Research on Catalysts for Online Ammonia Hydrogen Production in Marine Engines: Performance Evaluation and Reaction Kinetic Modeling
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Conversion Rates Under Different Temperatures and Space Velocities
2.2. Reaction Kinetic Parameters
2.3. Analysis of the Catalytic Reaction Process
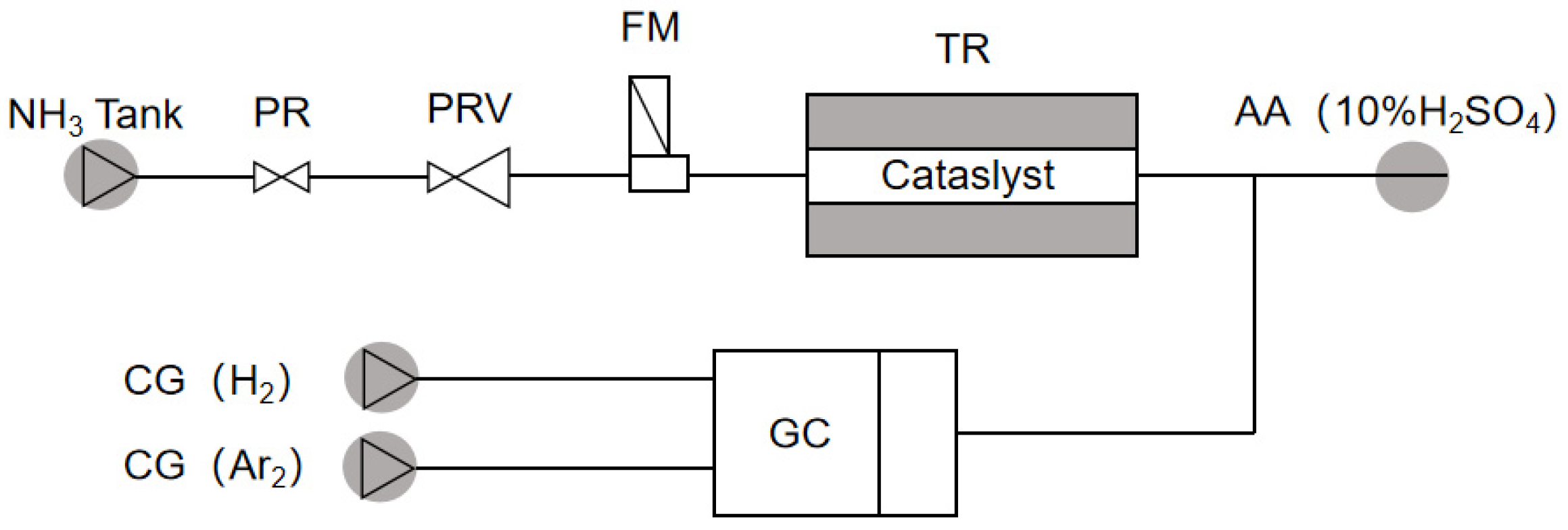
3. Experiment
3.1. Preparation of Catalysts
3.2. Catalytic Experiments
4. Kinetic Model
4.1. Law of Conservation of Mass Model
4.2. First-Order Reaction Model
4.3. Langmuir Model
4.4. Temkin–Pyzhev Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walker, T.R.; Adebambo, O.; Del Aguila Feijoo, M.C.; Elhaimer, E.; Hossain, T.; Edwards, S.J.; Morrison, C.E.; Romo, J.; Sharma, N.; Taylor, S.; et al. Environmental Effects of Marine Transportation. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 505–530. ISBN 978-0-12-805052-1. [Google Scholar]
- Hansson, J.; Brynolf, S.; Fridell, E.; Lehtveer, M. The Potential Role of Ammonia as Marine Fuel—Based on Energy Systems Modeling and Multi-Criteria Decision Analysis. Sustainability 2020, 12, 3265. [Google Scholar] [CrossRef]
- Chiong, M.-C.; Chong, C.T.; Ng, J.-H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of Combustion Technologies in the Ammonia-Fuelled Engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, Y. Numerical Investigation on the Combustion and Emission Characteristics of Ammonia in a Low-Speed Two-Stroke Marine Engine. Fuel 2022, 314, 122727. