Highly Dispersed Pt on TiOx Embedded in Porous Carbon as Electrocatalyst for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. The Synthesis of Pt-TiOx@C Electrocatalyst
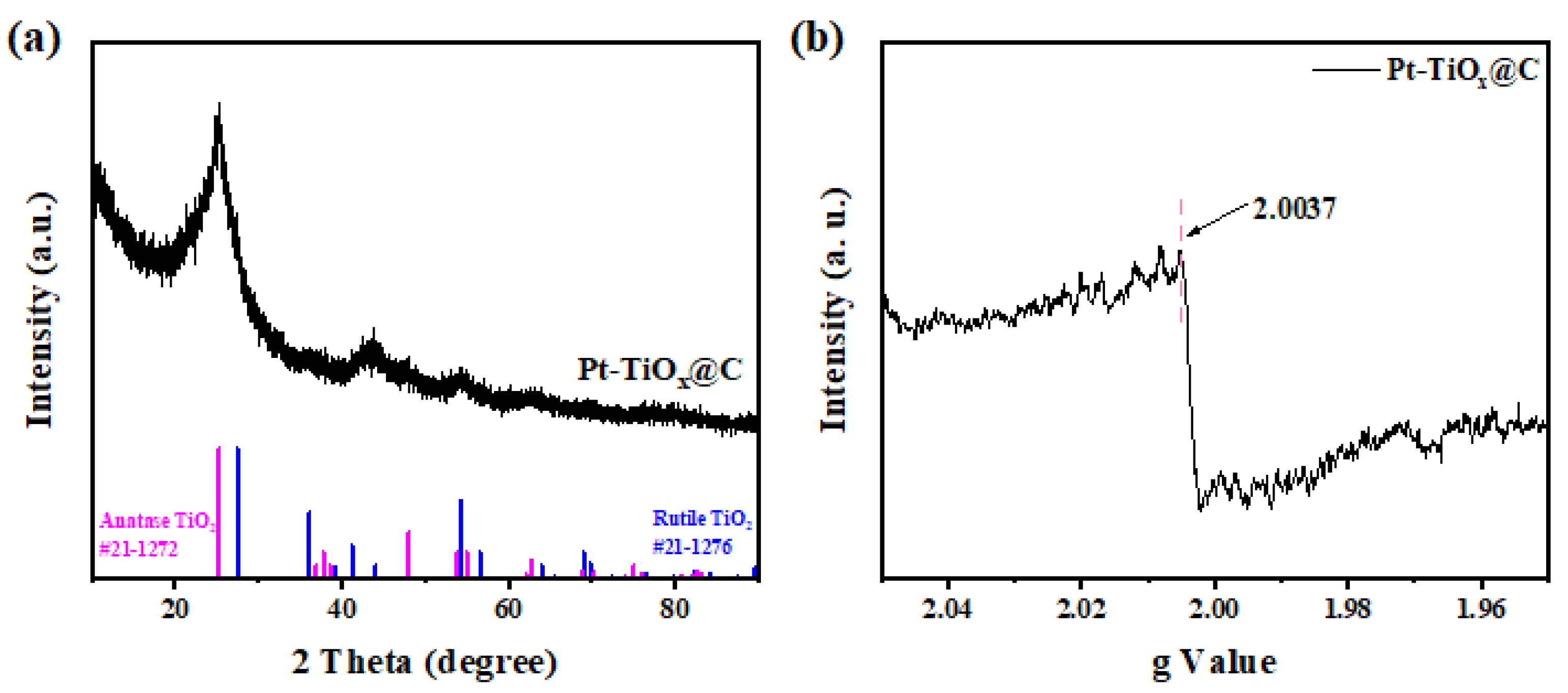
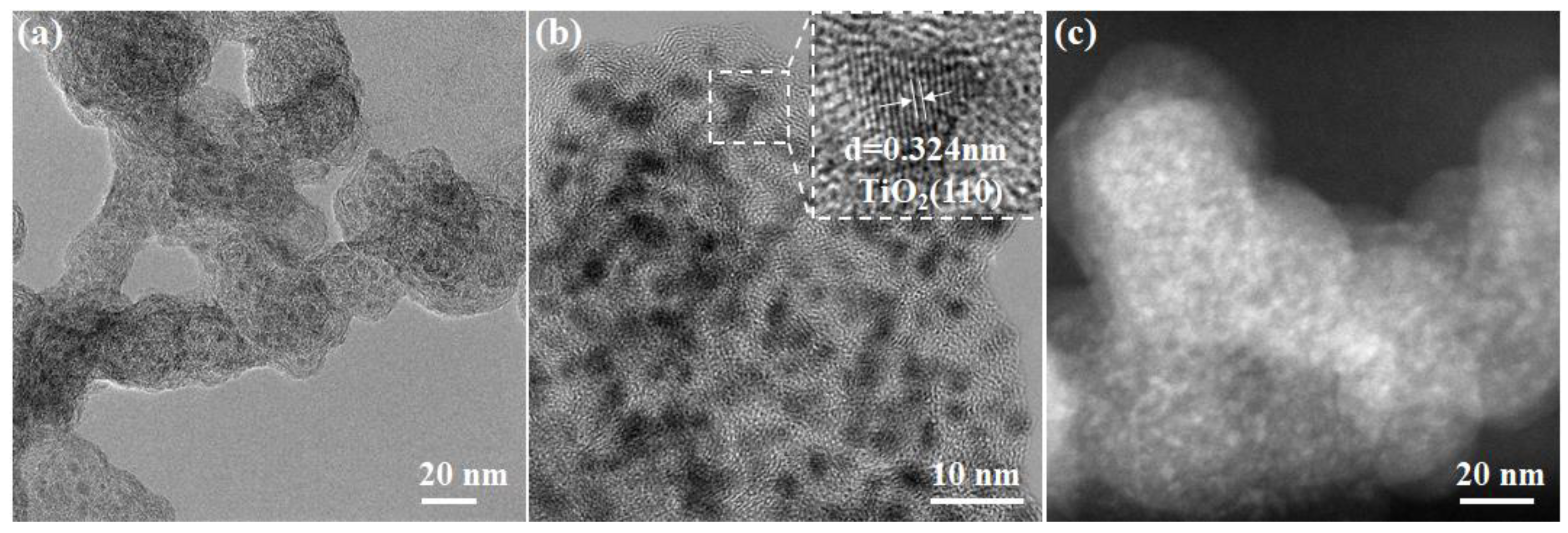
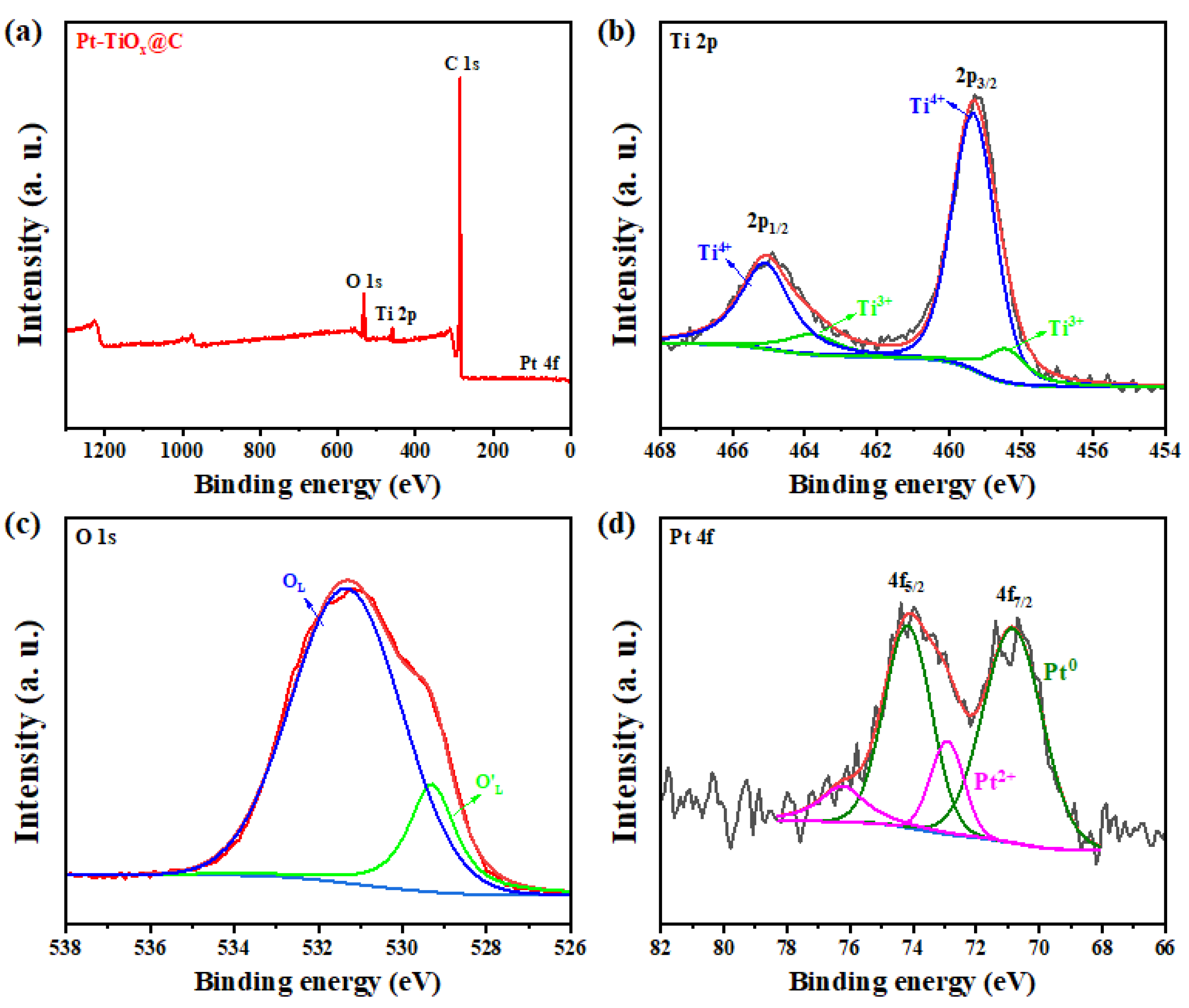
2.2. The Characterizations of Pt-TiOx@C Electrocatalyst
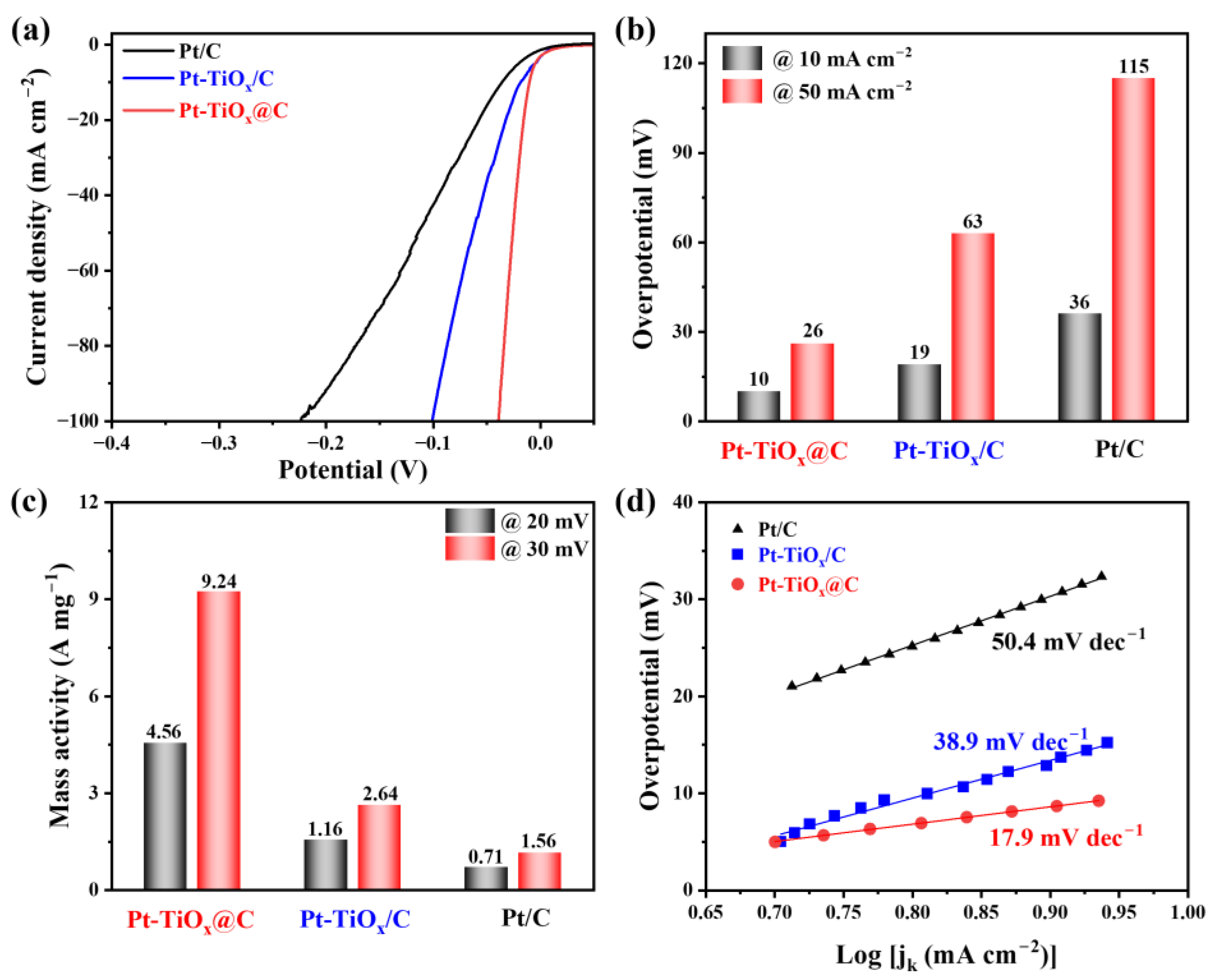
2.3. HER Performance of the Pt-TiOx@C Electrocatalyst
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of Pt-TiOx@C Catalyst
3.3. Synthesis of Pt-TiOx/C Catalyst
3.4. Material Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, L.; Zhao, Z.; Huang, Q.; Zhou, C.; Chen, W.; Wang, K.; Li, M.; Lin, F.; Luo, H.; Gu, Y.; et al. Single-atom Cr-N4 sites with high oxophilicity interfaced with Pt atomic clusters for practical alkaline hydrogen evolution catalysis. J. Am. Chem. Soc. 2023, 145, 21432–21441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Küspert, S.; Balaghi, S.E.; Hussein, H.E.M.; Ortlieb, N.; Knäbbeler-Buß, M.; Hügenell, P.; Pollitt, S.; Hug, N.; Melke, J.; et al. Ultrahigh mass activity Pt entities consisting of Pt single atoms, clusters, and nanoparticles for improved hydrogen evolution reaction. Small 2023, 19, 2205885. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhu, Y.; Chen, Y.; Wen, X.; Long, M.; Wu, X.; Hu, Z.; Guan, D.; Wang, X.; Zhou, C.; et al. Hydrogen spillover in complex oxide multifunctional sites improves acidic hydrogen evolution electrocatalysis. Nat. Commun. 2022, 13, 1189. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gu, W.; Zhang, B.; Zhao, Y.; Zhang, A.; Xiao, W.; Wei, S.; Pang, H. Research progress of non-noble metal-based self-supporting electrode for hydrogen evolution reaction at high current density. Adv. Funct. Mater. 2025, 2423760. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Chen, K.; Deng, S.; Lu, Y.; Gong, M.; Hu, Y.; Zhao, T.; Shen, T.; Wang, D. Molybdenum-doped titanium dioxide supported low-Pt electrocatalyst for highly efficient and stable hydrogen evolution reaction. Chin. Chem. Lett. 2021, 32, 765–769. [Google Scholar] [CrossRef]
