Abstract
Water pollution is a serious worldwide problem. Among its pollutants, dyes that are overused by various types of industries and are resistant to conventional effluent treatments stand out. In this study, mixed copper and nickel ferrites NixCu(1-x)Fe2O4 (x = 0, 0.2, 0.4, 0.6, 0.8 e 1.0), were produced by the EDTA-Citrate complexation method, characterized and applied in photocatalysis with methylene blue (MB) and rhodamine B (RhB) dyes, varying the reaction pH between 2, 6 and 10. The ferrites with the highest percentages of copper had a tetragonal structure, while those with the highest percentages of nickel had a cubic structure, all with inverse spinel and all presenting bandgap values lower than 2 eV. Samples with higher percentages of copper (x = 0 and 0.2) at pH 10 showed degradation of approximately 55% for RhB and 40% for MB. A mixture of MB and RhB was also evaluated, showing a greater removal of methylene blue due to its preferential adsorption on the surface of the material. In this way, mixed copper and nickel ferrites proved promising as catalysts in photocatalytic processes.
1. Introduction
Water contamination by various types of pollutants is one of the main current problems. The use of dyes in different types of industries, such as food [1], textile [2], pharmaceutical [3], paper and cellulose [4], among others, makes them potential polluters of aquatic environments.
Synthetic dyes, most used today, are organic in nature, causing difficulties in removal by conventional effluent treatments such as physical-chemical and biological processes [5,6]. The presence of dyes in the aquatic environment seriously affects fauna and flora and can even cause mortality [7].
Advanced Oxidative Processes (AOPs) appear as an effective alternative for the degradation of organic pollutants, such as dyes, and can be combined with conventional effluent treatments. AOPs generate reactive oxygen species, such as hydroxyl radicals, with high redox potential, attacking organic pollutants and mineralizing them [8]. Among the AOPs, we can mention the Fenton process, photocatalysis and photo-Fenton, which involve the use of semiconductor catalysts, in particular, metal oxides that have low solubility [9].
The greatest benefit of using semiconductor catalysts occurs because they have well-defined band energy, in addition to being stable, low cost and high activity in the UV-Vis region [10]. Spinel ferrites are ceramic materials with the general formula MFe2O4, where M and Fe are divalent and trivalent transition metals, respectively [11]. Ferrites have low bandgap values, in the range from 1.1 eV to 2.3 eV, making them potential catalysts for AOPs, in addition to being magnetically separable, facilitating the extraction of the catalyst after the reaction process [12].
Copper ferrites are highlighted because of their crystallographic change between tetragonal and cubic, which occurs due to their synthesis method and calcination temperature, which may be used to control their properties and to direct them toward specific applications [13]. Nickel ferrites have ferrimagnetic magnetic properties with high curie temperatures (843 K) [14] and present potential activity towards photocatalytic applications involving the degradation of organic matter. These ferrites have promising potential as photocatalysts due to their ability to produce radicals, especially hydroxyl radicals, which are extremely reactive and may serve as active species in different chemical reactions [15,16,17,18,19]. Several physical-chemistry properties of these materials can be modified through the replacement of atoms in the sublattices, leading to a synergetic effect that surpasses its intrinsic nature while maintaining the simplicity of its production. Nickel-copper mixed ferrite has been poorly explored in the field of photocatalysis in spite of its potential [20,21,22]. Therefore, this work aims to study mixed copper and nickel ferrites NixCu(1-x)Fe2O4 (x = 0, 0.2, 0.4, 0.6, 0.8 and 1), named X0, X20, X40, X60, X80 and X100, their structural properties and evaluate the photocatalytic performance in the degradation of methylene blue (MB) and rhodamine B (RhB) dyes.
2. Results and Discussions
The XRD patterns of the NixCu(1-x)Fe2O4 samples, named X0, X20, X40, X60, X80 and X100 are shown in Figure 1. Samples X0 (Figure 1a) and X20 (Figure 1b) were identified as tetragonal CuFe2O4 spinel and in agreement with JCPDS n° 34-0425. That implies that the heat treatment unfavored the formation of the cubic spinel structure, while for other concentrations of Cu(II) and Ni(II) (Figure 1c–f), the temperature of treatment allowed the formation of a cubic spinel structure typically of NiFe2O4. Therefore, all the x-ray diffraction peaks from samples X40 to X100 are identified as belonging to the aforementioned structure in agreement with the JCPDS n° 74-2081 pattern. The substitution of Cu by Ni ions favored the shift of diffraction peak for samples X40 to X80 in relation to X100. A peak shift is related to modifications on the atomic interplanar distancing of the crystal and, thus, on its lattice parameter. Clearly, NiFe2O4 presents different lattice parameters than samples X40 to X80, which then would not be sensitive to the substitution of copper atoms in this regard. Therefore, one can conclude that the partial substitution of copper atoms by nickel atoms reaches a thermodynamically stable state from the crystalline structure point of view, presenting no pertinent variations on unit cell volume.
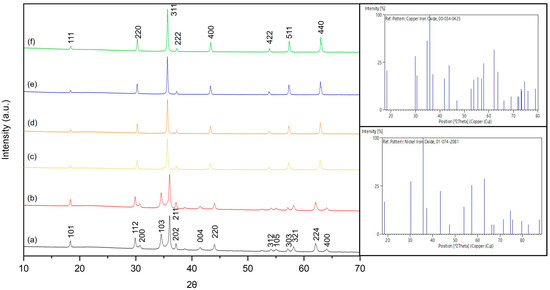
Figure 1.
XRD patterns of NixCu(1-x)Fe2O4: (a) X0, (b) X20, (c) X40, (d) X60, (e) X80 and (f) X100.
