Plasma–Liquid Synthesis of Titanium- and Molybdenum-Containing MXenes and Their Photocatalytic Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Process of Plasma–Liquid Synthesis of MXenes
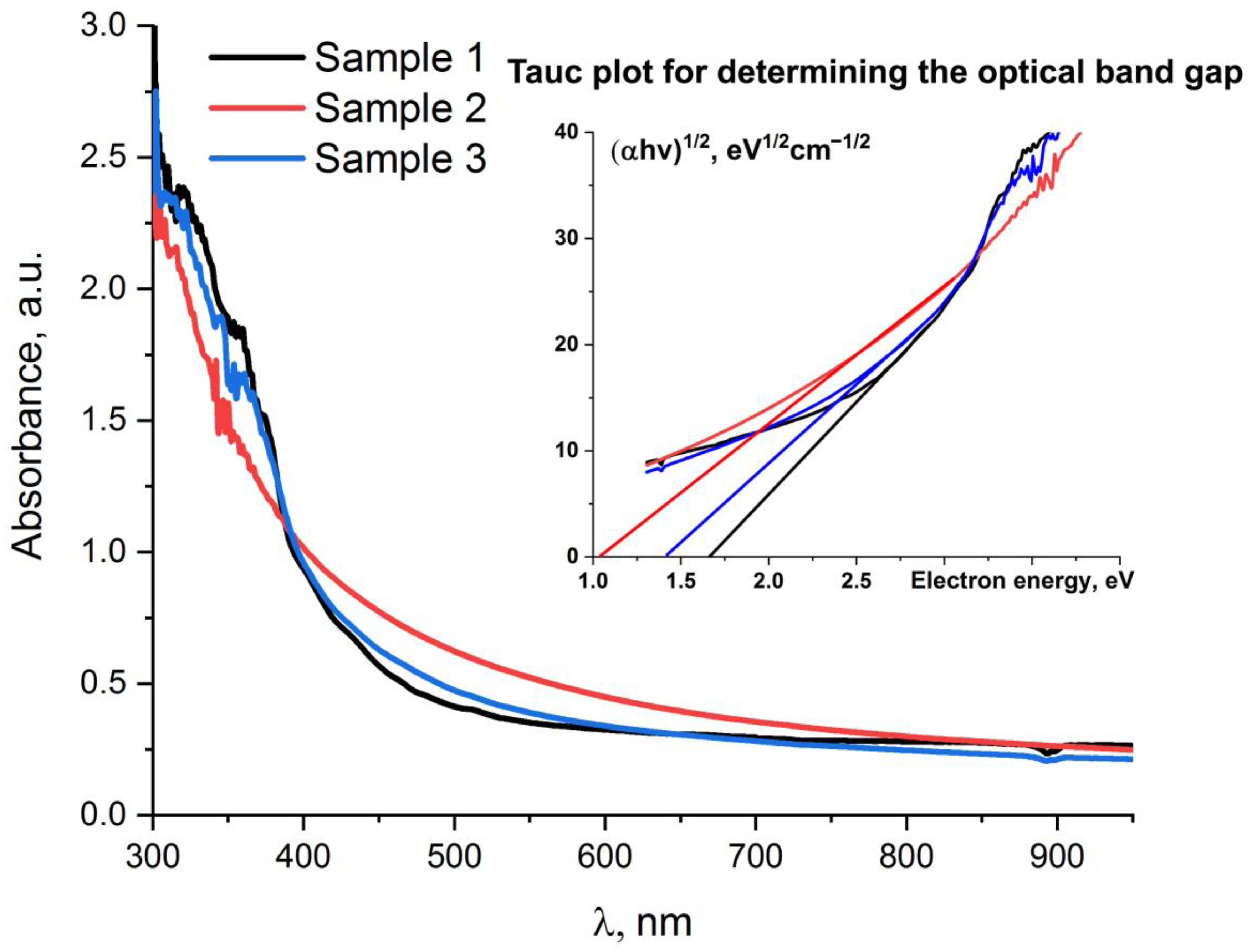
2.2. UV-Vis Spectra of Synthesized MXenes
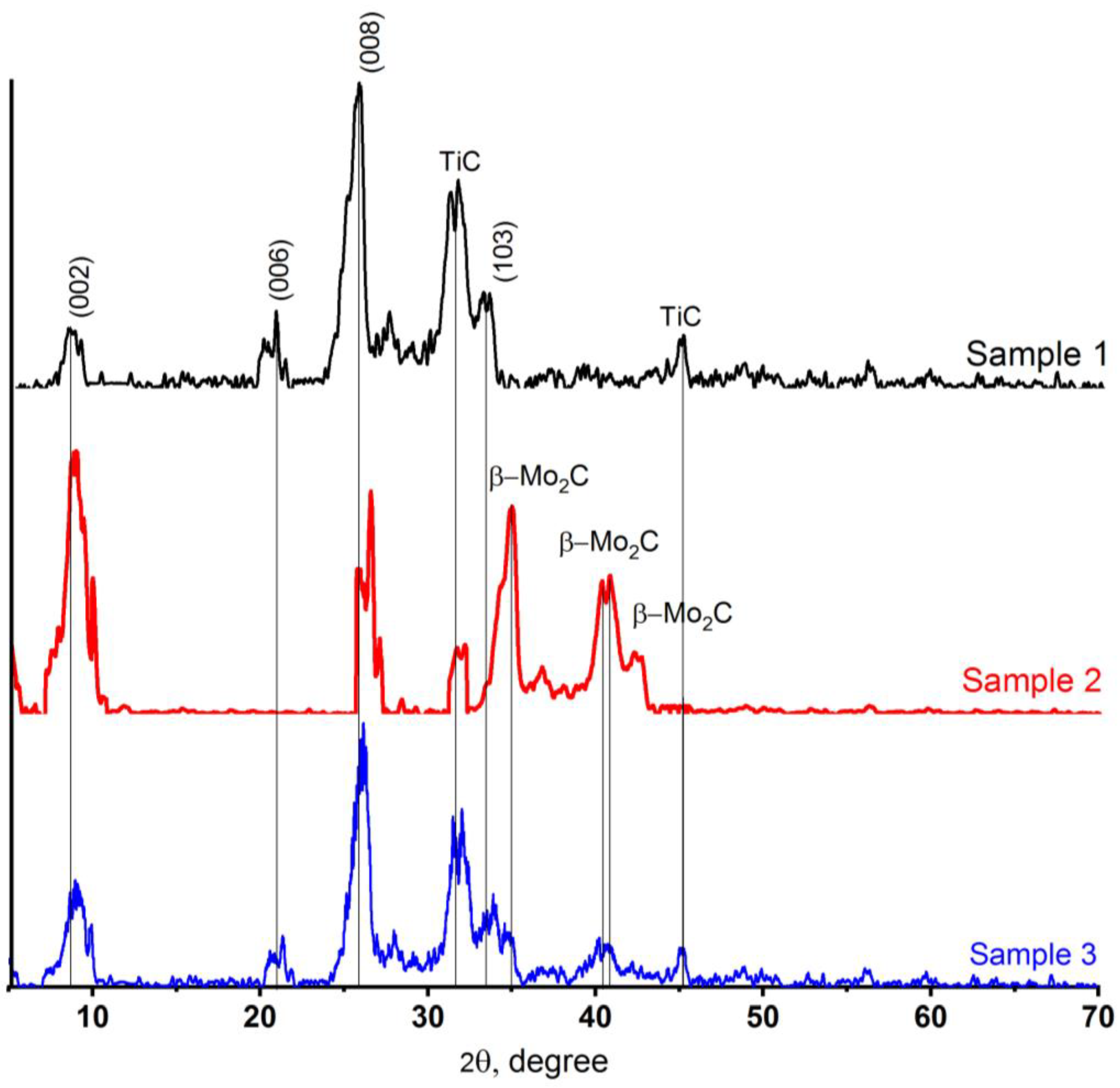
2.3. XRD Patterns of Synthesized MXenes
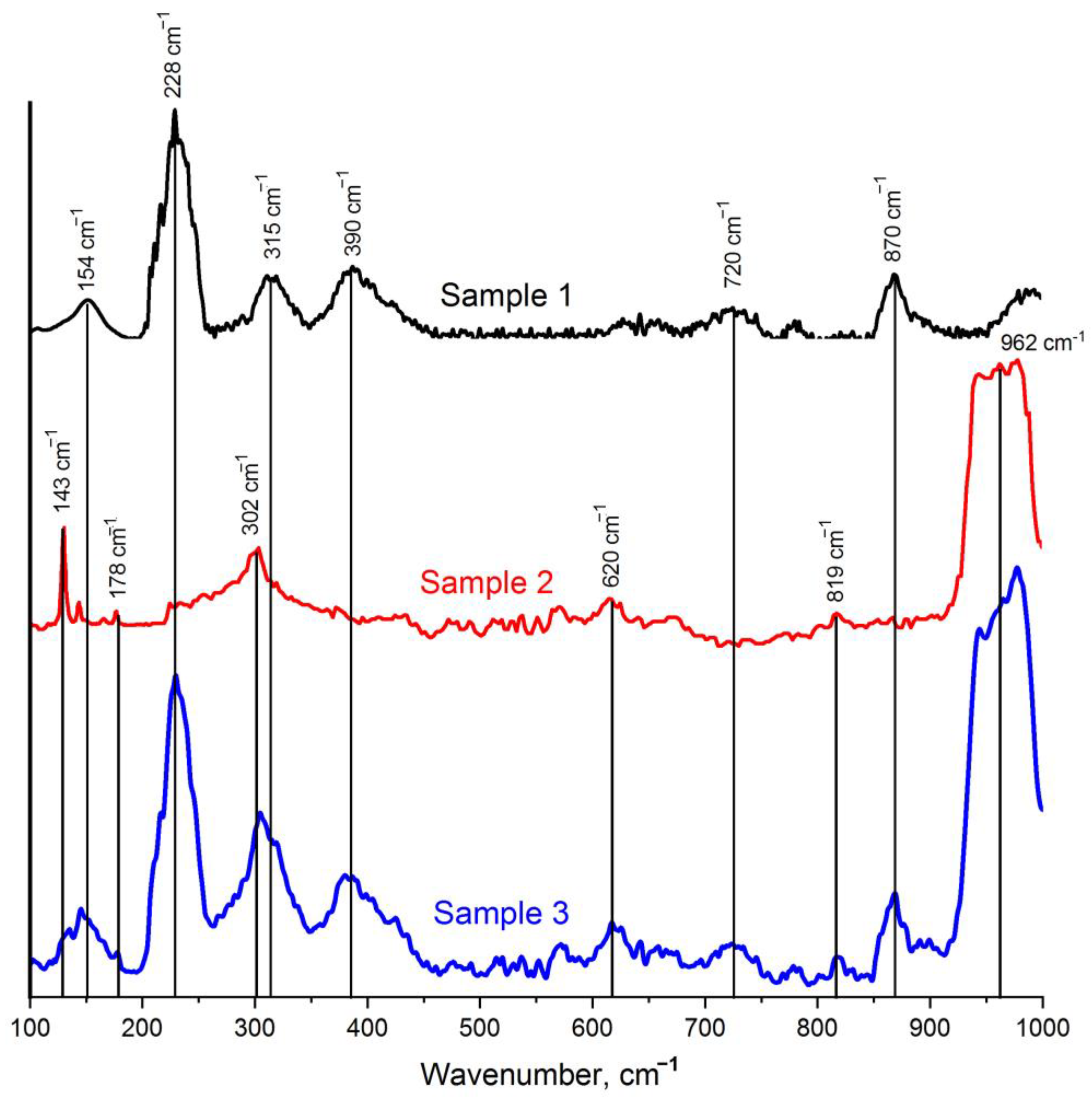
2.4. Raman Studies of Synthesized MXenes
2.5. Microscopic Studies of Samples
2.6. Photocatalytic Activity of Samples
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals: Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2 (Adv. Mater. 37/2011). Adv. Mater. 2011, 23, 4207. [Google Scholar] [CrossRef]
- Akhter, R.; Maktedar, S.S. MXenes: A comprehensive review of synthesis, properties, and progress in supercapacitor applications. J. Materiomics 2023, 9, 1196–1241. [Google Scholar] [CrossRef]
- Sun, S.; Liao, C.; Hafez, A.M.; Zhu, H.; Wu, S. Two-Dimensional MXenes for Energy Storage. Chem. Eng. J. 2018, 338, 27–45. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for Sensing Applications: RecentProgress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Liu, J.; Zheng, J.; Feng, X.; Liu, L.; Fang, Y. Photocatalytic water splitting from MXene and substoichiometric molybdenum oxide ultrathin nanowire Schottky heterojunction. Int. J. Hydrogen Energy 2023, 48, 34272–34285. [Google Scholar] [CrossRef]
- Swapnalin, J.; Koneru, B.; Pothu, R.; Banerjee, P.; Boddula, R.; Radwan, A.B.; Al-Qahtani, N. Surface modification of Ti3C2Tx using terminal groups and heteroatoms with excellent electrochemical performance in supercapacitors. Appl. Phys. Lett. 2023, 122, 161902. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tuan Nguyen, D.M.; Tran, D.L.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q. Van MXenes: Applications in Electrocatalytic, Photocatalytic Hydrogen Evolution Reaction and CO2 Reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Zhang, D.; Wang, S.; Qiu, M.; Song, G.; Fu, D.; Hu, B.; Wang, X. MXenes as Emerging Nanomaterials in Water Purification and Environmental Remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. MXenes: Trends, Growth, and Future Directions. Graphene 2D Mater. 2022, 7, 75–79. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, G.; Zuo, X.; Tang, H.; Yang, Q.; Li, G. Computational Studies on the Structural, Electronic and Optical Properties of Graphene-like MXenes (M2CT2, M = Ti, Zr, Hf; T = O, F, OH) and Their Potential Applications as Visible-Light Driven Photocatalysts. J. Mater. Chem. A 2016, 4, 12913–12920. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2Tx) in Photocatalysis: A Review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Qu, J.; Teng, D.; Zhang, X.; Yang, Q.; Li, P.; Cao, Y. Preparation and Regulation of Two-Dimensional Ti3C2Tx MXene for Enhanced Adsorption–Photocatalytic Degradation of Organic Dyes in Wastewater. Ceram. Int. 2022, 48, 14451–14459. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.-N.; Park, C.M.; Jang, M.; Taheri-Qazvini, N.; Yoon, Y. Effect of Single and Multilayered Ti3C2TX MXene as a Catalyst and Adsorbent on Enhanced Sonodegradation of Diclofenac and Verapamil. J. Hazard. Mater. 2022, 426, 128120. [Google Scholar] [CrossRef] [PubMed]
