Synergistic MoS2–Gold Nanohybrids for Sustainable Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
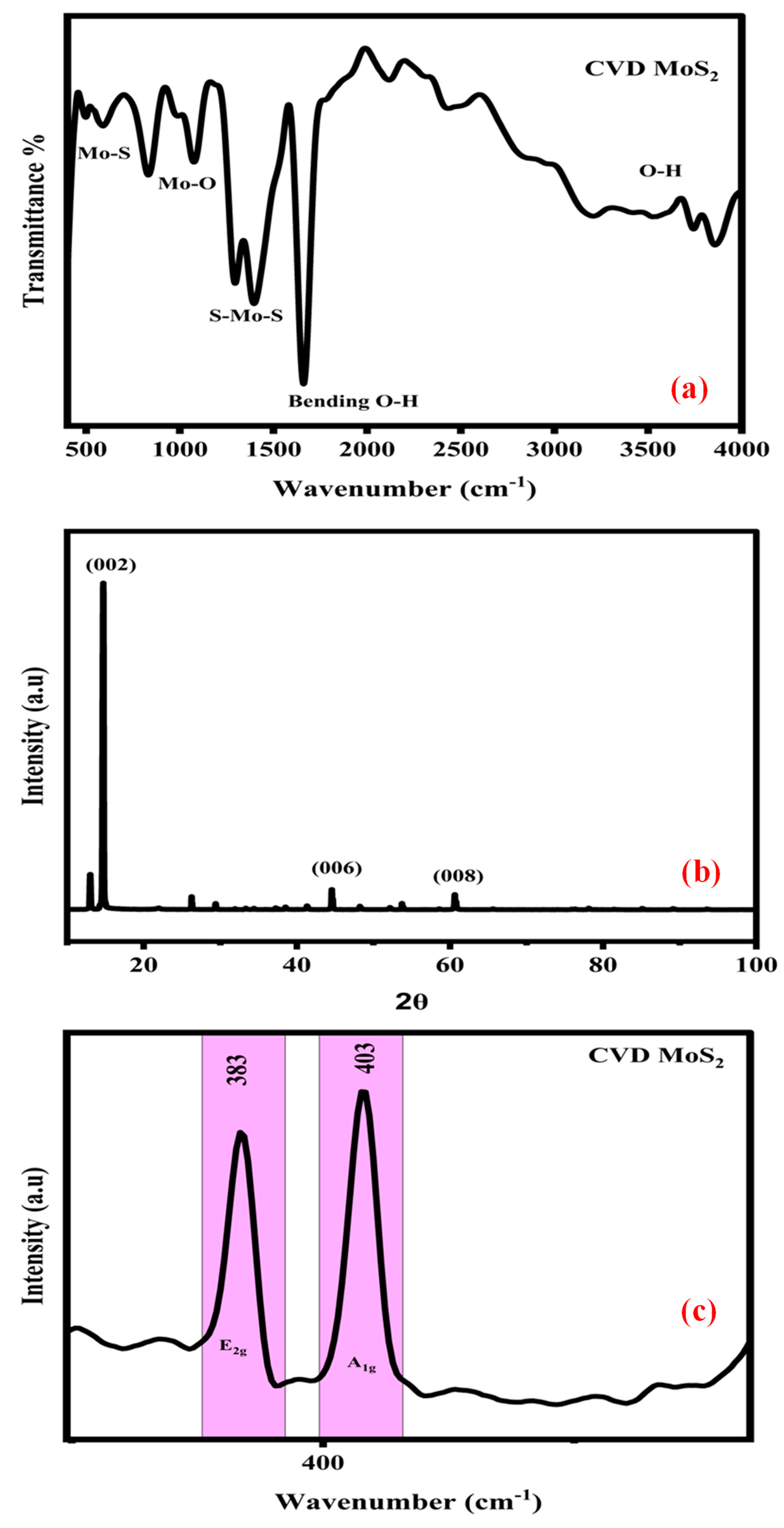
2.1. FTIR Spectra
2.2. XRD Spectra
2.3. Raman Spectra
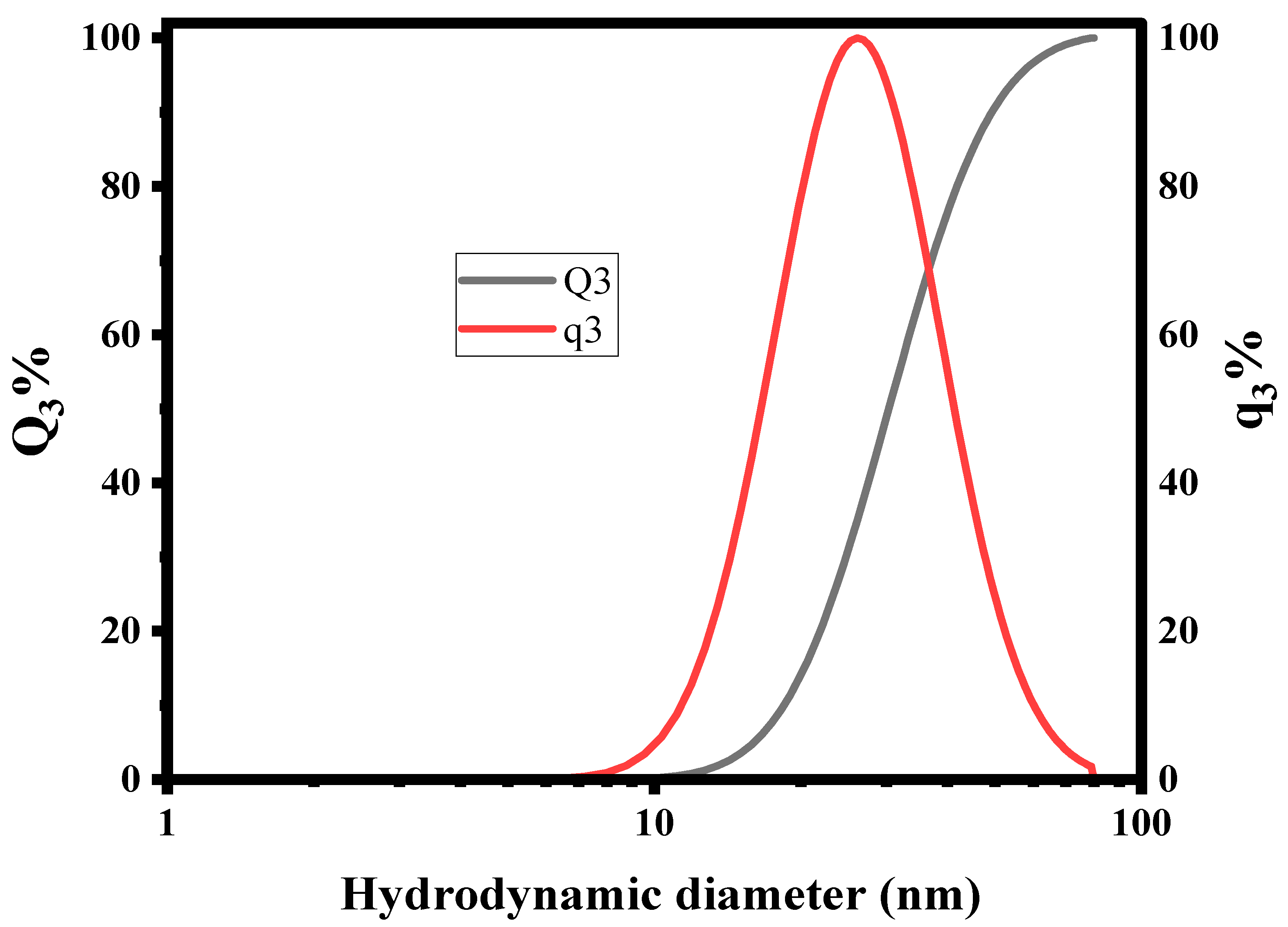
2.4. Dynamic Light Scattering (DLS)
2.5. SEM and EDS
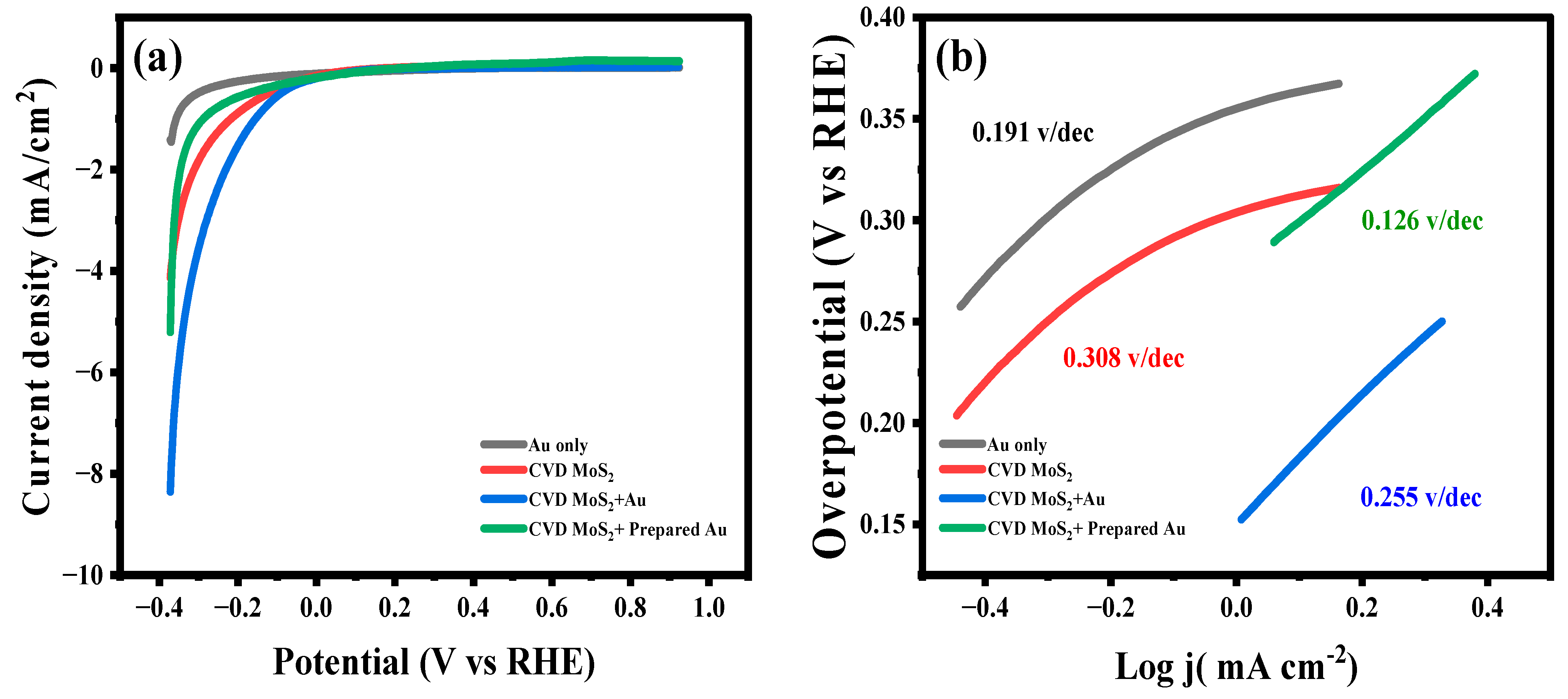
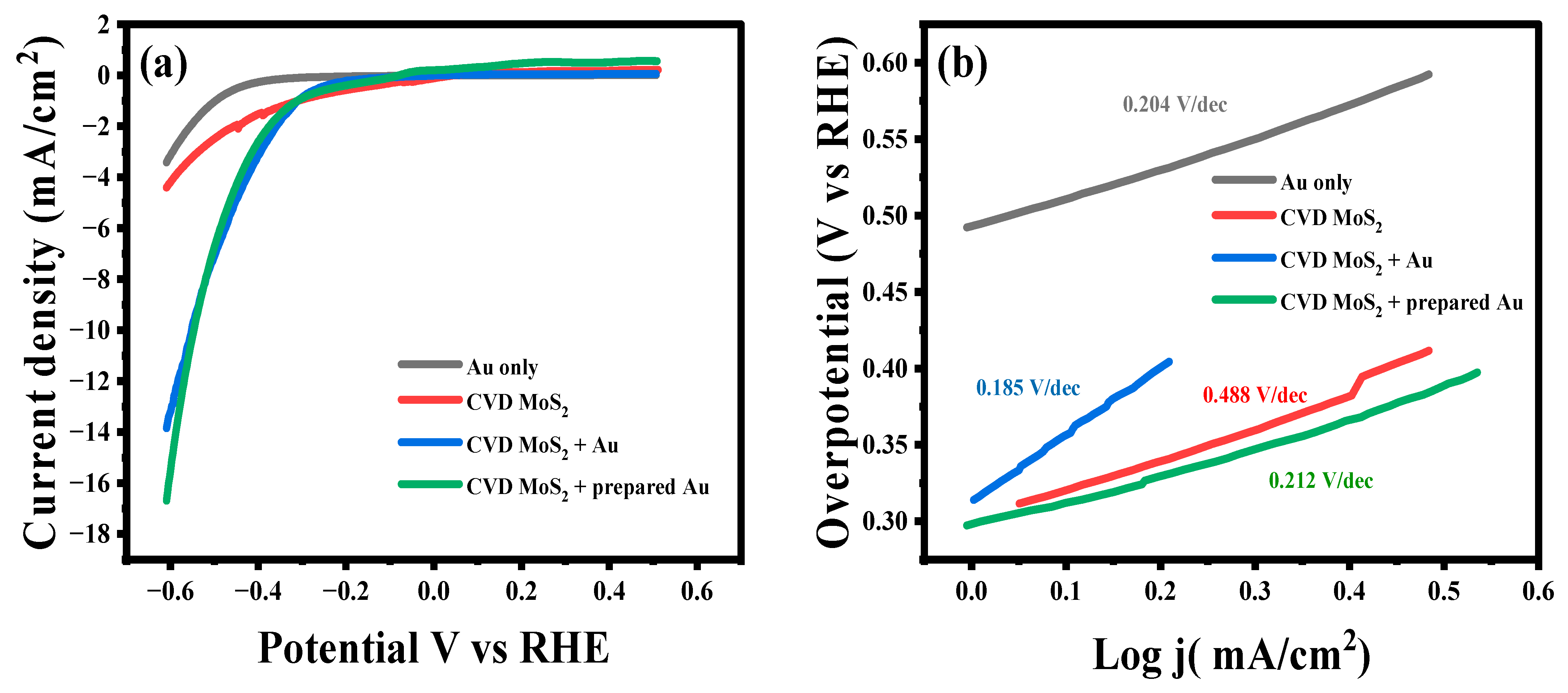
2.6. HER Activity of CVD-MoS2/Au in Neutral Medium
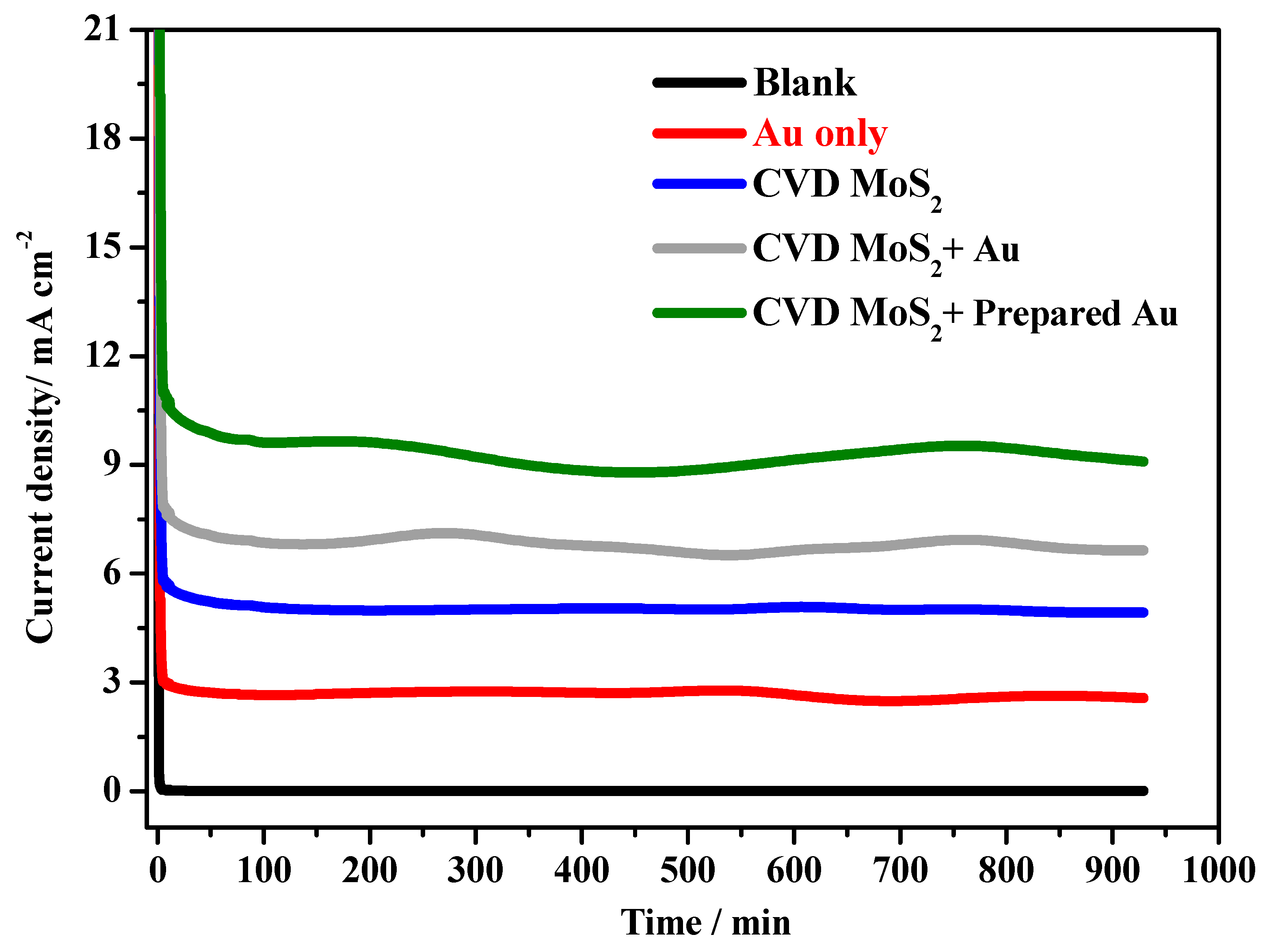
2.7. Chronoamperometry Studies of the MoS2+ Au Sample
2.8. Comparative Study of Recent Literature on the Use of MoS2/Au as a Catalyst for the HER
3. Experimental Procedures
3.1. Materials and Reagent
3.2. Synthesis of Au
3.3. Synthesis of CVD-MoS2
3.4. Fabrication of MoS2 Modified GCE
3.5. Samples Characterization
3.6. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, S.C.; Seok, J.H.; Manh, H.N.; Seol, J.H.; Lee, C.H.; Lee, S.U. Expanding the frontiers of electrocatalysis: Advanced theoretical methods for water splitting. Nano Convergence 2025, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Manyepedza, T.; Courtney, J.M.; Snowden, A.; Jones, C.R.; Rees, N.V. Impact Electrochemistry of MoS2: Electrocatalysis and Hydrogen Generation at Low Overpotentials. J. Phys. Chem. C. 2022, 126, 17942–17951. [Google Scholar] [CrossRef]
- Al-Amer, K.; Laradhi, S.S.; Aleithan, S.H.; Abd El-Lateef, H.M. Synthesis, Characterization, and Hydrogen Evolution Reaction Activity of MoS2 Nanostructures Prepared Using Nonionic Surfactant. Catalysts 2024, 14, 804. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Manjunath, K.; Samrat, D.; Reddy, V.; Ramakrishnappa, T.; Nagaraju, D.H. Hydrothermal synthesis of 2D MoS2 nanosheets for electrocatalytic hydrogen evolution reaction. RSC Adv. 2015, 5, 89389–89396. [Google Scholar] [CrossRef]
