Enhancing Propane Dehydrogenation Performance on Cerium-Modified PtSnIn/Al Trimetallic Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of PtSnIn/xCe-Al Catalysts
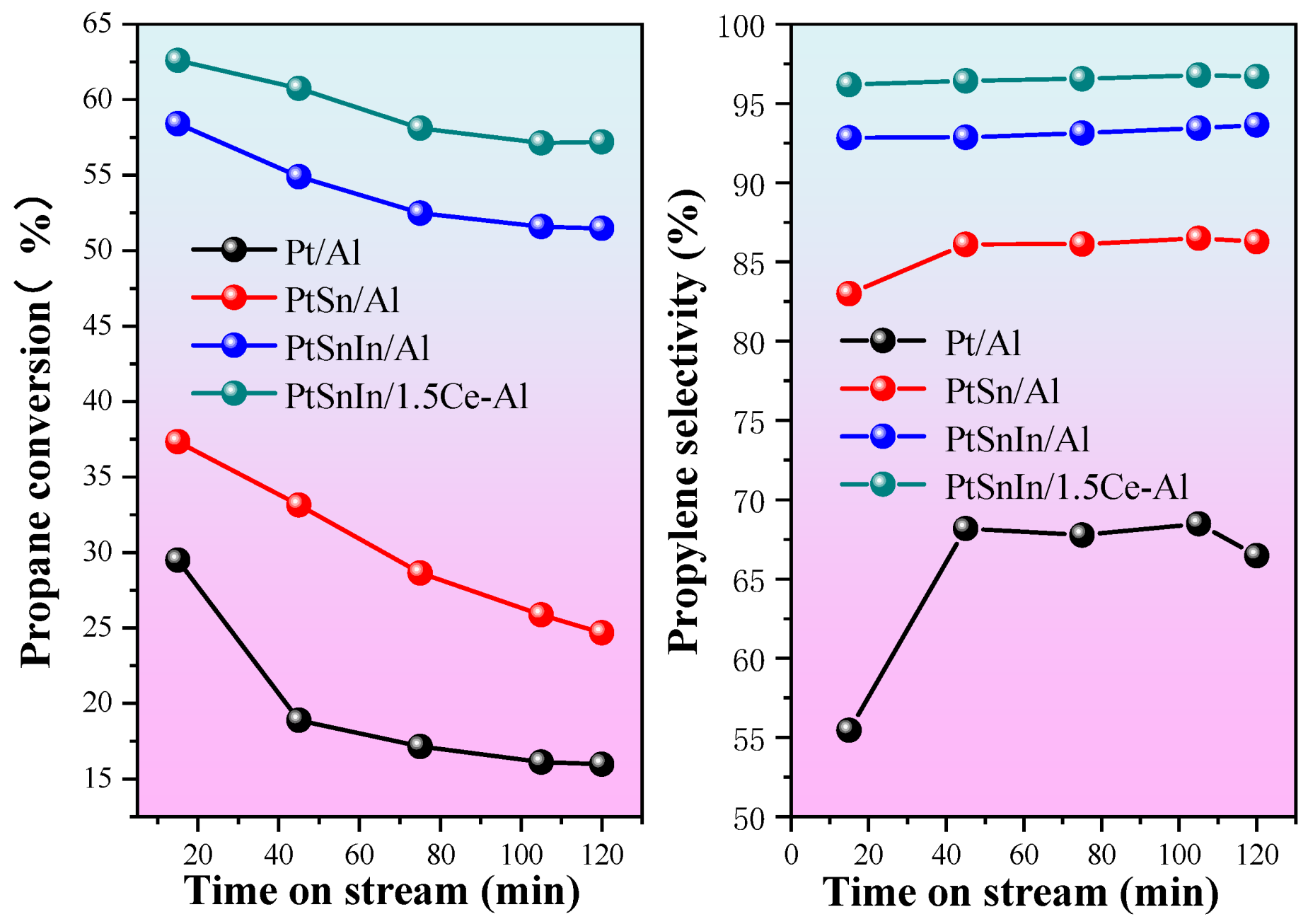
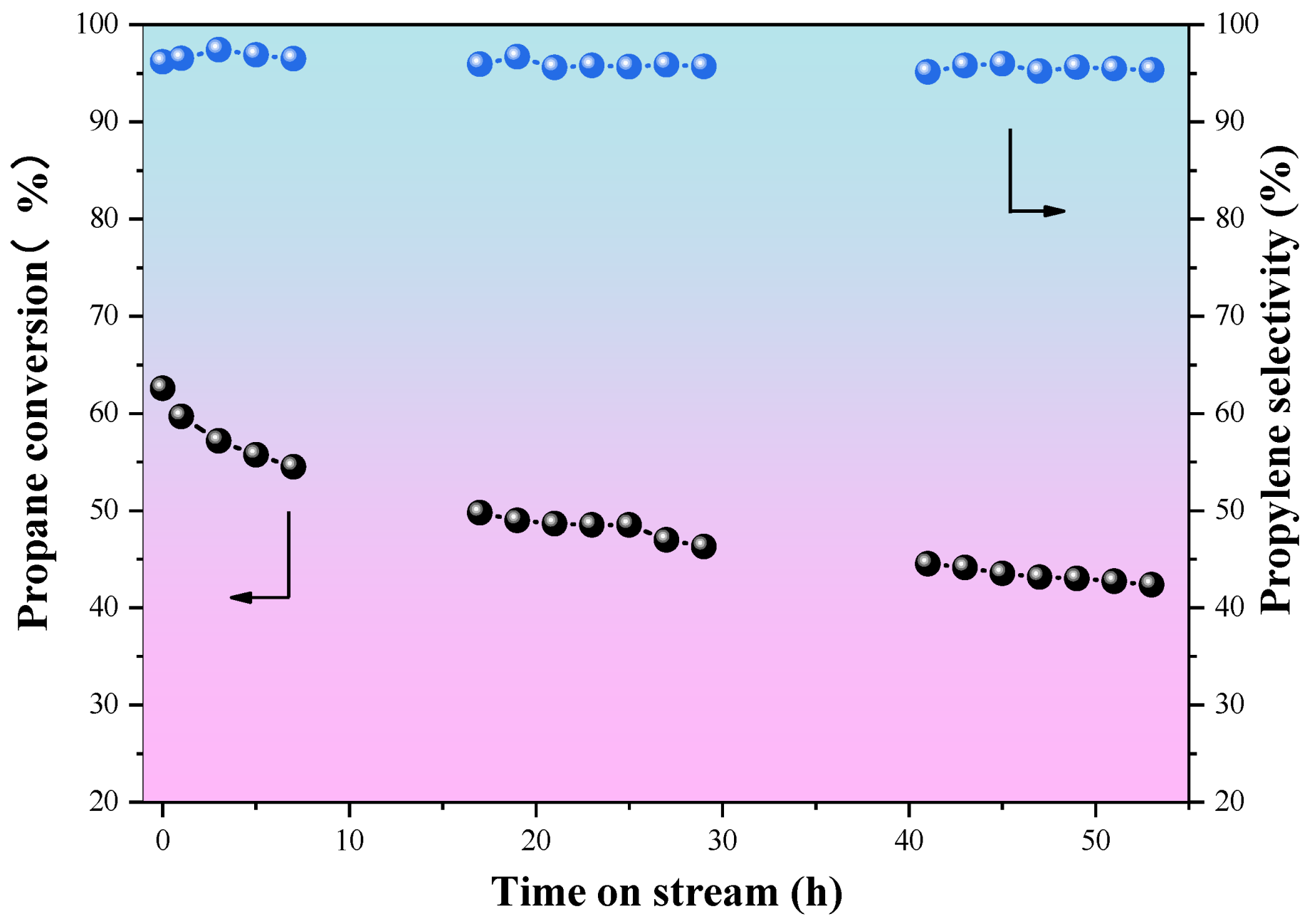
2.2. Catalytic Performances
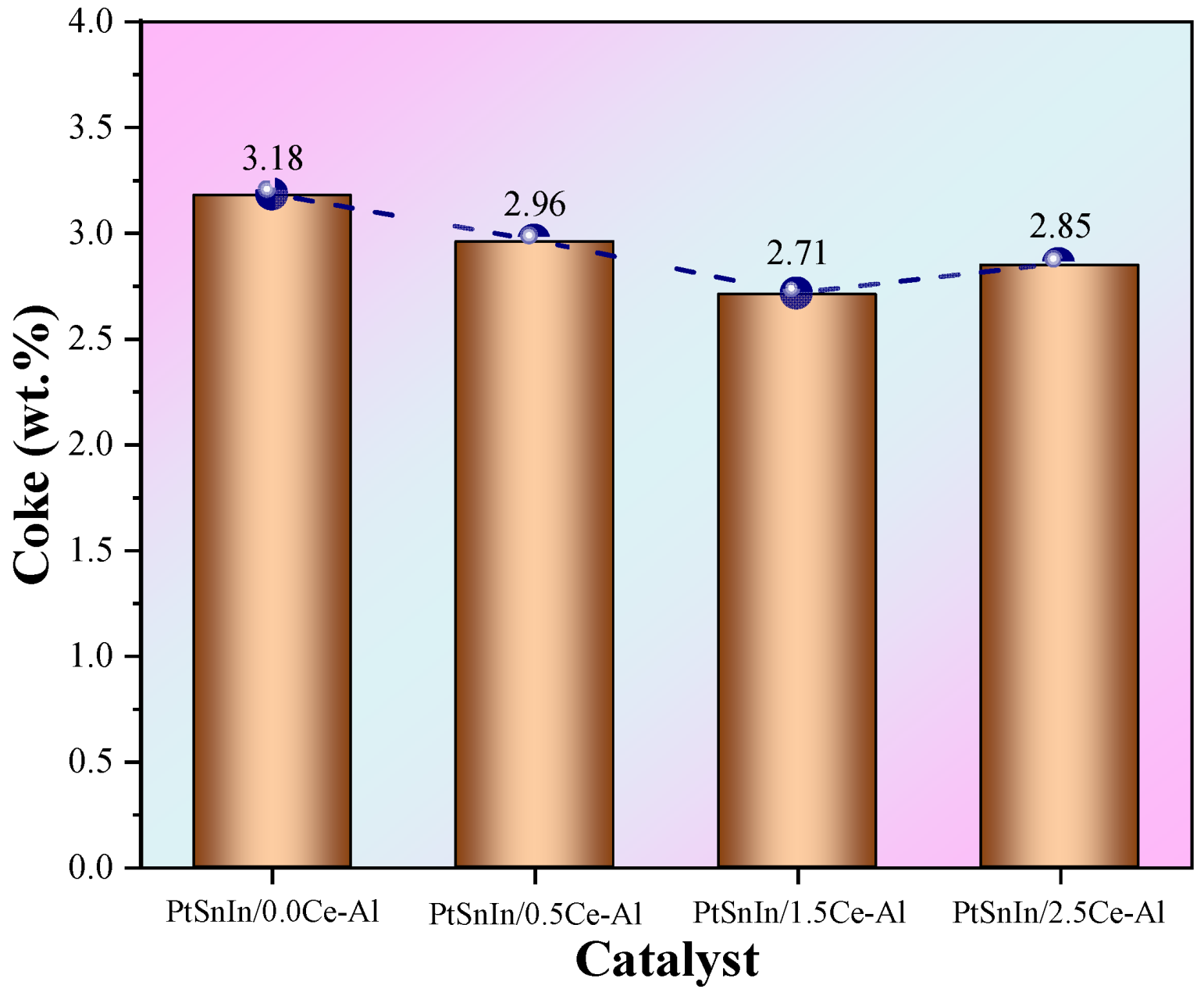
2.3. Coke Analysis
3. Experimental Section
3.1. Catalyst Preparation
- Ce Impregnation: The γ-Al2O3 support was first impregnated with an aqueous solution of Ce(NO3)3.
- Sn and In Impregnation: The Ce-impregnated support was then treated with a mixed ethanol solution containing SnCl2 (≥98.0%) and In(NO3)3 (≥99.5%).
- Pt Impregnation: Finally, the sample was impregnated with an aqueous solution of H2PtCl6.
