Sustainable MgO Nanocatalyst Additives for Boosting Performance and Mitigating Emissions of Used Cooking Oil Biodiesel–Diesel Blends in Compression Ignition Engines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Setup and Measurement Technology
2.2. Performance Parameters
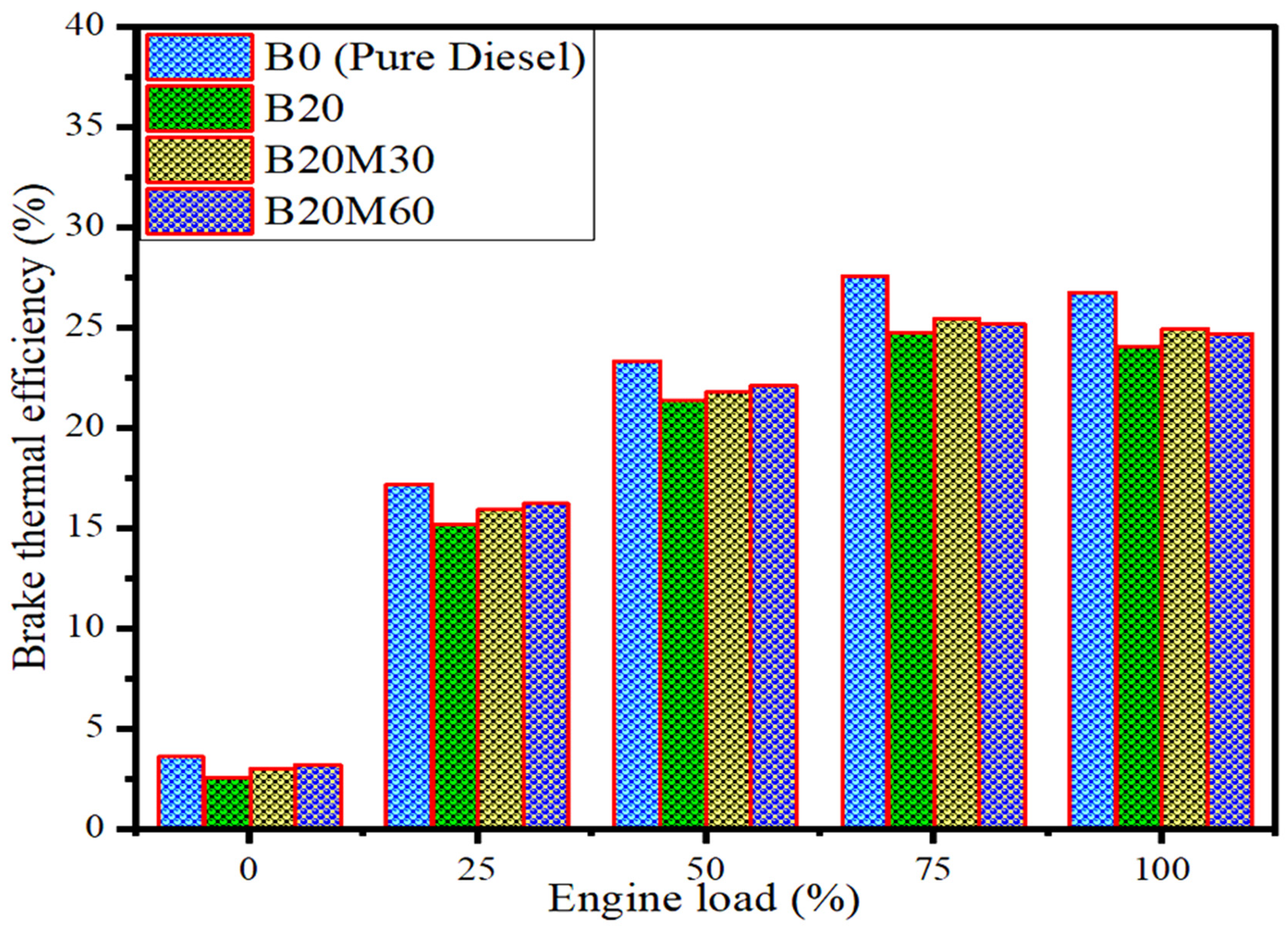
2.2.1. Brake Thermal Efficiency (BTE)
2.2.2. Brake-Specific Fuel Consumption (BSFC)
2.2.3. Exhaust Gas Temperature
2.3. Emission Parameters
2.3.1. Brake-Specific Unburned Hydrocarbons (HCs)
2.3.2. Brake-Specific Carbon Monoxide (CO)
2.4. Brake-Specific Oxides of Nitrogen
Smoke Opacity
3. Materials, Methods, and Characterization
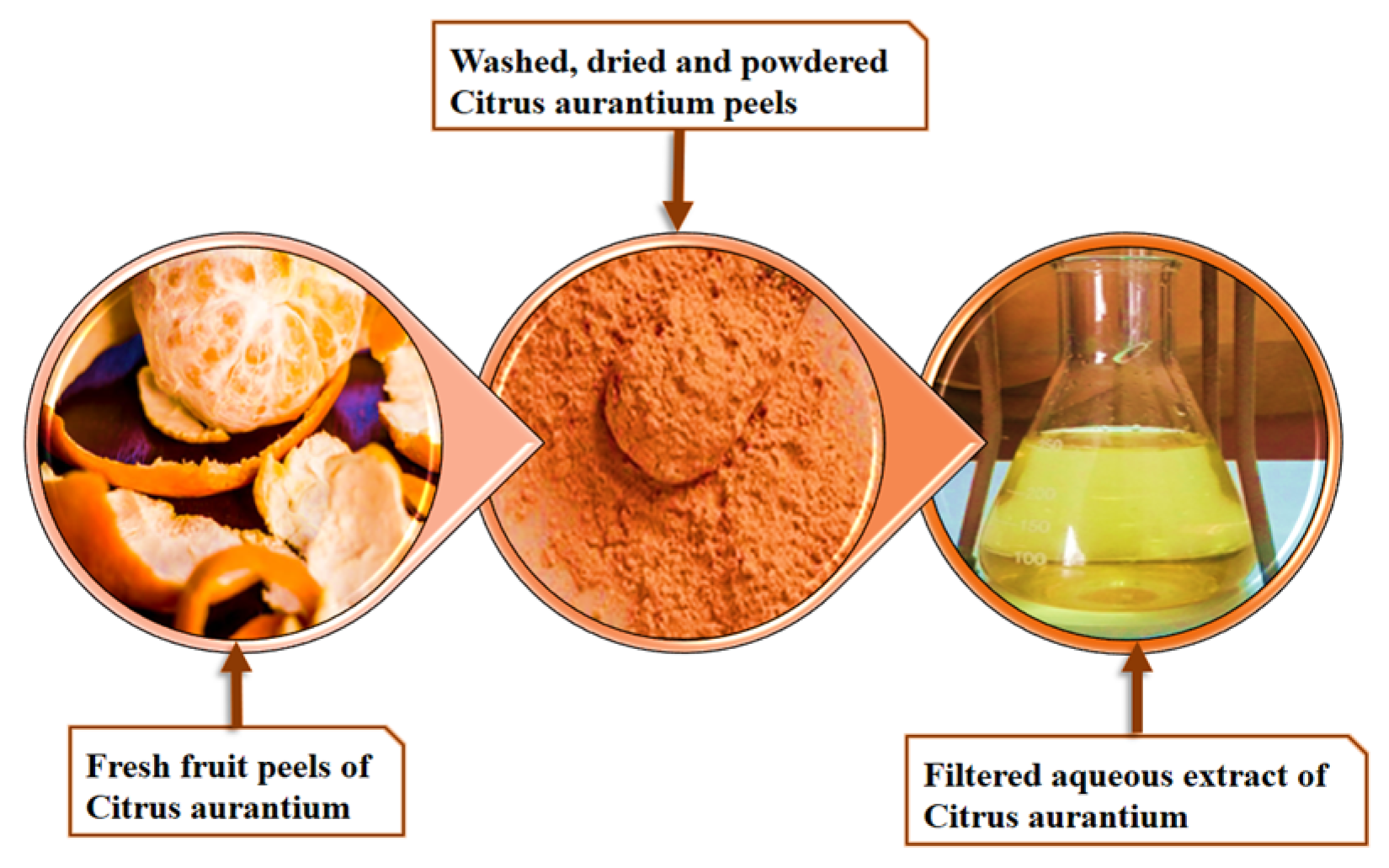
3.1. Preparation of Aqueous Extract from Citrus Aurantium Fruit Peel
3.2. Synthesis of MgO Particles
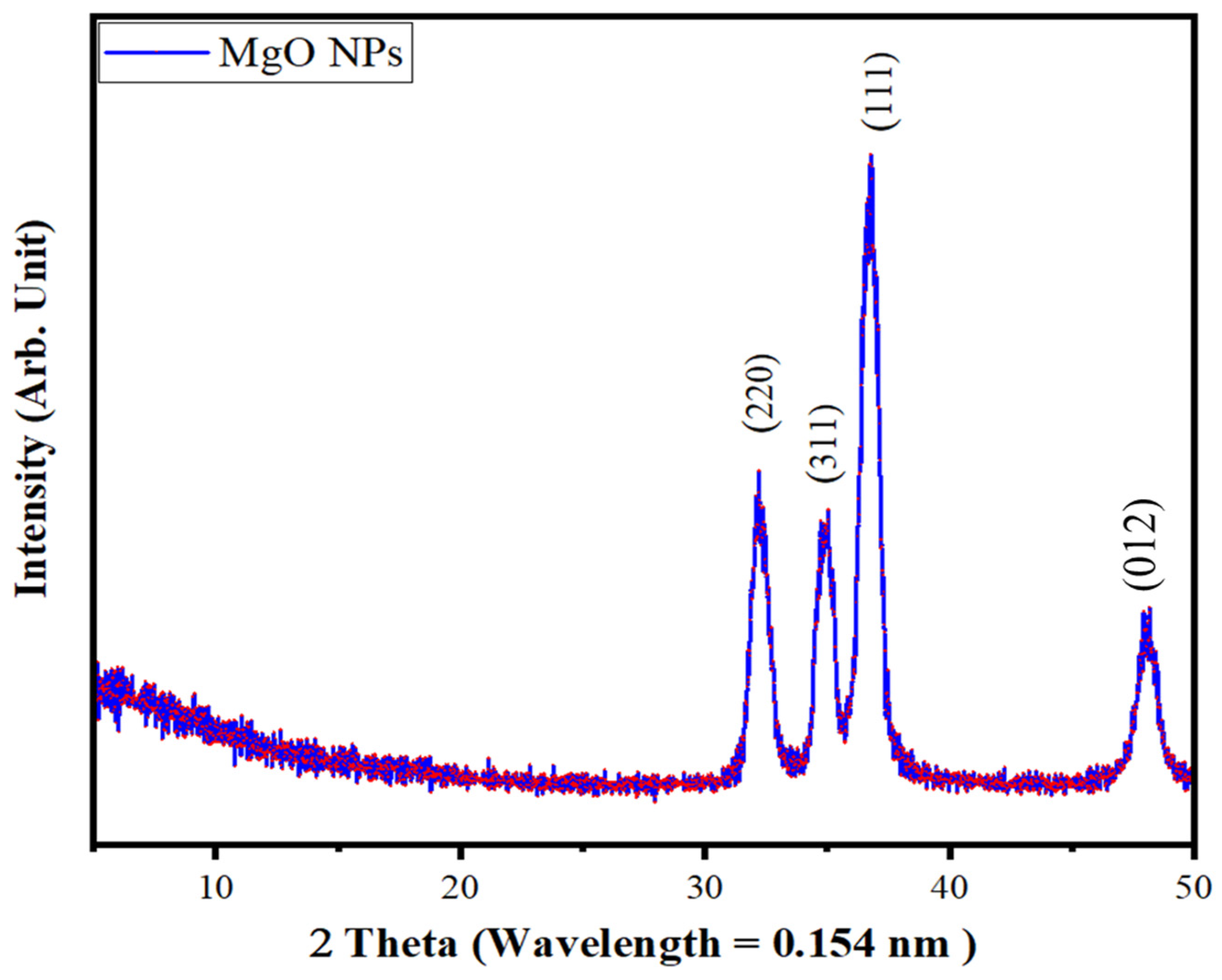
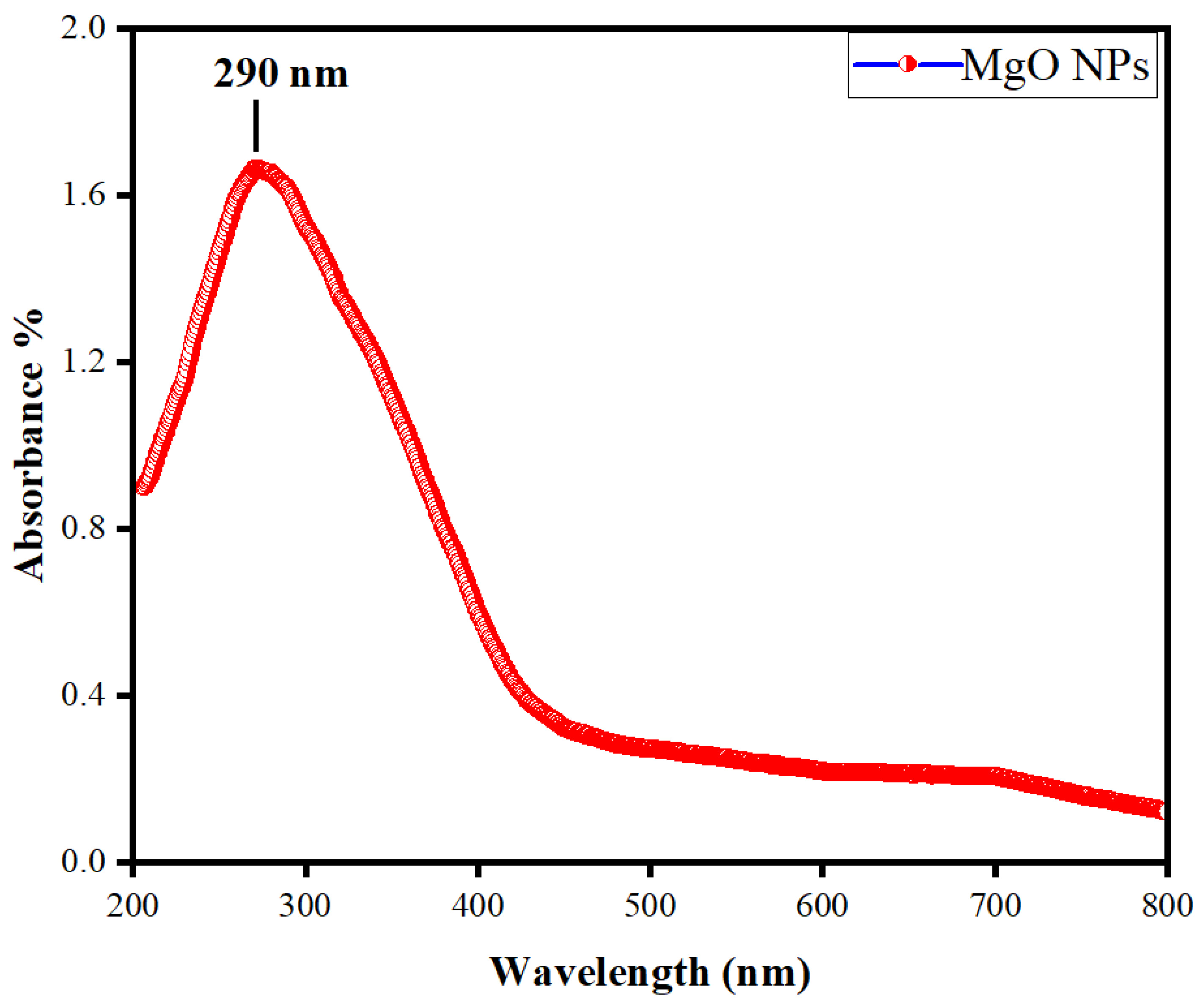
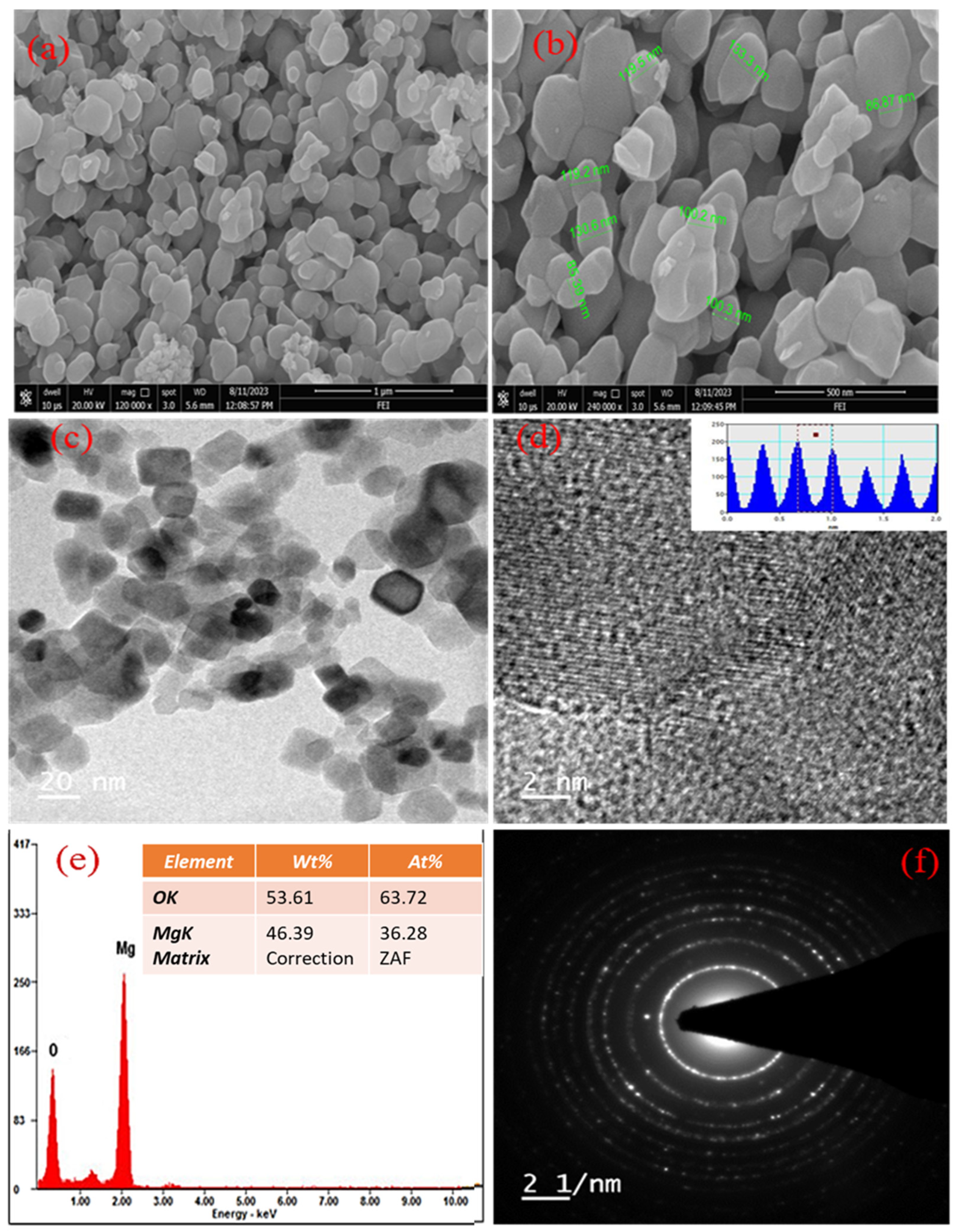
3.3. Characterization of MgO Particles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Deng, Z.; Davis, S.J.; Giron, C.; Ciais, P. Monitoring Global Carbon Emissions in 2021. Nat. Rev. Earth Environ. 2022, 3, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Mandal, B.K. A Comprehensive Review of Biodiesel as an Alternative Fuel for Compression Ignition Engine. Renew. Sustain. Energy Rev. 2016, 57, 799–821. [Google Scholar] [CrossRef]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on Feedstock, Technologies, Catalyst and Reactor for Sustainable Biodiesel Production: A Review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Leong, W.H.; Lim, J.W.; Lam, M.K.; Uemura, Y.; Ho, Y.C. Third Generation Biofuels: A Nutritional Perspective in Enhancing Microbial Lipid Production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2023, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Sorate, K.A.; Bhale, P.V. Biodiesel Properties and Automotive System Compatibility Issues. Renew. Sustain. Energy Rev. 2015, 41, 777–798. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.; Gao, Y. Effect of Adding Biodiesel to Diesel on the Physical and Chemical Properties and Engine Performance of Fuel Blends. J. Biobased Mater. Bioenergy 2016, 10, 34–43. [Google Scholar] [CrossRef]
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The Meaning of Net Zero and How to Get It Right. Nat. Clim. Change 2022, 12, 15–21. [Google Scholar] [CrossRef]
- Roy, M.M.; Chandra, A. Promoting Biofuels: The Case of Ethanol Blending Initiative in India. Clean Technol. Environ. Policy 2019, 21, 953–965. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on Transesterification of Non-Edible Sources for Biodiesel Production with a Focus on Economic Aspects, Fuel Properties and by-Product Applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Farooq, M.U.; Jamil, M.A.; Kausar, Z.; Sabah, N.U.; Shah, M.F.; Rehman, H.Z.U.; Rehman, A.U. Potential of Waste Cooking Oil Biodiesel as Renewable Fuel in Combustion Engines: A Review. Energies 2021, 14, 2565. [Google Scholar] [CrossRef]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel—Indian Perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- Kiran, C.; Nilesh P., S.; Vijay R., D. A Comprehensive Review on Performance Improvement of Diesel and Biodiesel Fueled CI Engines Using Additives. Int. J. Perform. Eng. 2021, 17, 815. [Google Scholar] [CrossRef]
