Synergistic Cu-Pd Nanocatalysts on MOF-Derived N-Doped Carbon for Selective Hydrogenolysis of Lignin to Aromatic Monomers
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Hydrogenolysis of Eucalyptus Wood Lignin
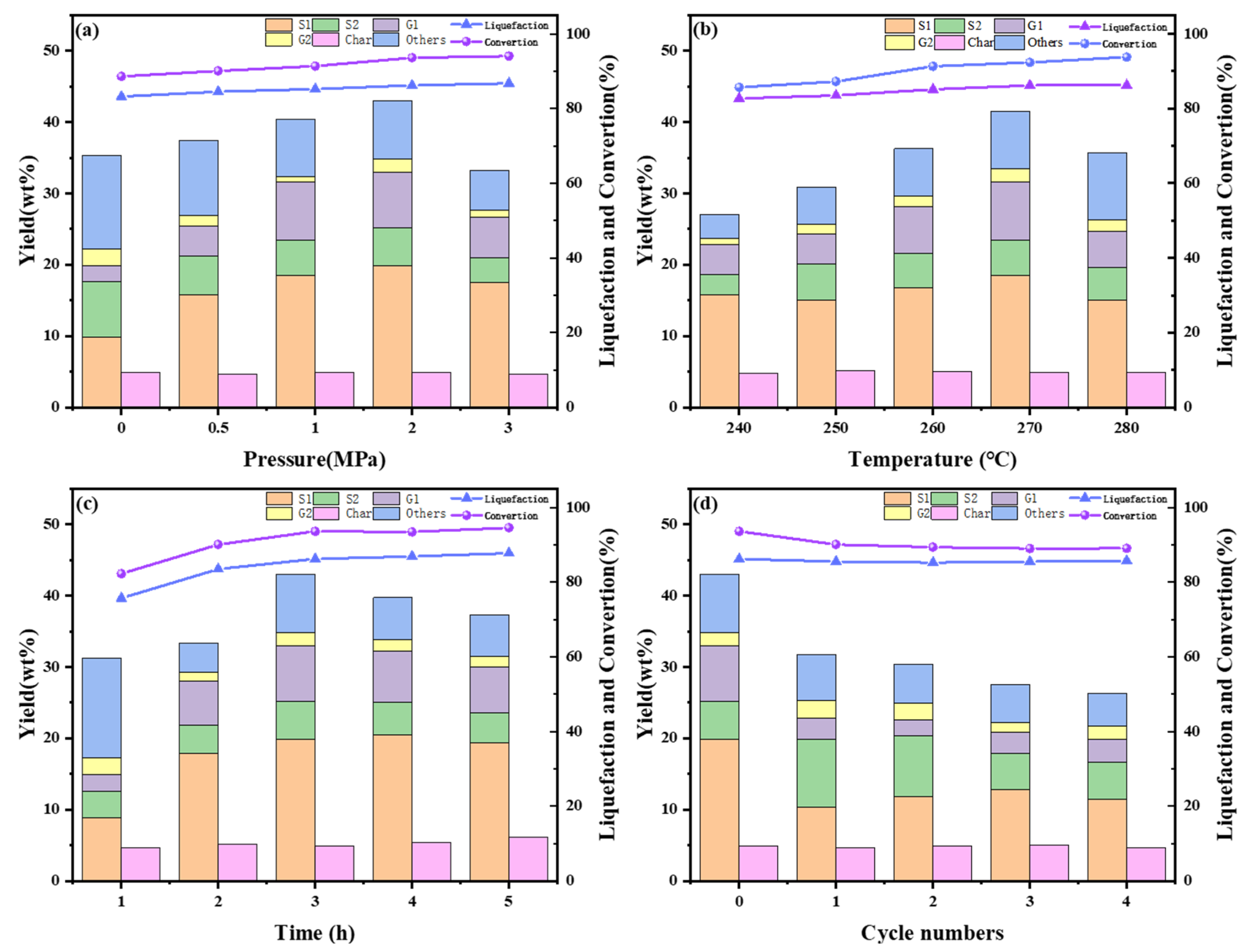
2.3. Investigation of Reaction Conditions
2.3.1. Effect of Hydrogen Pressure
2.3.2. Effect of Reaction Temperature
2.3.3. Effect of Reaction Time
2.3.4. Catalyst Cycling Performance
2.4. Analysis of Products
2.5. Mechanism of Lignin Hydrogenolysis
3. Experimental Section
3.1. Materials
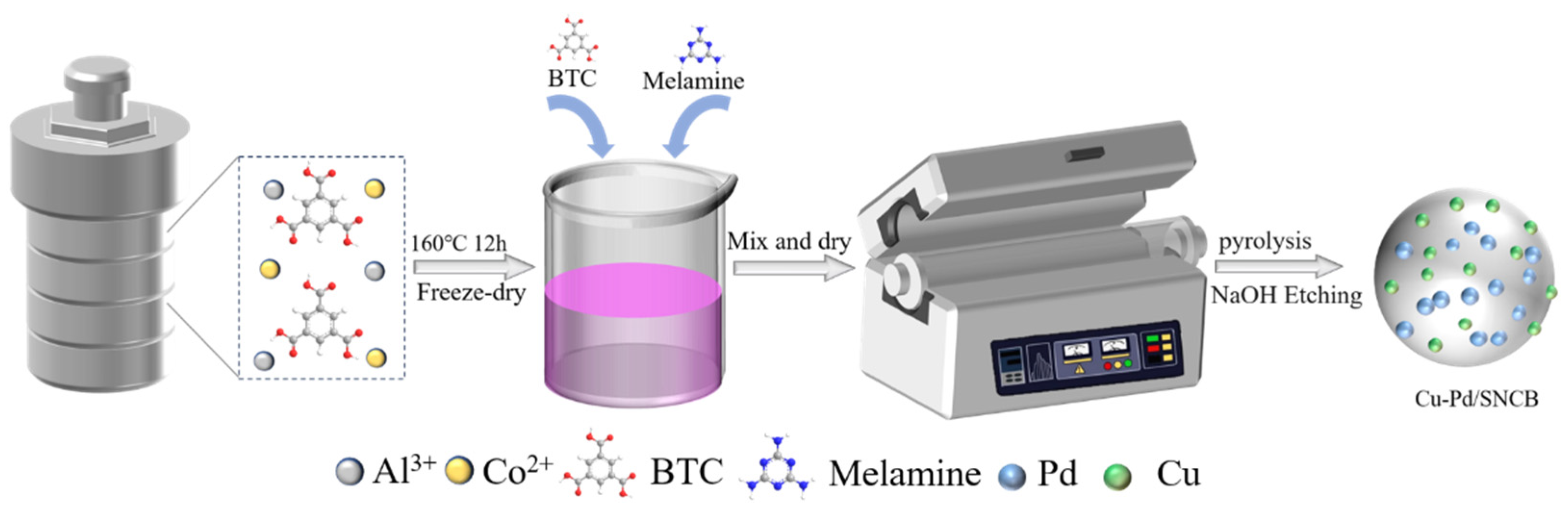
3.2. Catalyst Preparation
3.2.1. Preparation of Al/Co-BTC
3.2.2. Preparation of Al/Co-BTC-Derived Carbon Materials
3.2.3. Preparation of Catalyst Cu-Pd@SNCB
3.3. Hydrogenolysis of Lignin
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiu, S.; Fu, Y.; Lei, W.; Chang, J. Preparation of nitrogen and sulfur co-doped tubular porous carbon derived from Ceiba speciosa flowers for supercapacitors. J. Energy Storage 2025, 112, 115536. [Google Scholar] [CrossRef]
- Cabral Almada, C.; Kazachenko, A.; Fongarland, P.; Da Silva Perez, D.; Kuznetsov, B.N.; Djakovitch, L. Supported-metal catalysts in upgrading lignin to aromatics by oxidative depolymerization. Catalysts 2021, 11, 467. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Chen, Z.; Li, P.; Ji, X.; Zhi, C. Anion chemistry in energy storage devices. Nat. Rev. Chem. 2023, 7, 616–631. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastava, R. Selective C-O bond cleavage in diphenyl ether via catalytic transfer hydrogenolysis over Ru-decorated nanocrystalline H-ZSM-5. Sustain. Energy Fuels 2023, 7, 5714–5732. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Sánchez, C.; González Alriols, M.; Labidi, J. Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 2017, 26, 622–631. [Google Scholar] [CrossRef]
- Wang, H.-M.; Yuan, T.-Q.; Song, G.-Y.; Sun, R.-C. Advanced and versatile lignin-derived biodegradable composite film materials toward a sustainable world. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- Guiton, M.; Suárez-Montes, D.; Sánchez, R.; Baustert, P.; Soukoulis, C.; Okan, B.S.; Serchi, T.; Cambier, S.; Benetto, E. Comparative Life Cycle Assessment of a microalgae-based oil metal working fluid with its petroleum-based and vegetable-based counterparts. J. Clean. Prod. 2022, 338, 130506. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Wang, H.-M.D.; Shieh, C.-J.; Liu, Y.-C.; Kuo, C.-H. Biocatalysis for lignin conversion and valorization: Driving sustainability in the circular economy. Catalysts 2025, 15, 91. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, L.; Xu, Y.; Yan, X.; Zhang, C.; Sun, Z.; Lin, X.; Duan, Y.; Zhang, H. Ultralow loaded waste polystyrene derived hyper-cross-linked polymer for incineration flue gas demercuration: Synergistic effect of transition metal and halogen species. Chem. Eng. J. 2023, 471, 144732. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Manaenkov, O.V.; Markova, M.E.; Sidorov, A.I.; Bykov, A.V.; Sulman, M.G.; Kiwi-Minsker, L. Lignin hydrogenolysis over bimetallic Ni–Ru nanoparticles supported on SiO2@HPS. Catalysts 2023, 13, 856. [Google Scholar] [CrossRef]
- Yan, J.; Meng, Q.; Shen, X.; Chen, B.; Sun, Y.; Xiang, J.; Liu, H.; Han, B. Selective valorization of lignin to phenol by direct transformation of Csp2–Csp3 and C-O bonds. Sci. Adv. 2020, 6, eabd1951. [Google Scholar] [CrossRef]
- Shukla, R.K.; Chaturvedi, A.K.; Volla, C.M.R. Catalytic Cascade Cyclization and Regioselective Hydroheteroarylation of Unactivated Alkenes. ACS Catal. 2021, 11, 7750–7761. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, C.; Tian, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Constructing ultrafine lithiophilic layer on MXene paper by sputtering for stable and flexible 3D lithium metal anode. Chem. Eng. J. 2021, 421, 129685. [Google Scholar] [CrossRef]
- Xiao, Z.; Shen, J.; Zhang, J.; Li, D.; Li, Y.; Wang, X.; Zhang, Z. Intermediate stabilization for tuning photocatalytic selective oxidation of CH4 to CH3OH over Co3O4/ZnO. J. Catal. 2022, 413, 20–30. [Google Scholar] [CrossRef]
- Meng, H.; Yang, Y.; Shen, T.; Yin, Z.; Wang, L.; Liu, W.; Yin, P.; Ren, Z.; Zheng, L.; Zhang, J.; et al. Designing Cu0−Cu+ dual sites for improved C−H bond fracture towards methanol steam reforming. Nat. Commun. 2023, 14, 7980. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.N.; Qu, Y.B.; Wang, Z.L.; Jiang, Q. Hydrogen spillover in alkaline solutions for effective nitrogen fixation. Chem. Eng. J. 2023, 471, 144589. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, T.; Shi, H.; Pan, H.; Kang, P. Grafting amine-functionalized ligand layer on catalyst for electrochemical CO2 capture and utilization. Appl. Catal. B Environ. 2024, 343, 123456. [Google Scholar] [CrossRef]
- Diao, Z.J.; Huang, L.Q.; Chen, B.; Gao, T.; Cao, Z.Z.; Ren, X.D.; Zhao, S.J.; Li, S. Amorphous Ni-Ru bimetallic phosphide composites as efficient catalysts for the hydrogenolysis of diphenyl ether and lignin. Fuel 2022, 324, 124489. [Google Scholar] [CrossRef]
