Graphene Oxide-Supported Metal Catalysts for Selective Hydrogenation of Cinnamaldehyde: Impact of Metal Choice and Support Structure
Abstract
1. Introduction
2. Results
2.1. Catalyst Characterization
2.1.1. X-Ray Fluorescence
2.1.2. X-Ray Diffraction
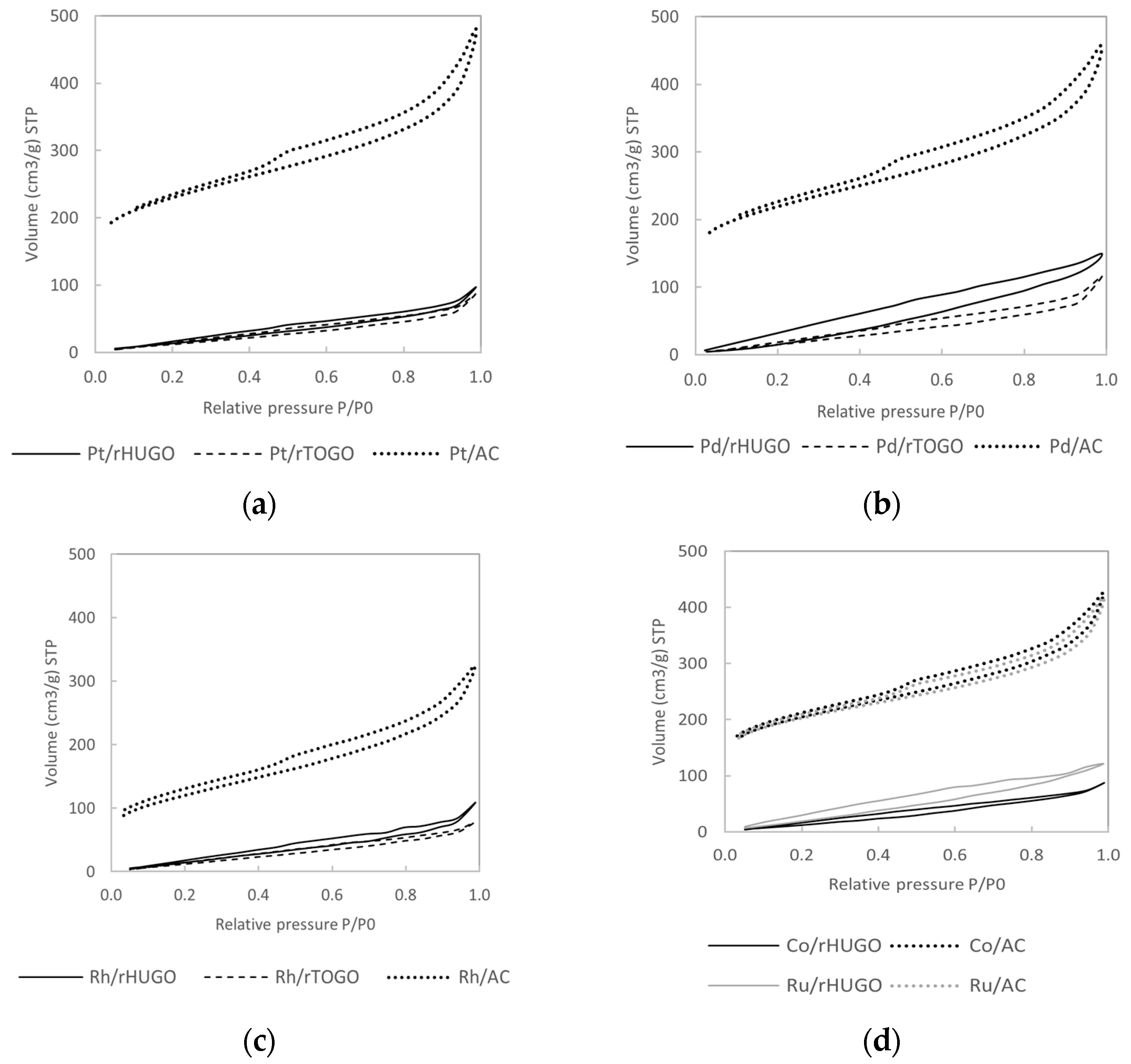
2.1.3. Nitrogen Physisorption
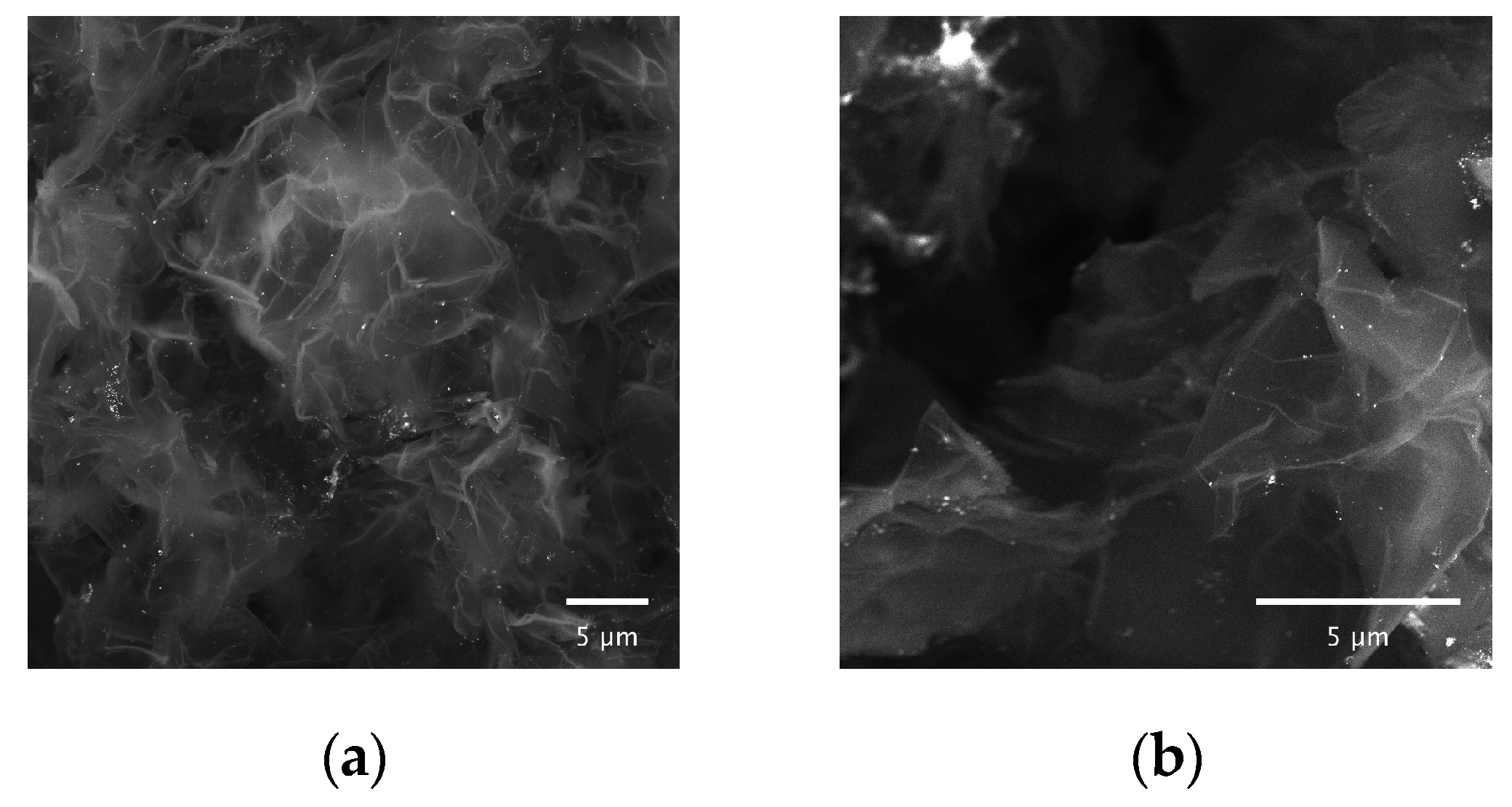
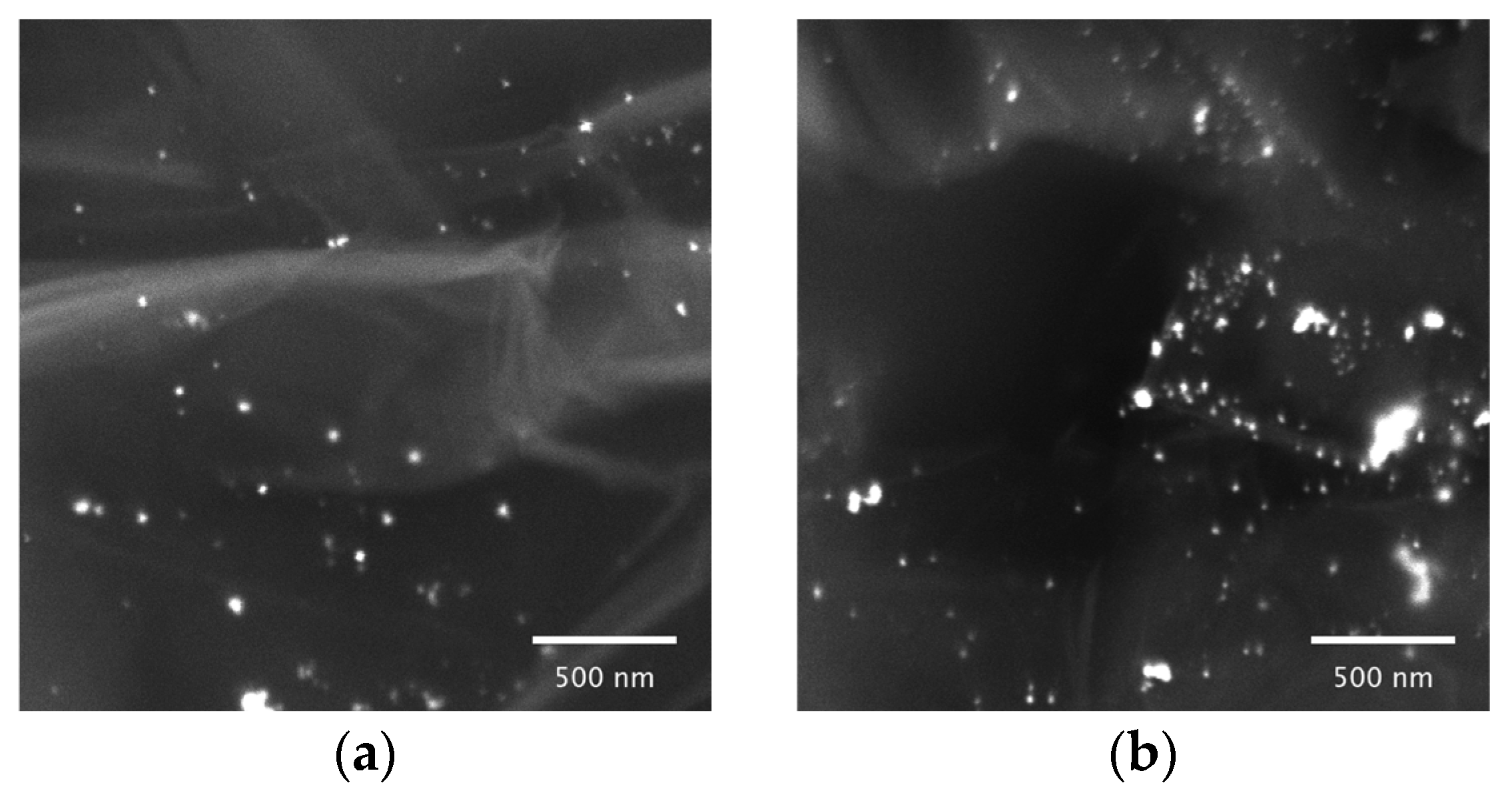
2.1.4. Scanning Electron Microscopy
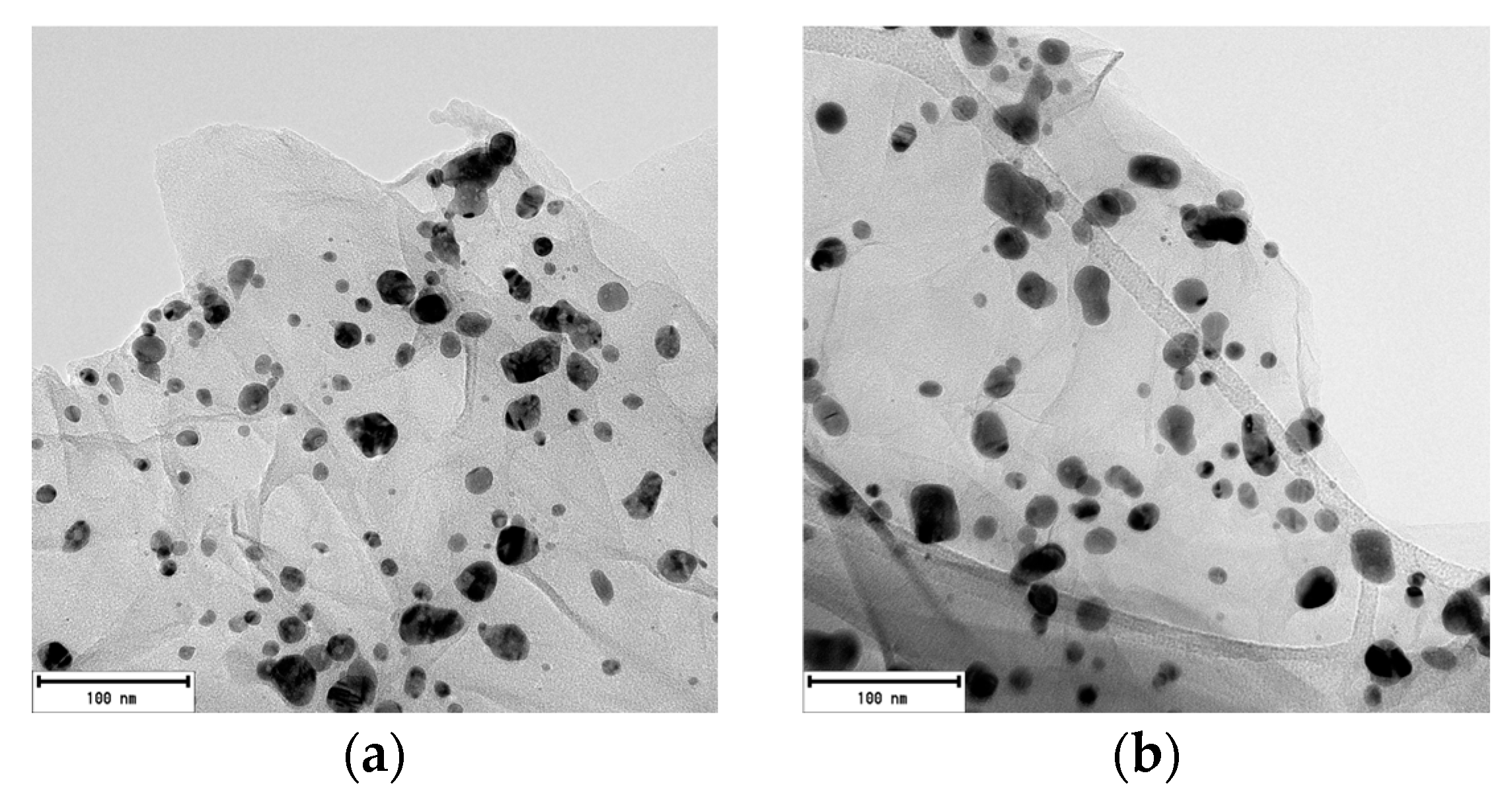
2.1.5. Transmission Electron Microscopy
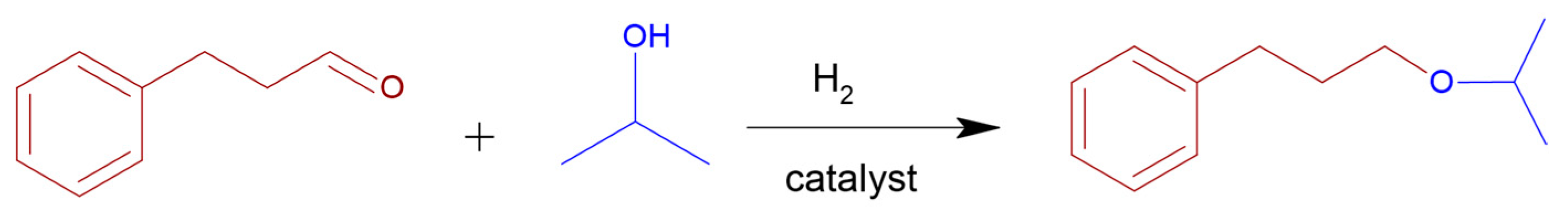
2.2. Cinnamaldehyde Hydrogenation
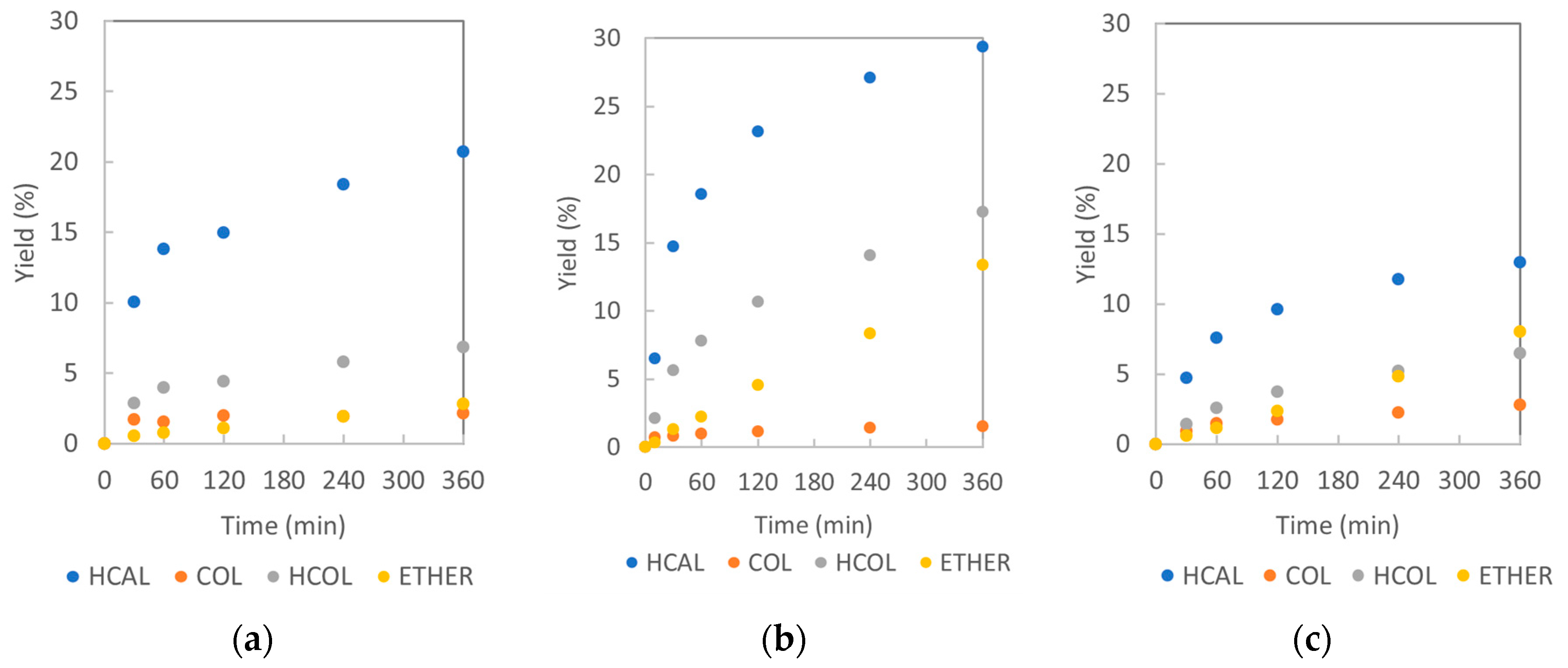
2.2.1. Catalyst Activities: Reaction Rates and Conversions of Cinnamaldehyde
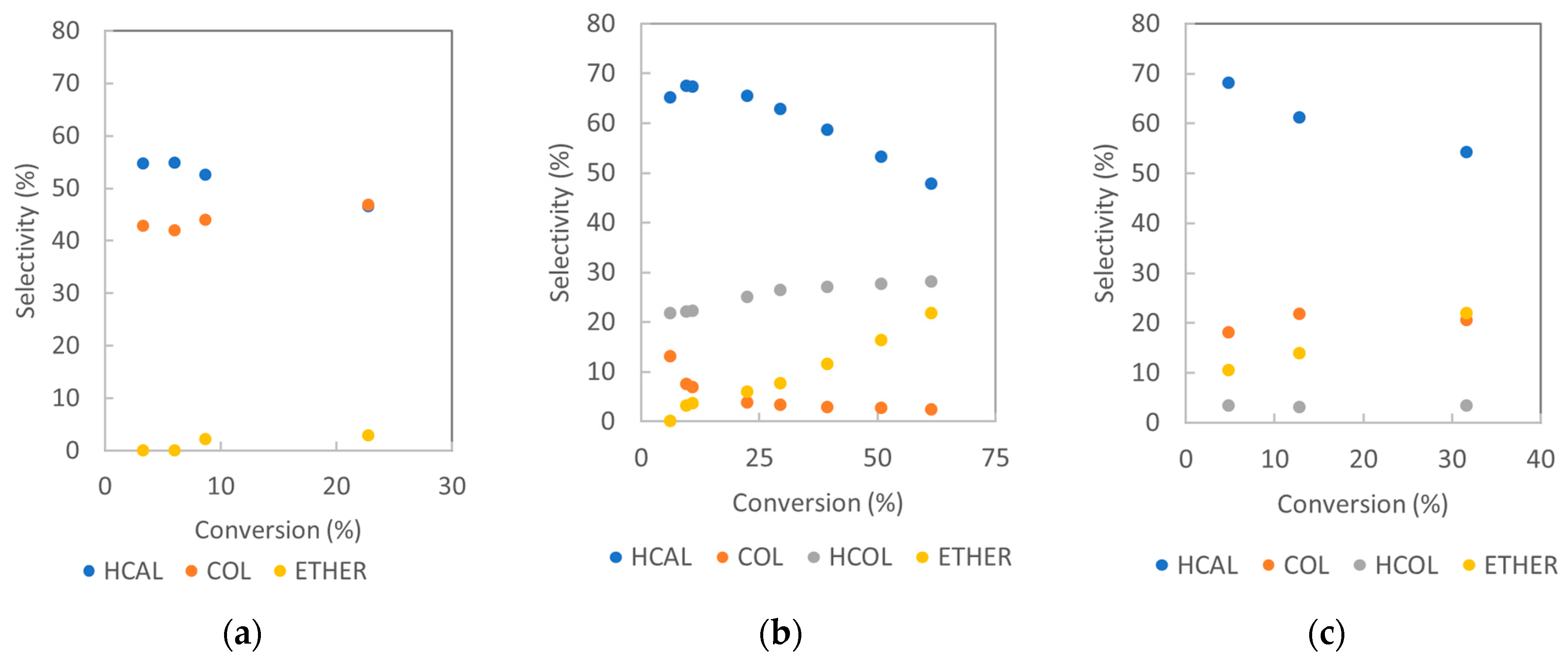
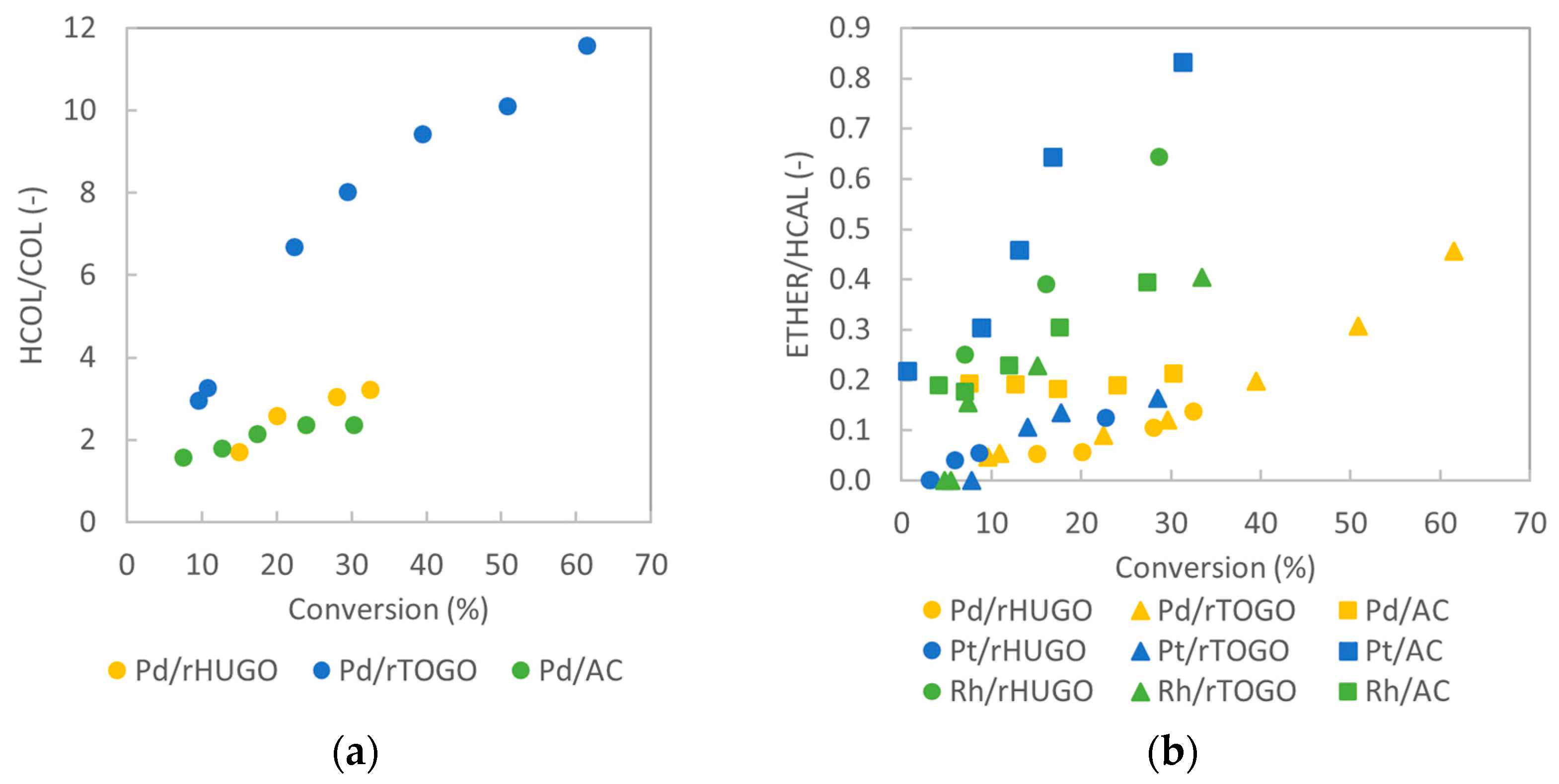
2.2.2. Catalysts’ Selectivities: Formation of Hydrogenation and Side Products
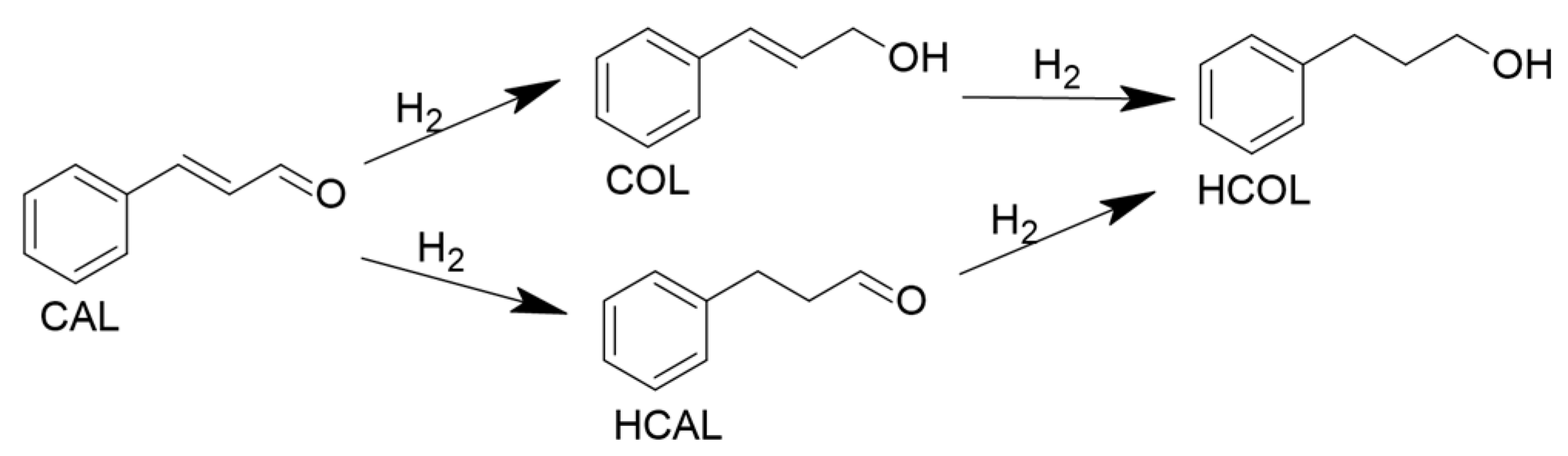
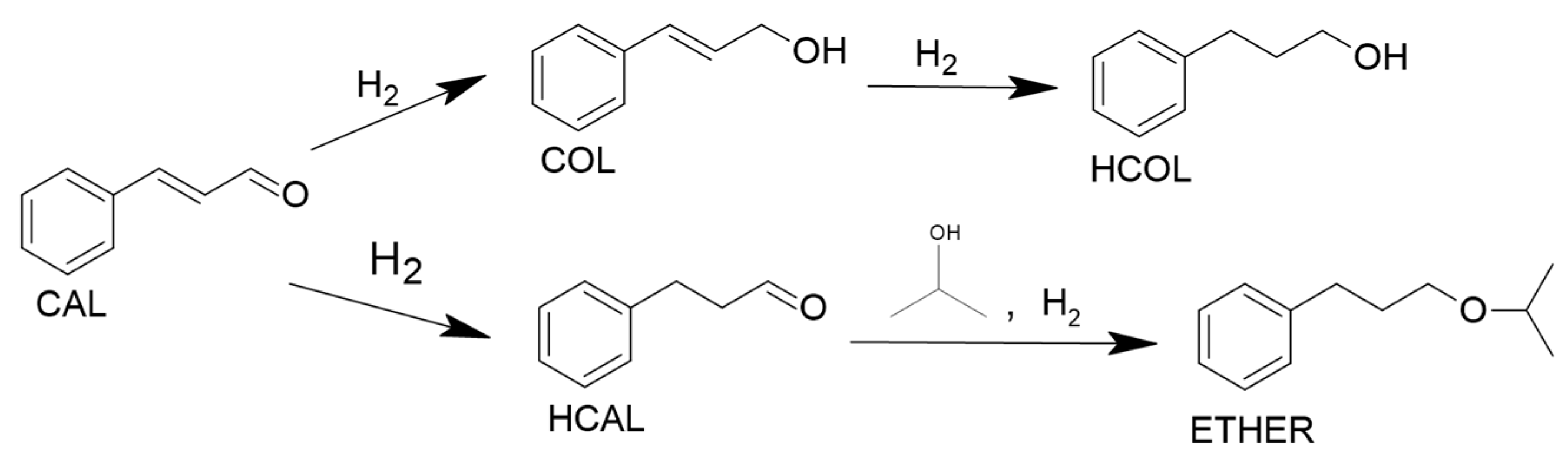
2.2.3. Reaction Pathway
2.3. Catalyst Regeneration and Reuse
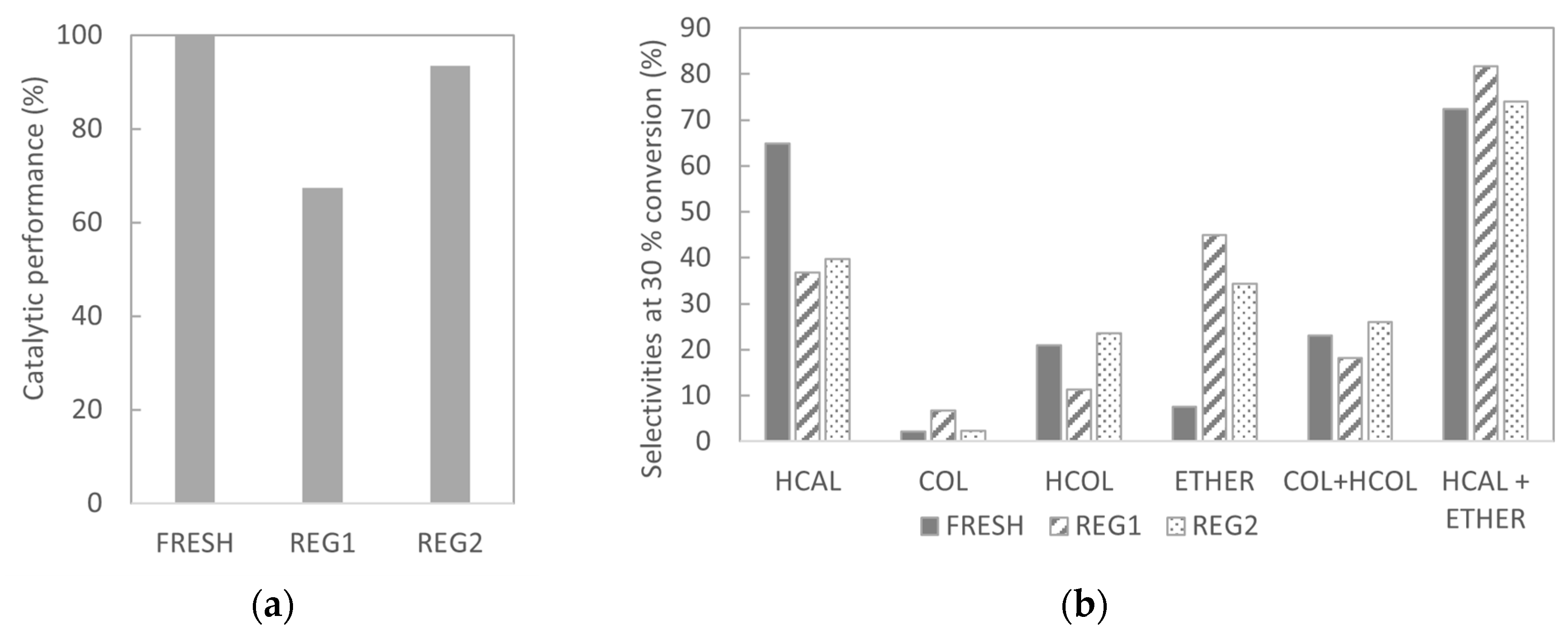
2.3.1. Regenerated Catalysts: Characterization
2.3.2. Regenerated Catalysts: Cinnamaldehyde Hydrogenation
3. Materials and Methods
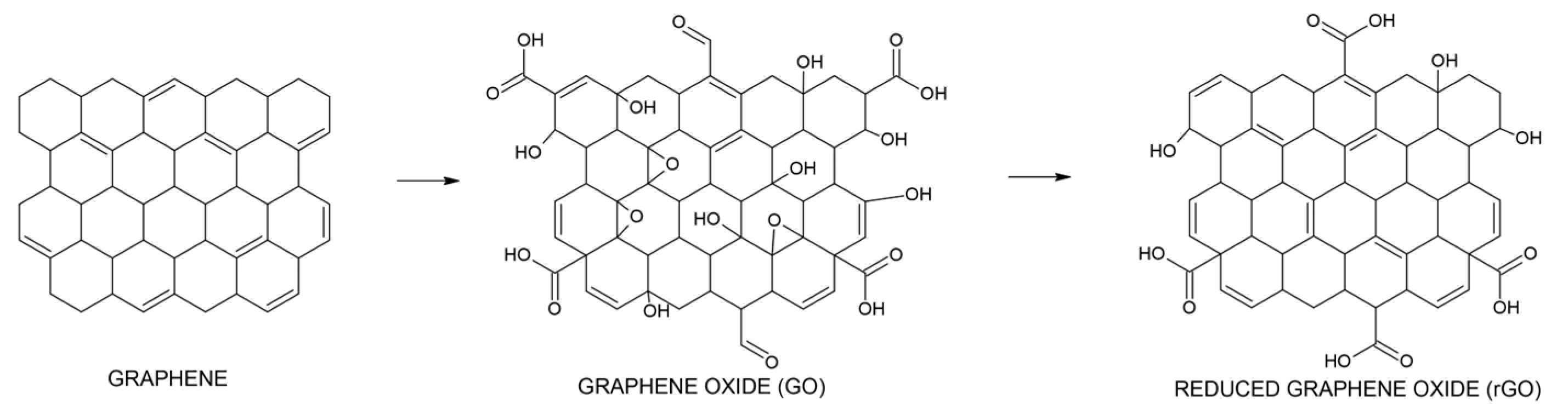
3.1. HUGO and TOGO Syntheses
3.2. Synthesis of Metal Supported Catalysts
3.3. Catalyst Characterization
3.4. Cinnamaldehyde Hydrogenation
3.5. Catalyst Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAL | cinnamaldehyde |
| COL | cinnamylalcohol |
| HCAL | hydrocinnamaldehyde |
| HCOL | hydrocinnamal alcohol |
| ETHER | 3-isopropoxy-propan-1-yl benzene |
| GO | graphene oxide |
| rGO | Reduced graphene oxide |
| HUGO | graphene oxide prepared by Hummer’s method |
| TOGO | graphene oxide prepared by Tour’s method |
References
- Taylor, H.S. Fourth Report of the Committee on Contact Catalysis. J. Phys. Chem. 1926, 30, 145–171. [Google Scholar] [CrossRef]
- Kačer, P.; Červený, L. Structure effects in hydrogenation reactions on noble metal catalysts. Appl. Catal. A Gen. 2002, 229, 193–216. [Google Scholar] [CrossRef]
- Mao, S.; Wang, Z.; Chen, Z.; Wu, K.; Zhang, K.; Li, Q.; Yan, H.; Lü, G.; Huang, G.; Wang, Y. Towards the selectivity distinction of phenol hydrogenation on noble metal catalysts. Nano Mater. Sci. 2023, 5, 91–100. [Google Scholar] [CrossRef]
- Singh, U.K.; Vannice, M.A. Liquid-Phase Citral Hydrogenation over SiO2-Supported Group VIII Metals. J. Catal. 2001, 199, 73–84. [Google Scholar] [CrossRef]
