One-Step Carbonization of Monosaccharide and Dicyandiamide to Oxygen and Nitrogen Co-Doped Carbon Nanosheets for Electrocatalytic O2 Reduction to H2O2
Abstract
1. Introduction
2. Results and Discussion
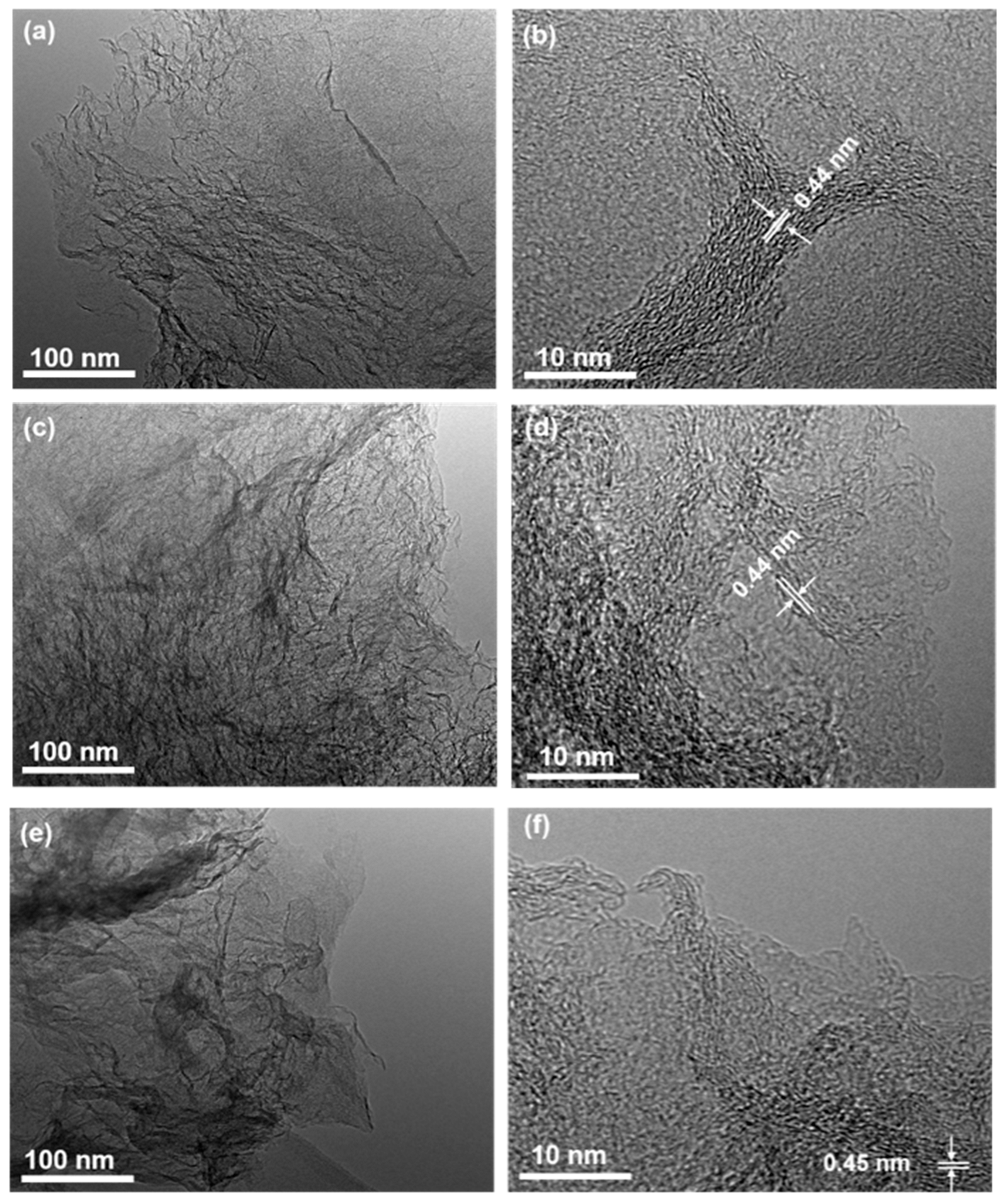
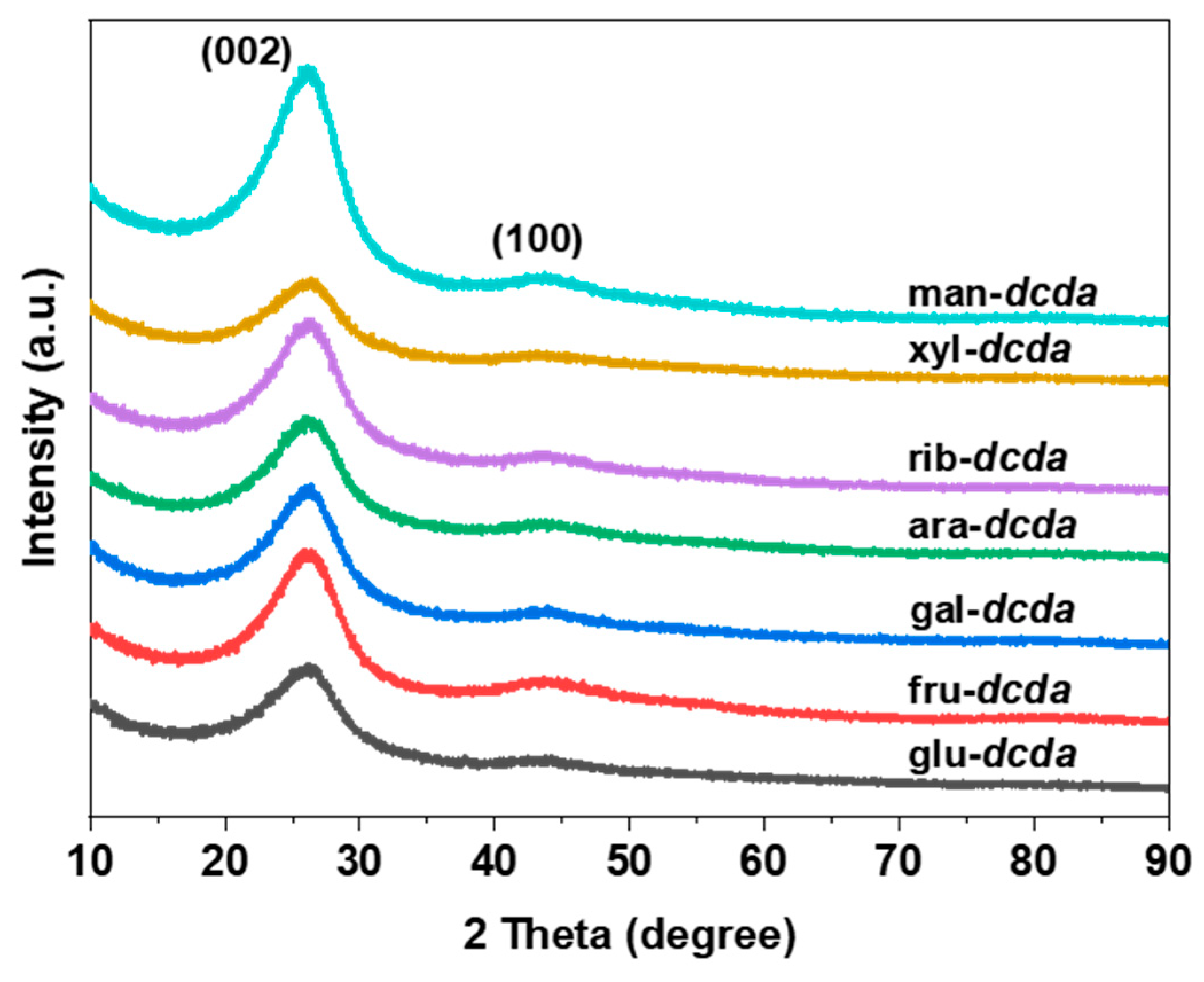
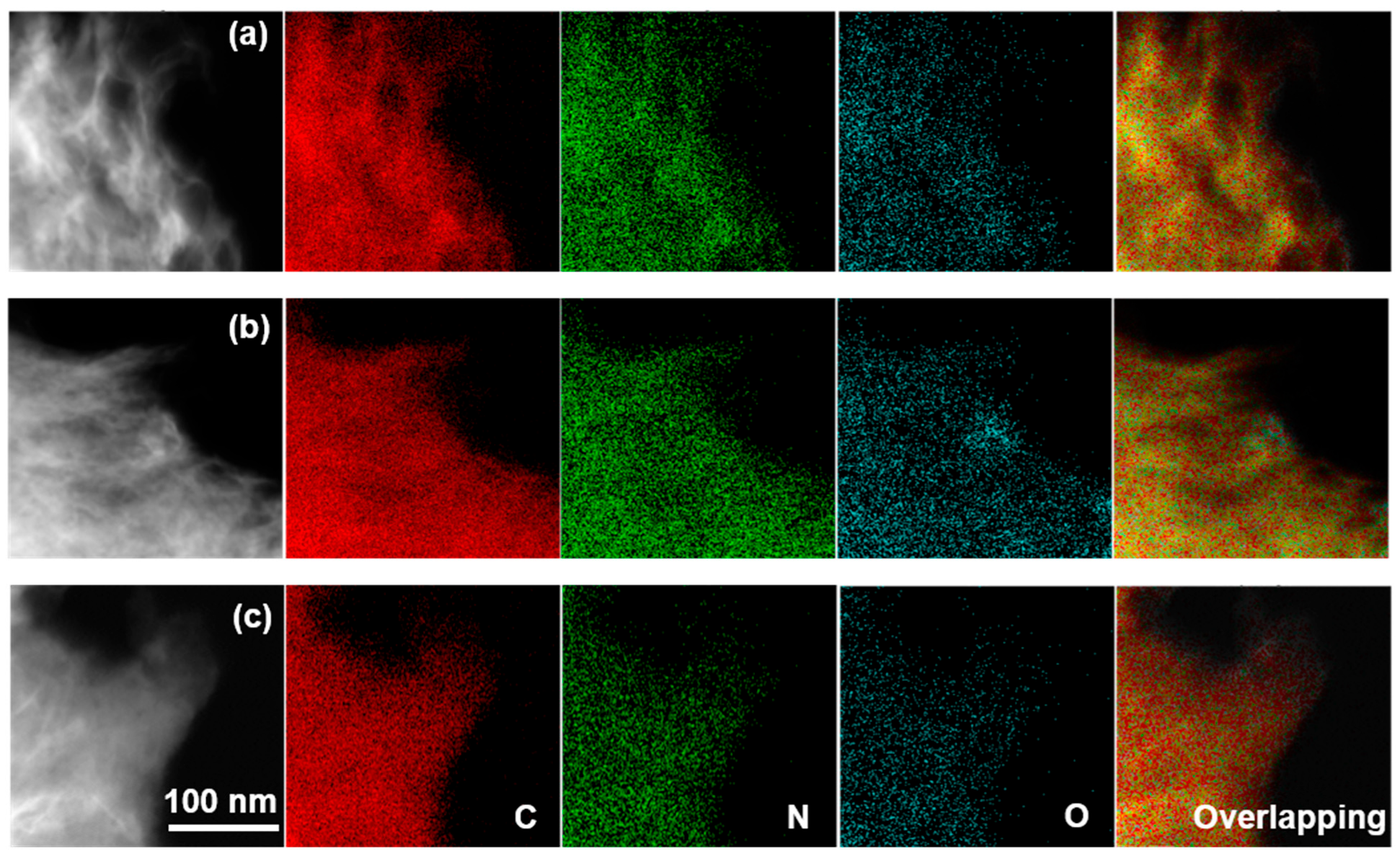
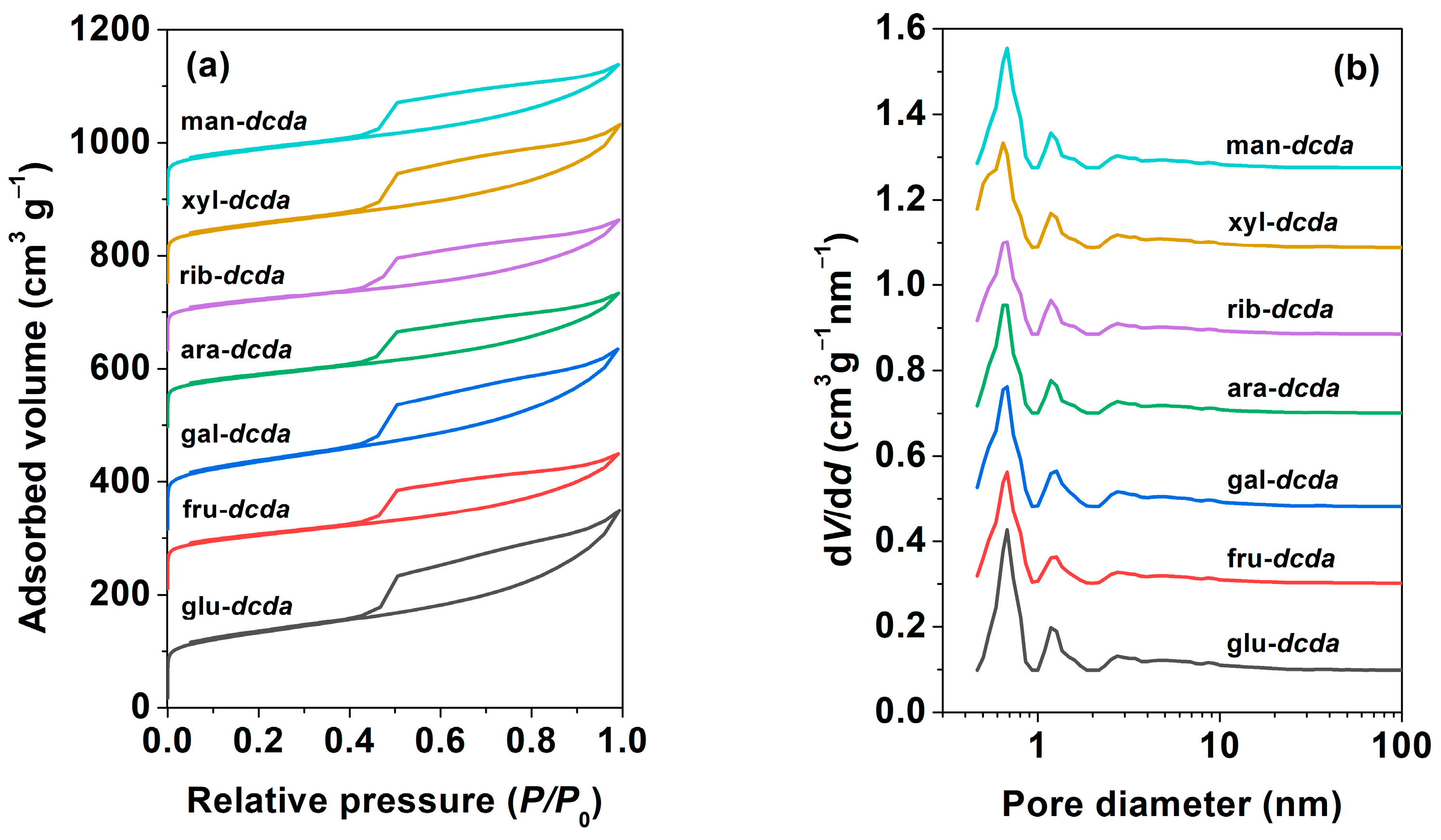
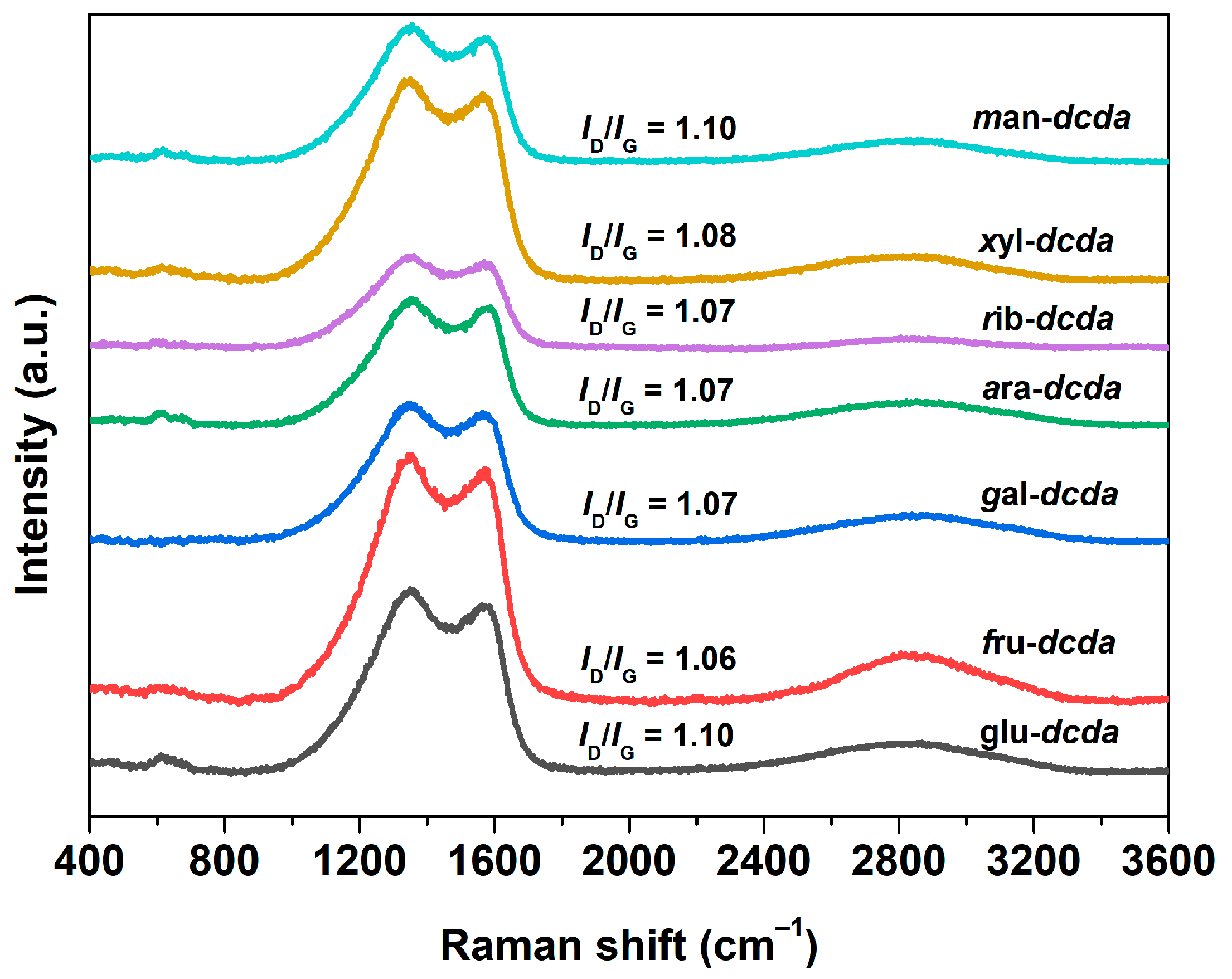
2.1. Basic Physicochemical Properties
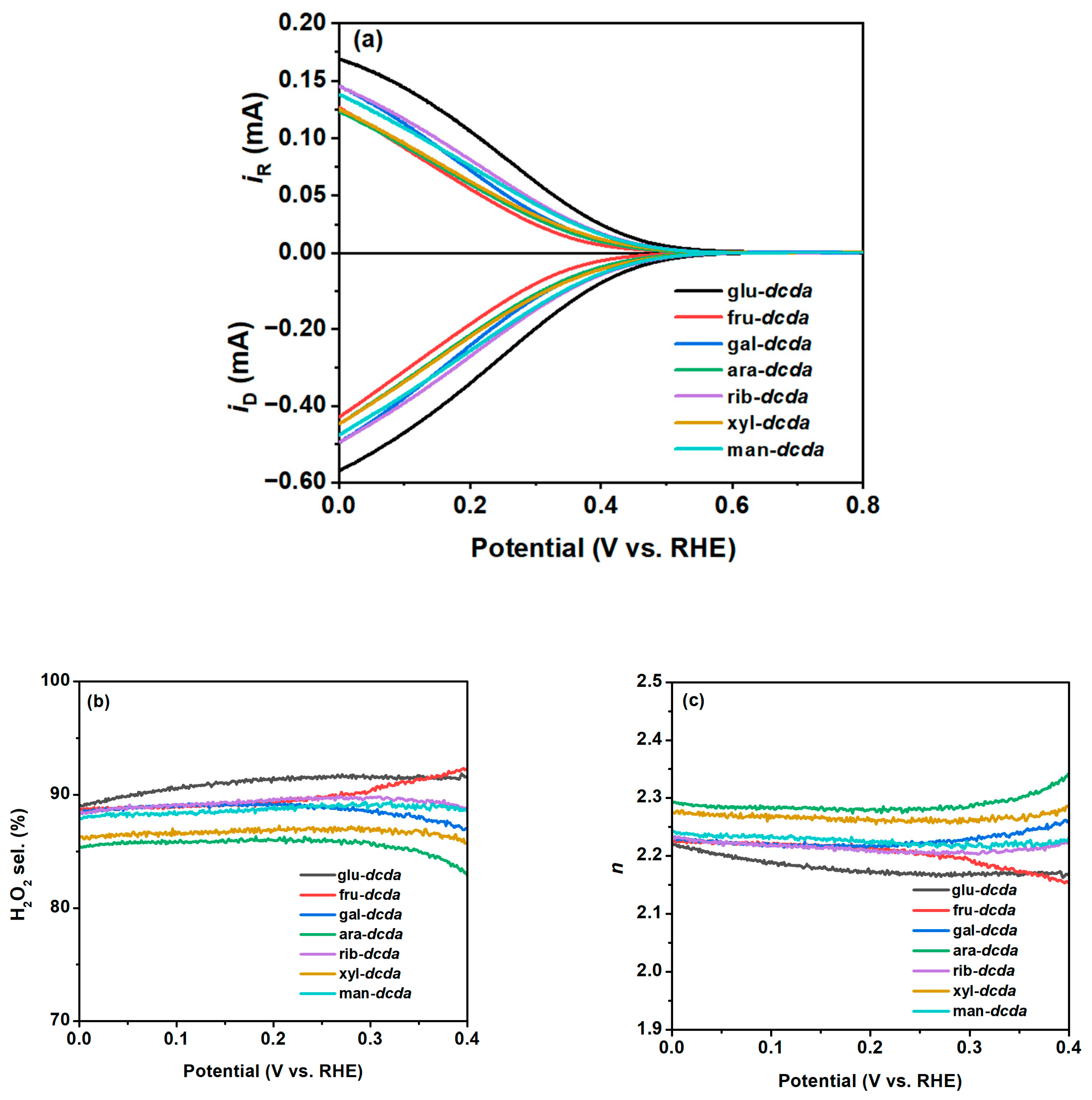
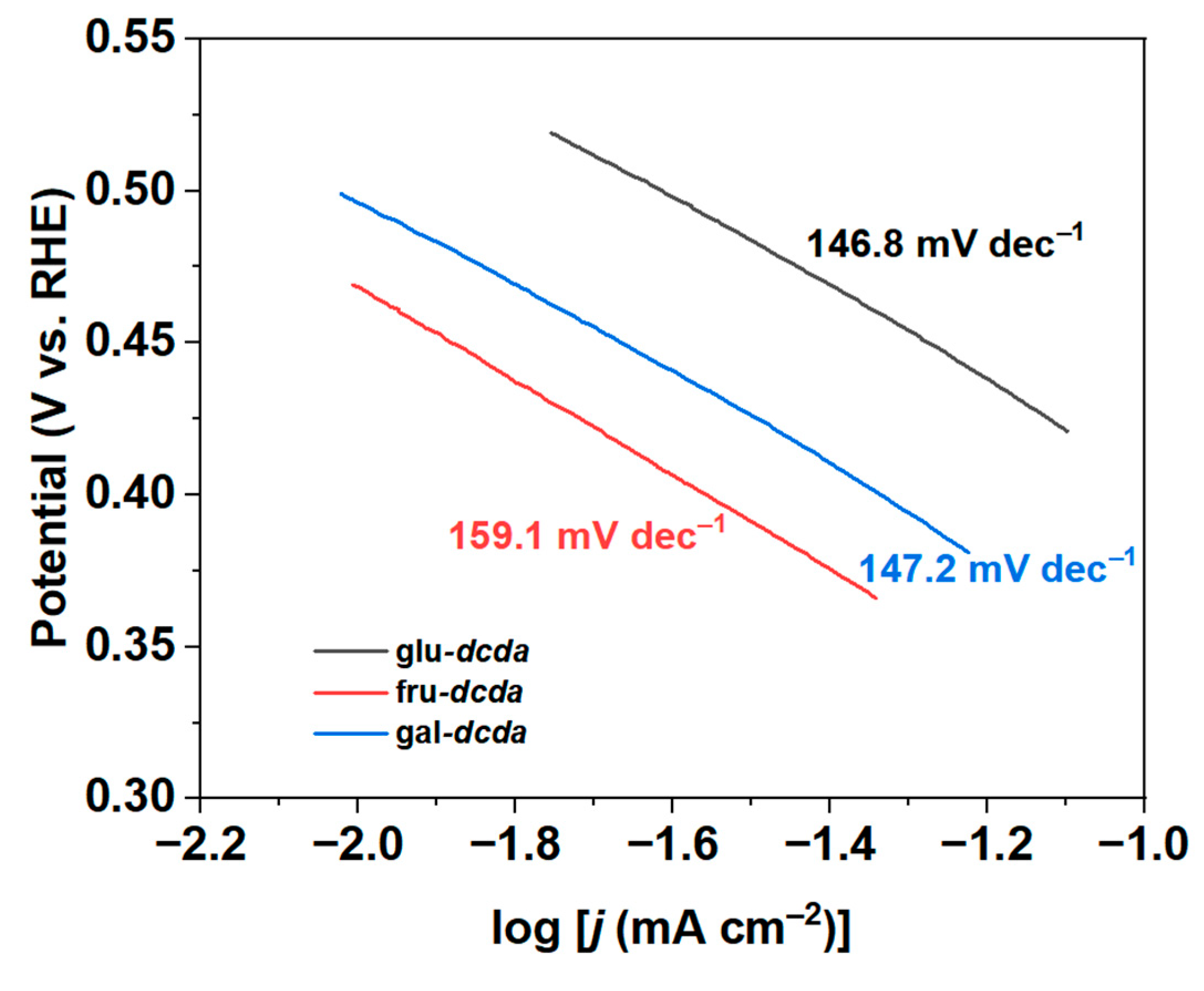
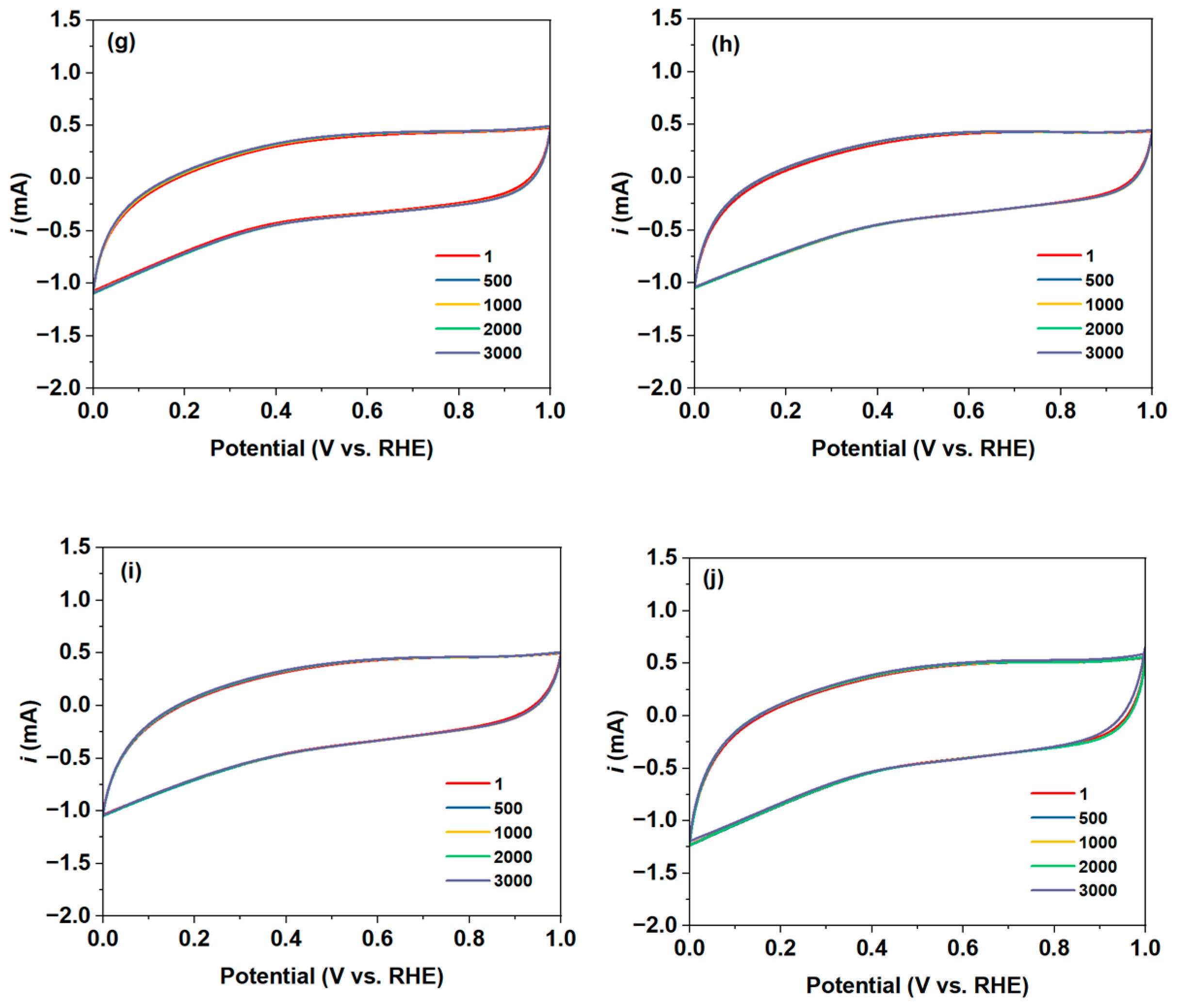
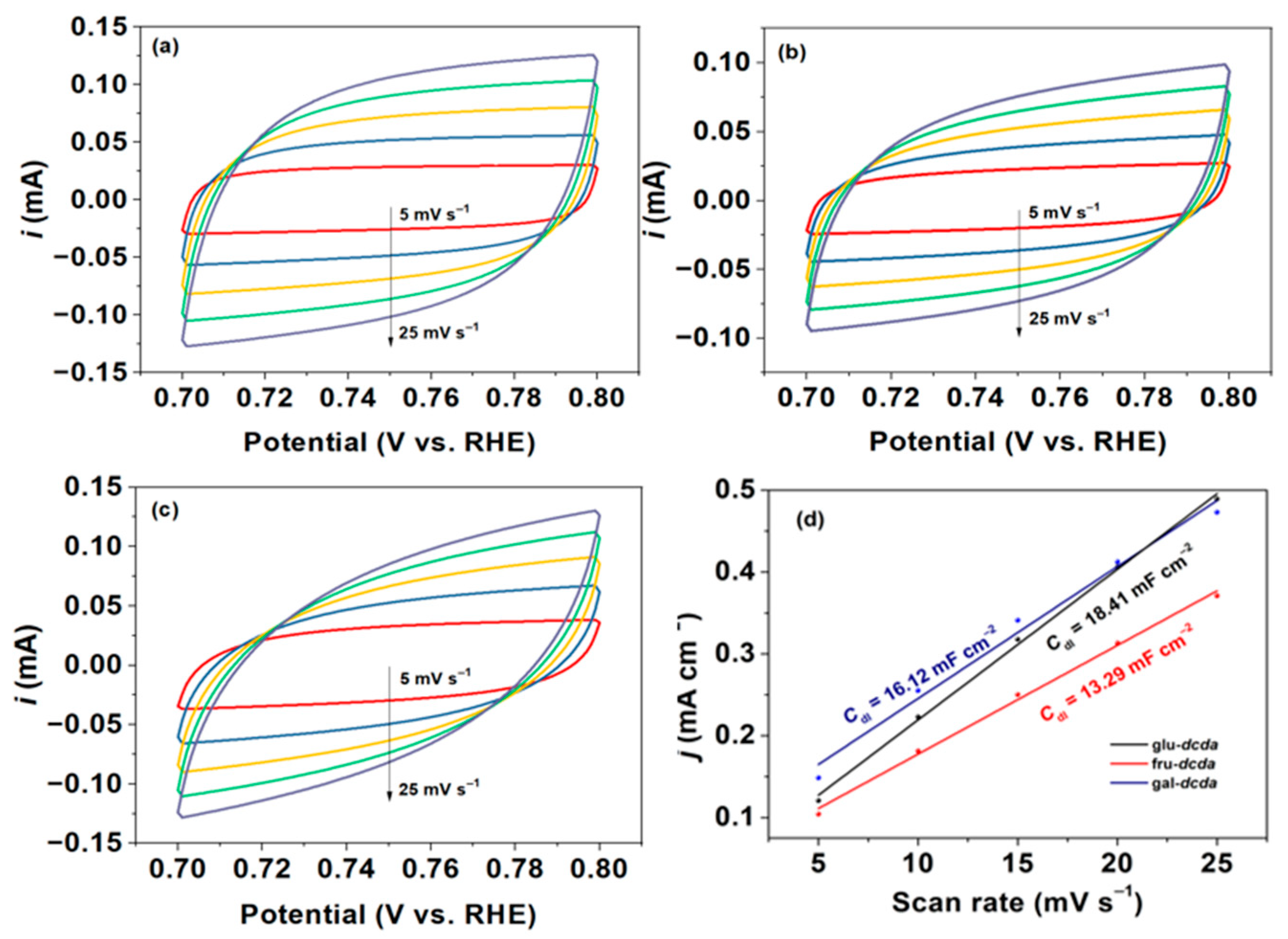
2.2. 2e-ORR Catalytic Performance
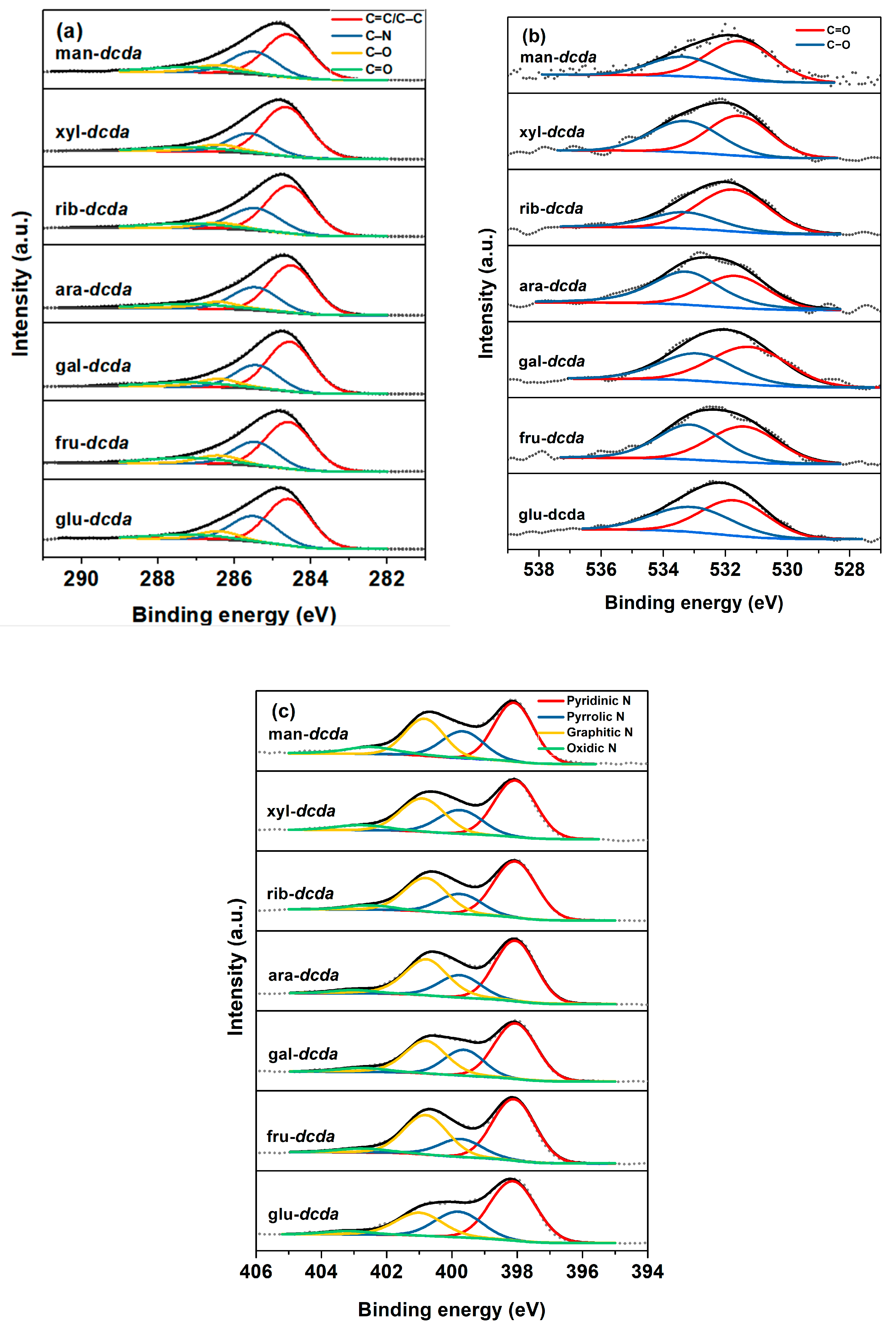
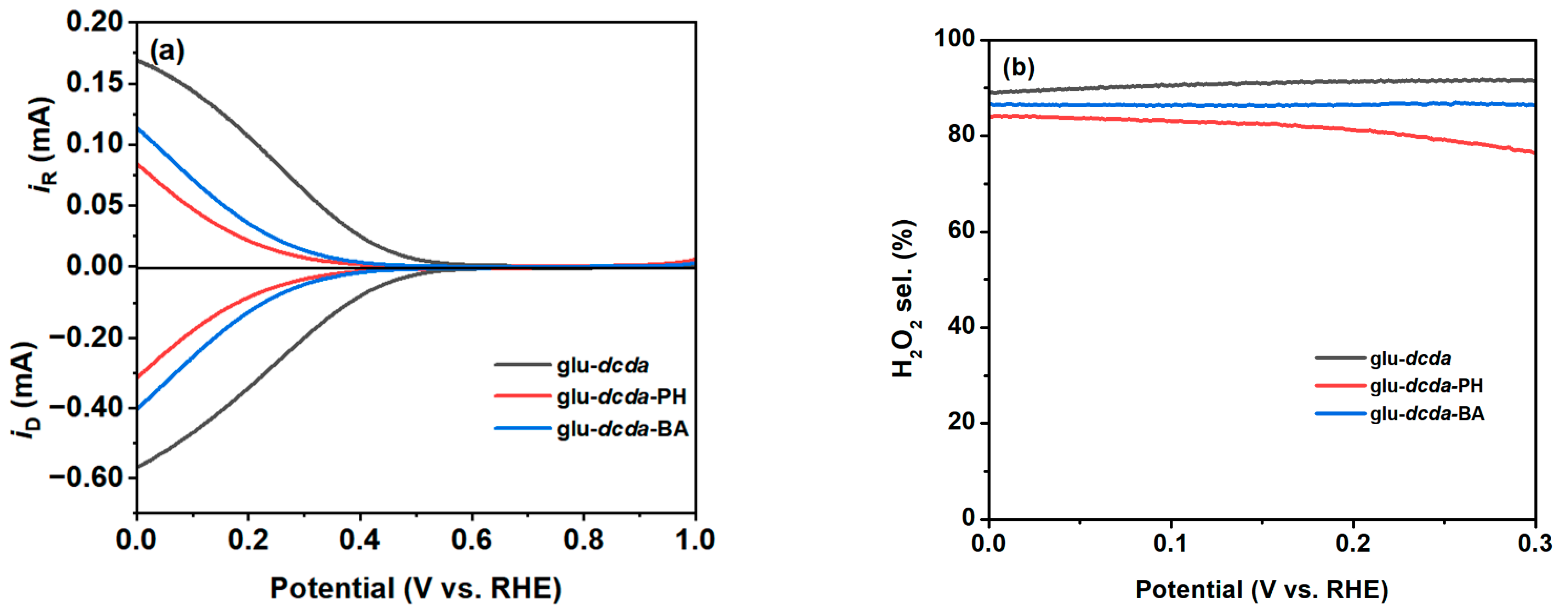
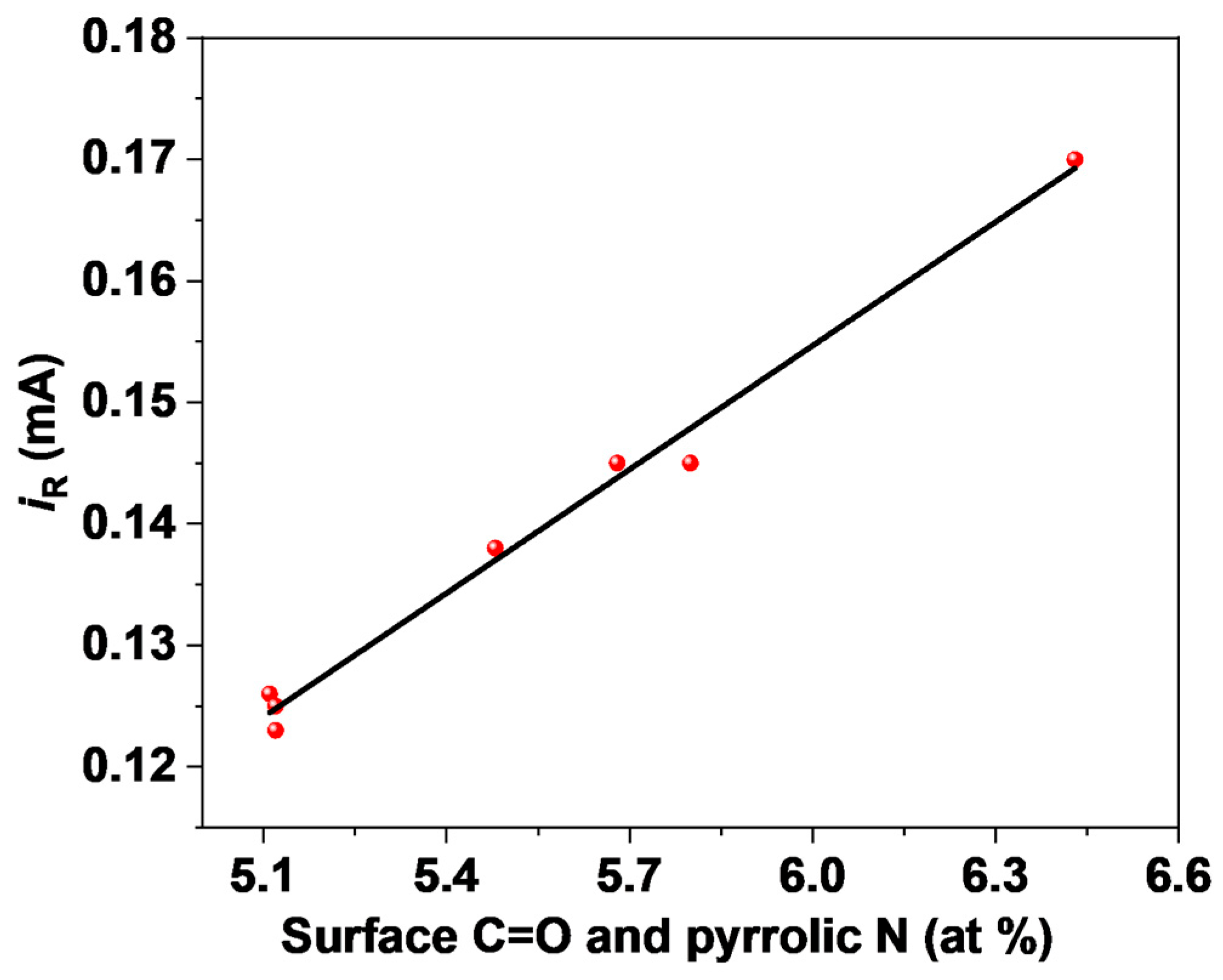
2.3. Elucidation of Surface Active Sites
3. Materials and Methods
3.1. Chemicals
3.2. Preparation
3.3. Electrocatalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P.; Prato, M. The Rise of Hydrogen Peroxide as the Main Product by Metal-Free Catalysis in Oxygen Reductions. Adv. Mater. 2019, 31, 1802920. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Ni, P.J.; Chen, C.X.; Lu, Y.Z.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H.T. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231. [Google Scholar] [CrossRef]
- Chen, Z.H.; Chen, S.C.; Siahrostami, S.; Chakthranont, P.; Hahn, C.; Nordlund, D.; Dimosthenis, S.; Nørskov, J.K.; Bao, Z.N.; Jaramillo, T.F. Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2. React. Chem. Eng. 2017, 2, 239–245. [Google Scholar] [CrossRef]
- Hansen, H.A.; Viswanathan, V.; Nørskov, J.K. Unifying Kinetic and Thermodynamic Analysis of 2e− and 4e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. C 2014, 118, 6706–6718. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.-L.; Hou, Z.; Ikeda, T.; Terakura, K. Two-Electron Oxygen Reduction on Carbon Materials Catalysts: Mechanisms and Active Sites. J. Phys. Chem. C 2017, 121, 14524–14533. [Google Scholar] [CrossRef]
