Abstract
Hydroxymethylfurfural (HMF) is a substance produced in sugar-rich foods through the Maillard reaction or thermal degradation. It has been shown that when HMF content reaches a certain dose, it causes harm to human health. In many food quality tests, the content of HMF can be used as an important indicator. Therefore, when the content of hydroxymethylfurfural in food is too high, it will cause damage to the human body. But to conserve resources, hydroxymethylfurfural in food can be converted into valuable chemicals, so as to achieve the effective use of resources. It has been shown that foods rich in fructose and glucose can be easily transformed into HMF. Therefore, it is necessary and important to study the conversion pathway of hydroxymethylfurfural in foods. 2, 5 furandicarboxylic acid (FDCA) can be obtained through the HMF oxidation reaction. Due to the similarity of its structure to the polymer monomer terephthalic acid, it can be used as a renewable substitute monomer of petroleum-based terephthalic acid in the process of synthesizing food-contact materials. Therefore, it is very significant to explore the oxidation process of HMF to FDCA.
1. Introduction
The chemical structure of Hydroxymethylfurfural (HMF) consists of a furan ring, a hydroxymethyl group and an aldehyde group, and the molecular formula is abbreviated as C6H6O3 [1]. Its molecular structure formula is shown in Figure 1. There are two main ways to generate HMF in food. The first way is the Maillard reaction between carbonyl compounds (especially reducing sugars) and amines, peptides, amino acids and other amino compounds [2,3,4,5,6]. The other is when HMF is produced by thermal decomposition during the storage process of foods with a high sugar content. Foods rich in fructose and glucose can easily be converted to produce HMF [7]. However, when the content of HMF reaches a certain dose, it will cause harm to human health. For example, HMF is considered to be a weakly carcinogenic cytotoxin, which can harm respiratory tract, skin and mucous membranes and produce adverse effects at high concentrations [8]. Therefore, in the quality testing of sugar-containing foods, such as milk, honey, juice and pharmaceuticals, HMF content is an important indicator of food quality [9].
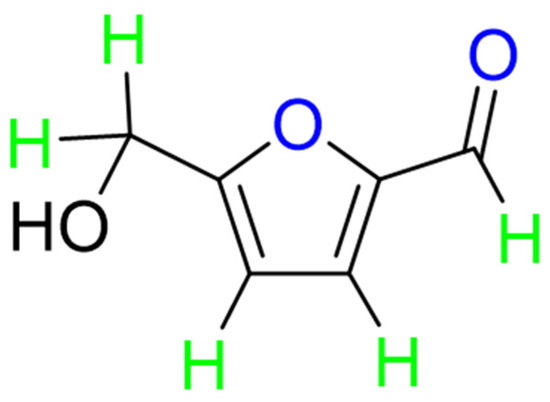
Figure 1.
Molecular formula and atom labels in 5-hydroxymethylfurfural (HMF). The color code is introduced for clarity to identify the type of atoms discussed in the structural assessment: O atoms, potential H-bond acceptor sites, are in blue; H atoms, including OH and aldehyde H, are in green; the carbon skeleton is in black.
Among them, the content of HMF in food is easily affected by factors such as pH value, pressure, time, temperature and even food composition in the food storage environment [10]. When the content of hydroxymethylfurfural in food is too high, for health and safety considerations, food cannot be eaten by human beings. The limits of furfural in some foods stipulated by the national standard are as follows: soft drinks 4.0 mg/kg, cold drinks 13.0 mg/kg, confectionery 12.0 mg/kg, baked food 17.0 mg/kg, pudding 0.8 mg/kg, gum candy 45.0 mg/kg, wine 10.0 mg/kg, syrup 30.0 mg/kg [11]. In addition, in view of the characteristics of the platform compound of hydroxymethylfurfural, it has been widely used as a raw material for the preparation of biomass-based fuels and polymeric monomers in recent years [12]. Therefore, fructose and glucose waste with a high content of HMF can be converted into hydroxymethylfurfural, and the excellent chemical intermediates of HMF can be converted into useful chemicals and applied to other industries [13,14,15,16,17]. For example, the downstream oxidation product of HMF, 2, 5-furanodiformic acid (FDCA), can be used as a monomer of food-contact polymeric material, and the prepared food-contact material is degradable, in line with the principle of green environmental protection. FDCA can be used as an important renewable structural unit for the synthesis of polyester and its copolymers, the synthesis of metal–organic skeleton materials, medicine, plasticizer, new semi-aromatic nylon, semi-biobased aramidans, spandex and polyethers and other products. Therefore, it is necessary and significant to study the production mechanism of hydroxymethylfurfural in food and its corresponding transformation path.
Electrochemical alcohol oxidation is considered a promising alternative to the oxygen evolution reaction due to the production of high-value products and early onset potential. NiOOH–Cu(OH)2 mixed electrodes showed a higher activity and faster conversion of HMF to FDCA than individual NiOOH or Cu(OH)2 electrodes. Electrochemical oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2, 5-furandicarboxylic acid (FDCA) is an intriguing way of achieving biomass conversion. The optimized Ni2S3/NF electrode exhibits a nearly 100% conversion of HMF, a 98% yield of FDCA, and a high faradaic efficiency of 94%. This material is stable and retains activity after six consecutive measurements [18].
2. Properties, Synthetic Methods and Transformation Pathways of Hydroxymethylfurfural
2.1. Research Progress of Catalytic Conversion of Fructose to HMF
Fructose, also known as hexulose, can easily remove three water molecules to produce hydroxymethylfurfural under the action of a catalyst [18,19]. In the process of fructose dehydration to hydroxymethylfurfural, the catalyst can be divided into a homogeneous catalyst and heterogeneous catalyst. Homogeneous catalysts mainly include inorganic acids, organic acids, and metal chlorides. As shown in Figure 2, Yuriy used hydrochloric acid as a catalyst to catalyze the dehydration of fructose to prepare HMF [20] in a water/methyl isobutyl ketone two-phase reaction system, and added the additives polyethylpyrrolide (PVP) and DMSO to the system. At the reaction temperature of 453 K for 5 min, a 90% fructose conversion and 72% HMF yield were obtained [20]. Tong used FeCl3 and Et4NBr as co-catalysts to catalyze fructose in an NMP single-phase system, at a 363 K reaction temperature and with a 2 h reaction time, and obtained an 86% HMF yield [21]. Del becq et al. used formic acid as a catalyst to catalyze the dehydration of fructose to prepare HMF in a water/methyl isobutyl ketone two-phase reaction system, and the HMF yield was 82% after a reaction at 463 K for 40 min [22]. Although the homogeneous catalyst has the advantages of a simple operation and small investment requirement, it also has the disadvantages of a difficult separation from the product, there being difficulty in reusing the catalyst, large environmental pollution, and serious corrosion occurring to the equipment. Heterogeneous catalysts have made up for the above shortcomings and were gradually selected by scholars as catalysts for HMF synthesis [23,24,25,26,27].
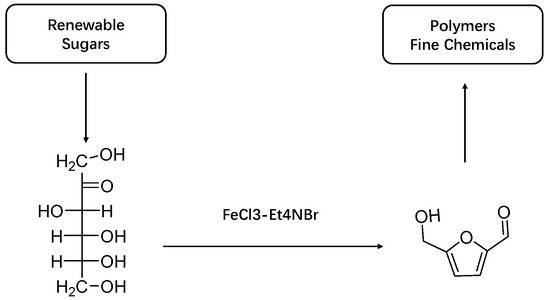
Figure 2.
A highly efficient iron-catalyzed production of 5-hydroxymethylfurfural (HMF) from sugar is reported.
Heterogeneous catalysts include heteropolyacid, ion exchange resin, and molecular sieves, amongst others. Gomes selected a supported heteropoly acid HPW/MCM catalyst to catalyze the conversion of fructose in DMSO solvent at a reaction temperature of 393 K and with a reaction time of 60 min, and obtained a 100% fructose conversion and 80% HMF yield. After repeating use four times, the conversion rate is still 75% [28]. In a single-phase DMSO system, Morales et al. used sulfonic acid resin Amberlyst-70 as a catalyst to catalyze the conversion of fructose into HMF by dehydration, and the fructose was completely transformed within 60 min, obtaining a 93% HMF yield [29]. Matharu et al. used Fe-NHC/HACS as a catalyst to react in a DMSO solvent system at 373 K for 30 min; the fructose conversion reached 87%, and the selectivity of the product HMF reached 99% [30]. In the single-phase reaction system of DMSO, Dong et al. used sulfonated HCP as a catalyst to catalyze the dehydration of fructose to prepare HMF, and the HMF was completely transformed within 30 min, and 96.7% HMF was obtained at the same time. After three reuses, the conversion rate was still 82% [31]. Simeonov et al. used the ion exchange resin Amberlyst-15 (10 wt%) to catalyze fructose, and the mixed solution of water and TEAB was used as the solvent. Finally, the HMF yield reached 92% and the fructose conversion rate reached 94% in just 15 min [32].
