Biosynthesis of Fe3O4 Nanoparticles Using Egg Albumin: Antifungal, Dielectric Analysis and Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. X-Ray Diffraction (XRD)
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
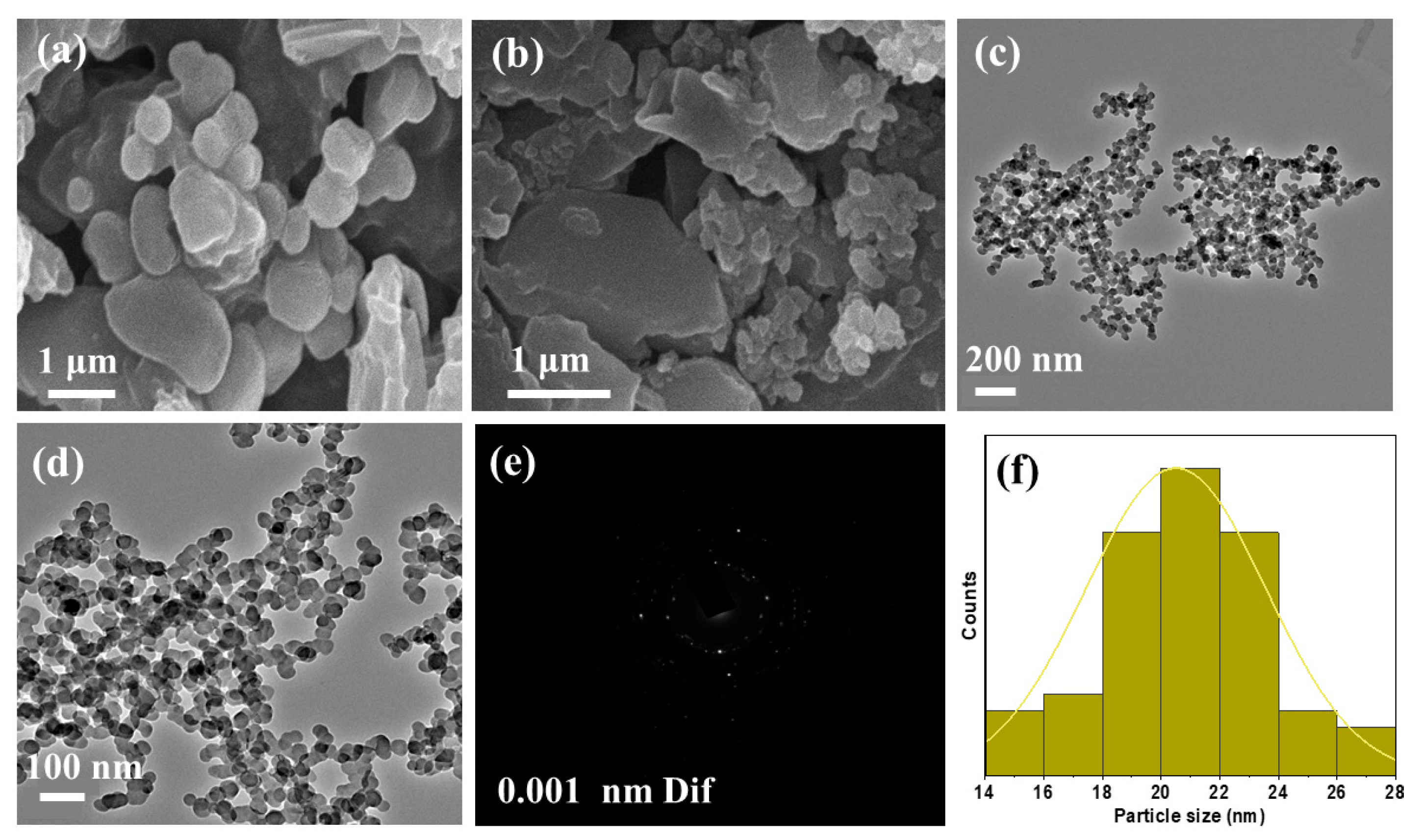
2.3. Scanning Electron Microscopy (SEM)
2.4. Transmission Electron Microscopy (TEM)
2.5. UV-Visible Spectroscopy
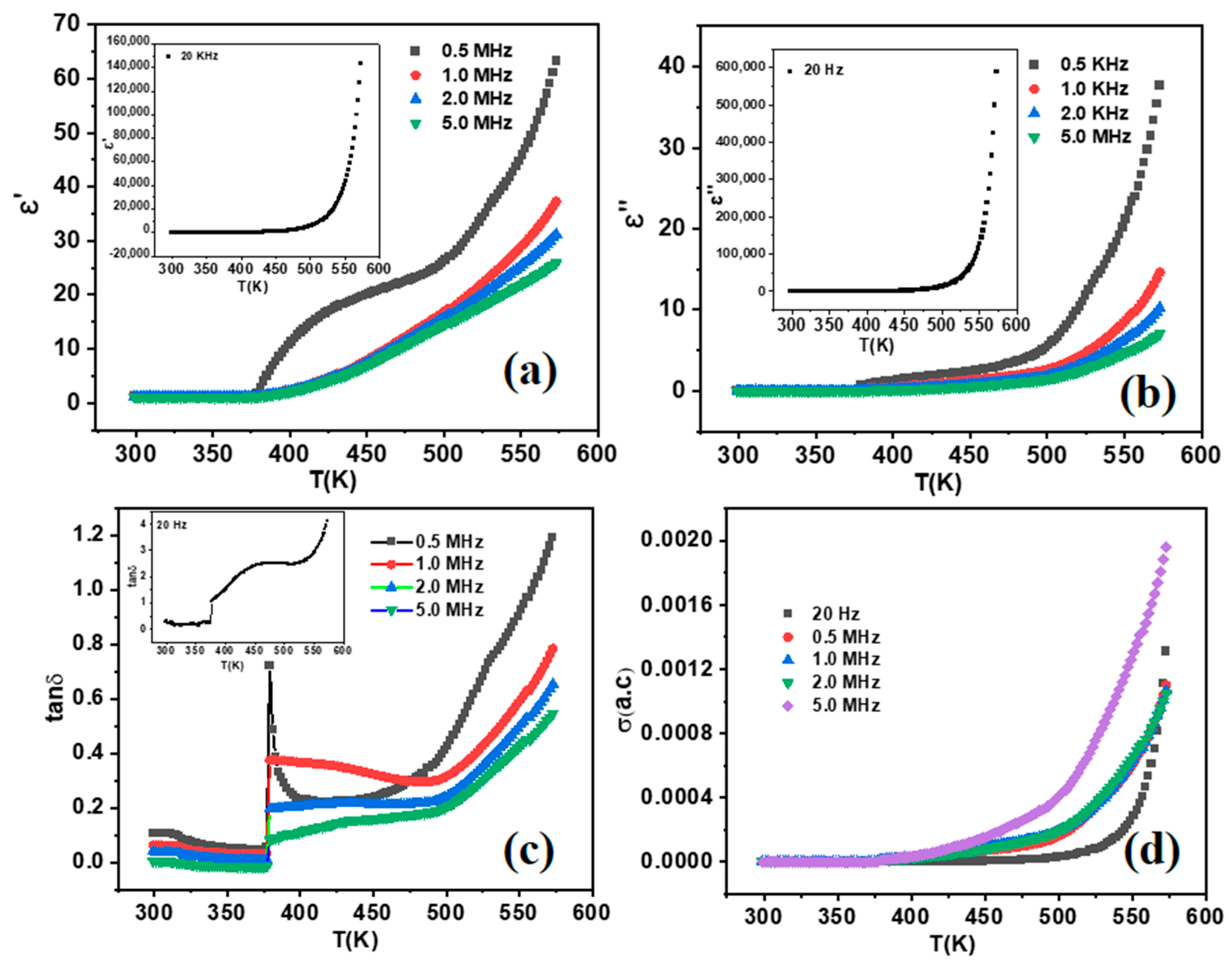
2.6. Dielectric Study of Fe3O4 NPs
2.7. Factors Affecting Biosynthesis of Fe3O4 NPs
2.7.1. pH
2.7.2. Temperature
2.7.3. Concentration of Reactants
2.7.4. Reaction Time
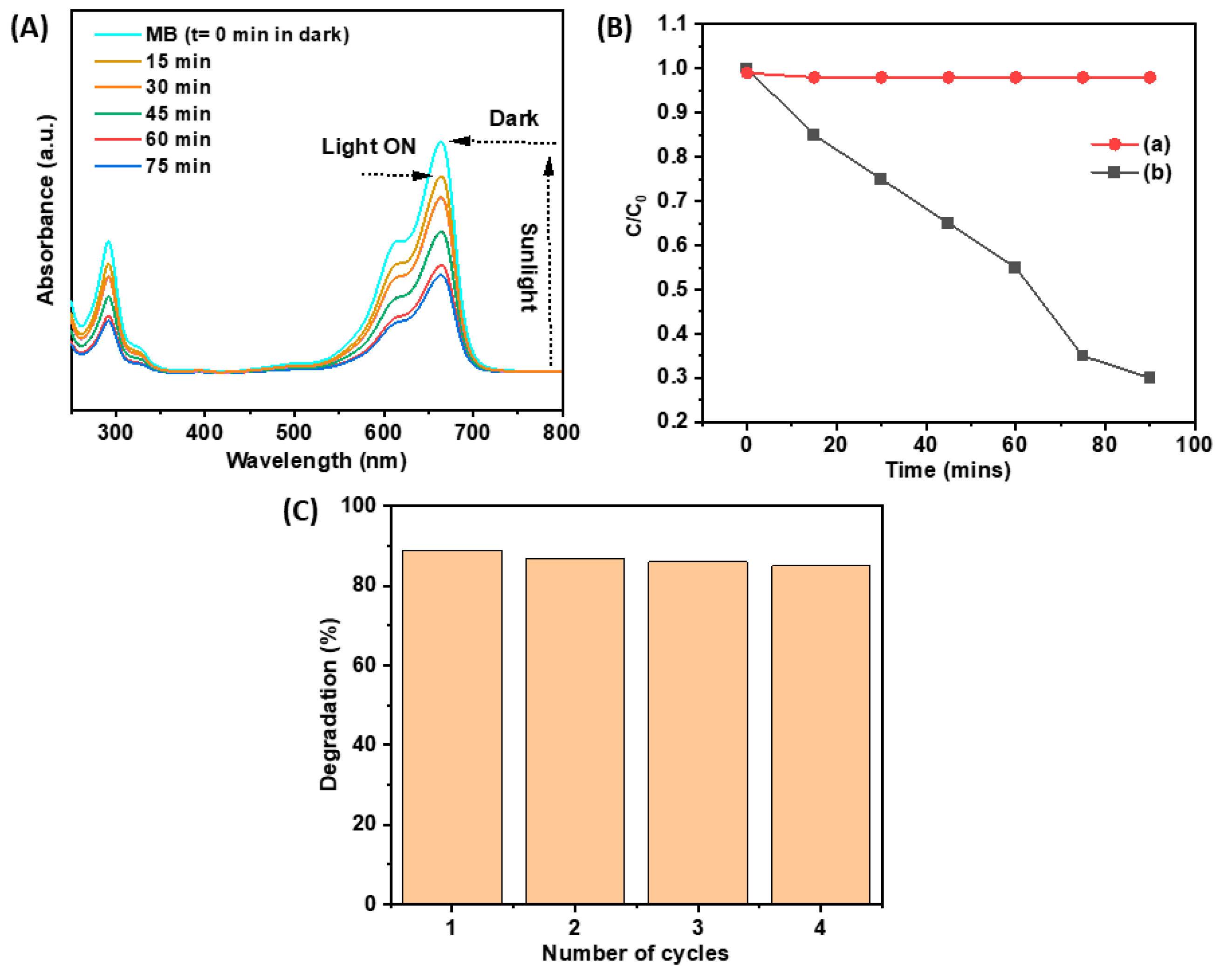
2.8. Photocatalytic Study of Fe3O4 NPs Under Solar Light
2.9. In Vitro Assessment of Fe3O4 NPs Against M. phaseolina
3. Materials and Methods
3.1. Materials
3.2. Biosynthesis Synthesis of Fe3O4 NPs
3.3. Characterization of Fe3O4 NPs
3.4. Temperature-Dependent Dielectric Studies of Fe3O4 NPs
3.5. Photocatalytic Study
3.6. In Vitro Assessment of Fe3O4 NPs Against Pathogenic Fungus and Growth Inhibition
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; De, B.; Mukherjee, S.; Negi, D.S.; Ranjan, P. An electrochemical bio-electronic tongue based on borophene/PPy@ITO hybrid for selective caffeine identification. Analyst 2025, 150, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Ahmed, S.; Mashniwi, M.H.; Shinde, S.M.; Khan, A.; Ranjan, P.; Negi, D.S. Recent advancements in photodetection utilizing inorganic, organic low-dimensional materials and their hybrids. J. Mater. Sci. 2025, 60, 2711–2743. [Google Scholar] [CrossRef]
- Ali, S.K.; Hakami, O.; Zelai, T.; Imran, M.; Manqari, H.M.A.; Alamri, A.A.; Ghazwani, N.A.; Ahmed, S. Pioneering photocatalysts: WSe2/ZIF-9 Z-scheme nanocomposites for efficient hexavalent chromium removal. Inorg. Chem. Commun. 2025, 172, 113733. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Li, Z.; Mazumdar, H.; Siddiqui, M.A.; Khan, A.; Ranjan, P.; Kaushik, A.; Vinu, A.; Kumar, P. Point-of-Care Health Diagnostics and Food Quality Monitoring by Molecularly Imprinted Polymers-Based Histamine Sensors. Adv. Sens. Res. 2025, 4, 2400132. [Google Scholar] [CrossRef]
- Ansari, A.; Ahmed, S.; Rehman, B.; Ali, S.K.; Azooz, R.E.; Hassan, K.F.; Khan, A.; Ranjan, P.; Negi, D.S. A synergistic effect of ZnO low dimensional rods/PEDOT:PSS hybrid structure for UV radiation detection. Appl. Phys. A 2024, 130, 692. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Ali, S.K.; Patil, B.R.; Riyaz, F.; Khan, A.; Ranjan, P. Pioneering Sensing Technologies Using Borophene-Based Composite/Hybrid Electrochemical Biosensors for Health Monitoring: A Perspective. Anal. Sens. 2024, 4, e202400034. [Google Scholar] [CrossRef]