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Elishav, O.; Mosevitzky Lis, B.; Miller, E.M.; Arent, D.J.; Valera-Medina, A.; Grinberg Dana, A.; Shter, G.E.; Grader, G.S. Progress and Prospective of Nitrogen-Based Alternative Fuels. Chem. Rev. 2020, 120, 5352–5436. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gersen, S.; Glarborg, P.; Mokhov, A.; Levinsky, H. Autoignition Studies of NH3/CH4 Mixtures at High Pressure. Combust. Flame 2020, 218, 19–26. [Google Scholar] [CrossRef]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/Hydrogen Mixtures in an SI-Engine: Engine Performance and Analysis of a Proposed Fuel System. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using Ammonia as a Sustainable Fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Bartels, J.R. A Feasibility Study of Implementing an Ammonia Economy. Master’s Thesis, Iowa State University, Digital Repository, Ames, IA, USA, 2008; p. 2807317. [Google Scholar]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Van Der Gryp, P.; Bessarabov, D.G. Reactor Technology Options for Distributed Hydrogen Generation via Ammonia Decomposition: A Review. Int. J. Hydrogen Energy 2013, 38, 14968–14991. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for Hydrogen Storage; A Review of Catalytic Ammonia Decomposition and Hydrogen Separation and Purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Schüth, F.; Palkovits, R.; Schlögl, R.; Su, D.S. Ammonia as a Possible Element in an Energy Infrastructure: Catalysts for Ammonia Decomposition. Energy Environ. Sci. 2012, 5, 6278–6289. [Google Scholar] [CrossRef]