2.2. Advances in Catalytic Conversion of Glucose to HMF
Although fructose catalyzed dehydration can obtain a very high yield of HMF, most of the current studies have used fructose conversion to synthesize HMF, but food waste with a high fructose content is limited as a source of synthetic raw materials. Glucose is abundant and easy to obtain, but its synthesis process is more complicated than that of fructose. Glucose needs to be isomerized to fructose first and then converted to hydroxymethylfurfural by dehydration, resulting in a decrease in conversion and yield [33,34,35,36]. The conversion of glucose into HMF can be mainly divided into two reaction solvent systems. The first is a single-phase system, which is a single aqueous solvent or organic solvent system and produces more side reactions [37,38,39,40]. Dou et al. used Aquivion PFSA@SiO2 solid acid as catalyst to catalyze glucose to prepare HMF in a single-phase reaction system of DMSO. At the reaction temperature of 363 K, the yield of HMF product reached 85% [41]. Zhang et al. catalyzed the conversion of glucose into HMF with 70 mol% TEAB and 12 mol% CrCl3 as catalysts in a DMAC single-phase reaction system, and the reaction temperature was 393 K for 90 min, obtaining a 72.7% HMF yield, as shown in Figure 3. As shown in [42], Zhao et al. used sulfonated carbon solid acid as catalyst and DMSO as reaction solvent in a reaction at 433 K for 1.5 h and obtained a 90% HMF yield [43]. The second reaction system is the double-phase reaction system. Compared with the single-phase system, it has an advantage that it can react in a single integrated phase first, and the generated products will then be transferred to the other integrated phase, which is conducive to the separation and purification of products and the purpose of reducing side reactions.
Figure 3.
Synthesis of HMF and its further derivatization into important chemicals.
Mukundan et al. used nano-level phosphosalated TiO2 as catalyst, a water/butanol biphase system as the reaction solvent, and a reaction temperature of 448 K for 3 h; the final glucose conversion rate was 97%, and the HMF yield was 81% [44]. Atanda et al. catalyzed glucose conversion to prepare HMF in a double-phase reaction system of water/THF, using phosphosalated TiO2 as a catalyst and adding the additive methylpyrrolidone to the reaction system, and the yield of HMF reached 90% at the reaction temperature of 448 K [45]. In addition, in addition to these two systems, there are also solution systems such as supercritical fluids and ionic liquids. Using 7 mol% CrCl3·6H2O as a catalyst and [C4C1im] Cl as a reaction system, Sanan et al. catalyzed glucose to prepare HMF at a 393 K reaction temperature, and the yield of the HMF product reached 90% within 30 min [46]. Li et al. selected sulfonated resin (SPPS) containing acidic sites as the catalyst to catalyze the preparation of HMF from glucose, and [EMIM]Br as the reaction solvent. The reaction temperature was 373 K and the reaction time was 4 h, and the product yield of HMF reached 87.2% [47]. Han et al. used reduced graphene oxide-supported tungsten trioxide (WO3/RGO) as a catalyst to produce 94% HMF in the [BMIM]Cl reaction system at a reaction temperature of 393 K and a reaction time of 2 h [48].
2.3. The Transformation Pathway of Hydroxymethylfurfural
The conversion reactions of HMF can be divided into three main categories (as shown in Figure 4). The first type of ring-opening reaction is the ring-opening of hydroxymethylfurfural into levulinic acid. Using Amberlyst 70 as catalyst, Zhang et al. catalyzed the dehydration of fructose into hydroxymethylfurfural and then into levulinic acid in a DMSO solvent system at a reaction temperature of 368 K and with a reaction time of 2 h, and obtained 50% levulinic acid [49]. The second type of reaction is the reduction reaction, and hydroxymethylfurfural can generate 2, 5-dimethylfuran (DMF), 2, 5-dimethyltetrahydrofuran (DMTHF) and other compounds through a hydrogenation reduction reaction, among which DMF has a strong market prospect as a liquid fuel. Nishimura et al. used a Pd50Au50/C catalyst to catalyze the hydrogenation reduction of HMF to produce DMF, and took hydrochloric acid as an auxiliary agent. Under atmospheric hydrogen pressure, the reaction temperature was 333 K and the reaction time was 12 h, and more than 99% DMF was produced [50].
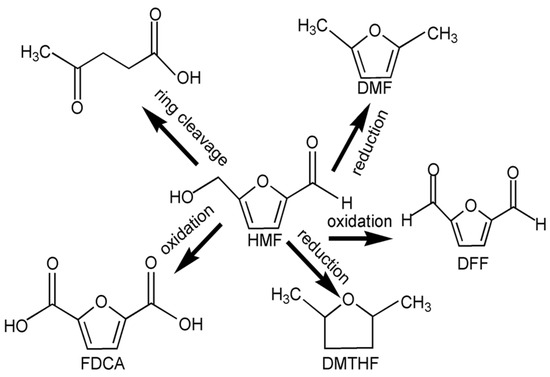
Figure 4.
Transormation path of HMF.
Using NC-Cu/MgAlO as a catalyst, Gao et al. reacted with cyclohexanone as a hydrogen source in a N2 atmosphere at 493 K for 200 min, and obtained 94.6% DMTHF [51]. The third type of reaction is an oxidation reaction; HMF can be oxidized to 2, 5-furan diformaldehyde (DFF), 2, 5-furan diformic acid (FDCA) and other substances. Ghosh et al. used melamine-formaldehyde polymer-supported ruthenium nanoparticles (Ru@mPMF) as the catalyst to catalyze the oxidation of HMF in a toluene reaction system. The conversion rate of HMF was 99.6% at an oxygen pressure of 2 MPa and reaction temperature of 373 K for 12 h, and the product DFF selectivity was 84.66%. The conversion rate was 64% after five reuses [52].
3. Research Progress in the Preparation of 2, 5-Furan Dicarboxylic Acid by Catalytic Oxidation of Hydroxymethylfurfural
3.1. Properties of 2, 5-Furandicarboxylic Acid
2, 5-Furandicarboxylic acid (FDCA) is a furan derivative produced by catalytic oxidation of HMF. Its chemical structure formula is shown in the figure above, which mainly includes furan rings and carboxyl groups. In recent years, FDCA has been proved to be a substitute for p-Phthalic acid (PTA) in the production of polyester materials, because it has similar structural and chemical properties to PTA [53,54,55,56,57]. In 2012, Avantium developed a polyFDCA glycol ester material with renewable and degradable properties [58]. Ji Junhui et al. also developed polyester materials—butanediol and FDCA copolymers that can be degraded by ordinary lipase [59]. In addition, FDCA can also be used for food-contact materials, and the European Union has issued opinions on the safety of FDCA used in food-contact materials: after verification by animal experiments, it is thought that when the content added to food is below a certain limit, it will not cause damage to human body [60].
In order to achieve sustainable economic development and reduce environmental pollution, Genovese et al. developed a furan-based polyester material–polyneopentyl glycol furanate (PNF), which is synthesized from 2, 5-furanediic acid, a traditional petrochemical raw material and a substitute for terephthalic acid. The material has good thermal stability. As a food packaging material, it does not show obvious penetration behavior [61]. As shown in Figure 5, the chemical structure of PBF and P(BFxBDGy) copolymers take 2, 5-furanodicarboxylic acid, diglycolic acid and 1, 4-butanediol as raw materials, and n-butyl titanate and titanium tetraisopropanol as catalysts. Polybutenofuranoate material was produced by secondary melt polycondensation reaction, which was applied in the field of food packaging materials due to its good thermal stability and biodegradability [62]. Therefore, if FDCA can become a substitute for PTA with a high annual output, it will play an extraordinary role in product industrialization [63,64,65,66,67].
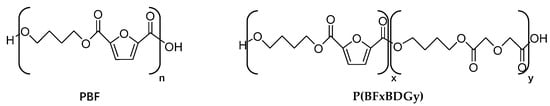
Figure 5.
Chemical structure of PBF and P(BFxBDGy) copolymers.
Although FDCA polyester material is degradable, it has good food safety properties as a packaging material. However, it has not been put into large-scale production, mainly because of the high production cost of the food-contact material monomer FDCA and the immature conversion technology. Therefore, the research on the process route of the catalytic oxidation of HMF to FDCA has great market application potential [68,69,70,71].
3.2. Product Distribution and Transformation Mechanism of HMF Oxidation
HMF can be oxidized to in two different ways under the action of the corresponding catalysts: 5-Diformylfuran (2, 5-Diformylfuran, DFF), 5-formyl2-Furan-carboxylic acid, 5-formyl2-Furan-carboxylic acid, FFCA and 5-Hydroxymethyl-2-furancarboxylic acid (HFCA) [72]. During the oxidation of HMF, two intermediate products, DFF or HFAC, are produced, and there are two conversion paths (see Figure 6).
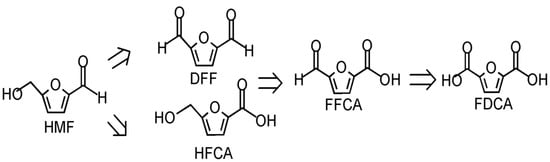
Figure 6.
Oxidation path of HMF.
The intermediate product is the process route of HFCA. Based on previous studies, Davis et al. revealed the oxidation reaction mechanism of supported Pt and Au nanoparticles in the reaction system, adding a certain homogeneous base to the system, with the intermediate oxidation product being HFCA [73,74]. The main reactions are as follows: the aldehyde group in HMF is oxidized first to form an intermediate of amido glycol. The intermediate of the diol continues to dehydrogenate to carboxylic acid, producing HFCA. The hydroxyl group in HFCA is further oxidized to the carbonyl group to form FFCA. Finally, the aldehyde group in FFCA is oxidized to carboxylic acid to produce FDCA (see Figure 7 for the mechanism).
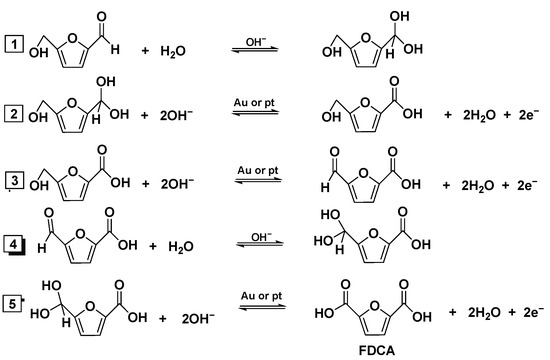
Figure 7.
