Performance and Mechanism Study of Simultaneous Removal of Carbamazepine and Ammonia from Water Using UV/Peroxymonosulfate Process
Abstract
1. Introduction
2. Results and Discussion
2.1. The Removal Performance of CBZ and Ammonia in UV/PMS Process
2.2. Influence of Different Parameters
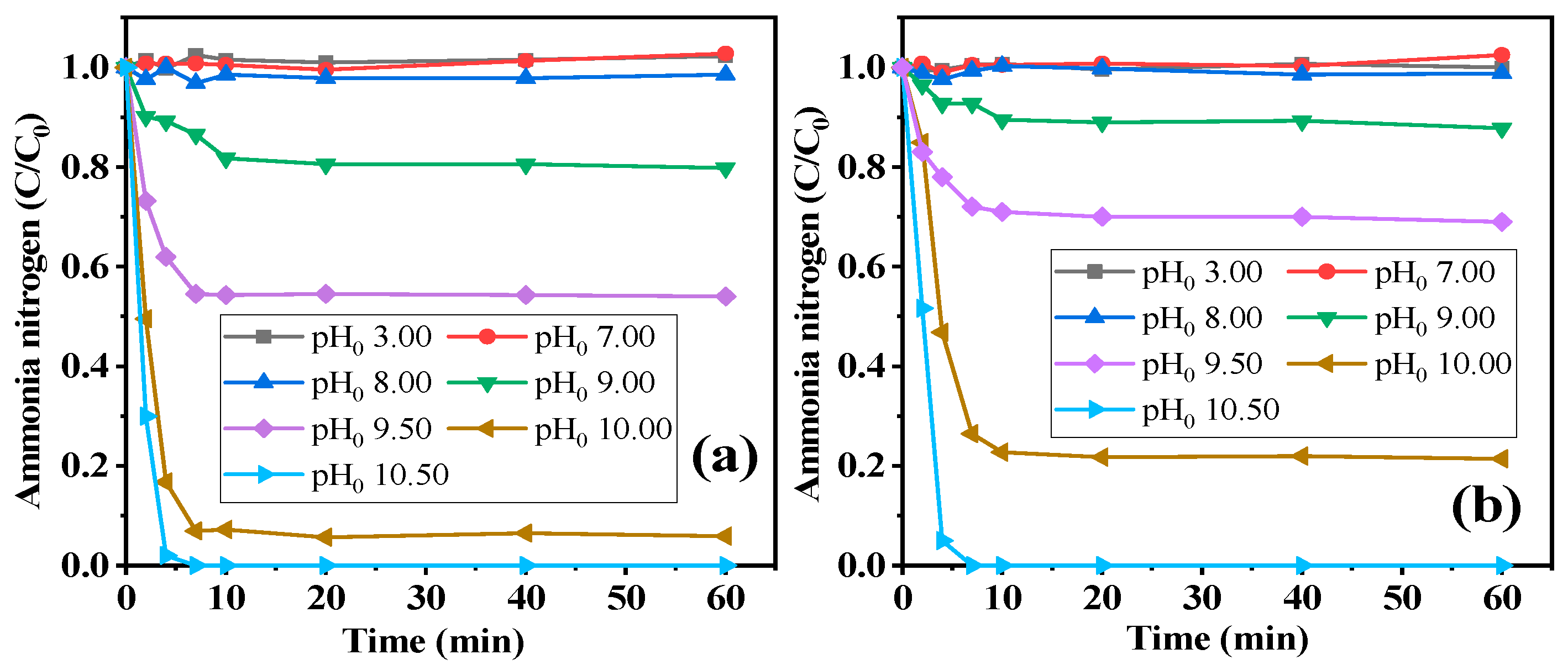
2.2.1. Influence of Solution pH
2.2.2. Influence of PMS Concentration
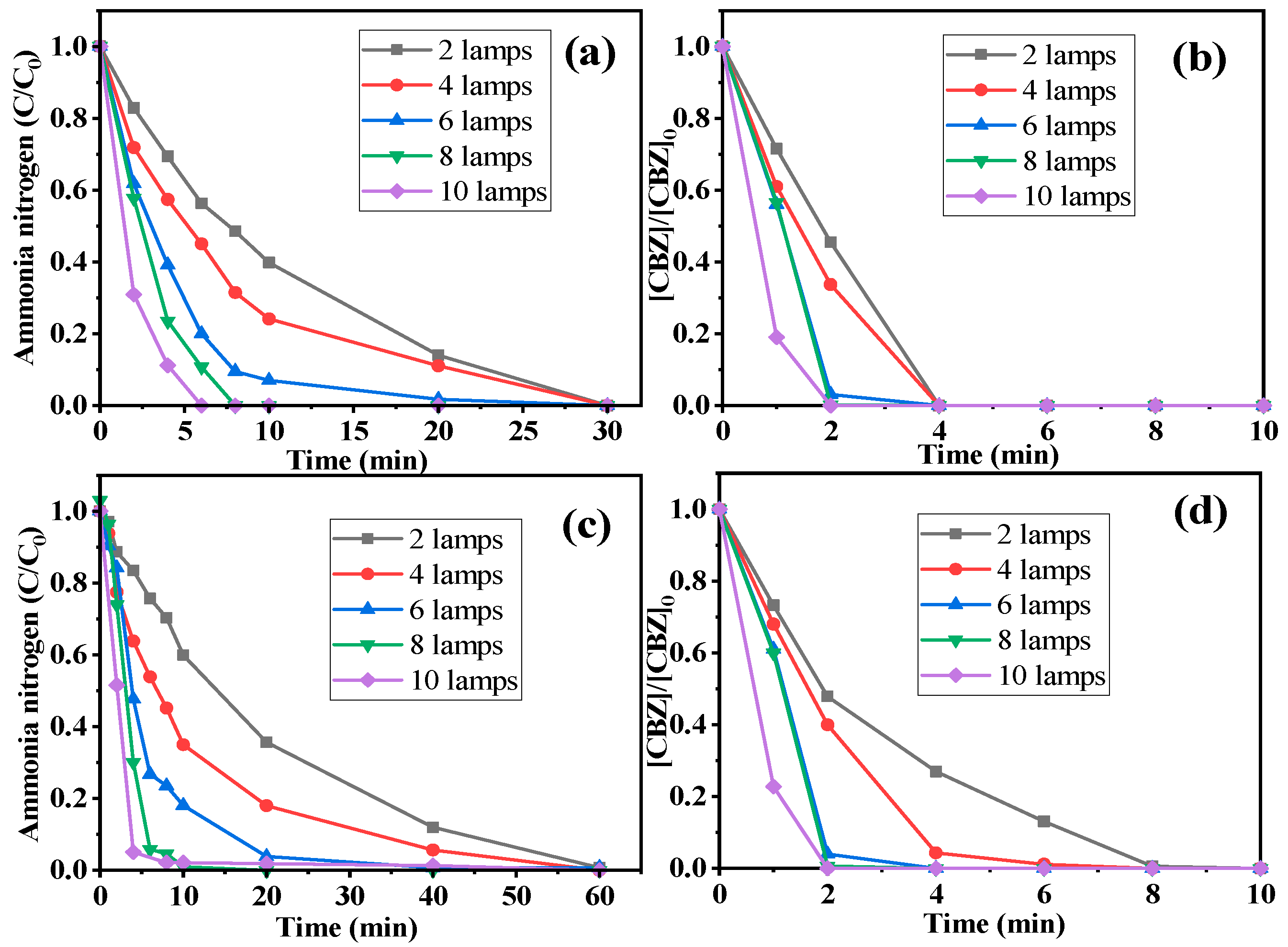
2.2.3. Influence of Light Intensity
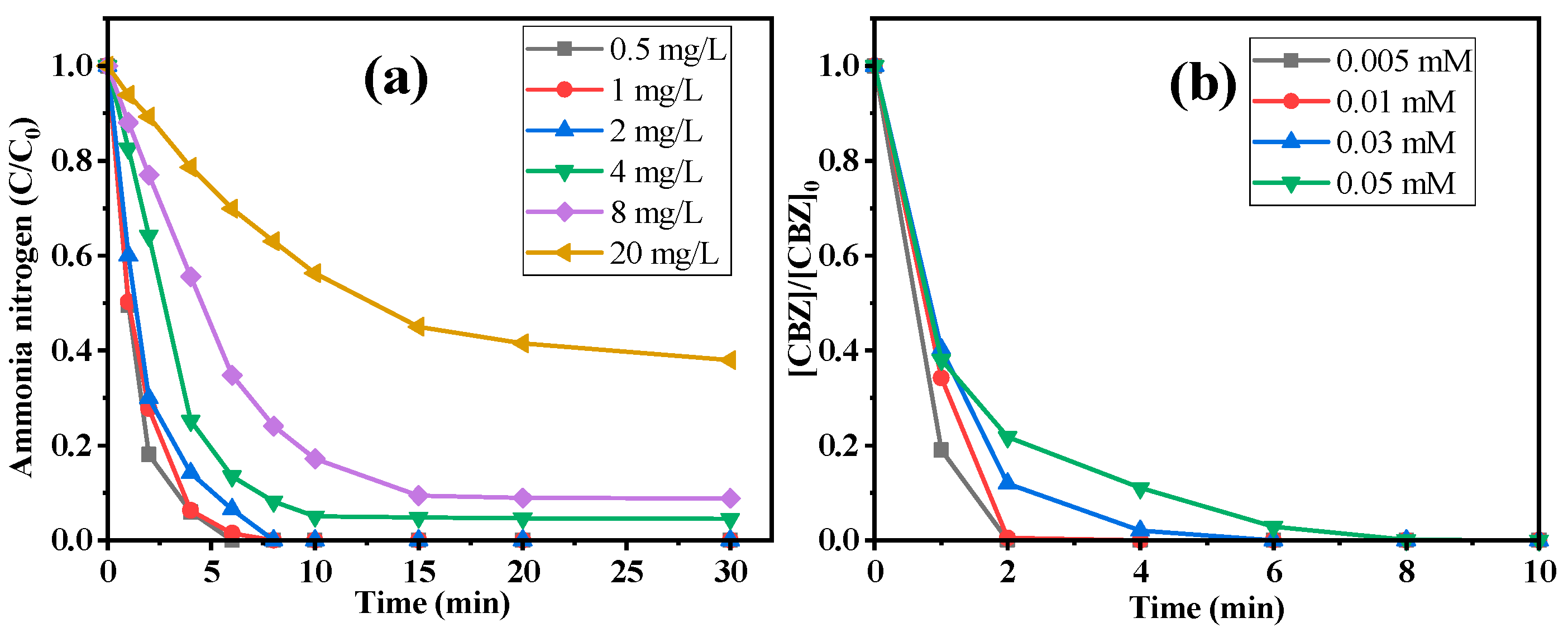
2.2.4. Influence of Pollutant Concentration
2.2.5. Influence of Background Anions
2.3. The Removal Mechanisms of Ammonia and CBZ
3. Chemicals and Methods
3.1. Chemicals
3.2. The Photochemical Reaction Apparatus and Experimental Conditions
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, W.; Fang, H.; Lei, Z.; Wang, R.; Fu, C.; Wang, F.; Fang, Y.; Du, X.; Wang, Z.; Zhao, Z. Insight into homogeneous activation of sodium hypochlorite by dithionite coupled with dissolved oxygen (DO@NaClO/DTN) for carbamazepine degradation. Water Res. 2025, 277, 123312. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Durães, L.; Simões, N.E.; Pereira, A.M.; Silva, L.J.; Feio, M.J. Pharmaceuticals in urban streams: A review of their detection and effects in the ecosystem. Water Res. 2024, 268, 122657. [Google Scholar] [CrossRef]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J. Toxic effects and metabolic fate of carbamazepine in diatom Navicula sp. as influenced by humic acid and nitrogen species. J. Hazard. Mater. 2019, 378, 120763. [Google Scholar] [CrossRef]
- Muschket, M.; Neuwald, I.J.; Zahn, D.; Seelig, A.H.; Kuckelkorn, J.; Knepper, T.P.; Reemtsma, T. Fate of persistent and mobile chemicals in the water cycle: From municipal wastewater discharges to river bank filtrate. Water Res. 2024, 266, 122436. [Google Scholar] [CrossRef]