- Zhao, X.; He, D.-W.; Wang, Y.-S.; Fu, C. In situ growth of different numbers of gold nanoparticles on MoS2 with enhanced electrocatalytic activity for hydrogen evolution reaction. Chinese Phys. B 2018, 27, 068103. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Papers Energy 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Lu, K.; Liu, J.; Dai, X.; Zhao, L.; Yang, Y.; Li, H.; Jiang, Y. Construction of a Au@MoS2 composite nanosheet biosensor for the ultrasensitive detection of a neurotransmitter and understanding of its mechanism based on DFT calculations. RSC Adv. 2022, 12, 798–809. [Google Scholar] [CrossRef]
- Chen, B.; Hu, P.; Yang, F.; Hua, X.; Yang, F.F.; Zhu, F.; Sun, R.; Hao, K.; Wang, K.; Yin, Z. In Situ Porousized MoS2 Nano Islands Enhance HER/OER Bifunctional Electrocatalysis. Small 2023, 19, 2207177. [Google Scholar] [CrossRef]
- Bar-Ziv, R.; Ranjan, P.; Lavie, A.; Jain, A.; Garai, S.; Bar Hen, A.; Popovitz-Biro, R.; Tenne, R.; Arenal, R.; Ramasubramaniam, A.; et al. Au-MoS2 Hybrids as Hydrogen Evolution Electrocatalysts ACS Appl. Energy Mater. 2019, 2, 6043–6050. [Google Scholar]
- Li, Y.; Majewski, M.B.; Islam, S.M.; Hao, S.; Murthy, A.A.; DiStefano, J.G.; Hanson, E.D.; Xu, Y.; Wolverton, C.; Kanatzidis, M.G.; et al. Morphological Engineering of Winged Au@MoS2 Heterostructures for Electrocatalytic Hydrogen Evolution. Nano Lett. 2018, 18, 7104–7110. [Google Scholar]