- Wen, S.; Huang, J.; Li, T.; Chen, W.; Chen, G.; Zhang, Q.; Zhang, X.; Qian, Q.; Ostrikov, K.K. Multiphase nanosheet-nanowire cerium oxide and nickel-cobalt phosphide for highly-efficient electrocatalytic overall water splitting. Appl. Catal. B Environ. 2022, 316, 121678. [Google Scholar] [CrossRef]
- De Falco, A.; Caruso, F.; Su, X.-D.; Iavarone, A.; Ceccarelli, M. A variational algorithm to detect the clonal copy number substructure of tumors from scRNA-seq data. Nat. Commun. 2023, 14, 1074. [Google Scholar] [CrossRef]
- Lin, F.; Ma, Y.; Sun, Y.; Zhao, K.; Gao, T.; Zhu, Y. Heterogeneous Ni–Ru/H-ZSM-5 one-pot catalytic conversion of lignin into monophenols. Renew. Energy 2021, 170, 1070–1080. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, M.; Jiang, B.; Wu, S.; Lu, P. Catalytic Transfer Hydrogenolysis of Native Lignin to Monomeric Phenols over a Ni–Pd Bimetallic Catalyst. Energy Fuels 2020, 34, 9754–9762. [Google Scholar] [CrossRef]
- Zeng, Z.; Xie, J.; Guo, Y.; Rao, R.; Chen, B.; Cheng, L.; Xie, Y.; Ouyang, X. Hydrogenolysis of lignin to produce aromatic monomers over FePd bimetallic catalyst supported on HZSM-5. Fuel Process. Technol. 2021, 213, 106713. [Google Scholar] [CrossRef]
- Zou, W.; Li, H.; Liu, M.; Lv, Y. High-performance depolymerization of lignin by bimetallic Cu-Ni@C catalysts prepared with MOF as a carrier under mild conditions. Appl. Catal. A Gen. 2023, 656, 119120. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Catalytic Depolymerization of Lignin in Supercritical Ethanol. ChemSusChem 2014, 7, 2276–2288. [Google Scholar] [CrossRef]
- Yangcheng, R.; Li, J.; He, J.; Zheng, Y.; Yu, H.; Chen, C.; Wang, J. Carboxyl-Decorated UiO-66 Supporting Pd Nanoparticles for Efficient Room-Temperature Hydrodeoxygenation of Lignin Derivatives. Small 2024, 20, 2309821. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, Q.; Gao, L.; Liu, J.; Ge, J.; Liu, C.; Xing, W. Metal organic framework derived nitrogen-doped carbon anchored palladium nanoparticles for ambient temperature formic acid decomposition. Int. J. Hydrogen Energy 2019, 44, 28402–28408. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Sun, Y.; Hua, D.; Yang, S.; Sun, L.; Li, T.; Chen, L. Co-pyrolysis induced strong metal-support interaction in N-doped carbon supported Ni catalyst for the hydrogenolysis of lignin. Chem. Eng. J. 2023, 473, 145182. [Google Scholar] [CrossRef]
- Duan, J.; Liang, Q.; Fu, Y.; Chang, J. Sea urchin-like nitrogen doped hierarchically carbon materials derived from bimetallic-MOF for fast and efficient hydrogen production of formic acid. Fuel 2024, 361, 130674. [Google Scholar] [CrossRef]