- Hanada, N.; Hino, S.; Ichikawa, T.; Suzuki, H.; Takai, K.; Kojima, Y. Hydrogen Generation by Electrolysis of Liquid Ammonia. Chem. Commun. 2010, 46, 7775–7777. [Google Scholar] [CrossRef] [PubMed]
- Yuzawa, H.; Mori, T.; Itoh, H.; Yoshida, H. Reaction Mechanism of Ammonia Decomposition to Nitrogen and Hydrogen over Metal Loaded Titanium Oxide Photocatalyst. J. Phys. Chem. C 2012, 116, 4126–4136. [Google Scholar] [CrossRef]
- Ban, J.-Y.; Kim, H.I.; Choung, S.-J.; Jeong, H.; Kang, M. NH3 Removal Using the Dielectric Barrier Discharge Plasma-V-TiO2 Photocatalytic Hybrid System. Korean J. Chem. Eng. 2008, 25, 780–786. [Google Scholar] [CrossRef]
- Son, Y.-S.; Kim, K.-H.; Kim, K.-J.; Kim, J.-C. Ammonia Decomposition Using Electron Beam. Plasma Chem. Plasma Process. 2013, 33, 617–629. [Google Scholar] [CrossRef]
- Smith, C.; Essex, H. Effect of Electric Fields on the Decomposition of Ammonia by Alpha-Rays. J. Chem. Phys. 1938, 6, 188–196. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Xu, H.; Li, W. NH3 Decomposition Kinetics on Supported Ru Clusters: Morphology and Particle Size Effect. Catal. Lett. 2007, 119, 311–318. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Zhou, X.P.; Au, C.T. Carbon Nanotubes-Supported Ru Catalyst for the Generation of COX-Free Hydrogen from Ammonia. Catal. Today 2004, 93–95, 27–38. [Google Scholar] [CrossRef]
- Lucentini, I.; Casanovas, A.; Llorca, J. Catalytic Ammonia Decomposition for Hydrogen Production on Ni, Ru and Ni Ru Supported on CeO2. Int. J. Hydrogen Energy 2019, 44, 12693–12707. [Google Scholar] [CrossRef]