- Ansari, A.; Ahmed, S.; Siddiqui, M.A.; Khan, A.; Tailor, S.; Kumar, P.; Ranjan, P.; Negi, D.S. PEDOT:PSS, TiO2 nanoparticles, and carbon quantum dot composites as ultraviolet sensors. ACS Appl. Nano Mater. 2024, 7, 9789–9799. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Bishwanathan, S.; Siddiqui, M.A.; Tailor, S.; Gupta, P.K.; Negi, D.S.; Ranjan, P. Electronic tongue based on ZnO/ITO@glass for electrochemical monitoring of spiciness levels. Langmuir 2024, 40, 4434–4446. [Google Scholar] [CrossRef]
- Ansari, A.; Ahmed, S.; Siddiqui, M.A.; Khan, A.; Banerjee, A.; Negi, D.S.; Ranjan, P. Investigation of complex hybrids in lithium salt under ultraviolet energy source. J. Mater. Sci. Mater. Electron. 2024, 35, 108. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Siddiqui, M.A.; Imran, M.; Kumari, B.; Khan, A.; Ranjan, P. Electrochemical and optical-based systems for SARS-COV-2 and various pathogens assessment. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 033001. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Mohseniazar, M.; Barin, M.; Zarredar, H.; Alizadeh, S.; Shanehbandi, D. Potential of Microalgae and Lactobacilli in biosynthesis of silver nanoparticles. BioImpacts 2011, 1, 149–152. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Khan, M.M. Selected nanotechnology and nanostructure for drug delivery, nanomedicine and cure. Bioprocess Biosyst. Eng. 2020, 43, 1339–1357. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, A.I.; Sehgal, S.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. The dichotomy of nanotechnology as the cutting edge of agriculture: Nano-farming as an asset versus nanotoxicity. Chemosphere 2022, 288, 132533. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, V.; Kumar, S.; Shahi, S.K. Plant development and crop protection using phyto nanotechnology: A new window for sustainable agriculture. Chemosphere 2022, 299, 134465. [Google Scholar] [CrossRef]
- Kumar, S.; Vishnoi, V.K.; Kumar, P.; Dubey, R.C. Survival of Macrophomina phaseolina in plant tissues and soil. In Macrophomina Phaseolina. Ecobiology, Pathology and Management; Kumar, P., Dubey, R.C., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 205–224. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S.; Brar, S.K.; Vallad, G.E.; Chand, R.; Chauhan, V.B. Emerging phytopathogen Macrophomina phaseolina: Biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 2012, 38, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Moshafi, M.H.; Ranjbar, M.; Ilbeigi, G. Biotemplate of albumen for synthesized iron oxide quantum dots nanoparticles (QDNPs) and investigation of antibacterial effect against pathogenic microbial strains. Int. J. Nanomed. 2019, 14, 3273–3282. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Zhang, Y.; Xu, C.; Chen, H. Egg albumin-assisted hydrothermal synthesis of Co3O4 quasi-cubes as superior electrode material for supercapacitors with excellent performances. Nanoscale Res. Lett. 2019, 14, 340. [Google Scholar] [CrossRef]
