Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization
Abstract
1. Introduction
2. Nanoconfined Catalysis
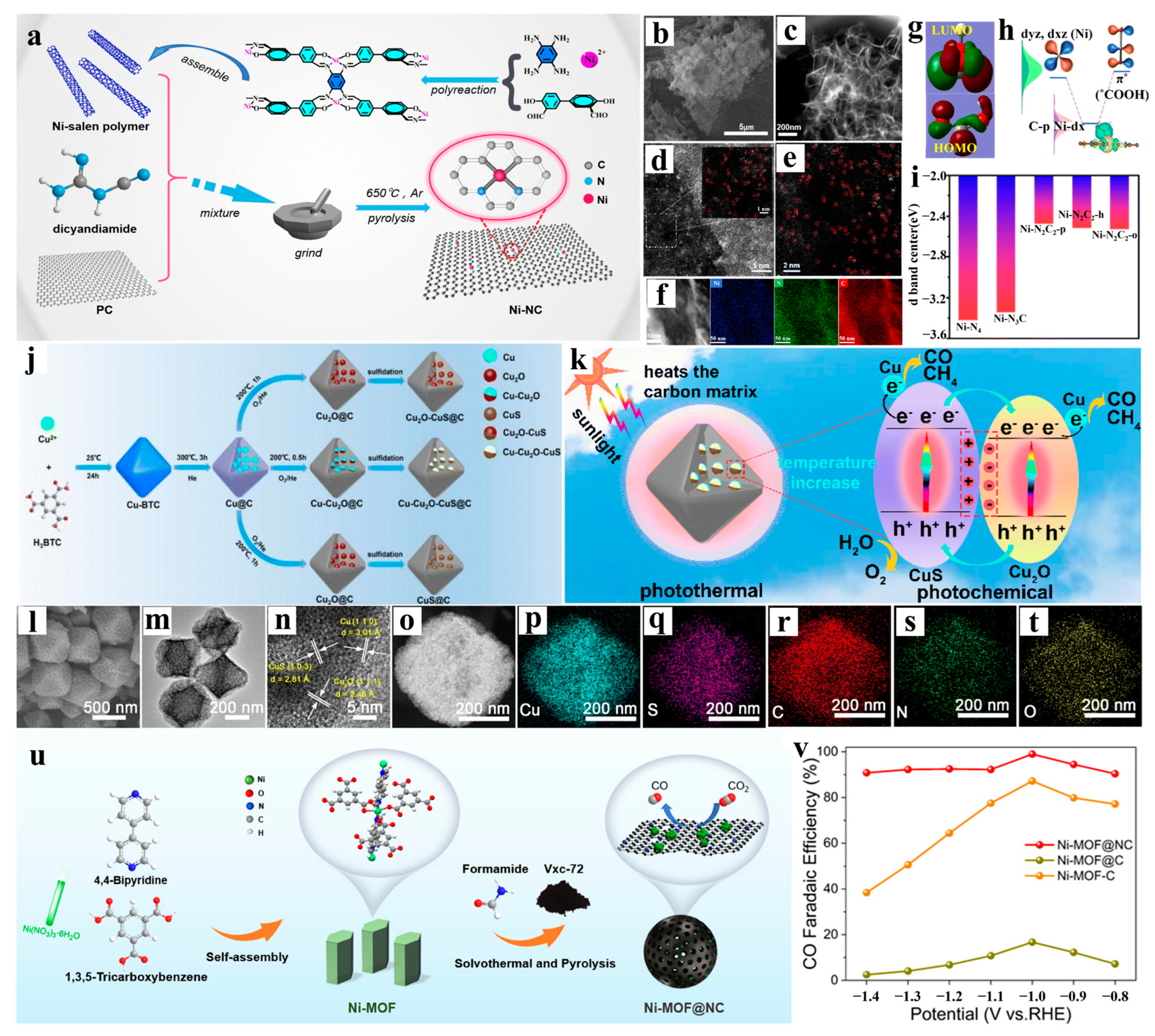
2.1. One-Dimensional Nanoconfined Catalysis
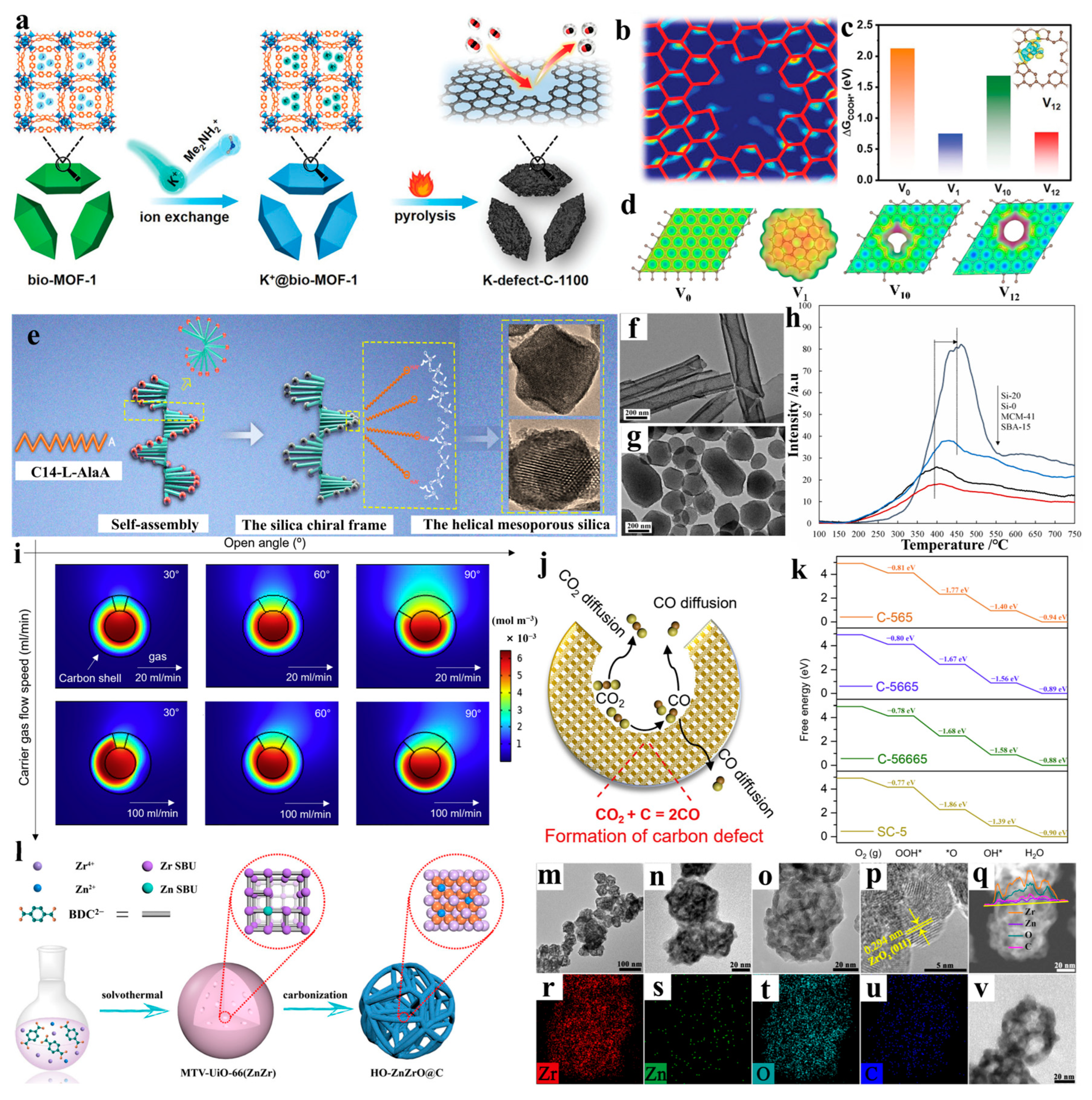
2.2. Two-Dimensional Nanoconfined Catalysis
2.3. Three-Dimensional Nanoconfined Catalysis
2.4. Interfacial Nanoconfined Catalysis
2.5. Systematic Evaluation
3. Synthesis and Optimization Strategy for Nanoconfined Catalysts
3.1. Heteroatom Doping Engineering
3.2. Defect Engineering
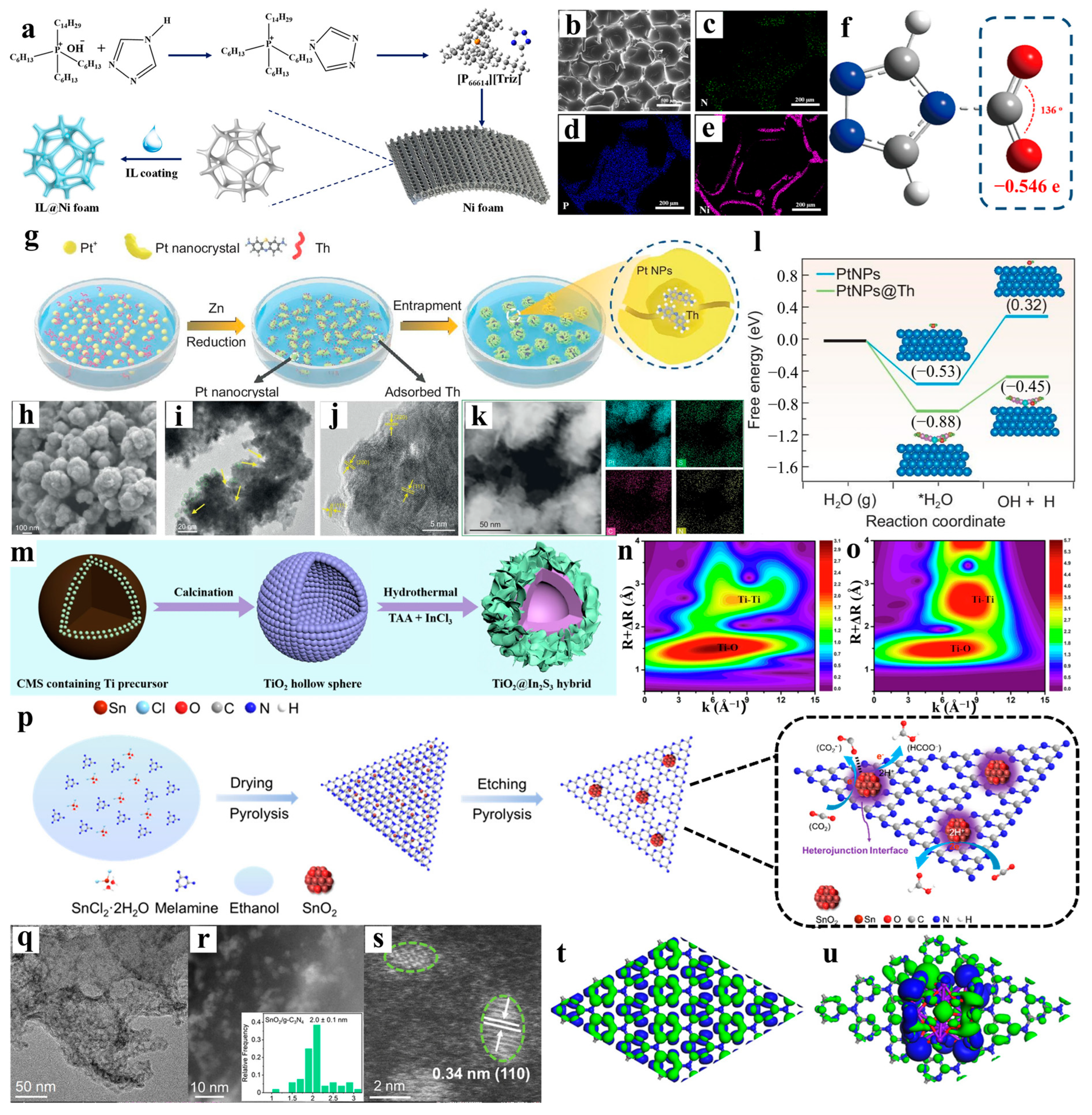
3.3. Surface Modification Engineering
4. Application and Analysis of Nanoconfined Catalysis in Various Catalytic Fields
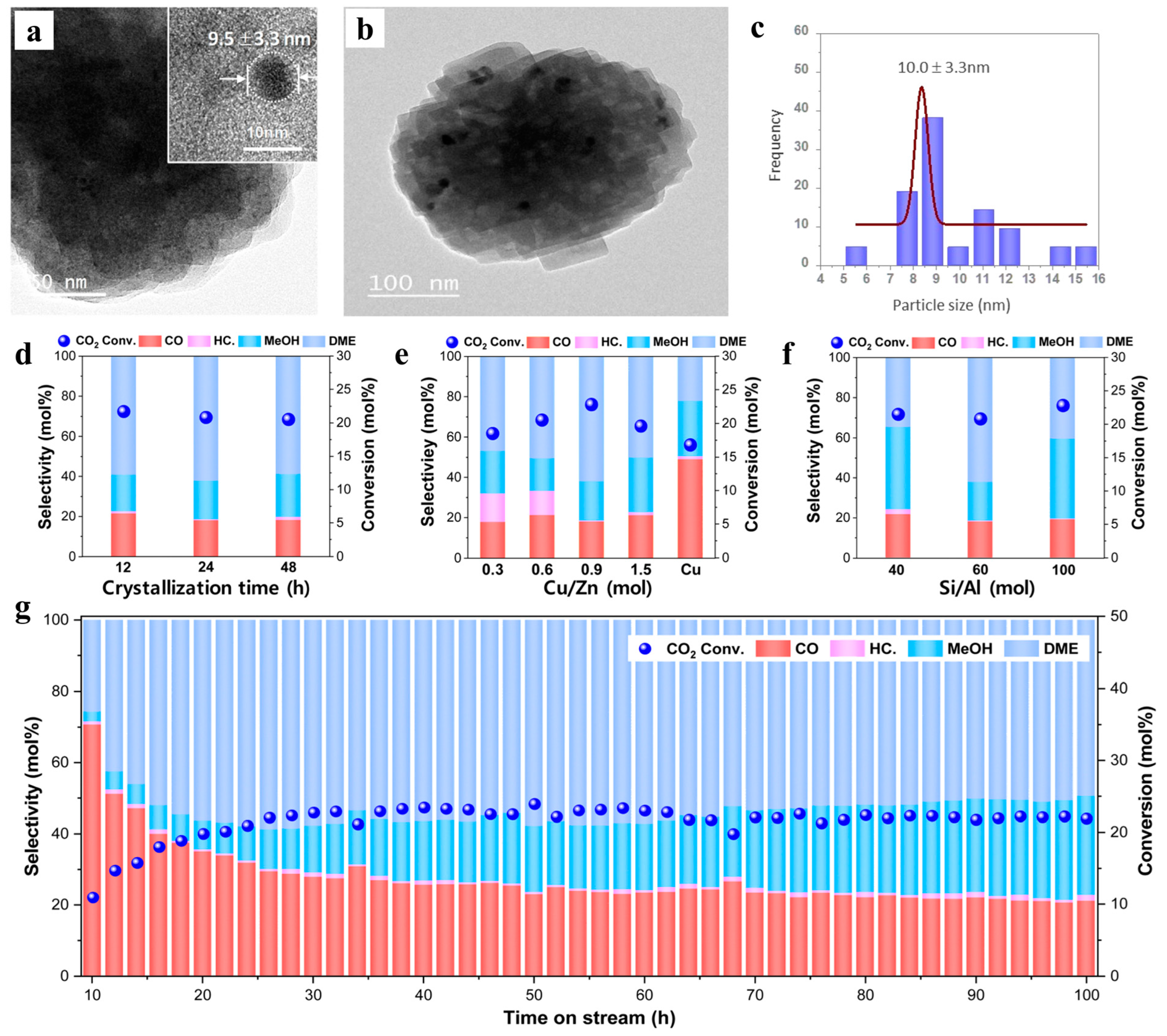
4.1. Thermal Catalysis
4.2. Photocatalysis
4.3. Electrocatalysis
5. Summary and Outlook
- (I)
- Although various structures have been developed to meet the demand for nanoconfined materials, creating nanoconfined materials that are easy to prepare, have excellent properties, exhibit structural stability, and can be applied in various environments remains a major challenge.
- (II)
- The coupled mechanisms of mass transfer, electronic effects, and interfacial chemistry in confined microenvironments have not been clarified, leading to difficulties in theoretical design.
- (III)
- Existing synthetic methods make it difficult to precisely control the size, shape and active site distribution of the confined cavity (e.g., uneven mass transfer due to the deviation of one-dimensional channel diameters), and the structure is prone to collapse at high temperature/pressure.
- (IV)
- Although the nanoconfined space can enrich reactants, the narrow channels tend to lead to increased mass transfer resistance (e.g., decreased CO2 diffusion rate in 3D MOF pores), limiting the overall reaction rate.
- (V)
- Laboratory-scale nanoconfined catalysts are complicated to synthesize (e.g., growing a graphene cover layer by the CVD method) and have high precious metal dependence (e.g., Pt-based confined systems), which makes it challenging to meet the demand for scale-up applications.
- (VI)
- Existing studies have mainly focused on single gas (e.g., CO2 or CH4) conversion and are not sufficiently adapted to real industrial exhaust gases (with SOx/NOx impurities) or liquid reaction systems (e.g., biomass-derived liquids).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RWGS | Reverse water gas shift |
| MOF | Metal organic framework |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectroscopy |
| TEM | Transmission electron microscope |
| HR-TEM | High-resolution transition electron microscopy |
| EDX | Energy dispersive X-ray spectroscopy |
| DFT | Density functional theory |
| HAADF-STEM | High angle angular dark field-scanning transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| ORR | Oxygen reduction reaction |
| DME | Dimethyl ether |
| LSV | Linear sweep voltammetry |
| FE | Faradaic efficiency |
| RHE | Reversible hydrogen electrode |
| EIS | Electrochemical impedance spectroscopy |
| TOF | Turnover frequency |
| CNTs | Carbon nanotubes |
| GDY | Graphdiyne |
| CVD | Chemical vapor deposition |
| CZZ | Cu-ZnO-ZrO2 |
| MMMs | Mixed matrix membranes |
| SPS | Spark plasma sintering |
| DRIFTS | Diffuse reflectance infrared Fourier transform spectroscopy |
| TPSR-MS | Temperature-programmed surface reaction mass spectrometry |
| PDOS | Phononic density of states |
| PL | Photoluminescence |
| WT | Wavelet transform |
| EXAFS | Extended X-ray absorption fine structure |
| FFT | Fast Fourier transform |
| AC | Aberration-corrected |
| HER | Hydrogen evolution reaction |
| PCN | Porous carbon nitride |
| MSS | Mesoporous silica shell |
| C2+ | Multi-carbon products |
| FTS | Fischer–Tröpsch synthesis |
References
- Dorian, J.P.; Franssen, H.T.; Simbeck, D.R. Global challenges in energy. Energy Policy 2006, 34, 1984–1991. [Google Scholar] [CrossRef]
- Khan, H.; Weili, L.; Khan, I.; Oanh, L.t.K. Recent advances in energy usage and environmental degradation: Does quality institutions matter? A worldwide evidence. Energy Rep. 2021, 7, 1091–1103. [Google Scholar] [CrossRef]
- Yalew, S.G.; van Vliet, M.T.H.; Gernaat, D.E.H.J.; Ludwig, F.; Miara, A.; Park, C.; Byers, E.; De Cian, E.; Piontek, F.; Iyer, G.; et al. Impacts of climate change on energy systems in global and regional scenarios. Nat. Energy 2020, 5, 794–802. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, W.; Wang, P.; D’Imperio, L.; Liu, Y.; Elberling, B. Predicting CO2 and CH4 fluxes and their seasonal variations in a subarctic wetland under two shared socioeconomic pathway climate scenarios. Agric. For. Meteorol. 2025, 362, 110359. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2014, 17, 15–27. [Google Scholar] [CrossRef]