Mechanism of HMF → HFCA → FFCA → FDCA (1. The aldehyde group in HMF is oxidized to form a gem-diol intermediate. 2. The gem-diol intermediate undergoes dehydrogenation to produce a carboxylic acid, yielding HFCA. 3. The hydroxyl group in HFCA is oxidized to a carbonyl group, forming FFCA. 4. The aldehyde group in FFCA is oxidized to a gem-diol intermediate. 5. The gem-diol intermediate is dehydrogenated to generate a carboxylic acid, producing FDCA. Note: HMF is 5-hydroxymethylfurfural; HFCA is 5-hydroxymethyl-2-furancarboxylic acid; FFCA is 5-formyl-2-furancarboxylic acid; FDCA is 2, 5-furandicarboxylic acid).
Davis et al. [73] also believed that O2 had an obvious effect on the oxidation of HMF into FDCA, and that O2 could remove electrons on the catalyst surface to make the reaction continue downward [73]. Most of the reactions which catalyzed the oxidation of HMF to FDCA use oxygen as the oxidant, and some choose to use tert-butyl hydrogen peroxide, hydrogen peroxide, sodium periodate and other strong oxidants as oxidants. Gao et al. used tert-butyl hydrogen peroxide as an oxidizing agent to react in the acetonitrile reaction system at 373 K for 24 h, and obtained a 95.6% FDCA yield [75]. Wang et al. used t-BuOOH as an oxidizing agent. It reacts at 353 K in Dimethyl sulfoxide solvent system. And only 1% FDCA yield was obtained [76].
HMF → DFF → FFAC → FDCA process circuit: Siankevich et al. revealed the reaction mechanism in which Pt/PVP nanoparticles were used as catalysts, there was no base in the reaction system, and the intermediate by-product was DFF [77]. The main reactions are as follows: the hydroxyl side chain in HMF is first deprotonated to produce DFF. The aldehyde group in DFF quickly generates the amido–glycol intermediate, and the amido–glycol intermediate dehydrogenates to form carboxylic acid and FFCA. Finally, the aldehyde group in FFCA continues to be oxidized to produce the final product FDCA (see Figure 8 for the mechanism).
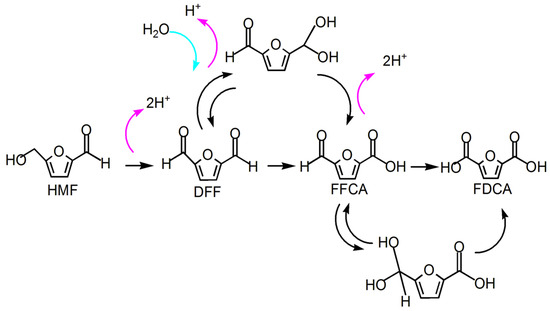
Figure 8.
Mechanism of HMF→DFF→FFCA→FDCA(The pink arrow represents the process of deprotonation (removal of a proton, H⁺). The green arrow represents the formation of a gem-diol intermediate from water).
3.3. Research Progress on Catalytic System and Process of Conversion of HMF to FDCA
In the process system for the oxidation of HMF to FDCA, the choice of catalyst and process conditions plays an important role. The selection of catalyst goes through the following stages: 1. The oxidizer KMnO4 is used for the HMF oxidation reaction, and the final product selectivity was low [78]. 2. A Co2+/Mn2+/Br− isohomogeneous catalyst is used in the reaction system of HMF oxidation to FDCA, and the homogeneous catalyst cannot be separated from the reaction system and reused [79]. 3. Supported metal catalysts were used to make up for the shortcomings of the above-mentioned catalysts [80]. Therefore, the optimization of process conditions will improve the efficiency of catalytic oxidation of HMF to FDCA.
For the process system of the catalytic oxidation of hydroxymethylfurfural used to synthesize 2, 5-furan dicarboxylic acid, a large number of studies have shown that the addition of a base can promote the formation of FDCA [81,82,83,84,85]. Among them, the types of alkali added include sodium hydroxide, potassium carbonate, and sodium bicarbonate, and the amount of alkali added is also determined by the specific reaction system [86,87,88,89]. For example, Sahu et al. used Pt/γ-Al2O3 as a catalyst, oxygen pressure was 1 MPa, and the reaction took place at 373 K for 24 h without adding an alkali, and only a 49% HMF conversion and 5% FDCA yield were achieved. Under the same reaction condition, 63.49 mg NaOH was added to the reaction system to obtain a 98% HMF conversion and 18% FDCA yield [90]. Hayashi et al. used MnO2 as a catalyst to catalyze the oxidation of HMF to FDCA, with an oxygen pressure of 1 MPa, reaction temperature of 373 K, and a reaction time of 24 h. When no alkali was added to the system, a 100% HMF conversion rate and 65% FDCA yield were obtained [91]. Under the same conditions, 50.41 mg NaHCO3 was added to the system, and the conversion rate of 100% HMF and the yield of 91% FDCA were obtained. After repeated use for four times, the conversion rate was still 81%. However, the homogeneous base remained in the reaction system because it contains metal ions, which makes the oxidation products in the reaction system difficult to separate and purify, and causes pollution to the environment. In recent years, some researchers have started from the catalyst itself, and achieved the aim of not adding a homogeneous base to the reaction system by designing the basic site of the catalyst. For example, Han et al. used a Pt/C-O-Mg catalyst to catalyze hydroxymethylfurfural. The formation of the C-O-Mg bond resulted in a Lewis alkaline on the catalyst surface, and the entire reaction system did not require additional lye. Under the temperature of 283 K and the oxygen pressure of 1 MPa, an FDCA yield of more than 99% could be obtained for 12 h [92].
3.4. Research Progress of Catalyst Selection in Synthesis of 2, 5-Furan Dicarboxylic Acid
3.4.1. Research on Supported Precious Metal Catalysts
Supported catalysts in an FDCA synthesis system are the most common, and supported palladium (Pd), platinum (Pt), gold (Au) and Ruthenium (Ru) catalysts are the most common in the reaction of HMF oxidation catalyzed by supported noble metal catalysts.
First, the supported Pd catalyst showed good catalytic activity in the HMF oxidation reaction system. Liu et al. used Pd/C@Fe3O4 as the catalyst to catalyze the oxidation of HMF. The oxygen flow rate of the oxidizer was 30 mL·min−1, the reaction temperature was 353 K, and the reaction time was 6 h. Finally, the conversion rate of HMF reached 98.4% and the yield of FDCA was 86.7% in the aqueous-phase reaction system. Catalysts can be separated by magnets and reused [93]. Davis compared the product distribution of reaction systems using supported Pt, Pd and Au nanoparticles as catalysts, and found that supported Au catalysts were more likely to oxidize aldehyde groups than hydroxyl groups, resulting in a large amount of the intermediate product HFCA. Under the same conditions, supported Pt and Pd catalysts can more efficiently convert HFCA into FDCA [94]. Wan et al. took bimetallic Au-Pd as the catalytic active center and loaded it onto CNT support to form a supported bimetallic catalyst, which reacted at a 373 K temperature for 12 h and a 0.5 MPa oxygen pressure in the aqueous system, and completely transformed HMF, and the yield of FDCA was 91% [95].
The supported Pt catalyst also has a high catalytic efficiency during HMF oxidation. Using Pt/ZrO2 as catalyst, Rass et al., under a 4 MPa oxygen pressure, a reaction temperature of 373 K, a reaction time of 24 h, reached an HMF conversion of 97% and an FDCA yield of 95% [96]. Niu et al., using Pt/RGO as a catalyst, water as a solvent, oxygen as an oxidizer and an oxygen flow rate at 50 mL·min−1, at 298 K for 24 h, obtained a 100% HMF conversion and an 84% FDCA yield [97]. Zhou et al. compared the catalytic efficiency of supported Pt, Au and Pd catalysts, and found that Pt/CNT had the highest catalytic efficiency. Under an oxygen pressure of 0.5 MPa, with water as the solvent, and a reaction temperature of 368 K for 14 h, the FDCA yield was as high as 98% [98].
3.4.2. Characteristics of Different Supported Noble Metal Catalysts
Compared with other supported noble metal catalysts, Au-based catalysts are more likely to oxidize aldehyde groups and easily generate HFCA intermediates [99]. Albonetti et al. found that under the reaction conditions of a 343 K reaction temperature with the catalyst Au/CeO2, a 1 MPa oxygen pressure, 4 h reaction time, and a molar ratio of HMF: Au: NaOH at 1:0.01:4, the maximum yield of FDCA was 92% [100]. Casanova et al. obtained a nearly 100% FDCA yield [101] under the conditions of a 338 K temperature, 10 bar oxygen pressure, using Au-CeO2 as catalyst, water as a solvent, and with the molar ratio of NaOH/HMF as 4. Donoeva et al. used Au/SAG-N as a catalyst, water as a solvent, an oxygen pressure of 1 MPa, a reaction temperature of 363 K for 12 h, and obtained 100% HMF conversion and a 75% FDCA yield [102]. The catalytic effects of different catalysts are shown in Table 1. With the right choice of biomimetic catalyst under specific conditions, the conversion and yield of HMF can be effectively improved.

Table 1.
Catalytic effect of different catalysts.