Regarding the size and the intrinsic strain effects on XRD peak broadening, the diffractograms of all samples were fitted with four different models for determining their crystalline size and strain, considering peaks with relative intensity equal to or greater than 10%. Scherrer and UDM Williamson-Hall (UDM-WH) generally provided poor adjustment to the experimental data based on the R2 parameter. Only the Size-Strain Plot (SSP) and Halder-Wagner-Langford (HWL) models were able to fit the XRD data with an acceptable margin of error, as can be seen in Table 1. Both SSP and HWL differ from the Scherrer model in considering the effect of strain on the XRD peak broadening. Thus, all samples are believed to contain a certain level of intrinsic strain, which can be associated with point defects, grain boundaries, triple junctions or stacking faults. Nanometric and sub-micrometric ferrites produced by coprecipitation usually present point defects due to size confinement [23]. The presence of defects can also be provoked by the substitution of atomic elements, as is the case in samples X20 to X80. For both substituted and non-substituted samples, the fact that the UDM-WH model does not fit the data implies that in these samples, the strain is not uniformly present throughout the crystallographic directions, which is due to the preferable occupancy of atomic positions by copper and nickel atoms on the unit cell. In this case, size and strain can be better analyzed by dealing with the XRD peak profile instead of the peak position. Both models, SSP and HWL, assume different functions for this task—the Voigt function is used by HWL for both size and strain, while Lorentz and Gauss functions are considered separately for the contributions of size and strain on the SSP model, respectively. Therefore, for the purpose of this work, both models (SSP and HWL) are used indistinctly. Table 1 presents the R2 values obtained from fittings using the different models and also presents the crystallite size and strain for all samples obtained by the better-fitting model.

Table 1.
Correlation coefficient (R2) values for different models used to fit XRD experimental data, crystallite size and strain calculation.
Copper atom substitution by nickel directly affects the crystallite size of samples. At first, a 20% substitution would cause a small reduction of it associated with a greater intrinsic strain related to the accommodation of nickel atoms in a tetragonal unit cell. As substitution increases to 40% and further until 80%, a proportional increase in the crystallite size is observed in the now cubic unit cell. These changes and nonlinear behavior can be associated with the atomic nature of Ni and Cu. Nickel(II) presents a greater atomic radius (78 pm) than Cu(II) (69 pm), which would justify the greater crystallite sizes when it partially substitutes copper in a spinel ferrite structure.
Figure 2 shows the infrared spectra of NixCu(1-x)Fe2O4 samples at room temperature. The high-frequency bands, for all samples, are positioned at a narrow range of wavenumbers (see the insert in Figure 2), which are characteristic of the formation of spinel ferrite structures [24]. The high-frequency band between 600 and 500 cm−1 is related to the stretching of metals in tetrahedral sites, while the high-frequency band close to 400 cm−1 corresponds to the stretching vibration in octahedral sites [25,26]. Both copper and nickel ferrites must have an inverse spinel structure, where preferably Fe occupies tetrahedral sites, reflecting the proximity of the intensity of the first high-frequency band for all samples. The octahedral sites are divided by Fe, Cu and Ni, and the change in the amount of metal in each sample reflects the change in the intensity of the second high-frequency bands.
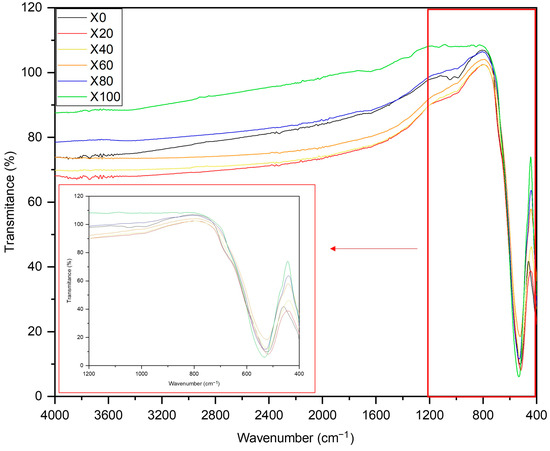
Figure 2.
FTIR spectra of NixCu(1-x)Fe2O4: X0, X20, X40, X60, X80 and X100.
The Mossbauer spectra were recorded at 300 K and are shown on Figure 3. The spectra were fitted using two sextets, S1 and S2; the first sextet is ascribed to Fe3+ in tetrahedral sites, and the second sextet is ascribed to Fe3+ in octahedral sites. The sextet with the larger isomer shift (IS) and hyperfine magnetic field (Bhf) is related to Fe3+ in octahedral sites [27]. The samples with larger Cu concentrations have smaller Bhf. For both sites is noticeable the increase of the Bhf with the increase of Ni concentration. Since the spectra do not have paramagnetic components, we can conclude that all samples are thermally blocked at room temperature. The χ2 for all spectra have fairly good values ranging from 0.99 to 1.89.
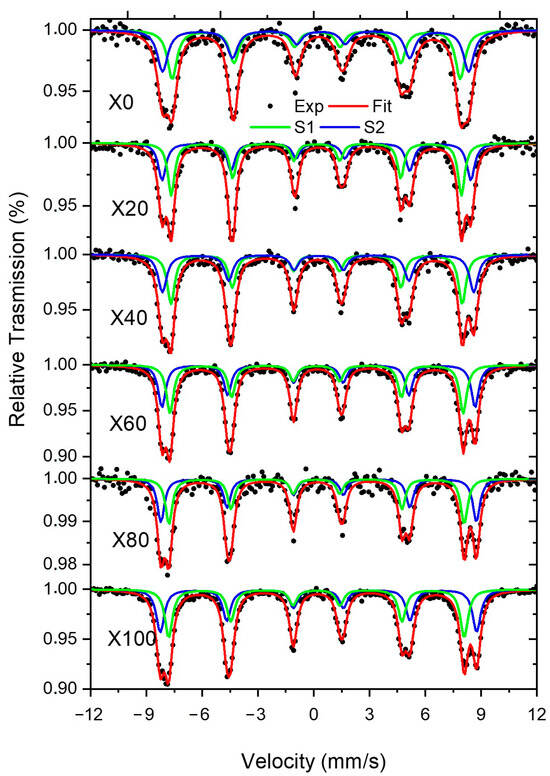
Figure 3.
Mössbauer spectra recorded at 12 K for samples X0, X20, X40, X60, X80 and X100.