- Huang, M.; Zhai, P. Achieving paris agreement temperature goals requires carbon neutrality by middle century with far-reaching transitions in the whole society. Adv. Clim. Chang. Res. 2021, 12, 281–286. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Chen, R.; Mao, X.; Qi, X. The United States and China on the paths and policies to carbon neutrality. J. Environ. Manag. 2022, 320, 115785. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, H.; Zeng, X.; Zhou, C.; Shu, Z.; Li, H.; Fei, Z.; Liu, G. Research on regional economic development and natural disaster risk assessment under the goal of carbon peak and carbon neutrality: A case study in Chengdu-Chongqing economic circle. Land Use Pol. 2024, 143, 107206. [Google Scholar] [CrossRef]
- Pan, X.; Xu, J.; Wang, Y.; Ma, M.; Liao, H.; Sun, H.; Fan, M.; Wang, K.; Sun, K.; Jiang, J. A new perspective on hydrogenation of CO2 into methanol over heterogeneous catalysts. Prog. Nat. Sci. Mater. Int. 2024, 34, 482–494. [Google Scholar] [CrossRef]
- Taherian, Z.; Shahed Gharahshiran, V.; Wei, X.; Khataee, A.; Yoon, Y.; Orooji, Y. Revisiting the mitigation of coke formation: Synergism between support & promoters’ role toward robust yield in the CO2 reformation of methane. Nano Mater. Sci. 2024, 6, 536–547. [Google Scholar]
- Wang, W.; Han, Z.; Wang, H.; Wei, X.; Zhong, R.; Qi, J. Construction of Co/Mn-based nanowires with adjustable surface state for boosting lean methane catalytic oxidation. Ceram. Int. 2024, 50, 2293–2302. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, R.; Li, C.; Zhong, R.; Wang, H.; Qi, J. Advancing catalytic oxidation of lean methane over cobalt-manganese oxide via a phase-engineered amorphous/crystalline interface. Chem. Commun. 2024, 60, 8896–8899. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, R.; Zhu, Q.; Fu, Z.; Zhong, R.; Wang, H.; Qi, J. ZIF-67-derived hollow dodecahedral Mn/Co3O4 nanocages with enrichment effect and good mass transfer for boosting low temperature catalytic oxidation of lean methane. J. Environ. Chem. Eng. 2024, 12, 113783. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, C.; Tsubaki, N. Recent advancements and perspectives of the CO2 hydrogenation reaction. Green Carbon 2023, 1, 133–145. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.; Qi, J.; Wan, J.; Wang, Z.; Yu, R.; Wang, D. Mass transfer modulation by hollow multi-shelled structures for high space-time yield synthesis of light olefins from syngas. Adv. Funct. Mater. 2024, 34, 2316547. [Google Scholar] [CrossRef]
- Hu, K.; Tian, J.; Zhou, Z.; Zhao, D.; Guan, X. Direct Z-scheme photocatalytic systems based on vdW heterostructures for water splitting and CO2 reduction: Fundamentals and recent advances. Microstructures 2024, 4, 2024021. [Google Scholar] [CrossRef]
- Ji, W.; Liu, J.; Sha, C.; Yong, Y.; Jiang, Y.; Fang, Z. Nanomaterial-biological hybrid systems: Advancements in solar-driven CO2-to-chemical conversion. Green Carbon 2024, 2, 322–336. [Google Scholar] [CrossRef]
- Li, H.; Du, H.; Luo, H.; Wang, H.; Zhu, W.; Zhou, Y. Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance. Microstructures 2023, 3, 2023024. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, Y.; Guo, Y.; Zhang, S.; Song, H.; Li, X.; Gao, Q.; Shang, W.; Hu, S.; et al. MXene Ti3C2 decorated g-C3N4/ZnO photocatalysts with improved photocatalytic performance for CO2 reduction. Nano Mater. Sci. 2023, 5, 237–245. [Google Scholar] [CrossRef]
- Liao, H.; Huang, K.; Hou, W.; Guo, H.; Lian, C.; Zhang, J.; Liu, Z.; Wang, L. Atmosphere engineering of metal-free Te/C3N4 p-n heterojunction for nearly 100% photocatalytic converting CO2 to CO. Adv. Powder Mater. 2024, 3, 100243. [Google Scholar] [CrossRef]
- Ren, Q.; He, Y.; Wang, H.; Sun, Y.; Dong, F. Rapid energy exchange between in situ formed bromine vacancies and CO2 molecules enhances CO2 photoreduction. Research 2023, 6, 0244. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, S.; Jing, W.; Qiu, H.; Liu, Y.; Guo, L. Double Z-scheme in SnO2/SnS2/Cu2SnS3 heterojunction for photocatalytic reduction of CO2 to ethanol. J. Mater. Sci. Technol. 2024, 189, 146–154. [Google Scholar] [CrossRef]
- Yang, X.; Wang, T.; Ma, H.; Shi, W.; Xia, Z.; Yang, Q.; Zhang, P.; Ma, R.; Xie, G.; Chen, S. Matched micro-geometrical configuration leading to hetero-interfacial intimate contact of MoS2@UiO-66-NH2 Z-scheme heterojunction for efficient photocatalytic CO2 reduction. J. Mater. Sci. Technol. 2024, 182, 210–219. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, S.; Zuo, S.; Zhang, H.; Chen, B.; Huang, J.; Wu, X.; Xu, Q.; Zhu, Q. Si doping-induced electronic structure regulation of single-atom Fe sites for boosted CO2 electroreduction at low overpotentials. Research 2023, 6, 0079. [Google Scholar] [CrossRef]
- Deng, C.; Qi, C.; Wu, X.; Jing, G.; Zhao, H. Unveiling the relationship between structural evaluation and catalytic performance of InOOH during electroreduction of CO2 to formate. Green Carbon 2024, 2, 124–130. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Wang, X.; Zhou, J.; Liang, H. Bi2S3/In(OH)3 heterojunction for enhanced CO2 electroreduction to formic acid. Prog. Nat. Sci. Mater. Int. 2024, 34, 1167–1172. [Google Scholar] [CrossRef]
- Tu, X.; Liu, X.; Zhang, Y.; Zhu, J.; Jiang, H. Advances in Sn-based oxide catalysts for the electroreduction of CO2 to formate. Green Carbon 2024, 2, 131–148. [Google Scholar] [CrossRef]