- Zhou, Q.; Hu, H.; Chen, Z.; Ren, X.; Ma, D. Enhancing electrocatalytic hydrogen evolution via engineering unsaturated electronic structures in MoS2. Chem. Sci. 2025, 16, 1597–1616. [Google Scholar] [CrossRef] [PubMed]
- Obiang Nsang, G.E.; Ullah, B.; Hua, S.; Shah, S.A.; Ullah, N.; Ullah, N.; Dike, F.N.; Yaseen, W.; Yuan, A.; Khan, N.; et al. NiS nanoparticle-MoS2 nanosheet core-shell spheres: PVP-assisted synthesis and efficient electrocatalyst for hydrogen evolution reaction. Energy Mater. 2025, 5, 500047. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, R.; Wang, Q.; Wang, X. Gold Nanoclusters Grown on MoS2 Nanosheets by Pulsed Laser Deposition: An Enhanced Hydrogen Evolution Reaction. Molecules 2021, 26, 7503. [Google Scholar] [CrossRef]
- Kim, J.; Byun, S.; Smith, A.J.; Yu, J.; Huang, J. Enhanced Electrocatalytic Properties of Transition-Metal Dichalcogenides Sheets by Spontaneous Gold Nanoparticle Decoration. J. Phys. Chem. Lett. 2013, 4, 1227–1232. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, R.; Song, Y.; Zhang, H.; House, S.D.; Yang, J.C.; Jin, R. Atomically Precise Gold Nanoclusters Accelerate Hydrogen Evolution over MoS2 Nanosheets: The Dual Interfacial Effect. Small 2017, 13, 1701519. [Google Scholar] [CrossRef]
- Shakya, J.; Patel, A.S.; Singh, F.; Mohanty, T. Composition dependent Fermi level shifting of Au decorated MoS2 nanosheets. Applied Physics Letters. 2016, 108, 013103. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, L.; Du, K.; Liu, W.; Zhu, Z.; Li, M. Molybdenum disulfide and Au ultrasmall nanohybrids as highly active electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A. 2017, 5, 4122–4128. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.