- Yue, C.; Feng, C.; Sun, G.; Liu, N.; Hao, H.; Bao, W.; Zhang, X.; Sun, F.; Zhang, C.; Bi, J.; et al. Hierarchically stabilized Pt single-atom catalysts induced by an atomic substitution strategy for an efficient hydrogen evolution reaction. Energy Environ. Sci. 2024, 17, 5227–5240. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, Y.; Yu, Y.; Chen, Y.; Liu, Q.; Bao, H.; Luo, F.; Pan, S.; Yang, Z. Electronic metal-support interaction induces hydrogen spillover and platinum utilization in hydrogen evolution reaction. Angew. Chem. Int. Edit. 2025, 64, e202413417. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Sun, H.; Yu, N.; Ni, G. Effect of bias in argon plasma on electronic structure of electrocatalyst Pt/CNT for hydrogen evolution reaction. Catal. Lett. 2025, 155, 110. [Google Scholar] [CrossRef]
- Ma, Z.; Cano, Z.P.; Yu, A.; Chen, Z.; Jiang, G.; Fu, X.; Yang, L.; Wu, T.; Bai, Z.; Lu, J. Enhancing oxygen reduction activity of Pt-based electrocatalysts: From theoretical mechanisms to practical methods. Angew. Chem. Int. Edit. 2020, 59, 18334–18348. [Google Scholar] [CrossRef]
- Jauhar, A.M.; Ma, Z.; Xiao, M.; Jiang, G.; Sy, S.; Li, S.; Yu, A.; Chen, Z. Space-confined catalyst design toward ultrafine Pt nanoparticles with enhanced oxygen reduction activity and durability. J. Power Sources 2020, 473, 228607. [Google Scholar] [CrossRef]
- Yu, F.; Gao, Y.; Lang, Z.; Ma, Y.; Yin, L.; Du, J.; Tan, H.; Wang, Y.; Li, Y. Electrocatalytic performance of ultrasmall Mo2C affected by different transition metal dopants in hydrogen evolution reaction. Nanoscale 2018, 10, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Ultrafine Pt-based nanowires for advanced catalysis. Adv. Funct. Mater. 2020, 30, 2000793. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Cui, X.; Chen, B.; Zhu, Y.; Gong, Y.; Saleem, F.; Xi, S.; Du, Y.; Borgna, A.; et al. Crystal phase and architecture engineering of lotus-thalamus-shaped Pt-Ni anisotropic superstructures for highly efficient electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1801741. [Google Scholar] [CrossRef]