- Wei, W.; Liu, H.; Zhu, Q. Electrocatalytic CO2 reduction in acids: A groundbreaking approach to converting CO2 into fuels and feedstocks. Research 2024, 8, 0589. [Google Scholar] [CrossRef]
- Du, P.; Deng, G.; Li, Z.; Sun, J.; Wang, L.; Yang, Y.; Wang, J.; Li, Y.; Xu, X.; Zhang, Y.; et al. Effective CO2 activation of enriched oxygen vacancies for photothermal CO2 methanation. J. Mater. Sci. Technol. 2024, 189, 203–210. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, J.; Zhu, T. Enhanced thermal-assisted photocatalytic CO2 reduction by RGO/H-CN two-dimensional heterojunction. J. Mater. Sci. Technol. 2024, 176, 36–47. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Wang, G.; Wang, K.; Li, J.; Zhao, L. In situ irradiated XPS investigation on S-scheme TiO2/Bi2S3 photocatalyst with high interfacial charge separation for highly efficient photothermal catalytic CO2 reduction. J. Mater. Sci. Technol. 2024, 189, 86–95. [Google Scholar] [CrossRef]
- Li, C.; Guo, R.; Zhang, Z.; Wu, T.; Pan, W. Converting CO2 into value-added products by Cu2O-based catalysts: From photocatalysis, electrocatalysis to photoelectrocatalysis. Small 2023, 19, 202207875. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.; Das, S.; Choudhary, S.; Venkanna, G.; Sharma, B.; Afroz, M.A.; Tailor, N.K.; Joshi, R.; Satapathi, S.; Tripathi, K. Enigma of sustainable CO2 conversion to renewable fuels and chemicals through photocatalysis, electrocatalysis, and photoelectrocatalysis: Design strategies and atomic level insights. Small 2025, 21, 2408981. [Google Scholar] [CrossRef]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Cai, H.; Yang, H.; Feng, J.; Zhou, K.; Liu, C.; Hu, Q.; He, C. Ionic liquid-induced product switching in CO2 electroreduction on copper reaction interface. Adv. Funct. Mater. 2024, 34, 2404102. [Google Scholar] [CrossRef]
- Yang, H.; Cai, H.; Li, D.; Kong, Y.; Feng, S.; Jiang, X.; Hu, Q.; He, C. Molecular modification enables CO2 electroreduction to methane on platinum surface in acidic media. Natl. Sci. Rev. 2024, 11, nwae361. [Google Scholar] [CrossRef]
- Yu, M.; Li, M.; Zhang, X.; Ge, Z.; Xu, E.; Wang, L.; Yin, B.; Dou, Y.; Yang, Y.; Zhang, X.; et al. Coupling photocatalytic reduction and biosynthesis towards sustainable CO2 upcycling. Angew. Chem. Int. Edit. 2025, 137, e202423995. [Google Scholar]
- Hu, B.; Li, Z.; Wang, B.; Chen, L.; Wang, X.; Hu, X.; Bai, Z.; Li, Y.; Chen, G.; Luo, X.; et al. Construction of Co-In dual single-atom catalysts for photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. Energy 2025, 371, 125196. [Google Scholar] [CrossRef]
- Zou, S.; Wang, L.; Wang, H.; Zhang, X.; Sun, H.; Liao, X.; Huang, J.; Masri, A.R. Structure-performance correlation on bimetallic catalysts for selective CO2 hydrogenation. Energy Environ. Sci. 2023, 16, 5513–5524. [Google Scholar] [CrossRef]
- Jiao, F.; Mu, R.; Xu, S.; Liu, H.; Yu, L.; Gao, D.; Song, Y.; Deng, D.; Wang, G.; Zheng, H.; et al. A career in catalysis: Xinhe Bao. ACS Catal. 2025, 15, 3061–3084. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, L. Microdroplet assisted hollow ZnCdS@PDA nanocages’ synergistic confinement effect for promoting photocatalytic H2O2 production. Mater. Horizons 2024, 11, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhi, Y.; Liu, Z.; Yuan, J.; Liu, W.; Zhang, W.; Xu, Z.; Zheng, A.; Wei, Y.; Liu, Z. Multiscale dynamical cross-talk in zeolite-catalyzed methanol and dimethyl ether conversions. Natl. Sci. Rev. 2022, 9, nwac151. [Google Scholar] [CrossRef]
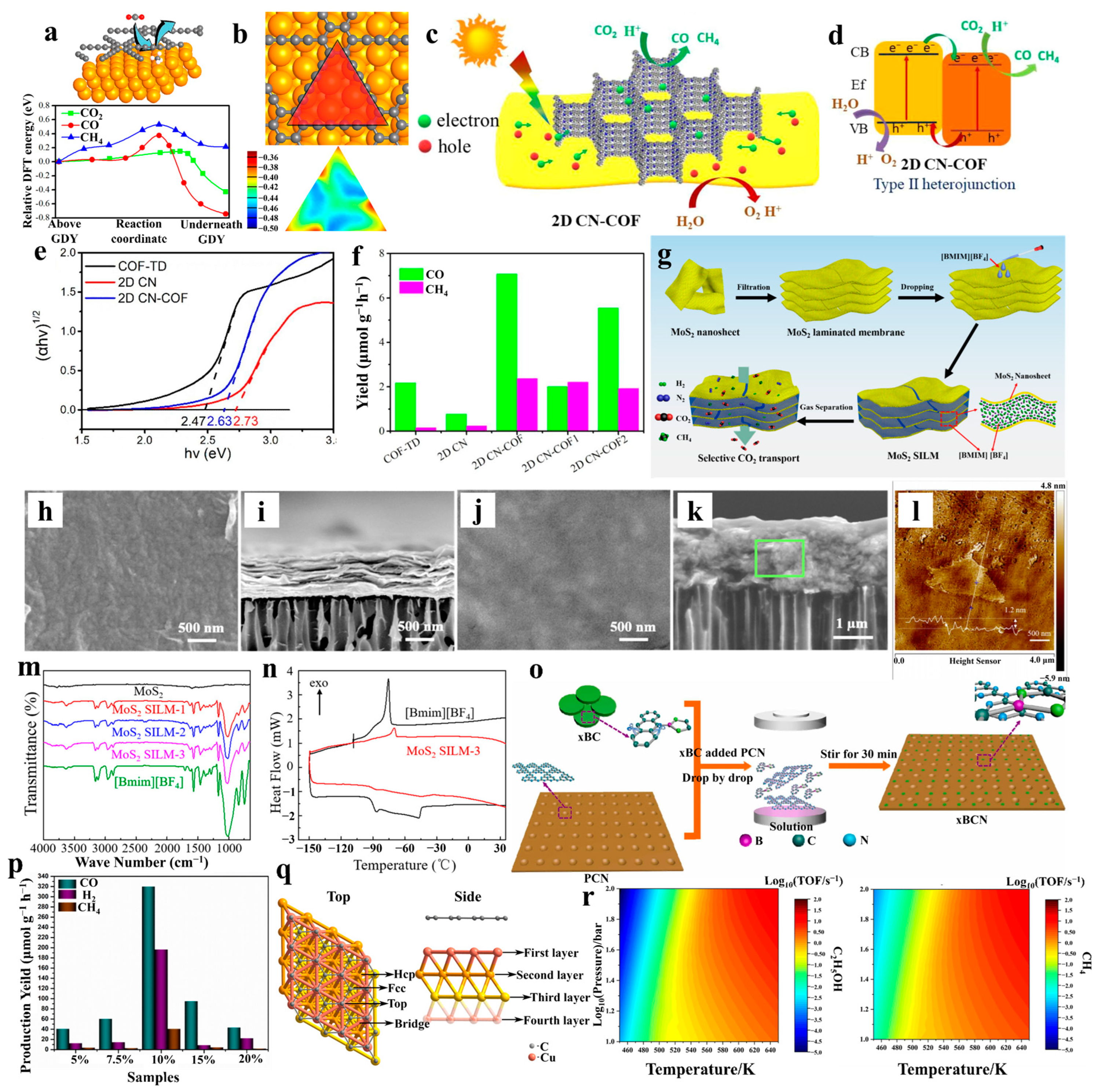
- Yang, Y.; He, D.; Feng, X.; Xiao, X. Low-dimension confinement effect in COF-based hetero-photocatalyst for energy-conversion application. SmartMat 2023, 5, e1223. [Google Scholar] [CrossRef]
- Tao, M.; Jia, Z.; He, H.; Han, Y.; Yu, X.; Ren, Y.; Wu, Y.; Lin, Y.; Shi, Z.; Zhao, Z.; et al. Ti F bridged IL-CuCQDs-F/TiO2 inverse opal composite for boosting CO2 visible-photo reduction via slow photon effect. J. Colloid Interface Sci. 2025, 678, 45–56. [Google Scholar] [CrossRef]
- Wang, G.; Luo, R.; Yang, C.; Song, J.; Xiong, C.; Tian, H.; Zhao, Z.; Mu, R.; Gong, J. Active sites in CO2 hydrogenation over confined VOx-Rh catalysts. Sci. China Chem. 2019, 62, 1710–1719. [Google Scholar] [CrossRef]
- Xu, Z.; Han, Y.; Sun, J.; Xu, M.; Zhao, W.; Wang, Q. Confinement and interface engineering of CuSiO3 nanotubes for enhancing CO2 electroreduction to C2+ products. Electrochim. Acta 2023, 470, 143291. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, S.; Li, N.; Chen, J.; Zhou, X.; Qian, Y.; Huang, C.; Zhang, Y. Water decontamination via nonradical process by nanoconfined Fenton-like catalysts. Nat. Commun. 2023, 14, 2881. [Google Scholar] [CrossRef]
- Peng, M.; Li, C.; Wang, Z.; Wang, M.; Zhang, Q.; Xu, B.; Li, M.; Ma, D. Interfacial catalysis at atomic level. Chem. Rev. 2025, 125, 2371–2439. [Google Scholar] [CrossRef]
- Fan, J.; Liang, S.; Zhu, K.; Mao, J.; Cui, X.; Ma, C.; Yu, L.; Deng, D. Boosting room-temperature conversion of methane via confining Cu atoms in ultrathin Ru nanosheets. Chem. Catal. 2022, 2, 2253–2261. [Google Scholar] [CrossRef]