- Hambly, B.; Guzinski, M.; Perez, F.; Pendley, B.; Lindner, E. Deposition of EDOT-Decorated Hollow Nanocapsules into PEDOT Films for Optical and Electrochemical Sensing. ACS Appl. Nano Mater. 2020, 3, 6328–6335. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Li, Z.; Chen, W.; Xu, Q.; He, D.; Xi, D.; Zhang, Q.; Yuan, T.; Qu, Y.; et al. Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction. Joule 2019, 3, 584–594. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef]
- Li, F.; Zhu, W.; Jiang, S.; Wang, Y.; Song, H.; Li, C. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over Fe3O4 modified Ru/Carbon nanotubes catalysts. Int. J. Hydrogen Energy 2020, 45, 1981–1990. [Google Scholar] [CrossRef]
- Su, C.; Cheng, H.; Li, W.; Liu, Z.; Li, N.; Hou, Z.; Bai, F.; Zhang, H.; Ma, T. Atomic Modulation of FeCo–Nitrogen–Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc–Air Battery. Adv. Energy Mater. 2017, 7, 1602420. [Google Scholar] [CrossRef]
- Sun, H.; Wang, G.; Ge, J.; Wei, N.; Sui, W.; Chen, Z.; Jia, H.; Parvez, A.M.; Si, C. Reduction of lignin heterogeneity for improved catalytic performance of lignin nanosphere supported Pd nanoparticles. Ind. Crops Prod. 2022, 180, 114685. [Google Scholar] [CrossRef]
- Li, L.; Kong, J.; Zhang, H.; Liu, S.; Zeng, Q.; Zhang, Y.; Ma, H.; He, H.; Long, J.; Li, X. Selective aerobic oxidative cleavage of lignin CC bonds over novel hierarchical Ce-Cu/MFI nanosheets. Appl. Catal. B Environ. 2020, 279, 119343. [Google Scholar] [CrossRef]
- Mauriello, F.; Paone, E.; Pietropaolo, R.; Balu, A.M.; Luque, R. Catalytic transfer hydrogenolysis of lignin-derived aromatic ethers promoted by bimetallic pd/Ni systems. ACS Sustain. Chem. Eng. 2018, 6, 9269–9276. [Google Scholar] [CrossRef]
- Wang, M.; Ma, D. Upcycling contaminated plastics. Nat. Sustain. 2023, 6, 1151–1152. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; An, Y.; Wang, Z.; Wang, M.; Bao, X.; Zheng, L.; Cheng, H.; Wang, P.; Liu, Y.; Zheng, Z.; et al. Stress-induced BiVO4 photoanode for enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2022, 304, 121012. [Google Scholar] [CrossRef]
- Kaiho, A.; Kogo, M.; Sakai, R.; Saito, K.; Watanabe, T. In situ trapping of enol intermediates with alcohol during acid-catalysed de-polymerisation of lignin in a nonpolar solvent. Green Chem. 2015, 17, 2780–2783. [Google Scholar] [CrossRef]
- Questell-Santiago, Y.M.; Galkin, M.V.; Barta, K.; Luterbacher, J.S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 2020, 4, 311–330. [Google Scholar] [CrossRef]