- Bachheti, R.K.; Konwarh, R.; Gupta, V.; Husen, A.; Joshi, A. Green synthesis of iron oxide nanoparticles: Cutting edge technology and multifaceted applications. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 239–259. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave Synthesis of Nanoparticles and Their Antifungal Activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Setiabudidaya, D. Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. Int. J. Environ. Sci. Dev. 2013, 4, 336. [Google Scholar] [CrossRef]
- Chinnadurai, G.; Subramanian, R.; Selvi, P. Fish mucus stabilized iron oxide nanoparticles: Fabrication, DNA damage and bactericidal activity. Inorg. Nano-Met. Chem. 2021, 51, 550–559. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Slimani, S.; Talone, A.; Abdolrahimi, M.; Imperatori, P.; Barucca, G.; Fiorani, D.; Peddis, D. Morpho-structural and magnetic properties of CoFe2O4/SiO2 nanocomposites: The effect of the molecular coating. J. Phys. Chem. C 2023, 127, 8840–8849. [Google Scholar] [CrossRef]
- Frolova, L.; Sukhyy, K. Study of the photocatalytic degradation of furacilin from aqueous solutions using the biochar/Fe3O4 nanocomposite. Appl. Nanosci. 2023, 13, 6895–6904. [Google Scholar] [CrossRef]
- Koli, R.R.; Phadatare, M.R.; Sinha, B.B.; Sakate, D.M.; Ghule, A.V.; Ghodake, G.S.; Deshpande, N.G.; Fulari, V.J. Gram bean extract-mediated synthesis of Fe3O4 nanoparticles for tuning the magneto-structural properties that influence the hyperthermia performance. J. Taiwan Inst. Chem. Eng. 2019, 95, 357–368. [Google Scholar] [CrossRef]
- Cai, W.; Weng, X.; Chen, Z. Highly efficient removal of antibiotic rifampicin from aqueous solution using green synthesis of recyclable nano-Fe3O4. Environ. Pollut. 2019, 247, 839–846. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Rashid, T.; Raza, A.; Saleh, H.A.M.; Khan, S.; Rahaman, S.; Pandey, K.; AlDamen, M.A.; Sama, F.; Ahmad, A.; Shahid, M.; et al. Green Synthesis of Ni/Fe3O4/rGO Nanocomposites for Desulfurization of Fuel. ACS Appl. Nano Mater. 2023, 6, 18905–18917. [Google Scholar] [CrossRef]
- Shehu, Z.; Nyakairu, G.W.A.; Tebandeke, E.; Odume, O.N. Circular Economy Approach for Treatment of Water-Containing Diclofenac Using Recyclable Magnetic Fe3O4 Nanoparticles: A Case Study of Real Water Sample from Lake Victoria. J. Pharm. Res. Int. 2023, 35, 66–81. [Google Scholar] [CrossRef]
- Falck, R. Wachtumgesetze, wachstumLaktorehnund temperature wertderholzersterenden. Myceture 1907, 32, 38–39. [Google Scholar]
- Sharma, A.; Sahu, G.; Mahapatra, S.P. Synthesis, characterization, and dielectric studies of magnetic iron oxide nanoparticles. Ferroelectrics 2024, 618, 11–24. [Google Scholar] [CrossRef]
- Sitorus, F.S.; Tumbelaka, R.M.; Istiqomah, N.I.; Nuswantari, A.; Suharyadi, E. Dielectrics and Optical Properties of Green-Synthesized Fe3O4 Nanoparticles. Key Eng. Mater. 2023, 941, 173–181. [Google Scholar] [CrossRef]
- Wang, H.; Li, H. Fe3O4 microplate filled PEI matrix composite with remarkable nonlinear conductive characteristics, dielectric property, and low percolation threshold. Heliyon 2023, 9, e22514. [Google Scholar] [CrossRef]