- He, D.; Zhang, Y.; Li, T.; Chen, D.; Wang, H.; Zhang, L.; Liu, J.; Cao, X.; Lu, J.; Luo, Y. Designing ultra-stable and surface-exposed Ni nanoparticles with dually confined microenvironment for high-temperature methane dry reforming. Adv. Funct. Mater. 2025, 35, 2412895. [Google Scholar] [CrossRef]
- Mo, Q.; Chen, C.; Li, S.; Song, H.; Zhang, L. Highly dispersed single clusters supported porphyrinic metal–organic frameworks for synergetic CO2 electroreduction to CH4. Small 2025, 21, 2411926. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Meng, S.; Cui, Z.; Li, S.; Li, D.; Gao, X.; Guo, H.; Bogaerts, A.; Yi, Y. Plasma-catalytic direct oxidation of methane to methanol over Cu-MOR: Revealing the zeolite-confined Cu2+ active sites. Chem. Eng. J. 2024, 496, 154337. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Sun, H.; Zhao, J.; Zhang, Y.; Wang, Y.; Zhu, C.; Gao, D.; Tuo, Y.; Zeng, J.; et al. Confinement catalysis of reaction intermediates in Ag@Cu2O cascade nanoreactors toward boosted electrochemical C–C coupling. ACS Catal. 2024, 14, 14744–14753. [Google Scholar] [CrossRef]
- Yang, C.; Fu, X.; Luan, D.; Xiao, J. Towards rational design of confined catalysis in carbon nanotube by machine learning and grand canonical monte carlo simulations. Angew. Chem. Int. Edit. 2025, 64, e202421552. [Google Scholar] [CrossRef]
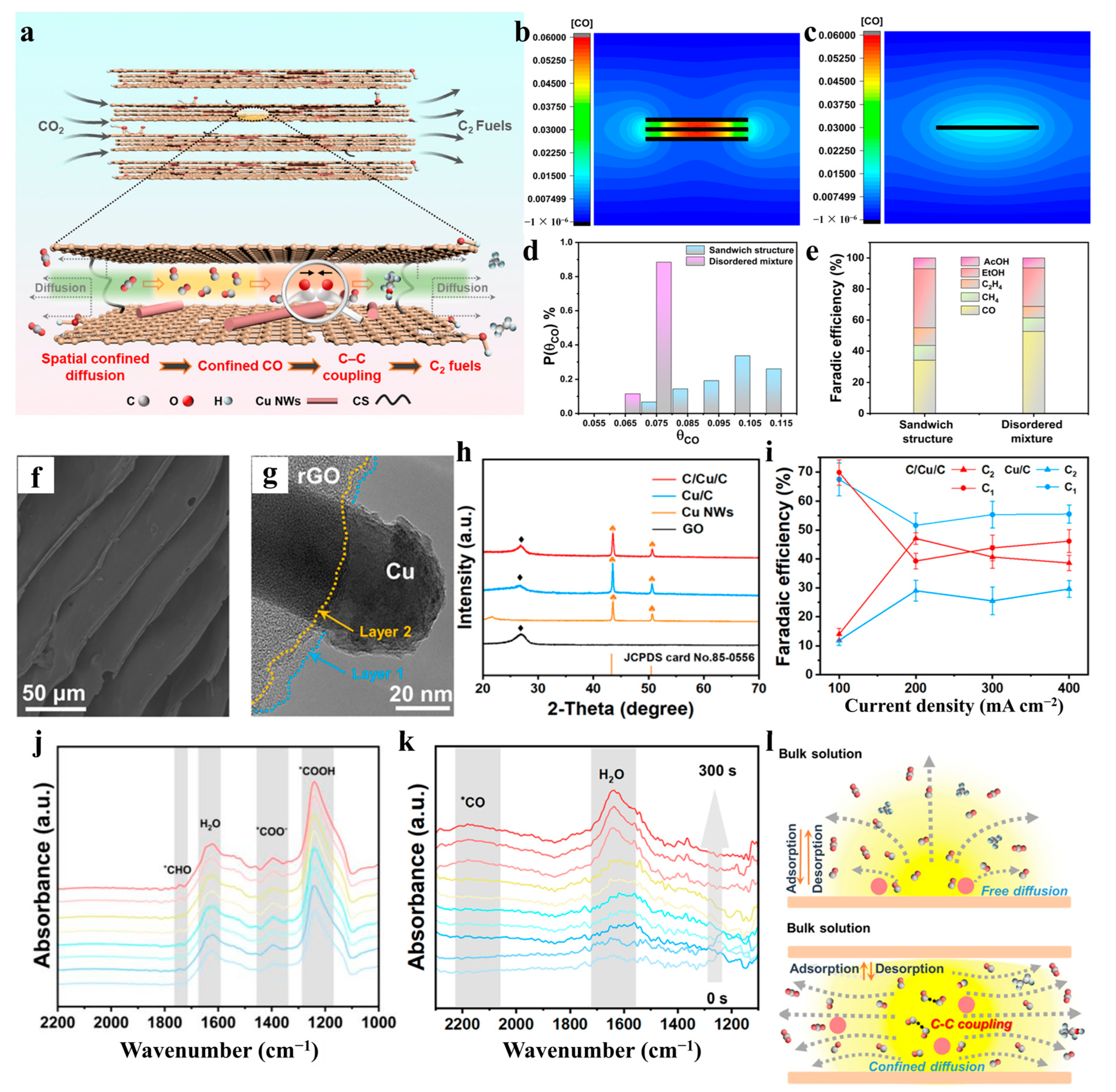
- Fan, W.; Liu, Y.; Zhang, C.; Chen, X.; He, D.; Li, M.; Hu, Q.; Jiao, X.; Chen, Q.; Xie, Y. Confined CO in a sandwich structure promotes C–C coupling in electrocatalytic CO2 reduction. Mater. Horizons 2024, 11, 4183–4189. [Google Scholar] [CrossRef]
- Xie, G.; Bai, X.; Niu, Y.; Zhang, R.; Liu, J.; Yang, Q.; Wang, Z. Highly dispersed AuCu nanoparticles confined in Zr-MOFs for efficient methanol synthesis from CO2 hydrogenation. ACS Appl. Mater. Interfaces 2024, 16, 70626–70633. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, G.; Dong, C.; Fan, Y.; Yang, S.; Chen, M.; Guo, X.; Mu, R.; Ning, Y.; et al. Confinement-induced indium oxide nanolayers formed on oxide support for enhanced CO2 hydrogenation reaction. J. Am. Chem. Soc. 2024, 146, 5523–5531. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, H. Metal@hollow carbon sphere nanoreactors for sustainable biomass and CO2 valorization. J. Mater. Chem. A 2022, 10, 7557–7603. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Pan, X.; Bao, X. Effect of confinement in carbon nanotubes on the activity of fischer−tropsch iron catalyst. J. Am. Chem. Soc. 2008, 130, 9414–9419. [Google Scholar] [CrossRef]
- Das, R.; Muthukumar, D.; Pillai, R.S.; Nagaraja, C.M. Rational design of a ZnII MOF with multiple functional sites for highly efficient fixation of CO2 under mild conditions: Combined experimental and theoretical investigation. Chem. Eur. J. 2020, 26, 17445–17454. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 6, 507–511. [Google Scholar] [CrossRef] [PubMed]
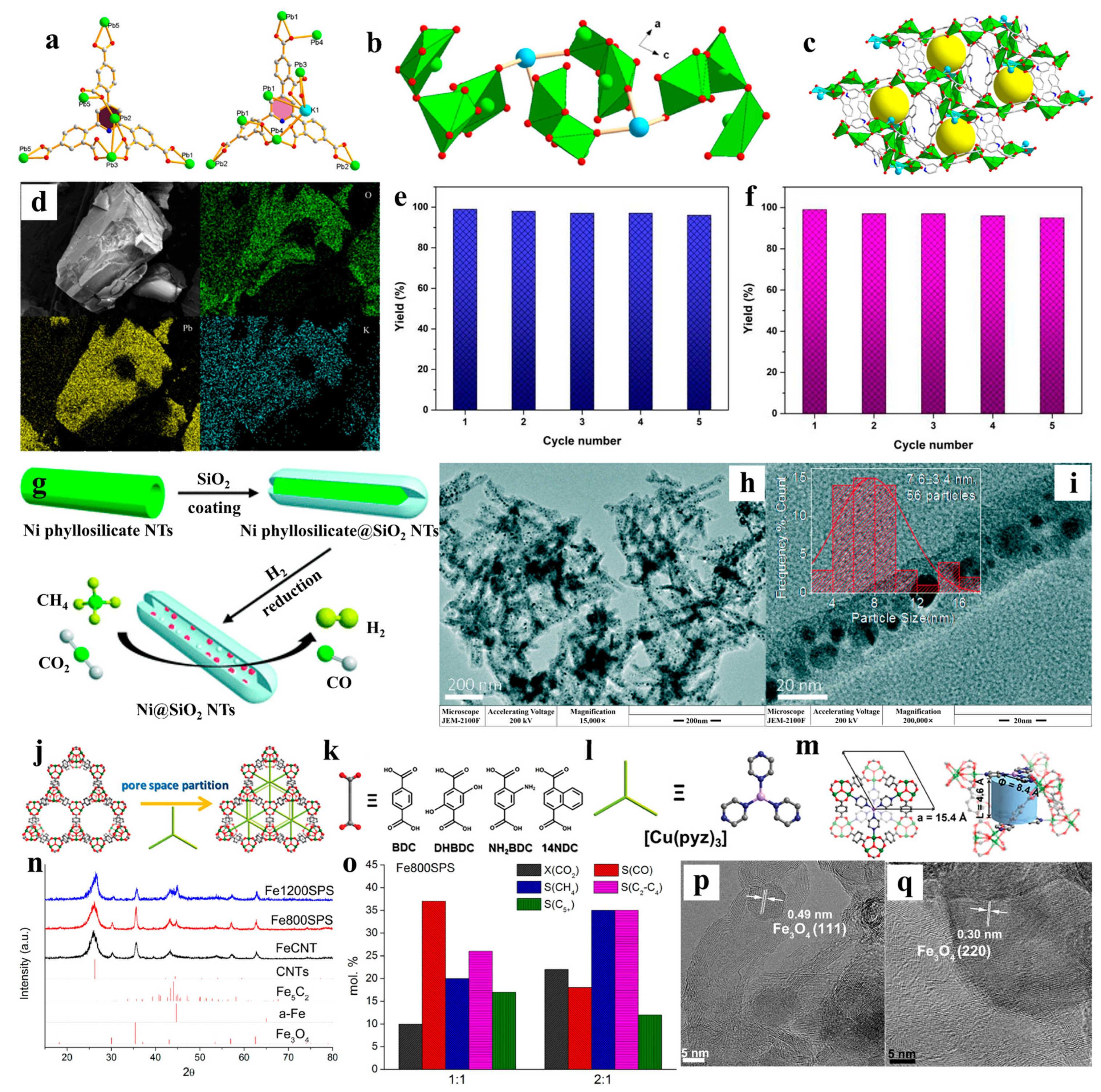
- Liu, S.; Chen, H.; Zhang, X. Bifunctional {Pb10K2}–organic framework for high catalytic activity in cycloaddition of CO2 with epoxides and knoevenagel condensation. ACS Catal. 2022, 12, 10373–10383. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Jiang, B.; Kawi, S. Sintering resistant Ni nanoparticles exclusively confined within SiO2 nanotubes for CH4 dry reforming. Catal. Sci. Technol. 2018, 8, 3363–3371. [Google Scholar] [CrossRef]
- Xue, Y.; Lei, J.; Lv, H.; Liang, P.; Li, L.; Zhai, Q. Spatially confined ?-complexation within pore-space-partitioned metal-organic frameworks for enhanced light hydrocarbon separation and purification. Small 2024, 20, 2311555. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Stolbov, D.N.; Maksimov, S.V.; Maslakov, K.I.; Chernavskii, P.A.; Pokusaeva, Y.A.; Koklin, A.E.; Bogdan, V.I.; Savilov, S.V. Sintered Fe/CNT framework catalysts for CO2 hydrogenation into hydrocarbons. Carbon 2020, 168, 475–484. [Google Scholar] [CrossRef]
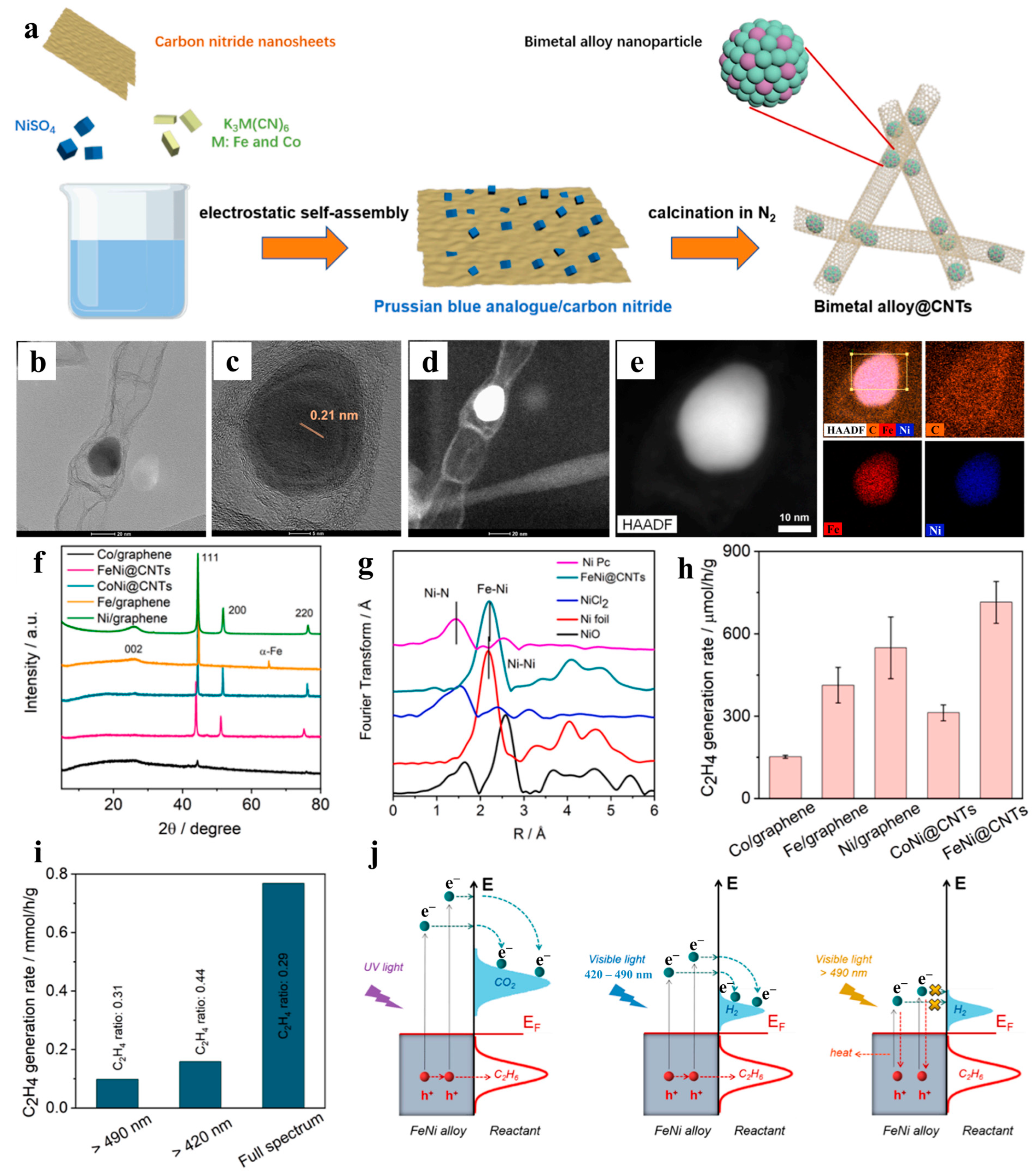
- Zhang, J.; Li, M.; Tan, X.; Shi, L.; Xie, K.; Zhao, X.; Wang, S.; Zhao, S.; Zhang, H.; Duan, X.; et al. Confined FeNi alloy nanoparticles in carbon nanotubes for photothermal oxidative dehydrogenation of ethane by carbon dioxide. Appl. Catal. B-Environ. 2023, 339, 123166. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, C.; Wang, Y.; Fang, D.; Zhang, L.; Wang, C. Confining chainmail-bearing Ni nanoparticles in N-doped carbon nanotubes for robust and efficient electroreduction of CO2. ChemSusChem 2021, 14, 1140–1154. [Google Scholar] [CrossRef]
- Fu, Q.; Bao, X. Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 2017, 46, 1842–1874. [Google Scholar] [CrossRef]
- Mu, R.; Fu, Q.; Jin, L.; Yu, L.; Fang, G.; Tan, D.; Bao, X. Visualizing chemical reactions confined under graphene. Angew. Chem. Int. Edit. 2012, 51, 4856–4859. [Google Scholar] [CrossRef]