- Corvaisier, F.; Schuurman, Y.; Fecant, A.; Thomazeau, C.; Raybaud, P.; Toulhoat, H.; Farrusseng, D. Periodic trends in the selective hydrogenation of styrene over silica supported metal catalysts. J. Catal. 2013, 307, 352–361. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Porras, A.; Urbano, F.J. Selective Liquid-Phase Hydrogenation of Citral over Supported Palladium. J. Catal. 1997, 172, 46–54. [Google Scholar] [CrossRef]
- Wei, Z.; Shao, F.; Wang, J. Recent advances in heterogeneous catalytic hydrogenation and dehydrogenation of N-heterocycles. Chin. J. Catal. 2019, 40, 980–1002. [Google Scholar] [CrossRef]
- Kluson, P.; Cerveny, L. Selective hydrogenation over ruthenium catalysts. Appl. Catal. A Gen. 1995, 128, 13–31. [Google Scholar] [CrossRef]
- Mercadante, L.; Neri, G.; Milone, C.; Donato, A.; Galvagno, S. Hydrogenation of α,β-unsaturated aldehydes over Ru/Al2O3 catalysts. J. Mol. Catal. A Chem. 1996, 105, 93–101. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Zhu, C.; Zhao, M.; Ni, Z.-H.; Zhao, X.-Y.; Xie, J.-X.; Zhao, L.; Zhao, Y.-P.; Bai, H.-C. Catalytic hydrogenation of aromatic ring over ruthenium nanoparticles supported on α-Al2O3 at room temperature. Appl. Catal. B Environ. 2022, 307, 121137. [Google Scholar] [CrossRef]
- Yoshida, H.; Onodera, Y.; Fujita, S.-i.; Kawamori, H.; Arai, M. Solvent effects in heterogeneous selective hydrogenation of acetophenone: Differences between Rh/C and Rh/Al2O3 catalysts and the superiority of water as a functional solvent. Green Chem. 2015, 17, 1877–1883. [Google Scholar] [CrossRef]
- Yan, P.; Tian, P.; Li, K.; Cohen Stuart, M.A.; Wang, J.; Yu, X.; Zhou, S. Rh nanoclusters encaged in hollow mesoporous silica nanoreactors with enhanced catalytic performance for phenol selective hydrogenation. Chem. Eng. J. 2020, 397, 125484. [Google Scholar] [CrossRef]
- Audemar, M.; Ciotonea, C.; De Oliveira Vigier, K.; Royer, S.; Ungureanu, A.; Dragoi, B.; Dumitriu, E.; Jérôme, F. Selective Hydrogenation of Furfural to Furfuryl Alcohol in the Presence of a Recyclable Cobalt/SBA-15 Catalyst. ChemSusChem 2015, 8, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Rösler, S.; Obenauf, J.; Kempe, R. A Highly Active and Easily Accessible Cobalt Catalyst for Selective Hydrogenation of C=O Bonds. J. Am. Chem. Soc. 2015, 137, 7998–8001. [Google Scholar] [CrossRef]
- Volf, J.; Pašek, J. Chapter 4 Hydrogenation of Nitriles. In Studies in Surface Science and Catalysis; Cerveny, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 105–144. [Google Scholar]
- Mukherjee, A.; Srimani, D.; Chakraborty, S.; Ben-David, Y.; Milstein, D. Selective Hydrogenation of Nitriles to Primary Amines Catalyzed by a Cobalt Pincer Complex. J. Am. Chem. Soc. 2015, 137, 8888–8891. [Google Scholar] [CrossRef]