The amount of Ni in the samples is given by x = (0, 0.2, 0.4, 0.6, 0.8, 1.0). Thus, the distribution of ions in the tetrahedral and octahedral sites would be [Cu(1-λ-t)Ni(t)Fe(λ)] {Cu(λ+t-x)Ni(x-t)Fe(2-λ)}O4 where λ and t are the amounts of Fe and Ni in tetrahedral sites. The square and curly brackets indicate the ions in tetrahedral and octahedral sites, respectively. The Fe distribution between these both sites can be determined from the relative absorption areas (AREA) obtained from the fittings. It is clear that the AREAs for S1 and S2 have similar values. Thus, we can conclude that each site has half of the total Fe, i.e., λ ≈ 1. Therefore, the Ni and Cu occupy only octahedral sites, i.e., t ≈ 0. In this case, the samples are inverse spinels, i.e., [Fe(1)] {Cu(1-x)Ni(x)Fe(1)}O4. This result is somehow expected because Ni-ferrite is usually inverse spinel. For instance, Silva et al. [28] produced NiFe2O4 nanoparticles using the complexation EDTA citrate method under acidic and basic conditions, and they concluded their samples were inverse spinels. Thanh et al. [29] studied the cation distribution of Ni-doped CuFe2O4 samples synthesized by using the spray co-precipitation method at an annealing temperature of 900 °C in the air for 5 h. They used Synchrotron X-ray powder diffraction experiments, and Fourier transforms of EXAFS data to characterize the Cu(1-y)Ni(y)Fe2O4 samples and found that for y > 0.7, the samples are inversed spinels [26]. Thanh reported that Cu2+ ions occupy both the tetrahedral and octahedral sites, but Ni2+ prefer to reside in the octahedral sites.
In Table 2, it is noticed that the Bhf for both sextets increases with the amount of Ni in the samples; it is because Ni has a magnetic moment while Cu is not magnetic, as also was discussed by Zaki et al. [30] in Cu2+ ions substituted Ni-ferrite having the general formula Ni(1-x)Cu(x)Fe2O4 (where x = 0.0, 0.2, 0.4, 0.6, 0.8, 1) were prepared by the solid-state reaction method.

Table 2.
Hyperfine parameters obtained from fits of Mossbauer spectra recorded at 300 K. The X0, X20, X40, X60, X80, X100 are samples with NixCu(1-x)Fe2O4, x = (0, 0.2, 0.4, 0.6, 0.8, 1.0) respectively.
Figure 4 shows high-resolution scanning electron microscopy images of all samples. The samples with the highest percentage of copper X0 (a), X20 (b), and X40 (c) present irregular agglomerates with different shapes and sizes. Sample X60 (d), which has a higher percentage of Ni compared with Cu, presents aggregated particles with morphologies similar to triangular prisms and octahedrons, the presence of those becoming more important as the percentage of Ni increases in samples X80 (e) and X100 (f). One can also notice the shape evolution from triangular prisms to octahedrons as the substitution of Cu by Ni is total (X100). In Figure 4f, the rugosity of the surface of the grain is evident, indicating the total formation of the octahedron shape is not yet complete. This shape is energetically favored on cubic spinel structures; thus, the rugosity of grains implies the thermodynamic equilibrium was not achieved, and some triangular prisms remain as a result. Those grains’ characteristics are due to the synthesis method [23,31]. In all samples, one can also observe the presence of two different groups concerning the sizes of the grains. The XRD peak analysis allows the determination of an average size. Clearly, the substitution of Cu atoms by Ni atoms favors the formation of the two distinct families of grains, specifically the grains with bigger sizes. It implies that the substitution of atoms affects not only the crystallographic characteristics of samples but also their grain size and morphology, which are in constant transformation during the heat treatment, whilst their general crystalline structures are already stable.

Figure 4.
Micrographs of NixCu(1-x)Fe2O4: (a) X0, (b) X20, (c) X40, (d) X60, (e) X80 and (f) X100.
Along with the SEM-FEG analyses, energy dispersive spectroscopy (EDS) measurements were also obtained, where the chemical elements were mapped according to Figure 5. In all samples, it was possible to identify regions of high point density, indicating the presence of elements in high concentrations. The green color is attributed to oxygen, yellow to iron, red to copper and fuchsia to nickel.
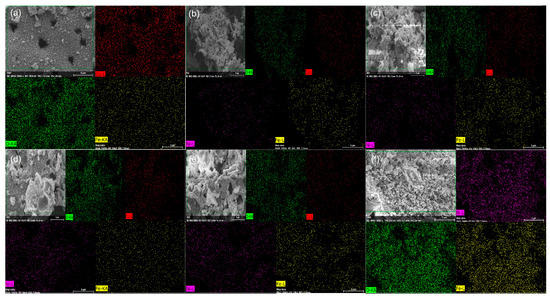
Figure 5.
Elementary mapping of NixCu(1-x)Fe2O4: (a) X0, (b) X20, (c) X40, (d) X60, (e) X80 and (f) X100.
In Figure 5a, there is a significant homogenous presence of the three elements (oxygen, iron and copper) throughout the sample. In Figure 5b, it is noted that the copper intensity was reduced, and nickel appeared in small quantities. As the proportion of nickel increases, the density of fuchsia dots also increases, and copper decreases. In Figure 5e, copper is seen in small quantities. This proves that there is a variation in copper and nickel amounts, forming the expected fractions. We did not observe any regions where the atomic fractions were in disagreement with the theoretical ones.
One of the pillars for proving the functioning of photocatalysts is the identification of their band gap energy. Figure 6 presents the diffuse reflectance spectroscopy for the NixCu(1-x)Fe2O4 samples and their band gap energy values obtained from the extrapolation of the linear region with the steepest curve using the Kulbeka-Monk (Equation (1)) and Wood and Tauc (Equation (2)) equations [32].
where: h, ν, α, R, A are the Planck constant, frequency, absorption coefficient, diffusion reflectance, a transition probability constant, respectively. n is related to the direct or indirect electronic transition.
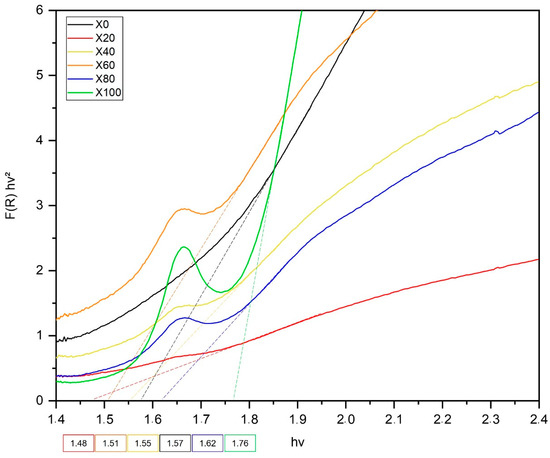
Figure 6.
UV-Vis diffuse reflectance spectra and optical band gap energies of NixCu(1-x)Fe2O4 samples.