- Praus, P.; Smýkalová, A.; Škuta, R.; Koštejn, M.; Pavlovský, J.; Tokarský, J.; Foniok, K.; Filip Edelmannová, M.; Kočí, K. Graphitic C3N4 and Ti3C2 Nanocomposites for the Enhanced Photocatalytic Degradation of Organic Compounds and the Evolution of Hydrogen under Visible Irradiation. J. Photochem. Photobiol. A Chem. 2024, 447, 115260. [Google Scholar] [CrossRef]
- Downes, M.; Shuck, C.E.; McBride, B.; Busa, J.; Gogotsi, Y. Comprehensive Synthesis of Ti3C2Tx from MAX Phase to MXene. Nat. Protoc. 2024, 19, 1807–1834. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of Two-Dimensional Ti3C2Tx MXene Using HCl+LiF Etchant: Enhanced Exfoliation and Delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Numan, A.; Rafique, S.; Khalid, M.; Zaharin, H.A.; Radwan, A.; Mokri, N.A.; Ching, O.P.; Walvekar, R. Microwave-Assisted Rapid MAX Phase Etching and Delamination: A Paradigm Shift in MXene Synthesis. Mater. Chem. Phys. 2022, 288, 126429. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Sirotkin, N.A.; Titov, V.A.; Khlyustova, A.V. Low-Temperature Underwater Plasma as an Instrument to Manufacture Inorganic Nanomaterials. Russ. J. Inorg. Chem. 2022, 67, 253–261. [Google Scholar] [CrossRef]
- Indarto, A.; Yang, D.R.; Choi, J.-W.; Lee, H.; Song, H.K. CCl4 decomposition by gliding arc plasma: Role of C2 compounds on products distribution. Chem. Eng. Commun. 2007, 194, 1111–1125. [Google Scholar] [CrossRef]
- Batukaev, T.; Bilera, I.; Krashevskaya, G.; Lebedev, Y. Physical and Chemical Phenomena during the Production of Hydrogen in the Microwave Discharge Generated in Liquid Hydrocarbons with the Barbotage of Various Gases. Processes 2023, 11, 2292. [Google Scholar] [CrossRef]
- Sirotkin, N.; Khlyustova, A.; Agafonov, A. Plasma-Liquid Synthesis as a New Method for the Production of MXenes. Plasma Chem. Plasma Process. 2024, 44, 1853–1866. [Google Scholar] [CrossRef]