-K.; Jin, L.; Hsu, Y.-T.; Yu, S.F.; Li, L.-J.; Yang, H.Y. Selective Decoration of Au Nanoparticles on Monolayer MoS2 Single Crystals. Sci. Rep. 2013, 3, 1839. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Yang, S.; Zheng, H.; Li, Y. Facile synthesis of MoS2 nanosheet-silver nanoparticles composite for surface enhanced Raman scattering and electrochemical activity. J. Alloys Compd. 2013, 559, 87–91. [Google Scholar] [CrossRef]
- Shomalian, K.; Bagheri-Mohagheghim, M.-M.; Ardyanian, M. Characterization and study of reduction and sulfurization processing in phase transition from molybdenum oxide (MoO2) to molybdenum disulfide (MoS2) chalcogenide semiconductor nanoparticles prepared by one-stage chemical reduction method. Appl. Phys. A. 2017, 123, 93. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Thalji, M.R.; Soh, W.C.; Algarni, H.; Chong, K.F. One-step electrochemical synthesis of MoS2/graphene composite for supercapacitor application. J. Solid State Electrochem. 2020, 24, 25–34. [Google Scholar] [CrossRef]
- Venkateshwaran, S.; Senthil Kumar, S.M. Provoking Metallic 1T Phase Conversion of 2H-MoS2 via an Effectual Solvothermal Route for Electrocatalytic Water Reduction in Acid. ACS Sustainable Chem. Eng. 2022, 10, 5258–5267. [Google Scholar] [CrossRef]
- Saadati, M.; Akhavan, O.; Fazli, H. Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features. Catalysts 2021, 11, 1445. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, S.; Zhang, N.; Chen, Y.; Gao, H.; Zhang, X. Synthesis and characterization of three-dimensional MoS2@carbon fibers hierarchical architecture with high capacity and high mass loading for Li-ion batteries. J. Colloid Interface Sci. 2018, 510, 327–333. [Google Scholar] [CrossRef]