- Im, J.; Son, H.; Kang, Y.; Zoh, K. Carbamazepine Degradation by Photolysis and Titanium Dioxide Photocatalysis. Water Environ. Res. 2012, 84, 554–561. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, S.; Hu, Y.; Gong, S.; Bao, S.; Li, H.; Zhang, X.; Yuan, Z.; Wu, X. Activation of Peroxymonosulfate by Fe, O Co-Embedded Biochar for the Degradation of Tetracycline: Performance and Mechanisms. Catalysts 2024, 14, 556. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.J.; García-Cañibano, C.; Lepistö, R.-J.; Encinas, Á.; Pellinen, J.; Marugán, J. Intensification of UV-C tertiary treatment: Disinfection and removal of micropollutants by sulfate radical based Advanced Oxidation Processes. J. Hazard. Mater. 2019, 372, 94–102. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Zhou, A.; Liu, Y. Rapid oxidation of ammonia nitrogen to nitrogen gas by UV-activated persulfate with calcium oxide. Environ. Sci. Tech. Eng. 2024, 4, 1092–1101. [Google Scholar] [CrossRef]
- Amanollahi, H.; Moussavi, G.; Giannakis, S. Enhanced vacuum UV-based process (VUV/H2O2/PMS) for the effective removal of ammonia from water: Engineering configuration and mechanistic considerations. J. Hazard. Mat. 2021, 402, 123789. [Google Scholar] [CrossRef]
- Zhang, G.; Ruan, J.; Du, T. Recent advances on photocatalytic and electrochemical oxidation for ammonia treatment from water/wastewater. Environ. Sci. Technol. 2021, 1, 310–325. [Google Scholar] [CrossRef]
- Heeb, M.B.; Kristiana, I.; Trogolo, D.; Arey, J.S.; von Gunten, U. Formation and reactivity of inorganic and organic chloramines and bromamines during oxidative water treatment. Water Res. 2017, 110, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.J.; Doucette, W.J.; Moore, M.T. Contaminants of emerging concern (CECs) in Zea mays: Uptake, translocation and distribution tissue patterns over the time and its relation with physicochemical properties and plant transpiration rate. Chemosphere 2022, 288, 132480. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhao, S.; Mao, R.; Zhao, X. Activation of peroxymonosulfate by cobalt doped graphitic carbon nitride for ammonia removal in chloride-containing wastewater. Sep. Purif. Technol. 2021, 271, 118858. [Google Scholar] [CrossRef]
- Liu, G.; You, S.; Tan, Y.; Ren, N. In Situ Photochemical Activation of Sulfate for Enhanced Degradation of Organic Pollutants in Water. Environ. Sci. Techn. 2017, 51, 2339–2346. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, F.; Tan, W.; Feng, X. New insight into wastewater treatment by activation of sulfite with humic acid under visible light irradiation. Water Res. 2024, 258, 121773. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W.; Xu, B. Catalytic degradation of caffeine in aqueous solutions by cobalt-MCM41 activation of peroxymonosulfate. Appl. Catal. B Environ. 2013, 134–135, 324–332. [Google Scholar] [CrossRef]
- Xu, L.; Sun, Y.; Gan, L.; Han, J.; Wang, P.; Yu, L.; Mei, X.; Li, W.; Lyu, B.; Pei, C.; et al. Utilization of photochemical circulation between NO3− and NO2− in water to degrade photoinert dimethyl phthalate: Influence of organic media and mechanism study. Appl. Catal. B Environ. 2019, 259, 117958. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Wang, S.; Liu, W.; Zhou, G.; Xie, Y.; Guan, X. Both Fe(IV) and Radicals Are Active Oxidants in the Fe(II)/Peroxydisulfate Process. Environ. Sci. Technol. Lett. 2020, 7, 219–224. [Google Scholar] [CrossRef]