Spinel ferrites have low band gap (Egap) values, approximately 2 eV, making it possible to absorb light in the visible range [19]. The Egap values obtained for all samples are 1.57 eV, 1.48 eV, 1.55 eV, 1.51 eV, 1.62 eV and 1.76 eV, for X0 to X100, respectively. They all fall within the visible light spectrum range. The variation in band gap values is normally associated with properties such as molecular composition and crystallite size, besides crystallographic intrinsic arrangements [18]. In order to establish if the bandgap trend found for our series of samples could be related to their crystallite sizes, we measured the statistical correlation between the crystallite size obtained by XRD and the optical band gaps for all samples. Neither Pearson nor Spearman functions indicated the existence of such a correlation, as both R2 parameters were 0.25 and 0.51, respectively. Thus, one can conclude that by the synthesis procedure used, the crystallite size plays a secondary role of influence on the levels of the optical band gap in this series of samples, which would then be mainly determined by the atom substitution in the lattice.
Linked to the bandgap, it is necessary to establish the values of the valence and the conduction band energies, which should provide the oxidation of OH- generating OH, having a vacancy greater than the standard redox potential OH-/•OH = 1.99 eV (vs. NHE) [20,21]. Table 3 shows the energies of the conduction band (ECB) and valence band (EVB) calculated by the empirical equations (Equations (3) and (4)) [22,33], showing that all samples present sufficient levels for promoting the oxidation of hydroxyls radicals.

Table 3.
The energy of conduction and valence bands of NixCu(1-x)Fe2O4 samples.
Figure 7 shows the degradation efficiency of methylene blue (MB) dye using mixed Cu and Ni ferrites, as well as photolysis, at pH 2 (a), pH 6 (b) and pH 10 (c). For the reactions at pH 2, only adsorption was observed during the first 30 min, except for X20. When changing the pH to 6 and 10, an increase in adsorption is noted during the initial minutes followed by the photocatalysis reactions until the end of 240 min. Samples with higher percentages of copper X0 and X20 showed better degradation results, reaching approximately 40% removal at basic pH.
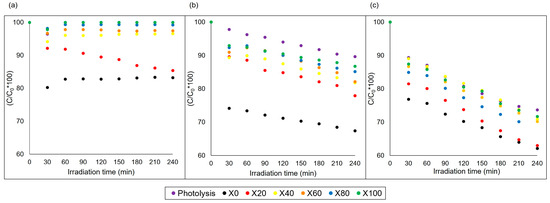
Figure 7.
Photodegradation of MB in the presence of NixCu(1-x)Fe2O4 photocatalysts: (a) pH 2, (b) pH 6 and (c) pH 10.
In previous studies [34] with the rhodamine B dye (RhB), it was found that samples CuFe2O4 and Ni0.2Cu0.8Fe2O4 (for x = 0 and 0.2) showed higher removal percentages at basic pH, reaching approximately 55% removal. In order to study the photodegradation of RhB, we have performed tests using samples X40 and X60 (Figure 8). We found a photocatalytic reaction at acidic pH (a), highlighting the concentration of X60, which showed similar removal at basic pH (c). Sample X40 behaved similarly to the other samples, with increased activity as the pH increased.
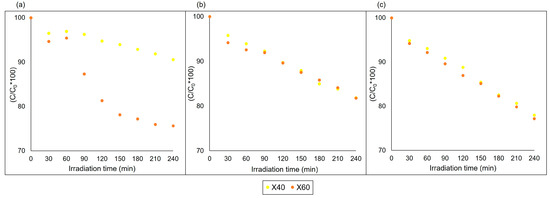
Figure 8.
Photodegradation of RhB in the presence of X40 and X60: (a) pH 2, (b) pH 6 and (c) pH 10.
Considering all the degradations using MB and RhB, it is noted that the reaction pH is a significant factor during heterogeneous photocatalysis due to the surface charge of the catalysts (pHPZC = 6.4 for NiFe2O4 [35] and 5.4 for CuFe2O4 [36]) and directly influencing the generation of hydroxyl radicals as well as the electrostatic attraction with the dyes [37,38]. At pHs below pHPZC, the photocatalyst’s surfaces are positively charged, thus repulsing positively-charged molecules, as is the case for both MB and RhB, while above pHPZC, the photocatalysts would have a negatively charged surface, which has then the potential for attracting positively charged molecules. In this perspective, at pH < 5.4, the photocatalytic mechanism is based on the production of radicals on the surface of samples followed by the reaction of dyes with those radicals on the bulk of the reactor, while at pH > 6.4, reactions at the surface of the photocatalysts are also expected to occur in addition to those at the bulk of the reactor.
After the photocatalytic reactions, EDS analyses were carried out to prove the integrity of the materials. All samples presented percentages of Fe, Cu and Ni close to theoretical values after the reactions, indicating the absence of leaching during the experiments.
As the reactions at pH 2 for methylene blue did not show interesting results, the kinetics studies were carried out only for the reactions at pH 6 and pH 10. Considering that the first 30 min of an adsorption reaction associated with photocatalysis occurs due to not having an adsorption equilibrium time before starting the process, the initial time was disregarded [38,39]. Zero-order, first- and second-order kinetics were evaluated, obtaining a better first-order result for both pHs, described by Equation (5) and presented in Figure 9. Photocatalysis for rhodamine B also follows first-order kinetics.
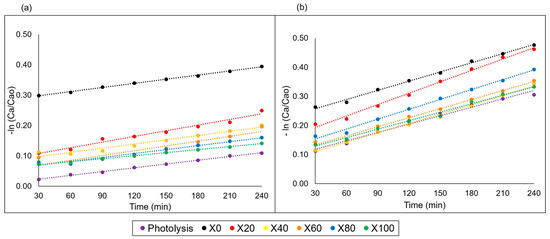
Figure 9.
Kinetic behavior of MB degradation at pH 6 (a) and pH 10 (b).
Table 4 shows the kinetic speed constants. The highest rate constants were linked to the basic pH, where pHPZC indicates the negatively charged surface of samples, as well as for samples with higher percentages of copper. We see that some compositions are optimal from a kinetic point of view while others present virtually the same or even lower kinetic rate as achieved by photolysis.

Table 4.
Values of apparent rate constants for MB photodegradation at pH 6 and pH 10, using samples NixCu(1-x)Fe2O4.