- Yao, Y.; Fu, Q.; Zhang, Y.Y.; Weng, X.; Li, H.; Chen, M.; Jin, L.; Dong, A.; Mu, R.; Jiang, P.; et al. Graphene cover-promoted metal-catalyzed reactions. Proc. Natl. Acad. Sci. USA 2014, 111, 17023–17028. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, J.; Fu, Q.; Bao, X. Confined catalysis under two-dimensional materials. Proc. Natl. Acad. Sci. USA 2017, 114, 5930–5934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Z.; Ju, M.; Guo, L. Confined electrochemical catalysis under cover: Enhanced CO2 reduction at the interface between graphdiyne and Cu surface. Appl. Surf. Sci. 2019, 479, 685–692. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Zhang, X.; Li, X.; Zhu, Z.; Ma, C.; Yan, Y.; Huo, P.; Yang, G. Boosting charge carriers separation and migration efficiency via fabricating all organic van der Waals heterojunction for efficient photoreduction of CO2. Chem. Eng. J. 2021, 408, 127292. [Google Scholar] [CrossRef]
- Chen, D.; Ying, W.; Guo, Y.; Ying, Y.; Peng, X. Enhanced gas separation through nanoconfined ionic liquid in laminated MoS2 membrane. ACS Appl. Mater. Interfaces 2017, 9, 44251–44257. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, H.; Song, T.; Yin, S.; Long, B.; Ali, A.; Deng, G. Fine-tuning the conjugate system of 0D heteroborane to construct an all-organic 0D/2D heterojunction for photocatalytic CO2 reduction. J. Environ. Chem. Eng. 2021, 9, 106642. [Google Scholar] [CrossRef]
- Wei, Z.; Bai, B.; Bai, H.; Duan, Y.; Yang, M.; Cao, H.; Zuo, Z.; Zuo, J.; Wang, Q.; Huang, W. Mechanistic exploration of syngas conversion at the interface of graphene/Cu(111): Identifying the effect of promoted electron transfer on the product selectivity. Catal. Sci. Technol. 2023, 13, 6527–6540. [Google Scholar] [CrossRef]
- An, B.; Ma, Y.; Han, X.; Schröder, M.; Yang, S. Activation and catalysis of methane over metal–organic framework materials. Acc. Mater. Res. 2024, 6, 77–88. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Guo, S.; Ma, Z.; Li, Y.; Ma, L.; Zhang, K. Abundant hydrogen production over well dispersed nickel nanoparticles confined in mesoporous metal oxides in partial oxidation of methane. Int. J. Hydrog. Energy 2019, 44, 30171–30184. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Q. Three-dimensional networked Ni-phyllosilicate catalyst for CO2 methanation: Achieving high dispersion and enhanced stability at high Ni loadings. ACS Sustain. Chem. Eng. 2020, 8, 6753–6766. [Google Scholar] [CrossRef]
- Wu, T.; Lin, J.; Cheng, Y.; Tian, J.; Wang, S.; Xie, S.; Pei, Y.; Yan, S.; Qiao, M.; Xu, H.; et al. Porous graphene-confined Fe–K as highly efficient catalyst for CO2 direct hydrogenation to light olefins. ACS Appl. Mater. Interfaces 2018, 10, 23439–23443. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Ichibha, T.; Qin, K.S.; Muraoka, K.; Vequizo, J.J.M.; Hibino, K.; Kuriki, R.; Yamashita, S.; Hongo, K.; Uchiyama, T.; et al. Undoped layered perovskite oxynitride Li2LaTa2O6N for photocatalytic CO2 reduction with visible light. Angew. Chem. Int. Edit. 2018, 57, 8154–8158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Wan, S.; Zhang, C.; Li, Z.; Zhang, S.; Zhong, Q. ZIF-67-derived ultrathin Co-Ni layered double hydroxides wrapped on 3D g-C3N4 with enhanced visible-light photocatalytic performance for greenhouse gas CO2 reduction. J. Environ. Chem. Eng. 2022, 10, 108119. [Google Scholar] [CrossRef]
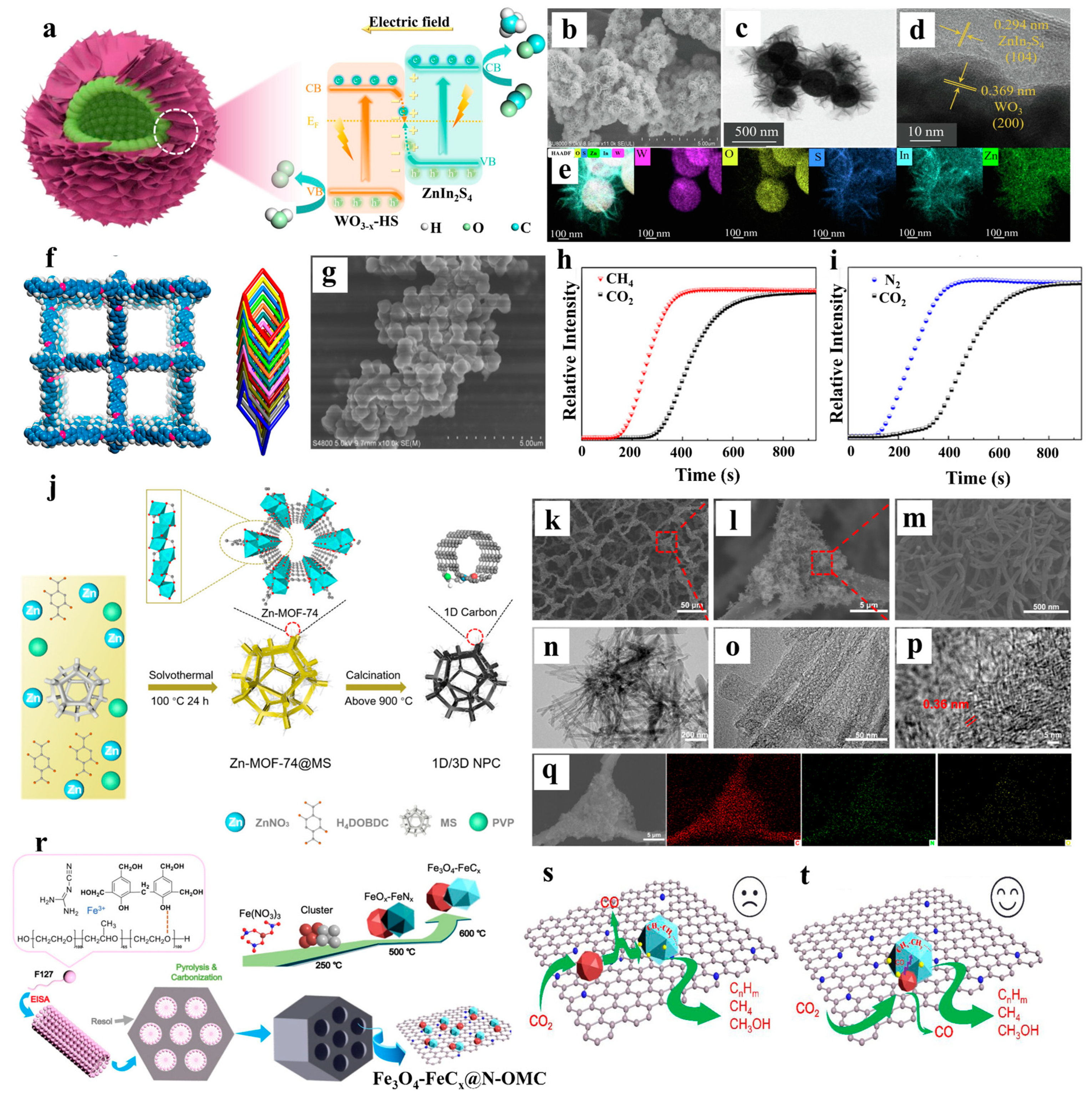
- Xia, Y.; Xiao, J.; Zhang, J.; Huang, R.; Huang, X.; Yan, G.; Zheng, L. Construction of a sunflower-like S-scheme WO3/ZnIn2S4 heterojunction with spatial charge transfer for enhanced photocatalytic CO2 reduction. Fuel 2025, 382, 133779. [Google Scholar] [CrossRef]
- Guan, X.; Ma, Y.; Li, H.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Feng, D.; Wu, C.; Sun, C.; Jia, B.; Liu, X.; Ma, T. MOF-derived 1D/3D N-doped porous carbon for spatially confined electrochemical CO2 reduction to adjustable syngas. Carbon Energy 2024, 6, e461. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Yan, J.; Qiao, X.; Guan, Q.; Li, W. N-doped ordered mesoporous carbon (N-OMC) confined Fe3O4-FeCx heterojunction for efficient conversion of CO2 to light olefins. Appl. Catal. B-Environ. 2021, 299, 120639. [Google Scholar] [CrossRef]
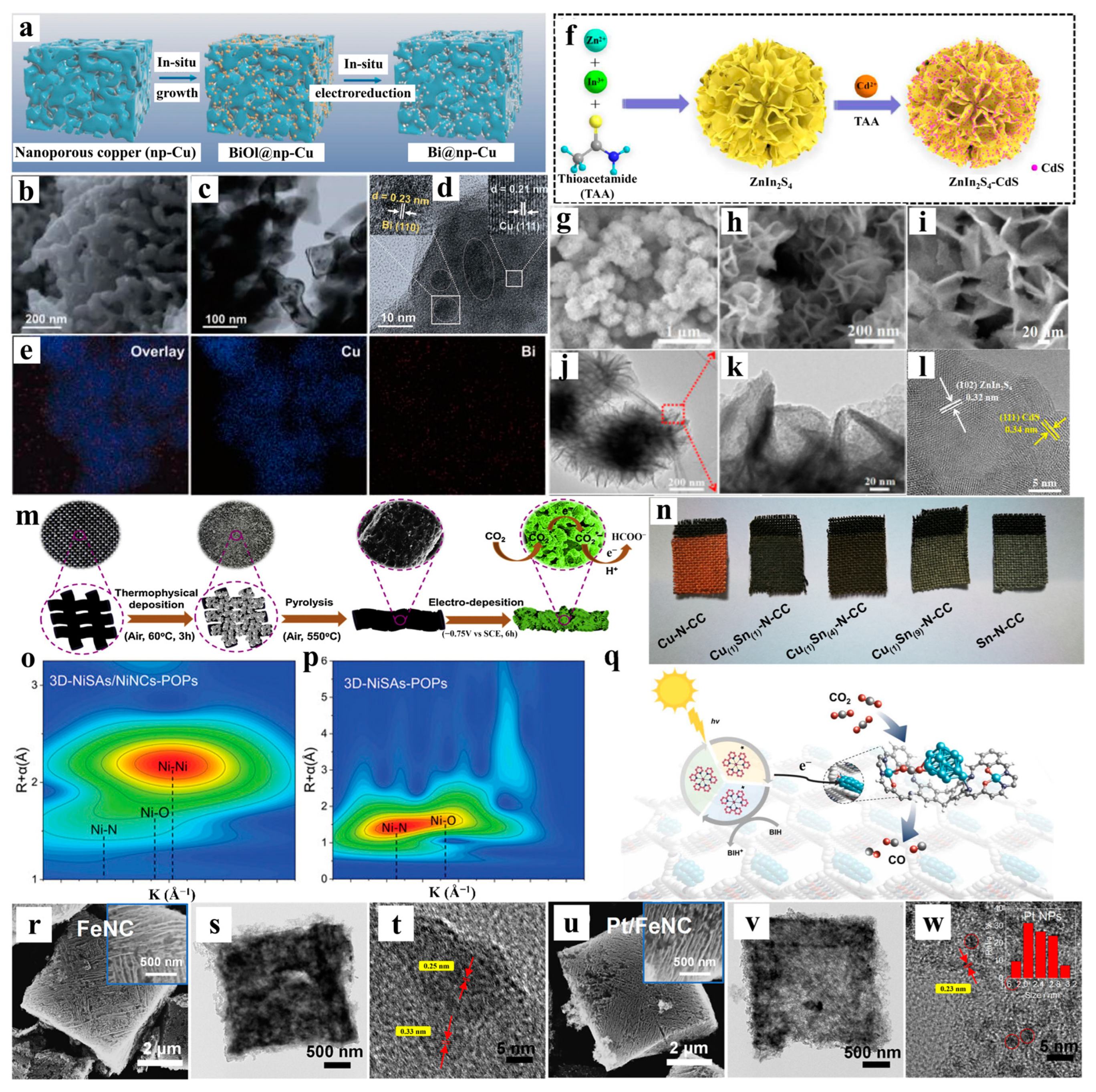
- Zhao, Q.; Wang, J.; Zhuang, Y.; Gong, L.; Zhang, W.; Fan, W.; Lu, Z.; Zhang, Y.; Fujita, T.; Zhang, P.; et al. In situ reconstruction of Bi nanoparticles confined within 3D nanoporous Cu to boost CO2 electroreduction. Sci. China Mater. 2024, 67, 796–803. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Qu, Y.; Zhou, F.; Wang, Z.; Wang, W.; Zhao, C.; Wang, H.; Li, L.; Yao, Y.; et al. A hierarchical heterostructure of CdS QDs confined on 3D ZnIn2S4 with boosted charge transfer for photocatalytic CO2 reduction. Nano Res. 2020, 14, 81–90. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Masood, I.; Zhou, B.; Wang, Y.; Lin, J.; Qiao, J.; Zhang, F. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction. Appl. Catal. B-Environ. 2020, 264, 118447. [Google Scholar] [CrossRef]