- Li, L.Q.; Yang, H.B.; Miao, J.W.; Zhang, L.P.; Wang, H.-Y.; Zeng, Z.P.; Huang, W.; Dong, X.C.; Liu, B. Unraveling Oxygen Evolution Reaction on Carbon-Based Electrocatalysts: Effect of Oxygen Doping on Adsorption of Oxygenated Intermediates. ACS Energy Lett. 2017, 2, 294–300. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadmap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.Y.; Yang, T.; Qin, Y.; Xie, J.C.; Wei, Z.X.; Zhao, S.L. Epoxidized Single-Atom Co–N–C Catalysts Promote the Oxygen Reduction Reaction via a Two-Electron Pathway. ACS Appl. Mater. Interfaces 2024, 16, 68221–68228. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, B.Q.; Liu, Y.; Wang, C.B.; Ye, C.L.; Yang, W.J. A Ni single-atom catalyst for advanced environmental disinfection based on electrochemical production of hydrogen peroxide. J. Mater. Chem. A 2025, 13, 10683–10693. [Google Scholar] [CrossRef]
- Choi, J.W.; Byeon, A.; Kim, S.; Hwang, C.K.; Zhang, W.; Lee, J.; Yun, W.C.; Paek, S.Y.; Kim, J.H.; Jeong, G.; et al. Mesoporous Boron-Doped Carbon with Curved B4C Active Sites for Highly Efficient H2O2 Electrosynthesis in Neutral Media and Air-Supplied Environments. Adv. Mater. 2025, 37, 2415712. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, X.; Wang, G.F.; Zhou, L.L.; Zhu, X.X.; Pan, J.Q.; Yu, W.; Xia, C.G.; Tian, C.C. Pyrolysis-Free Mechanochemical Conversion of Small Organic Molecules into Metal-Free Heteroatom-Doped Mesoporous Carbons for Efficient Electrosynthesis of Hydrogen Peroxide. ACS Materials Lett. 2023, 5, 379–387. [Google Scholar] [CrossRef]
- Qu, J.T.; Long, G.F.; Luo, L.; Yang, Y.; Fan, W.J.; Zhang, F.X. Electrosynthesis of H2O2 Promoted by π–π Interaction on a Metal-Free Carbon Catalyst. Small 2024, 20, 2400695. [Google Scholar] [CrossRef]
- Wu, F.M.; Nan, J.; Ge, Z.C.; Wang, T.Z.; Zhang, Y.B.; Ye, X.S.; Liu, B.H. S, N, and O tri-doped graphite nanosheets-based gas diffusion electrode boosts highly selective electrosynthesis of H2O2. Sep. Purif. Technol. 2024, 347, 127599. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Chen, Y.G.; Pan, J.; Daiyan, R.; Lovell, E.C.; Yun, J.; Amal, R.; Lu, X.Y. Electrosynthesis of Hydrogen Peroxide through Selective Oxygen Reduction: A Carbon Innovation from Active Site Engineering to Device Design. Small 2023, 19, 2302338. [Google Scholar] [CrossRef]