- Sirotkin, N.A.; Khlyustova, A.V.; Titov, V.A.; Krayev, A.S.; Nikitin, D.I.; Dmitrieva, O.A.; Agafonov, A.V. Synthesis and Photocatalytic Activity of WO3 Nanoparticles Prepared by Underwater Impulse Discharge. Plasma Chem. Plasma Process. 2020, 40, 571–587. [Google Scholar] [CrossRef]
- Sirotkin, N.; Khlyustova, A.; Titov, V.; Agafonov, A. Plasma-assisted Synthesis and Deposition of Molybdenum Oxide Nanoparticles on Polyethylene Terephthalate for Photocatalytic Degradation of Rhodamine B. Plasma Process. Polym. 2020, 17. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Meiling, T.T.; Cywiński, P.J.; Löhmannsröben, H.-G. Two-Photon Excitation Fluorescence Spectroscopy of Quantum Dots: Photophysical Properties and Application in Bioassays. J. Phys. Chem. C 2018, 122, 9641–9647. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Ghorbani-Asl, M.; Arai, M.; Sasaki, T.; Liang, Y.; Yunoki, S. Nearly Free Electron States in MXenes. Phys. Rev. B 2016, 93, 205125. [Google Scholar] [CrossRef]
- Roy, C.; De, S.K.; Banerjee, P.; Pradhan, S.; Bhattacharyya, S. Investigating Suitable Medium for the Long-Duration Storage of Ti2CTx MXene. J. Alloys Compd. 2023, 938, 168471. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, S.; Wang, L.; He, P.; Hu, Q.; Fan, L.-Z.; Zhou, A. Synthesis of Two-Dimensional Carbide Mo2CTx MXene by Hydrothermal Etching with Fluorides and Its Thermal Stability. Ceram. Int. 2020, 46, 19550–19556. [Google Scholar] [CrossRef]