- Lee, C.C.; Tran, M.V.; Tan, B.T.; Scribano, G.; Chong, C.T. A Comprehensive Review on the Effects of Additives on Fundamental Combustion Characteristics and Pollutant Formation of Biodiesel and Ethanol. Fuel 2021, 288, 119749. [Google Scholar] [CrossRef]
- Venkatesan, H.; Sivamani, S.; Sampath, S.; Gopi, V.; Dinesh Kumar, M. A Comprehensive Review on the Effect of Nano Metallic Additives on Fuel Properties, Engine Performance and Emission Characteristics. Int. J. Renew. Energy Res. 2017, 7, 825–843. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Ciprioti, S.V. Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method. Materials 2018, 11, 2326. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, V.K.; Patil, S.R.; Suryawanshi, K.E.; Isai, K.A.; Sonawane, M.S.; Patil, P.N.; Nandre, S.S. Synthesis and Characterization of CdS Nanoparticles and Study of Its Antibacterial Activity Against Methicillin-Resistant Staphylococcus Aureus (MRSA). Chem. Afr. 2023, 6, 2537–2659. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Abdel-Rahman, A.A.; Elwardany, A.E. Analysis of the Impact of Different Nanoparticle Metal Oxides as Fuel Additives in Compression Ignition Engine Performance. Energy Rep. 2020, 6, 99–105. [Google Scholar] [CrossRef]
- Sivaram, A.R.; Prabhu, N.; Praveen Kumar, R.; Navaneethakrishnan, G.; Palanisamy, R.; AboRas, K.M.; Bajaj, M.; Djidimbele, R.; Dieudonné, K.K.; Vinodhan, V.L. Performance Analysis of Compression Ignition Engines Using Emulsion Fuel Blended with Aluminium Oxide Nanoparticles. Adv. Mater. Sci. Eng. 2023, 2023, 1–6. [Google Scholar] [CrossRef]
- Tamrat, S.; Ancha, V.R.; Gopal, R.; Nallamothu, R.B.; Seifu, Y. Emission and Performance Analysis of Diesel Engine Running with CeO2 Nanoparticle Additive Blended into Castor Oil Biodiesel as a Substitute Fuel. Sci. Rep. 2024, 14, 7634. [Google Scholar] [CrossRef]
- Koca, S.; Zincirci, O.; Aktaş, F. Investigation of the Effect of TiO2 Nanoparticles on Engine Performance and Emission Characteristics in Diesel Engines. Int. J. Automot. Sci. Technol. 2024, 8, 242–251. [Google Scholar] [CrossRef]
- Sarvestani, N.S.; Tabasizadeh, M.; Abbaspour Fard, M.H.; Nayebzadeh, H.; Van, T.C.; Jafari, M.; Bodisco, T.A.; Ristovski, Z.; Brown, R.J. Effects of Enhanced Fuel with Mg-Doped Fe3O4 Nanoparticles on Combustion of a Compression Ignition Engine: Influence of Mg Cation Concentration. Renew. Sustain. Energy Rev. 2021, 141, 110817. [Google Scholar] [CrossRef]