- Pinzón, M.; Romero, A.; De Lucas Consuegra, A.; De La Osa, A.R.; Sánchez, P. Hydrogen Production by Ammonia Decomposition over Ruthenium Supported on SiC Catalyst. J. Ind. Eng. Chem. 2021, 94, 326–335. [Google Scholar] [CrossRef]
- Kocer, T.; Oztuna, F.E.S.; Kurtoğlu, S.F.; Unal, U.; Uzun, A. Graphene Aerogel-Supported Ruthenium Nanoparticles for COX-Free Hydrogen Production from Ammonia. Appl. Catal. Gen. 2021, 610, 117969. [Google Scholar] [CrossRef]
- Yan, H.; Xu, Y.-J.; Gu, Y.-Q.; Li, H.; Wang, X.; Jin, Z.; Shi, S.; Si, R.; Jia, C.-J.; Yan, C.-H. Promoted Multimetal Oxide Catalysts for the Generation of Hydrogen via Ammonia Decomposition. J. Phys. Chem. C 2016, 120, 7685–7696. [Google Scholar] [CrossRef]
- Sato, K.; Abe, N.; Kawagoe, T.; Miyahara, S.; Honda, K.; Nagaoka, K. Supported Ni Catalysts Prepared from Hydrotalcite-like Compounds for the Production of Hydrogen by Ammonia Decomposition. Int. J. Hydrogen Energy 2017, 42, 6610–6617. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Weng, C.-C.; Chen, C.; Yuan, Z.-Y. Catalytic Decomposition of Ammonia to COX-Free Hydrogen over Ni/ZSM-5 Catalysts: A Comparative Study of the Preparation Methods. Appl. Catal. Gen. 2018, 562, 49–57. [Google Scholar] [CrossRef]
- Yu, Y.; Gan, Y.-M.; Huang, C.; Lu, Z.-H.; Wang, X.; Zhang, R.; Feng, G. Ni/La2O3 and Ni/MgO–La2O3 Catalysts for the Decomposition of NH3 into Hydrogen. Int. J. Hydrogen Energy 2020, 45, 16528–16539. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, Y.; Long, Z.; Zhao, S.; Wang, Y.; Zhang, W. One-Pot Synthesis of Supported Ni@Al2O3 Catalysts with Uniform Small-Sized Ni for Hydrogen Generation via Ammonia Decomposition. Int. J. Hydrogen Energy 2021, 46, 4045–4054. [Google Scholar] [CrossRef]
