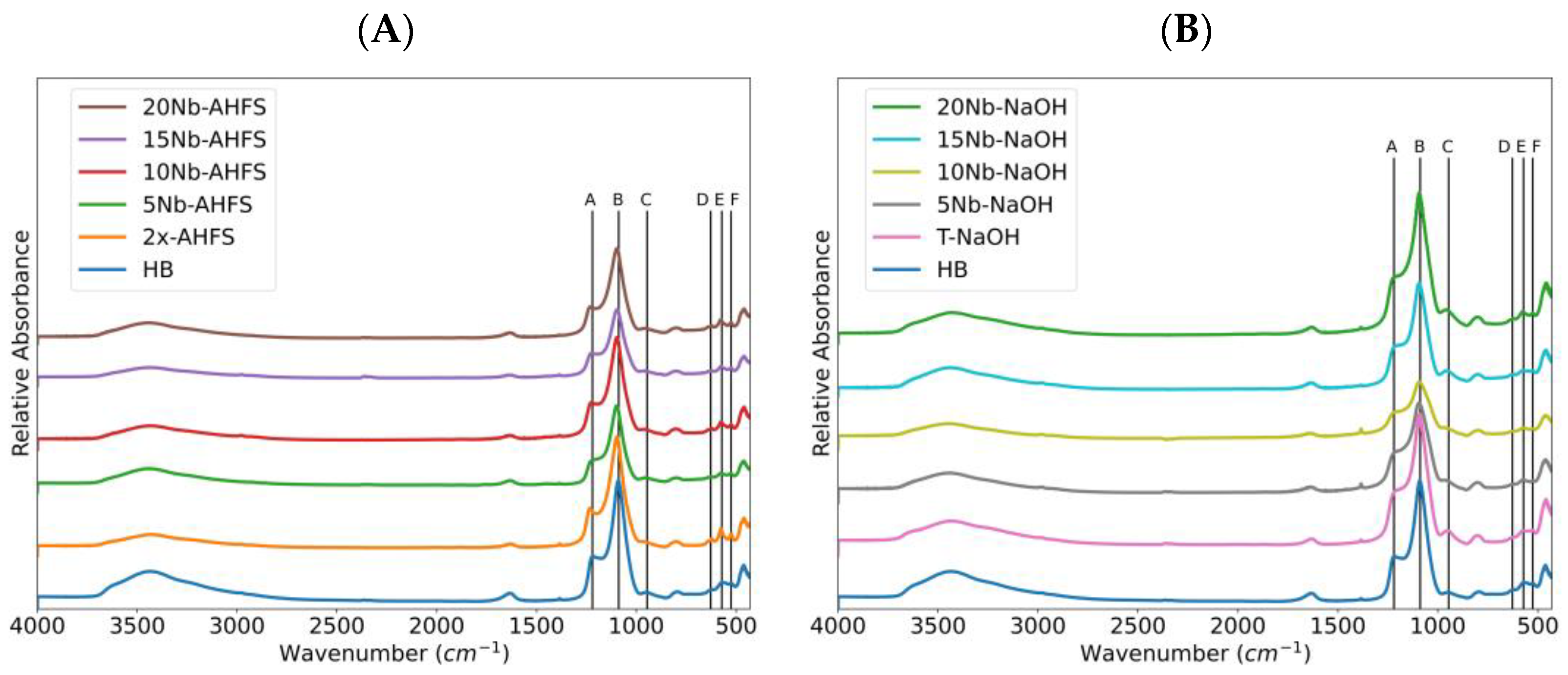


| Catalyst | %C a | 2θ (°) b | β (rad) b | D (nm) c | L (nm) d | Strain e | |
|---|---|---|---|---|---|---|---|
| HB | 25 | 100 | 22.52 | 0.0105 | 13.1 | 4.6 | 0.0007 |
| 2x-AHFS | 44 | 102 | 22.63 | 0.0115 | 11.9 | 5.2 | 0.0009 |
| 5Nb-AHFS | 35 | 100 | 22.60 | 0.0119 | 11.6 | 6.5 | 0.0009 |
| 10Nb-AHFS | 33 | 100 | 22.50 | 0.0119 | 11.6 | 6.7 | 0.0009 |
| 15Nb-AHFS | 35 | 95 | 22.50 | 0.0108 | 12.7 | 6.6 | 0.0008 |
| 20Nb-AHFS | 36 | 96 | 22.50 | 0.0112 | 12.3 | 6.6 | 0.0008 |
| T-NaOH | 39 | 88 | 22.57 | 0.0136 | 10.1 | 5.0 | 0.0012 |
| 5Nb-NaOH | 42 | 87 | 22.77 | 0.0199 | 6.9 | 7.7 | 0.0023 |
| 10Nb-NaOH | 41 | 89 | 22.69 | 0.0182 | 7.6 | 8.6 | 0.0020 |
| 15Nb-NaOH | 41 | 100 | 22.67 | 0.0140 | 9.8 | 6.2 | 0.0013 |
| 20Nb-NaOH | 38 | 106 | 22.72 | 0.0157 | 8.7 | 5.8 | 0.0015 |
| Catalyst | SBET a (m2/g) | SExt b (m2/g) | SMicro c (m2/g) | SMeso d (m2/g) | VMicro e (cm3/g) | Vp f (cm3/g) | VMeso g (cm3/g) |
|---|---|---|---|---|---|---|---|
| HB | 649 | 190 | 459 | 188 | 0.19 | 0.85 | 0.66 |
| 2x-AHFS | 577 | 193 | 384 | 211 | 0.16 | 0.87 | 0.71 |
| 5Nb-AHFS | 462 | 170 | 292 | 193 | 0.12 | 0.67 | 0.55 |
| 10Nb-AHFS | 448 | 156 | 292 | 179 | 0.12 | 0.63 | 0.51 |
| 15Nb-AHFS | 484 | 163 | 321 | 199 | 0.13 | 0.67 | 0.54 |
| 20Nb-AHFS | 512 | 183 | 330 | 211 | 0.13 | 0.77 | 0.64 |
| T-NaOH | 607 | 220 | 387 | 239 | 0.16 | 0.97 | 0.81 |
| 5Nb-NaOH | 391 | 222 | 169 | 151 | 0.07 | 0.68 | 0.61 |
| 10Nb-NaOH | 347 | 138 | 209 | 137 | 0.08 | 0.51 | 0.43 |
| 15Nb-NaOH | 484 | 163 | 320 | 198 | 0.13 | 0.76 | 0.63 |
| 20Nb-NaOH | 488 | 168 | 321 | 199 | 0.13 | 0.76 | 0.63 |
| Catalyst | δ Al-Td (ppm) | Area (%) | δ Al-Oh (ppm) | Area (%) |
|---|---|---|---|---|
| HB | 56 | 62 | −4.0 | 38 |
| 2x-AHFS | 56 | 65 | −4.0 | 35 |