3.2. Catalyst Characterizations
3.3. Propane Dehydrogenation Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Song, M.; Xu, M.; Liu, G. Recent Advances in Metal−Zeolite Catalysts for Direct Propane Dehydrogenation. Energy Fuels 2023, 37, 19419–19432. [Google Scholar] [CrossRef]
- Wang, P.; Liao, H.; Yang, M.; Wu, L.; Tang, Y.; Tan, L. Constructing rich-Zn Pt1Zn1 alloy over the BEA zeolite as a selective catalyst for propane dehydrogenation. Fuel 2023, 354, 129421. [Google Scholar] [CrossRef]
- Zuo, C.; Su, Q. Research Progress on Propylene Preparation by Propane Dehydrogenation. Molecules 2023, 28, 3594. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane dehydrogenation: Catalyst development; new chemistry; emerging technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar]
- Song, S.; Sun, Y.; Yang, K.; Fo, Y.; Ji, X.; Su, H.; Li, Z.; Xu, C.; Huang, G.; Liu, J.; et al. Recent Progress in Metal-Molecular Sieve Catalysts for Propane Dehydrogenation. ACS Catal. 2023, 13, 6044–6067. [Google Scholar] [CrossRef]
- Chen, S.; Pei, C.; Sun, G.; Zhao, Z.-J.; Gong, J. Nanostructured Catalysts toward Efficient Propane Dehydrogenation. Acc. Mater. Res. 2020, 1, 30–40. [Google Scholar] [CrossRef]
- Zha, S.; Sun, G.; Wu, T.; Zhao, J.; Zhao, Z.-J.; Gong, J. Identification of Pt-based catalysts for propane dehydrogenation via a probability analysis. Chem. Sci. 2018, 9, 3925–3931. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Jiang, J.; Sui, Z.; Zhu, Y.; Chen, D.; Zhou, X. Size Dependence of Pt Catalysts for Propane Dehydrogenation: From Atomically Dispersed to Nanoparticles. ACS Catal. 2020, 10, 12932–12942. [Google Scholar] [CrossRef]
- Martino, M.; Meloni, E.; Festa, G.; Palma, V. Propylene Synthesis: Recent Advances in the Use of Pt-Based Catalysts for Propane Dehydrogenation Reaction. Catalysts 2021, 11, 1070. [Google Scholar] [CrossRef]
- Wang, P.; Yao, J.; Jiang, Q.; Gao, X.; Lin, D.; Yang, H.; Wu, L.; Tang, Y.; Tan, L. Stabilizing the isolated Pt sites on PtGa/Al2O3 catalyst via silica coating layers for propane dehydrogenation at low temperature. Appl. Catal. B Environ. 2022, 300, 120731. [Google Scholar] [CrossRef]
- Lian, Z.; Si, C.; Jan, F.; Zhi, S.; Li, B. Coke Deposition on Pt-Based Catalysts in Propane Direct Dehydrogenation: Kinetics, Suppression, and Elimination. ACS Catal. 2021, 11, 9279–9292. [Google Scholar] [CrossRef]
- Bariås, O.A.; Holmen, A.; Blekkan, E.A. Propane dehydrogenation over supported platinum catalysts: Effect of tin as a promoter. Catal. Today 1995, 24, 361–364. [Google Scholar] [CrossRef]
