Interfacial Engineering of 0D/2D Cu2S/Ti3C2 for Efficient Photocatalytic Synchronous Removal of Tetracycline and Hexavalent Chromium
Abstract
:1. Introduction
2. Results and Discussion
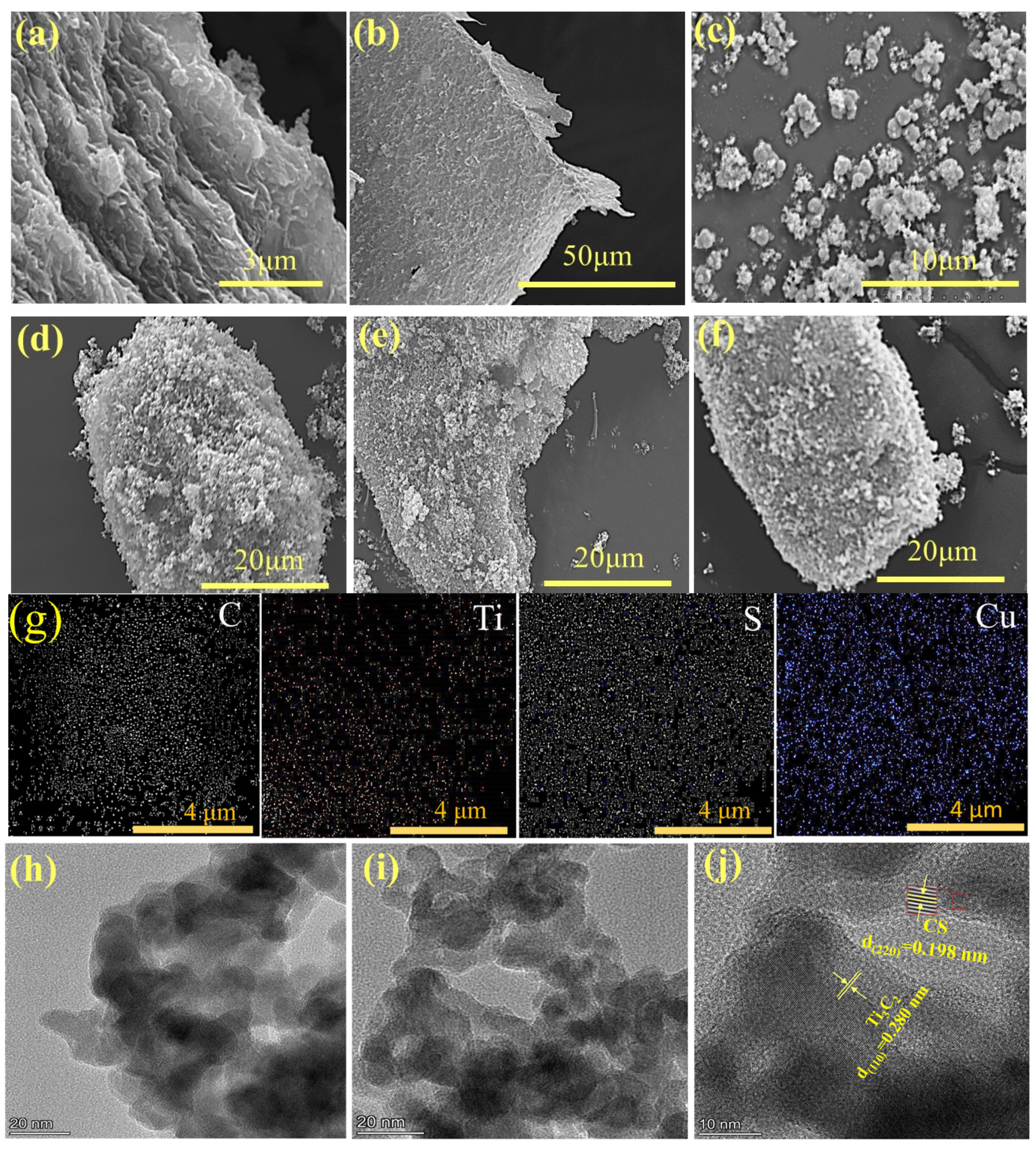
2.1. Characterization of All Samples
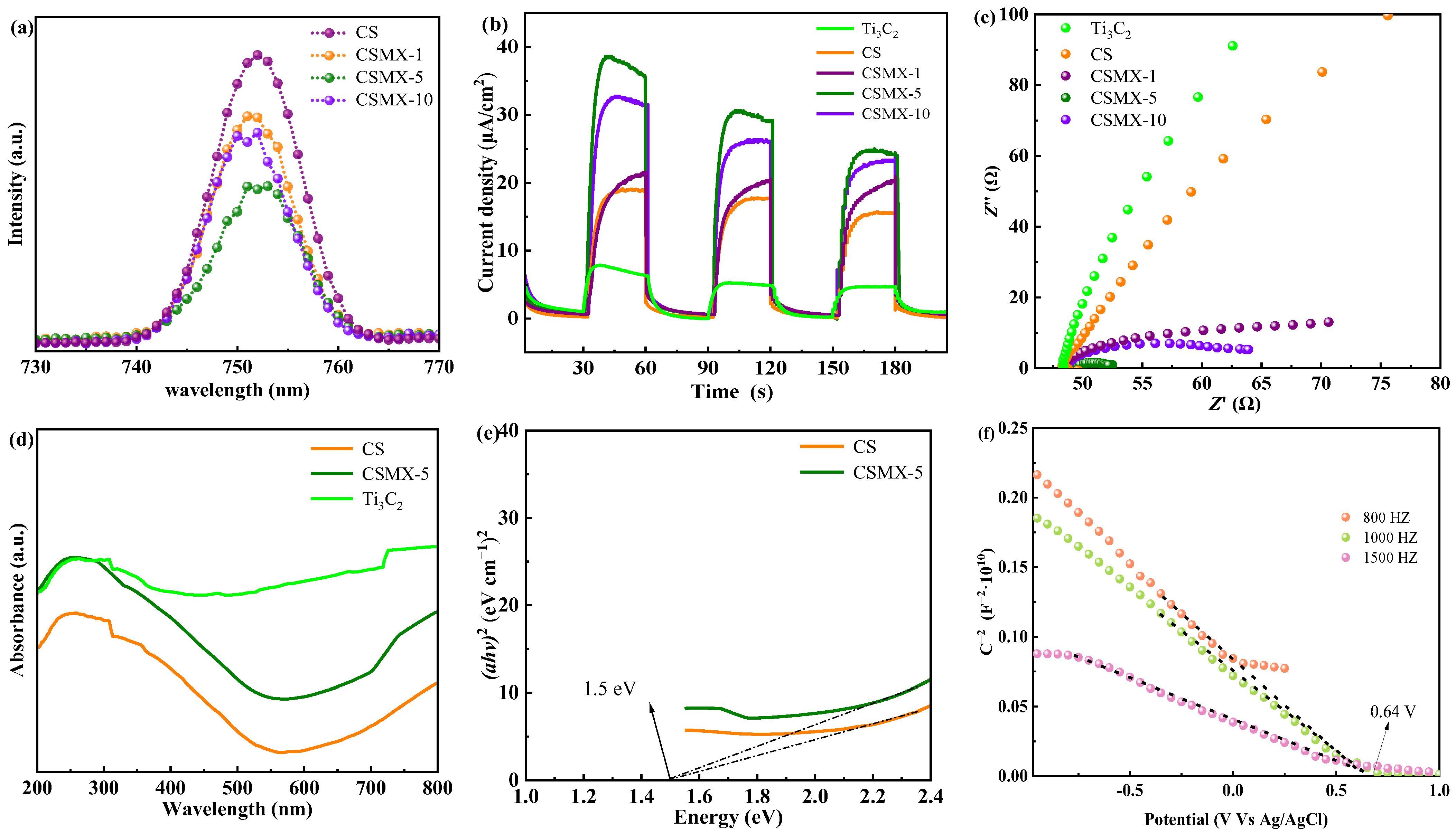
2.2. Photoelectrochemical Characterization Analysis
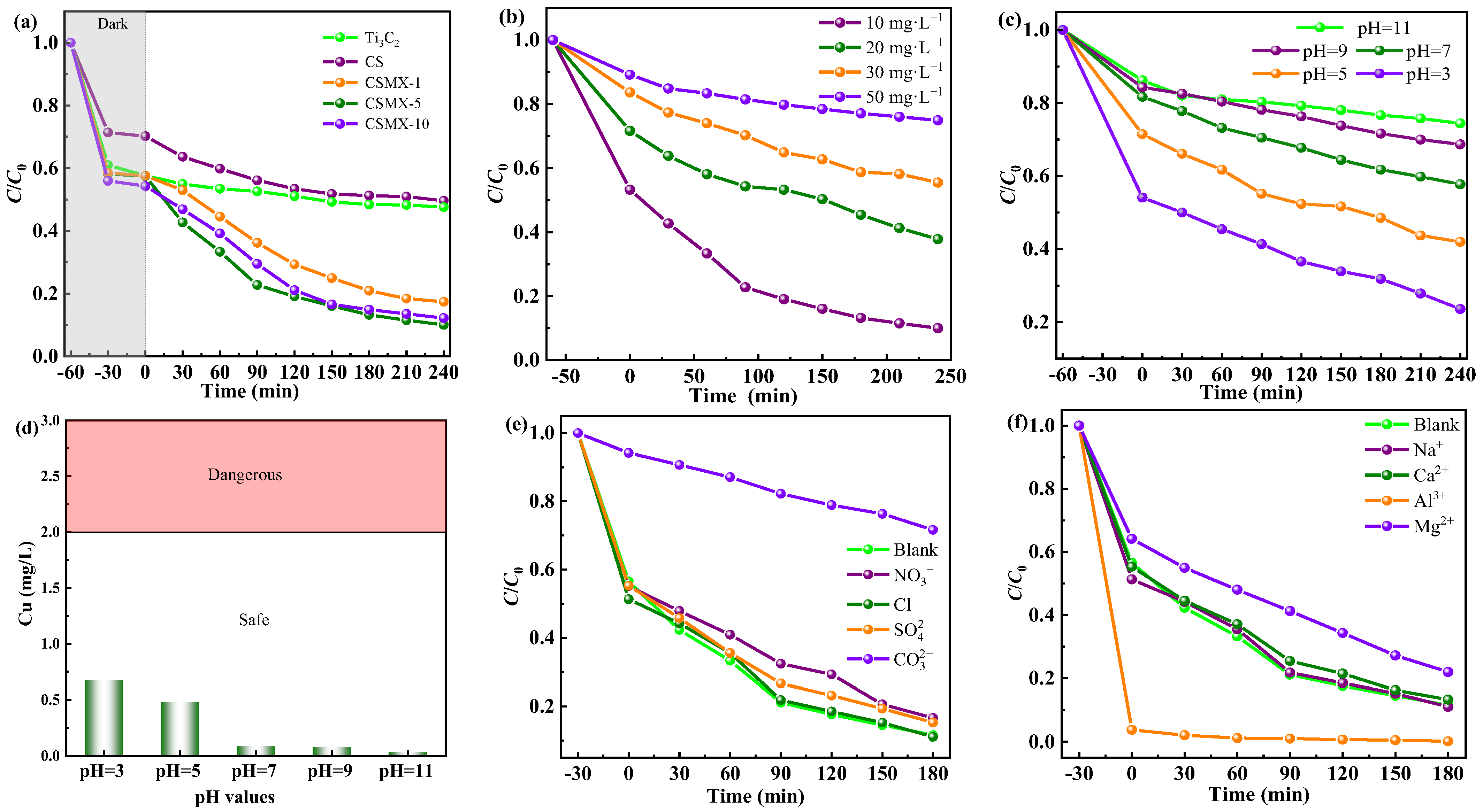
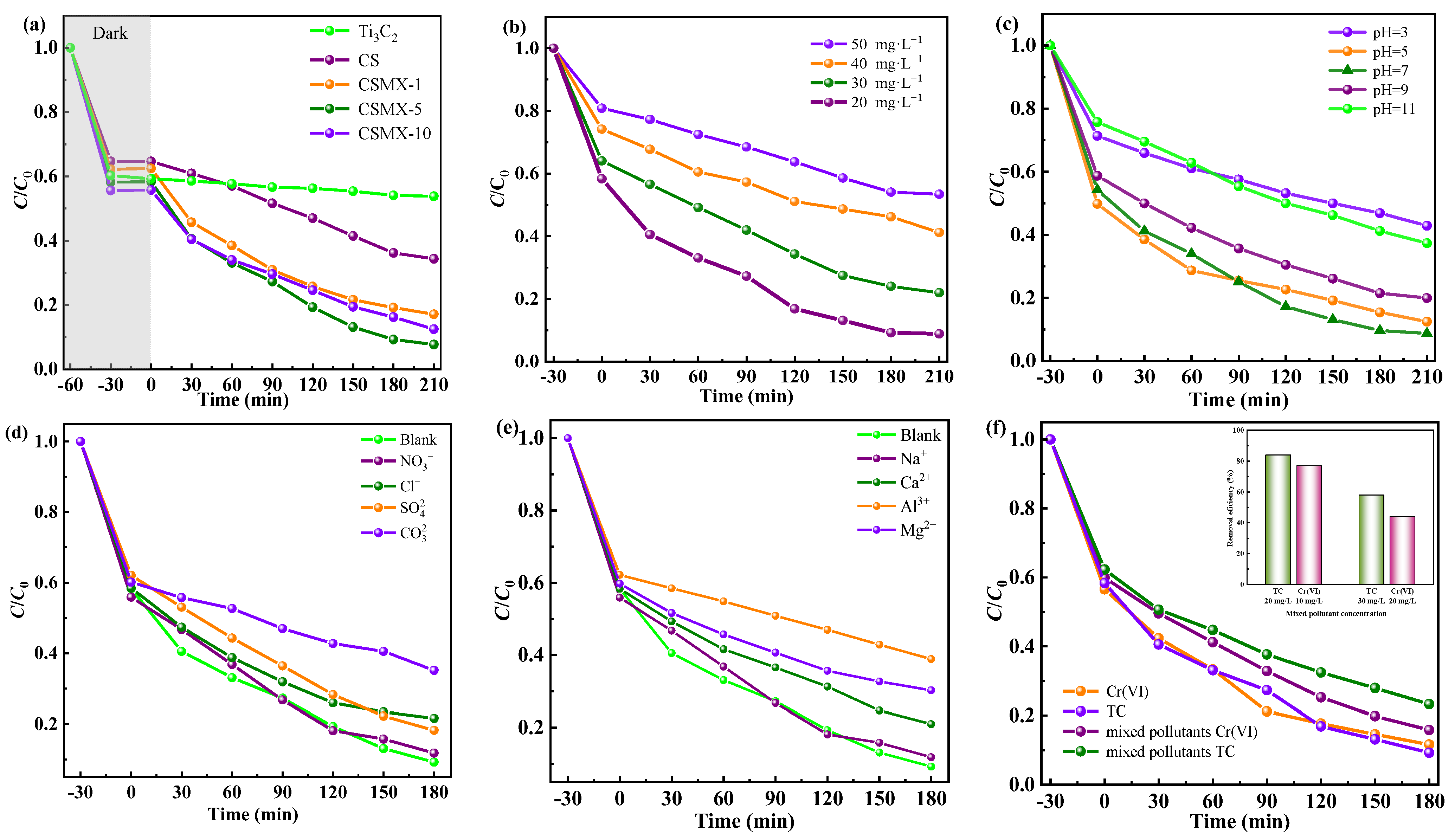
2.3. Photocatalytic Reduction of Cr(VI)
Photocatalytic Reduction Activities
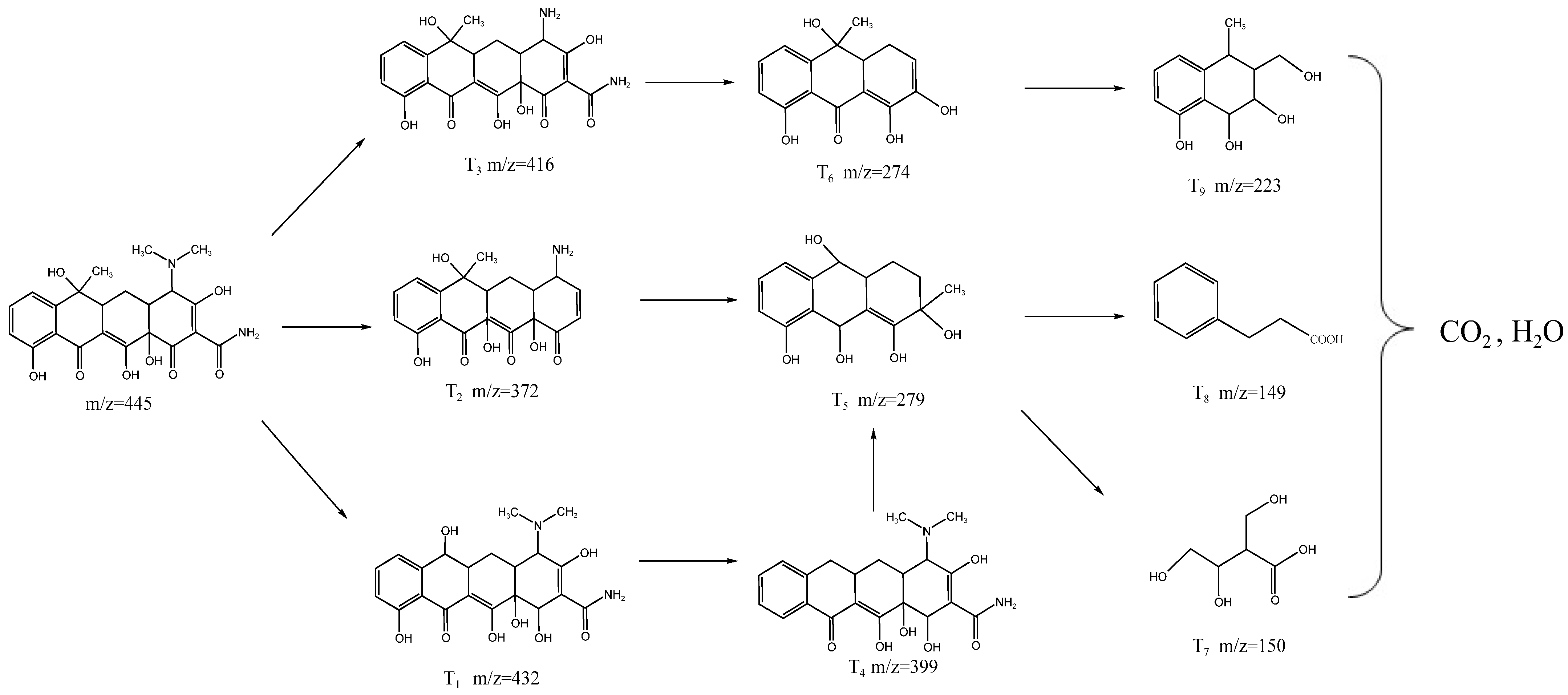
2.4. Photocatalytic Degradation of TC
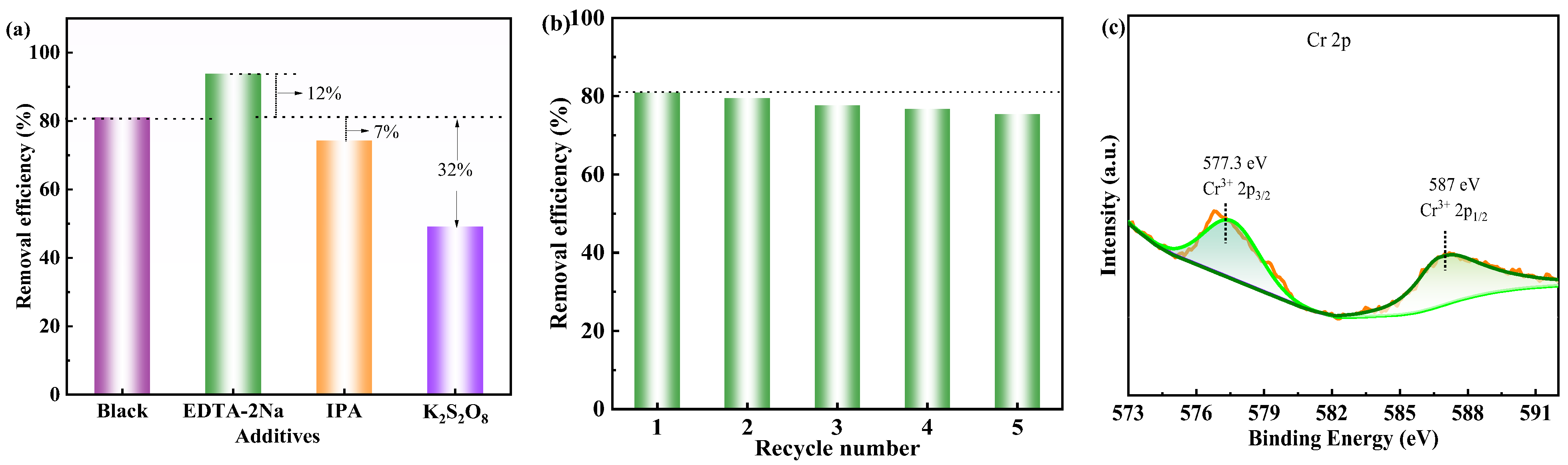
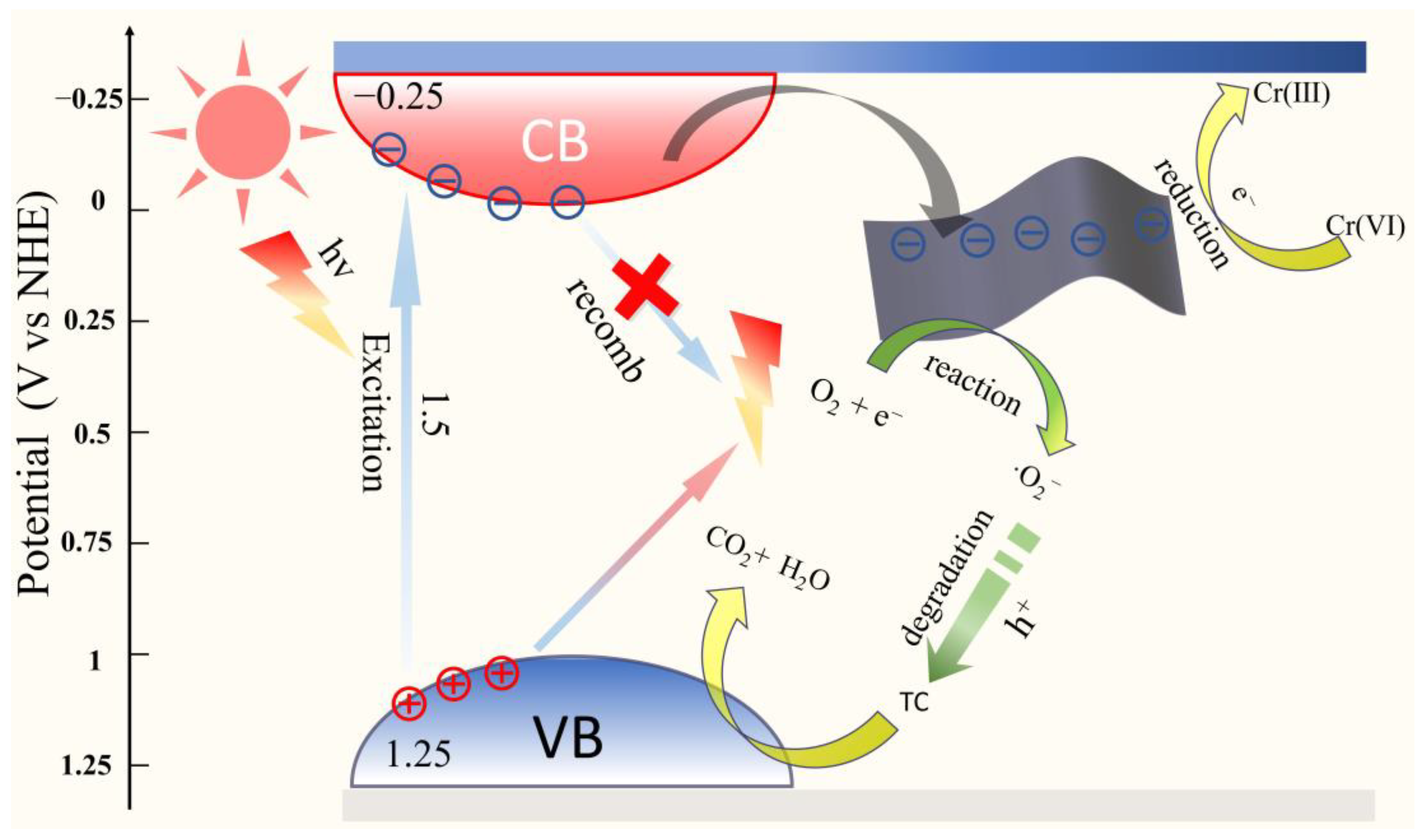
2.4.1. Photocatalytic Reduction Mechanism
2.4.2. Photocatalytic Degradation Mechanism
2.5. Photocatalytic Removal of Mixed TC and Cr(VI) Pollutants
3. Materials and Methods
3.1. Characterization of Photocatalysts
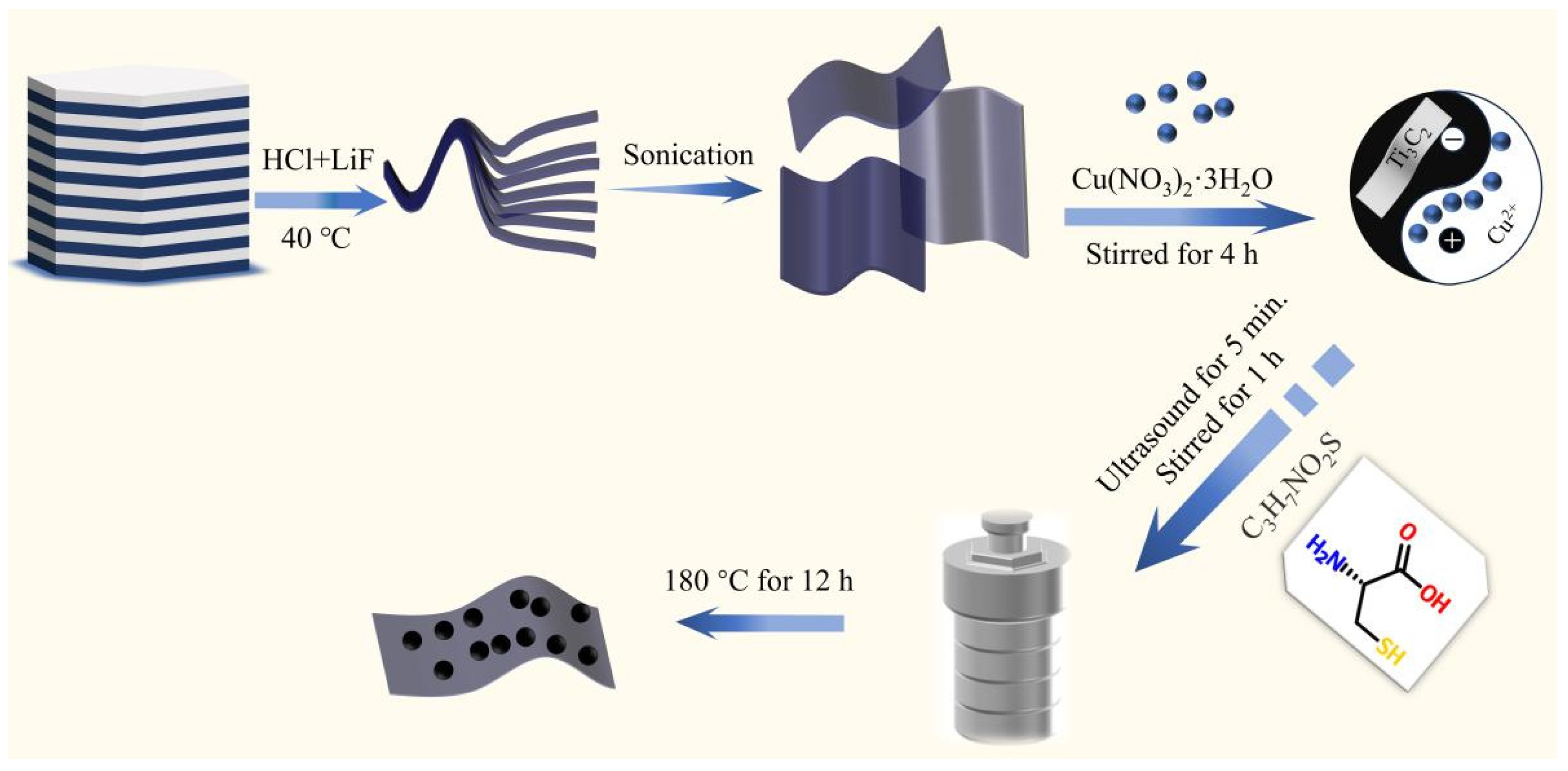
3.2. Preparation of Photocatalysts
3.3. Evaluation of the Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; Xie, Y.; He, F.; Ling, Y.; Zhao, J.; Ye, H.; Li, S.; Wang, J.; Hou, Y. Facile synthesis of GO as middle carrier modified flower-like BiOBr and C3N4 nanosheets for simultaneous treatment of chromium(VI) and tetracycline. Chin. Chem. Lett. 2021, 32, 2187–2191. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Z.; Liang, J.; Liang, B.; Huang, H.; Huang, G.; Junaid, M.; Wang, J.; Huang, K. Simultaneous photocatalytic removal of tetracycline and hexavalent chromium by BiVO4/0.6CdS photocatalyst: Insights into the performance, evaluation, calculation and mechanism. J. Colloid Interface Sci. 2024, 667, 650–662. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Huang, Z.; Wu, S.; Dai, X.; Zhang, L.; Cui, L. Effect of reduced graphene oxide doping on photocatalytic reduction of Cr(VI) and photocatalytic oxidation of tetracycline by ZnAlTi layered double oxides under visible light. Chemosphere 2019, 227, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.H.; Begum, W.; Nasrollahzadeh, M.; Ghorbannezhad, F.; Antoniadis, V.; Levizou, E.; Saha, B. A comprehensive review on chromium chemistry along with detection, speciation, extraction and remediation of hexavalent chromium in contemporary science and technology. Vietnam J. Chem. 2021, 59, 711–732. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.C.; Li, Q.; Jiang, S. Effects of Elevated Tetracycline Concentrations on Aerobic Composting of Human Feces: Composting Behavior and Microbial Community Succession. Indian J. Microbiol. 2018, 58, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Sun, P.; Li, D.; Huang, C.-H. Transformation of Tetracycline Antibiotics and Fe(II) and Fe(III) Species Induced by Their Complexation. Environ. Sci. Technol. 2016, 50, 145–153. [Google Scholar] [CrossRef]