- Ding, E.; Bai, B.; Wang, X.; Zhang, W.; Liu, W.; Zhang, L. Enhanced microwave absorption performance of geopolymer composites by incorporating hollow Fe3O4 nanospheres. Int. J. Appl. Ceram. Technol. 2024, 21, 806–817. [Google Scholar] [CrossRef]
- Sadat, M.E.; Bud’ko, S.L.; Ewing, R.C.; Xu, H.; Pauletti, G.M.; Mast, D.B.; Shi, D. Effect of dipole interactions on blocking temperature and relaxation dynamics of super paramagnetic iron oxide (Fe3O4) nanoparticle systems. Materials 2023, 16, 496. [Google Scholar] [CrossRef]
- Hayat, K.; Khan, K.; Bibi, F.; Sfina, N.; Elhadi, M.; Safeen, K.; Abdullaev, S.; Rahman, N.; Shah, S.K. Investigation of size-dependent electrical, dielectric, and magnetic properties of iron oxide nanostructures. Mater. Chem. Phys. 2024, 315, 128882. [Google Scholar] [CrossRef]
- Laid, T.M.; Abdelhamid, K.; Eddine, L.S.; Abderrhmane, B. Optimizing the biosynthesis parameters of iron oxide nanoparticles using central composite design. J. Mol. Struct. 2021, 1229, 129497. [Google Scholar] [CrossRef]
- Kaushal, P.; Verma, N.; Kaur, K.; Sidhu, A.K. Green synthesis: An eco-friendly route for the synthesis of iron oxide nanoparticles. Front. Nanotechnol. 2021, 3, 655062. [Google Scholar] [CrossRef]
- Radon, A.; Łukowiec, D.; Kremzer, M.; Mikuła, J.; Włodarczyk, P. Electrical conduction mechanism and dielectric properties of spherical shaped Fe3O4 nanoparticles synthesized by co-precipitation method. Materials 2018, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Afzia, M.; Khan, R.A.; Ismail, B.; Zaki, M.E.A.; Althagafi, T.M.; Alanazi, A.A.; Khan, A.U. Correlation between magnetic and dielectric response of CoFe2O4: Li1+/Zn2+ nanopowders having improved structural and morphological properties. Molecules 2023, 28, 2824. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Eddine, L.S.; Abderrhmane, B.; Alonso-González, M.; Guerrero, A.; Romero, A. Green synthesis and characterization of iron oxide nanoparticles by Pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 2020, 17, 100280. [Google Scholar] [CrossRef]
- Demirezen, D.A.; Yılmaz, Ş.; Yılmaz, D.D.; Yıldız, Y.Ş. Green synthesis of iron oxide nanoparticles using Ceratonia siliqua L. aqueous extract: Improvement of colloidal stability by optimizing synthesis parameters, and evaluation of antibacterial activity against Gram-positive and Gram-negative bacteria. Int. J. Mat. Res. 2022, 113, 849–861. [Google Scholar] [CrossRef]
- Sidkey, N. Biosynthesis, characterization and antimicrobial activity of iron oxide nanoparticles synthesized by fungi. Al-Azhar J. Pharm. Sci. 2020, 62, 164–179. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, L.; Xu, C.; Zhao, J.; Pan, H.; Chen, M.; Xu, Q.; Diao, G. Preparation of highly efficient and magnetically recyclable Fe3O4@C@Ru nanocomposite for the photocatalytic degradation of methylene blue in visible light. Appl. Surf. Sci. 2019, 483, 241–251. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Porrawatkul, P.; Chanthai, S.; Sricharoen, P.; Limchoowong, N. Enhanced photocatalytic degradation of methylene blue using Fe2O3/graphene/CuO nanocomposites under visible light. J. Environ. Chem. Eng. 2019, 7, 103438. [Google Scholar] [CrossRef]