- Chellappa, A.S.; Fischer, C.M.; Thomson, W.J. Ammonia Decomposition Kinetics over Ni-Pt/Al2O3 for PEM Fuel Cell Applications. Appl. Catal. Gen. 2002, 227, 231–240. [Google Scholar] [CrossRef]
- Maleki, H. Kinetic Assessment of H2 Production from NH3 Decomposition over CoCeAlO Catalyst in a Microreactor: Experiments and CFD Modelling. Chem. Eng. J. 2021, 411, 128595. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Chen, Y.-C.; Chang, C.-S.; Chung, J.N. Numerical Modeling of Hydrogen Production from Ammonia Decomposition for Fuel Cell Applications. Int. J. Hydrogen Energy 2010, 35, 589–597. [Google Scholar] [CrossRef]
- Deshmukh, S.R.; Mhadeshwar, A.B.; Vlachos, D.G. Microreactor Modeling for Hydrogen Production from Ammonia Decomposition on Ruthenium. Ind. Eng. Chem. Res. 2004, 43, 2986–2999. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Yang, J.; Yan, Y.; Wang, D.; Hu, F.; Wang, X.; Zhang, R.; Feng, G. Ru/La2O3 Catalyst for Ammonia Decomposition to Hydrogen. Appl. Surf. Sci. 2019, 476, 928–936. [Google Scholar] [CrossRef]
- Mao, Z.; Campbell, C.T. Apparent Activation Energies in Complex Reaction Mechanisms: A Simple Relationship via Degrees of Rate Control. ACS Catal. 2019, 9, 9465–9473. [Google Scholar] [CrossRef]
- Vilekar, S.A.; Fishtik, I.; Datta, R. The Peculiar Catalytic Sequence of the Ammonia Decomposition Reaction and Its Steady-State Kinetics. Chem. Eng. Sci. 2012, 71, 333–344. [Google Scholar] [CrossRef]