- Park, J.; Gasparrini, A.J.; Reck, M.R.; Symister, C.T.; Elliott, J.L.; Vogel, J.P.; Wencewicz, T.A.; Dantas, G.; Tolia, N.H. Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat. Chem. Biol. 2017, 13, 730–736. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, J.; Zhang, M.; Dionysiou, D.D. Size-Tunable Hydrothermal Synthesis of SnS2 Nanocrystals with High Performance in Visible Light-Driven Photocatalytic Reduction of Aqueous Cr(VI). Environ. Sci. Technol. 2011, 45, 9324–9331. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q.; Du, M.; Zeng, X.; Zhong, G.; Qiu, B.; Zhang, J. Recent Progress of Metal Sulfide Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, 2202929. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Staiger, M.; Gartsman, K.; Thomsen, C.; Lorenz, T.; Seifert, G.; Tenne, R. Synthesis of copious amounts of SnS2 and SnS2/SnS nanotubes with ordered superstructures. Angewandte Chemie 2011, 50, 12316–12320. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Zhang, S.; Guan, G.-W.; Yang, Q.-Y.; Wang, J. Ti3C2Tx MXene coupled with CdS for enhanced photocatalytic H2 production. Int. J. Hydrogen Energy 2025, 101, 1191–1200. [Google Scholar] [CrossRef]
- Cui, Z.-G.; Ahmed, K.; Zaidi, S.F.; Muhammad, J.S. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat. Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Sun, Y.; Hu, Y.; Fan, J.; Wang, Z.; Wu, P.; Cai, C. A Tumor Microenvironment-Triggered and Photothermal-Enhanced Nanocatalysis Multimodal Therapy Platform for Precise Cancer Therapy. Chem. Mater. 2021, 33, 9334–9347. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Pan, L.; Son, M.-K.; Mayer, M.T.; Zhang, W.-D.; Hagfeldt, A.; Luo, J.; Grätzel, M. Solution-Processed Cu2S Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2018, 3, 760–766. [Google Scholar] [CrossRef]
- Wang, G.; Quan, Y.; Yang, K.; Jin, Z. EDA-assisted synthesis of multifunctional snowflake-Cu2S/CdZnS S-scheme heterojunction for improved the photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2022, 121, 28–39. [Google Scholar] [CrossRef]
- Hiragond, C.B.; Powar, N.S.; Kim, H.; In, S.-I. Unlocking solar energy: Photocatalysts design for tuning the CO2 conversion into high-value (C2+) solar fuels. EnergyChem 2024, 6, 100130. [Google Scholar] [CrossRef]
- Hiragond, C.B.; Powar, N.S.; Lee, J.; In, S.-I. Single-Atom Catalysts (SACs) for Photocatalytic CO2 Reduction with H2O: Activity, Product Selectivity, Stability, and Surface Chemistry. Small 2022, 18, 2201428. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Tian, J.; Sun, B.; Liang, Z.; Xu, X.; Cui, H. Controllable growth of MoS2 nanosheets on novel Cu2S snowflakes with high photocatalytic activity. Appl. Catal. B 2018, 232, 355–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Wang, Y.; Zhang, P.; Liu, D.; Li, Y.; Jin, Z.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Insight into l-cysteine-assisted growth of Cu2S nanoparticles on exfoliated MoS2 nanosheets for effective photoreduction removal of Cr(VI). Appl. Surf. Sci. 2020, 518, 146191. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Tong, X.; Yu, P.; Xu, J.-Y.; Wu, J.; Wang, Z.M.; Lou, J.; Chueh, Y.-L. A Critical Review on Enhancement of Photocatalytic Hydrogen Production by Molybdenum Disulfide: From Growth to Interfacial Activities. Small 2019, 15, 1900578. [Google Scholar] [CrossRef]