- Dagher, S.; Soliman, A.; Ziout, A.; Tit, N.; Hilal-Alnaqbi, A.; Khashan, S.; Alnaimat, F.; Abu Qudeiri, J. Photocatalytic removal of methylene blue using titania-and silica-coated magnetic nanoparticles. Mater. Res. Exp. 2018, 5, 065518. [Google Scholar] [CrossRef]
- Imran, M.; Alam, M.M.; Hussain, S.; Ali, M.A.; Shkir, M.; Mohammad, A.; Ahamad, T.; Kaushik, A.; Irshad, K. Highly photocatalytic active r-GO/Fe3O4 nanocomposites development for enhanced photocatalysis application, A facile low-cost preparation and characterization. Ceram. Int. 2021, 47, 31973–31982. [Google Scholar] [CrossRef]
- Tseng, W.J.; Chuang, Y.C.; Chen, Y.A. Mesoporous Fe3O4@Ag@TiO2 nanocomposite particles for magnetically recyclable photocatalysis and bactericide. Adv. Powder Technol. 2018, 29, 664–671. [Google Scholar] [CrossRef]
- Hussain, S.; Alam, M.M.; Imran, M.; Ali, M.S.; Ahamad, T.; Haidyrah, A.S.; Alotaibi, S.M.A.R.; Naik, M.U.D.; Shariq, M. A facile low-cost scheme for highly photoactive Fe3O4-MWCNTs nanocomposite material for degradation of methylene blue. Alex. Eng. J. 2022, 61, 9107–9117. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Li, M.; Shakoor, N.; Adeel, M.; Zhou, P.; Guo, M.; Jiang, Y.; Zhao, W.; Lou, B.; et al. Application and mechanisms of metal-based nanoparticles in the control of bacterial and fungal crop diseases. Pest Manag. Sci. 2023, 79, 21–36. [Google Scholar] [CrossRef]
- Athie-García, M.S.; Piñón-Castillo, H.A.; Muñoz-Castellanos, L.N.; Ulloa-Ogaz, A.L.; Martínez-Varela, P.I.; Quintero-Ramos, A.; Duran, R.; Murillo-Ramirez, J.G.; Orrantia-Borunda, E. Cell wall damage and oxidative stress in Candida albicans ATCC10231 and Aspergillusniger caused by palladium nanoparticles. Toxicology 2018, 48, 111–120. [Google Scholar] [CrossRef]
- Babele, P.K.; Thakre, P.K.; Kumawat, R.; Tomar, R.S. Zinc oxide nanoparticles induce toxicity by affecting cell wall integrity pathway, mitochondrial function and lipid homeostasis in Saccharomyces cerevisiae. Chemosphere 2018, 213, 65–75. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, K.S.; Lamsal, K.; Kim, Y.J.; Kim, S.B.; Jung, M.Y.; Sim, S.J.; Kim, H.S.; Chang, S.J.; Kim, J.K.; et al. An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J. Microbiol. Biotechnol. 2009, 19, 760–764. [Google Scholar] [PubMed]
- Ouda, S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternariaalternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34. [Google Scholar] [CrossRef]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic anocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef]