- García-García, F.R.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Goguet, A.; Shekhtman, S.O.; Hardacre, C. TAP Studies of Ammonia Decomposition over Ru and Ir Catalysts. Phys. Chem. Chem. Phys. 2011, 13, 12892–12899. [Google Scholar] [CrossRef]
- Duan, X.; Ji, J.; Qian, G.; Fan, C.; Zhu, Y.; Zhou, X.; Chen, D.; Yuan, W. Ammonia Decomposition on Fe(1 1 0), Co(1 1 1) and Ni(1 1 1) Surfaces: A Density Functional Theory Study. J. Mol. Catal. Chem. 2012, 357, 81–86. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Liu, C.; Gong, W.; Guo, H. Plasma Driven Ammonia Decomposition on a Fe-Catalyst: Eliminating Surface Nitrogen Poisoning. Chem. Commun. 2013, 49, 3787. [Google Scholar] [CrossRef]
- Miao, M.; Gong, X.; Lei, S.; Wang, L.; Sha, M.; Meng, Q. The Graphene-Supported Non-Noble Metal Catalysts Activate Ammonia Decomposition: A DFT Study. Chem. Phys. 2021, 548, 111249. [Google Scholar] [CrossRef]
- Armenise, S.; García-Bordejé, E.; Valverde, J.L.; Romeo, E.; Monzón, A. A Langmuir–Hinshelwood Approach to the Kinetic Modelling of Catalytic Ammonia Decomposition in an Integral Reactor. Phys. Chem. Chem. Phys. 2013, 15, 12104. [Google Scholar] [CrossRef]


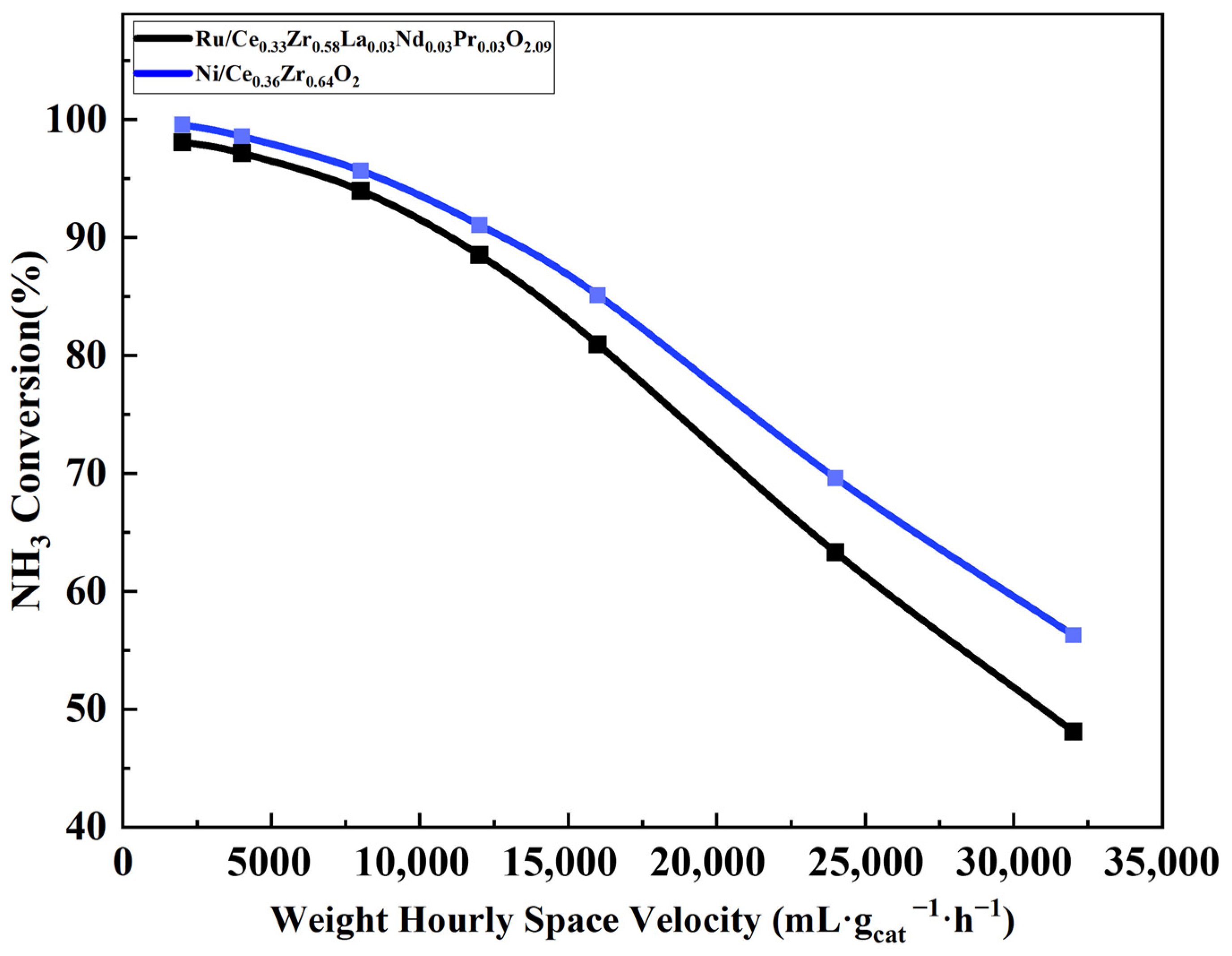
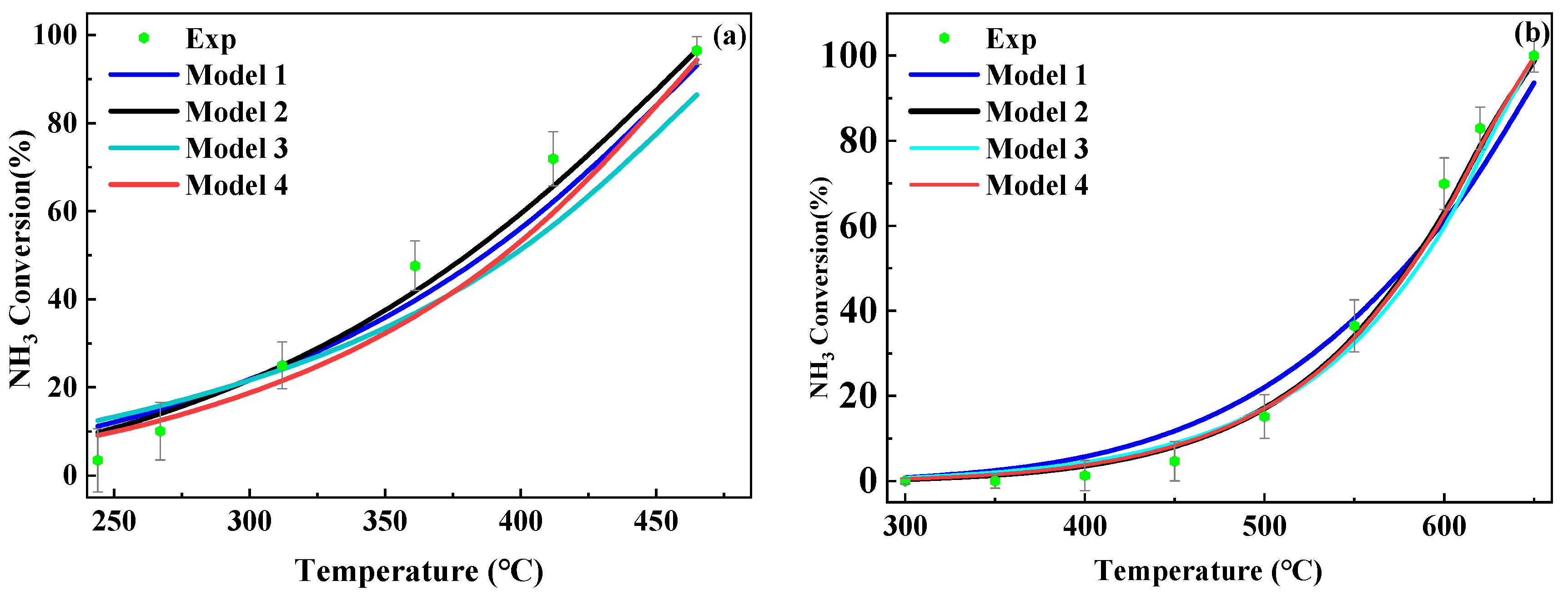
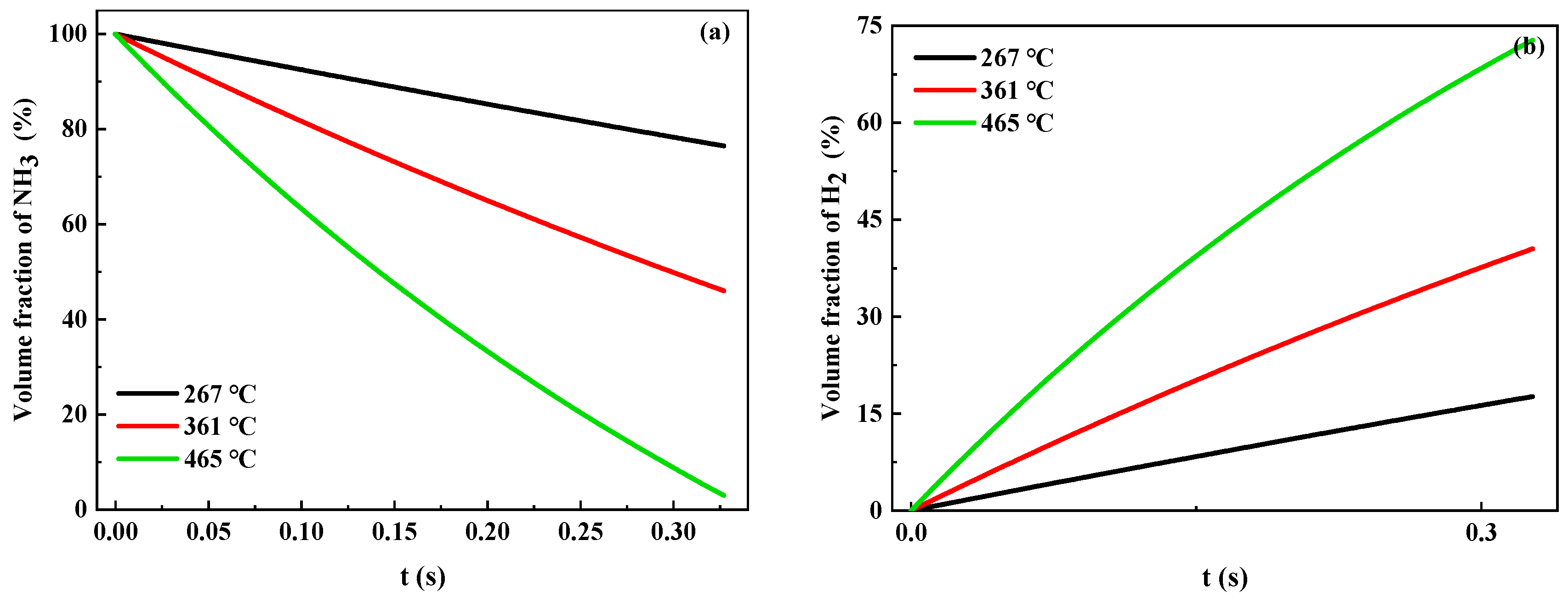

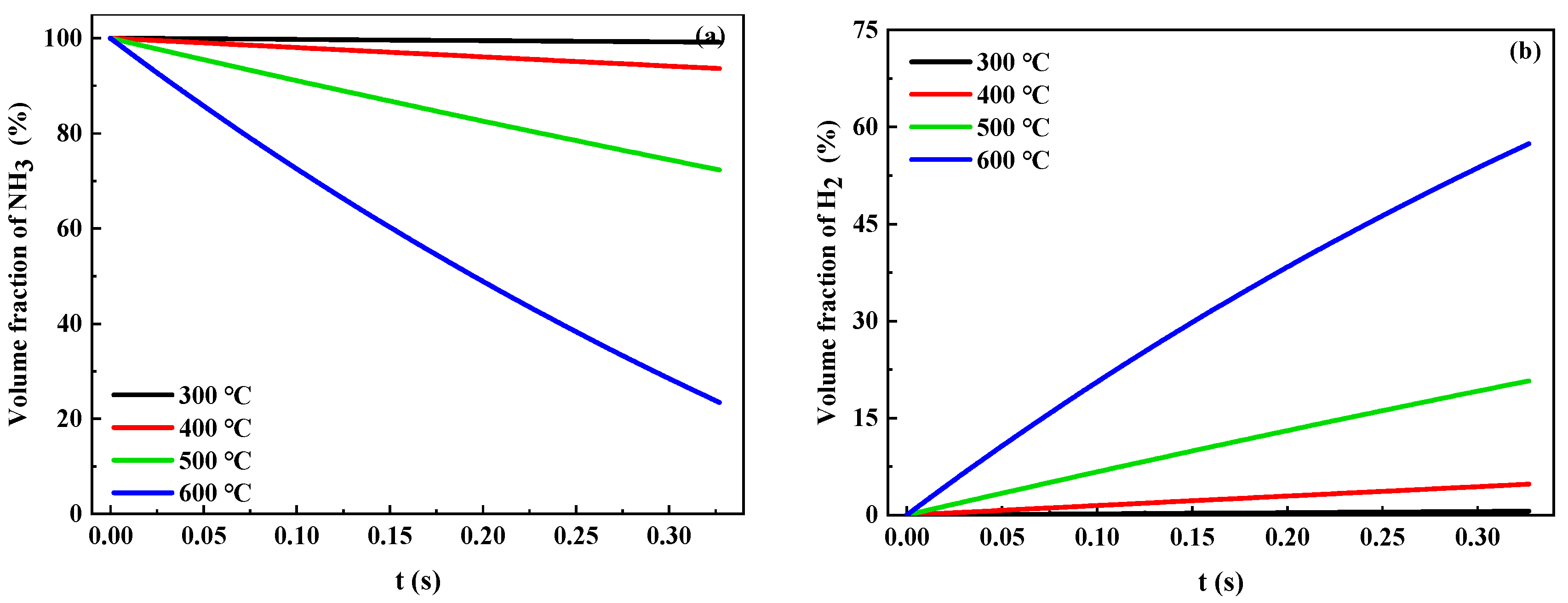

| Ru/Ce0.33Zr0.58La0.03Nd0.03Pr0.03O2.09 | Ni/Ce0.36Zr0.64O2 | |
|---|---|---|
| Model l | 0.959 | 0.992 |
| Model 2 | 0.968 | 0.993 |
| Model 3 | 0.943 | 0.983 |
| Model 4 | 0.939 | 0.991 |
| Ru/Ce0.33Zr0.58La0.03Nd0.03Pr0.03O2.09 | Ni/Ce0.36Zr0.64O2 | |