- Yan, F.; Dong, X.; Wang, Y.; Wang, Q.; Wang, S.; Zang, S. Asymmetrical interactions between Ni single atomic sites and Ni clusters in a 3D porous organic framework for enhanced CO2 photoreduction. Adv. Sci. 2024, 11, 2401508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhuo, H.; Dong, Y.; Zhou, Y.; Li, Y.; Hao, H.; Li, D.; Shi, W.; Zeng, S.; Xu, S.; et al. Pt Nanoparticles confined in a 3D porous FeNC matrix as efficient catalysts for rechargeable Li-CO2/O2 batteries. ACS Appl. Mater. Interfaces 2023, 15, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, D.; Zhang, W.; Wang, L. Graphene-supported isolated platinum atoms and platinum dimers for CO2 hydrogenation: Catalytic activity and selectivity variations. Chin. Chem. Lett. 2025, 36, 110611. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Li, Y.; Wang, J.; Zhang, G. Efficient photocatalytic CO2 conversion over 2D/2D Ni-doped CsPbBr3/Bi3O4Br Z-scheme heterojunction: Critical role of Ni doping, boosted charge separation and mechanism study. Appl. Catal. B-Environ. 2022, 319, 121895. [Google Scholar] [CrossRef]
- Kumar, P.; Hu, J.; Kibria, M.G. Edge-confined under-coordinated Cu atoms on Ru nanosheets enable efficient CH4 activation. Chem. Catal. 2022, 2, 2118–2120. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Kochubei, A.; Lee, C.; Wagner, P.; Chen, Z.; Jiang, Y.; Yan, W.; Wallace, G.G.; Wang, C. Copper/polyaniline interfaces confined CO2 electroreduction for selective hydrocarbon production. ChemSusChem 2024, 17, e202400209. [Google Scholar] [CrossRef]
- Kang, X.; Liu, J.; Xie, Y.; Wang, D.; Liu, Q.; Yu, P.; Tian, C.; Fu, H. Pt single atoms in oxygen vacancies boost reverse water-gas shift reaction by enhancing hydrogen spillover. Sci. China Mater. 2024, 67, 3579–3588. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.M.; Won, J.M.; Yang, H.J.; Hong, S.C. Effect of Ce/Ti ratio on the catalytic activity and stability of Ni/CeO2–TiO2 catalyst for dry reforming of methane. Chem. Eng. J. 2015, 280, 433–440. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Senftle, T.P.; Zhu, J.; Zhang, G.; Guo, X.; Song, C. Variation in the In2O3 crystal phase alters catalytic performance toward the reverse water gas shift reaction. ACS Catal. 2019, 10, 3264–3273. [Google Scholar] [CrossRef]
- Cannizzaro, F.; Kurstjens, S.; van den Berg, T.; Hensen, E.J.M.; Filot, I.A.W. A computational study of CO2 hydrogenation on single atoms of Pt, Pd, Ni and Rh on In2O3(111). Catal. Sci. Technol. 2023, 13, 4701–4715. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, Z.; Yan, J.; Ge, Q.; Liu, C. Reverse water gas shift over In2O3–CeO2 catalysts. Catal. Today 2016, 259, 402–408. [Google Scholar] [CrossRef]
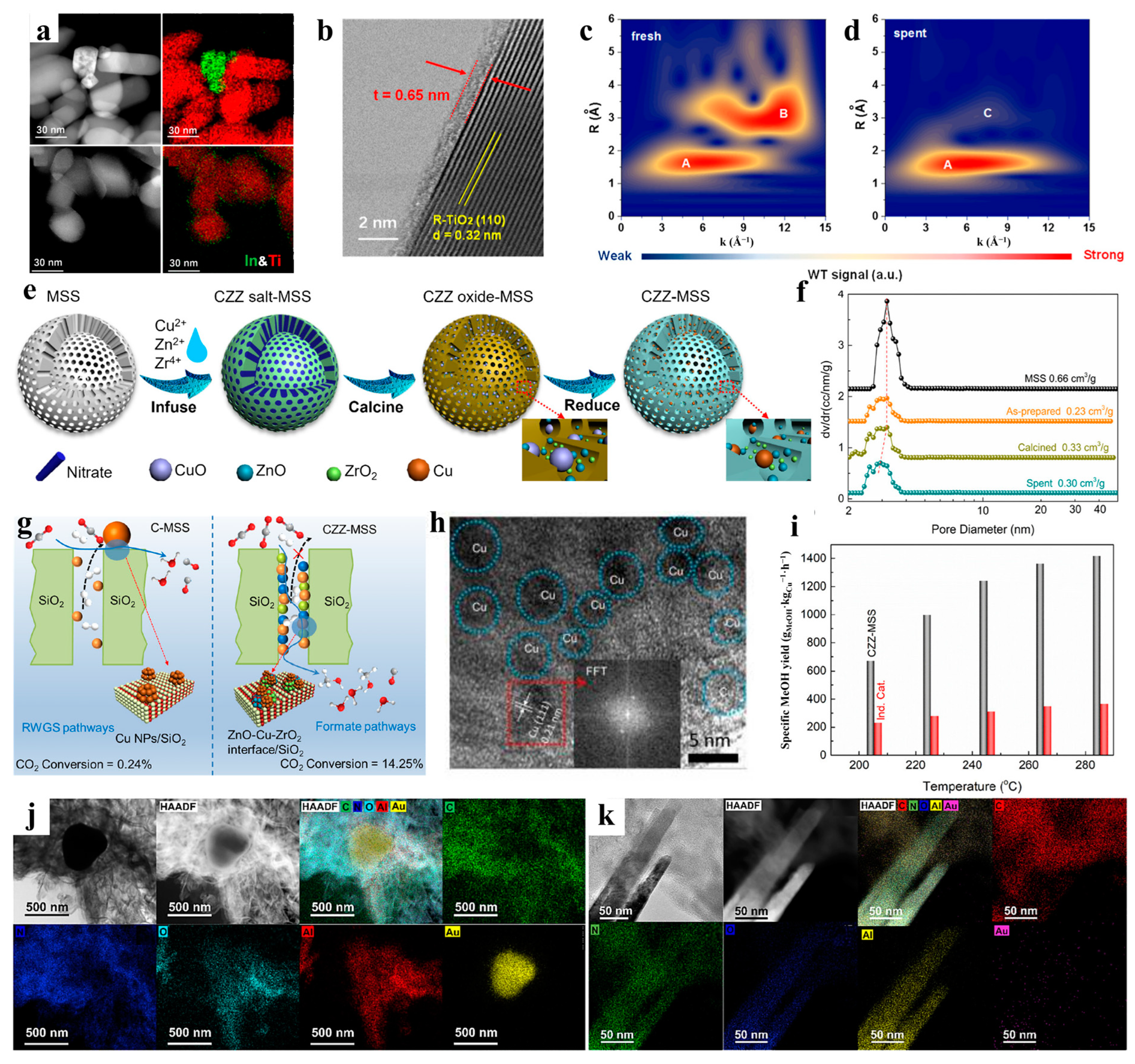
- Chen, C.; Kosari, M.; Xi, S.; Lim, A.M.H.; He, C.; Zeng, H.C. Optimizing the interfacial environment of triphasic ZnO-Cu-ZrO2 confined inside mesoporous silica spheres for enhancing CO2 hydrogenation to methanol. ACS EST Eng. 2023, 3, 638–650. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Gu, F.; Tong, L.; Jiang, J.; Zuo, Y.; Dong, H. Atomically dispersed Au confined by oxygen vacancies in Au-θ-Al2O3/Au/PCN hybrid for boosting photocatalytic CO2 reduction driven by multiple built-in electric fields. Chem. Eng. J. 2023, 476, 146514. [Google Scholar] [CrossRef]
- Bao, C.; Huo, Y.; Li, Y.; Yang, S.; Li, W.; Hu, T. Anchoring Cu on Zirconium-oxo nodes in a pore-confined metal-organic framework for CO2 hydrogenation to methanol. Chem. Eng. J. 2025, 503, 158610. [Google Scholar] [CrossRef]
- Bu, K.; Kuboon, S.; Deng, J.; Li, H.; Yan, T.; Chen, G.; Shi, L.; Zhang, D. Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts. Appl. Catal. B-Environ. 2019, 252, 86–97. [Google Scholar] [CrossRef]
- Long, X.; Feng, C.; Liu, M.; Li, X.; Huang, Z.; Du, Y.; Chen, Y.; Yang, S.; Wang, G.; Ding, D.; et al. PMS activation by hollow sulfur doped multilayer carbon shell wrapped CoFe alloy: Confined adsorption and surface activation. J. Clean Prod. 2023, 425, 138672. [Google Scholar] [CrossRef]
- Meng, J.; Wang, K.; Wang, Y.; Ma, J.; Ban, C.; Feng, Y.; Zhang, B.; Zhou, K.; Gan, L.; Han, G.; et al. Bismuth clusters pinned on TiO2 porous nanowires boosting charge transfer for CO2 photoreduction to CH4. Nano Res. 2023, 17, 1190–1198. [Google Scholar] [CrossRef]
- Wang, X.; Woo, H.Y.; Shen, D.; Park, M.J.; Ali, M.; Zafar, F.; Kang, K.Y.; Choi, J.; Jang, E.; Bae, J.W. Cu–ZnO nanoparticles encapsulated in ZSM-5 for selective conversion of carbon dioxide into oxygenates. EES Catal. 2025, 3, 163–350. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Ren, C.; Wu, L.; Yao, Y.; Zhong, S.; Lin, H.; Zhao, L.; Gao, Y.; Bai, S. Modulating the selectivity of CO2 photoreduction by regulating the location of PtCu in a UiO-66@ZnIn2S4 core–shell nanoreactor. ACS Catal. 2024, 15, 828–840. [Google Scholar] [CrossRef]
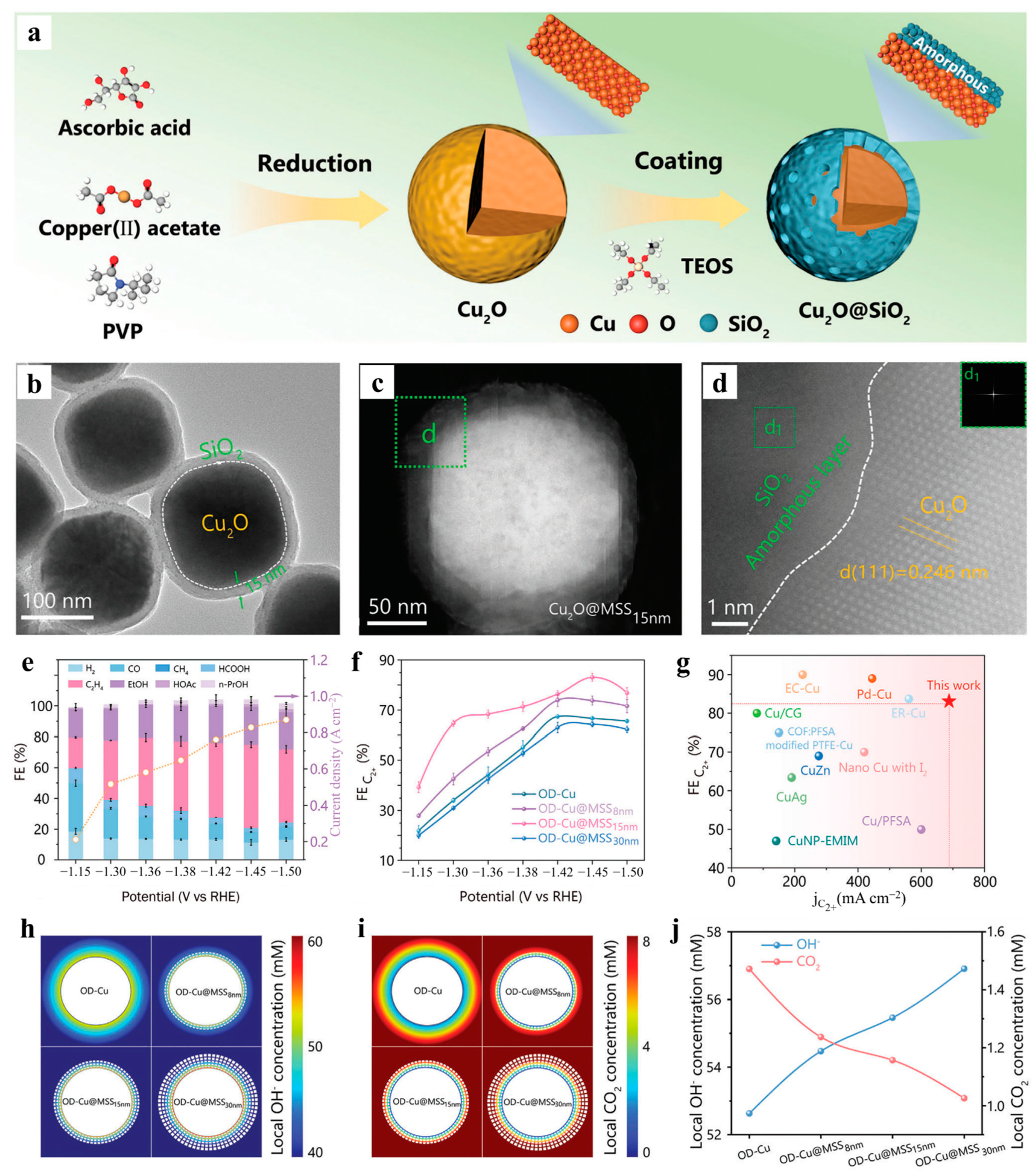
- Wang, Y.; Lai, W.; Tao, H.; Qiao, Y.; Chen, X.; Lian, C.; Ge, J.; Li, J.; Huang, H. CO2 electroreduction to multicarbon products over Cu2O@mesoporous SiO2 confined catalyst: Relevance of the shell thickness. Adv. Energy Mater. 2024, 15, 2404606. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, Y.; Han, W.; Li, W.; Yu, X.; Tian, G. Cu-BTC-confined synthesis of Cu-Cu2O-CuS nanojunctions embedded in a porous carbon matrix for remarkable photothermal CO2 conversion. J. Mater. Chem. A 2023, 11, 8342–8351. [Google Scholar] [CrossRef]