- Li, Y.; Lawson, T.; Hou, Y.; Dai, L.M. Multifunctional Carbon-Based Metal-Free Catalysts for Cascade Electrochemical-Chemical Coupling Catalyses. Adv. Funct. Mater. 2025, 2423960. Available online: https://advanced.onlinelibrary.wiley.com/doi/10.1002/adfm.202423960 (accessed on 20 January 2025). [CrossRef]
- Sa, Y.J.; Kim, J.H.; Joo, S.H. Active Edge-Site-Rich Carbon Nanocatalysts with Enhanced Electron Transfer for Efficient Electrochemical Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2019, 58, 1100–1105. [Google Scholar] [CrossRef]
- Dong, K.; Liang, J.; Wang, Y.Y.; Xu, Z.Q.; Liu, Q.; Luo, Y.L.; Li, T.S.; Li, L.; Shi, X.F.; Asiri, A.M.; et al. Honeycomb Carbon Nanofibers: A Superhydrophilic O2-Entrapping Electrocatalyst Enables Ultrahigh Mass Activity for the Two-Electron Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 10583–10587. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, Z.H.; Siahrostami, S.; Kim, T.R.; Nordlund, D.; Sokaras, D.; Nowak, S.; To, J.W.F.; Higgins, D.; Sinclair, R.; et al. Defective Carbon-Based Materials for the Electrochemical Synthesis of Hydrogen Peroxide. ACS Sustainable Chem. Eng. 2018, 6, 311–317. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Zhang, J.J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide. J. Mater. Chem. A 2020, 8, 20849–20869. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, J.H.; Woo, J.; Baek, D.; Ihm, K.; Shin, T.J.; Sa, Y.J.; Joo, S.H. Designing highly active nanoporous carbon H2O2 production electrocatalysts through active site identification. J. Chem. 2021, 7, 3114–3130. [Google Scholar] [CrossRef]
- Han, G.F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.P.; Kim, S.W.; Kim, S.J.; Bu, Y.F.; Fu, Z.P.; Lu, Y.L.; et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Chen, S.Y.; Luo, T.; Chen, K.J.; Lin, Y.Y.; Fu, J.W.; Liu, K.; Cai, C.; Wang, Q.Y.; Li, H.J.W.; Li, X.Q.; et al. Chemical Identification of Catalytically Active Sites on Oxygen-doped Carbon Nanosheet to Decipher the High Activity for Electro-synthesis Hydrogen Peroxide. Angew. Chem. Int. Ed. 2021, 60, 16607–16614. [Google Scholar] [CrossRef]