Some scholars have also studied the catalytic effect of a supported Ru catalyst in a reaction system where HMF is converted via oxidation to FDCA. Nie et al. used Ru/C as a catalyst, with a 2 MPa oxygen pressure, water as the reaction solvent, and a 403 K temperature for 8 h to obtain a 75.3% FDCA yield [103]. Tim et al. used Ru(OH)x/La2O3 as a catalyst, oxygen as the oxidant, the oxygen pressure was 3 MPa, and [EMIm][OAc] ionic liquid was used as a solvent at a 373 K temperature, obtaining a 48% FDCA yield [104].
In addition, some researchers have applied supported non-precious-metal catalysts in HMF oxidation process systems due to their low cost, but their catalytic activity cannot be compared with traditional precious metal catalysts. Using Fe3O4-CoOx as a catalyst and t-BuOOH as an oxidizing agent, with the reaction occurring at 353 K for 15 h, Wang et al. obtained a 68.6% FDCA yield, but the strong oxidizing agent also caused certain harm to the environment [76]. Hansen et al. used CuCl/t-BuOOH as a catalyst, air as an oxidant and acetonitrile as a reaction solvent to obtain a 50% FDCA yield; however, the organic solvent acetonitrile could aggravate environmental pollution [105]. Liu et al. used SBA-NH2-Cu2+ and SBA-NH2-VO2+ as co-catalysts to catalyze the oxidation of HMF, using toluene as the reaction solvent, at an oxygen flow rate of 20 mL·min−1 and at a temperature of 383 K for 12 h, and obtained a 99.1% HMF conversion and a 26.9% FDCA yield [106]. Wang et al. used magnetic γ-Fe2O3@HAP-Mo as a catalyst, and the oxygen flow rate was 20 mL·min, as shown in Figure 9. It was shown that after the reaction at 393 K for 12 h in the p-chlorotoluene solvent system, a 95.8% HMF conversion and 19.6% FDCA yield were obtained [107].
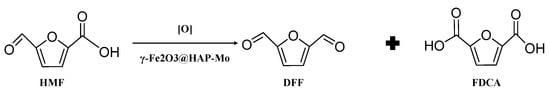
Figure 9.
Mo-hydroxyapatite-encapsulated magnetic γ-Fe2O3 showed high catalytic activity in the aerobic oxidation of HMF under atmospheric oxygen pressure.
3.5. Study on Conversion of Selective Electrooxidation HMF to FDCA
Electrooxidation of 5-hydroxymethylfurfural (HMF) to 2, 5-furan dicarboxylic acid (FDCA) is a promising value-added biomass strategy. However, there is a significant carbon loss due to spontaneous HMF degradation in alkaline electrolytes, especially at high concentrations, which is a major challenge. The carbon loss during the electrochemical conversion of HMF to FDCA was reduced by inhibiting non-Faraday degradation by low-temperature electrolysis. Notably, with carbon losses of up to 25% at conventional room temperature, this strategy effectively addresses the long-standing challenge of balancing enhanced electrooxidation kinetics of HMF with accelerated degradation as the concentration of HMF increases in alkaline media. The results highlight the critical role of the inhibition of non-Faraday degradation in the effective conversion of HMF and demonstrate that low-temperature electrolysis offers a viable solution to the challenge of adding value to industrial-scale electrochemical materials.
4. Research Progress of Novel and Efficient Catalysts for Preparation of FDCA by Catalytic Oxidation of HMF
Metalloporphyrin is the catalytic activity center of cytochrome P450 monooxygenase, which can independently simulate oxidase to realize the activation of molecular oxygen under mild conditions, and has stable chemical properties, a strong specificity and a cheap availability. At present, there have been successful cases of using metalloporphyrin simulation enzymes for the industrial oxidation of cyclohexane in China [108,109,110]. Professor Ji Hongbing of South China University of Technology has also studied and shown that metal porphyrins are efficient catalysts for the oxidation of benzyl alcohol and benzaldehyde, and the conversion and selectivity of the reaction with oxygen at room temperature and atmospheric pressure both exceed 95% [111,112]. How to improve the activity and stability of a metal porphyrin mimicase catalyst and make it conform to the current process has been the focus of research in the field of bionic chemistry. For example, Santos J et al. changed the outer ring substituents and central metal ions of metal porphyrins, and found that the electron absorption effect was produced after chlorophenyl substitution, and highly active species (such as Fe4+=O) could be produced more rapidly, significantly increasing activity [113]. Although the purpose of changing catalytic properties can be achieved by changing the structure of metal porphyrins, the disadvantage of this method is that it cannot effectively prevent metal porphyrins from forming dimers, nor can it solve the problem that metal porphyrins are difficult to separate, recover and reuse from the reaction system [113,114].
However, further loading of metal porphyrins on the surface of mesoporous materials solves the above problems. A mesoporous molecular sieve has been proved to be an excellent metal porphyrin carrier material due to its large specific surface area, adjustable pore size (2–50 nm) and modifiable pore surface [115]. However, in cytochrome P450 enzymes, catalytic oxidation occurs when metalloporphyrins and protein-formed complexes work together. Metalloporphyrins self-assemble in the pores of unmodified mesoporous materials, and it is difficult for highly active species to maintain stability due to the lack of a natural hydrophobic microenvironment provided by the cytochrome P450 enzyme protein. On the other hand, based on the Pauling stable transition state theory, the simulated enzyme first binds to the substrate, and then stabilizes in the transition state to reduce the activation energy of the reaction, while the surface properties of the enzyme immobilized carrier have a significant impact on the mass transfer of the substrate [115]. Therefore, in addition to the consideration of catalytic groups, the design of the microenvironment around the active center, that is, the surface properties of the carrier, is also crucial during the immobilization of metal porphyrins. However, the properties of existing modifiers are very different, are not designable, and cannot meet the fine tuning of the pore environment. Moreover, the screening of modifiers is relatively blind, and a fast and effective screening method is lacking [116].
In recent years, a large number of studies have found that when designed ionic liquids are used as solvents, their functional groups form a tight electron transport chain with metal porphyrins through fine-adjustment of the arm end, which realizes the regeneration and closed cycle of catalyst electrons, and improves the activity and stability of the catalyst [117,118]. However, ionic liquid is used as a solvent, the cost is high, and the ionic liquid is difficult to separate from the product; the high viscosity limits the mass transfer, amongst other things. Combined with these research results, we can start from the structure and catalytic mechanism of natural P450 enzymes, take mesoporous materials as the structural framework, use the designability of ionic liquids to functionalize the material surface, and quickly and directionally introduce ionic liquid cations of protein-like functional groups (such as hydrophilic and hydrophobic groups, charged groups or hydrogen bonding groups) on the surface of mesoporous materials. This provides a biomimetic microenvironment for metal porphyrins and improves the ability of simulating the enzyme activation of molecular oxygen. At the same time, in addition to the characteristics of the HMF oxidation process, the aldehyde group can be rapidly converted into a polyhydroxyl intermediate in an alkaline environment to facilitate the formation of FDCA [119,120], the rapid transformation of the substrate is promoted through the anion modulation reaction of the acid–alkaline environment in the ionic liquid, and efficient catalysts are developed for the selective oxidation of the HMF-to-FDCA process.
5. Research Significance of Biomimetic Enzyme Applied to HMF Oxidation Conversion Process
5.1. Significance and Challenge of Research
FDCA is prepared by a catalytic reaction of molecular oxygen with HMF in a water phase. The atomic utilization rate is high and the by-product is water, which is an economical atomic route. The core technology of this process is the development of a highly efficient selective oxidation catalyst. The existing catalysts have some problems, such as a low substrate recognition, harsh reaction conditions and low recycling and reuse, which lead to great environmental pollution in the reaction process and limit their application in the HMF oxidation process. Biomimetic enzymes can alleviate the above problems due to their excellent characteristics, such as their mild reaction conditions, strong specificity, and being green as regards pollution, and are suitable for the HMF oxidation process [121,122]. In addition, the catalyst used in the HMF oxidation reaction system requires an alkaline environment to promote the rapid transformation of HMF raw materials into product FDCA. At present, most of the added homogeneous bases in the reaction system will pollute the environment, and the product separation and purification are difficult [123,124]. Designing a heterogeneous base can avoid the addition of a homogeneous base in the reaction system. Therefore, it is of great significance to explore the catalytic oxidation of HMF by a biomimetic enzyme to prepare FDCA under the conditions of a low temperature, normal pressure and no homogeneous base. In the process of the catalytic oxidation of HMF to FDCA, an immobilized metal porphyrin simulated-enzyme alkaline catalyst was designed and innovatively applied in the HMF oxidation reaction, which reduced the activation energy of the HMF reaction and made HMF efficiently and rapidly, with a directional conversion to FDCA under mild conditions [125,126].
HMF is an important biomass-derived chemical with a wide range of potential applications, especially in the production of renewable energy, green chemicals and high-value chemicals. HMF is not only a promising bio-based chemical intermediate, but also can be used to produce some important chemicals, such as hydroquinone and succinic acid, amongst others. However, there are some challenges in the practical application of the oxidation conversion process of HMF, including a poor selectivity, low catalytic efficiency and insufficient catalyst stability. The oxidation of HMF usually involves complex chemical transformations that may result in the formation of multiple by-products. Although natural enzymes exhibit high levels of activity and selectivity during catalysis, they are prone to inactivation under conditions such as a high temperature, high concentration of reactants and extreme pH. With the decrease in the dependence on fossil fuel energy and the increasing emphasis on biomass resources, the use of renewable resources (such as lignocellulose, sugar, etc.) to produce HMF and its derivatives has become an important research direction.