- Patel, T.A.; Panda, E. Copper deficiency induced varying electronic structure and optoelectronic properties of Cu2−xS thin films. Appl. Surf. Sci. 2019, 488, 477–484. [Google Scholar] [CrossRef]
- Shi, X.; Dai, W.; Li, X.; Bai, Y.; Ren, Q.; Lei, Y.; Dong, X.a. Internal electric field modulation by copper vacancy concentration of cuprous sulfide nanosheets for enhanced selective CO2 photoreduction. J. Energy Chem. 2024, 91, 324–330. [Google Scholar] [CrossRef]
- Mao, P.; Yang, X.; Wang, Z.; Ping, X.; Tian, Y.; Chen, Y.; Chen, Z.; Zhang, J.; Shen, J.; Sun, A. Interfacial engineering of 0D/2D Cd0.5Zn0.5S/Ti3C2 for efficient photocatalytic synergistic removal of antibiotics and Cr(VI). J. Environ. Chem. Eng. 2024, 12, 111649. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Hang, T.; Wu, L.; Yang, X. Roles of MXenes in Photocatalysis. Sol. RRL 2024, 8, 2300691. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zou, Y.; Cao, Y.; Cui, W.; Dong, F.; Zhou, Y. Ti3C2 MXene modified g-C3N4 with enhanced visible-light photocatalytic performance for NO purification. J. Colloid Interface Sci. 2020, 575, 443–451. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Wu, C.; Zhang, H.; Sun, J.; Li, Q. Delaminating Ti3C2 MXene by blossom of ZnIn2S4 microflowers for noble-metal-free photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 120, 89–98. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl+LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, W.; Liu, X.; Wang, Q.; Hu, J.; Zhang, S.; Xu, J.; Zhang, Q.; Zou, Z. Rationally designed Ti3C2 MXene/CaIn2S4 Schottky heterojunction for enhanced photocatalytic Cr(VI) reduction: Performance, influence factors and mechanism. J. Mater. Sci. Technol. 2023, 161, 123–135. [Google Scholar] [CrossRef]
- Ren, T.; Huang, H.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. 3D hollow MXene@ZnIn2S4 heterojunction with rich zinc vacancies for highly efficient visible-light photocatalytic reduction. J. Colloid Interface Sci. 2021, 598, 398–408. [Google Scholar] [CrossRef]
- He, L.; Zhou, D.; Lin, Y.; Ge, R.; Hou, X.; Sun, X.; Zheng, C. Ultrarapid in Situ Synthesis of Cu2S Nanosheet Arrays on Copper Foam with Room-Temperature-Active Iodine Plasma for Efficient and Cost-Effective Oxygen Evolution. ACS Catal. 2018, 8, 3859–3864. [Google Scholar] [CrossRef]
- Mao, P.; Liu, K.; Sun, A.; Yang, X.; Ping, X.; Zhang, J.; Shen, J.; Li, Y.; Teng, J.; Yang, Y. Controlled synthesis of 3D marigold-like ZnIn2S4/Ti3C2 for rapid and efficient removal of antibiotics. Arabian J. Chem. 2023, 16, 104883. [Google Scholar] [CrossRef]