- Fellinger, T.P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef]
- Li, L.Q.; Tang, C.; Zheng, Y.; Xia, B.Q.; Zhou, X.L.; Xu, H.L.; Qiao, S.Z. Tailoring Selectivity of Electrochemical Hydrogen Peroxide Generation by Tunable Pyrrolic-Nitrogen-Carbon. Adv. Energy Mater. 2020, 10, 2000789. [Google Scholar] [CrossRef]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; et al. N-Doped Graphitized Carbon Nanohorns as a Forefront Electrocatalyst in Highly Selective O2 Reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Sinev, I.; Ju, W.; Bergmann, A.; Dresp, S.; Kuehl, S.; Spoeri, C.; Schmies, H.; Wang, H.; Bernsmeier, D.; et al. Efficient Electrochemical Hydrogen Peroxide Production from Molecular Oxygen on Nitrogen-Doped Mesoporous Carbon Catalysts. ACS Catal. 2018, 8, 2844–2856. [Google Scholar] [CrossRef]
- Wang, D.; Feng, B.; Zhang, X.X.; Liu, Y.N.; Pei, Y.; Qiao, M.H.; Zong, B.N. Nitrogen-doped Carbon Pyrolyzed from ZIF-8 for Electrocatalytic Oxygen Reduction to Hydrogen Peroxide. Acta Chim. Sinica 2022, 80, 772–780. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Shen, X.Q.; Chen, Y.; Zhang, S.N.; Gao, P.; Zhen, X.J.; Li, X.H.; Zhao, G.H. A COOH-terminated nitrogen-doped carbon aerogel as a bulk electrode for completely selective two-electron oxygen reduction to H2O2. Chem. Commun. 2019, 55, 6173–6176. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.T.; Zhang, X.X.; Feng, B.; Pei, Y.; Zhu, Y.F.; Xu, W.; Li, Z.H.; Qiao, M.H.; Zong, B.N. Pyrolyzed polydopamine-modified carbon black for selective and durable electrocatalytic oxygen reduction to hydrogen peroxide in acidic medium. Appl. Catal. B 2022, 305, 121036. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef]
- Li, X.H.; Kurasch, S.; Kaiser, U.; Antonietti, M. Synthesis of Monolayer-Patched Graphene from Glucose. Angew. Chem. Int. Ed. 2012, 51, 9689–9692. [Google Scholar] [CrossRef]