The stability and activity of biomimetic enzymes may be poor under these extreme reaction conditions. Like natural enzymes, bionic enzymes may have the problem of catalyst activity decline. The synthesis of biomimetic enzymes often requires a high cost, especially in mass production processes. The oxidative transformation of HMF is a complex process involving multiple reaction steps. Whether biomimetic enzymes can catalyze these steps accurately and avoid unnecessary side reactions requires in-depth understanding of the reaction mechanism. These challenges point the way for research.
The application of biomimetic enzymes in the HMF oxidation conversion process has important research significance: it can not only improve catalytic efficiency and selectivity, but also reduce the reaction condition requirements, enhance the stability of the catalyst, and promote the development of green chemistry. In addition, the versatility and sustainability of biomimetic enzymes offer great potential for the industrial production of HMF and its derivatives. The deepening of this research direction will open up new ways for the production of green chemistry and bio-based chemicals.
5.2. Outlook and Prospects
With the development of renewable resources and green chemistry, the production of bio-based chemicals has become an important research direction. In this context, HMF (5-hydroxymethylfurfural), as a key chemical intermediate derived from biomass, has become a research hotspot for its efficient and selective oxidation. Biomimetic enzymes, as a new type of catalyst that simulate the catalytic properties of natural enzymes, show great potential in the oxidation conversion process of HMF with their high catalytic efficiency, mild reaction conditions, good selectivity and environmental friendliness. The future prospects of biomimetic enzymes in this field are discussed from multiple dimensions. The design and modification of the catalyst to enhance its activity and stability in multiple catalytic reactions will greatly improve the economy and practicability of biomimetic enzyme-catalyzed HMF conversion. In addition, the integrated reaction system catalyzed by biomimetic enzymes may also make the HMF conversion process more efficient, economical and sustainable.
In the HMF production process, bionic enzymes can replace traditional chemical catalysts, thereby reducing production costs, reducing pollutant emissions and improving the yield and purity of HMF products. The application prospects of biomimetic enzymes in green chemistry, sustainable energy production and environmentally friendly technology are very broad. Through industrial application, bionic enzyme technology can not only effectively reduce production costs, but also push the industry in a more environmentally friendly and sustainable direction. The continuous promotion of multi-disciplinary collaboration and technological innovation will accelerate the application and development of bionic enzyme catalysis technology, which has huge market potential in the future of the bio-based chemical industry, green chemistry and in other fields.
In the future, it is necessary to further optimize the design of immobilized metal porphyrin analog enzyme catalysts, such as in providing other effective carriers for metal porphyrin analog enzymes, or changing the surface modifier of the carrier to provide a different bionic environment for metal porphyrin, and changing the fixed load of metal porphyrin. Further study should explore the thermodynamics of the high-efficiency catalytic oxidation of HMF with new catalysts under mild conditions, clarifying the interaction mechanism between metal porphyrin bionic catalysts and the HMF substrate, and further providing theoretical guidance for process optimization.
Author Contributions
L.Q.: conceptualization, methodology, validation, writing—original draft, writing—review and editing. F.K.: conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, writing–review and editing. X.C.: investigation, resources, writing—review and editing. Y.Z. (Yuyang Zhang): investigation, resources, writing—review and editing. Z.L.: investigation, resources, writing—review and editing. X.N.: investigation, resources, writing—review and editing. X.Z.: investigation, resources, writing—review and editing. Q.L.: investigation, resources, writing—review and editing. Y.Z. (Yani Zhao): investigation, resources, writing—review and editing. B.Z.: conceptualization, supervision, methodology, investigation, resources, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Scientific Research Startup Funding Project for Introduced Talents of Wuhu Institute of Technology (No. wzyrc202403), the Wuhu Science and Technology Innovation Strategy Research Special Project (No. 2024swwzx07), the Wuhu Institute of Technology Food Nutrition and Health Innovation Team (No. 2023jxtd06), and the Natural Science Research Project of Anhui Universities and Colleges of Anhui Provincial Department of Education (No. KJ2021ZD0153).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nzediegwu, E.; Pérez-Venegas, M.; Auclair, K.; Dumont, M.-J. Semisynthetic production of hydroxymethylfurfural and furfural: The benefits of an integrated approach. J. Environ. Chem. Eng. 2022, 10, 108515. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, Q.; Fan, D.; Xiao, J.; Zhao, Y.; Cheng, K.W.; Wang, M. Novel roles of hydrocolloids in foods: Inhibition of toxic maillard reaction products formation and attenuation of their harmful effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Thermal and non-thermal processing affect Maillard reaction products, flavor, and phytochemical profiles of Ginkgo biloba seed. Food Biosci. 2021, 41, 101044. [Google Scholar] [CrossRef]
- Chen, X.; Zou, Y.; Wang, D.; Xiong, G.; Xu, W. Effects of ultrasound pretreatment on the extent of Maillard reaction and the structure, taste and volatile compounds of chicken liver protein. Food Chem. 2020, 331, 127369. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zeng, X.A.; Brennan, C.S.; Ma, H.; Aadil, R.M. Preparation and characterisation of novelty food preservatives by Maillard reaction between ε-polylysine and reducing sugars. Int. J. Food Sci. Technol. 2019, 54, 1824–1835. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Zhang, L.; Yu, X.; Liu, H.; Yagoub, A.E.; Zhou, C. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020, 149, 112361. [Google Scholar] [CrossRef]
- Lin, N.; Liu, T.; Lin, L.; Lin, S.; Zang, Q.; He, J.; Abliz, Z.; Li, C.; Wang, A.; Jin, H. Comparison of in vivo immunomodulatory effects of 5-hydroxymethylfurfural and 5, 5′-oxydimethylenebis (2-furfural). Regul. Toxicol. Pharmacol. 2016, 81, 500–511. [Google Scholar] [CrossRef]
- Chen, W.; Wu, S.; Zhang, J.; Yu, F.; Miao, X.; Tu, X. Salting-out-assisted liquid–liquid extraction of 5-hydroxymethylfurfural from honey and the determination of 5-hydroxymethylfurfural by high-performance liquid chromatography. Anal. Methods 2019, 11, 4835–4841. [Google Scholar] [CrossRef]
- Song, Y.; Ding, Z.; Peng, Y.; Wang, J.; Zhang, T.; Yu, Y.; Wang, Y. Acrylamide formation and aroma evaluation of fried pepper sauce under different exogenous Maillard reaction conditions. Food Chem. X 2022, 15, 100413. [Google Scholar] [CrossRef]
- Bhavana, B.K.; Mudliar, S.N.; Bokade, V.V.; Debnath, S. Effect of furfural, acetic acid and 5-hydroxymethylfurfural on yeast growth and xylitol fermentation using Pichia stipitis NCIM 3497. Biomass Convers. Biorefin. 2022, 14, 4909–4923. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Long, Y.; Chen, Q.; Zhang, W.; Liu, G. Food additives: From functions to analytical methods. Crit. Rev. Food Sci. Nutr. 2021, 62, 8497–8517. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, Y.; Xiao, J.; Zhou, Q.; Wang, M. The occurrence and stability of Maillard reaction products in various traditional Chinese sauces. Food Chem. 2021, 342, 128319. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, S.; Chen, C.; Liu, X.; Cai, J. Identification of eggshell crack for hen egg and duck egg using correlation analysis based on acoustic resonance method. J. Food Process. Eng. 2020, 43, e13430. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, X.; Ruan, S.; Zhou, A.; Huang, S.; Ma, H. The Preparation and Identification of Characteristic Flavour Compounds of Maillard Reaction Products of Protein Hydrolysate from Grass Carp (Ctenopharyngodon idella) Bone. J. Food Qual. 2021, 2021, 8394152. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, S.; Pei, J.; Jiang, P.; Jin, W.; Gao, R. Antioxidative Activity and Volatile Profiles of Maillard Reaction Products between Giant Salamander (Andrias davidianus) Peptides and Glucose during the Heating Process. J. Food Qual. 2023, 2023, 8804009. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Mehmood, A.; Lu, T.; Chen, X. Unraveling the temporal changes of Maillard reaction products and aroma profile in coffee leaves during hot-air drying. J. Food Compos. Anal. 2024, 128, 106055. [Google Scholar] [CrossRef]