- Muniyappan, S.; Krishnaiah, R. Investigation on CuO Nanoparticle Enhanced Mahua Biodiesel/Diesel Fuelled CI Engine Combustion for Improved Performance and Emission Abetted by Response Surface Methodology. Sci. Rep. 2024, 14, 26882. [Google Scholar] [CrossRef]
- Bazooyar, B.; Hosseini, S.Y.; Moradi Ghoje Begloo, S.; Shariati, A.; Hashemabadi, S.H.; Shaahmadi, F. Mixed Modified Fe2O3-WO3 as New Fuel Borne Catalyst (FBC) for Biodiesel Fuel. Energy 2018, 149, 438–453. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Khan, N. Enhancement of Combustion Behaviour of Waste Cooking Oil Biodiesel Fuelled Engine Using Metallic Oxide Nanoparticle with Varying Volume Concentration: An Experimental Study. Int. J. Renew. Energy Res. 2021, 11, 101–107. [Google Scholar] [CrossRef]
- Jit Sarma, C.; Sharma, P.; Bora, B.J.; Bora, D.K.; Senthilkumar, N.; Balakrishnan, D.; Ayesh, A.I. Improving the Combustion and Emission Performance of a Diesel Engine Powered with Mahua Biodiesel and TiO2 Nanoparticles Additive. Alex. Eng. J. 2023, 72, 387–398. [Google Scholar] [CrossRef]
- Kalaimurugan, K.; Karthikeyan, S.; Periyasamy, M.; Mahendran, G.; Dharmaprabhakaran, T. Experimental Studies on the Influence of Copper Oxide Nanoparticle on Biodiesel-Diesel Fuel Blend in CI Engine. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 8997–9012. [Google Scholar] [CrossRef]
- Kanimozhi, B.; Karthikeyan, L.; Praveenkumar, T.R.; Ali Alharbi, S.; Alfarraj, S.; Gavurová, B. Evaluation of Karanja and Safflower Biodiesel on Engine’s Performance and Emission Characteristics along with Nanoparticles in DI Engine. Fuel 2023, 352, 129101. [Google Scholar] [CrossRef]
- Arun, S.B.; Karthik, B.M.; Yatish, K.V.; Prashanth, K.N.; Balakrishna, G.R. Green Synthesis of Copper Oxide Nanoparticles Using the Bombax Ceiba Plant: Biodiesel Production and Nano-Additive to Investigate Diesel Engine Performance-Emission Characteristics. Energy 2023, 274, 127354. [Google Scholar] [CrossRef]
- Rajesh Kana, S.; Shaija, A. Performance, Combustion and Emission Characteristics of a Diesel Engine Using Waste Avocado Biodiesel with Manganese-Doped Alumina Nanoparticles. Int. J. Ambient. Energy 2022, 43, 1437–1444. [Google Scholar] [CrossRef]
- Aalam, C.S.; Saravanan, C.G.; Kannan, M. Experimental Investigations on a CRDI System Assisted Diesel Engine Fuelled with Aluminium Oxide Nanoparticles Blended Biodiesel. Alex. Eng. J. 2015, 54, 351–358. [Google Scholar] [CrossRef]
- Sateesh, K.A.; Yaliwal, V.S.; Soudagar, M.E.M.; Banapurmath, N.R.; Fayaz, H.; Safaei, M.R.; Elfasakhany, A.; EL-Seesy, A.I. Utilization of Biodiesel/Al2O3 Nanoparticles for Combustion Behavior Enhancement of a Diesel Engine Operated on Dual Fuel Mode. J. Therm. Anal. Calorim. 2022, 147, 5897–5911. [Google Scholar] [CrossRef]
- Sundar, S.S.P.; Vijayabalan, P.; Sathyamurthy, R.; Kabeel, A.E.; Kamalakkannan, K. An Experimental Approach on the Utilization of Palm Oil Biodiesel with Higher Concentration of Al2O3 Nanoadditive for Performance Enhancement and Emission Reduction. Environ. Sci. Pollut. Res. 2022, 29, 89411–89425. [Google Scholar] [CrossRef] [PubMed]