- Hu, T.; Wang, J.; Zhang, H.; Li, Z.; Hu, M.; Wang, X. Vibrational Properties of Ti3C2 and Ti3C2T2 (T = O, F, OH) Monosheets by First-Principles Calculations: A Comparative Study. Phys. Chem. Chem. Phys. 2015, 17, 9997–10003. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, Q.; Liu, Z.; Shen, D.; Wang, T.; Huang, Q.; Du, S.; Jiang, N.; Lin, C.-T.; Yu, J. Enhanced Thermal Properties of Poly(Vinylidene Fluoride) Composites with Ultrathin Nanosheets of MXene. RSC Adv. 2017, 7, 20494–20501. [Google Scholar] [CrossRef]
- Bayhan, Z.; El-Demellawi, J.K.; Yin, J.; Khan, Y.; Lei, Y.; Alhajji, E.; Wang, Q.; Hedhili, M.N.; Alshareef, H.N. A Laser-Induced Mo2CTx MXene Hybrid Anode for High-Performance Li-Ion Batteries. Small 2023, 19, e2208253. [Google Scholar] [CrossRef]
- Sheng, M.; Bin, X.; Xiao, J.; Que, W. Fluoride-Free Synthesis of Two-Dimensional Mo2TiC2 MXene by Solvothermal Reaction. Mater. Lett. 2024, 371, 136936. [Google Scholar] [CrossRef]
- Tang, M.; Li, J.; Wang, Y.; Han, W.; Xu, S.; Lu, M.; Zhang, W.; Li, H. Surface Terminations of MXene: Synthesis, Characterization, and Properties. Symmetry 2022, 14, 2232. [Google Scholar] [CrossRef]
- Yu, C.; Wang, S.; Zhang, K.; Li, M.; Gao, H.; Zhang, J.; Yang, H.; Hu, L.; Jagadeesha, A.V.; Li, D. Visible-Light-Enhanced Photocatalytic Activity of BaTiO3/γ-Al2O3 Composite Photocatalysts for Photodegradation of Tetracycline Hydrochloride. Opt. Mater. 2023, 135, 113364. [Google Scholar] [CrossRef]
- Bury, D.; Jakubczak, M.; Purbayanto, M.A.K.; Wojciechowska, A.; Moszczyńska, D.; Jastrzębska, A.M. Photocatalytic activity of the oxidation stabilized Ti3C2Tx MXene in decomposing methylene blue, bromocresol green and commercial textile dye. Small Methods 2023, 7, 2201252. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K.; Babu, R.R.; Sethuraman, K.; Babu, S.M. Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 2017, 418, 138–146. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Iqbal, M.Z.; Akinwande, D.; Rizwan, S. Ti3C2-MXene/bismuth ferrite nanohybrids for efficient degradation of organic dyes and colorless pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Cao, X. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Grzegórska, A.; Gajewicz-Skretna, A.; Trykowski, G.; Sikora, K.; Zielińska-Jurek, A. Design and synthesis of TiO2/Ti3C2 composites for highly efficient photocatalytic removal of acetaminophen: The relationships between synthesis parameters, physicochemical properties, and photocatalytic activity. Catal. Today 2023, 413, 113980. [Google Scholar] [CrossRef]
- Venkadajapathy, V.R.T.; Sivaramakrishnan, S. Enhancing bacterial control and daylight-driven water remediation with chitosan-impregnated MoC nanosheets. Environ. Sci. Pollut. Res. 2024, 31, 44938–44951. [Google Scholar] [CrossRef] [PubMed]


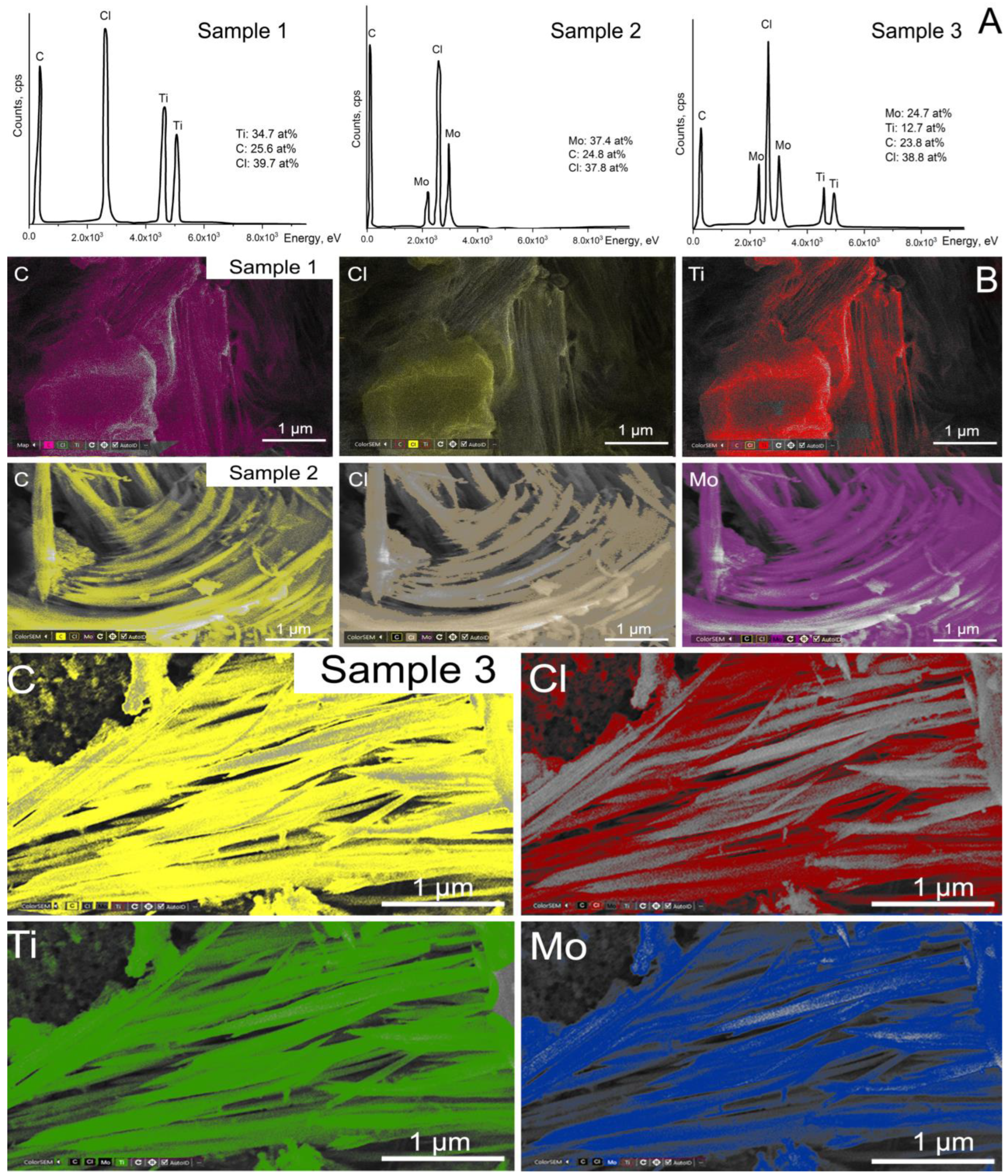
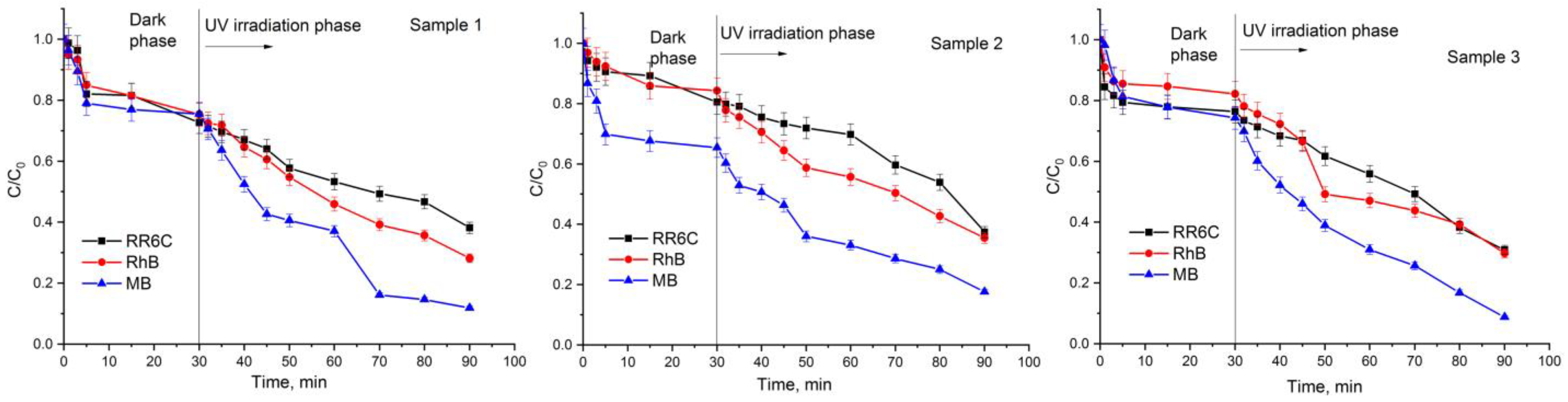
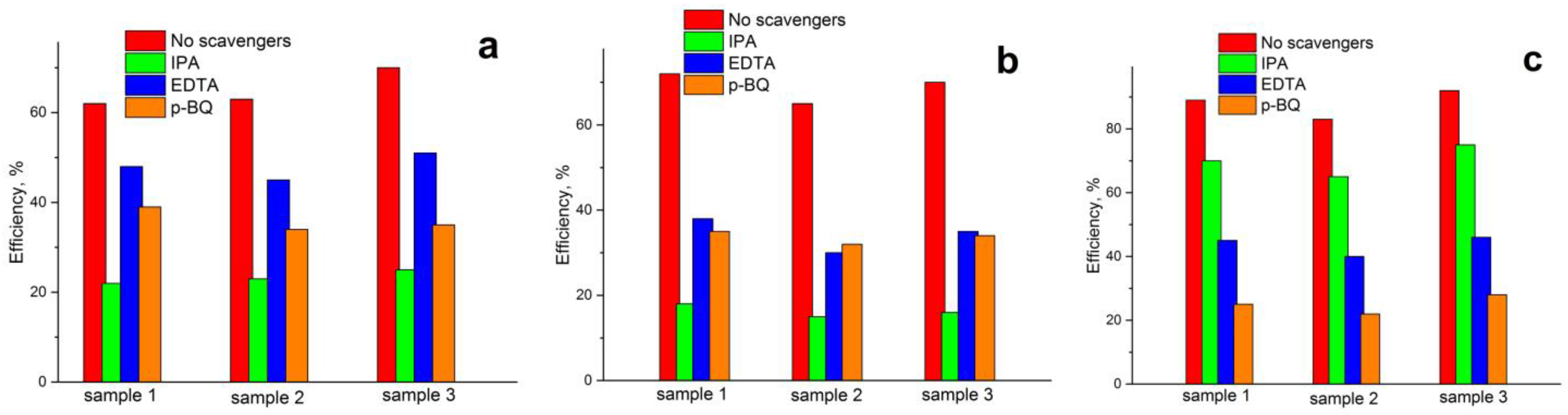
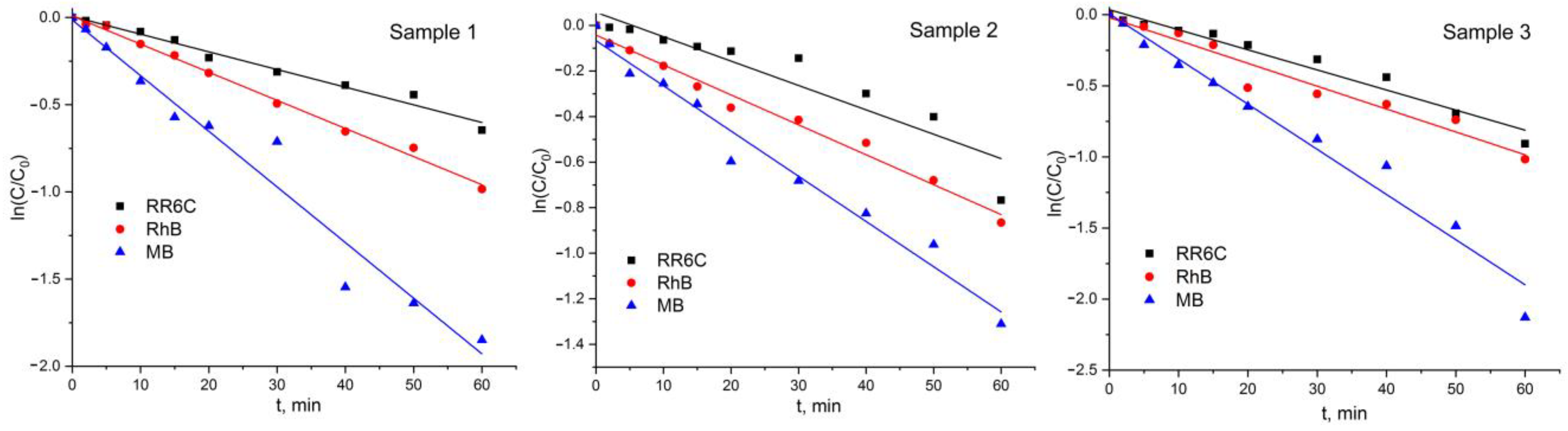

| Name | Experimental Conditions | Chemical Composition |
|---|---|---|
| Sample 1 | Underwater plasma in CCl4 at discharge current of 0.25 A; Ti—anode; Ti—cathode | Ti2CClx |
| Sample 2 | Underwater plasma in CCl4 at discharge current of 0.25 A; Mo—anode; Mo—cathode | Mo2CClx |
| Sample 3 | Underwater plasma in CCl4 at discharge current of 0.25 A; Ti—anode; Mo—cathode | Mo2TiC2Clx |
| Parameter | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| SBET, m2g−1 | 4.45 | 7.22 | 6.89 |
| SBJH, m2g−1 | 3.12 | 5.48 | 4.98 |
| Dp, nm | 12.04 | 13.14 | 12.97 |
| Vp, cm3g−1 | 0.027 | 0.036 | 0.031 |
| Photocatalytic Decomposition Efficiency, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | |||||||