- Azadi, S.; Amani, A.M.; Jangjou, A.; Vaez, A.; Zareshahrabadi, Z.; Zare, A.; Kasaee, S.R.; Kamyab, H.; Chelliapan, S.; Mosleh-Shirazi, S. Fe3O4@SiO2/Schiff-base/Zn (II) nanocomposite functioning as a versatile antimicrobial agent against bacterial and fungal pathogens. Sci. Rep. 2025, 15, 5694. [Google Scholar] [CrossRef]
- Abbas, H.S.; Krishnan, A. Magnetic nanosystems as a therapeutic tool to combat pathogenic fungi. Adv. Pharm. Bull. 2020, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Huo, D.; Li, H.; Zhang, B.; Li, Y.; Liang, A.; Wang, H.; Yu, Q.; Li, M. ROS-independent toxicity of Fe3O4 nanoparticles to yeast cells: Involvement of mitochondrial dysfunction. Chem. Biol. Interact. 2018, 287, 20–26. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, P.K.; Madheshiya, P.; Kumar, I. Role of Nanoparticles in Plant Disease Management. In Nanotechnology-based Sustainable Agriculture; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 347–376. [Google Scholar] [CrossRef]
- Sadat, M.; Patel, R.; Sookoor, J.; Bud’Ko, S.L.; Ewing, R.C.; Zhang, J.; Xu, H.; Wang, Y.; Pauletti, G.M.; Mast, D.B. Effect of spatial confinement on magnetic hyperthermia via dipolar interactions in Fe3O4 nanoparticles for biomedical applications. Mater. Sci. Eng. C 2014, 42, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.A.; Alghazaly, M.S.; Assem, Y.; Abou Zeid, A.A. Bioengineering and optimization of PEGylated zinc nanoparticles by simple green method using Monascuspurpureus, and their powerful antifungal activity against the most famous plant pathogenic fungi, causing food spoilage. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100543. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of chitosan-based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Iqbal, N.; Shoaib, A.; Fatima, Q.; Farah, M.A.; Raja, V. Synthesis and antifungal efficacy of chitosan nanoparticles against notorious mycotoxigenic phytopathogens. Plant Stress 2024, 14, 100614. [Google Scholar] [CrossRef]
- Jagathesan, G.; Rajiv, P. Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Eichhornia Crassipes Leaf Extract and Assessing Their Antibacterial Activity. Biocatal. Agric. Biotechnol. 2018, 13, 90–94. [Google Scholar] [CrossRef]
- Shahid, H.; Shah, A.A.; Shah Bukhari, S.N.U.; Naqvi, A.Z.; Arooj, I.; Javeed, M.; Aslam, M.; Chandio, A.D.; Farooq, M.; Gilani, S.J.; et al. Synthesis, Characterization, and Biological Properties of Iron Oxide Nanoparticles Synthesized from Apis mellifera Honey. Molecules 2023, 28, 6504. [Google Scholar] [CrossRef]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, Antioxidant and Anticancer Activities of Gold Nanoparticles Green Synthesized Using Mangifera indica Seed Aqueous Extract. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1315–1325. [Google Scholar] [CrossRef]