- Zamankhan, F.; Pirouzfar, V.; Ommi, F.; Valihesari, M. Investigating the Effect of MgO and CeO2 Metal Nanoparticle on the Gasoline Fuel Properties: Empirical Modeling and Process Optimization by Surface Methodology. Environ. Sci. Pollut. Res. 2018, 25, 22889–22902. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, E.; Dhairiyasamy, R.; Dixit, S.; Singh, S. The Impact of Nanoparticle-Diesel Blends on Fuel Properties, Combustion Efficiency, and Emissions. Case Stud. Therm. Eng. 2025, 69, 106070. [Google Scholar] [CrossRef]
- Çılğın, E. Synergistic Effects of SWCNT and MgO Nanoparticle Additives on Engine Performance and Emissions: A Laboratory Analysis Approach. Biofuels 2025, 1–21. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Microstructure, Optical and Photocatalytic Properties of MgO Nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Kumar, P.P.; Bhatlu, M.L.D.; Sukanya, K.; Karthikeyan, S.; Jayan, N. Synthesis of Magnesium Oxide Nanoparticle by Eco Friendly Method (Green Synthesis)–A Review. Mater. Today Proc. 2021, 37, 3028–3030. [Google Scholar] [CrossRef]
- Chaudhari, K.; Salunke, N.; Diware, V. Multi Objective Optimization of Diesel Engine Performance and Emission Characteristics Using Taguchi-Grey Relational Analysis. Int. J. Adv. Technol. Eng. Explor. 2023, 10, 124303. [Google Scholar] [CrossRef]
- Sudarsanam, M.; Jayaprabakar, J. Effect of Alumina and Bio-Based Calcium Oxide Nanoadditives on Reduction of Emissions and Performance Improvement in a Common Rail Direct Injection Diesel Engine Fueled with B20 Blend of Waste Cooking Oil Biodiesel. Energy Technol. 2024, 12, 2301107. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative Study of Nanoparticles and Alcoholic Fuel Additives-Biodiesel-Diesel Blend for Performance and Emission Improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Jegan, C.D.; Selvakumaran, T.; Karthe, M.; Hemachandu, P.; Gopinathan, R.; Sathish, T.; Ağbulut, Ü. Influences of Various Metal Oxide-Based Nanosized Particles-Added Algae Biodiesel on Engine Characteristics. Energy 2023, 284, 128633. [Google Scholar] [CrossRef]
- Mofijur, M.; Ahmed, S.F.; Ahmed, B.; Mehnaz, T.; Mehejabin, F.; Shome, S.; Almomani, F.; Chowdhury, A.A.; Kalam, M.A.; Badruddin, I.A.; et al. Impact of Nanoparticle-Based Fuel Additives on Biodiesel Combustion: An Analysis of Fuel Properties, Engine Performance, Emissions, and Combustion Characteristics. Energy Convers. Manag. X 2024, 21, 100515. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Rosen, M.A. Effect of Injection Pressure on the Combustion, Performance and Emission Characteristics of a Biodiesel Engine with Cerium Oxide Nanoparticle Additive. Energy 2019, 185, 1163–1173. [Google Scholar] [CrossRef]