- Hao, R.; Feng, Q.-L.; Wang, X.-J.; Zhang, Y.-C.; Li, K.-S. Morphology-controlled growth of large-area PtSe2 films for enhanced hydrogen evolution reaction. Rare Met. 2022, 41, 1314–1322. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, K.; Chen, Y.; Guo, X.-P.; Huang, B.; Liu, Z.-Q. In situ precise anchoring of Pt single atoms in spinel Mn3O4 for a highly efficient hydrogen evolution reaction. Energy Environ. Sci. 2022, 15, 4592–4600. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, S.; Yang, G.; Li, K.; Ding, L.; Wang, W.; Cullen, D.A.; Meyer, H.M.; Retterer, S.T.; Wu, Z.; et al. Ultrathin platinum nanowire based electrodes for high-efficiency hydrogen generation in practical electrolyzer cells. Chem. Eng. J. 2021, 410, 128333. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, J.; Ding, J.; Cheng, Y.; Wang, T.; Li, J.; Hu, F.; Yang, H.B.; Liu, B. Recent advances in carbon-supported noble-metal electrocatalysts for hydrogen evolution reaction: Syntheses, structures, and properties. Adv. Energy Mater. 2022, 12, 2200928. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Q.; Nichols, F.; Mayford, K.; Pan, D.; Kuo, H.-L.; Lu, J.Q.; Bridges, F.; Chen, S. Platinum-anchored iron oxide nanostructures for efficient hydrogen evolution reaction in acidic media. J. Phys. Chem. C 2023, 127, 3996–4005. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Zheng, L.; Yan, Y.; Zhang, Y.; Chen, G.; Sun, S.; Zhang, J. Highly active, stable oxidized platinum clusters as electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2017, 10, 2450–2458. [Google Scholar] [CrossRef]
- Liu, J.; Guo, P.; Liu, D.; Yan, X.; Tu, X.; Pan, H.; Wu, R. Activating TiO2 through the phase transition-mediated hydrogen spillover to outperform Pt for electrocatalytic pH-universal hydrogen evolution. Small 2024, 20, 2400783. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Han, X.; Ma, Z.; Zhang, B.; Zheng, X.; Liu, Y.; Gao, M.; Zhao, G.; Lin, Y.; Pan, H.; et al. Unraveling the unique strong metal-support interaction in titanium dioxide supported platinum clusters for the hydrogen evolution reaction. Angew. Chem. Int. Edit. 2024, 63, e202406728. [Google Scholar] [CrossRef]
- Zhang, W.; Du, M.; Xi, W.; Zhang, H.; Liu, S.F.; Yan, J. Platinum species on oxygen vacancy-rich titania for efficient basic electrocatalytic hydrogen evolution. Langmuir 2023, 39, 12715–12724. [Google Scholar] [CrossRef]