- Liu, X.; Lang, W.-Z.; Long, L.-L.; Hu, C.-L.; Chu, L.-F.; Guo, Y.-J. Improved catalytic performance in propane dehydrogenation of PtSn/γ-Al2O3 catalysts by doping indium. Chem. Eng. J. 2014, 247, 183–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Qiu, A.; Wang, Y.; Xu, Y.; Wu, P. Propane dehydrogenation on PtSn/ZSM-5 catalyst: Effect of tin as a promoter. Catal. Commun. 2006, 7, 860–866. [Google Scholar] [CrossRef]
- Wang, P.; Liao, H.; Chen, Y.; Tao, X.; Gan, Y.; Deng, H.; Fu, Y.; Tang, Y.; Wu, L.; Tan, L. Enhanced PtIn Catalyst via Ce-Assisted Confinement Effect in Propane Dehydrogenation. ACS Catal. 2024, 14, 8116–8129. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Shi, J.; Zhou, S.; Sheng, X.; Zhang, Z.; Xiang, S. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation. J. Mol. Catal. A Chem. 2014, 381, 138–147. [Google Scholar] [CrossRef]
- Zhu, Y.; An, Z.; Song, H.; Xiang, X.; Yan, W.; He, J. Lattice-Confined Sn (IV/II) Stabilizing Raft-Like Pt Clusters: High Selectivity and Durability in Propane Dehydrogenation. ACS Catal. 2017, 7, 6973–6978. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, Z.; Shishido, T. Behavior of active species on Pt-Sn/SiO2 catalyst during the dehydrogenation of propane and regeneration. Appl. Catal. A Gen. 2020, 606, 117826. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Ma, A.; Rong, J.; Da, Z.; Zheng, A.; Qin, L. Effects of Al2O3 phase and Cl component on dehydrogenation of propane. Appl. Surf. Sci. 2016, 368, 233–240. [Google Scholar] [CrossRef]
- Gong, N.; Zhao, Z. Efficient supported Pt-Sn catalyst on carambola-like alumina for direct dehydrogenation of propane to propene. Mol. Catal. 2019, 477, 110543. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, C.; Wang, X.; Sun, G.; Zhao, Z.-J.; Gong, J. The role of pentacoordinate Al3+ sites of Pt/Al2O3 catalysts in propane dehydrogenation. Fundam. Res. 2024, 4, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Lang, W.-Z.; Li, P.-P.; Long, L.-L.; Yan, X.; Guo, Y.-J. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O- x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Long, L.-L.; Xia, K.; Lang, W.-Z.; Shen, L.-L.; Yang, Q.; Yan, X.; Guo, Y.-J. The comparison and optimization of zirconia, alumina, and zirconia-alumina supported PtSnIn trimetallic catalysts for propane dehydrogenation reaction. J. Ind. Eng. Chem. 2017, 51, 271–280. [Google Scholar] [CrossRef]
- Long, L.-L.; Lang, W.-Z.; Yan, X.; Xia, K.; Guo, Y.-J. Yttrium-modified alumina as support for trimetallic PtSnIn catalysts with improved catalytic performance in propane dehydrogenation. Fuel Process. Technol. 2016, 146, 48–55. [Google Scholar] [CrossRef]