|---|---|---|
| Ea (kJ·mol−1) | 37.7 | 66.0 |
| A (s−1) | 128.0 | 1621.2 |
| Simulation Conditions | |
|---|---|
| Residence time, (s) | 0.327 |
| Catalyst volume, Vcat (cm3) | 0.727 |
| Catalyst density, (g∙cm−3) | 1.375 |
| Pressure, p (bar) | 1.01 |
| Inlet gas composition, α (%) | = 99.98%, = 0.01%, = 0.01% |
| Temperature, T (°C) | 240–650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Yang, L.; Xiang, C.; Liang, J.; Yang, H.; Li, D.; Sun, Y.; Lv, L.; Zhu, N. Research on Catalysts for Online Ammonia Hydrogen Production in Marine Engines: Performance Evaluation and Reaction Kinetic Modeling. Catalysts 2025, 15, 488. https://doi.org/10.3390/catal15050488
Wu J, Yang L, Xiang C, Liang J, Yang H, Li D, Sun Y, Lv L, Zhu N. Research on Catalysts for Online Ammonia Hydrogen Production in Marine Engines: Performance Evaluation and Reaction Kinetic Modeling. Catalysts. 2025; 15(5):488. https://doi.org/10.3390/catal15050488
Chicago/Turabian StyleWu, Jin, Liang Yang, Chuang Xiang, Junjie Liang, He Yang, Dilong Li, Ying Sun, Lin Lv, and Neng Zhu. 2025. "Research on Catalysts for Online Ammonia Hydrogen Production in Marine Engines: Performance Evaluation and Reaction Kinetic Modeling" Catalysts 15, no. 5: 488. https://doi.org/10.3390/catal15050488
APA StyleWu, J., Yang, L., Xiang, C., Liang, J., Yang, H., Li, D., Sun, Y., Lv, L., & Zhu, N. (2025). Research on Catalysts for Online Ammonia Hydrogen Production in Marine Engines: Performance Evaluation and Reaction Kinetic Modeling. Catalysts, 15(5), 488. https://doi.org/10.3390/catal15050488