- Yang, C.; Zhuang, C.; Zhai, Z.; Zhao, X.; Huang, D.; Tian, D.; Min, C.; Zhao, J.; Wang, Y. Phase regulation of Ni-based catalyst promotes selective hydrogenation of furfural: Effect of glycerol and Zn content. Appl. Catal. B Environ. 2023, 334, 122854. [Google Scholar] [CrossRef]
- Liang, Q.; Fu, Y.; Qiu, S.; Chang, J. Sustainable Production of High-Value Chemicals from Selective Hydrogenolysis of Lignin Catalyzed by Co–Pd Bimetallic Supported on Nitrogen-Doped Carbon Spheres. Energy Fuels 2024, 38, 10993–11005. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3 catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Wang, S.; Xing, Y.; Xu, H.; Zhang, S. MnO nanoparticles interdispersed in 3D porous carbon framework for high performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 12713–12718. [Google Scholar] [CrossRef]
- Yan, B.; Lin, X.; Chen, Z.; Cai, Q.; Zhang, S. Selective production of phenolic monomers via high efficient lignin depolymerization with a carbon based nickel-iron-molybdenum carbide catalyst under mild conditions. Bioresour. Technol. 2021, 321, 124503. [Google Scholar] [CrossRef]
- Shu, R.; Zhou, L.; Zhu, Z.; Luo, B.; You, H.; Zhong, Z.; He, Y. Enhanced hydrogenolysis of enzymatic hydrolysis lignin over in situ prepared RuNi bimetallic catalyst. Int. J. Hydrogen Energy 2022, 47, 41564–41572. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, S.; Li, Y. Selective hydrogenolysis of lignin and model compounds to monophenols over AuPd/CeO2. Mol. Catal. 2019, 462, 69–76. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, W.; Fu, Y.; Gu, S.; Qiu, S.; Chang, J. Synergistic Cu-Pd Nanocatalysts on MOF-Derived N-Doped Carbon for Selective Hydrogenolysis of Lignin to Aromatic Monomers. Catalysts 2025, 15, 455. https://doi.org/10.3390/catal15050455
Lei W, Fu Y, Gu S, Qiu S, Chang J. Synergistic Cu-Pd Nanocatalysts on MOF-Derived N-Doped Carbon for Selective Hydrogenolysis of Lignin to Aromatic Monomers. Catalysts. 2025; 15(5):455. https://doi.org/10.3390/catal15050455
Chicago/Turabian StyleLei, Wenjun, Yan Fu, Shipeng Gu, Shuaishuai Qiu, and Jie Chang. 2025. "Synergistic Cu-Pd Nanocatalysts on MOF-Derived N-Doped Carbon for Selective Hydrogenolysis of Lignin to Aromatic Monomers" Catalysts 15, no. 5: 455. https://doi.org/10.3390/catal15050455
APA StyleLei, W., Fu, Y., Gu, S., Qiu, S., & Chang, J. (2025). Synergistic Cu-Pd Nanocatalysts on MOF-Derived N-Doped Carbon for Selective Hydrogenolysis of Lignin to Aromatic Monomers. Catalysts, 15(5), 455. https://doi.org/10.3390/catal15050455