- Littlejohn, D.; Chang, S.-G. An XPS study of nitrogen-sulfur compounds. J. Electron. Spectrosc. Relat. Phenom. 1995, 71, 47–50. [Google Scholar] [CrossRef]
- Peng, B.; Lu, Y.; Luo, J.; Zhang, Z.; Zhu, X.; Tang, L.; Wang, L.; Deng, Y.; Ouyang, X.; Tan, J.; et al. Visible light-activated self-powered photoelectrochemical aptasensor for ultrasensitive chloramphenicol detection based on DFT-proved Z-scheme Ag2CrO4/g-C3N4/graphene oxide. J. Hazard. Mater. 2021, 401, 123395. [Google Scholar] [CrossRef]
- Li, H.; Deng, F.; Zheng, Y.; Hua, L.; Qu, C.; Luo, X. Visible-light-driven Z-scheme rGO/Bi2S3–BiOBr heterojunctions with tunable exposed BiOBr (102) facets for efficient synchronous photocatalytic degradation of 2-nitrophenol and Cr(vi) reduction. Environ. Sci. Nano 2019, 6, 3670–3683. [Google Scholar] [CrossRef]
- Zhu, K.; Ou-Yang, J.; Zeng, Q.; Meng, S.; Teng, W.; Song, Y.; Tang, S.; Cui, Y. Fabrication of hierarchical ZnIn2S4@CNO nanosheets for photocatalytic hydrogen production and CO2 photoreduction. Chin. J. Catal. 2020, 41, 454–463. [Google Scholar] [CrossRef]
- Riha, S.C.; Schaller, R.D.; Gosztola, D.J.; Wiederrecht, G.P.; Martinson, A.B.F. Photoexcited Carrier Dynamics of Cu2S Thin Films. J. Phys. Chem. Lett. 2014, 5, 4055–4061. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, J.; Lu, S.; Cen, C.; Chen, Y.; Ren, L.; Feng, W.; Fang, Z. Zirconium metal-organic framework supported highly-dispersed nanosized BiVO4 for enhanced visible-light photocatalytic applications. J. Chem. Technol. Biotechnol. 2016, 91, 2785–2792. [Google Scholar] [CrossRef]
- Xiao, L.; Yuan, D.; Li, P.; Huang, L.; Mao, B.-W.; Zhan, D. Copper/cuprous sulfide electrode: Preparation and performance. Sci. China Chem. 2015, 58, 1039–1043. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2020, 268, 118382. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Cu2O/BiVO4 heterostructures: Synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Noble-metal-free ultrathin MXene coupled with In2S3 nanoflakes for ultrafast photocatalytic reduction of hexavalent chromium. Appl. Catal. B 2021, 284, 119754. [Google Scholar] [CrossRef]
- Fan, W.K.; Sherryna, A.; Tahir, M. Advances in Titanium Carbide (Ti3C2Tx) MXenes and Their Metal–Organic Framework (MOF)-Based Nanotextures for Solar Energy Applications: A Review. ACS Omega 2022, 7, 38158–38192. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, X.; Mu, M.; Jiang, Y.; Li, X.; Zhang, H.; Ouyang, T. Anatase TiO2@MIL-101(Cr) nanocomposite for photocatalytic degradation of bisphenol A. Colloids Surf. A 2020, 596, 124745. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Zhu, Z.; Yan, M.; Zhou, Y.; Wang, J.; Liu, Y.; Wang, J. Insight into highly efficient simultaneous photocatalytic removal of Cr (VI) and 2, 4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, Q.; Xing, H.; Chen, D.; Wang, H. Construction of Pillared-Layer MOF as Efficient Visible-Light Photocatalysts for Aqueous Cr(VI) Reduction and Dye Degradation. ACS Sustain. Chem. Eng. 2017, 5, 4449–4456. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Janini, A.C.P.; Gomes, B.P.; de-Jesus-Soares, A.; Marciano, M.A. Effects of Bismuth Exposure on the Human Kidney—A Systematic Review. Antibiotics 2022, 11, 1741. [Google Scholar] [CrossRef]