| 5Nb-AHFS | 57 | 70 | −0.9 | 30 |
| 10Nb-AHFS | 56 | 70 | −6.8 | 30 |
| 15Nb-AHFS | 57 | 69 | −5.2 | 31 |
| 20Nb-AHFS | 56 | 81 | −4.3 | 19 |
| T-NaOH | 57 | 76 | 1.2 | 24 |
| 5Nb-NaOH | 57 | 89 | −1.4 | 11 |
| 10Nb-NaOH | 57 | 91 | −2.2 | 9 |
| 15Nb-NaOH | 57 | 91 | −7.2 | 9 |
| 20Nb-NaOH | 57 | 79 | −4.9 | 21 |
| Catalyst | Q3 (ppm) | Area (%) | Q4 (ppm) | Area (%) | Q4 (ppm) | Area (%) |
|---|---|---|---|---|---|---|
| HB | −103 | 20 | −111 | 67 | −115 | 13 |
| 2x-AHFS | −102 | 8 | −112 | 83 | −115 | 9 |
| 5Nb-AHFS | −103 | 15 | −112 | 74 | −115 | 11 |
| 10Nb-AHFS | −103 | 20 | −112 | 72 | −115 | 8 |
| 15Nb-AHFS | −103 | 13 | −112 | 78 | −115 | 9 |
| 20Nb-AHFS | −103 | 14 | −112 | 76 | −114 | 10 |
| T-NaOH | −102 | 26 | −111 | 67 | −114 | 7 |
| 5Nb-NaOH | −103 | 23 | −112 | 66 | −115 | 12 |
| 10Nb-NaOH | −104 | 29 | −110 | 50 | −113 | 21 |
| 15Nb-NaOH | −103 | 10 | −112 | 84 | −115 | 6 |
| 20Nb-NaOH | −103 | 18 | −112 | 73 | −115 | 10 |
| Catalyst | B/L |
|---|---|
| HB | 1.1 |
| 2x-AHFS | 1.2 |
| 5Nb-AHFS | 1.1 |
| 10Nb-AHFS | 1.1 |
| 15Nb-AHFS | 1.1 |
| 20Nb-AHFS | 1.1 |
| T-NaOH | 1.1 |
| 5Nb-NaOH | 1.2 |
| 10Nb-NaOH | 1.2 |
| 15Nb-NaOH | 1.1 |
| 20Nb-NaOH | 1.2 |
| Code | Description |
|---|---|
| HB | Protonic *BEA zeolite |
| 2x-AHFS | HB dealuminated twice 70 mol% |
| 5Nb-AHFS | HB dealuminated twice 70 mol% and impregnated with 5 wt.% of Nb2O5 |
| 10Nb-AHFS | HB dealuminated twice 70 mol% and impregnated with 10 wt.% of Nb2O5 |
| 15Nb-AHFS | HB dealuminated twice 70 mol% and impregnated with 15 wt.% of Nb2O5 |
| 20Nb-AHFS | HB dealuminated twice 70 mol% and impregnated with 20 wt.% of Nb2O5 |
| T-NaOH | HB treated with NaOH and HCl |
| 5Nb-NaOH | HB treated with NaOH and HCl and impregnated with 5 wt.% of Nb2O5 |