- Zeng, H.; Ji, Y.; Wen, J.; Li, X.; Zheng, T.; Jiang, Q.; Xia, C. Pt nanocluster-catalyzed hydrogen evolution reaction: Recent advances and future outlook. Chin. Chem. Lett. 2025, 36, 109686. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Du, N.; Hou, W. Single platinum atoms immobilized on monolayer tungsten trioxide nanosheets as an efficient electrocatalyst for hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2009770. [Google Scholar] [CrossRef]
- Li, C.; Jang, H.; Kim, M.G.; Hou, L.; Liu, X.; Cho, J. Ru-incorporated oxygen-vacancy-enriched MoO2 electrocatalysts for hydrogen evolution reaction. Appl. Catal. B Environ. Energy 2022, 307, 121204. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Wu, L.; Song, L.; Jiang, G.; Liang, Z.; Su, D.; Zhu, Y.; Adzic, R.R.; Wang, J.X.; et al. NbOx nano-nail with a Pt head embedded in carbon as a highly active and durable oxygen reduction catalyst. Nano Energy 2020, 69, 104455. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; She, Z.; Pope, M.A.; Li, Y. Ultrasmall TiOx nanoparticles rich in oxygen vacancies synthesized through a simple strategy for ultrahigh-rate lithium-ion batteries. ChemElectroChem 2020, 7, 4124–4130. [Google Scholar] [CrossRef]
- Wei, Z.-W.; Wang, H.-J.; Zhang, C.; Xu, K.; Lu, X.-L.; Lu, T.-B. Reversed charge transfer and enhanced hydrogen spillover in platinum nanoclusters anchored on titanium oxide with rich oxygen vacancies boost hydrogen evolution reaction. Angew. Chem. Int. Edit. 2021, 60, 16622–16627. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.M.; Higuchi, E.; Inoue, H. Pt nanoparticle-decorated two-dimensional oxygen-deficient TiO2 nanosheets as an efficient and stable electrocatalyst for the hydrogen evolution reaction. Nanoscale 2020, 12, 11055–11062. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Leung, K.T.; Pradhan, D. Nitrogen doped reduced graphene oxide based Pt-TiO2 nanocomposites for enhanced hydrogen evolution. J. Phys. Chem. C 2015, 119, 19117–19125. [Google Scholar] [CrossRef]