- Sun, K.; He, E.; Van Gestel, C.A.M.; Cao, X.; Zhao, L.; Xu, X.; Peijnenburg, W.J.G.M.; Qiu, H. Biosafety of two-dimensional molybdenum disulfide nanomaterials: Natural and engineered transformation pathways and their role in the toxicity profile. Crit. Rev. Environ. Sci. Technol. 2024, 54, 840–863. [Google Scholar] [CrossRef]
- Luo, S.; Qin, F.; Ming, Y.a.; Zhao, H.; Liu, Y.; Chen, R. Fabrication uniform hollow Bi2S3 nanospheres via Kirkendall effect for photocatalytic reduction of Cr(VI) in electroplating industry wastewater. J. Hazard. Mater. 2017, 340, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shen, J.; Liu, R.; Liu, X. Synthesis of Novel CuS-Bi2WO6/CNFs Ternary Heterojunctions for the Photocatalytic Reduction of Cr (VI) in Wastewater. Water Air Soil Pollut. 2024, 235, 317. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.-F.; Zhang, X.; Feng, Y.; Li, Y.; Yang, L.; Lu, H.; Yao, J. Constructing Cd0.5Zn0.5S@ZIF-8 nanocomposites through self-assembly strategy to enhance Cr(VI) photocatalytic reduction. J. Hazard. Mater. 2018, 349, 234–241. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, C.; Li, X.; Hu, Q.; Wang, C. Ag NPs decorated C–TiO2/Cd0.5Zn0.5S Z-scheme heterojunction for simultaneous RhB degradation and Cr(VI) reduction. Environ. Pollut. 2021, 286, 117305. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Y.; Zhu, C.; Tian, F.; Zhang, X.; Huang, D.; Sheng, J.; Yu, Y.; Yang, W. Designing a novel dual Z-scheme Bi2S3-ZnS/MoSe2 photocatalyst for photocatalytic reduction of Cr(VI). Sep. Purif. Technol. 2022, 286, 120502. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, L.; Wang, T.; Bai, Y.; Guan, Y. Fabrication of highly efficient Bi2WO6/CuS composite for visible-light photocatalytic removal of organic pollutants and Cr(VI) from wastewater. Front. Environ. Sci. Eng. 2020, 15, 52. [Google Scholar] [CrossRef]
- Lu, S.; Yin, L.; Xin, C.; Yang, X.; Chi, M.; Wan, W.; Han, Y.; Zhang, L.; Zhang, P. Resin-derived carbon to in-situ support Cu-Cu2-xS heteroparticles for efficient photocatalytic reduction of Cr(VI). Mol. Catal. 2023, 542, 113137. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, Y.; Wei, W.; Cui, S.; Hou, H.; Chen, W.; Mi, L. One-Step Transformation from Cu2S Nanocrystal to CuS Nanocrystal with Photocatalytic Properties. ChemistrySelect 2019, 4, 7512–7522. [Google Scholar] [CrossRef]
- Han, Y.; Zhai, L.; Pei, W.-B.; Luo, J.; Yu, J.-M.; Li, H.; Hou, C.; Wang, Z.; Ma, J.; Hu, B.; et al. Fast photocatalytic Cr(VI) removal by an organic hybrid silver antimony sulfide coupled with PEDOT. J. Cleaner Prod. 2025, 489, 144703. [Google Scholar] [CrossRef]