- Chen, J.Z.; Talpade, A.; Canning, G.A.; Probus, P.R.; Ribeiro, F.H.; Datye, A.K.; Miller, J.T. Strong metal-support interaction (SMSI) of Pt/CeO2 and its effect on propane dehydrogenation. Catal. Today 2021, 371, 4–10. [Google Scholar] [CrossRef]
- Vu, B.K.; Song, M.B.; Ahn, I.Y.; Suh, Y.-W.; Suh, D.J.; Kim, W.-I.L.; Koh, H.-L.; Choi, Y.G.; Shin, E.W. Propane dehydrogenation over Pt–Sn/Rare-earth-doped Al2O3: Influence of La, Ce, or Y on the formation and stability of Pt–Sn alloys. Catal. Today 2011, 164, 214–220. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Li, J.; Wang, N.; An, C.; Sun, L. Propane dehydrogenation over Al2O3 supported Pt nanoparticles: Effect of cerium addition. Fuel Process. Technol. 2014, 128, 283–288. [Google Scholar] [CrossRef]
- Wang, H.; Pan, X.; Wang, Y.; Ng, B.K.Y.; Tsang, S.C.E. Lanthanum-group elements promoted PtGa catalysts for propane dehydrogenation: Exploring key performance descriptors. Appl. Catal. A Gen. 2025, 691, 120055. [Google Scholar] [CrossRef]
- Yu, C.; Ge, Q.; Xu, H.; Li, W. Effects of Ce addition on the Pt-Sn/γ-Al2O3 catalyst for propane dehydrogenation to propylene. Appl. Catal. A Gen. 2006, 315, 58–67. [Google Scholar] [CrossRef]
- Kwon, H.C.; Park, Y.; Park, J.Y.; Ryoo, R.; Shin, H.; Choi, M. Catalytic Interplay of Ga, Pt, and Ce on the Alumina Surface Enabling High Activity, Selectivity, and Stability in Propane Dehydrogenation. ACS Catal. 2021, 11, 10767–10777. [Google Scholar] [CrossRef]
- Naseri, M.; Zangeneh, F.T.; Taeb, A. The effect of Ce, Zn and Co on Pt-based catalysts in propane dehydrogenation. React. Kinet. Mech. Catal. 2018, 126, 477–495. [Google Scholar] [CrossRef]
- Damyanova, S.; Perez, C.A.; Schmal, M.; Bueno, J.M.C. Characterization of ceria-coated alumina carrier. Appl. Catal. A Gen. 2002, 234, 271–282. [Google Scholar] [CrossRef]
- Gorczyca, A.; Raybaud, P.; Moizan, V.; Joly, Y.; Chizallet, C. Atomistic models for highly–dispersed PtSn/γ-Al2O3 catalysts: Ductility and dilution affect the affinity for hydrogen. ChemCatChem 2019, 11, 3941–3951. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Lu, W.-D.; Hu, S.-Z.; Li, W.-C.; Lu, A.-H. Rod-shaped porous alumina-supported Cr2O3 catalyst with low acidity for propane dehydrogenation. Chin. J. Catal. 2019, 40, 184–191. [Google Scholar] [CrossRef]
- Riguetto, B.A.; Damyanova, S.; Gouliev, G.; Marques, C.M.P.; Petrov, L.; Bueno, J.M.C. Surface Behavior of Alumina-Supported Pt Catalysts Modified with Cerium as Revealed by X-ray Diffraction, X-ray Photoelectron Spectroscopy, and Fourier Transform Infrared Spectroscopy of CO Adsorption. J. Phys. Chem. B 2004, 108, 5349–5358. [Google Scholar] [CrossRef]