- Gallezot, P.; Richard, D. Selective Hydrogenation of α,β-Unsaturated Aldehydes. Catal. Rev. 1998, 40, 81–126. [Google Scholar] [CrossRef]
- Ide, M.S.; Hao, B.; Neurock, M.; Davis, R.J. Mechanistic Insights on the Hydrogenation of α,β-Unsaturated Ketones and Aldehydes to Unsaturated Alcohols over Metal Catalysts. ACS Catal. 2012, 2, 671–683. [Google Scholar] [CrossRef]
- Lan, X.; Wang, T. Highly Selective Catalysts for the Hydrogenation of Unsaturated Aldehydes: A Review. ACS Catal. 2020, 10, 2764–2790. [Google Scholar] [CrossRef]
- Englisch, M.; Jentys, A.; Lercher, J.A. Structure Sensitivity of the Hydrogenation of Crotonaldehyde over Pt/SiO2and Pt/TiO2. J. Catal. 1997, 166, 25–35. [Google Scholar] [CrossRef]
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Selective Hydrogenation of Crotonaldehyde to Crotyl Alcohol over Metal Oxide Modified Ir Catalysts and Mechanistic Insight. ACS Catal. 2016, 6, 3600–3609. [Google Scholar] [CrossRef]
- Mohr, C.; Hofmeister, H.; Claus, P. The influence of real structure of gold catalysts in the partial hydrogenation of acrolein. J. Catal. 2003, 213, 86–94. [Google Scholar] [CrossRef]
- Mohr, C.; Hofmeister, H.; Radnik, J.; Claus, P. Identification of Active Sites in Gold-Catalyzed Hydrogenation of Acrolein. J. Am. Chem. Soc. 2003, 125, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, T.B.L.W.; Ponec, V. A Study on the Selectivity in Acrolein Hydrogenation on Platinum Catalysts: A Model for Hydrogenation of α,β-Unsaturated Aldehydes. J. Catal. 1995, 156, 51–59. [Google Scholar] [CrossRef]
- Han, Q.; Liu, Y.; Wang, D.; Yuan, F.; Niu, X.; Zhu, Y. Effect of carbon nanosheets with different graphitization degrees as a support of noble metals on selective hydrogenation of cinnamaldehyde. RSC Adv. 2016, 6, 98356–98364. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Geng, P.; Li, Q. Recent Advances in Selective Hydrogenation of Cinnamaldehyde over Supported Metal-Based Catalysts. ACS Catal. 2020, 10, 2395–2412. [Google Scholar] [CrossRef]
- Hu, D.; Fan, W.; Liu, Z.; Li, L. Three-Dimensionally Hierarchical Pt/C Nanocomposite with Ultra-High Dispersion of Pt Nanoparticles as a Highly Efficient Catalyst for Chemoselective Cinnamaldehyde Hydrogenation. ChemCatChem 2018, 10, 779–788. [Google Scholar] [CrossRef]
- Plomp, A.J.; Vuori, H.; Krause, A.O.I.; de Jong, K.P.; Bitter, J.H. Particle size effects for carbon nanofiber supported platinum and ruthenium catalysts for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2008, 351, 9–15. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Wang, C.-X.; Liu, Z.-W.; Lu, J. Selective hydrogenation of cinnamaldehyde over Pt-supported multi-walled carbon nanotubes: Insights into the tube-size effects. Appl. Catal. A Gen. 2008, 344, 114–123. [Google Scholar] [CrossRef]