- Reddy, M.J.; Sai Rakesh, N.; Jayaraman, J.; Vijai Anand, K.; Appavu, P.; Arunkumar, T. Effect of Novel Bio-Waste Derived Nano Particles as Additives on the Performance of Diesel Engine Fuelled with Waste Cooking Oil Biodiesel Blends. Mater. Today Proc. 2021, 44, 3530–3535. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Wang, Y.; Yang, Z.; Zhao, H.; Ding, Y. Light-Duty Vehicle Organic Gas Emissions from Tailpipe and Evaporation: A Review of Influencing Factors. Sci. Total Environ. 2024, 947, 174523. [Google Scholar] [CrossRef]
- Manigandan, S.; Ponnusamy, V.K.; Devi, P.B.; Oke, S.A.; Sohret, Y.; Venkatesh, S.; Vimal, M.R.; Gunasekar, P. Effect of Nanoparticles and Hydrogen on Combustion Performance and Exhaust Emission of Corn Blended Biodiesel in Compression Ignition Engine with Advanced Timing. Int. J. Hydrogen Energy 2020, 45, 3327–3339. [Google Scholar] [CrossRef]
- Kumar, S.; Dinesha, P.; Bran, I. Influence of Nanoparticles on the Performance and Emission Characteristics of a Biodiesel Fuelled Engine: An Experimental Analysis. Energy 2017, 140, 98–105. [Google Scholar] [CrossRef]
- H S, A.P.; Mohanty, D.K. Enhancement of Combustion, Performance and Emission Characteristics of Diesel Engines Fuelled with Jatropha-Karanja Biodiesel Using EGM and TGME as Additive. Energy 2024, 300, 131523. [Google Scholar] [CrossRef]
- Bitire, S.O.; Jen, T.-C. Performance and Emission Analysis of a CI Engine Fueled with Parsley Biodiesel–Diesel Blend. Mater Renew Sustain. Energy 2022, 11, 143–153. [Google Scholar] [CrossRef]
- Tuan Hoang, A.; Xuan Le, M.; Nižetić, S.; Huang, Z.; Ağbulut, Ü.; Veza, I.; Said, Z.; Tuan Le, A.; Dung Tran, V.; Phuong Nguyen, X. Understanding Behaviors of Compression Ignition Engine Running on Metal Nanoparticle Additives-Included Fuels: A Control Comparison between Biodiesel and Diesel Fuel. Fuel 2022, 326, 124981. [Google Scholar] [CrossRef]
- Prabu, A. Nanoparticles as Additive in Biodiesel on the Working Characteristics of a DI Diesel Engine. Ain Shams Eng. J. 2018, 9, 2343–2349. [Google Scholar] [CrossRef]
- Hoang, A.T. Combustion Behavior, Performance and Emission Characteristics of Diesel Engine Fuelled with Biodiesel Containing Cerium Oxide Nanoparticles: A Review. Fuel Process. Technol. 2021, 218, 106840. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Mahalingam, A. Influence of Nano-Additive on Performance and Emission Characteristics of a Diesel Engine Running on Neat Neem Oil Biodiesel. Environ. Sci. Pollut. Res. 2018, 25, 26167–26172. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Garg, M. Comprehensive Review of Combustion, Performance and Emissions Characteristics of a Compression Ignition Engine Fueled with Hydroprocessed Renewable Diesel. Renew. Sustain. Energy Rev. 2018, 81, 2947–2954. [Google Scholar] [CrossRef]
- Bari, S.; Hossain, S.N.; Saad, I. A Review on Improving Airflow Characteristics inside the Combustion Chamber of CI Engines to Improve the Performance with Higher Viscous Biofuels. Fuel 2020, 264, 116769. [Google Scholar] [CrossRef]