- Cai, S.; Tao, S.; Chong, M.; Shi, Z.; Liu, X.; Cheng, D.; Chen, F. Ag nanoparticles-confined doped within triazine-based covalent organic frameworks for syngas production from electrocatalytic reduction of CO2. ACS Appl. Mater. Interfaces 2024, 16, 55267–55275. [Google Scholar] [CrossRef]
- Gong, S.; Hou, M.; Niu, Y.; Teng, X.; Liu, X.; Xu, M.; Xu, C.; Ka-Man Au, V.; Chen, Z. Molybdenum phosphide coupled with highly dispersed nickel confined in porous carbon nanofibers for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2022, 427, 131717. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Deng, C.; Cao, S.; Dai, X.; Guo, S.; Chen, Y.; Tan, Q.; Zhu, H.; Zhang, S.; et al. Ordered mesoporous carbon spheres assisted Ru nanoclusters/RuO2 with redistribution of charge density for efficient CO2 methanation in a novel H2/CO2 fuel cell. J. Energy Chem. 2022, 72, 116–124. [Google Scholar] [CrossRef]
- Sun, C.; Beaunier, P.; Da Costa, P. Effect of ceria promotion on the catalytic performance of Ni/SBA-16 catalysts for CO2 methanation. Catal. Sci. Technol. 2020, 10, 6330–6341. [Google Scholar] [CrossRef]
- Lan, X.; Li, Q.; Cao, L.; Du, C.; Ricardez-Sandoval, L.; Bai, G. Rebuilding supramolecular aggregates to porous hollow N-doped carbon tube inlaid with ultrasmall Ag nanoparticles: A highly efficient catalyst for CO2 conversion. Appl. Surf. Sci. 2020, 508, 145220. [Google Scholar] [CrossRef]
- Zou, X.; Li, A.; Ma, C.; Gao, Z.; Zhou, B.; Zhu, L.; Huang, Z. Nitrogen-doped carbon confined Cu-Ag bimetals for efficient electroreduction of CO2 to high-order products. Chem. Eng. J. 2023, 468, 143606. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, Z.; Wu, X.; Xiong, W.; Ding, J.; Zhang, Z.; Huang, W. Ni single atoms confined in nitrogen-doped carbon nanotubes for active and selective hydrogenation of CO2 to CO. ACS Catal. 2023, 13, 7132–7138. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, T.; Zheng, W.; Lei, C.; Yang, B.; Chen, J.; Li, Z.; Lei, L.; Yuan, C.; Hou, Y. Nanoconfined tin oxide within N-doped nanocarbon supported on electrochemically exfoliated graphene for efficient electroreduction of CO2 to formate and C1 products. ACS Appl. Mater. Interfaces 2020, 12, 16178–16185. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Yan, J.; Qiao, X.; Zhu, M.; Guan, Q.; Li, W. Heteroatom induced synthesis of FeO-Fe3C confined within F-doped graphene shell for efficient CO2 hydrogenation to light olefins. Chem. Eng. J. 2023, 477, 147153. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, G.; Li, B.; Zhang, X.; Jiao, M.; Wang, N.; Zhang, X.; Liu, L. In situ carbon encapsulation confined nickel-doped indium oxide nanocrystals for boosting CO2 electroreduction to the industrial level. ACS Catal. 2021, 11, 14596–14604. [Google Scholar] [CrossRef]
- Zang, D.; Gao, X.J.; Li, L.; Wei, Y.; Wang, H. Confined interface engineering of self-supported Cu@N-doped graphene for electrocatalytic CO2 reduction with enhanced selectivity towards ethanol. Nano Res. 2022, 15, 8872–8879. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, L.; Wu, C.; Wang, X.; Zhang, X. Confined pyrolysis transformation of ZIF-8 to hierarchically ordered porous Zn-N-C nanoreactor for efficient CO2 photoconversion under mild conditions. J. Catal. 2020, 390, 213–223. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A single-atom nanozyme for wound disinfection applications. Angew. Chem. Int. Edit. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, C.; Lin, C.; Jiang, H. Metal–organic-framework-derived hollow N-doped porous carbon with ultrahigh concentrations of single Zn atoms for efficient carbon dioxide conversion. Angew. Chem. Int. Edit. 2019, 58, 3511–3515. [Google Scholar] [CrossRef]
- Liu, X.; Liao, L.; Xia, G.; Yu, F.; Zhang, G.; Shu, M.; Wang, H. An accurate “metal pre-buried” strategy for constructing Ni–N2C2 single-atom sites with high metal loadings toward electrocatalytic CO2 reduction. J. Mater. Chem. A 2022, 10, 25047–25054. [Google Scholar] [CrossRef]
- Huang, H.; Guo, X.; Jiang, Q.; He, H.; Yang, L.; Zhang, C.; Ying, G.; Xu, X. Space-confined growth of monodisperse Pt within nitrogen-doped mesostructured hexagonal prism carbon nanoarchitectures for boosted methanol oxidation reaction. Chem. Eng. J. 2025, 511, 161570. [Google Scholar] [CrossRef]
- Liu, L.; Lu, J.; Yang, Y.; Ruettinger, W.; Gao, X.; Wang, M.; Lou, H.; Wang, Z.; Liu, Y.; Tao, X.; et al. Dealuminated Beta zeolite reverses Ostwald ripening for durable copper nanoparticle catalysts. Science 2024, 383, 94–101. [Google Scholar] [CrossRef]
- Mamatkulov, M.; Zhdanov, V.P. Ostwald ripening of supported metal nanoparticles: Role of dimers and other general trends. Chem. Eng. Sci. 2025, 308, 121373. [Google Scholar] [CrossRef]
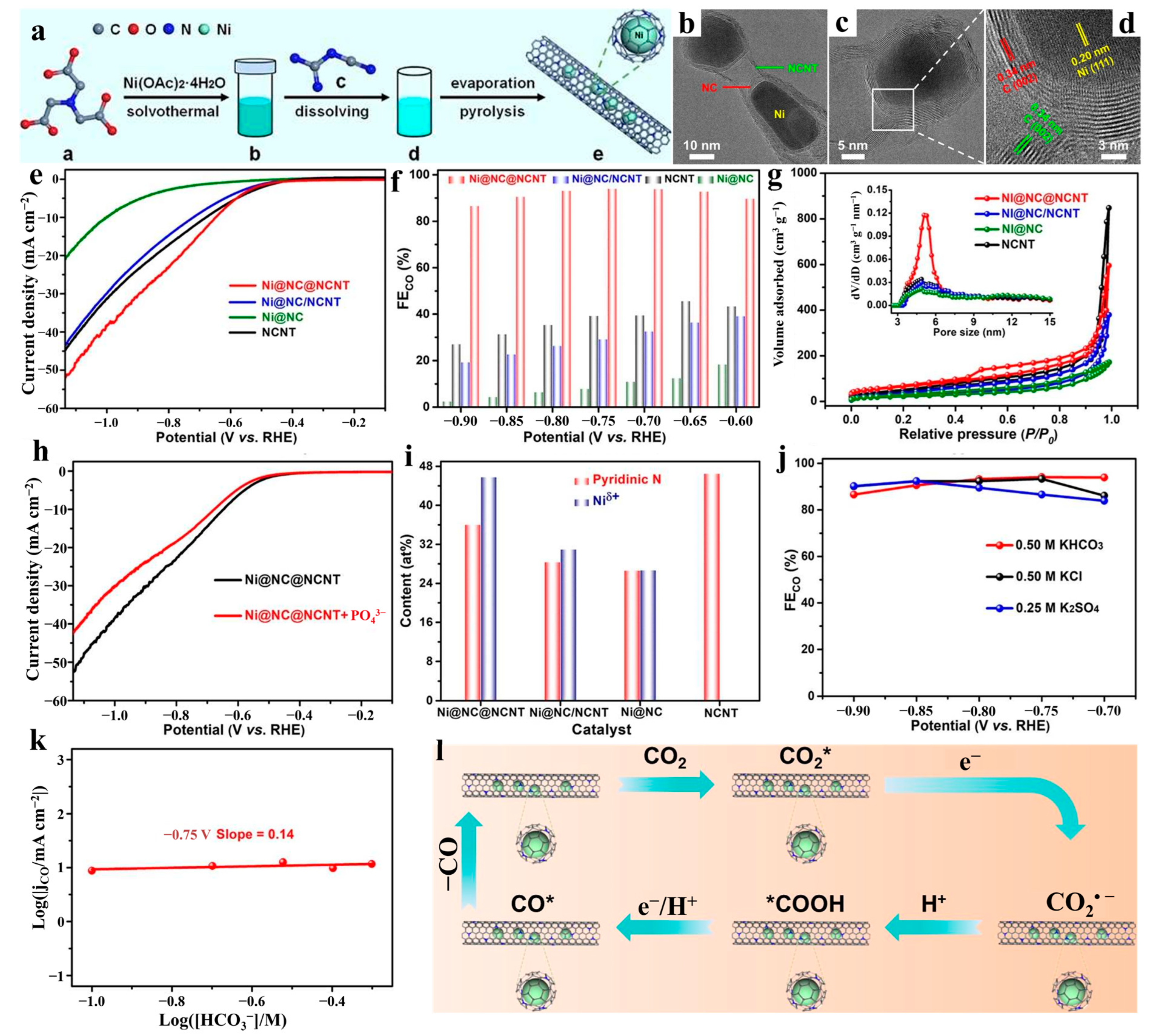
- Guo, M.; Du, S.; Yang, G.; Zhang, Q.; Liu, Z.; Peng, F. MOF-derived N-doped carbon-wrapped Ni electrocatalyst for highly efficient electrochemical CO2 reduction to CO. Energy Fuels 2024, 38, 11043–11050. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Wang, Q.; Wang, X.; Zuo, S.; Zhang, H.; Ren, X.; He, Y.; Ichihara, F.; Ye, J. Synergy between confined cobalt centers and oxygen defects on α-Fe2O3 platelets for efficient photocatalytic CO2 reduction. Sol. RRL 2021, 6, 2100833. [Google Scholar] [CrossRef]