As bang gaps of samples X0 to X80 are very close, other underlying factors also influenced the efficiency of the catalysts. Samples with higher percentages of copper present smaller crystallite sizes, which are known to positively affect the specific surface area of catalysts [40,41] and thus favor its activities due to an increase in the concentration of active sites per gram of catalyst. Also, the change in the oxidation state of Cu+/Cu2+, linked to the inverse spinel structure of ferrites, favors the occurrence of oxidation-reduction reactions, generating more hydroxyl radicals [42,43]. The combination of those facts is responsible for the greater photocatalytic activity of samples containing copper atoms.
In order to verify the real viability of sample X0, which is the most promising candidate for the photodegradation of both Methylene Blue and Rhodamine B, we performed a mixed dye photocatalytic experiment aiming to find the efficiency for possible industrial applications as, typically, different dye effluents are mixed together before treatment.
Figure 10 shows a photocatalysis reaction at pH 10 using a mixture of methylene blue and rhodamine B, both with 10 ppm concentration. All other experimental conditions used previously were maintained. During the initial 30 min, there is a sudden drop in the concentration of methylene blue dye, which is attributed to the adsorption process on the catalyst surface due to its cationic nature [44]. The level of adsorption contributed to circa 20% of dye removal, which is close to the level found in the absence of Rhodamine B. The concentration of Rhodamine B due to adsorption falls within the same level found in the absence of Methylene Blue. As this now consists of a competitive adsorption process, one can conclude that the active sites available for the adsorption of both MB and RhB are not of the same nature. Thus, the presence of one does not implicate the reduction of viable active sites to the other. After 30 min, the concentration of methylene blue reduces in a linear fashion to reach circa 60% after 240 min, similar to the kinetic found in the absence of RhB. On the opposite side, the levels of RhB photodegradation are not the same as those found in the absence of MB, reaching only circa 15% after 240 min of reaction instead of 45%. In addition, its kinetics are not as linear as before and reach a stable plateau after 180 min, indicating a kinetically reversible nature. In fact, R2 falls to 0.97 for first-order kinetics, while for MB, it continues to be greater than 0.99. The adsorption effect cannot explain this result for the reasons mentioned above. Thus, after adsorption, the production/consumption of hydroxyl radicals becomes the major factor affecting the reduction of both dyes’ concentrations. As for MB degradation, the reaction mechanism can be expected to be the same as in the absence of RhB, while for RhB, the presence of MB would alter its reaction mechanism, reducing the availability of key radicals which are found to be responsible for an important part of its degradation. Thus, both MB and RhB photodegradation using CuFe2O4 should follow a duo chemical-dependent major step, one of which relies on the abundancy of a reagent/radical while the other depends on a limiting reagent/radical that is primarily available to MB, leaving then a great part of RhB molecules unreacted.
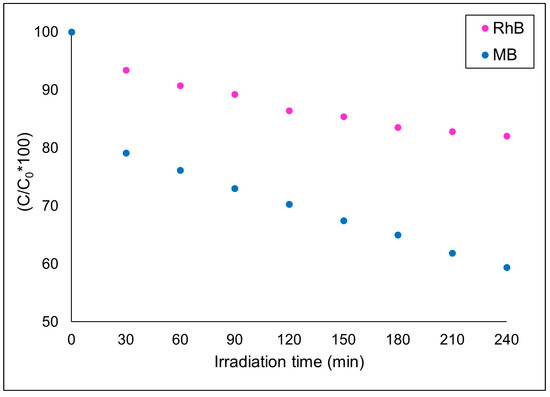
Figure 10.
Photodegradation of MB and RhB at pH 10.
Experiments with reactive species scavengers were conducted to understand the role of copper ferrite (X0) in the photocatalytic degradation of the mixture of dyes, RhB and MB. These results are presented in Figure 11. The presence of isopropanol promoted a small reduction in the percentage of methylene blue degradation, confirming that •OH acts in the photo performance of the catalyst for that contaminant but only to a small extent. Thus, the MB degradation occurs due to mechanisms not controlled by the presence of hydroxyl groups. However, for rhodamine B, an increase in degradation was observed. By scavenging •OH, isopropanol favors the decomposition of water on the surface of the catalyst, thus liberating electrons that could form other reactive oxygen species, which are clearly one path to the oxidation of RhB [45]. The addition of EDTA-2Na caused an increase in the photocatalytic efficiency of the reaction for both dyes, but RhB is most affected by its presence. EDTA-2Na acts by sequestering h+ at the valence band, thus reducing its availability for recombination, which leads to the production of greater quantities of reactive oxygen species at the conduction band of the catalyst. As clearly the decrease in the recombination rate of the e−/h+ pair promotes a higher photodegradation rate, we conclude that a drawback of this material is its high recombination rate, which can be viewed as a limiting step in the photodegradation process of both dyes.
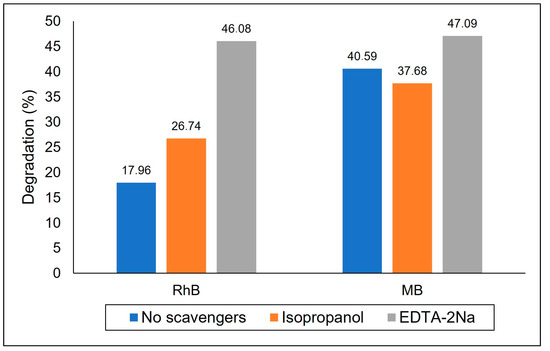
Figure 11.
Experiments with reactive species scavengers.
3. Materials and Methods
3.1. Reagents and Synthesis Procedure
The NixCu(1-x)Fe2O4 nanoferrites (x = 0, 0.2, 0.4, 0.6, 0.8 and 1) were synthesized using the combined EDTA-Citrate complexation method. Firstly, a solution of ammonium hydroxide (NH4OH, 27%, Synth, Zola Predosa, Italy) and EDTA acid (C10H16N2O8, 99.4%, Synth) in a proportion of 1 g:10 mL was prepared under constant stirring and controlled temperature (40 °C) for 15 min. Secondly, iron nitrate III (Fe(NO3)3.9H2O, 98%, Sigma Aldrich, Burlington, MA, USA), nickel nitrate II (Ni(NO3)2.6H2O, 97%, Isofar, Westerau, Germany) and copper nitrate II (Cu(NO3)2.9H2O, 98%, Synth) in stoichiometric proportions were dissolved separately in water and added to the EDTA- NH4OH solution, and maintained under stirring at 40 °C, along 15 min. The citric acid (C6H8O7, 99.5%, Synth), also in stoichiometric proportion, was diluted in water and added to the above solution, after which the pH was adjusted to 9, and the temperature increased to 80 °C until the formation of an organometallic gel. A thermal pre-treatment was applied (230 °C for 180 min) to eliminate moisture, thus forming the NixCu(1-x)Fe2O4 precursors. The resulting powders were ground to homogenize and then subjected to final heat treatment (700 °C for 240 min) to obtain the desired ferrites.