- Rathinam, S.; Sajin, J.B.; Subbiah, G.; Rajeev, A.; Prakash, S.; Christopherselvam, D. Assessment of the Emission Characteristics of the Diesel Engine with Nano-Particle in Neem Biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2623–2631. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Chintala, V.; Khatri, N.; Patel, A. Environment-Friendly Biodiesel/Diesel Blends for Improving the Exhaust Emission and Engine Performance to Reduce the Pollutants Emitted from Transportation Fleets. Int. J. Environ. Res. Public Health 2020, 17, 3896. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rasul, M.; Hassan, N.M.S.; Azad, A.K.; Uddin, M.N. Effect of Small Proportion of Butanol Additive on the Performance, Emission, and Combustion of Australian Native First- and Second-Generation Biodiesel in a Diesel Engine. Environ. Sci. Pollut. Res. 2017, 24, 22402–22413. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.; Nagappan, B.; Ganesan, S. Detailed Study on the Effect of Different Ignition Enhancers in the Binary Blends of Diesel/Biodiesel as a Possible Substitute for Unaltered Compression Ignition Engine. Pet. Sci. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Pullagura, G.; Bikkavolu, J.; Vadapalli, S.; Chebattina, K.R.; Kuchipudi, V. Comparative Study of TiO2 Nanoparticles and Alcoholic Fuel Additives-Biodiesel-Diesel Blend for Combustion, Performance, and Emission Improvements. Int. J. Heat Technol. 2022, 40, 1249–1257. [Google Scholar] [CrossRef]
- Devarajan, Y.; Nagappan, B.; Subbiah, G. A Comprehensive Study on Emission and Performance Characteristics of a Diesel Engine Fueled with Nanoparticle-Blended Biodiesel. Environ. Sci. Pollut. Res. 2019, 26, 10662–10672. [Google Scholar] [CrossRef]
- Devaraj, A.; Devarajan, Y.; Vinoth Kanna, I. Investigation on Emission Pattern of Biodiesel and Nano-Particles. Int. J. Ambient. Energy 2021, 42, 1103–1107. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.; Mahalingam, A. Investigation on Behavior of Diesel Engine Performance, Emission, and Combustion Characteristics Using Nano-Additive in Neat Biodiesel. Heat Mass Transf. 2019, 55, 1641–1650. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Krishnan, P.L.N.; Rathinam, S.; R, T.; Devarajan, Y. Role of Nano-Additive Blended Biodiesel on Emission Characteristics of the Research Diesel Engine. Int. J. Green Energy 2019, 16, 435–441. [Google Scholar] [CrossRef]
- Sathish, T.; Giri, J.; Saravanan, R.; Zairov, R.; Hasnain, S.M.M. Nano-Fuels of Al2O3/SiO2/MgO/Tamarind Seed Oil Biodiesel for CI Engines: An Evaluation of Combustion Consumption and Emission Performance. Int. J. Thermofluids 2024, 23, 100815. [Google Scholar] [CrossRef]