- Jung, A.; Jess, A.; Schubert, T.; Schütz, W. Performance of carbon nanomaterial (nanotubes and nanofibres) supported platinum and palladium catalysts for the hydrogenation of cinnamaldehyde and of 1-octyne. Appl. Catal. A Gen. 2009, 362, 95–105. [Google Scholar] [CrossRef]
- Lenz, J.; Campo, B.C.; Alvarez, M.; Volpe, M.A. Liquid phase hydrogenation of α,β-unsaturated aldehydes over gold supported on iron oxides. J. Catal. 2009, 267, 50–56. [Google Scholar] [CrossRef]
- Toebes, M.L.; Prinsloo, F.F.; Bitter, J.H.; van Dillen, A.J.; de Jong, K.P. Influence of oxygen-containing surface groups on the activity and selectivity of carbon nanofiber-supported ruthenium catalysts in the hydrogenation of cinnamaldehyde. J. Catal. 2003, 214, 78–87. [Google Scholar] [CrossRef]
- Hájek, J.; Kumar, N.; Mäki-Arvela, P.; Salmi, T.; Murzin, D.Y.; Paseka, I.; Heikkilä, T.; Laine, E.; Laukkanen, P.; Väyrynen, J. Ruthenium-modified MCM-41 mesoporous molecular sieve and Y zeolite catalysts for selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2003, 251, 385–396. [Google Scholar] [CrossRef]
- Machado, B.F.; Gomes, H.T.; Serp, P.; Kalck, P.; Faria, J.L. Liquid-Phase Hydrogenation of Unsaturated Aldehydes: Enhancing Selectivity of Multiwalled Carbon Nanotube-Supported Catalysts by Thermal Activation. ChemCatChem 2010, 2, 190–197. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Gomez-Lopez, J.; Griffin, K.; Hayes, M. Steric effects in the selective hydrogenation of cinnamaldehyde to cinnamyl alcohol using an Ir/C catalyst. Appl. Catal. A Gen. 2004, 268, 267–274. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhang, Y.J.; Chen, F.; Xiang, Y.Z.; Zhang, B.; Xu, L.Y.; Zhang, T.R. Efficient selective hydrogenation of cinnamaldehyde over zeolite supported cobalt catalysts in water. React. Kinet. Mech. Catal. 2015, 115, 283–292. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Long, J.; Zhang, W.; Liu, X.; Wei, D. MOFs-Derived Co@CN bi-functional catalysts for selective transfer hydrogenation of α,β-unsaturated aldehydes without use of base additives. Mater. Chem. Front. 2017, 1, 2005–2012. [Google Scholar] [CrossRef]
- Gutiérrez, V.S.; Diez, A.S.; Dennehy, M.; Volpe, M.A. Cu incorporated MCM-48 for the liquid phase hydrogenation of cinnamaldehyde. Microporous Mesoporous Mater. 2011, 141, 207–213. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Mi, J.; Tan, X.; Song, Y. Synthesis of copper-containing ordered mesoporous carbons for selective hydrogenation of cinnamaldehyde. Catal. Commun. 2012, 21, 58–62. [Google Scholar] [CrossRef]
