Preparation of Metal-Hybridized Magnetic Nanocellulose for ω-Transaminase Immobilization
Abstract
1. Introduction
2. Results and Discussion
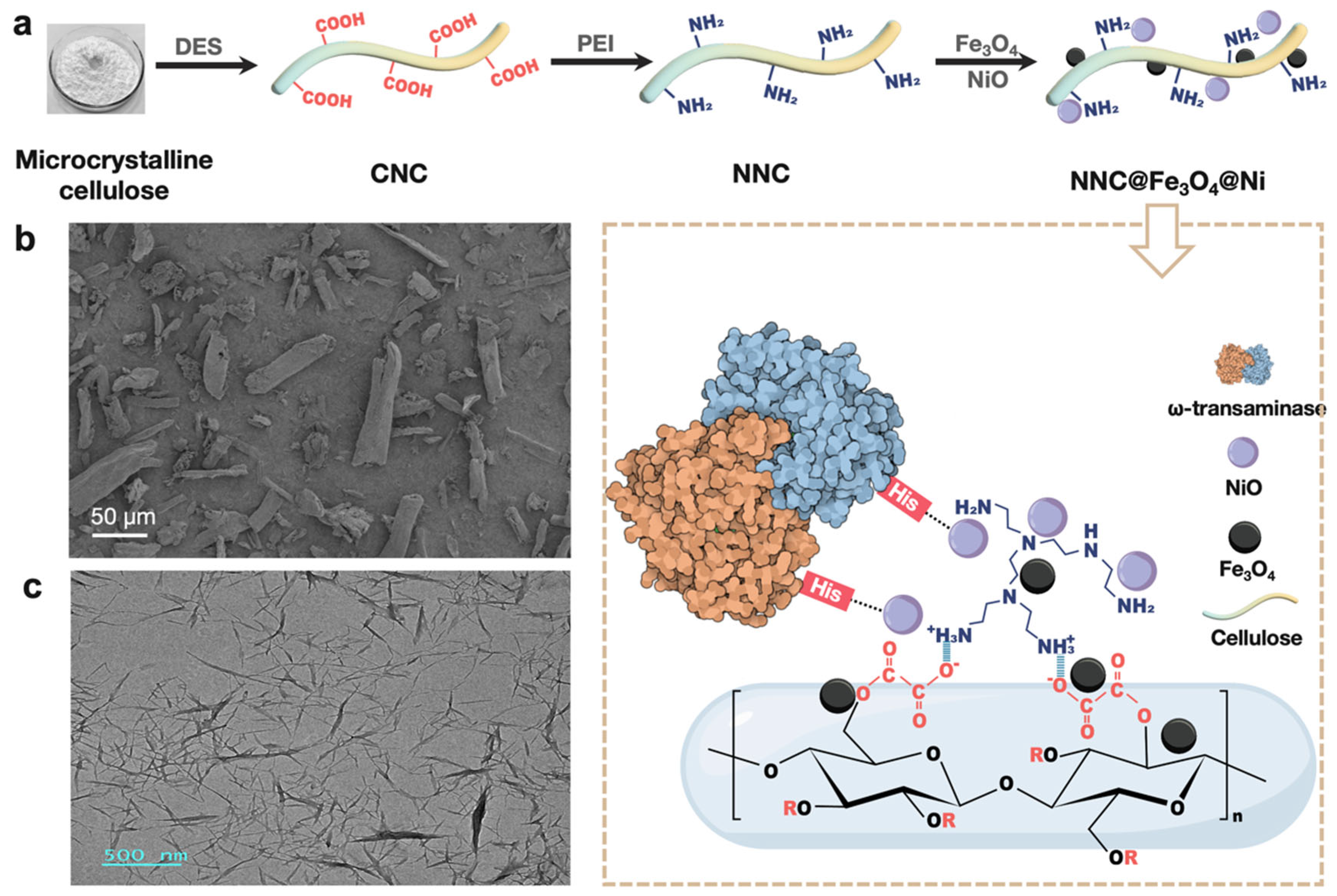
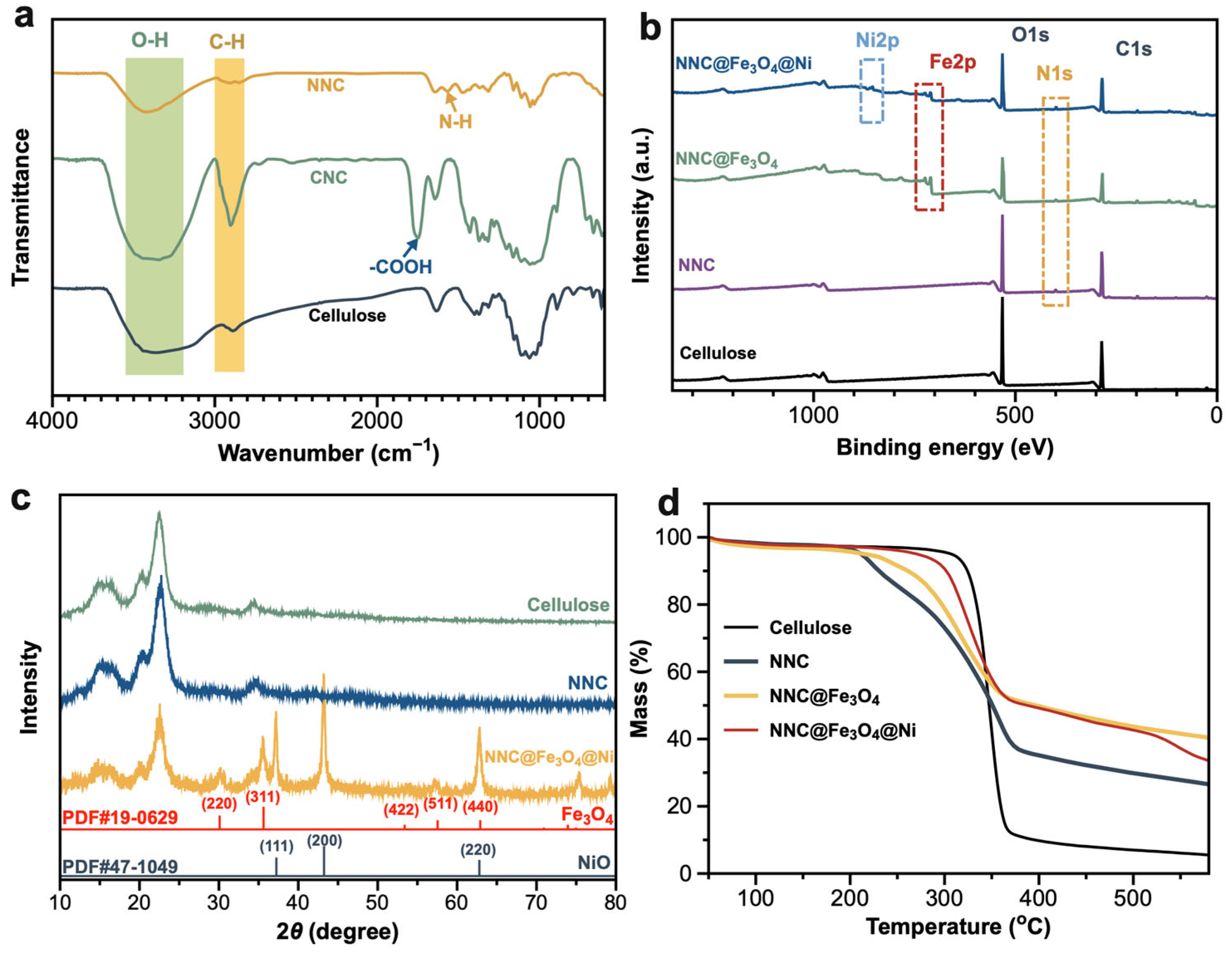
2.1. Structural and Characteristics of Nanocomposite
2.2. Specific Surface Area and Magnetic Properties of NNC@Fe3O4@Ni
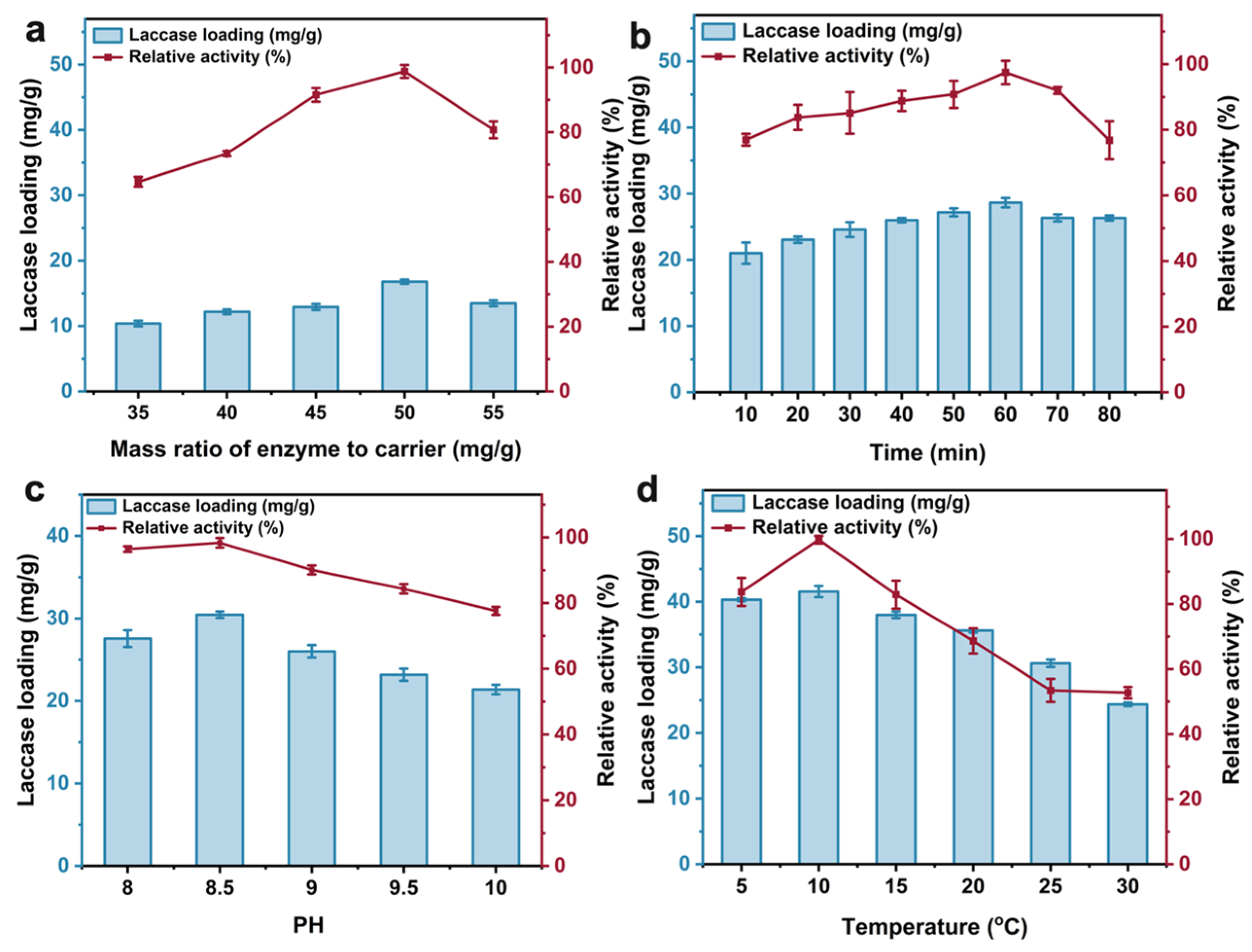
2.3. Optimization of Immobilization Reaction Conditions
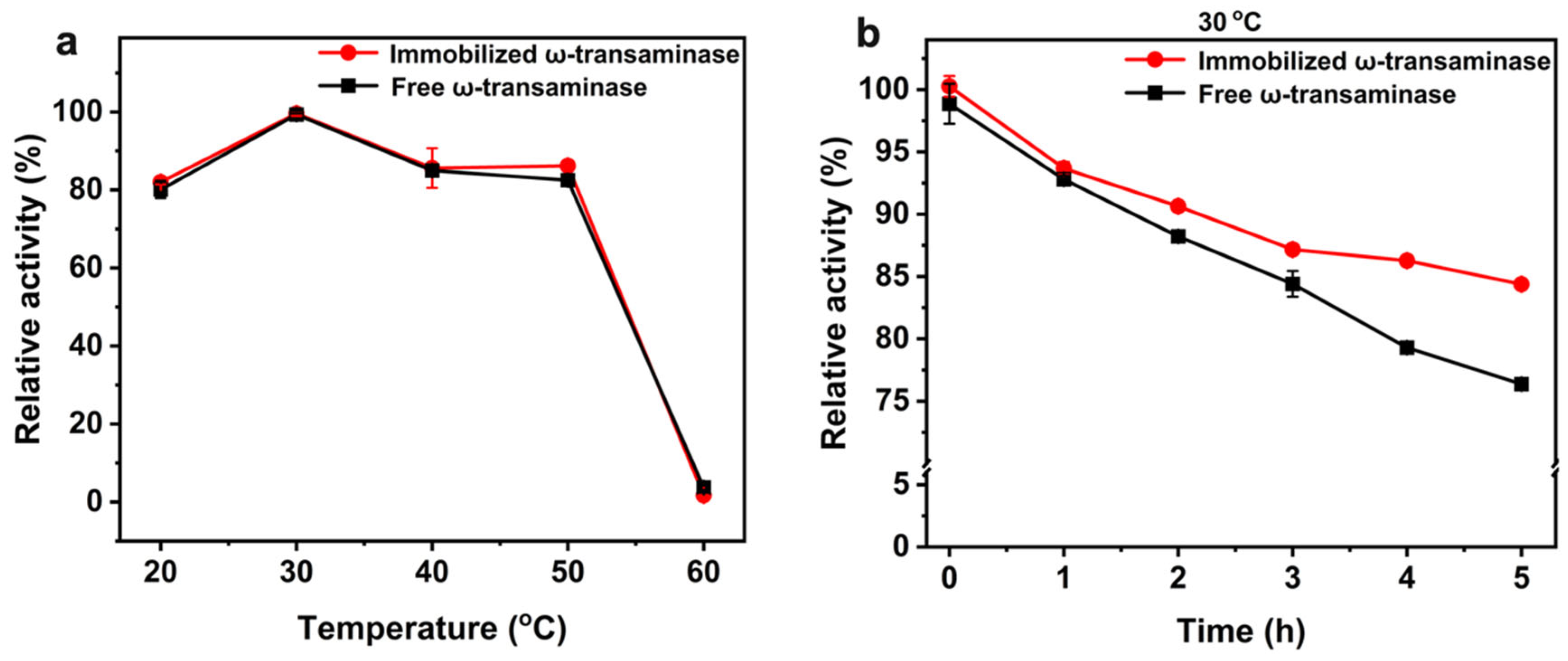
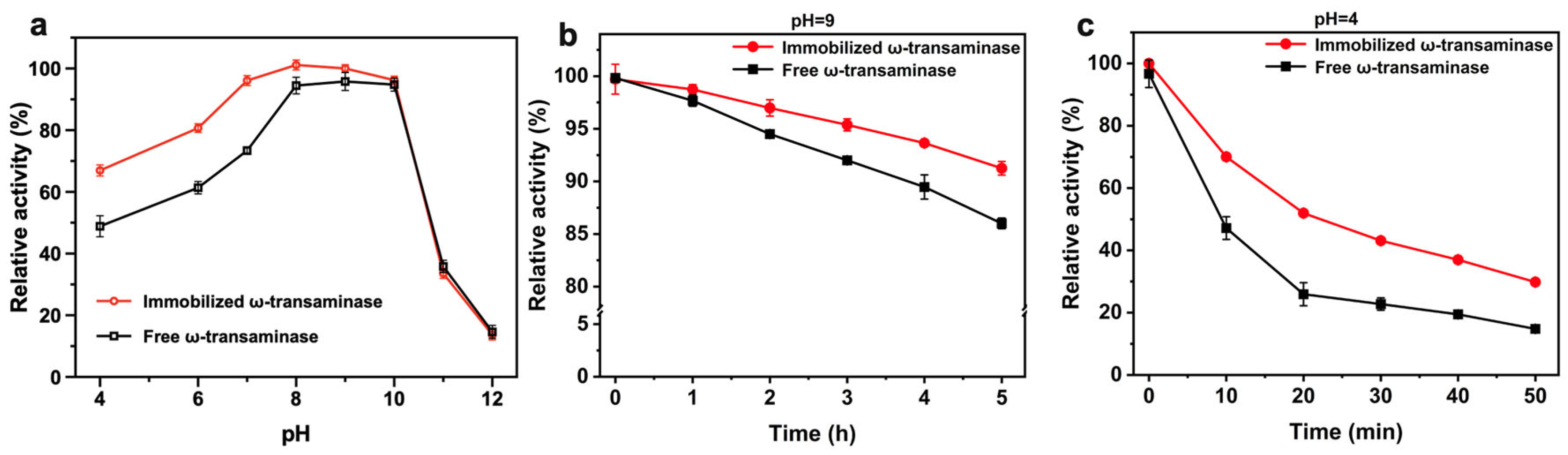
2.4. Enzymatic Properties of AtATA@NNC@Fe3O4@Ni
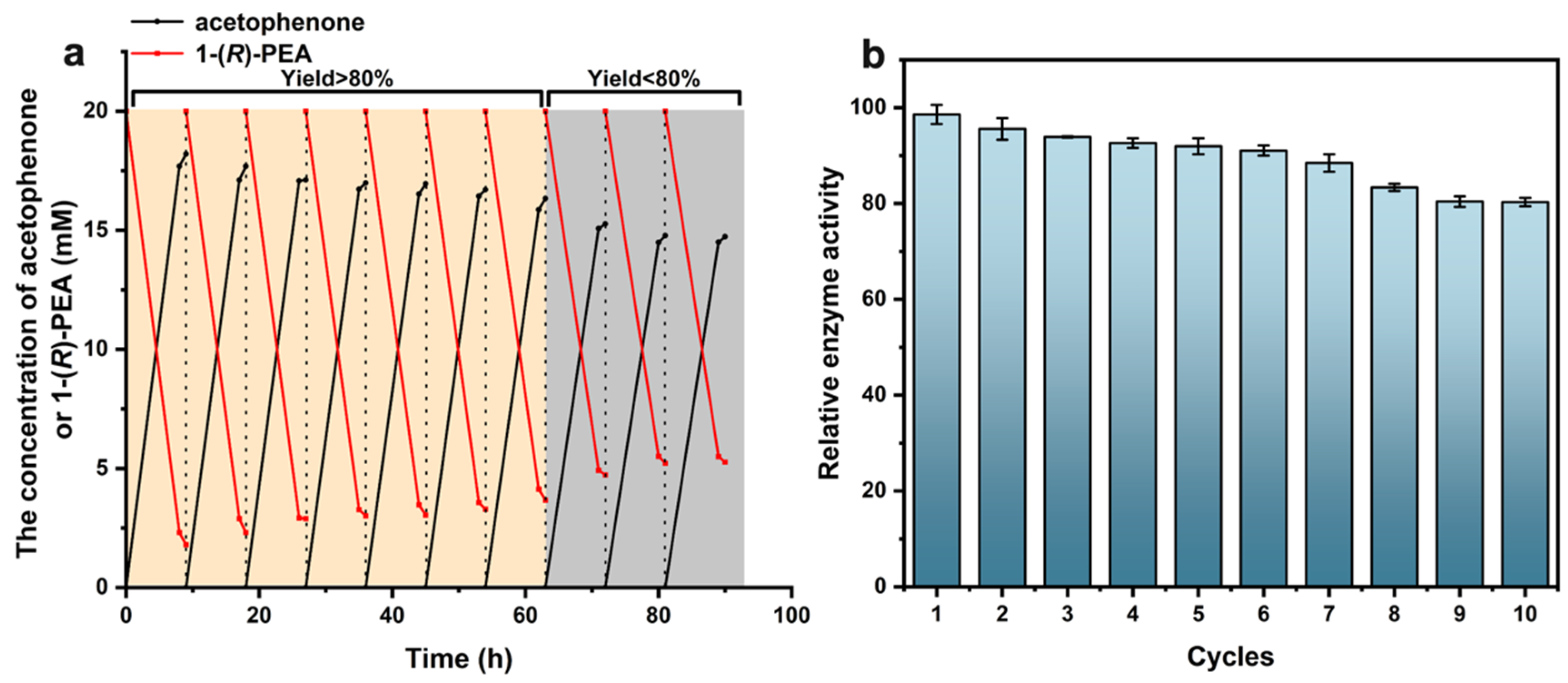
2.5. Recycling Capacity of AtATA@NNC@Fe3O4@Ni
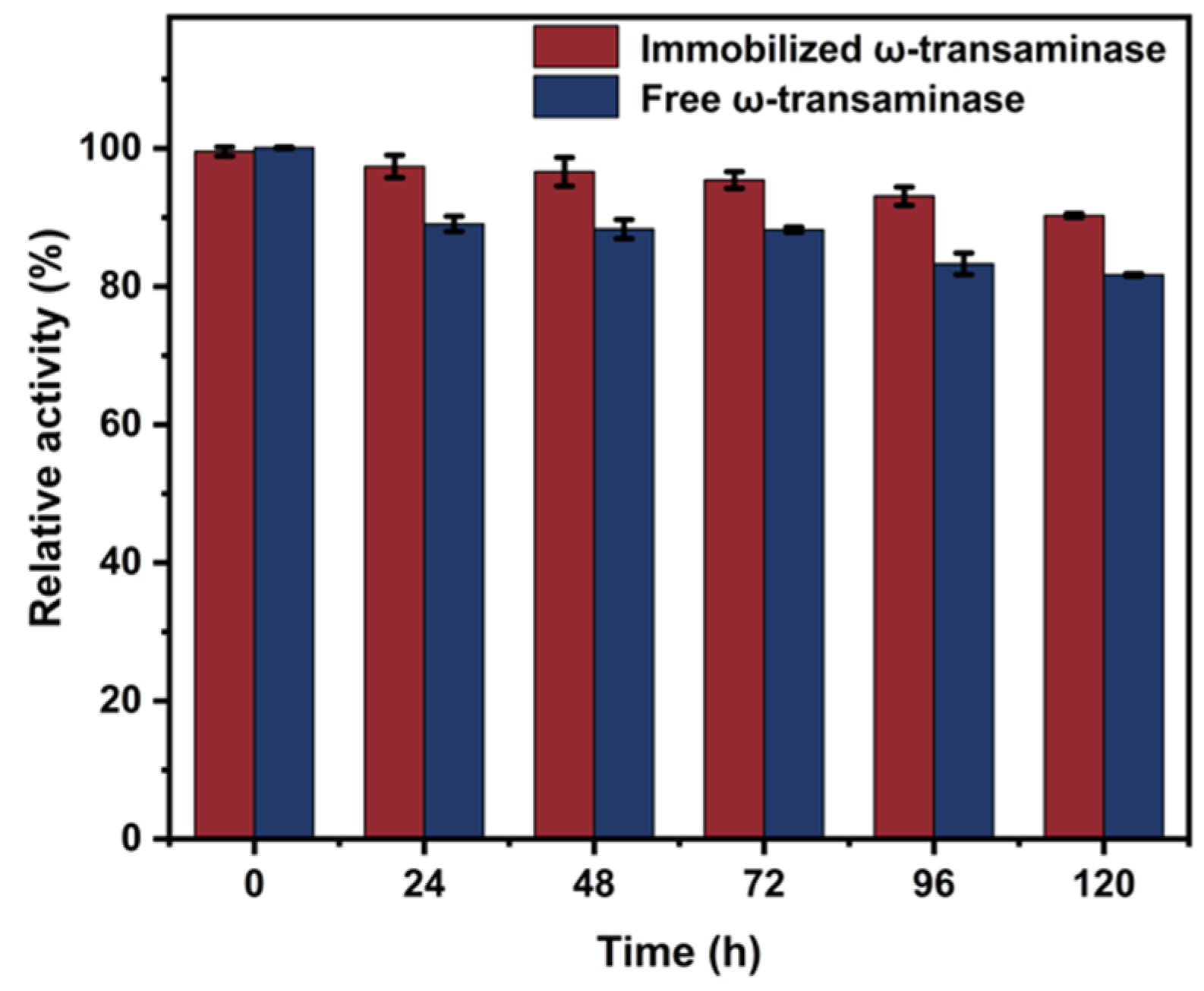
2.6. Storage Stability of AtATA@NNC@Fe3O4@Ni
2.7. Kinetics of Enzymatic Reactions of ω-TA
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Carboxylated Nanocellulose (CNC)
3.3. Preparation of Aminated Nanocellulose (NNC)
3.4. Preparation of NNC@Fe3O4@Ni
3.5. Characterization
3.6. Activity Assay of Immobilized Enzyme