- Gu, D.G.; Wang, F.F.; Yan, K.; Ma, R.G.; Wang, J.C. A Thermally Decomposable Template Route to Synthesize Nitrogen-Doped Wrinkled Carbon Nanosheets as Highly Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction. ACS Sustainable Chem. Eng. 2018, 6, 1951–1960. [Google Scholar] [CrossRef]
- He, Q.; Liu, D.B.; Lee, J.H.; Liu, Y.M.; Xie, Z.H.; Hwang, S.; Kattel, S.; Song, L.; Chen, J.G. Electrochemical Conversion of CO2 to Syngas with Controllable CO/H2 Ratios over Co and Ni Single-Atom Catalysts. Angew. Chem. Int. Ed. 2020, 59, 3033–3037. [Google Scholar] [CrossRef]
- de Andres, P.L.; Ramírez, R.; Vergés, J.A. Strong covalent bonding between two graphene layers. Phys. Rev. B 2008, 77, 045403. [Google Scholar] [CrossRef]
- Liu, Y.M.; Quan, X.; Fan, X.F.; Wang, H.; Chen, S. High-Yield Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction by Hierarchically Porous Carbon. Angew. Chem. Int. Ed. 2015, 54, 6837–6841. [Google Scholar] [CrossRef]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M.A. Highly Selective Two-Electron Oxygen Reduction Catalyzed by Mesoporous Nitrogen-Doped Carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, G.; Jin, S.Y.; Zhou, Y.J.; Ji, Q.H.; Lan, H.C.; Liu, H.J.; Qu, J.H. Graphitic N in nitrogen-Doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction. Carbon 2020, 163, 154–161. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, Z.H.; Siahrostami, S.; Higgins, D.; Nordlund, D.; Sokaras, D.; Kim, T.R.; Liu, Y.Z.; Yan, X.Z.; Nilsson, E.; et al. Designing Boron Nitride Islands in Carbon Materials for Efficient Electrochemical Synthesis of Hydrogen Peroxide. J. Am. Chem. Soc. 2018, 140, 7851–7859. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active Sites and Mechanism of Oxygen Reduction Reaction Electrocatalysis on Nitrogen-Doped Carbon Materials. Adv. Mater. 2019, 31, 1804297. [Google Scholar] [CrossRef]