| 10Nb-NaOH | HB treated with NaOH and HCl and impregnated with 10 wt.% of Nb2O5 |
| 15Nb-NaOH | HB treated with NaOH and HCl and impregnated with 15 wt.% of Nb2O5 |
| 20Nb-NaOH | HB treated with NaOH and HCl and impregnated with 20 wt.% of Nb2O5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Valadares, D.; de Carvalho, W.H.R.; Fonseca, A.L.F.; Machado, G.d.F.; Silva, M.R.; Campos, P.T.A.; Dias, J.A.; Dias, S.C.L. Different Routes for the Hierarchization of *BEA Zeolite, Followed by Impregnation with Niobium and Application in Ethanol and 1-Propanol Dehydration. Catalysts 2025, 15, 340. https://doi.org/10.3390/catal15040340
da Silva Valadares D, de Carvalho WHR, Fonseca ALF, Machado GdF, Silva MR, Campos PTA, Dias JA, Dias SCL. Different Routes for the Hierarchization of *BEA Zeolite, Followed by Impregnation with Niobium and Application in Ethanol and 1-Propanol Dehydration. Catalysts. 2025; 15(4):340. https://doi.org/10.3390/catal15040340
Chicago/Turabian Styleda Silva Valadares, Deborah, Willian Henrique Ribeiro de Carvalho, Ana Lívia Fernandes Fonseca, Guilherme de França Machado, Matheus Ramos Silva, Pablo Teles Aragão Campos, José Alves Dias, and Sílvia Cláudia Loureiro Dias. 2025. "Different Routes for the Hierarchization of *BEA Zeolite, Followed by Impregnation with Niobium and Application in Ethanol and 1-Propanol Dehydration" Catalysts 15, no. 4: 340. https://doi.org/10.3390/catal15040340
APA Styleda Silva Valadares, D., de Carvalho, W. H. R., Fonseca, A. L. F., Machado, G. d. F., Silva, M. R., Campos, P. T. A., Dias, J. A., & Dias, S. C. L. (2025). Different Routes for the Hierarchization of *BEA Zeolite, Followed by Impregnation with Niobium and Application in Ethanol and 1-Propanol Dehydration. Catalysts, 15(4), 340. https://doi.org/10.3390/catal15040340