- Piras, A.; Colussi, S.; Trovarelli, A.; Sergo, V.; Llorca, J.; Psaro, R.; Sordelli, L. Structural and Morphological Investigation of Ceria-Promoted Al2O3 under Severe Reducing/Oxidizing Conditions. J. Phys. Chem. B 2005, 109, 11110–11118. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Otto, K. Characterization of Pt/γ-alumina catalysts containing ceria. J. Catal. 1989, 115, 16–23. [Google Scholar] [CrossRef]
- Xue, M.; Zhou, Y.; Zhang, Y.; Liu, X.; Duan, Y.; Sheng, X. Effect of cerium addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. J. Nat. Gas Chem. 2012, 21, 324–331. [Google Scholar] [CrossRef]
- Yu, C.; Xu, H.; Ge, Q.; Li, W. Properties of the metallic phase of zinc-doped platinum catalysts for propane dehydrogenation. J. Mol. Catal. A Chem. 2007, 266, 80–87. [Google Scholar] [CrossRef]
- Damyanova, S.; Bueno, J.M.C. Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts. Appl. Catal. A Gen. 2003, 253, 135–150. [Google Scholar] [CrossRef]
- Ivanova, A.S. Physicochemical and catalytic properties of systems based on CeO2. Kinet. Catal. 2009, 50, 797–815. [Google Scholar] [CrossRef]
- Ballarini, A.D.; Ricci, C.G.; de Miguel, S.R.; Scelza, O.A. Use of Al2O3–SnO2 as a support of Pt for selective dehydrogenation of light paraffins. Catal. Today 2008, 133–135, 28–34. [Google Scholar] [CrossRef]
- Long, L.-L.; Lang, W.-Z.; Liu, X.; Hu, C.-L.; Chu, L.-F.; Guo, Y.-J. Improved catalytic stability of PtSnIn/xCa–Al catalysts for propane dehydrogenation to propylene. Chem. Eng. J. 2014, 257, 209–217. [Google Scholar] [CrossRef]
- Zhao, P.; Guan, Y.; Wang, Y.; Guo, X.; Zhang, J.; Du, Z.; Zhang, S.; Xie, Q.; Wu, S. Propane dehydrogenation over PtSnMg/Cr2O3·Al2O3 catalysts: Effect of the amount of Mg loading. IOP Conf. Ser. Mater. Sci. Eng. 2017, 167, 012053. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Huber, G.W.; Sánchez-Castillo, M.A.; Dumesic, J.A.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of Sn addition to Pt/CeO2–Al2O3 and Pt/Al2O3 catalysts: An XPS, 119Sn Mössbauer and microcalorimetry study. J. Catal. 2006, 241, 378–388. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Weber, W.H.; Gandhi, H.S. Surface characterization of alumina-supported ceria. J. Phys. Chem. 1988, 92, 4964–4970. [Google Scholar] [CrossRef]
- Park, P.W.; Ledford, J.S. Effect of Crystallinity on the Photoreduction of Cerium Oxide: A Study of CeO2 and Ce/Al2O3 Catalysts. Langmuir 1996, 12, 1794–1799. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S.I. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Hongmanorom, P.; Wang, Z.; Zhang, S.; Chen, J.; Zhong, Q.; Kawi, S. Active sites adjustable phosphorus promoted CeO2/TiO2 catalysts for selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2021, 409, 128242. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, X.; Zhang, Y.; Xie, C.; Zhou, S.; Wang, R.; Luo, S.-Z.; Jing, F.; Chu, W. Various Metals (Ce, In, La, and Fe) Promoted Pt/Sn-SBA-15 as Highly Stable Catalysts for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2019, 58, 10804–10818. [Google Scholar] [CrossRef]