- Tan, M.; Yang, G.; Wang, T.; Vitidsant, T.; Li, J.; Wei, Q.; Ai, P.; Wu, M.; Zheng, J.; Tsubaki, N. Active and regioselective rhodium catalyst supported on reduced graphene oxide for 1-hexene hydroformylation. Catal. Sci. Technol. 2016, 6, 1162–1172. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, Y.; Fan, G.; Yang, L.; Li, F. Structure-dependent selective hydrogenation of cinnamaldehyde over high-surface-area CeO2-ZrO2 composites supported Pt nanoparticles. Chem. Eng. J. 2017, 322, 234–245. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X.; Chu, X.; Jiang, C.; Yao, Y.; Guo, Z.; Zhou, C.; Wang, C.; Wang, H.; Yang, Y. On the role of water in selective hydrogenation of cinnamaldehyde to cinnamyl alcohol on PtFe catalysts. J. Catal. 2018, 364, 192–203. [Google Scholar] [CrossRef]
- Chang, S.; Meng, S.; Fu, X.; Zhang, S.; Zheng, X.; Chen, S. Hydrogenation of Cinnamaldehyde to Hydrocinnamyl Alcohol on Pt/Graphite Catalyst. ChemistrySelect 2019, 4, 2018–2023. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Chen, L.; Dong, C.; Yu, W.; Huang, T.; Qian, Y. Pt nanoparticles deposited over carbon nanotubes for selective hydrogenation of cinnamaldehyde. Catal. Commun. 2007, 8, 452–456. [Google Scholar] [CrossRef]
- Xu, Z.; Duong-Viet, C.; Liu, Y.; Baaziz, W.; Li, B.; Nguyen-Dinh, L.; Ersen, O.; Pham-Huu, C. Macroscopic graphite felt containing palladium catalyst for liquid-phase hydrogenation of cinnamaldehyde. Appl. Catal. B Environ. 2019, 244, 128–139. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Keller, N.; Ehret, G.; Charbonniere, L.c.J.; Ziessel, R.; Ledoux, M.J. Carbon nanofiber supported palladium catalyst for liquid-phase reactions: An active and selective catalyst for hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. J. Mol. Catal. A Chem. 2001, 170, 155–163. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Gurrala, L.; Gogoi, P.; Chilukuri, S.V. Hydrogenation of cinnamaldehyde to hydrocinnamaldehyde over Pd nanoparticles deposited on nitrogen-doped mesoporous carbon. RSC Adv. 2016, 6, 44333–44340. [Google Scholar] [CrossRef]
- Kumar, A.; Xu, Q. Two-Dimensional Layered Materials as Catalyst Supports. ChemNanoMat 2018, 4, 28–40. [Google Scholar] [CrossRef]
- Gusmão, R.; Veselý, M.; Sofer, Z. Recent Developments on the Single Atom Supported at 2D Materials Beyond Graphene as Catalysts. ACS Catal. 2020, 10, 9634–9648. [Google Scholar] [CrossRef]