- Nagarajan, T.; Khalid, M.; Sridewi, N.; Jagadish, P.; Shahabuddin, S.; Muthoosamy, K.; Walvekar, R. Tribological, oxidation and thermal conductivity studies of microwave synthesised molybdenum disulfide (MoS2) nanoparticles as nano-additives in diesel based engine oil. Sci. Rep. 2022, 12, 14108. [Google Scholar] [CrossRef]
- Hasmin, H.F.; Imawan, C.; Fauzia, V. The Role of Temperature in the Hydrothermal Synthesis on the Structural and Morphological Properties of MoS2. J. Phys. Conf. Ser. 2021, 1951, 012014. [Google Scholar]
- Zhang, J.; Han, D.; Wang, Y.; Wang, L.; Chen, X.; Qiao, X.; Yu, X. Synergy between nanozymes and natural enzymes on the hybrid MoS2 nanosheets/graphite microfiber for enhanced voltammetric determination of hydrogen peroxide. Microchim Acta 2020, 187, 321. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, K.; Qin, J.; Wang, S.; Wei, W.; Wang, J.; Shen, Q.; Qu, P.; Liu, D. Rux Se@MoS2 hybrid as a highly efficient electrocatalyst toward hydrogen evolution reaction. RSC Adv. 2019, 9, 13486–13493. [Google Scholar] [CrossRef]
- Biswas, S.; Ranwa, S. CVD-Grown MoS2 Nanosheets-Based Gas Sensor for Low-Limit Detection of NO2 Gas. In Proceedings of the 4th International Conference on Communication, Devices and Computing; Lecture Notes in Electrical Engineering; Sarkar, D.K., Sadhu, P.K., Bhunia, S., Samanta, J., Paul, S., Eds.; Springer: Singapore, 2023; Volume 1046, pp. 439–447. [Google Scholar]