- Woo, J.; Moon, B.C.; Lee, U.; Oh, H.-S.; Chae, K.H.; Jun, Y.; Min, B.K.; Lee, D.K. Collaborative Electrochemical Oxidation of the Alcohol and Aldehyde Groups of 5-Hydroxymethylfurfural by NiOOH and Cu(OH)2 for Superior 2,5-Furandicarboxylic Acid Production. ACS Catal. 2022, 12, 4078–4091. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Tong, X.; Li, M.; Yan, N.; Ma, Y.; Dyson, P.J.; Li, Y. Defunctionalization of fructose and sucrose: Iron-catalyzed production of 5-hydroxymethylfurfural from fructose and sucrose. Catal. Today 2011, 175, 524–527. [Google Scholar] [CrossRef]
- He, Q.; Lu, Y.; Peng, Q.; Chen, W.; Fan, G.; Chai, B.; Song, G. Synthesis of 5-hydroxymethylfurfural from fructose catalyzed by sulfonated carbon-based solid acid. Biomass Convers. Biorefin. 2021, 13, 9195–9203. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.T.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Morgan, H.M., Jr.; Liang, J.; Zhang, X.; Yan, L.; Mao, H. Thermal Behavior and Kinetic Study of the Effects of Zinc-Modified Biochar Catalyst on Lignin and Low-Density Polyethylene (LDPE) Co-Pyrolysis. Trans. ASABE 2018, 61, 1783–1793. [Google Scholar] [CrossRef]
- Lin, L.; Cui, H.; Vittayapadung, S.; Xiao, Z.; Zhang, A.; Liu, R.; Li, C. Synthesis of KF/CaO as a catalyst for the production of bio-fuel from cracking of Cornus wisoniana oil. Eur. J. Lipid Sci. Technol. 2014, 117, 406–410. [Google Scholar] [CrossRef]
- Shao, S.; Sun, T.; Li, X.; Wang, Y.; Ma, L.; Liu, Z.; Wu, S. Preparation of heavy bio-oil-based porous carbon by pyrolysis gas activation and its performance in the aldol condensation for aviation fuel as catalyst carrier. Ind. Crops Prod. 2024, 218, 118963. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bidgoli, N.S.S.; Shafiei, N.; Momenbeik, F. Biomass valorization: Sulfated lignin-catalyzed production of 5-hydroxymethylfurfural from fructose. Int. J. Biol. Macromol. 2021, 182, 59–64. [Google Scholar] [CrossRef]
- Tian, X.; Qin, X.; Jia, X.; Lyu, Q.; Li, S.; Jiang, L.; Chen, L.; Yan, Z.; Huang, J. Lignocellulose degradation and temperature adaptation mechanisms during composting of mushroom residue and wood chips at low temperature with inoculation of psychrotolerant microbial agent. Environ. Pollut. 2024, 363 Pt 2, 125156. [Google Scholar] [CrossRef]
- Gomes, F.N.D.C.; Mendes, F.M.T.; Souza, M.M.V.M. Synthesis of 5-hydroxymethylfurfural from fructose catalyzed by phosphotungstic acid. Catal. Today 2017, 279, 296–304. [Google Scholar] [CrossRef]
- Morales, G.; Melero, J.A.; Paniagua, M.; Iglesias, J.; Hernández, B.; Sanz, M. Sulfonic acid heterogeneous catalysts for dehydration of C6-monosaccharides to 5-hydroxymethylfurfural in dimethyl sulfoxide. Chin. J. Catal. 2014, 35, 644–655. [Google Scholar] [CrossRef]
- Matharu, A.S.; Ahmed, S.; Almonthery, B.; Macquarrie, D.J.; Lee, Y.S.; Kim, Y. Starbon/High Amylose Corn Starch-Supported N-Heterocyclic Carbene–Iron(III) Catalyst for Conversion of Fructose into 5-Hydroxymethylfurfural. ChemSusChem 2018, 11, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Zhang, J.; Luo, W.; Su, L.; Huang, Z. Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chem. Eng. J. 2018, 334, 1055–1064. [Google Scholar] [CrossRef]
- Salamone, M.; Bietti, M. Reaction Pathways of Alkoxyl Radicals. The Role of Solvent Effects on C–C Bond Fragmentation and Hydrogen Atom Transfer Reactions. Synlett 2014, 25, 1803–1816. [Google Scholar]
- Xiao, X.; Zhang, X.; Bai, J.; Li, J.; Zhang, C.; Zhao, Y.; Zhu, Y.; Zhang, J.; Zhou, X. Bisphenol S increases the obesogenic effects of a high-glucose diet through regulating lipid metabolism in Caenorhabditis elegans. Food Chem. 2021, 339, 127813. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. Chicoric acid promotes glucose uptake and Akt phosphorylation via AMP-activated protein kinase α-dependent pathway. J. Funct. Foods 2019, 59, 8–15. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Structure and functional properties of watermelon seed protein-glucose conjugates prepared by different methods. LWT 2021, 155, 113004. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; Li, J.; Zhang, Y.; Dong, Y.; Xiao, X. Effects of Bitter Melon Saponin on the Glucose and Lipid Metabolism in HepG2 Cell and C. elegans. J. Food Qual. 2020, 2020, 8860356. [Google Scholar] [CrossRef]
- Ullah, H.; Esposito, C.; Piccinocchi, R.; De Lellis, L.F.; Santarcangelo, C.; Di Minno, A.; Baldi, A.; Buccato, D.G.; Khan, A.; Piccinocchi, G.; et al. Postprandial Glycemic and Insulinemic Response by a Brewer’s Spent Grain Extract-Based Food Supplement in Subjects with Slightly Impaired Glucose Tolerance: A Monocentric, Randomized, Cross-Over, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3916. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Zhang, H.; Tian, Y.; Li, J.; Yun, J.; Zabed, H.M.; Qi, X. Efficient Fermentative Production of β-Alanine from Glucose through Multidimensional Engineering of Escherichia coli. J. Agric. Food Chem. 2024, 72, 14274–14283. [Google Scholar] [CrossRef]
- Gimbernat, A.; Heuson, E.; Dumeignil, F.; Delcroix, D.; Girardon, J.; Froidevaux, R. Reactor development for a one-step hybrid catalytic conversion of d-glucose to hmf. ChemCatChem 2024, 16, e202300713. [Google Scholar] [CrossRef]
- Lutz, M.D.R.; Roediger, S.; Rivero-Crespo, M.A.; Morandi, B. Mechanistic Investigation of the Rhodium-Catalyzed Transfer Hydroarylation Reaction Involving Reversible C–C Bond Activation. J. Am. Chem. Soc. 2023, 145, 26657–26666. [Google Scholar] [CrossRef]
- Dou, Y.; Zhou, S.; Oldani, C.; Fang, W.; Cao, Q. 5-Hydroxymethylfurfural production from dehydration of fructose catalyzed by Aquivion@silica solid acid. Fuel 2018, 214, 45–54. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, X.J.; Ai, N.; Nie, Y.; Ji, J.B. Conversion of Glucose to 5-hydroxymethylfurfural with Tetraethylammonium Bromide and Chromium (III) Chloride as Catalysts. Appl. Mech. Mater. 2013, 316–317, 157–160. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; He, C.; Dai, Y.; Jia, X.; Yang, Y. Efficient dehydration of fructose to 5-hydroxymethylfurfural over sulfonated carbon sphere solid acid catalysts. Catal. Today 2016, 264, 123–130. [Google Scholar] [CrossRef]
- Atanda, L.; Mukundan, S.; Shrotri, A.; Ma, Q.; Beltramini, J. Catalytic Conversion of Glucose to 5-Hydroxymethyl-furfural with a Phosphated TiO2 Catalyst. ChemCatChem 2015, 7, 781–790. [Google Scholar] [CrossRef]
- Atanda, L.; Shrotri, A.; Mukundan, S.; Ma, Q.; Konarova, M.; Beltramini, J. Direct Production of 5-Hydroxymethylfurfural via Catalytic Conversion of Simple and Complex Sugars over Phosphated TiO2. ChemSusChem 2015, 8, 2907–2916. [Google Scholar] [CrossRef]
- Eminov, S.; Brandt, A.; Wilton-Ely, J.D.; Hallett, J.P. The Highly Selective and Near-Quantitative Conversion of Glucose to 5-Hydroxymethylfurfural Using Ionic Liquids. PLoS ONE 2016, 11, e0163835. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Ren, J.; Yang, D.; Cheng, B.; Kim, C.K.; Yao, X. Direct catalytic conversion of glucose and cellulose. Green Chem. 2018, 20, 863–872. [Google Scholar] [CrossRef]
- Han, H.; Zhao, H.; Liu, Y.; Li, Z.; Song, J.; Chu, W.; Sun, Z. Efficient conversion of fructose into 5-hydroxymethylfurfural over WO3/reduced graphene oxide catalysts. RSC Adv. 2017, 7, 3790–3795. [Google Scholar] [CrossRef]
- Godan, T.K.; Devendra, L.P.; Alphy, M.P.; Rajesh, R.; Vivek, N.; Sindhu, R.; Awasthi, M.K.; Binod, P. Catalytic synthesis of 5-hydroxymethyl furfural from sorghum syrup derived fructose. Sustain. Energy Technol. Assess. 2022, 54, 102884. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Chen, L.; Zhang, X.; Liu, J.; Wang, C.; Zhang, Q.; Ma, L. Selective hydrodeoxygenation of 5-hydromethylfurfural to 2,5-dimethylfuran over PtFe/C catalyst. Mol. Catal. 2024, 561, 114169. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Fan, G.; Yang, L.; Li, F. Nitrogen-doped carbon-decorated copper catalyst for highly efficient transfer hydrogenolysis of 5-hydroxymethylfurfural to convertibly produce 2,5-dimethylfuran or 2,5-dimethyltetrahydrofuran. Appl. Catal. B Environ. 2018, 226, 523–533. [Google Scholar] [CrossRef]
- Ghosh, K.; Molla, R.A.; Iqubal, A.; Islam, S.M. Ruthenium nanoparticles supported on N-containing mesoporous polymer catalyzed aerobic oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF). Appl. Catal. A Gen. 2016, 520, 44–52. [Google Scholar] [CrossRef]