- Niu, W.-J.; He, J.-Z.; Gu, B.-N.; Liu, M.-C.; Chueh, Y.-L. Opportunities and Challenges in Precise Synthesis of Transition Metal Single-Atom Supported by 2D Materials as Catalysts toward Oxygen Reduction Reaction. Adv. Funct. Mater. 2021, 31, 2103558. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, A.; Kordy, M.G.M.; AlMohamadi, H.; Eissa, M.F.; Hamdy, H. Design and Performance of CuNi-rGO and Ag-CuNi-rGO Composite Electrodes for Use in Fuel Cells. Catalysts 2024, 14, 551. [Google Scholar] [CrossRef]
- Saeed, M.; Albadran, F.H.; Zahoor, A.F.; Nisar, A.; Al-Mutairi, A.A.; Al-Hussain, S.A.; Irfan, A.; Zaki, M.E.A. Co3O4-rGO—Synthesis, Characterization, and Evaluation of Photocatalytic Activities. Catalysts 2024, 14, 96. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sens. Actuators B Chem. 2014, 199, 190–200. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Wang, B.; Gao, T.; Wang, D. A reduced graphene oxide modified metallic cobalt composite with superior electrochemical performance for supercapacitors. RSC Adv. 2015, 5, 63553–63560. [Google Scholar] [CrossRef]
- Jose, P.P.A.; Kala, M.S.; Joseph, A.V.; Kalarikkal, N.; Thomas, S. Reduced graphene oxide/silver nanohybrid as a multifunctional material for antibacterial, anticancer, and SERS applications. Appl. Phys. A 2020, 126, 58. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Linder, C. Catalytic Reductive Etherification of Ketones with Alcohols at Ambient Hydrogen Pressure: A Practical, Waste-Minimized Synthesis of Dialkyl Ethers. Synlett 2006, 2006, 3489–3491. [Google Scholar] [CrossRef]
- Kalutharage, N.; Yi, C.S. Chemoselective formation of unsymmetrically substituted ethers from catalytic reductive coupling of aldehydes and ketones with alcohols in aqueous solution. Org. Lett. 2015, 17, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Seth, J.; Nepak, D.; Chaudhari, V.R.; Prasad, B.L.V. Preparation of MgO supported platinum nanoparticle catalyst using toluene dispersed platinum sol. Appl. Surf. Sci. 2017, 418, 87–91. [Google Scholar] [CrossRef]
- Xue, Y.; Xin, H.; Xie, W.; Wu, P.; Li, X. Pt nanoparticles supported on YCoxFe1−xO3 perovskite oxides: Highly efficient catalysts for liquid-phase hydrogenation of cinnamaldehyde. Chem. Commun. 2019, 55, 3363–3366. [Google Scholar] [CrossRef]
- Chen, L.; Zhan, W.; Fang, H.; Cao, Z.; Yuan, C.; Xie, Z.; Kuang, Q.; Zheng, L. Selective Catalytic Performances of Noble Metal Nanoparticle@MOF Composites: The Concomitant Effect of Aperture Size and Structural Flexibility of MOF Matrices. Chem. Eur. J. 2017, 23, 11397–11403. [Google Scholar] [CrossRef]