- Ji, Z.; Miao, D.; Gao, L.; Pan, X.; Bao, X. Effect of pH on the catalytic performance of PtSn/B-ZrO2 in propane dehydrogenation. Chin. J. Catal. 2020, 41, 719–729. [Google Scholar] [CrossRef]
- Wu, J.; Sharada, S.M.; Ho, C.; Hauser, A.W.; Head-Gordon, M.; Bell, A.T. Ethane and propane dehydrogenation over PtIr/Mg(Al)O. Appl. Catal. A Gen. 2015, 506, 25–32. [Google Scholar] [CrossRef]
- Xiong, H.; Lin, S.; Goetze, J.; Pletcher, P.; Guo, H.; Kovarik, L.; Artyushkova, K.; Weckhuysen, B.M.; Datye, A.K. Thermally Stable and Regenerable Platinum–Tin Clusters for Propane Dehydrogenation Prepared by Atom Trapping on Ceria. Angew. Chem. Int. Ed. 2017, 56, 8986–8991. [Google Scholar] [CrossRef]
- Festa, G.; Serrano-Lotina, A.; Meloni, E.; Portela, R.; Ruocco, C.; Martino, M.; Palma, V. Support Screening to Shape Propane Dehydrogenation SnPt-Based Catalysts. Ind. Eng. Chem. Res. 2024, 63, 16269–16284. [Google Scholar] [CrossRef]
- Jung, J.-W.; Kim, W.-I.; Kim, J.-R.; Oh, K.; Koh, H.L. Effect of Direct Reduction Treatment on Pt–Sn/Al2O3 Catalyst for Propane Dehydrogenation. Catalysts 2019, 9, 446. [Google Scholar] [CrossRef]
- Liu, L.; Lopez-Haro, M.; Lopes, C.W.; Li, C.; Concepcion, P.; Simonelli, L.; Calvino, J.J.; Corma, A. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 2019, 18, 866–873. [Google Scholar] [CrossRef]
- Chen, X.; Ge, M.; Li, Y.; Liu, Y.; Wang, J.; Zhang, L. Fabrication of highly dispersed Pt-based catalysts on γ-Al2O3 supported perovskite Nano islands: High durability and tolerance to coke deposition in propane dehydrogenation. Appl. Surf. Sci. 2019, 490, 611–621. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Q.; Chen, L.; Liu, Q.; Zhang, X.; Zhang, Q.; Ma, L.; Wang, C. Enhanced catalytic performance of PtSn catalysts for propane dehydrogenation by a Zn-modified Mg(Al)O support. Fuel Process. Technol. 2020, 198, 106222. [Google Scholar] [CrossRef]
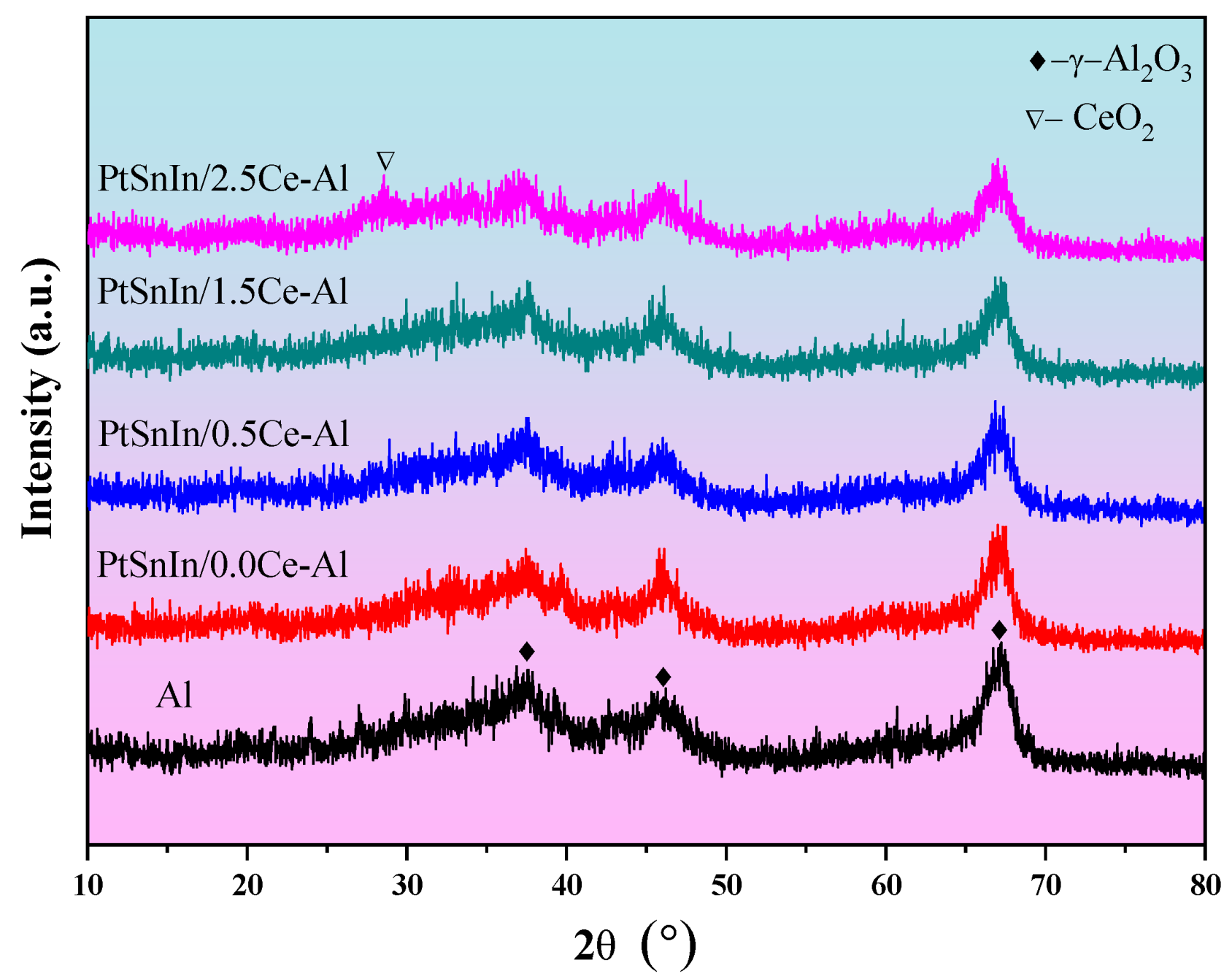
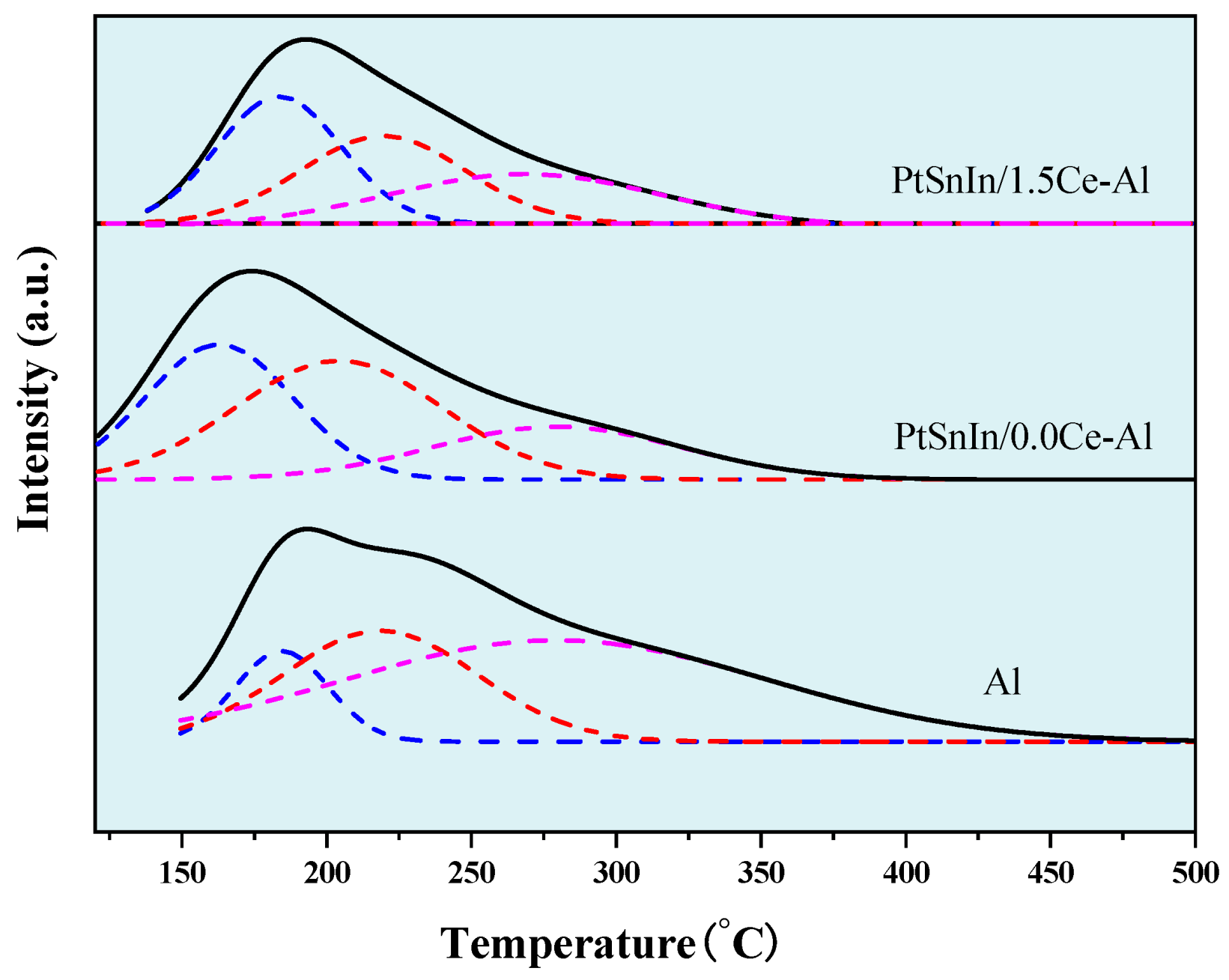
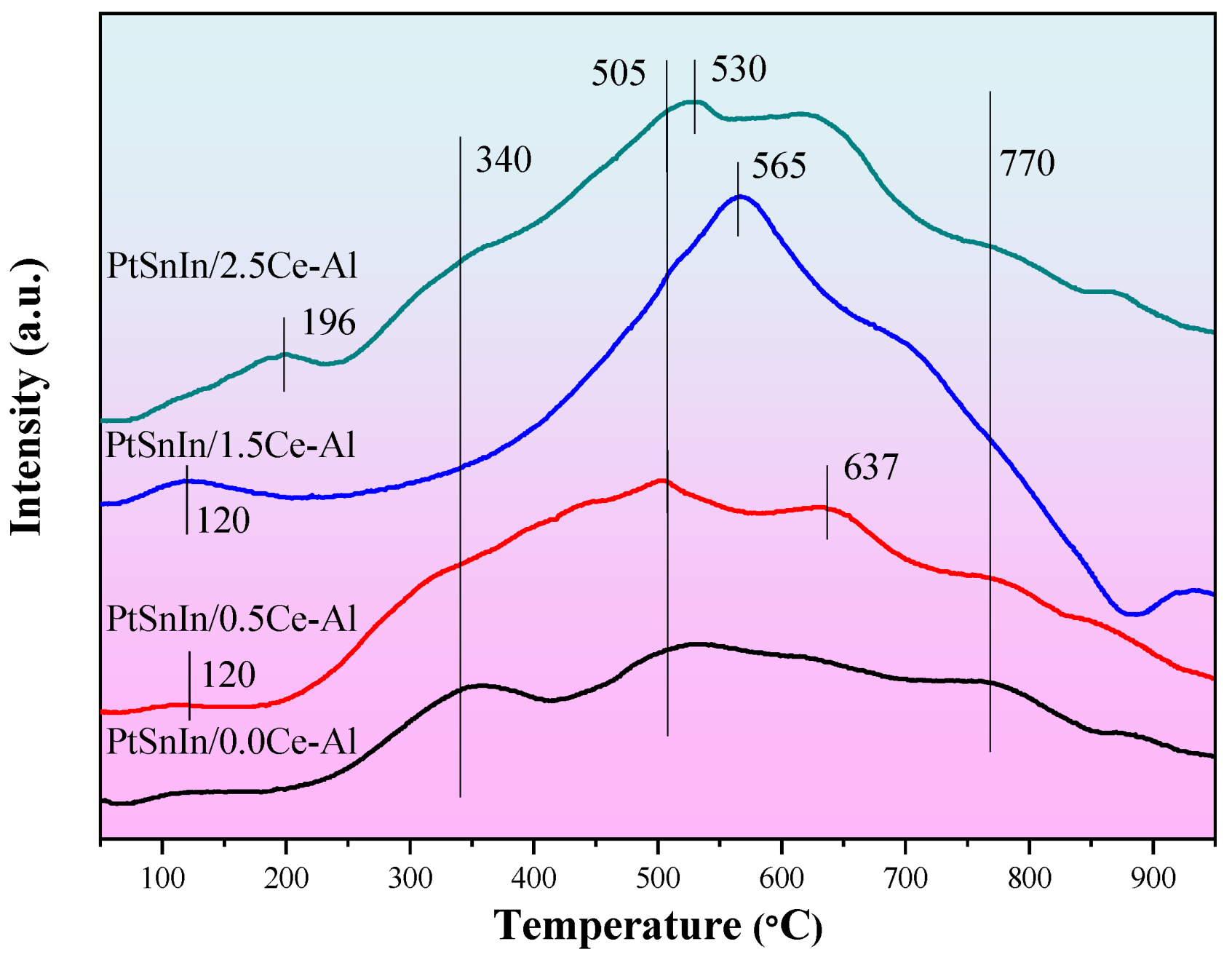


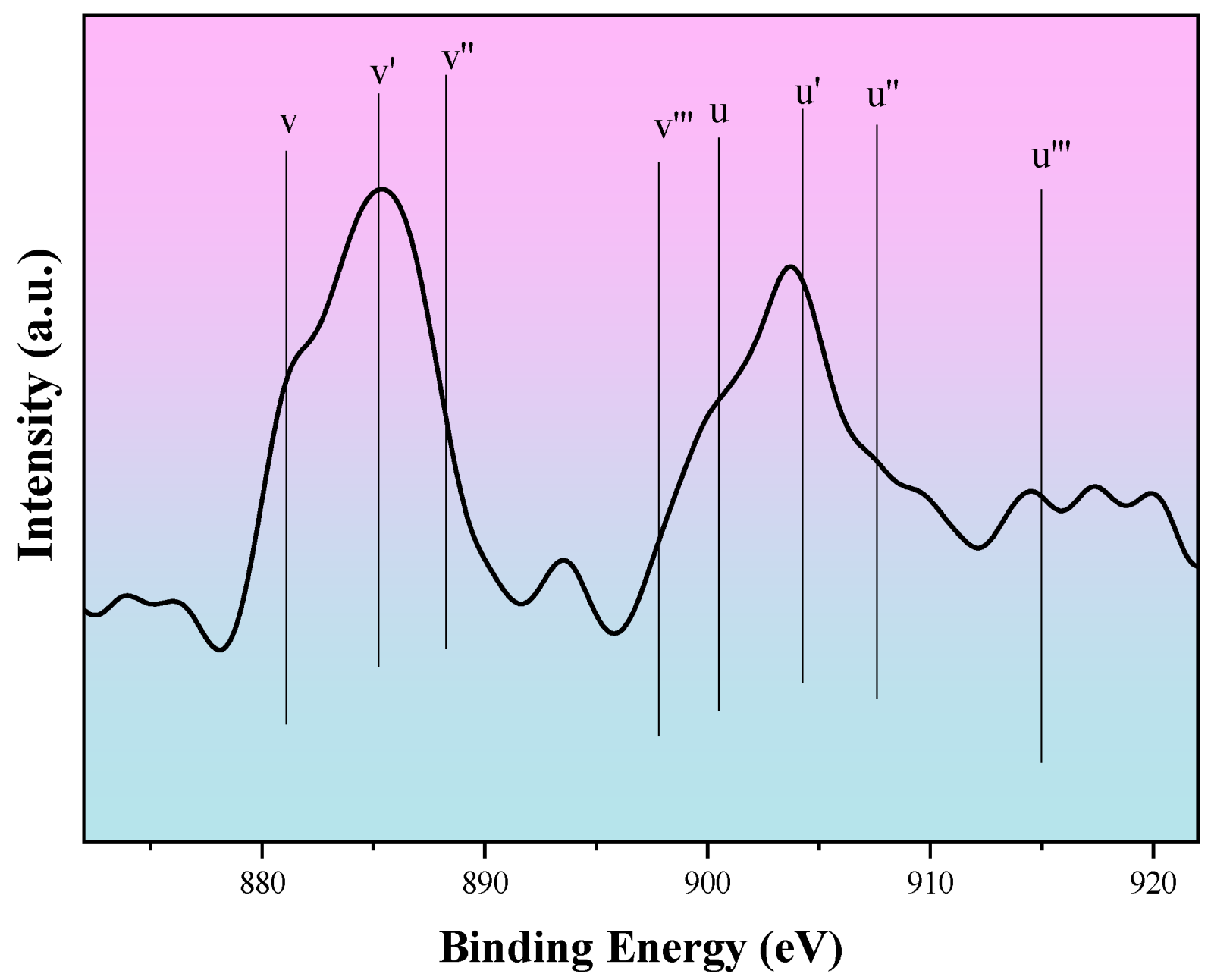
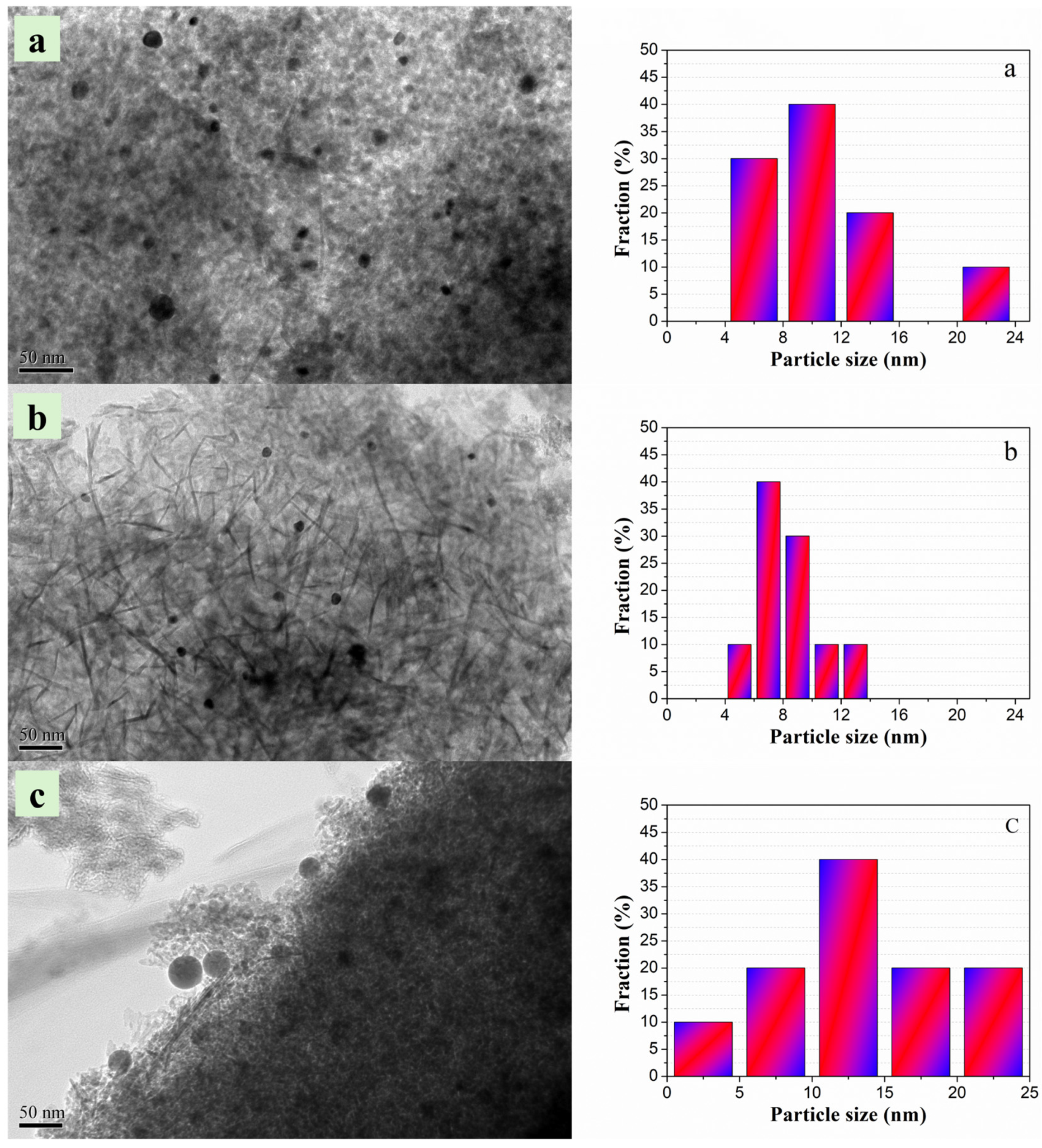


| Sample | S BET (m2g−1) | V P (cm3g−1) | Dpore (nm) | Average Pt Particle Size (nm) |
|---|---|---|---|---|
| Al | 200.844 | 0.472 | 6.655 | / |
| PtSnIn/0.0Ce-Al | 181.867 | 0.436 | 6.662 | 10.8 |
| PtSnIn/0.5Ce-Al | 188.047 | 0.489 | 5.980 | - |
