Rational Synthesis of a Dual Z-Scheme CdS/Ag2MoO4/β-Bi2O3 Heterojunction for the Deep Photodegradation of Methylene Blue and Analysis of Its Mechanisms
Abstract
1. Introduction
2. Results and Discussion
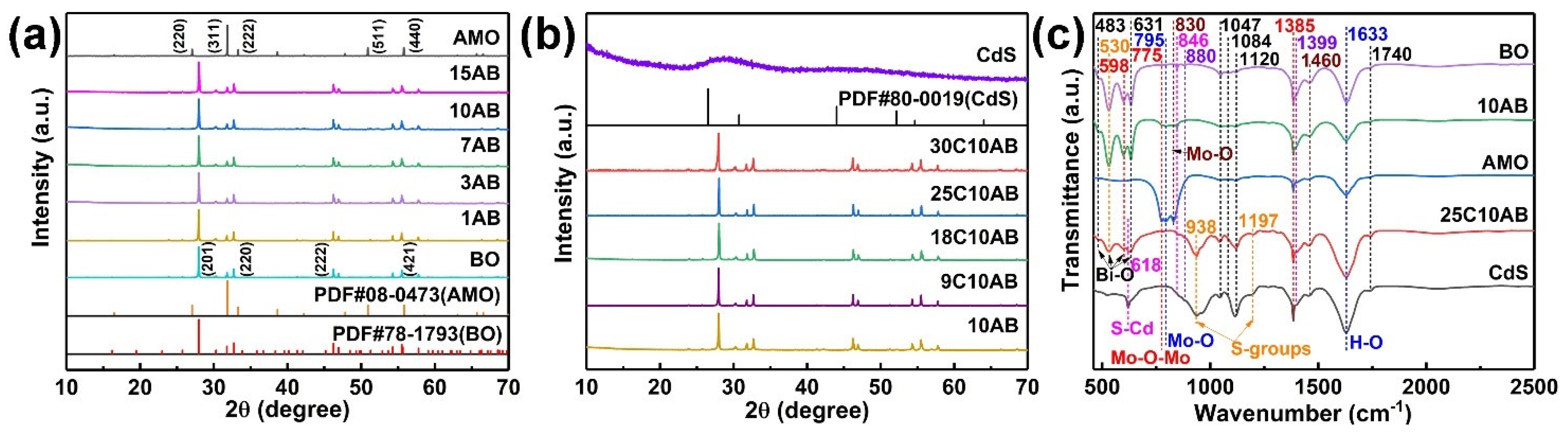
2.1. XRD and FTIR Analysis
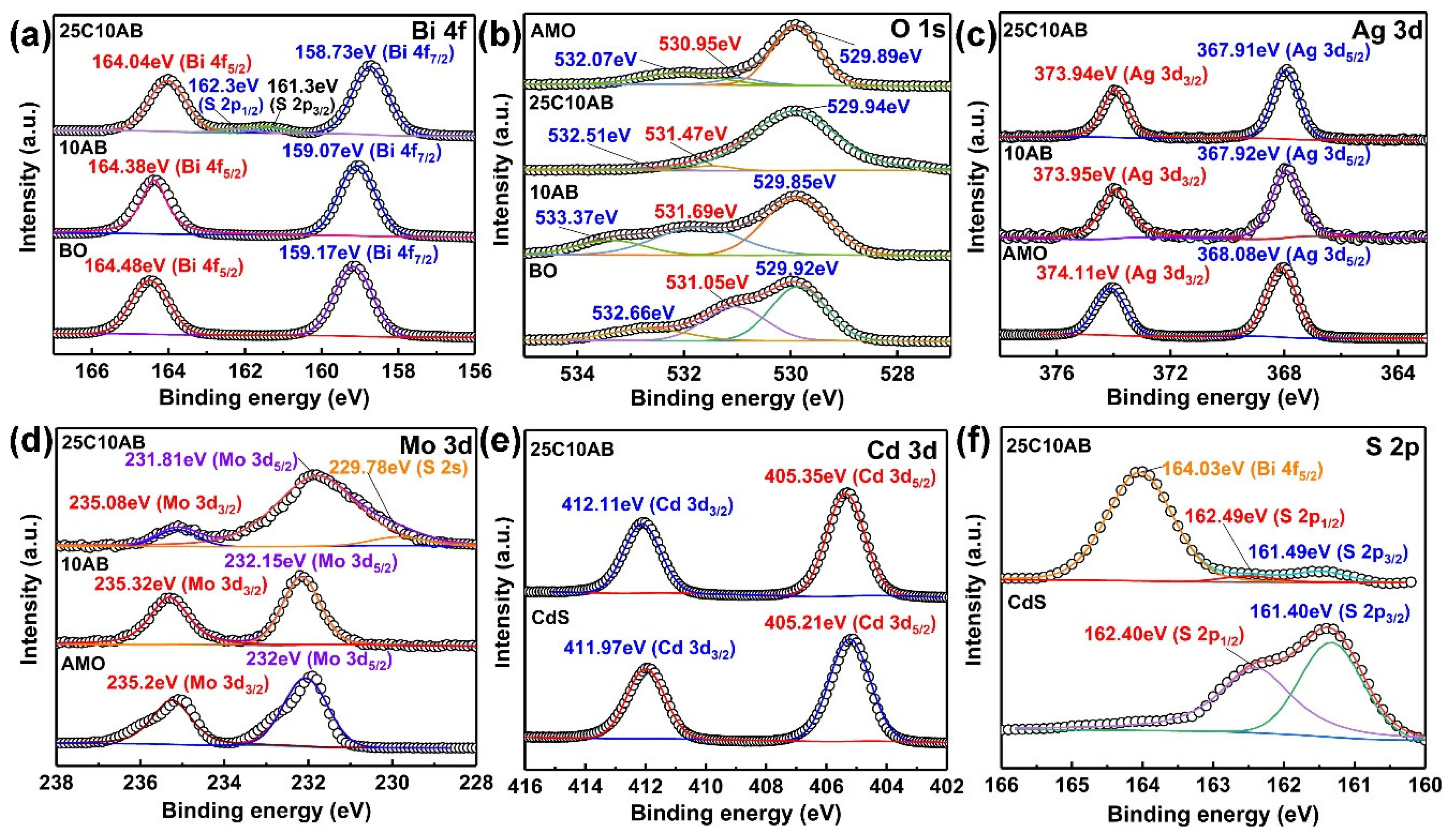
2.2. XPS Analysis
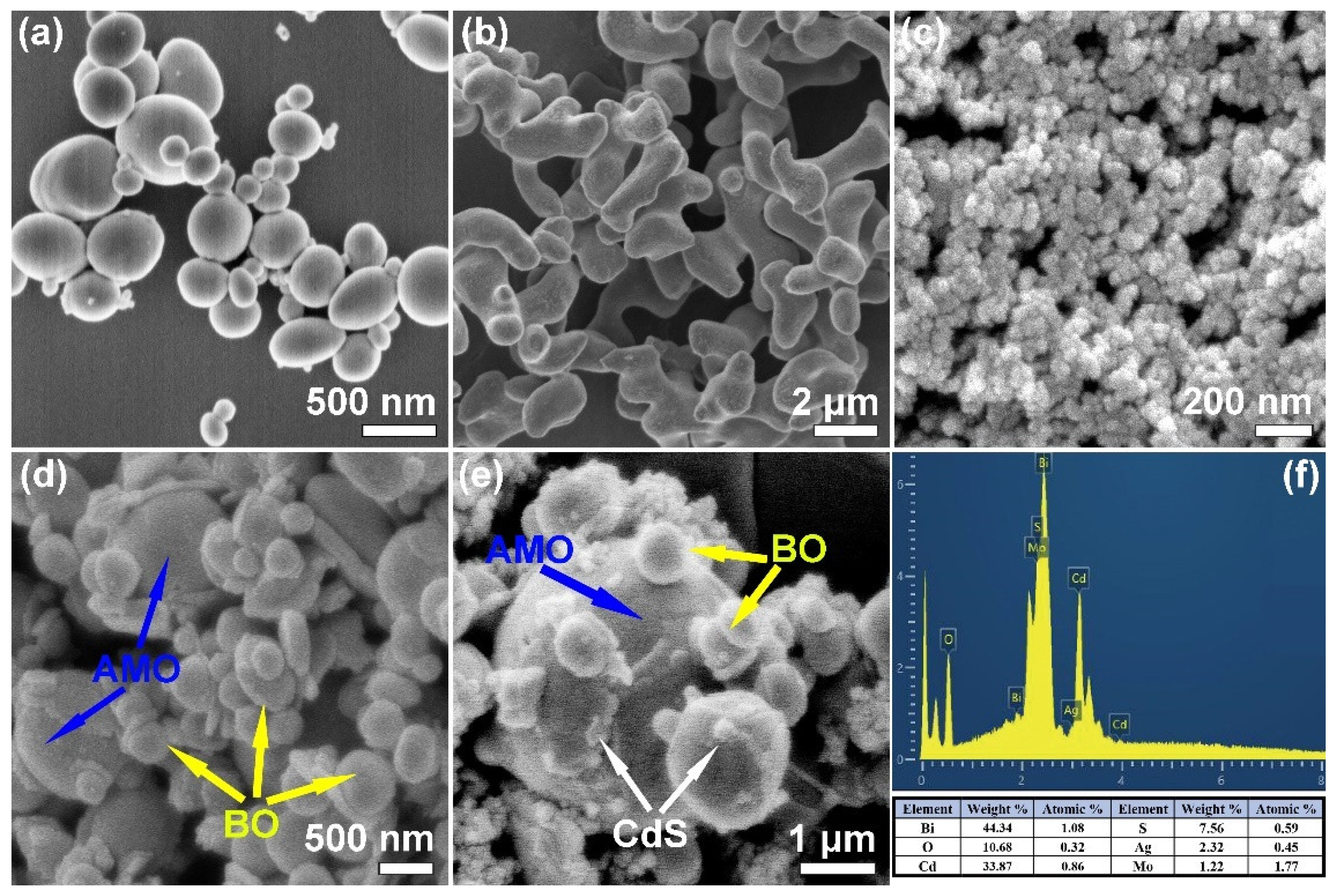
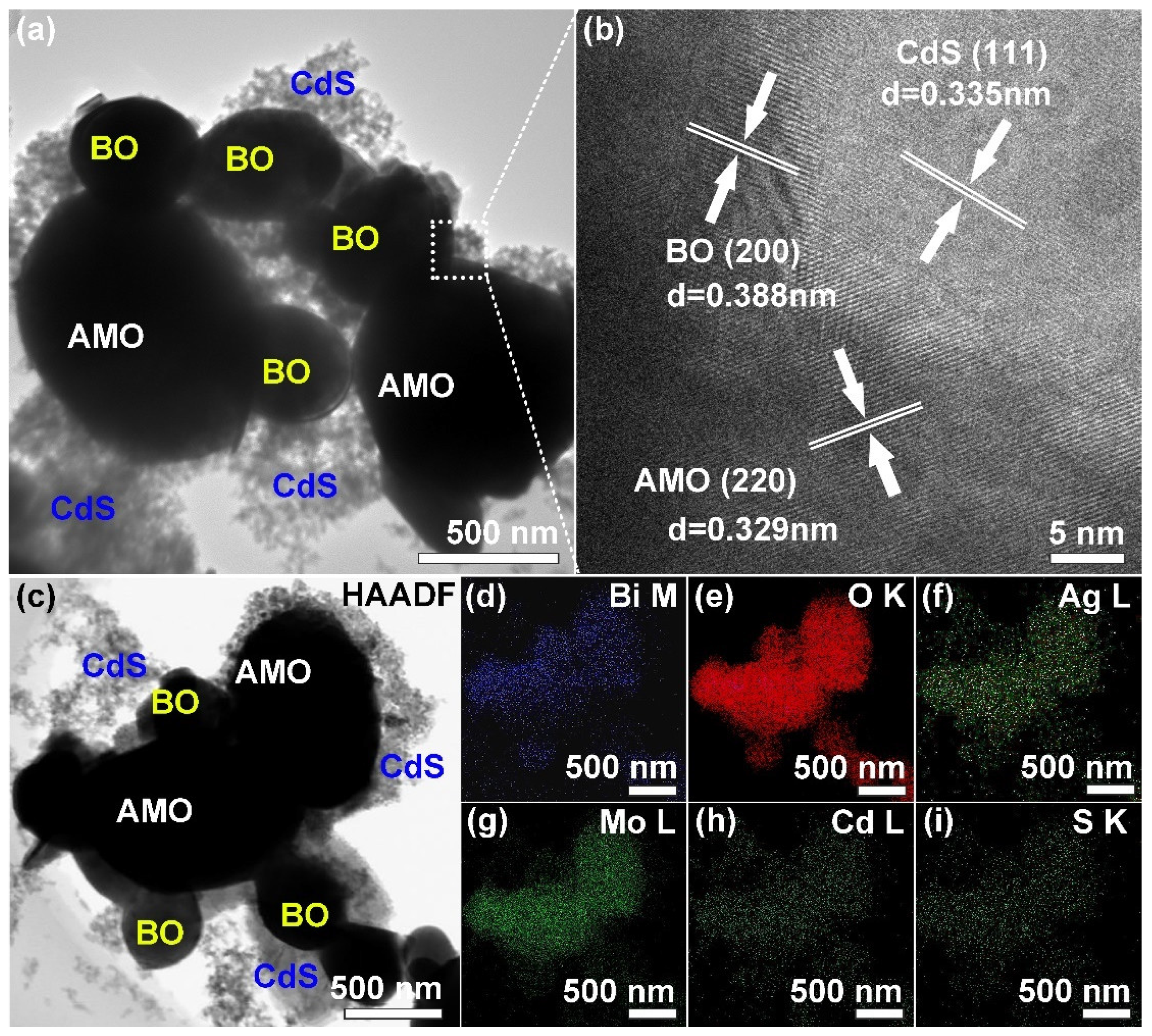
2.3. Morphology Observation
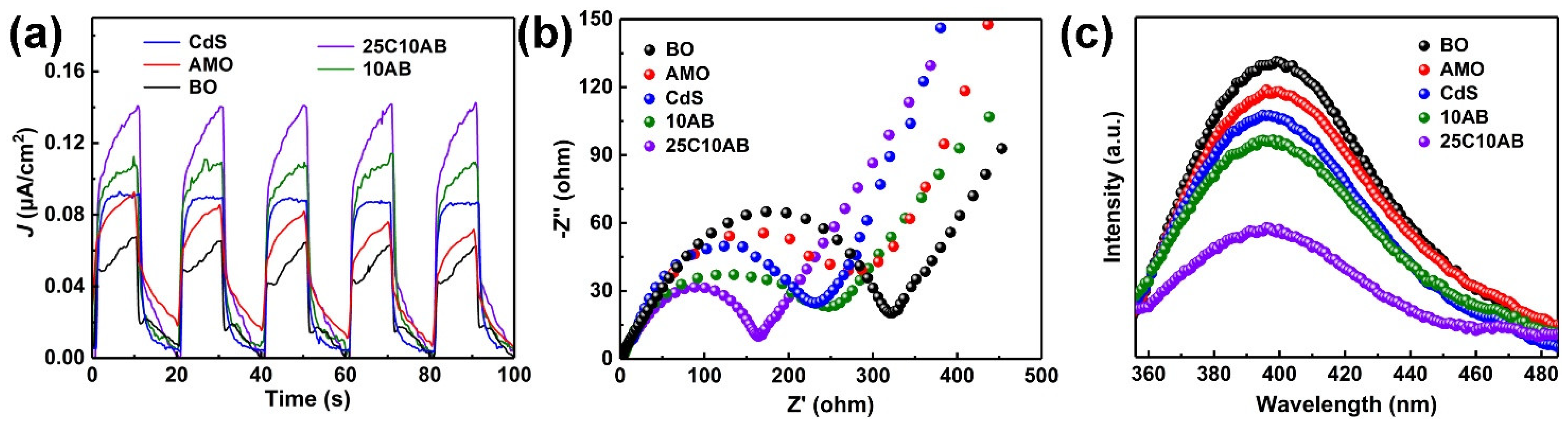
2.4. Photogenerated Charge Performance
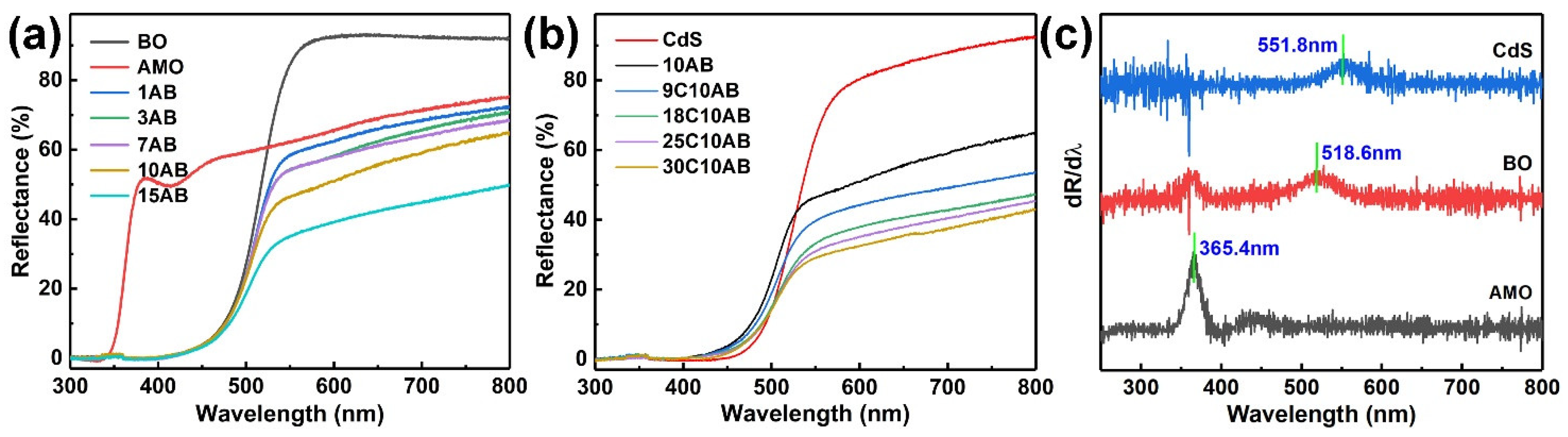
2.5. Optical Absorption Property
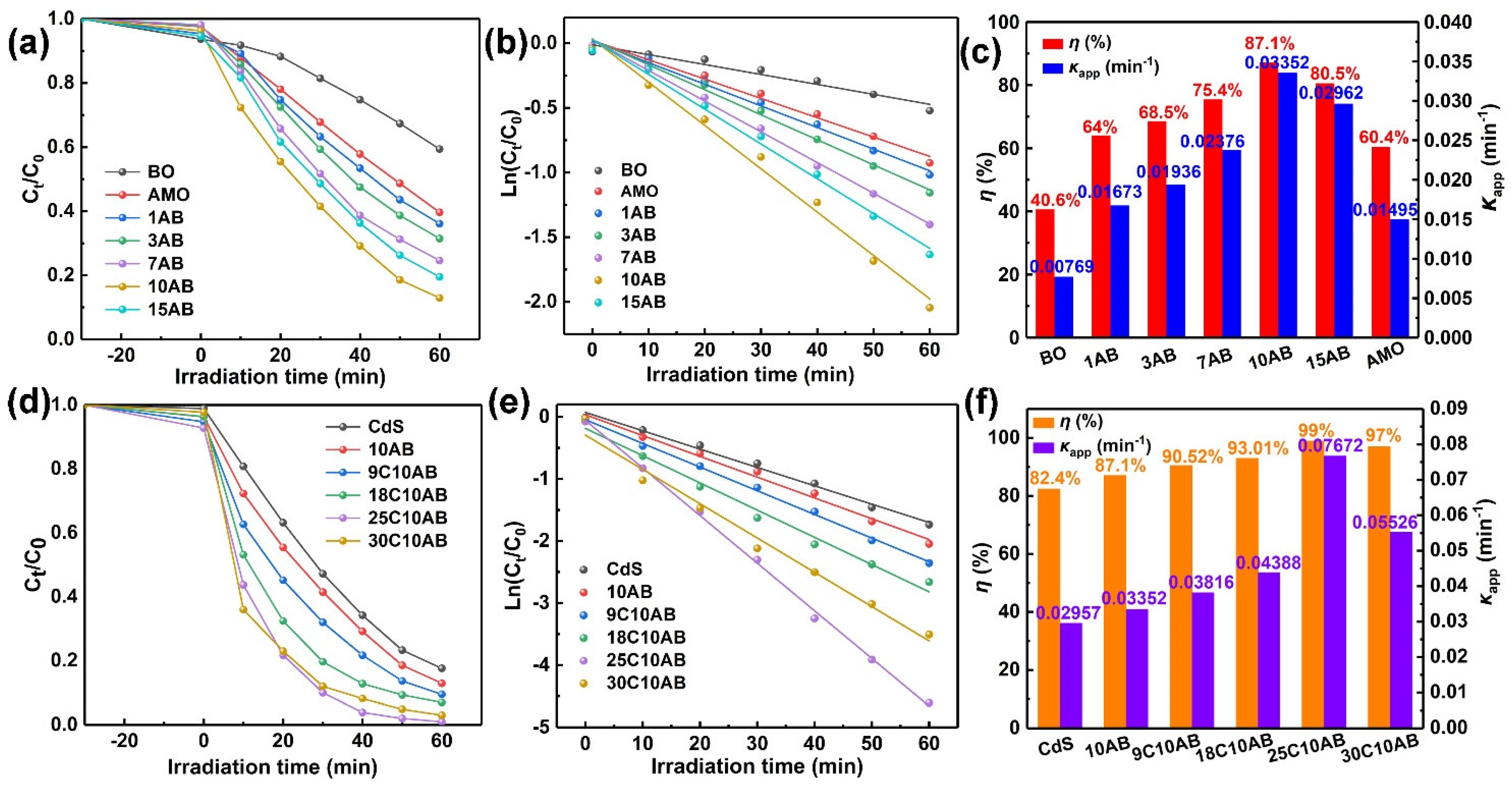
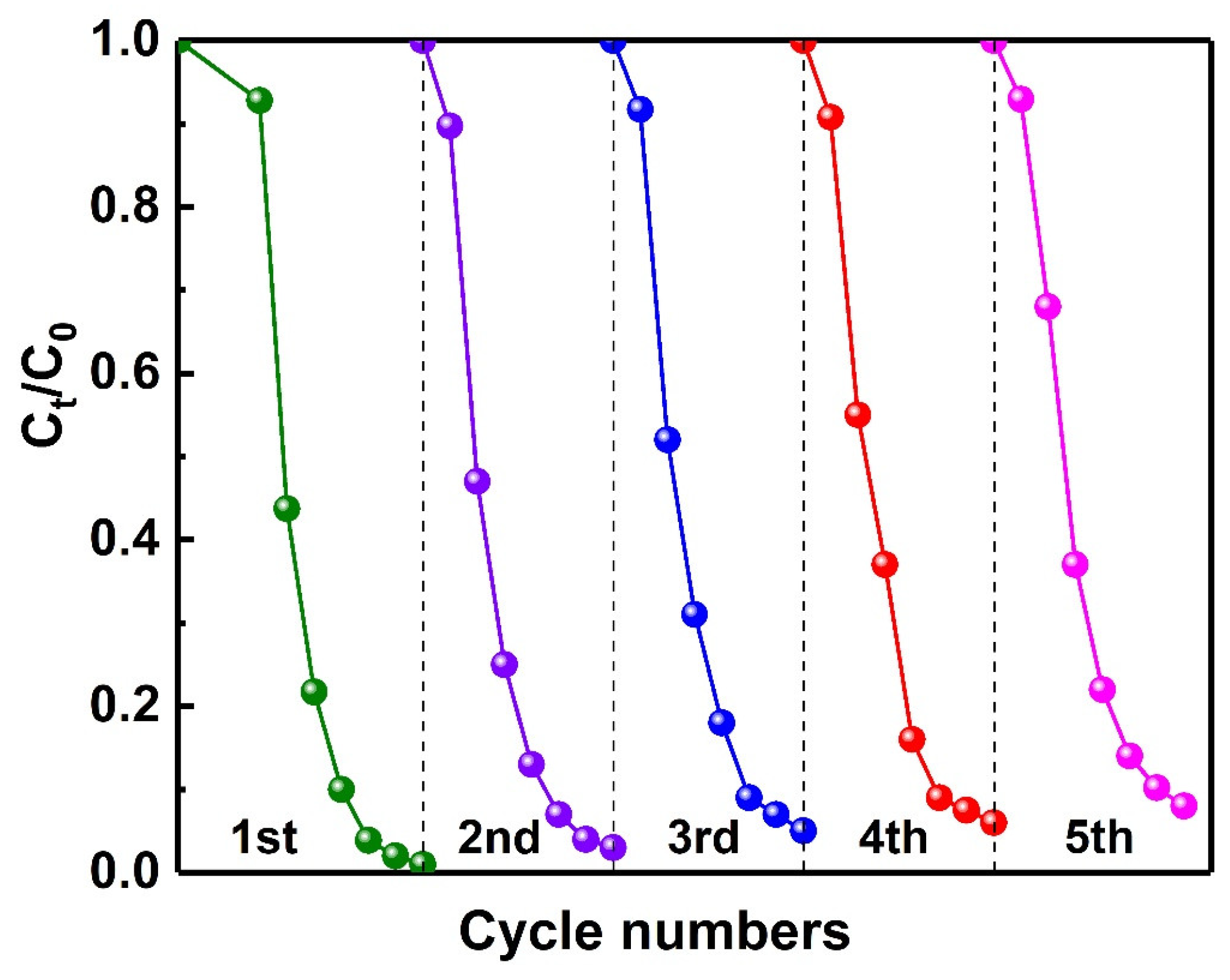
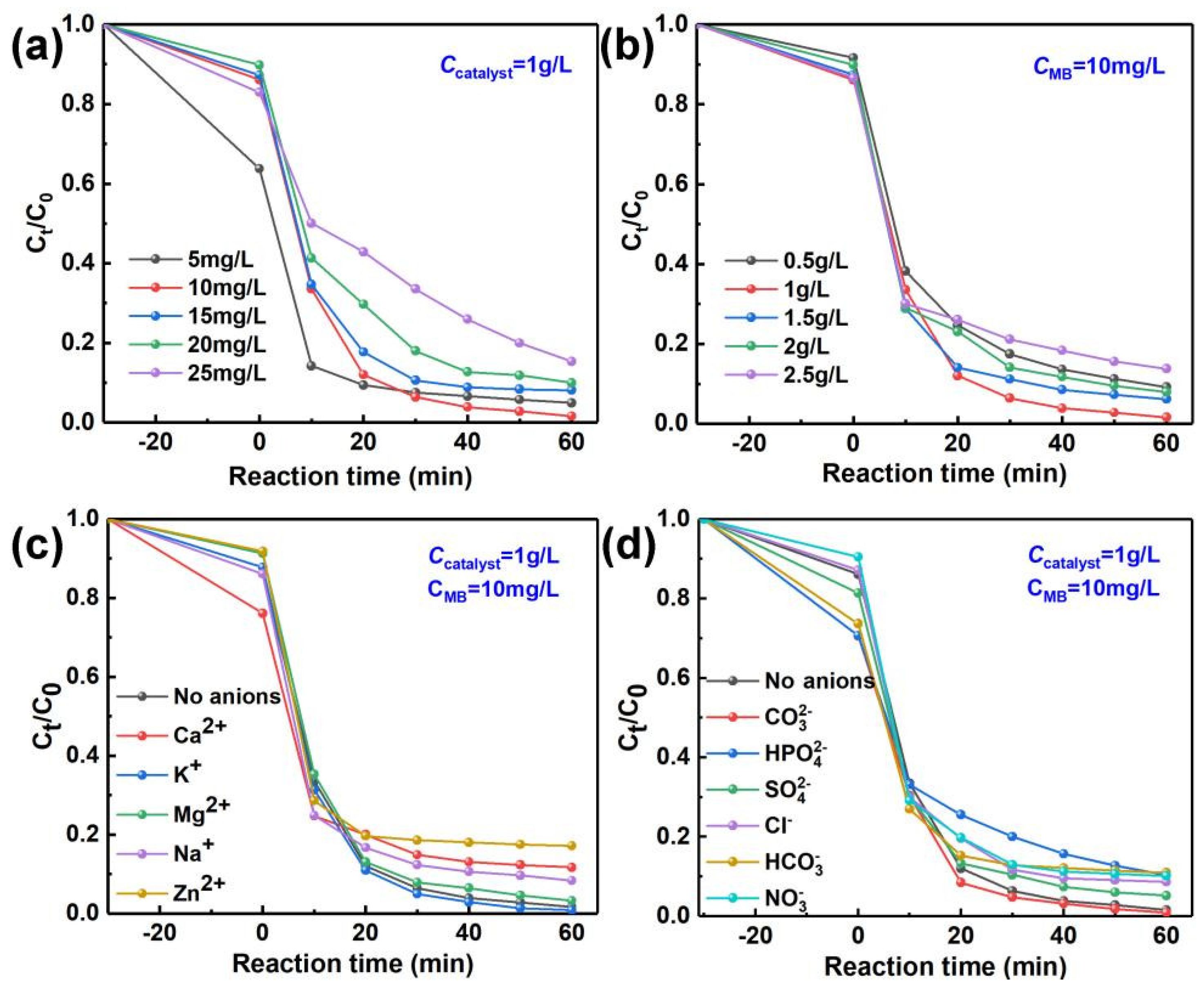