3.2. Characterizations
The ferrites were analyzed by X-ray diffraction (XRD) (XRD-7000, Shimadzu, Natal, Brazil) to identify crystallographic phases, concentrations, crystallite size and intrinsic microstrain. The size and intrinsic effects of strains on XRD peak broadening were evaluated with four different models: Scherrer (Equation (6)), Williamson-Hall Uniform Strain Model (UDM) (Equation (7)), Williamson-Hall (Equation (8)) and Halder Method-Wagner (Equation (9)), making it possible to determine the crystalline size and deformation [46].
where corresponds to the peak position in radians, λ is the wavelength of x-ray radiation, D is the average particle size, and βd is the corrected peak broadening.
where βhkl is the full width at half of the maximum intensity for different diffraction planes, and represents the strain associated with the peak broadening.
where dhkl is the distance between the (hkl) planes.
where β*hkl = βhkl.cosθ/λ and d*hkl = 2dhkl.sinθ/λ. The instrumental contribution was removed from the peak broadening prior to the calculations. The R2 correlation parameter was used as the reference for analyzing the ability of the models to describe the XRD peak broadening data.
To understand the molecular structures of the samples in terms of chemical bonds, Fourier transform infrared spectroscopy (FTIR) analyses were performed using an IRAffinity-1 equipment (Shimadzu), in the wavelength range of 4000 to 400 cm−1 with a resolution of 4 cm. High-resolution scanning electron microscopy (SEM-FEG) analyzes were performed on a FESEM equipment (Auriga, Carl Zeiss, Maple Grove, MN, USA), with EDS attached, model Supra 35-VP to analyze morphological parameters. Diffuse reflectance spectroscopies (ERD UV-Vis) were performed on a UV-2450 spectrometer (Shimadzu) in the range of 200–800 nm in order to determine the characteristic energy gap (bandgap) between the valence and conduction bands. The 57Fe Mössbauer spectroscopy study was performed in the transmission mode, at room temperature, using a constant acceleration drive and a 57Co(Rh) gamma-ray source. The spectrometer is from SEECo (Charlotte, NC, USA). The isomer shifts are reported in relation to α-Fe at 300 K. The spectra were fitted using the Normos software, version 95.
3.3. Photocatalytic Experiments
Heterogeneous photocatalytic experiments were performed on a homemade apparatus consisting of a closed, white-painted wood box equipped with a forced air evacuation system that efficiently removes the heat generated by the lamp. The reactor was placed in the center of the box and mounted over a heat exchanger that uses water as a cooling fluid. The temperature and flow of circulating water were controlled by an external apparatus that provided enough heat exchange rates to allow precise control of the reactor temperature during light exposure. All experiments were conducted at 25 °C. A constant flow of atmospheric air was injected into the reacting solution, which provided a homogenous degree of agitation and aided in maintaining a constant dissolved-O2 concentration in the liquid. In previous experiments, the constant concentration of O2 was proved to positively impact the photodegradation of dyes. We used an OSRAM ULTRA-VITALUX 300 W lamp that generates radiation similar to the solar spectrum. The intensity of the lamp was measured prior to the experiments to confirm they were all exposed to the same light conditions. The dyes we chose to work with were Methylene Blue (MB) and Rhodamine B (RhB), both at 10 ppm concentration. The catalyst concentration was optimized to 0.62 g/L in our previous works [34]. All experiments were performed to allow the determination of kinetic information at pHs 2, 6 and 10. For that, a maximum exposure time of 240 min was chosen, during which the adsorption effect was found to be negligible. Samples were collected every 30 min and analyzed with the aid of a UV-Vis spectrophotometer at an adequate wavelength. To understand the catalyst’s behavior, tests were carried out with reactive species scavengers. Isopropanol, as hydroxyl scavenger, and disodium EDTA (EDTA-2Na), h+ scavenger, was added at a concentration of 0.01 M according to [47].
4. Conclusions
Single phases of nickel and copper ferrites were efficiently produced by the combined EDTA-Citrate complexation method, presenting an inverse spinel structure for all cases, being tetragonal for samples with higher percentages of copper and cubic for those with higher percentages of nickel. Despite the low bandgap values for the majority of samples, copper has a greater photocatalyst character than nickel for both dyes, especially X0 and X20, attributed both to its variation in oxidation state and to the smaller crystallite sizes. In addition to the samples, pH also has a great influence on photocatalytic processes, where the basic pH provides a greater generation of hydroxyl radicals, increasing degradation. The photodegradation of a mixture of dyes was possible, reaching the same levels of degradation for methylene blue, while lower levels were found for rhodamine B, which is explained by the different photoreaction pathways of those dyes. CuFe2O4 was shown to be a promising material for the photodegradation of dyes.