- Subbiah, G.; Kumar, R.S.; Rangabashiam, D.; Janarathanam, H.; Ponnappan, V.S.; Nagappan, B.; Sreenivasan, K.S. Multi-Objective Optimization of VCR Diesel Engine Performance and Emissions Fueled with Diesel-Lime Steam Oil Blends Using Grey Relational Analysis. AIP Conf. Proc. 2020, 2311, 020034. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Naveen, V.; Danaraj, J. Green Synthesis of Magnesium Oxide Nanoparticles with Antioxidant Potential Using the Leaf Extract of Piper Nigrum. Jom 2022, 74, 4817–4822. [Google Scholar] [CrossRef]
- Patil, P.V.; Nerlekar, N.A.; Kuldeep, A.R.; Patil, P.P.; Dandge, P.B.; Dongale, T.D.; Dandge, P.B.; Rashinkar, G.S. Terminalia Bellirica (Gaertn.) Roxb. Extract-Mediated Green Synthesis of Magnesium Oxide Nanoparticles for Multifunctional Applications. Plant Nano Biol. 2024, 8, 100069. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Punitha, V.N.; Parameswari, N. Phytonanosynthesis of MgO Nanoparticles: Green Synthesis, Characterization and Antimicrobial Evaluation. Arab. J. Sci. Eng. 2022, 47, 6729–6734. [Google Scholar] [CrossRef]
- Moeini-Nodeh, S.; Rahimifard, M.; Baeeri, M.; Abdollahi, M. Functional Improvement in Rats’ Pancreatic Islets Using Magnesium Oxide Nanoparticles Through Antiapoptotic and Antioxidant Pathways. Biol. Trace Elem. Res. 2017, 175, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Chan, Y.S.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Abd Elkodous, M.; El-Sayyad, G.S. Response Surface Methodology Optimization of Mono-Dispersed MgO Nanoparticles Fabricated by Ultrasonic-Assisted Sol–Gel Method for Outstanding Antimicrobial and Antibiofilm Activities. J. Clust. Sci. 2020, 31, 367–389. [Google Scholar] [CrossRef]







| Kirloskar Engine Test Rig | AVL Digas 444N and AVL 437C Smoke Meter | ||
|---|---|---|---|
| Measurement | Uncertainty | Measurement | Uncertainty |
| Engine load | CO (0–15% volume) | ||
| Engine power | HC (0–20,000 ppm) | ||
| Engine speed | NOx (0–6000 ppm) | ||
| BTE | Smoke opacity (0–100 in %) | ||
| BSFC | |||
| Total Uncertainty = square root of addition of squares of discrete uncertainties | |||
| Parameter | Pure Diesel | B20 | B20M30 | B20M60 |
|---|---|---|---|---|
| BTE (%) | 26.8 | 24.1 | 24.68 | 24.92 |
| BSFC (kg/kWh) | 0.3072 | 0.3289 | 0.3241 | 0.3212 |
| EGT (°C) | 386 | 419 | 398 | 392 |
| HC (g/kWh) | 0.156 | 0.140 | 0.113 | 0.108 |
| CO (g/kWh) | 6.41 | 5.0 | 4.87 | 4.66 |
| NOx (g/kWh) | 6.728 | 6.848 | 6.615 | 6.535 |
| Smoke Intensity (%) | 82 | 79 | 75 | 78 |
| Engine | Biodiesel (Blend Ratio) | Additive | Results of the Study | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BTE | BSFC | HC | CO | NOx | Smoke | Reference | |||
| 1-cylinder, DI, WC, CI engine | Used Cooking oil 20% | NA | NA | +3.2% | −50% | −21.4% | 13.5% | −34.7% | [7] |
| 1-cylinder, DI, WC, CI engine | Microalgae biodiesel, (10%) | NA | Lower | +3.2% | −5.37% | −3.1% | 1.18% | −5.45% | [58] |
| 1-cylinder, DI, WC, CI engine | Microalgae biodiesel, (10%) | NA | +3.2% | −12.41% | −8.8% | 2.21% | −9.43% | 39 | |