- Ren, R.; Yang, X.; Xu, J.; Zhang, K.; Zhang, M.; Liu, G.; Yao, X.; Lou, L.; Xu, J.; Zhu, L.; et al. Genome-wide identification and analysis of promising GDSL-type lipases related to gummy stem blight resistance in watermelon (Citrullus lanatus). Sci. Hortic. 2021, 289, 110461. [Google Scholar] [CrossRef]
- Chen, G.; Khan, I.M.; He, W.; Li, Y.; Jin, P.; Campanella, O.H.; Zhang, H.; Huo, Y.; Chen, Y.; Yang, H.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef] [PubMed]
- He, W.-S.; Li, L.; Zhao, J.; Xu, H.; Rui, J.; Cui, D.; Li, H.; Zhang, H.; Liu, X. Candida sp. 99-125 lipase-catalyzed synthesis of ergosterol linolenate and its characterization. Food Chem. 2019, 280, 286–293. [Google Scholar] [CrossRef]
- Jiaojiao, X.; Bin, Z.; Ruoyu, Z.; Onyinye, A.I. Lipase nanogel catalyzed synthesis of vitamin E succinate in non-aqueous phase. J. Sci. Food Agric. 2021, 101, 3186–3192. [Google Scholar] [CrossRef]
- Zan, X.; Cui, F.; Sun, J.; Zhou, S.; Song, Y. Novel Dual-Functional Enzyme Lip10 Catalyzes Lipase and Acyltransferase Activities in the Oleaginous Fungus Mucor circinelloides. J. Agric. Food Chem. 2019, 67, 13176–13184. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.; Coelho, J.F.J. New copolyesters derived from terephthalic and 2, 5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Wen, M.; Zong, M.-H.; Li, N. Synergistic chemo/biocatalytic synthesis of 2, 5-furandicarboxylic acid from 5-hydroxymethylfurfural. Catal. Commun. 2020, 139, 105979. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(Neopentyl Glycol Furanoate): A Member of the Furan-Based Polyester Family with Smart Barrier Performances for Sustainable Food Packaging Applications. Materials 2017, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Manaresi, P.; Munari, A. Novel fully biobased poly(butylene 2,5-furanoate/diglycolate) copolymers containing ether linkages: Structure-property relationships. Eur. Polym. J. 2016, 81, 397–412. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Li, Z.; Yang, H.; Li, J.; Wang, Q.; Tan, C.; Zou, B. Enhanced antioxidant capacity of lipoic acid in different food systems through lipase-mediated esterification with phytosterols. J. Sci. Food Agric. 2022, 102, 7115–7125. [Google Scholar] [CrossRef]
- Huang, C.; Lin, Z.; Zhang, Y.; Liu, Z.; Tang, X.; Li, C.; Lin, L.; Huang, W.; Ye, Y. Structure-guided preparation of fuctional oil rich in 1,3-diacylglycerols and linoleic acid from Camellia oil by combi-lipase. J. Sci. Food Agric. 2022, 103, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, Y.; Hamadou, A.H.; Shen, Q.; Xu, B. Tempering–preservation treatment inactivated lipase in wheat bran and retained phenolic compounds. Int. J. Food Sci. Technol. 2021, 57, 2104–2112. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Liu, C.; Yang, H.; Jia, L.; Zhao, L.; Gong, F.; Tan, C.; Tao, H.; He, W. Improved antioxidant activity of rutin via lipase-mediated esterification with oleic acid. J. Sci. Food Agric. 2023, 103, 3489–3500. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Ma, H.; Dong, Y.; Fu, J.; Lai, X.; Yagoub, A.E.-G.A.; Peng, W.; Ni, H. Antioxidant and lipase inhibitory activities of Camellia pollen extracts: The effect of composition and extraction solvents. All Life 2022, 15, 1304–1314. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. Technol. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.K.; Miao, W.J.; Wu, Q.F.; Liu, Y.X.; Sun, Y.; Gao, C. Thermal versus Microwave Inactivation Kinetics of Lipase and Lipoxygenase from Wheat Germ. J. Food Process. Eng. 2015, 39, 247–255. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wang, L.; Liu, S.; Wang, K.; Sun, J.; Xu, B. Evaluation of the possible non-thermal effect of microwave radiation on the inactivation of wheat germ lipase. J. Food Process. Eng. 2016, 40, e12506. [Google Scholar] [CrossRef]
- He, W.-S.; Cui, D.-D.; Zhang, Y.-L.; Liu, Y.; Yin, J.; Chen, G.; Jia, C.-S.; Feng, B. Highly Efficient Synthesis of Phytosterol Linolenate Catalyzed by Candida Rugosa Lipase through Transesterification. Food Sci. Technol. Res. 2017, 23, 525–533. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Davis, S.E.; Benavidez, A.D.; Gosselink, R.W.; Bitter, J.H.; de Jong, K.P.; Datye, A.K.; Davis, R.J. Kinetics and mechanism of 5-hydroxymethylfurfural oxidation and their implications for catalyst development. J. Mol. Catal. A Chem. 2014, 388–389, 123–132. [Google Scholar] [CrossRef]
- Gao, L.; Deng, K.; Zheng, J.; Liu, B.; Zhang, Z. Efficient oxidation of biomass derived 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid catalyzed by Merrifield resin supported cobalt porphyrin. Chem. Eng. J. 2015, 270, 444–449. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Liu, B. Catalytic conversion of fructose and 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid over a recyclable Fe3O4–CoOx magnetite nanocatalyst. ACS Sustain. Chem. Eng. 2015, 3, 406–412. [Google Scholar] [CrossRef]
- Siankevich, S.; Savoglidis, G.; Fei, Z.; Laurenczy, G.; Alexander, D.T.; Yan, N.; Dyson, P.J. A novel platinum nanocatalyst for the oxidation of 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid under mild conditions. J. Catal. 2014, 315, 67–74. [Google Scholar] [CrossRef]
- Miura, T.; Kakinuma, H.; Kawano, T.; Matsuhisa, H. Method for Producing Furan-2,5-dicarboxylic Acid. U.S. Patent US-7411078, 12 August 2008. [Google Scholar]
- Lv, Y.; Zhang, L.; Xiao, Q.; Ye, X.; Zhang, P.; Yang, D.; Shang, Y. Recent advances of Ni-based electrocatalyst for driving selective electrooxidation of 5-hydroxymethylfurfural into 2,5-furandicarbox-ylic acid. Int. J. Hydrogen Energy 2024, 72, 149–165. [Google Scholar] [CrossRef]
- Padligur, M.C.; Westerfeld, P.; Linkhorst, J.; Wessling, M. Electrochemical oxidation of 5-hydroxymethylfurfural with flow electrodes: A novel approach for continuous synthesis of 2, 5-furandicarboxylic acid. Chem. Eng. J. 2025, 504, 158374. [Google Scholar] [CrossRef]
- Madadi, M.; Nazar, M.; Shah, S.W.; Li, N.; Imtiaz, M.; Zhong, Z.; Zhu, D. Green alkaline fractionation of sugarcane bagasse at cold temperature improves digestibility and delignification without the washing processes and release of hazardous waste. Ind. Crop. Prod. 2023, 200, 116815. [Google Scholar] [CrossRef]
- Tong, F.; Huang, Q.; Liu, L.; Fan, G.; Shi, G.; Lu, X.; Gao, Y. Interactive Effects of Inorganic–Organic Compounds on Passivation of Cadmium in Weakly Alkaline Soil. Agronomy 2023, 13, 2647. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Huang, A.-J.; Dong, X.-X.; Zhang, Y.-F.; Zhu, L.; Luo, L.; Xu, Z.-L.; Wang, H. A simple and sensitive fluoroimmunoassay based on the nanobody-alkaline phosphatase fusion protein for the rapid detection of fenitrothion. Front. Sustain. Food Syst. 2023, 7, 1320931. [Google Scholar] [CrossRef]
- Gao, X.; Yin, Y.; Yan, J.; Zhang, J.; Ma, H.; Zhou, C. Separation, biochemical characterization and salt-tolerant mechanisms of alkaline protease from Aspergillus oryzae. J. Sci. Food Agric. 2019, 99, 3359–3366. [Google Scholar] [CrossRef]
- Golly, M.K.; Ma, H.; Yuqing, D.; Wu, P.; Dabbour, M.; Sarpong, F.; Farooq, M. Enzymolysis of walnut (Juglans regia L.) meal protein: Ultrasonication-assisted alkaline pretreatment impact on kinetics and thermodynamics. J. Food Biochem. 2019, 43, e12948. [Google Scholar] [CrossRef]
- Ding, Q.; Li, Z.; Wu, W.; Su, Y.; Sun, N.; Luo, L.; Ma, H.; He, R. Physicochemical and functional properties of dietary fiber from Nannochloropsis oceanica: A comparison of alkaline and ultrasonic-assisted alkaline extractions. LWT 2020, 133, 110080. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Dabbour, M.; Mintah, B.K.; Chen, W.; Yang, F.; Zhang, Z.; Cheng, Y.; Dai, C.; He, R.; et al. Effect of alkaline pH-shifting process on extraction rate, structural, and functional properties of black soldier fly (Hermetia illucens) larvae protein. LWT 2023, 185, 115180. [Google Scholar] [CrossRef]
- Baba, W.N.; Mudgil, P.; Mac Regenstein, J.; Maqsood, S. Impact of quercetin conjugation using alkaline and free radical methods with tandem ultrasonication on the functional properties of camel whey and its hydrolysates. Food Res. Int. 2024, 190, 114562. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Qi, Y.; Tufail, T.; Xu, B. Demarcating quality improvement mechanisms in fresh yellow alkaline noodles: Insights into water-solid interaction, gluten network development, and starch properties during noodles resting. J. Cereal Sci. 2024, 117, 103935. [Google Scholar] [CrossRef]