- Qi, W.; Liu, W.; Zhang, B.S.; Gu, X.M.; Guo, X.L.; Su, D.S. Oxidative Dehydrogenation on Nanocarbon: Identification and Quantification of Active Sites by Chemical Titration. Angew. Chem. Int. Ed. 2013, 52, 14224–14228. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhao, A.D.; Gao, N.; Li, K.; Ren, J.S.; Qu, X.G. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef]
- Rao, C.V.; Cabrera, C.R.; Ishikawa, Y. In Search of the Active Site in Nitrogen-Doped Carbon Nanotube Electrodes for the Oxygen Reduction Reaction. J. Phys. Chem. Lett. 2010, 1, 2622–2627. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Kondo, T.; Casolo, S.; Suzuki, T.; Shikano, T.; Sakurai, M.; Harada, Y.; Saito, M.; Oshima, M.; Trioni, M.I.; Tantardini, G.F.; et al. Atomic-scale characterization of nitrogen-doped graphite: Effects of dopant nitrogen on the local electronic structure of the surrounding carbon atoms. Phys. Rev. B 2012, 86, 035436. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Silvioli, L.; Sahraie, N.R.; Ju, W.; Li, J.K.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.L.; et al. Activity−Selectivity Trends in the Electrochemical Production of Hydrogen Peroxide over Single-Site Metal−Nitrogen−Carbon Catalysts. J. Am. Chem. Soc. 2019, 141, 12372–12381. [Google Scholar] [CrossRef]
- Pobiner, H. Determination of Hydroperoxides in Hydrocarbon by Conversion to Hydrogen Peroxide and Measurement by Titanium Complexing. Anal. Chem. 1961, 33, 1423–1426. [Google Scholar] [CrossRef]
- Perazzolo, V.; Daniel, G.; Brandiele, R.; Picelli, L.; Rizzi, G.A.; Isse, A.A.; Durante, C. PEO-b-PS Block Copolymer Templated Mesoporous Carbons: A Comparative Study of Nitrogen and Sulfur Doping in the Oxygen Reduction Reaction to Hydrogen Peroxide. Chem. A Eur. J. 2020, 27, 1002–1014. [Google Scholar] [CrossRef]
- Zhao, K.; Su, Y.; Quan, X.; Liu, Y.; Chen, S.; Yu, H. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon. J. Catal. 2018, 357, 118–126. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, K.; Xie, H.; Sun, Y.; Titirici, M.M.; Chai, G.L. Mesoporous carbon hollow spheres as efficient electrocatalysts for oxygen reduction to hydrogen peroxide in neutral electrolytes. ACS Cataly. 