| RR6C | RhB | MB | RR6C | RhB | MB | RR6C | RhB | MB | |
| Sample 1 | 61.8 | 71.8 | 88.1 | 56.2 | 68.2 | 84.6 | 55.1 | 66.2 | 82.0 |
| Sample 2 | 62.6 | 64.5 | 82.4 | 57.6 | 59.3 | 75.0 | 54.7 | 55.8 | 72.0 |
| Sample 3 | 69.1 | 70.2 | 97.4 | 65.6 | 67.4 | 93.5 | 63.0 | 64.7 | 90.7 |
| Dye | k, min−1 | knorm, min−1·m−2 | R2 |
|---|---|---|---|
| Sample 1 | |||
| RR6C | 0.01013 ± 4.8·10−4 | 0.07588 ± 0.00359 | 0.98 |
| RhB | 0.01613 ± 3.9·10−4 | 0.12082 ± 0.00292 | 0.99 |
| MB | 0.03191 ± 0.00021 | 0.23902 ± 0.00149 | 0.96 |
| Sample 2 | |||
| RR6C | 0.01071 ± 0.0015 | 0.04944 ± 0.00692 | 0.86 |
| RhB | 0.01313 ± 5.8·10−4 | 0.06062 ± 0.00267 | 0.98 |
| MB | 0.01985 ± 0.0011 | 0.09164 ± 0.00507 | 0.97 |
| Sample 3 | |||
| RR6C | 0.01413 ± 9.8·10−4 | 0.06836 ± 0.00474 | 0.96 |
| RhB | 0.01609 ± 0.0012 | 0.07784 ± 0.00581 | 0.95 |
| MB | 0.03177 ± 0.0019 | 0.15371 ± 0.00918 | 0.97 |
| MXene | Synthesis Conditions | Eg, eV | SBET, m2/g | Photocatalysis Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Ti3C2Tx | Etching using HF-forming etchants (LiF and HCl) | - | 6.138 | Visible light, t = 120 min, Cdye = 10 mg/L, mph = 30 mg | MB—81.2% RhB—17.3% MO—2.8% | [14] |
| Ti3C2Tx | MXene synthesized with HF/tetramethylammonium hydroxide | 1.05 | - | Visible light, t = 180 min, Cdye = 25 mg/L, mph = 3.2 mg | MB—100% BG—100% | [38] |
| Ti3C2Tx | Used the HCl/LiF method, known as minimally intensive layer delamination | 0.97 | - | Visible light, t = 180 min, Cdye = 25 mg/L, mph = 3.2 mg | MB—100% BG—100% | [38] |
| Ti3C2 | HF treatment | - | - | UV light, t = 60 min, Cdye = 10 mg/L, mph = 50 mg | MB—60% | [39] |
| Ti3C2 | Etching using HF | 2.01 | Visible light, t = 120 min, Cdye = 100 mg/L, mph = 100 mg | CR—80% | [40] | |