- Thangappan, R.; Dhinesh Kumar, R.; Jayavel, R. Facile Synthesis and Characterization of Molybdenum Oxide (MoO3) Nanofibers and Submicron Rods by Electrospinning Technique for Potential Application in Photocatalytic Activity. J. Clust Sci. 2022, 33, 2209–2214. [Google Scholar] [CrossRef]
- Solanki, K.N.; Cadambi, R.M.; Srikari, S.; Shankapal, S.R. Synthesis of Nanostructured Molybdenum Disulfide by Chemical Vapour Deposition. Mater. Today Proc. 2017, 4, 11134–11140. [Google Scholar] [CrossRef]
- Ghahramanifard, F.; Rouhollahi, A.; Fazlolahzadeh, O. Synthesis of n-type Cu-doped ZnO Nanorods onto FTO by Electrodeposition Method and Study its Electrocatalytic Properties toward CO2 Reduction. Anal. Bioanal. Electrochem. 2018, 10, 362–371. [Google Scholar]
- Singh, S.; Deb, J.; Sarkar, U.; Sharma, S. MoS2/MoO3 Nanocomposite for Selective NH3 Detection in a Humid Environment. ACS Sustain. Chem. Eng. 2021, 9, 7328–7340. [Google Scholar] [CrossRef]
- Aleithan, S.H.; Al-Amer, K.; Alabbad, Z.H.; Khalaf, M.M.; Alam, K.; Alhashem, Z.; Abd El-Lateef, H.M. Highly scalable synthesis of MoS2 thin films for carbon steel coatings: Influence of synthetic route on the nanostructure and corrosion performance. J. Mater. Res. Technol. 2023, 23, 1239–1251. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Wang, C.; Zhai, T.-T.; Bao, W.-J.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Hot Electron of Au Nanorods Activates the Electrocatalysis of Hydrogen Evolution on MoS2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 7365–7370. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. KONA 2020, 37, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of Various Size Gold Nanoparticles by Chemical Reduction Method with Different Solvent Polarity. Nanoscale Res. Lett. 2020, 15, 140. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Aleithan, S.H.; Al-Amer, K.; Alhashem, Z.; Alati, N.A.; Alabbad, Z.H.; Alam, K. Growth of MoS2 films: High-quality monolayered and multilayered material. AIP Adv. 2022, 12, 075220. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Chen, L.; Huang, J.; Shi, X.; Han, D.; Wen, J.; Yan, H. Preparation of MoS2 @AuNP nanocomposite by a self-reduction method and its application for electrochemical glucose sensing. Mater. Adv. 2022, 3, 8677–8683. [Google Scholar] [CrossRef]
- Zeng, X.; Niu, L.; Song, L.; Wang, X.; Shi, X.; Yan, J. Effect of Polymer Addition on the Structure and Hydrogen Evolution Reaction Property of Nanoflower-Like Molybdenum Disulfide. Metals 2015, 5, 1829–1844. [Google Scholar] [CrossRef]
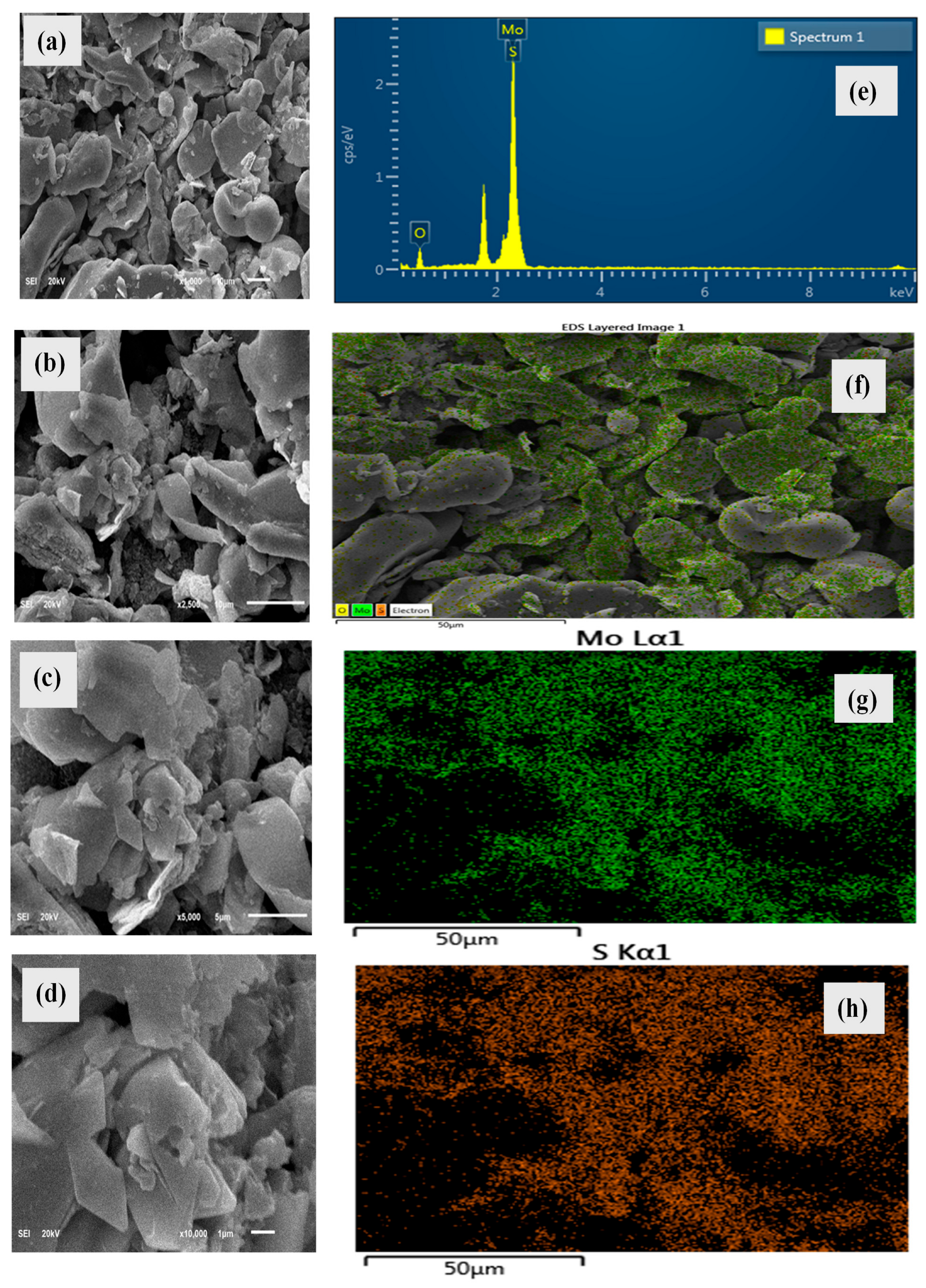
| Element | At. No. | Mass [%] | Atom [%] |
|---|---|---|---|
| Oxygen | 8 | 28.88 | 2.24 |
| Sulfur | 8 | 22.35 | 1.59 |
| Molybdenum | 42 | 48.76 | 2.63 |
| Sum | 100.00 |
| Catalyst | Onset Potential (V) | Cathodic Current * (mA/ cm 2) | Tafel (sV/dec) | Exchange Current mA cm−2 (j0) |
|---|---|---|---|---|
| Au | 0.357 | 1.4 | 0.191 | 0.111 |
| CVD-MoS2 | 0.215 | 4.3 | 0.308 | 0.149 |
| CVD-MoS2 + prepared Au | 0.289 | 5.2 | 0.126 | 0.186 |
| CVD-MoS2 + Au | 0.152 | 8.4 | 0.255 | 0.22 |
| * at 0.372 V | ||||
| Catalyst | Onset Potential (V) | Cathodic Current * (mA/ cm 2) | Tafel (V/dec) | Exchange Current mA cm−2 (j0) |
|---|---|---|---|---|
| Au | 0.492 | 1.15 | 0.204 | 0.0027 |
| CVD-MoS2 | 0.311 | 2.6 | 0.488 | 0.11 |