- Liu, X.; Xu, P.; Fu, Q.; Li, R.; He, C.; Yao, W.; Wang, L.; Xie, S.; Xie, Z.; He, Q.; et al. Ferric ion promoted degradation of acetaminophen with zero−valent copper activated peroxymonosulfate process. Chem. Eng. J. 2021, 426, 131679. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J. Cobalt-based catalyst to activate PMS for selective ammonium oxidation in PMS/chloridion system. J. Environ. Chem. Eng. 2024, 12, 112513. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahdavianpour, M. The selective direct oxidation of ammonium in the contaminated water to nitrogen gas using the chemical-less VUV photochemical continuous-flow reactor. Chem. Eng. J. 2016, 295, 57–63. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Peng, J.; Zhang, H.; Zhang, Y.; Lai, B. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation. Chem. Eng. J. 2019, 359, 1097–1110. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Huo, Y.; Zhang, Y.; Xu, L.; Gan, L. Performance and Mechanism Study of Simultaneous Removal of Carbamazepine and Ammonia from Water Using UV/Peroxymonosulfate Process. Catalysts 2025, 15, 468. https://doi.org/10.3390/catal15050468
Yuan S, Huo Y, Zhang Y, Xu L, Gan L. Performance and Mechanism Study of Simultaneous Removal of Carbamazepine and Ammonia from Water Using UV/Peroxymonosulfate Process. Catalysts. 2025; 15(5):468. https://doi.org/10.3390/catal15050468
Chicago/Turabian StyleYuan, Shiqi, Yudong Huo, Ying Zhang, Lijie Xu, and Lu Gan. 2025. "Performance and Mechanism Study of Simultaneous Removal of Carbamazepine and Ammonia from Water Using UV/Peroxymonosulfate Process" Catalysts 15, no. 5: 468. https://doi.org/10.3390/catal15050468
APA StyleYuan, S., Huo, Y., Zhang, Y., Xu, L., & Gan, L. (2025). Performance and Mechanism Study of Simultaneous Removal of Carbamazepine and Ammonia from Water Using UV/Peroxymonosulfate Process. Catalysts, 15(5), 468. https://doi.org/10.3390/catal15050468