Author Contributions
Conceptualization, A.L.L.M. and I.G.D.D.d.A.; methodology, A.L.L.M., C.P.d.S., I.G.D.D.d.A. and M.A.M.T.; software, I.G.D.D.d.A. and M.A.M.T.; validation, A.L.L.M., C.P.d.S. and M.A.M.T.; formal analysis, A.L.L.M. and M.A.M.T.; investigation, A.L.L.M., I.G.D.D.d.A. and M.A.M.T.; resources, A.L.L.M., C.P.d.S. and M.A.M.T.; writing—original draft preparation, A.L.L.M., I.G.D.D.d.A. and M.A.M.T.; writing—review and editing, A.L.L.M., I.G.D.D.d.A. and M.A.M.T.; visualization, I.G.D.D.d.A. and M.A.M.T.; supervision, A.L.L.M., C.P.d.S. and M.A.M.T.; project administration, A.L.L.M.; funding acquisition, A.L.L.M., C.P.d.S. and M.A.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Graduate Program in Chemical Engineering from the Universidade Federal do Rio Grande do Norte (PPGEQ/UFRN) for their financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry; IntechOpen: London, UK, 2020; pp. 1–14. [Google Scholar]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Gičević, A.; Hindija, L.; Karačić, A. Toxicity of Azo Dyes in Pharmaceutical Industry. In CMBEBIH 2019, Proceedings of the International Conference on Medical and Biological Engineering, Banja Luka, Bosnia and Herzegovina, 16–18 May 2019; Springer: Cham, Switzerland, 2020; pp. 581–587. [Google Scholar]
- Singh, A.K.; Chandra, R. Pollutants Released from the Pulp Paper Industry: Aquatic Toxicity and Their Health Hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Aguiar, A. Presença de Metais Pesados e Processos Convencionais Para o Tratamento de Efluentes de Indústrias Têxteis Brasileiras. In Engenharia Química: Inovação e Tradição em Tempos de Pandemia; Even3 Publicações: Recife, Brazil, 2021; pp. 806–819. ISBN 9786559412624. [Google Scholar]
- Ramos, M.D.N.; Lima, J.P.P.; de Aquino, S.F.; Aguiar, A. A Critical Analysis of the Alternative Treatments Applied to Effluents from Brazilian Textile Industries. J. Water Process Eng. 2021, 43, 102273. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef]
- Kowalska, K.; Maniakova, G.; Carotenuto, M.; Sacco, O.; Vaiano, V.; Lofrano, G.; Rizzo, L. Removal of Carbamazepine, Diclofenac and Trimethoprim by Solar Driven Advanced Oxidation Processes in a Compound Triangular Collector Based Reactor: A Comparison between Homogeneous and Heterogeneous Processes. Chemosphere 2020, 238, 124665. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of Heterogeneous Nano-Semiconductors for Photocatalytic Advanced Oxidation of Organic Compounds: A Review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Xie, X.; Wang, B.; Wang, Y.; Ni, C.; Sun, X.; Du, W. Spinel Structured MFe2O4 (M = Fe, Co, Ni, Mn, Zn) and Their Composites for Microwave Absorption: A Review. Chem. Eng. J. 2022, 428, 131160. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; John Kennedy, L.; Judith Vijaya, J. Photocatalytic Degradation of Rhodamine B under Visible Light Using Nanostructured Zinc Doped Cobalt Ferrite: Kinetics and Mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Rashad, M.M.; Mohamed, R.M.; Ibrahim, M.A.; Ismail, L.F.M.; Abdel-Aal, E.A. Magnetic and Catalytic Properties of Cubic Copper Ferrite Nanopowders Synthesized from Secondary Resources. Adv. Powder Technol. 2012, 23, 315–323. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and Characterization of Nickel Ferrite Magnetic Nanoparticles. Mater. Res. Bull. 2011, 46, 2208–2211. [Google Scholar] [CrossRef]
- Priya, R.; Stanly, S.; Anuradha, R.; Sagadevan, S. Evaluation of Photocatalytic Activity of Copper Ferrite Nanoparticles. Mater. Res. Express 2019, 6, 095014. [Google Scholar] [CrossRef]
- Karcıoğlu Karakaş, Z. A Comprehensive Study on the Production and Photocatalytic Activity of Copper Ferrite Nanoparticles Synthesized by Microwave-Assisted Combustion Method as an Effective Photocatalyst. J. Phys. Chem. Solids 2022, 170, 110927. [Google Scholar] [CrossRef]
- Vosoughifar, M. Preparation and Application of Copper Ferrite Nanoparticles for Degradation of Methyl Orange. J. Mater. Sci. Mater. Electron. 2016, 27, 10449–10453. [Google Scholar] [CrossRef]
- Oliveira, T.P.; Rodrigues, S.F.; Marques, G.N.; Viana Costa, R.C.; Garçone Lopes, C.G.; Aranas, C.; Rojas, A.; Gomes Rangel, J.H.; Oliveira, M.M. Synthesis, Characterization, and Photocatalytic Investigation of CuFe2O4 for the Degradation of Dyes under Visible Light. Catalysts 2022, 12, 623. [Google Scholar] [CrossRef]
- Ismael, M. Ferrites as Solar Photocatalytic Materials and Their Activities in Solar Energy Conversion and Environmental Protection: A Review. Sol. Energy Mater. Sol. Cells 2021, 219, 110786. [Google Scholar] [CrossRef]
- Behera, A.; Kandi, D.; Majhi, S.M.; Martha, S.; Parida, K. Facile Synthesis of ZnFe2O4 Photocatalysts for Decolourization of Organic Dyes under Solar Irradiation. Beilstein J. Nanotechnol. 2018, 9, 436–446. [Google Scholar] [CrossRef]
- Jarusheh, H.S.; Yusuf, A.; Banat, F.; Haija, M.A.; Palmisano, G. Integrated Photocatalytic Technologies in Water Treatment Using Ferrites Nanoparticles. J. Environ. Chem. Eng. 2022, 10, 108204. [Google Scholar] [CrossRef]
- Dhiwahar, A.T.; Maruthamuthu, S.; Marnadu, R.; Sundararajan, M.; Manthrammel, M.A.; Shkir, M.; Sakthivel, P.; Minnam Reddy, V.R. Improved Photocatalytic Degradation of Rhodamine B under Visible Light and Magnetic Properties Using Microwave Combustion Grown Ni Doped Copper Ferrite Spinel Nanoparticles. Solid State Sci. 2021, 113, 106542. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Raimundo, R.A.; Silva, T.R.; Araújo, A.J.M.; Macedo, D.A.; Morales, M.A.; Souza, C.P.; Santos, A.G.; Lopes-Moriyama, A.L. Morphology-Controlled NiFe2O4 Nanostructures: Influence of Calcination Temperature on Structural, Magnetic and Catalytic Properties towards OER. J. Electroanal. Chem. 2023, 933, 117277. [Google Scholar] [CrossRef]