2.6. Photocatalytic Activity of Photocatalysts
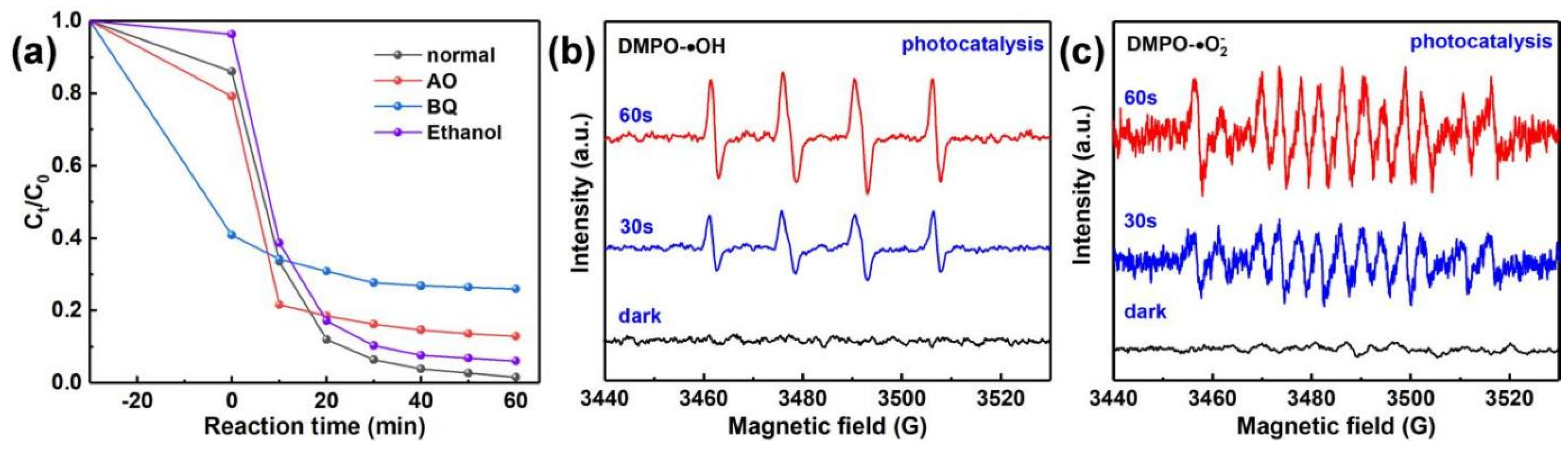
2.7. Capture Experiment and EPR Testing
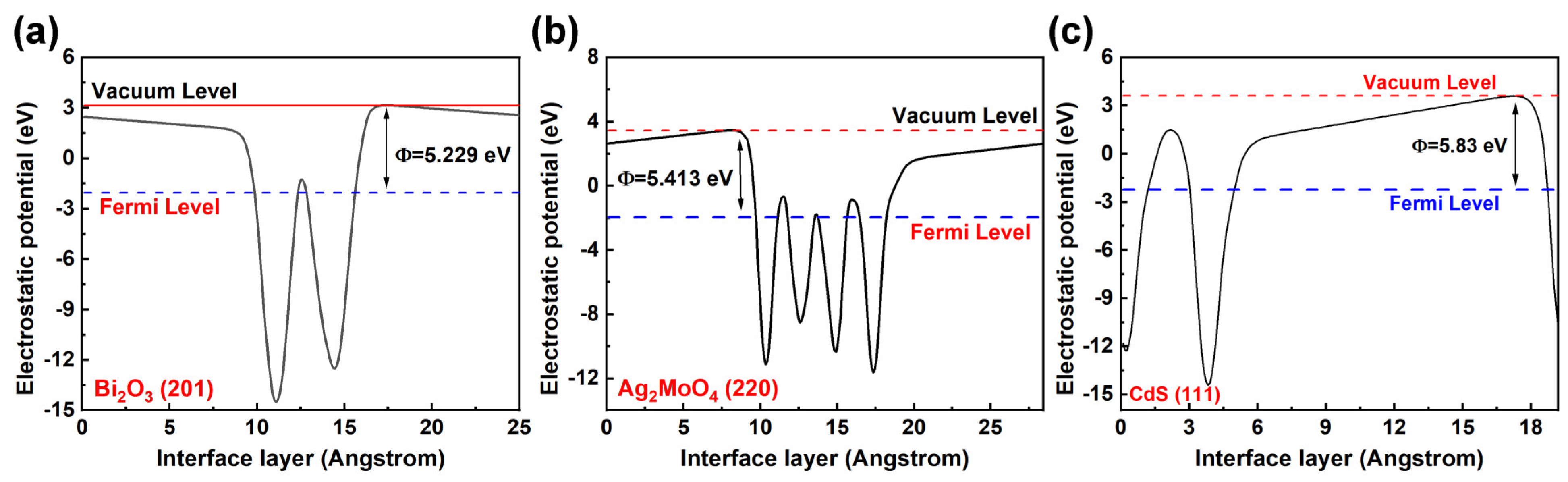
2.8. DFT Calculation
2.9. Photocatalytic Mechanism
3. Experimental Section
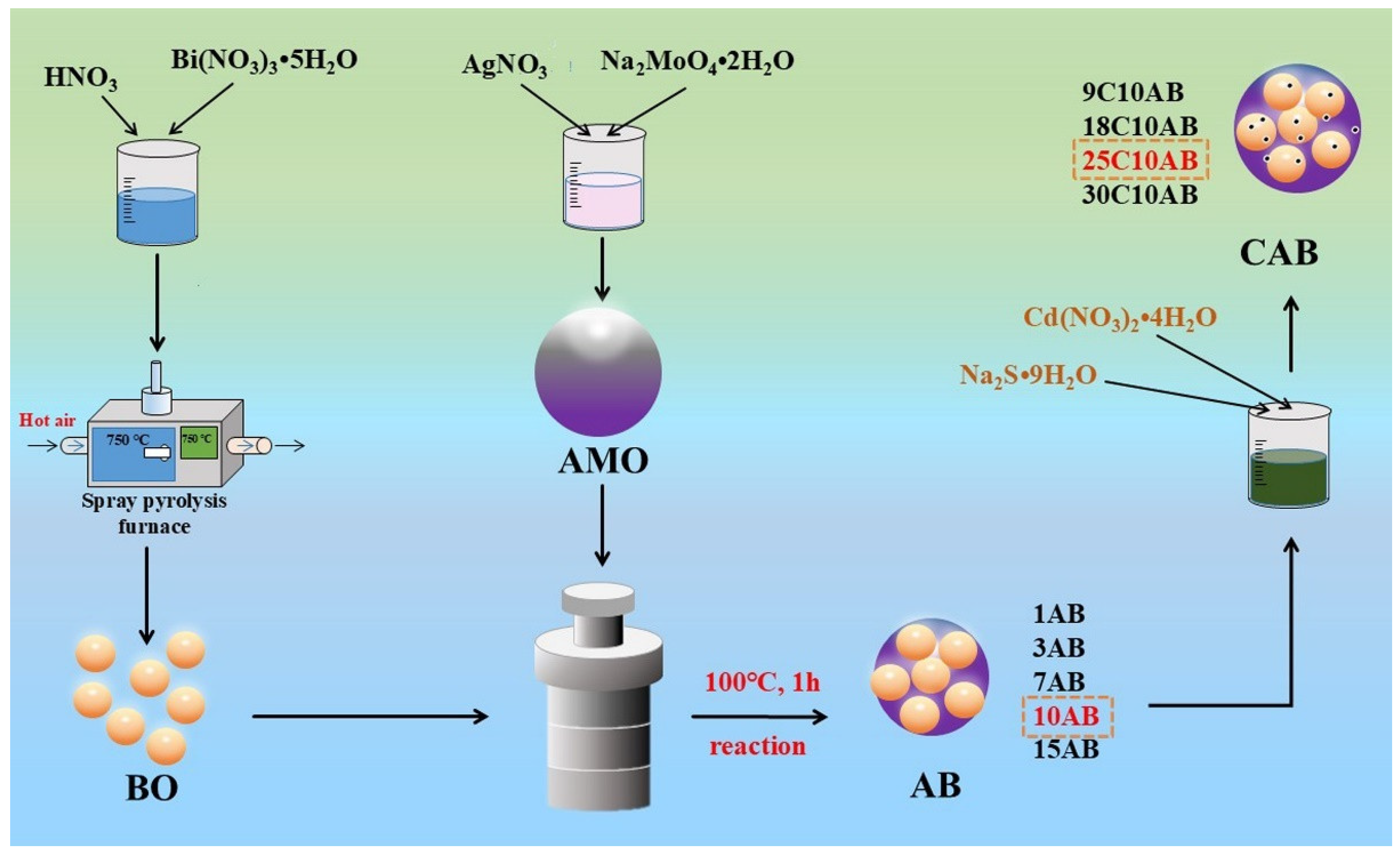
3.1. Fabrication of BO
3.2. Fabrication of AMO
3.3. Fabrication of AMO/BO (AB)
3.4. Fabrication of CdS/AMO/BO (CAB)
3.5. Characterizations
3.6. Electrochemical Testing
3.7. DFT Calculations
3.8. Photocatalytic Performance Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes. China Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, F.; Han, Z. In situ fabrication of a direct Z-scheme