| CATALYST | SBET (m2/g) | ||
|---|---|---|---|
| HUGO | TOGO | AC | |
| Pure | 548 | 559 | 669 |
| Pt | 82 | 70 | 743 |
| Pd | 110 | 81 | 721 |
| Rh | 83 | 72 | 418 |
| Ru | 136 | x | 646 |
| Co | 79 | x | 649 |
| Metal | Support | Median (nm) | Particle Size Distribution (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 nm | 2–4 nm | 4–6 nm | 6–8 nm | 8–10 nm | 10–12 nm | 12–14 nm | 14–16 nm | 16–18 nm | 18–20 nm | >20 nm | |||
| Pt | rHUGO | 3.5 | 5 | 63 | 22 | 6 | 3 | 0 | 2 | 0 | 0 | 0 | 0 |
| rTOGO | 3.9 | 2 | 52 | 30 | 11 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | |
| AC | 7.8 | 0 | 0 | 21 | 34 | 18 | 21 | 5 | 0 | 0 | 0 | 0 | |
| Pd | rHUGO | 15.5 | 0 | 0 | 0 | 0 | 10 | 11 | 18 | 13 | 16 | 7 | 25 |
| rTOGO | 21.4 | 0 | 0 | 0 | 3 | 4 | 4 | 4 | 4 | 8 | 9 | 63 | |
| AC | 13.9 | 0 | 0 | 0 | 1 | 11 | 21 | 25 | 14 | 11 | 7 | 10 | |
| Rh | rHUGO | 6.6 | 0 | 13 | 29 | 27 | 11 | 7 | 2 | 7 | 2 | 2 | 0 |
| rTOGO | 3.0 | 15 | 68 | 16 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AC | 3.7 | 0 | 64 | 33 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ru | rHUGO | 1.8 | 68 | 30 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AC | 3.1 | 6 | 65 | 14 | 12 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Co | rHUGO | 24.0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 9 | 11 | 76 |
| AC | 18.0 | 0 | 0 | 0 | 6 | 10 | 12 | 4 | 10 | 6 | 8 | 44 | |
| Metal | Support | CAL Conversion (%) | r0 (mmol·L−1·min−1) | rt (mmol·L−1·min−1) |
|---|---|---|---|---|
| Pt | rHUGO | 22.8 | 0.57 | 0.37 |
| rTOGO | 28.6 | 1.43 | 0.33 | |
| AC | 25.8 | 0.81 | 0.29 | |
| Pd | rHUGO | 32.6 | 2.83 | 0.23 |
| rTOGO | 61.5 | 3.79 | 0.46 | |
| AC | 30.4 | 1.49 | 0.32 | |
| Rh | rHUGO | 28.8 | 0.54 | 0.35 *(110 °C) |
| rTOGO | 33.5 | 0.83 | 0.57 *(110 °C) | |
| AC | 27.5 | 0.78 | 0.37 *(110 °C) | |
| Ru | rHUGO | 14.4 | 0.54 | 0.13 |
| AC | 13.8 | 0.65 | 0.17 | |
| Co | rHUGO | 5.0 | 0.44 | 0.06 |
| AC | 6.5 | 0.42 | 0.09 |
| CATALYST | Selectivity at 10%/30% Conversion of CAL (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCAL | COL | HCOL | ETHERS | Other | SUM of H2 Products | |||||||
| 10% | 30% | 10% | 30% | 10% | 30% | 10% | 30% | 10% | 30% | 10% | 30% | |
| Pt/rHUGO | 52.0 | 46.5 γ | 44.3 | 46.8 γ | 0.0 | 0.0 γ | 2.2 | 5.8 γ | 0.8 | 0.9 γ | 96.3 | 93.3 γ |
| Pt/rTOGO | 52.8 | 43.8 δ | 36.2 | 46.4 δ | 0.0 | 0.0 δ | 7.0 | 7.2 δ | 4.1 | 2.6 δ | 89.0 | 90.2 δ |
| Pt/AC | 49.0 | 39.0 | 27.1 | 26.6 | 0.0 | 0.0 | 17.1 | 31.7 | 7.0 | 2.9 | 76.1 | 65.6 |
| Pd/rHUGO | 66.2 α | 64.8 | 11.1 α | 2.1 | 18.7 α | 20.9 | 3.5 α | 7.6 | 0.7 α | 0.4 | 96.0 α | 87.8 |