- Nihore, A.; Aziz, F.; Oswal, N.; Jain, P.; Subohi, O.; Gupta, N. Synthesis and Characterization of Copper Doped Nickel Ferrite Prepared by Sol-Gel Method. Mater. Today Proc. 2019, 18, 3651–3656. [Google Scholar] [CrossRef]
- Anjana, V.; John, S.; Prakash, P.; Nair, A.M.; Nair, A.R.; Sambhudevan, S.; Shankar, B. Magnetic Properties of Copper Doped Nickel Ferrite Nanoparticles Synthesized by Co Precipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 012024. [Google Scholar] [CrossRef]
- Gayathri Manju, B.; Raji, P. Biological Synthesis, Characterization, and Antibacterial Activity of Nickel-Doped Copper Ferrite Nanoparticles. Appl. Phys. A 2019, 125, 313. [Google Scholar] [CrossRef]
- Greenwood, N.N. Mössbauer Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 94-009-5697-5. [Google Scholar]
- Silva, M.M.S.; Raimundo, R.A.; Ferreira, L.S.; Macedo, D.A.; Morales, M.A.; Souza, C.P.; Santos, A.G.; Lopes-Moriyama, A.L. Effects of Morphology on the Electrochemical Performance of NiFe2O4 Nanoparticles with Battery-Type Behavior. Int. J. Appl. Ceram. Technol. 2022, 19, 2016–2028. [Google Scholar] [CrossRef]
- Thanh, N.K.; Loan, T.T.; Anh, L.N.; Duong, N.P.; Soontaranon, S.; Thammajak, N.; Hien, T.D. Cation Distribution in CuFe2O4 Nanoparticles: Effects of Ni Doping on Magnetic Properties. J. Appl. Phys. 2016, 120, 142115. [Google Scholar] [CrossRef]
- Zaki, H.M. Structure, Analysis and Some Magnetic Properties for Low Temperature Fired Ni–Cu Ferrite. Phys. B Condens. Matter 2012, 407, 2025–2031. [Google Scholar] [CrossRef]
- Rodrigues, M.V. Síntese e Caracterização de Ferritas Mistas de Níquel e Cobre Pelo Método de Complexação Combinado EDTA/Citrato. Master Thesis, Engenharia Química, Natal, Brasil, 2020. [Google Scholar]
- Yousaf, M.; Noor, A.; Xu, S.; Akhtar, M.N.; Wang, B. Magnetic Characteristics and Optical Band Alignments of Rare Earth (Sm+3, Nd+3) Doped Garnet Ferrite Nanoparticles (NPs). Ceram. Int. 2020, 46, 16524–16532. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wen, T.; Zhang, S.; Chang, B.; Guo, Y.; Yang, B. Mesoporous Cd1−xZnxS Microspheres with Tunable Bandgap and High Specific Surface Areas for Enhanced Visible-Light-Driven Hydrogen Generation. J. Colloid Interface Sci. 2016, 467, 97–104. [Google Scholar] [CrossRef]
- De Azevedo, I.G.D.D.; Rodrigues, M.V.; Gomes, Y.F.; de Araújo, C.P.B.; de Souza, C.P.; Moriyama, A.L.L. Photocatalytic Degradation of the Rhodamine B Dye Under Visible Light Using NixCu(1−x)Fe2O4 Synthesized by EDTA-Citrate Complexation Method. Mater. Res. 2023, 26 (Suppl. 1), e20230061. [Google Scholar] [CrossRef]
- Fathy, M.A.; Kamel, A.H.; Hassan, S.S.M. Novel magnetic nickel ferrite nanoparticles modified with poly(aniline-co-o-toluidine) for the removal of hazardous 2,4-dichlorophenol pollutant from aqueous solutions. RSC Adv. 2022, 12, 7433. [Google Scholar] [CrossRef]
- Vergis, B.R.; Krishna, R.H.; Kottam, N.; Nagabhushana, B.M.; Sharath, R.; Darukaprasad, B. Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. J. Nanostructure Chem. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Ali, D.; Bahnemann, D.; Muneer, M. Comparative Photocatalytic Activity of Sol–Gel Derived Rare Earth Metal (La, Nd, Sm and Dy)-Doped ZnO Photocatalysts for Degradation of Dyes. RSC Adv. 2018, 8, 17582–17594. [Google Scholar] [CrossRef] [PubMed]
- Soltani, T.; Entezari, M.H. Photolysis and Photocatalysis of Methylene Blue by Ferrite Bismuth Nanoparticles under Sunlight Irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Makofane, A.; Motaung, D.E.; Hintsho-Mbita, N.C. Photocatalytic Degradation of Methylene Blue and Sulfisoxazole from Water Using Biosynthesized Zinc Ferrite Nanoparticles. Ceram. Int. 2021, 47, 22615–22626. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and Photocatalytic Activity of Ferrites under Visible Light: A Review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.; Hu, Y.; Qiu, S.; Min, G.; Song, Z.; Sun, Z.; Li, C. General Flame Approach to Chainlike MFe2O4 Spinel (M= Cu, Ni, Co, Zn) Nanoaggregates for Reduction of Nitroaromatic Compounds. Ind. Eng. Chem. Res. 2015, 54, 9750–9757. [Google Scholar] [CrossRef]
- Goyal, A.; Bansal, S.; Singhal, S. Facile Reduction of Nitrophenols: Comparative Catalytic Efficiency of MFe2O4 (M= Ni, Cu, Zn) Nano Ferrites. Int. J. Hydrogen Energy 2014, 39, 4895–4908. [Google Scholar] [CrossRef]
- Sharma, R.; Bansal, S.; Singhal, S. Tailoring the Photo-Fenton Activity of Spinel Ferrites (MFe2O4) by Incorporating Different Cations (M = Cu, Zn, Ni and Co) in the Structure. RSC Adv. 2015, 5, 6006–6018. [Google Scholar] [CrossRef]
- Harraz, F.A.; Mohamed, R.M.; Rashad, M.M.; Wang, Y.C.; Sigmund, W. Magnetic Nanocomposite Based on Titania–Silica/Cobalt Ferrite for Photocatalytic Degradation of Methylene Blue Dye. Ceram. Int. 2014, 40, 375–384. [Google Scholar] [CrossRef]
- Mancipe, S.; Martínez, J.J.; Pinzon, C.; Rojas, H.; Solis, D.; Gomez, R. Effective photocatalytic degradation of Rhodamine B using tin semiconductors over hydrotalcite-type materials under sunlight driven. Catal. Today 2021, 372, 191–197. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Brasileiro, I.L.O. Desenvolvimento de Heterojunções Constituídas de ZnO/g-C3N4 e ZnFe2O4/g-C3N4 Para Fotodegradação de Contaminantes Orgânicos Emergentes. Ph.D. Thesis, Engenharia Química, Natal, Brasil, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).