- Saha, B.; Dutta, S.; Abu-Omar, M.M. Aerobic oxidation of 5-hydroxylmethylfurfural with homogeneous and nanoparticulate catalysts. Catal. Sci. Technol. 2011, 2, 79–81. [Google Scholar] [CrossRef]
- Hayashi, E.; Komanoya, T.; Kamata, K.; Hara, M. Heterogeneously-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid with MnO2. ChemSusChem 2017, 10, 815. [Google Scholar] [CrossRef]
- Han, X.; Geng, L.; Guo, Y.; Jia, R.; Liu, X.; Zhang, Y.; Wang, Y. Base-free aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over a Pt/C–O–Mg catalyst. Green Chem. 2015, 18, 1597–1604. [Google Scholar] [CrossRef]
- Liu, B.; Ren, Y.; Zhang, Z. Aerobic oxidation of 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid in water under mild conditions. Green Chem. 2015, 17, 1610–1617. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, C.; Chen, J.; Deng, W.; Zhang, Q.; Yang, Y.; Wang, Y. Base-Free Aerobic Oxidation of 5-Hydroxymethyl-furfural to 2,5-Furandicarboxylic Acid in Water Catalyzed by Functionalized Carbon Nanotube-Supported Au–Pd Alloy Nanoparticles. ACS Catal. 2014, 4, 2175–2185. [Google Scholar] [CrossRef]
- Ait Rass, H.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2, 5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2- and ZrO2- Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Wang, D.; Yang, G.; Sun, J.; Wu, M.; Yoneyama, Y.; Tsubaki, N. Pt Nanoparticles Loaded on Reduced Graphene Oxide as an Effective Catalyst for the Direct Oxidation of 5-Hydroxymethylfurfural (HMF) to Produce 2,5-Furandicarboxylic Acid (FDCA) under Mild Conditions. Bull. Chem. Soc. Jpn. 2014, 87, 1124–1129. [Google Scholar] [CrossRef]
- Zhou, C.; Deng, W.; Wan, X.; Zhang, Q.; Yang, Y.; Wang, Y. Functionalized Carbon Nanotubes for Biomass Conversion: The Base-Free Aerobic Oxidation of 5-Hydroxymethylfurfural to 2, 5-Furandicarboxylic Acid over Platinum Supported on a Carbon Nanotube Catalyst. ChemCatChem 2015, 7, 2722. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Huai, L.; Hao, P.; Zhao, X.; Wang, Y.; Zhang, B.; Chen, C.; Zhang, J. Oxidation of 2,5-bis(hydroxymethyl)furan to 2, 5-furandicarboxylic acid catalyzed by carbon nanotube-supported Pd catalysts. Chin. J. Catal. 2022, 43, 793–801. [Google Scholar] [CrossRef]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over Au-based catalysts: Optimization of active phase and metal-support interaction. Appl. Catal. B Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into Chemicals: Aerobic Oxidation of 5-Hydroxymethyl-2-furfural into 2,5-Furandicarboxylic Acid with Gold Nanoparticle Catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef]
- Donoeva, B.; Masoud, N.; de Jongh, P.E. Carbon Support Surface Effects in the Gold-Catalyzed Oxidation of 5-Hydroxymethylfurfural. ACS Catal. 2017, 7, 4581–4591. [Google Scholar] [CrossRef]
- Nie, J.; Xie, J. Activated carbon-supported ruthenium as an efficient catalyst for selective aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Chin. J. Catal. 2013, 34, 871–875. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Eyjólfsdóttir, E.; Gorbanev, Y.Y.; Sádaba, I.; Riisager, A. Aerobic Oxidation of 5-(Hydroxymethyl)furfural in Ionic Liquids with Solid Ruthenium Hydroxide Catalysts. Catal. Lett. 2012, 142, 1089–1097. [Google Scholar] [CrossRef]
- Hansen, T.S.; Sádaba, I.; García-Suárez, E.J.; Riisager, A. Cu catalyzed oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran and 2, 5-furandicarboxylic acid under benign reaction conditions. Appl. Catal. A Gen. 2013, 456, 44–50. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Ding, H.; Zhong, W.; Xu, Q.; Su, S.; Yin, D. Catalytic aerobic oxidation of 5-hydroxymethylfurfural over VO2+, and Cu2+, immobilized on amino functionalized SBA-15. Chem. Eng. J. 2016, 283, 1315–1321. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Yuan, Z.; Zhang, Z. Aerobic oxidation of 5-hydroxymethylfurfural into furan compounds over Mo-hydroxyapatite-encapsulated magnetic γ-Fe2O3. J. Taiwan Inst. Chem. Eng. 2016, 58, 92–96. [Google Scholar] [CrossRef]
- de Ortiz, M.P.R. Cytochrome P450: Structure, Mechanism, and Biochemistry; Plenum Press: New York, NY, USA, 2015. [Google Scholar]
- Wang, T.; Ke, H.; Chen, S.; Wang, J.; Yang, W.; Cao, X.; Liu, J.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Porous protoporphyrin IX-embedded cellulose diacetate electrospun microfibers in antimicrobial photodynamic inactivation. Mater. Sci. Eng. C 2021, 118, 111502. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.V.D.; González, B.; Vicente, M.A.; Trujillano, R.; Nassar, E.J.; Gil, A.; Santamaría, L.; Korili, S.A.; Marçal, L.; de Faria, E.H.; et al. Multifunctional heterogeneous catalysts: Tetrakis(pentafluorophenyl)porphinato]iron(III) immobilized on amine–functionalized Diatomaceous Earth for catalytic and adsorption applications. J. Environ. Chem. Eng. 2023, 11, 109729. [Google Scholar] [CrossRef]
- Das, A.K.; Nandy, S.; Bhar, S. Chemoselective and ligand-free aerobic oxidation of benzylic alcohols to carbonyl compounds using alumina-supported mesoporous nickel nanoparticle as an efficient recyclable heterogeneous catalyst. Appl. Organomet. Chem. 2021, 35, e6282. [Google Scholar] [CrossRef]
- Tekuri, C.S.; Singh, P.; Nath, M. Unravelling a trichloroacetic acid-catalyzed cascade access to benzo[f]chromeno [2,3-h]quinoxalinoporphyrins. Beilstein J. Org. Chem. 2023, 19, 1216–1224. [Google Scholar] [CrossRef]
- Balogh, G.T.; Decsi, B.; Krammer, R.; Kenéz, B.; Ender, F.; Hergert, T.; Balogh-Weiser, D. Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin. Metabolites 2022, 12, 1269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; He, B.; Zhang, Y.; Ni, J.-Y.; Liu, Q.-P.; Wang, M.; Shen, H.-M.; She, Y.-B. Selective and Efficient Catalytic Oxygenation of Alkyl Aromatics Employing H2O2 Catalyzed by Simple Porphyrin Iron(II) under Mild Conditions. Processes 2023, 11, 1187. [Google Scholar] [CrossRef]
- Abdrassilova, A.; Vassilina, G.; Abdildina, K.; Briones, L.; Peral, A.; Escola, J. The notable features of mesoporous aluminosilicates as catalytic supports for hydrodearomatization and hydrodesulfurization of fuels. Microporous Mesoporous Mater. 2024, 384, 113457. [Google Scholar] [CrossRef]
- Yuan, H.; Su, T.; Li, G.; Li, K. Numerical simulation investigation on aerodynamic characteristics of rotor in plateau environment. Aerosp. Sci. Technol. 2024, 155, 109628. [Google Scholar] [CrossRef]
- Hu, H.; Hu, Y.; Kong, W.; Tao, Y.; Jiang, Q.; Wang, J.; Li, C.; Xie, H.; Shi, Y.; Li, Y.; et al. The photocatalytic mineralization of phenolic wastewater via self-generation and -activation of H2O2 technology. J. Environ. Chem. Eng. 2023, 11, 111108. [Google Scholar] [CrossRef]
- Hussain, S.; Dong, H.; Zeng, S.; Ahmad, M.U.; Shehzad, F.K.; Wu, H.; Zhang, Y. Investigation uncovered the impact of anions on CO2 absorption by low viscous ether functionalized pyridinium ionic liquids. J. Mol. Liq. 2021, 336, 116362. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Fu, J.; Miao, C.; Jia, S.; Yan, P.; Huang, J. Highly selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan over metal-oxide supported Pt catalysts: The role of basic sites. Appl. Catal. A Gen. 2022, 643, 118762. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Y.; Chen, H.; Tang, Y.; Li, Y. The application of graphitic nitrogen from corn stover for the selective catalytic oxidation of 5-hydroxymethyl furfural. Catal. Today 2025, 446, 115113. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Peng, M.; Zhang, Q.; Liu, J.; Zhang, J.; Fu, Y.; Li, W. Highly selective electrocatalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-dihydroxymethylfuran over AgCu nanoalloys. Int. J. Hydrogen Energy 2022, 47, 28904–28914. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Ma, L.; Zhang, Q.; Wang, C.; Chen, Y. Controllable production of guaiacols and phenols from lignin depolymerization using Pd/C catalyst cooperated with metal chloride. Chem. Eng. J. 2018, 338, 457–464. [Google Scholar] [CrossRef]
- Movahed, S.K.; Jafari, P.; Mallakpour, S. Ruthenium nickel bimetallic nanoparticles embedded in nitrogen-doped carbon mesoporous spheres as a superior catalyst for the hydrogenation of toxic nitroarenes. J. Environ. Chem. Eng. 2023, 11, 110426. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Sun, H.; Xu, R.; Jia, X.; Liu, Z.; Chen, H.; Lu, T. Recent advances in the photocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Biomass Convers. Biorefin. 2023, 14, 26707–26724. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Yu, L.; Wang, S.; Ma, X.; Wang, C.; Li, Y. Hydroxyapatite Supported Manganese Oxide as a Heterogeneous Catalyst for the Synthesis of 2, 5-Diformylfuran. Catal. Lett. 2022, 152, 3716–3724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).