| CVD-MoS2 + prepared Au | 0.304 | 7.48 | 0.212 | 0.199 |
| CVD-MoS2 + Au | 0.304 | 7.27 | 0.185 | 0.0123 |
| Catalyst | Supporting Electrode | Electrolyte | HER onset (V) | Tafel (mV/dec) | Ref |
|---|---|---|---|---|---|
| MoS2-Au | GC | H2SO4 | 0.2 | 50 | [11] |
| MoS2-Au | GC | KCl | 0.27 | 71 | [37] |
| MoS2-Au | Si substrate | H2SO4 | 0.340 | 99.8 | [12] |
| W- MoS2-Au | Si substrate | H2SO4 | 0.190 | 86.9 | [12] |
| CVD-MoS2 | GC | H2SO4 | 0.31 | 488 | This work |
| CVD-MoS2/Au | GC | H2SO4 | 0.304 | 185 | This work |
| CVD-MoS2/Prepared Au | GC | H2SO4 | 0.306 | 212 | This work |
| CVD-MoS2 | GC | KCl | 0.215 | 308 | This work |
| CVD-MoS2/Au | GC | KCl | 0.152 | 255 | This work |
| CVD-MoS2/Prepared Au | GC | KCl | 0.289 | 126 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleithan, S.H.; Laradhi, S.S.; Al-Amer, K.; El-Lateef, H.M.A. Synergistic MoS2–Gold Nanohybrids for Sustainable Hydrogen Production. Catalysts 2025, 15, 550. https://doi.org/10.3390/catal15060550
Aleithan SH, Laradhi SS, Al-Amer K, El-Lateef HMA. Synergistic MoS2–Gold Nanohybrids for Sustainable Hydrogen Production. Catalysts. 2025; 15(6):550. https://doi.org/10.3390/catal15060550
Chicago/Turabian StyleAleithan, Shrouq H., Shroq S. Laradhi, Kawther Al-Amer, and Hany M. Abd El-Lateef. 2025. "Synergistic MoS2–Gold Nanohybrids for Sustainable Hydrogen Production" Catalysts 15, no. 6: 550. https://doi.org/10.3390/catal15060550
APA StyleAleithan, S. H., Laradhi, S. S., Al-Amer, K., & El-Lateef, H. M. A. (2025). Synergistic MoS2–Gold Nanohybrids for Sustainable Hydrogen Production. Catalysts, 15(6), 550. https://doi.org/10.3390/catal15060550