2020, 10, 7434–7442. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, W.; Guo, K.; Xiong, M.; Zhang, J.; Lu, X. A pentagonal defect-rich metal-free carbon electrocatalyst for boosting acidic O2 reduction to H2O2 production. J. Am. Chem. Soc. 2023, 145, 11589–11598. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Wang, M.; Chai, G.; Guan, L. Pyrimidine-assisted synthesis of S, N-codoped few-layered graphene for highly efficient hydrogen peroxide production in acid. Chem Catal. 2022, 2, 1450–1466. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Chen, G.; Wu, Y.; Tian, M.; Peng, Y.; Dou, S.; Li, L.; Sun, J. Refining Metal-Free Carbon Nanoreactors through Electronic and Geometric Comodification for Boosted H2O2 Electrosynthesis toward Efficient Water Decontamination. Environ. Sci. Technol. 2024, 58, 21893–21903. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, S.; Jovanov, Z.P.; Bernsmeier, D.; Wang, H.; Paul, B.; Wang, X.; Kühl, S.; Strasser, P. Structure, Activity, and Faradaic Efficiency of Nitrogen-Doped Porous Carbon Catalysts for Direct Electrochemical Hydrogen Peroxide Production. ChemSusChem 2018, 11, 3388–3395. [Google Scholar] [CrossRef] [PubMed]


| Sample | Bulk Composition (wt %) | SBET (m2 g−1) | Vpore (cm3 g−1) | dpore (nm) | |||
|---|---|---|---|---|---|---|---|
| C | H | O | N | ||||
| glu-dcda | 68.56 | 2.51 | 11.54 | 17.39 | 400 | 0.52 | 5.2 |
| fru-dcda | 59.91 | 2.13 | 18.68 | 19.28 | 325 | 0.38 | 4.6 |
| gal-dcda | 66.66 | 2.39 | 12.04 | 18.91 | 416 | 0.50 | 4.8 |
| ara-dcda | 70.06 | 2.42 | 8.46 | 19.06 | 317 | 0.37 | 4.7 |
| rib-dcda | 69.31 | 2.41 | 8.84 | 19.44 | 303 | 0.36 | 4.8 |
| xyl-dcda | 68.89 | 2.36 | 10.74 | 18.01 | 355 | 0.44 | 4.9 |
| man-dcda | 69.00 | 2.43 | 9.73 | 18.84 | 338 | 0.34 | 4.1 |
| Sample | Surface O (at %) | Percentage in Surface O (%) | Surface C=O (at %) | Surface Pyrrolic N + C=O (at %) | |
|---|---|---|---|---|---|
| C=O | C–O | ||||
| glu-dcda | 4.93 | 53.7 | 46.3 | 2.65 | 6.43 |
| fru-dcda | 4.64 | 49.1 | 50.9 | 2.28 | 5.11 |