- Wei, H.; Hou, C.; Zhang, Y.; Nan, Z. Scalable low temperature in air solid phase synthesis of porous flower-like hierarchical nanostructure SnS2 with superior performance in the adsorption and photocatalytic reduction of aqueous Cr(VI). Sep. Purif. Technol. 2017, 189, 153–161. [Google Scholar] [CrossRef]
- Araña, J.; Martínez Nieto, J.L.; Herrera Melián, J.A.; Doña Rodríguez, J.M.; González Díaz, O.; Pérez Peña, J.; Bergasa, O.; Alvarez, C.; Méndez, J. Photocatalytic degradation of formaldehyde containing wastewater from veterinarian laboratories. Chemosphere 2004, 55, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, L.; Wang, J.; Zhu, Y.; Pu, Y.; Dai, W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B 2020, 277, 119122. [Google Scholar] [CrossRef]
- Tan, B.; Fang, Y.; Chen, Q.; Ao, X.; Cao, Y. Construction of Bi2O2CO3/Ti3C2 heterojunctions for enhancing the visible-light photocatalytic activity of tetracycline degradation. J. Colloid Interface Sci. 2021, 601, 581–593. [Google Scholar] [CrossRef]
- Zare, E.N.; Iftekhar, S.; Park, Y.; Joseph, J.; Srivastava, V.; Khan, M.A.; Makvandi, P.; Sillanpaa, M.; Varma, R.S. An overview on non-spherical semiconductors for heterogeneous photocatalytic degradation of organic water contaminants. Chemosphere 2021, 280, 130907. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Shi, W.; Zhang, J.; Bi, S. DFT Studies on the Water-Assisted Synergistic Proton Dissociation Mechanism for the Spontaneous Hydrolysis Reaction of Al3+ in Aqueous Solution. ACS Earth Space Chem. 2018, 2, 269–277. [Google Scholar] [CrossRef]
- Xu, H.-T.; Fu, X.-H.; Feng, W.-H.; Wang, T. Photo-Degradation Mechanism and Pathway for Tetracycline in Simulated Seawater Under Irradiation of Visible Light. Huan Jing Ke Xue 2023, 44, 3260–3269. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, Y.; Zhou, E.; Zhang, W.; Liu, Y.; Zhang, J.; Wu, X.; Qi, Q.; Liu, Z. In-suit fabricating an efficient electronic transport channels via S-scheme polyaniline/Cd0.5Zn0.5S heterojunction for rapid removal of tetracycline hydrochloride and hydrogen production. J. Mater. Sci. Technol. 2023, 153, 205–218. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Y.; Wang, B.; Guan, Z.; Yin, G.; Zheng, H.; Li, Y.; Cao, X.; Zheng, S. Constructing hollow core–shell Z-scheme heterojunction CdS@ CoTiO3 nanorods for enhancing the photocatalytic degradation of 2, 4-DCP and TC. PCCP 2024, 26, 14194–14204. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Xu, M.; Chu, Q.; Gong, Y.; Meng, M.; Cui, H.; Feng, Y. Construction of multi-functional S-Vacancy-Zn3In2S6/Bi-MOF S-scheme heterojunction containing interfacial chemical bonds for the efficient photocatalytic production of H2O2 and excellent photocatalytic degradation of OTC and TC. Colloids Surf. A 2024, 702, 135169. [Google Scholar] [CrossRef]
- Tan, J.; Wei, G.; Wang, Z.; Su, H.; Liu, L.; Li, C.; Bian, J. Application of Zn1− xCdxS photocatalyst for degradation of 2-CP and TC, catalytic mechanism. Catalysts 2022, 12, 1100. [Google Scholar] [CrossRef]