3.7. Response Surface Methodology for Optimization of Immobilized ω-TA Activity
3.8. Enzymatic Properties of Immobilized ω-TA
3.8.1. Effect of Temperature on the Activity of Immobilized ω-TA
3.8.2. Effect of pH on Immobilized ω-TA Activity
3.8.3. Evaluation of Recycling Ability of Immobilized Enzyme
3.8.4. Storage Stability Assessment of Immobilized Enzymes
3.9. Kinetic Analysis of ω-TA Enzymatic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sang-Woo, H.; Eul-Soo, P.; Joo-Young, D.; Jong-Shik, S. Expanding substrate specificity of ω-transaminase by rational remodeling of a large substrate-binding pocket. Adv. Synth. Catal. 2015, 357, 2712–2720. [Google Scholar] [CrossRef]
- Park, E.-S.; Dong, J.-Y.; Shin, J.-S. ω-Transaminase-catalyzed asymmetric synthesis of unnatural amino acids using isopropylamine as an amino donor. Org. Biomol. Chem. 2013, 11, 6929–6933. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent advances in ω-transaminase-mediated biocatalysis for the enantioselective synthesis of chiral amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Ao, Y.-F.; Pei, S.; Xiang, C.; Menke, M.J.; Shen, L.; Sun, C.; Dörr, M.; Born, S.; Höhne, M.; Bornscheuer, U.T. Structure- and data-driven protein engineering of transaminases for improving activity and stereoselectivity. Angew. Chem. Int. Ed. 2023, 62, e202301660. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y.; Masuku, M.V.; Cao, J.; Yang, J.; Huang, K.; Ge, Y.; Yu, Y.; Xiao, Z.; Kuang, Y. Effects of deep eutectic solvents on the biotransformation efficiency of ω-transaminase. J. Mol. Liq. 2023, 377, 121379. [Google Scholar] [CrossRef]
- Wang, H.; Masuku, M.V.; Tao, Y.; Yang, J.; Kuang, Y.; Lyu, C.; Huang, J.; Yang, S. Improved Stability and Catalytic Efficiency of ω-Transaminase in Aqueous Mixture of Deep Eutectic Solvents. Molecules 2023, 28, 3895. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, M.-C.; Liu, R.-L. Recent developments in carriers and non-aqueous solvents for enzyme immobilization. Catalysts 2019, 9, 647. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Machorro-García, G.; López-Legarrea, A.; Trejo-Ayala, D.; de Jesús Rostro-Alanis, M.; Sánchez-Sánchez, M.; Blanco, R.M.; Rodríguez-Rodríguez, J.; Parra-Saldívar, R. Metal-organic frameworks for enzyme immobilization and nanozymes: A laccase-focused review. Biotechnol. Adv. 2024, 70, 108299. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Jackson, E.; Ripoll, M.; López-Gallego, F.; Betancor, L. Stabilization of ω-transaminase from Pseudomonas fluorescens by immobilization techniques. Int. J. Biol. Macromol. 2020, 164, 4318–4328. [Google Scholar] [CrossRef] [PubMed]
- Koszelewski, D.; Müller, N.; Schrittwieser, J.H.; Faber, K.; Kroutil, W. Immobilization of ω-transaminases by encapsulation in a sol–gel/celite matrix. J. Mol. Catal. B Enzym. 2010, 63, 39–44. [Google Scholar] [CrossRef]
- Marina Simona, R.; Teodora, B. A comprehensive guide to enzyme immobilization: All you need to know. Molecules 2025, 30, 939. [Google Scholar] [CrossRef] [PubMed]
- Guiru, C.; Ran, S.; Hongyu, W.; Leiyu, H.; Luying, W.; Jiandu, L. Construction of hierarchical porous MIL-100(Fe) for horseradish peroxidase immobilization and its degradation performance of bisphenol A. J. Clean. Prod. 2024, 481, 144178. [Google Scholar] [CrossRef]
- Lau, E.C.H.T.; Dodds, K.C.; McKenna, C.; Cowan, R.M.; Ganin, A.Y.; Campopiano, D.J.; Yiu, H.H.P. Direct purification and immobilization of his-tagged enzymes using unmodified nickel ferrite NiFe2O4 magnetic nanoparticles. Sci. Rep. 2023, 13, 21549. [Google Scholar] [CrossRef]
- Ma, M.; Chen, X.; Yue, Y.; Wang, J.; He, D.; Liu, R. Immobilization and property of penicillin G acylase on amino functionalized magnetic Ni0.3Mg0.4Zn0.3Fe2O4 nanoparticles prepared via the rapid combustion process. Front. Bioeng. Biotechnol. 2023, 11, 1108820. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, T.; Cheng, Y.; Zhao, K.; Meng, Z.; Huang, J.; Cai, W.; Lai, Y. Recent advances in functional cellulose-based materials: Classification, properties, and applications. Adv. Fiber Mater. 2024, 6, 1343–1368. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-based films and membranes: A comprehensive review on preparation and applications. Chem. Eng. J. 2024, 49, 153500. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, H.; Zhang, H.; Wang, X.; Fu, Y.; Wang, Q.; Liu, H.; Yong, Y.-c.; Guo, J.; Liu, J. Biosafety consideration of nanocellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2024, 265, 130900. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Lu, Z.; Ji, L.; Nian, B.; Hu, Y. Ionic liquid interface modification of magnetic carboxymethyl cellulose-immobilized lipase: Improvement of lid and tunnel structures to enhance stability and catalytic performance. Chem. Eng. J. 2025, 507, 160647. [Google Scholar] [CrossRef]
- Verma, N.K.; Raghav, N. In-silico identification of lysine residue for α-Amylase immobilization on dialdehyde cellulose. Int. J. Biol. Macromol. 2022, 200, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Goel, N.; Shelkar, S.; Varshney, L.; Kumar, V. Catalase immobilized-radiation grafted functional cellulose matrix: A novel biocatalytic system. J. Mol. Catal. B Enzym. 2016, 133, S172–S178. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced flame-retardant methods for polymeric materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Qiu, S.; Cui, Y.-T.; Wang, T.-T.; Fan, F.-F.; Lyu, C.-J.; Huang, J. Stereoselective synthesis of (R)-(+)−1-(1-naphthyl)ethylamine by ω-amine transaminase immobilized on amino modified multi-walled carbon nanotubes and biocatalyst recycling. Enzym. Microb. Technol. 2023, 174, 110378. [Google Scholar] [CrossRef]
- Yang, Z.; Si, S.; Zhang, C. Study on the activity and stability of urease immobilized onto nanoporous alumina membranes. Microporous Mesoporous Mater. 2008, 111, 359–366. [Google Scholar] [CrossRef]

| Name | kcatpyruvate (min−1) | Kmpyruvate (mM) | kcat/Kmpyruvate (L·(min.mmol)−1) | kcat1-(R)-PEA (min−1) | Km1-(R)-PEA (mM) | kcat/Km1-(R)-PEA (L·(min.mmol)−1) |
|---|---|---|---|---|---|---|
| AtATA | 176.68 ± 59.93 | 2.70 ± 1.28 | 65.44 ± 38.14 | 60.25 ± 0.95 | 0.31 ± 0.11 | 194.35 ± 69.03 |