| 1-cylinder, DI, WC, CI engine | Karanja biodiesel 45% | Di-Tetra-Butyl-Phenol, (DTBP) 10% | −0.2% | +0.3 kg/kWhr | −4.9% | −7.6% | +1.8% | −3.2% | [59] |
| 1-cylinder, DI, WC, CI engine | Karanja biodiesel 45% | 1-Pentadecanol 10% | +0.4% | +0.3 kg/kWhr | −3.3% | −4.9% | +3.1% | −4.9% | |
| 4-cylinder, DI, WC, CI engine | Biodiesel 20% | Butanol, 5% | −6% | Higher than diesel | Slight rise in HC | −50% | −2% | −15% | [60] |
| 1-cylinder, DI, WC, CI engine | Tamarind oil methyl ester 20% | TiO2 50 ppm | +6.13% | −17.64% | −7.81% | −9.49% | −6.53% | NA | [61] |
| 1-cylinder, DI, AC, CI engine | Mahua oil | Copper oxide, 100 ppm | +0.9% | −2.1 g/kWh | −5.6% | −4.9% | −3.9% | −2.8% | [62] |
| 2-cylinder, DI, WC, CI engine | Mustard oil biodiesel | Aluminum oxide, 100 ppm | NA | NA | −2.2% | −4.3% | −4.8% | −3.4% | [63] |
| 1-cylinder, DI, AC, CI engine | Neat palm stearin biodiesel | Silver oxide, 10 ppm | +2.4% | −2.7% | −8.8% | −11.9% | −14.4% | NA | [64] |
| 1-cylinder, DI, AC, CI engine | Neem biodiesel | Carbon nano tubes, 100 ppm | NA | NA | −6.7% | −5.9% | −9.2% | −7.8% | [65] |
| 1-cylinder, DI, WC, CI engine | Waste cooking oil biodiesel 20% | Magnesium oxide, 30 ppm | −7.01% | 5.23% | −38.3% | −29.9% | +1.7% | −28.13% | Present work |
| 1-cylinder, DI, WC, CI engine | Waste cooking oil biodiesel 20% | Magnesium oxide, 60 ppm | −7.84% | 4.37% | −43.5% | −35.4% | +2.33% | −46.43% | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhari, K.; Salunke, N.; Ateequr Raheman, S.R.; Ansari, K.B.; Saner, K.A.; Suryawanshi, V.K.; Shah, M. Sustainable MgO Nanocatalyst Additives for Boosting Performance and Mitigating Emissions of Used Cooking Oil Biodiesel–Diesel Blends in Compression Ignition Engines. Catalysts 2025, 15, 489. https://doi.org/10.3390/catal15050489
Chaudhari K, Salunke N, Ateequr Raheman SR, Ansari KB, Saner KA, Suryawanshi VK, Shah M. Sustainable MgO Nanocatalyst Additives for Boosting Performance and Mitigating Emissions of Used Cooking Oil Biodiesel–Diesel Blends in Compression Ignition Engines. Catalysts. 2025; 15(5):489. https://doi.org/10.3390/catal15050489
Chicago/Turabian StyleChaudhari, Kiran, Nilesh Salunke, Shakeelur Raheman Ateequr Raheman, Khursheed B. Ansari, Kapil Ashokrao Saner, Vijay Kashinath Suryawanshi, and Mumtaj Shah. 2025. "Sustainable MgO Nanocatalyst Additives for Boosting Performance and Mitigating Emissions of Used Cooking Oil Biodiesel–Diesel Blends in Compression Ignition Engines" Catalysts 15, no. 5: 489. https://doi.org/10.3390/catal15050489
APA StyleChaudhari, K., Salunke, N., Ateequr Raheman, S. R., Ansari, K. B., Saner, K. A., Suryawanshi, V. K., & Shah, M. (2025). Sustainable MgO Nanocatalyst Additives for Boosting Performance and Mitigating Emissions of Used Cooking Oil Biodiesel–Diesel Blends in Compression Ignition Engines. Catalysts, 15(5), 489. https://doi.org/10.3390/catal15050489