- Kameli, S.; Mehrizad, A. Ultrasound-assisted Synthesis of Ag-ZnS/rGO and its Utilization in Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation. Photochem. Photobiol. 2019, 95, 512–521. [Google Scholar] [CrossRef]
- Li, Y.; Fu, M.; Lu, P.; Hu, X.; Wang, R.; Bai, J.; He, Y. Visible light photocatalytic abatement of tetracycline over unique Z-scheme ZnS/PI composites. Appl. Surf. Sci. 2022, 575, 151798. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Wang, W.; Cai, Y.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. J. Hazard. Mater. 2020, 384, 121302. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Ye, B.; Zheng, X. Snowflake-like In2S3/Cu2S heterojunction for simultaneous photocatalytic persulfate oxidation of tetracycline hydrochloride and CO2 photoreduction. Sep. Purif. Technol. 2025, 357, 130242. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, R.; Dong, J.; Yu, J.; Zhang, D.; Liu, J.; Cai, P.; Pu, X. BiOBr/Cu2S p-n heterojunction for efficient photodegradation of ciprofloxacin and tetracycline under visible light. J. Alloys Compd. 2024, 990, 174463. [Google Scholar] [CrossRef]
- Luo, C.-W.; Jiang, L.; Xie, C.; Huang, D.-G.; Jiang, T.-J. LED illumination-assisted Activation of Peroxydisulfate by Heterogeneous Cu2S under Alkaline Condition for Efficient Organic Pollutants Removal. Environ. Res. 2024, 268, 120634. [Google Scholar] [CrossRef]
- Cao, Y.; Yue, L.; Li, Z.; Han, Y.; Lian, J.; Qin, H.; He, S. Construction of Sn-Bi-MOF/Ti3C2 Schottky junction for photocatalysis of tetracycline: Performance and degradation mechanism. Appl. Surf. Sci. 2023, 609, 155191. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, Y.; Chen, X.; Liao, B.; Yu, Y.; Hou, S.; Fan, X. Internal electric field and oxygen vacancies synergistically optimized Ba2+ doped SrBi2B2O7 for photocatalytic tetracycline degradation from water. Chem. Eng. J. 2022, 433, 134580. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, K.; Jiang, H.; Zhang, H.; Liu, Q.; You, T. Photoelectrochemical aptasensor for sensitive detection of tetracycline in soil based on CdTe-BiOBr heterojunction: Improved photoactivity enabled by Z-scheme electron transfer pathway. J. Hazard. Mater. 2022, 424, 127498. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Jin, X.; Zhong, J.; Li, J. One-pot hydrothermal synthesis of MXene Ti3C2/TiO2/BiOCl ternary heterojunctions with improved separation of photoactivated carries and photocatalytic behavior toward elimination of contaminants. Colloids Surf. A 2020, 603, 125239. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Lei, X.; Tan, B.; Hu, X.; Liu, B.; Chen, Q. Fabrication of novel CuFe2O4/MXene hierarchical heterostructures for enhanced photocatalytic degradation of sulfonamides under visible light. J. Hazard. Mater. 2020, 387, 122021. [Google Scholar] [CrossRef] [PubMed]