- van der Heijden, O.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.M. Tafel slope plot as a tool to analyze electrocatalytic reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Deng, J.; Xu, Y.; Tao, W.; Wang, X.; Lin, Z.; Zhang, Q.; Gu, L.; Zhong, W. Pollen-like self-supported FeIr alloy for improved hydrogen evolution reaction in acid electrolyte. J. Energy Chem. 2022, 66, 560–565. [Google Scholar] [CrossRef]
- Yang, J.; Ning, G.; Yu, L.; Wang, Y.; Luan, C.; Fan, A.; Zhang, X.; Liu, Y.; Dong, Y.; Dai, X. Morphology controllable synthesis of PtNi concave nanocubes enclosed by high-index facets supported on porous graphene for enhanced hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 17790–17796. [Google Scholar] [CrossRef]
- Wang, J.; Zang, W.; Liu, X.; Sun, J.; Xi, S.; Liu, W.; Kou, Z.; Shen, L.; Wang, J. Switch Volmer-Heyrovsky to Volmer-Tafel pathway for efficient acidic electrocatalytic hydrogen evolution by correlating Pt single atoms with clusters. Small 2024, 20, 2309427. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Tang, Z.; Wang, Y.; Wu, T.; Huang, M.; Zhao, L.; Zhao, Z.; Liu, H.; Xu, C.; et al. Enhanced interfacial stability of Pt/TiO2/Ti via Pt-O bonding for efficient acidic electrolyzer. Chem. Eng. J. 2024, 492, 152339. [Google Scholar] [CrossRef]
- Cho, S.; Yim, G.; Koh, J.; Jang, H.; Park, J.T. One-pot synthesis of Pt@TiO2 core-shell nanoparticles for stable hydrogen evolution reaction in acidic and alkaline media. Mater. Today Chem. 2023, 32, 101644. [Google Scholar] [CrossRef]
- Luo, X.; Tan, X.; Ji, P.; Chen, L.; Yu, J.; Mu, S. Surface reconstruction-derived heterostructures for electrochemical water splitting. EnergyChem 2023, 5, 100091. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H.; et al. Ultrahigh Pt-mass-activity hydrogen evolution catalyst electrodeposited from bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Zhu, Y.a.; Luo, Y.; Yao, J.; Dai, W.; Zhong, X.; Lu, T.; Pan, Y. Atomically dispersed Pt-O coordination boosts highly active and durable acidic hydrogen evolution reaction. Chem. Eng. J. 2022, 440, 135957. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, B.; Cheng, B.; Macyk, W.; Kuang, P.; Yu, J. Hollow carbon sphere-supported Pt/CoOx hybrid with excellent hydrogen evolution activity and stability in acidic environment. Appl. Catal. B Environ. 