- Yang, X.; Liu, T.; Zhang, M.; Song, B.; Li, Q.; Yang, J. Interfacial dual vacancies modulating electronic structure to promote the separation of photogenerated carriers for efficient CO2 photoreduction. Appl. Surf. Sci. 2021, 551, 149305. [Google Scholar] [CrossRef]
- Guo, D.; Lu, Y.; Ruan, Y.; Zhao, Y.; Zhao, Y.; Wang, S.; Ma, X. Effects of extrinsic defects originating from the interfacial reaction of CeO2-x-nickel silicate on catalytic performance in methane dry reforming. Appl. Catal. B-Environ. 2020, 277, 119278. [Google Scholar] [CrossRef]
- Karmakar, S.; Barman, S.; Rahimi, F.A.; Rambabu, D.; Nath, S.; Maji, T.K. Confining charge-transfer complex in a metal-organic framework for photocatalytic CO2 reduction in water. Nat. Commun. 2023, 14, 4508. [Google Scholar] [CrossRef]
- Ling, L.; Jiao, L.; Liu, X.; Dong, Y.; Yang, W.; Zhang, H.; Ye, B.; Chen, J.; Jiang, H. Potassium-assisted fabrication of intrinsic defects in porous carbons for electrocatalytic CO2 reduction. Adv. Mater. 2022, 34, 2205933. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, L.; Zhang, Y.; Wu, L.; Chen, H.; Li, C. Fabrication of polyimide mixed matrix membranes with asymmetric confined mass transfer channels for improved CO2 separation. J. Membr. Sci. 2021, 637, 119653. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, Y.; Liu, Q.; Mao, X.; Guo, Q.; Yan, X.; Zhao, J.; Liu, F.; Du, A.; Yao, X. Ultra-dense carbon defects as highly active sites for oxygen reduction catalysis. Chem 2022, 8, 2715–2733. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, W.; Chen, Z.; Chen, J.; Li, X.; Li, Y. Advanced 3D hollow-out ZnZrO@C combined with hierarchical zeolite for highly active and selective CO hydrogenation to aromatics. ACS Catal. 2020, 10, 7177–7187. [Google Scholar] [CrossRef]
- Shi, H.; Du, J.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Solar-driven CO2 conversion over Co2+ doped 0D/2D TiO2/g-C3N4 heterostructure: Insights into the role of Co2+ and cocatalyst. J. CO2 Util. 2020, 38, 16–23. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Wu, H.; Xie, C.; Hua, M.; Hu, Y.; Han, B. Ordered-mesoporous-carbon-confined Pb/PbO composites: Superior electrocatalysts for CO2 reduction. ChemSusChem 2020, 13, 6346–6352. [Google Scholar] [CrossRef]
- Shingdilwar, S.; Dolui, S.; Kumar, D.; Banerjee, S. Facile access to template-shape-replicated nitrogen-rich mesoporous carbon nanospheres for highly efficient CO2 capture and contaminant removal. Mater. Adv. 2022, 3, 665–671. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Fan, X.; Yang, H.; Nie, D.; Qi, L. Alkaline-promoted Co-Ni bimetal ordered mesoporous catalysts with enhanced coke-resistant performance toward CO2 reforming of CH4. J. CO2 Util. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, K.; Liu, C.; Hu, Q.; Fang, H.; Yang, H.; He, C. Superbase and hydrophobic ionic liquid confined within Ni foams as a free-standing catalyst for CO2 electroreduction. ACS Appl. Mater. Interfaces 2022, 14, 38717–38726. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Zhou, T.; Li, J.; Huang, S.; Chang, C.; Fan, X.; Zhang, H.; Ma, X.; Gao, D.; Qi, J.; et al. Rich oxygen vacancies in confined heterostructured TiO2@In2S3 hybrid for boosting solar-driven CO2 reduction. J. Colloid Interface Sci. 2024, 660, 77–86. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, M.; Yuan, C.; Sun, Q.; Huang, B.; Dong, H.; Zhang, Y. Strong electronic coupling effects at the heterojunction interface of SnO2 nanodots and g-C3N4 for enhanced CO2 electroreduction. ACS Catal. 2023, 13, 7055–7066. [Google Scholar] [CrossRef]







| Dimension | Typical Structure | Common Advantage | Limitation |
|---|---|---|---|
| 1D | Carbon nanotube | Mass transfer effect | Diffusion issues caused by non-uniform pore sizes |
| 2D | Graphene overlayer | Spatial compartmentation effect | The overlayer requires high mechanical stability |
| 3D | MOFs | Surface modification effect | High-temperature structural collapse risk |
| Interface | Metal–oxide heterojunction | Metal size effect | Scalability challenges in manufacturing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Sun, Q.; Ge, J.; Wang, H.; Qi, J. Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization. Catalysts 2025, 15, 477. https://doi.org/10.3390/catal15050477
Fang Q, Sun Q, Ge J, Wang H, Qi J. Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization. Catalysts. 2025; 15(5):477. https://doi.org/10.3390/catal15050477
Chicago/Turabian StyleFang, Qimin, Qihan Sun, Jinming Ge, Haiwang Wang, and Jian Qi. 2025. "Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization" Catalysts 15, no. 5: 477. https://doi.org/10.3390/catal15050477
APA StyleFang, Q., Sun, Q., Ge, J., Wang, H., & Qi, J. (2025). Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization. Catalysts, 15(5), 477. https://doi.org/10.3390/catal15050477