| gal-dcda | 4.39 | 55.5 | 44.5 | 2.44 | 5.68 |
| ara-dcda | 4.21 | 44.9 | 55.1 | 1.89 | 5.22 |
| rib-dcda | 4.11 | 67.2 | 32.8 | 2.76 | 5.80 |
| xyl-dcda | 3.38 | 53.5 | 46.5 | 1.81 | 5.12 |
| man-dcda | 2.99 | 66.5 | 33.5 | 1.99 | 5.48 |
| Sample | Surface N (at %) | Percentage in Surface N (%) | Surface Pyrrolic N (at %) | |||
|---|---|---|---|---|---|---|
| Pyridinic N | Pyrrolic N | Graphitic N | Oxidic N | |||
| glu-dcda | 16.6 | 50.9 | 22.8 | 22.3 | 4.0 | 3.78 |
| fru-dcda | 18.3 | 47.5 | 15.5 | 33.0 | 4.0 | 2.83 |
| gal-dcda | 16.1 | 46.0 | 20.1 | 30.0 | 3.9 | 3.24 |
| ara-dcda | 18.2 | 46.0 | 18.3 | 32.8 | 2.9 | 3.33 |
| rib-dcda | 17.7 | 48.6 | 17.2 | 30.3 | 3.9 | 3.04 |
| xyl-dcda | 17.1 | 46.9 | 19.4 | 28.5 | 5.2 | 3.31 |
| man-dcda | 16.6 | 43.3 | 21.1 | 27.0 | 8.6 | 3.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, Y.; Wan, K.; Feng, D.; Pei, Y.; Qiao, M.; Zhang, X.; Zong, B. One-Step Carbonization of Monosaccharide and Dicyandiamide to Oxygen and Nitrogen Co-Doped Carbon Nanosheets for Electrocatalytic O2 Reduction to H2O2. Catalysts 2025, 15, 459. https://doi.org/10.3390/catal15050459
Wang D, Liu Y, Wan K, Feng D, Pei Y, Qiao M, Zhang X, Zong B. One-Step Carbonization of Monosaccharide and Dicyandiamide to Oxygen and Nitrogen Co-Doped Carbon Nanosheets for Electrocatalytic O2 Reduction to H2O2. Catalysts. 2025; 15(5):459. https://doi.org/10.3390/catal15050459
Chicago/Turabian StyleWang, Dan, Yanan Liu, Kun Wan, Danning Feng, Yan Pei, Minghua Qiao, Xiaoxin Zhang, and Baoning Zong. 2025. "One-Step Carbonization of Monosaccharide and Dicyandiamide to Oxygen and Nitrogen Co-Doped Carbon Nanosheets for Electrocatalytic O2 Reduction to H2O2" Catalysts 15, no. 5: 459. https://doi.org/10.3390/catal15050459
APA StyleWang, D., Liu, Y., Wan, K., Feng, D., Pei, Y., Qiao, M., Zhang, X., & Zong, B. (2025). One-Step Carbonization of Monosaccharide and Dicyandiamide to Oxygen and Nitrogen Co-Doped Carbon Nanosheets for Electrocatalytic O2 Reduction to H2O2. Catalysts, 15(5), 459. https://doi.org/10.3390/catal15050459