2022, 314, 121503. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, P.; Li, Q.; Xiao, W.; Li, Z.; Xu, G.; Liu, F.; Jia, B.; Ma, T.; Feng, S.; et al. Microwave synthesis of Pt clusters on black TiO2 with abundant oxygen vacancies for efficient acidic electrocatalytic hydrogen evolution. Angew. Chem. Int. Edit. 2023, 62, e202300406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; An, J.; Shi, H.; Dai, L.; Jiao, S. Ultra-stable Ti vacancies-Pt atomic clusters structure on titanium oxycarbide supports for high current density hydrogen evolution reaction. Small 2024, 20, 2309823. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, L.; Wang, J.; Cheng, L.; Chen, R.; Ni, H. Binary electrocatalyst composed of Mo2C nanocrystals with ultra-low Pt loadings anchored in TiO2 nanotube arrays for hydrogen evolution reaction. Appl. Surf. Sci. 2020, 509, 144679. [Google Scholar] [CrossRef]
- Yan, J.; Xi, Z.; Cong, L.; Lv, K.; Xin, R.; Cao, B.; Liu, B.; He, J.; Zhang, J. Synergy of platinum single atoms and platinum atomic clusters on sulfur-doped titanium nitride nanotubes for enhanced hydrogen evolution reaction. Small 2022, 18, 2205603. [Google Scholar] [CrossRef]
- Wang, S.; Sang, L.; Jiao, Z.; Zhang, F.; Wang, K.; Xu, B.; Zhang, P.; Liu, B.; Wang, Y.; Li, Y.; et al. Pt nanoparticles supported on in-situ growth titanium dioxide nanowire arrays with oxygen vacancies for hydrogen evolution reaction. Appl. Surf. Sci. 2025, 687, 162257. [Google Scholar] [CrossRef]
- Samanta, R.; Mishra, R.; Barman, S. Interface-engineered porous Pt–PdO nanostructures for highly efficient hydrogen evolution and oxidation reactions in base and acid. ACS Sustain. Chem. Eng. 2022, 10, 3704–3715. [Google Scholar] [CrossRef]
- Pham, H.Q.; Huynh, T.T.; Huynh, Q. Mixed-oxide-containing composite-supported MoPt with ultralow Pt content for accelerating hydrogen evolution performance. Chem. Comm. 2023, 59, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Roy, S.B.; Moon, S.; Yoo, S.; Choi, H.; Parale, V.G.; Kim, Y.; Lee, J.; Jun, S.C.; Kang, K.; et al. Highly dispersed Pt clusters on F-doped tin(IV) oxide aerogel