- Miri, A.; Najafzadeh, H.; Darroudi, M.; Miri, M.J.; Kouhbanani, M.A.J.; Sarani, M. Iron oxide nanoparticles: Biosynthesis, magnetic behavior, cytotoxic effect. Chem. Open 2021, 10, 327–333. [Google Scholar] [CrossRef]
- Salama, A.; Abedin, R.; Elwakeel, K. Influences of greenly synthesized iron oxide nanoparticles on the bioremediation of dairy effluent using selected microbial isolates. Int. J. Environ. Sci. Technol. 2022, 19, 7019–7030. [Google Scholar] [CrossRef]
- Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, V.S.R.R. Synthesis and Characterization of Iron Nanoparticles from a Bioflocculant Produced by Pichiakudriavzevii Isolated from Kombucha Tea SCOBY. Bioengineering 2024, 11, 1091. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Al-Askar, A.A.; Alharbi, R.I. Green Synthesis, Characterization, and Antifungal Efficiency of Biogenic Iron Oxide Nanoparticles. Appl. Sci. 2023, 13, 9942. [Google Scholar] [CrossRef]
- Mutalib, M.A.; Aziz, F.; Jamaludin, N.A.; Yahya, N.; Ismail, A.F.; Mohamed, M.A.; Yusop, M.Z.M.; Salleh, W.N.W.; Jaafar, J.; Yusof, N. Enhancement in photocatalytic degradation of methylene blue by LaFeO3-GO integrated photocatalyst-adsorbents under visible light irradiation. Korean J. Chem. Eng. 2018, 35, 548–556. [Google Scholar] [CrossRef]
- Raza, A.; Shoeb, M.; Mashkoor, F.; Rahaman, S.; Mobin, M.; Jeong, C.; Yusuf Ansari, M.; Ahmad, A. Phoenix dactylifera Mediated Green Synthesis of Mn Doped Nanoparticles and its Adsorption Performance for Methyl Orange dye Removal: A Comparative Study Mater. Chem. Phys. 2022, 286, 126173. [Google Scholar] [CrossRef]
- Donta, P. Structural and temperature dependent dielectric behaviour of BxFe(3−x)O4 nanoferrite particles. Micro Nano Lett. 2024, 19, e12194. [Google Scholar] [CrossRef]
- Siragam, S.; Dubey, R.S.; Pappula, L. Synthesis and investigation of dielectric properties of nanoceramic composite material for microwave applications. Micro Nano Lett. 2020, 15, 1156–1161. [Google Scholar] [CrossRef]
- Vincent, J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef]
- Sheoran, O.P.; Tonk, D.S.; Kaushik, L.S.; Hasija, R.C.; Pannu, R.S. Statistical Software Package for Agricultural Research Workers. In Recent Advances in Information Theory, Statistics & Computer Applications; Hooda, D.S., Hasija, R.C., Eds.; Department of Mathematics Statistics, CCS HAU: Hisar, India, 1998; pp. 139–143. [Google Scholar]
- Majid, F.; Bashir, M.; Bibi, I.; Ayub, M.; Khan, B.S.; Somaily, H.H.; Al-Mijalli, S.H.; Nazir, A.; Iqbal, S.; Iqbal, M. Green synthesis of magnetic Fe3O4 nanoflakes using vegetables extracts and their magnetic, structural and antibacterial properties evaluation. Z. Für Phys. Chem. 2023, 237, 1345–1360. [Google Scholar] [CrossRef]


| Parameter | Tested Values | Particle Size (nm) | Crystallinity (XRD FWHM) | Optimum Condition |
|---|---|---|---|---|
| pH | 6, 7, 8, 9 | 40.2, 28.5, 20.6, 25.3 | Broad, Medium, Sharp, Broad | pH 8 |