| Pd/rTOGO | 67.3 | 62.7 | 6.8 | 3.3 | 22.2 | 26.4 | 3.7 | 7.6 | 0.0 | 0.0 | 96.3 | 92.4 |
| Pd/AC | 60.6 | 42.6 | 11.7 | 9.1 | 19.1 | 21.3 | 8.0 | 26.4 | 0.9 | 0.6 | 91.4 | 73.0 |
| Rh/rHUGO | 66.8 | 55.3 δ | 4.6 | 6.3 δ | 0.0 | 0.0 δ | 26.0 | 37.6 δ | 3.1 | 3.0 δ | 71.4 | 59.6 δ |
| Rh/rTOGO | 63.0 | 54.2 | 21.8 | 20.5 | 3.1 | 3.4 | 12.8 | 21.9 | 0.0 | 0.0 | 87.9 | 78.1 |
| Rh/AC | 72.0 | 54.7 ε | 10.0 | 21.8 ε | 0.0 | 0.0 ε | 14.8 | 21.8 ε | 3.1 | 2.0 ε | 82.0 | 76.5 ε |
| Ru/rHUGO | 50.0 | 46.4 ζ | 5.1 | 15.5 ζ | 0.0 | 0.0 ζ | 14.5 | 7.0 ζ | 28.7 | 31.1 ζ | 55.1 | 61.9 ζ |
| Ru/AC | 59.3 | 47.3 ζ | 11.5 | 25.4 ζ | 0.0 | 0.0 ζ | 24.7 | 21.7 ζ | 4.8 | 5.6 ζ | 70.8 | 72.7 ζ |
| Co/rHUGO | 30.3 β | x | 0.0 β | x | 0.0 β | x | 69.7 β | x | 0.0 β | x | 30.3 β | x |
| Co/AC | 69.5 β | x | 30.5 β | x | 0.0 β | x | 0.0 β | x | 0.0 β | x | 100 β | x |
| Catalyst | Support | Median (nm) | Particle Size Distribution (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 nm | 2–4 nm | 4–6 nm | 6–8 nm | 8–10 nm | 10–12 nm | 12–14 nm | 14–16 nm | 16–18 nm | 18–20 nm | >20 nm | |||
| Pd/rTOGO | Fresh | 21.4 | 0 | 0 | 0 | 3 | 4 | 4 | 4 | 4 | 8 | 9 | 63 |
| Reg1 | 9.9 | 0 | 20 | 7 | 5 | 20 | 20 | 5 | 10 | 7 | 2 | 3 | |
| Reg2 | 13.8 | 0 | 2 | 5 | 5 | 4 | 14 | 21 | 7 | 5 | 7 | 29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitínová, M.; Danylo, I.; Shafiq, A.; Hartman, T.; Khover, M.; Sevemez, B.; Koláčný, L.; Veselý, M. Graphene Oxide-Supported Metal Catalysts for Selective Hydrogenation of Cinnamaldehyde: Impact of Metal Choice and Support Structure. Catalysts 2025, 15, 470. https://doi.org/10.3390/catal15050470
Pitínová M, Danylo I, Shafiq A, Hartman T, Khover M, Sevemez B, Koláčný L, Veselý M. Graphene Oxide-Supported Metal Catalysts for Selective Hydrogenation of Cinnamaldehyde: Impact of Metal Choice and Support Structure. Catalysts. 2025; 15(5):470. https://doi.org/10.3390/catal15050470
Chicago/Turabian StylePitínová, Martina, Iryna Danylo, Ayesha Shafiq, Tomáš Hartman, Mariia Khover, Berke Sevemez, Lukáš Koláčný, and Martin Veselý. 2025. "Graphene Oxide-Supported Metal Catalysts for Selective Hydrogenation of Cinnamaldehyde: Impact of Metal Choice and Support Structure" Catalysts 15, no. 5: 470. https://doi.org/10.3390/catal15050470
APA StylePitínová, M., Danylo, I., Shafiq, A., Hartman, T., Khover, M., Sevemez, B., Koláčný, L., & Veselý, M. (2025). Graphene Oxide-Supported Metal Catalysts for Selective Hydrogenation of Cinnamaldehyde: Impact of Metal Choice and Support Structure. Catalysts, 15(5), 470. https://doi.org/10.3390/catal15050470