- GB 7467–1987; Water Quality—Determination of Chromium (VI)—1,5 Diphenylcarbohydrazide Spectrophotometric Method. State Environmental Protection Administration: Beijing, China, 1987.


| Photocatalyst | Light Source | Amount (mg) | Concentration (mg·L−1) | Solid-Liquid Ratio | Light Time (min) | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| CSMX-5 | 300 W | 10 | 10 | 1:4 | 240 | 90% | This work |
| 2-Cu2S-MoS2 | 500 W | 10 | 10 | 1:4 | 240 | 80% | [19] |
| Bi2S3 nanospheres | 500 W | 20 | 40 | 1:2 | 120 | 100% | [51] |
| 1%CuS-Bi2WO6/CNFs | Xenon lamp | 80 | 10 | 1:1 | 180 | 100% | [52] |
| Cd0.5Zn0.5S@ZIF-8 | 300 W | 40 | 20 | 1:1 | 30 | 100% | [53] |
| Ag@C-TiO2/Cd0.5Zn0.5S | 500 W | 50 | 5 | 1:2 | 120 | 98% | [54] |
| ZnS | 500 W | 40 | 40 | 1:1 | 180 | 53% | [55] |
| Bi2WO6/CuS | 500 W | 100 | 15 | 2:5 | 90 | 95% | [56] |
| Cu-Cu2−xS/C | 300 W | 20 | 10 | 1:2 | 180 | 90% | [57] |
| Cu2S@CT | 500 W | 14 | 50 | 7:15 | 120 | 19.24% | [58] |
| Photcatalyst | Light Source | Amount (mg) | Antibiotics (mg·L−1) | Solid-Liquid Ratio | Light Time (min) | Efficiency | Ref |
|---|---|---|---|---|---|---|---|
| CSMX-5 | 300 W | 20 | TC (20) | 1:4 | 180 | 92% | This work |
| PANI/Cd0.5Zn0.5S | 300 W | 20 | TC (15) | 1:3 | 120 | 84.9% | [67] |
| CdS@CoTiO3 | 500 W | 30 | TC (10) | 3:5 | 80 | 91.8% | [68] |
| Zn3In2S6/Bi-MOF | Xenon lamp | 10 | TC (60) | 1:5 | 60 | 60% | [69] |
| Zn1−xCdxS | 300 W | 50 | TC (50) | 1:2 | 120 | 91% | [70] |
| Ag-ZnS/rGO | 300 W | 125 | TC (10) | 1.25:1 | 110 | 86.78% | [71] |
| ZnS/PI | LED light | 25 | TC (20) | 1:2 | 240 | 84% | [72] |
| Cu2O/Cu2S | 300 W | 10 | TC (50) | 1:10 | 120 | 84.8% | [73] |
| In2S3/Cu2S | 300 W | 20 | TC (120) | 2:5 | 150 | 78.38% | [74] |
| BiOBr/Cu2S | 1000 W | 50 | TC (10) | 1:2 | 60 | 88.5% | [75] |
| Cu2S/PSD | LED light | 10 | TC (20) | 1:5 | 90 | 93.9% | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lv, Z.; Zeng, B.; Wang, F.; Yang, X.; Mao, P. Interfacial Engineering of 0D/2D Cu2S/Ti3C2 for Efficient Photocatalytic Synchronous Removal of Tetracycline and Hexavalent Chromium. Catalysts 2025, 15, 458. https://doi.org/10.3390/catal15050458
Wang Z, Lv Z, Zeng B, Wang F, Yang X, Mao P. Interfacial Engineering of 0D/2D Cu2S/Ti3C2 for Efficient Photocatalytic Synchronous Removal of Tetracycline and Hexavalent Chromium. Catalysts. 2025; 15(5):458. https://doi.org/10.3390/catal15050458
Chicago/Turabian StyleWang, Zengyu, Zhiwei Lv, Bowen Zeng, Fafa Wang, Xiaoyu Yang, and Ping Mao. 2025. "Interfacial Engineering of 0D/2D Cu2S/Ti3C2 for Efficient Photocatalytic Synchronous Removal of Tetracycline and Hexavalent Chromium" Catalysts 15, no. 5: 458. https://doi.org/10.3390/catal15050458
APA StyleWang, Z., Lv, Z., Zeng, B., Wang, F., Yang, X., & Mao, P. (2025). Interfacial Engineering of 0D/2D Cu2S/Ti3C2 for Efficient Photocatalytic Synchronous Removal of Tetracycline and Hexavalent Chromium. Catalysts, 15(5), 458. https://doi.org/10.3390/catal15050458