| Temperature (°C) | 30, 50, 85, 100 | 35.6, 30.4, 20.6, 22.8 | Broad, Medium, Sharp, Sharp | 85 °C |
| Iron Salt Conc. (M) | 0.1, 0.2, 0.3, 0.4 | 20.6, 50.3,35.8, 18.4 | Sharp, Broad, Medium, Broad | 0.1 M |
| Reaction Time (hrs) | 1.5, 4, 8, 24 | 20.6, 45.2, 30.8, 22.5 | Sharp, Medium, Sharp, Broad | 1.5 h |
| Calcination Temp (°C) | 300, 400, 500, 600 | 35.2, 28.4, 20.6, 22.3 | Broad, Medium, Sharp, Sharp | 500 °C |
| Calcination Time (hrs) | 3, 4, 5, 6 | 32.4,20.6, 28.6, 21.8 | Broad, Sharp, Medium, Sharp | 4 h |
| Frequency | ε′ | ε″ | Tanδ | σ (AC) (S·m−1) |
|---|---|---|---|---|
| 20 Hz | 145,000 | 600,000 | 4.00 | 0.0065 |
| 0.50 MHz | 67 | 38 | 1.20 | 0.0024 |
| 1.0 MHz | 37 | 14 | 0.80 | 0.0016 |
| 2.0 MHz | 31 | 10 | 0.60 | 0.0021 |
| 5.0 MHz | 25 | 07 | 0.40 | 0.0027 |
| Nanomaterial | MB Photodegradation (%) | Time (min) | K (min−1) | Reference |
|---|---|---|---|---|
| Fe3O4 | 89 | 75 | 0.0133 | This work |
| Fe3O4@C@Ru | 92.70 | 140 | 0.0176 | [50] |
| Fe2O3/graphene/CuO | 94.27 | 40 | 0.0725 | [51] |
| Fe3O4@SiO2 | 97 | 140 | 0.0474 | [52] |
| rGO-Fe3O4 | 98.30 | 80 | 0.0464 | [53] |
| Fe3O4@Ag@TiO2 | 79.90 | 120 | 0.0120 | [54] |
| Fe3O4/MWCNT | 98.40 | 60 | 0.7398 | [55] |
| GO-LaFeO3 | 91.20 | 150 | 0.0137 | [56] |
| Treatment | Concentrations (ppm) | Inhibition Zone (%) of M. phaseolina After Seven Days |
|---|---|---|
| Fe3O4 NPs | 300 | 51.5 a |
| 200 | 34.3 b | |
| 100 | 21.5 c | |
| Control | 0 d | |
| ANOVA | DF | 3 |
| Sum of Squares | 4238 | |
| Mean Squares | 1412.66 | |
| F-Calculated | 209.98 | |
| Significance | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Khadrawy, S.M.; Ahmad, I.; Imran, M.; Khuwaja, G.; Parveen, H.; Mukhtar, S.; Patil, B.R.; Allam, A.A.; Rudayni, H.A.; et al. Biosynthesis of Fe3O4 Nanoparticles Using Egg Albumin: Antifungal, Dielectric Analysis and Photocatalytic Activity. Catalysts 2025, 15, 505. https://doi.org/10.3390/catal15060505
Raza A, Khadrawy SM, Ahmad I, Imran M, Khuwaja G, Parveen H, Mukhtar S, Patil BR, Allam AA, Rudayni HA, et al. Biosynthesis of Fe3O4 Nanoparticles Using Egg Albumin: Antifungal, Dielectric Analysis and Photocatalytic Activity. Catalysts. 2025; 15(6):505. https://doi.org/10.3390/catal15060505
Chicago/Turabian StyleRaza, Azam, Sally Mostafa Khadrawy, Irfan Ahmad, Mohd Imran, Gulrana Khuwaja, Humaira Parveen, Sayeed Mukhtar, Bhagyashree R. Patil, Ahmed A. Allam, Hassan A. Rudayni, and et al. 2025. "Biosynthesis of Fe3O4 Nanoparticles Using Egg Albumin: Antifungal, Dielectric Analysis and Photocatalytic Activity" Catalysts 15, no. 6: 505. https://doi.org/10.3390/catal15060505
APA StyleRaza, A., Khadrawy, S. M., Ahmad, I., Imran, M., Khuwaja, G., Parveen, H., Mukhtar, S., Patil, B. R., Allam, A. A., Rudayni, H. A., Ali, S. K., & Ahmad, A. (2025). Biosynthesis of Fe3O4 Nanoparticles Using Egg Albumin: Antifungal, Dielectric Analysis and Photocatalytic Activity. Catalysts, 15(6), 505. https://doi.org/10.3390/catal15060505