| PtSnIn/1.5Ce-Al | 191.794 | 0.481 | 6.620 | 8.0 |
| PtSnIn/2.5Ce-Al | 184.899 | 0.466 | 5.450 | 11.3 |
| Sample | Tm (°C) | Total Area (a.u.) | Peak Area Fraction (%) | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | ||
| Al | 184 | 222 | 285 | 120.58 | 12 | 29 | 59 |
| PtSnIn/0.0Ce-Al | 163 | 204 | 279 | 91.94 | 35 | 42 | 23 |
| PtSnIn/1.5Ce-Al | 184 | 220 | 269 | 70.55 | 36 | 33 | 31 |
| Catalyst | Binding Energy (eV) | |
|---|---|---|
| Sn 3d5/2 | In 3d5/2 | |
| PtSnIn/Al | 485.7 (26%); 486.6 (40%); 487.5 (34%); | 444.1 (30%) 445.2 (70%) |
| PtSnIn/1.5Ce-Al | 485.2 (14%); 486.7 (50%); 487.6 (36%); | 444.4 (31%) 445.3 (69%) |
| Catalyst | Pt wt. % | Reaction Conditions | Time on Stream Test (h) | Propane Conversion (%) | Propylene Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|
| InPt/Sn-SBA-15 | 1 | 580 °C C3H8/Ar = 1/4 WHSV = 4.05 h−1 | 33 | 40.9 | 99.0 | [50] |
| Pt-Sn/B-ZrO2-10 | 0.35 | 550 °C C3H8/H2/N2 = 1/1/8 WHSV = 3 h−1 | 5 | 35.0 | 99.5 | [51] |
| Pt-Ir/Mg(Al)O | 1.91 | 600 °C C3H8/H2/He = 1/1/8 WHSV = 51.9 h−1 | 0.5 | 17.5 | 88.7 | [52] |
| Pt-Sn/CeO2 | 1 | 680 °C C3H8/H2 = 16.7/83.3 WHSV = 2.2 h−1 | 6 | 34.6 | 85.7 | [53] |
| SnPt_10B20S70A | 0.5 | 550 °C C3H8/H2O = 4/1 WHSV = 4 h−1 | 24 | 21.9 | 96.2 | [54] |
| Pt-Sn/Al2O3 | 3 | 600 °C C3H8/H2/N2 = 3/3/7 WHSV = 8.9 h−1 | 5 | 32.9 | 94.0 | [55] |
| Pt-Sn-K-Co0.3-Zn0.7/γ-Al2O3 | 0.5 | 620 °C C3H8/H2 = 1.2/1 WHSV = 2 h−1 | 10 | 42.7 | 90.0 | [31] |
| K-PtSn@MFI | 0.4 | 600 °C C3H8/N2 = 5/16 WHSV = 1.85 h−1 | 65 | 47.2 | 97.5 | [56] |
| PtIn/LaAlO/AlO | 0.6 | 600 °C C3H8/H2/N2 = 8/7/35 WHSV = 3 h−1 | 16 | 27.8 | 90.0 | [57] |
| PtSn-Mg(3Zn)AlO | 0.5 | 550 °C C3H8/H2/N2 = 20/3/80 WHSV = 6.3 h−1 | 14 | 43.7 | 99.5 | [58] |
| PtSnIn/1.5Ce-Al | 0.3 | 620 °C C3H8/H2/Ar = 8/7/35 WHSV = 3.3 h−1 | 53 | 40.0 | 96.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xia, K.; Zhang, F. Enhancing Propane Dehydrogenation Performance on Cerium-Modified PtSnIn/Al Trimetallic Catalysts. Catalysts 2025, 15, 506. https://doi.org/10.3390/catal15050506
Liu J, Xia K, Zhang F. Enhancing Propane Dehydrogenation Performance on Cerium-Modified PtSnIn/Al Trimetallic Catalysts. Catalysts. 2025; 15(5):506. https://doi.org/10.3390/catal15050506
Chicago/Turabian StyleLiu, Jinbao, Ke Xia, and Fen Zhang. 2025. "Enhancing Propane Dehydrogenation Performance on Cerium-Modified PtSnIn/Al Trimetallic Catalysts" Catalysts 15, no. 5: 506. https://doi.org/10.3390/catal15050506
APA StyleLiu, J., Xia, K., & Zhang, F. (2025). Enhancing Propane Dehydrogenation Performance on Cerium-Modified PtSnIn/Al Trimetallic Catalysts. Catalysts, 15(5), 506. https://doi.org/10.3390/catal15050506