matrix: An ultra-robust hybrid catalyst for enhanced hydrogen evolution. ACS Nano 2022, 16, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Q.; Shao, Y.; Wang, R.; Cheng, M.; Hu, J.; Wei, T.; Liu, B.; Jiang, H.; Qi, L.; et al. Single-atom nickel encapsulated in nanosheet-coiled rGO-CTAB-MoS2 nanoflowers for high-efficiency and long-term hydrogen evolution in acidic medium. Adv. Funct. Mater. 2025, 2425826. [Google Scholar] [CrossRef]
- Su, Y.; Wu, H.; Wang, S.; Hu, Z.; Li, J.; Chang, J.; Yin, G.; Lu, S. Enhanced overall water splitting by CQDs-coupled RuO2-IrO2 heterojunction in acidic media. J. Energy Chem. 2025, 106, 331–339. [Google Scholar] [CrossRef]
- Jian, C.; Yuan, J.; Wang, T.; Zeng, L.; Liu, W. Construction of Ru single-atom and cluster/np-Mo synergistic active sites with hydrogen spillover for efficient hydrogen evolution reaction. Chem. Eng. J. 2025, 510, 161683. [Google Scholar] [CrossRef]
- Kim, T.; Jung, H.; Choi, H.; Lee, W.; Patil, U.M.; Parale, V.G.; Kim, Y.; Kim, J.; Kim, S.-H.; Park, H.-H. Partially oxidized inter-doped RuNi alloy aerogel for the hydrogen evolution reaction in both alkaline and acidic media. Mater. Horiz. 2024, 11, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, Y.; Li, J.; Feng, K.; Zhong, J.; Huang, H.; Shao, M.; Fan, Z.; Liao, F.; Liu, Y.; et al. Enhanced acidic hydrogen evolution reaction kinetics via nitrogen-doped iridium nanosheet with optimized hydrogen adsorption energy. Chem. Eng. J. 2024, 495, 153214. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Pan, L.F.; Wang, H.F.; Yang, Z.Z.; Zheng, L.R.; Hu, P.; Zhao, H.J.; Gu, L.; Yang, H.G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Comm. 2015, 6, 8064. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, C.; Xiao, L.; Han, C.; Zhao, X.; Yin, P.; Dong, C.; Liu, H.; Du, X.; Yang, J. Construction of Co–Se–W at interfaces of phase-mixed cobalt selenide via spontaneous phase transition for platinum-like hydrogen evolution activity and long-term durability in alkaline and acidic media. Adv. Mater. 2024, 36, 2401880. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energ. Mater. 2020, 10, 2002260. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, K.; Yang, S.; Xiao, J.; Xiao, F.; Zhao, X.; Wang, S. Optimizing the electronic structure of atomically dispersed Ru sites with CoP for highly efficient hydrogen evolution in both alkaline and acidic media. Small 2023, 19, 2301403. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yin, D.; Zhang, H.; Liu, D.; Yang, Y.; Yu, W.; Dong, X. CoN/MoC embedded in nitrogen-doped multi-channel carbon nanofibers as an efficient acidic and alkaline hydrogen evolution reaction electrocatalysts. Renew. Sust. Energ. Rev. 2023, 181, 113354. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, Y.; Zhang, F.; Luo, X.; Li, K.; Shen, L.; Shi, H.; Kang, D.J.; Pang, H. In situ construction of layered transition metal phosphides/sulfides heterostructures for efficient hydrogen evolution in acidic and alkaline media. Chem. Eng. J. 2024, 490, 151693. [Google Scholar] [CrossRef]
- Jung, K.; Pratama, D.S.A.; Haryanto, A.; Jang, J.I.; Kim, H.M.; Kim, J.C.; Lee, C.W.; Kim, D.W. Iridium-cluster-implanted ruthenium phosphide electrocatalyst for hydrogen evolution reaction. Adv. Fiber Mater. 2024, 6, 158–169. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Chen, X.; Diao, P.; Liao, J.; Chong, Z.; Yao, C.; Ma, Z.; Li, G. Highly Dispersed Pt on TiOx Embedded in Porous Carbon as Electrocatalyst for Hydrogen Evolution Reaction. Catalysts 2025, 15, 487. https://doi.org/10.3390/catal15050487
Wei Z, Chen X, Diao P, Liao J, Chong Z, Yao C, Ma Z, Li G. Highly Dispersed Pt on TiOx Embedded in Porous Carbon as Electrocatalyst for Hydrogen Evolution Reaction. Catalysts. 2025; 15(5):487. https://doi.org/10.3390/catal15050487
Chicago/Turabian StyleWei, Zihan, Xin Chen, Pengfei Diao, Jiayi Liao, Zhaonan Chong, Change Yao, Zhong Ma, and Guisheng Li. 2025. "Highly Dispersed Pt on TiOx Embedded in Porous Carbon as Electrocatalyst for Hydrogen Evolution Reaction" Catalysts 15, no. 5: 487. https://doi.org/10.3390/catal15050487
APA StyleWei, Z., Chen, X., Diao, P., Liao, J., Chong, Z., Yao, C., Ma, Z., & Li, G. (2025). Highly Dispersed Pt on TiOx Embedded in Porous Carbon as Electrocatalyst for Hydrogen Evolution Reaction. Catalysts, 15(5), 487. https://doi.org/10.3390/catal15050487