| AtATA@NNC@Fe3O4@Ni | 182.20 ± 57.18 | 1.67 ± 0.78 | 109.10 ± 61.10 | 169.86 ± 4.87 | 0.76 ± 0.05 | 223.5 ± 16.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, X.; Wang, H.; Huang, J. Preparation of Metal-Hybridized Magnetic Nanocellulose for ω-Transaminase Immobilization. Catalysts 2025, 15, 510. https://doi.org/10.3390/catal15060510
Yang J, Wang X, Wang H, Huang J. Preparation of Metal-Hybridized Magnetic Nanocellulose for ω-Transaminase Immobilization. Catalysts. 2025; 15(6):510. https://doi.org/10.3390/catal15060510
Chicago/Turabian StyleYang, Jiayao, Xingxing Wang, Hongpeng Wang, and Jun Huang. 2025. "Preparation of Metal-Hybridized Magnetic Nanocellulose for ω-Transaminase Immobilization" Catalysts 15, no. 6: 510. https://doi.org/10.3390/catal15060510
APA StyleYang, J., Wang, X., Wang, H., & Huang, J. (2025). Preparation of Metal-Hybridized Magnetic Nanocellulose for ω-Transaminase Immobilization. Catalysts, 15(6), 510. https://doi.org/10.3390/catal15060510