| Ti3C2 | Etching using HF | - | - | UV light, t = 45 min, Cdye = 20 mg/L, mph = 100 mg | MO—60% | [41] |
| Ti3C2 | Etching using HF | - | 0.37 | Solar light, t = 480 min, Cdye = 10 mg/L, mph = 100 mg | RhB—30% | [42] |
| Mo2CTx | Etching using HF-forming etchants (LiF and HCl) | -- | - | Visible light, t = 300 min, Cdye = 5 mg/L, mph = 25 mg | MB—75% | [43] |
| Ti3C2Tx | Plasma–liquid synthesis | 1.71 | 4.45 | UV light, t = 60 min, Cdye = 1.2 mg/L, mph = 30 mg | MB—89% RR6C—73% RhB −65% | This work |
| Mo2CTx | Plasma–liquid synthesis | 1.07 | 7.22 | MB—83% RR6C—66% RhB—65% | ||
| Mo2TiC2Clx | Plasma–liquid synthesis | 1.42 | 6.89 | MB—98% RR6C—69% RhB—66% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirotkin, N.; Khlyustova, A.; Shibaeva, V.; Agafonov, A. Plasma–Liquid Synthesis of Titanium- and Molybdenum-Containing MXenes and Their Photocatalytic Properties. Catalysts 2025, 15, 445. https://doi.org/10.3390/catal15050445
Sirotkin N, Khlyustova A, Shibaeva V, Agafonov A. Plasma–Liquid Synthesis of Titanium- and Molybdenum-Containing MXenes and Their Photocatalytic Properties. Catalysts. 2025; 15(5):445. https://doi.org/10.3390/catal15050445
Chicago/Turabian StyleSirotkin, Nikolay, Anna Khlyustova, Valeriya Shibaeva, and Alexander Agafonov. 2025. "Plasma–Liquid Synthesis of Titanium- and Molybdenum-Containing MXenes and Their Photocatalytic Properties" Catalysts 15, no. 5: 445. https://doi.org/10.3390/catal15050445
APA StyleSirotkin, N., Khlyustova, A., Shibaeva, V., & Agafonov, A. (2025). Plasma–Liquid Synthesis of Titanium- and Molybdenum-Containing MXenes and Their Photocatalytic Properties. Catalysts, 15(5), 445. https://doi.org/10.3390/catal15050445