photocatalyst by immobilizing CdS quantum dots in the channels of graphene-hybridized and supported mesoporous titanium nanocrystals for high photocatalytic performance under visible light. RSC Adv. 2018, 73, 42233–42245. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, S.; Gao, H.; Chen, X.; Liu, H.; Yu, C.; Yin, Z.; Zhao, X.; Pan, X.; Yang, H. Microstructure, optical, photoluminescence properties and the intrinsic mechanism of photoluminescence and photocatalysis for the BaTiO3, BaTiO3/TiO2 and BaTiO3/TiO2/CeO2 smart composites. Opt. Mater. 2021, 118, 111273. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, B.; Yang, Z.; Zhou, B.; Xi, F.; Dong, X. Graphitic carbon nitride-BiVO4 heterojunctions: Simple hydrothermal synthesis and high photocatalytic performances. RSC Adv. 2014, 4, 4187–4193. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, Y.; Ju, P.; Long, Y.; Zhang, D. Facile fabrication of AgI/BiVO4 composites with enhanced visible photocatalytic degradation and antibacterial ability. J. Alloys Compd. 2017, 712, 622–627. [Google Scholar] [CrossRef]
- Gómez-Cerezo, M.N.; Munoz-Batista, M.J.; Tudela, D.; Fernández-Garcíaa, M.; Kubackaa, A. Composite Bi2O3-TiO2 catalysts for toluene photo-degradation: Ultraviolet and visible light performances. Appl. Catal. B Environ. 2014, 156–157, 307–313. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Li, J.; Wang, Y.; Chen, C.; Yu, X.; Tang, S.; Zhao, X.; Sun, G.; Li, D. Comparative study of the photoluminescence performance and photocatalytic activity of CeO2/MgAl2O4 composite materials with an n-n heterojunction prepared by one-step synthesis and two-step synthesis methods. J. Phys. Chem. Solids 2021, 150, 109891. [Google Scholar] [CrossRef]
- Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Direct sunlight driven photocatalytic activity of GeO2/monoclinic-BiVO4 nanoplate composites. Sol. Energy 2017, 148, 87–97. [Google Scholar] [CrossRef]
- Fan, T.; Chen, C.; Tang, Z. Hydrothermal synthesis of novel BiFeO3/BiVO4 heterojunctions with enhanced photocatalytic activities under visible light Irradiation. RSC Adv. 2016, 6, 9994–10000. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, D.; Sun, H.; Liang, P.; Duan, X.; Tian, W.; Tadé, M.O.; Liu, S.; Wang, S. Oxygen vacancies in shape controlled Cu2O/reduced graphene oxide/In2O3 hybrid for promoted photocatalytic water oxidation and degradation of environmental pollutants, ACS Appl. Mater. Interfaces 2017, 9, 11678–11688. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ke, J.; Ma, W.; Chen, X.; Yi, Z.; Yang, H. Influence of pH adjustment and surfactant addition on the particle morphology and photocatalytic performance of hydrothermally synthesized BiVO4. Opt. Mater. 2023, 146, 114532. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wang, W.; Pei, D.; Huang, G.; Chen, J.; Zhang, X.; Yu, H. Enhanced photocatalytic degradation of bisphenol A by Co-doped BiOCl nanosheets under visible light irradiation. Appl. Catal. B Environ. 2018, 221, 320–328. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Fang, L.; Sun, G.; Chen, X.; Tang, S.; Yang, H.; Sun, G.; Li, D. Nanostructured spinel-type M (M = Mg, Co, Zn) Cr2O4 oxides: Novel adsorbents for aqueous Congo red removal. Mater. Today Chem. 2021, 22, 100593. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.; Luo, X.; Luo, S.; Dionysiou, D.D. Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Dong, F.; Li, M.; Zhang, T.; Huang, H. Readily achieving concentration-tunable oxygen vacancies in Bi2O2CO3: Triple-functional role for efficient visible-light photocatalytic redox performance. Appl. Catal. B Environ. 2018, 226, 441–450. [Google Scholar] [CrossRef]
- Duan, Q.; Jia, J.; Hong, X.; Fu, Y.; Wang, C.; Zhou, K.; Liu, X.; Yang, H.; Wang, Z. Design of hole-transport-material free CH3NH3PbI3/CsSnI3 allperovskite heterojunction efficient solar cells by device simulation. Sol. Energy 2020, 201, 555–560. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Wang, S.; Wang, X.; Yi, Z.; Yang, H. Photocatalytic activity tuning in a novel Ag2S/CQDs/CuBi2O4 composite: Synthesis and photocatalytic mechanism. Mater. Res. Bull. 2019, 115, 140–149. [Google Scholar] [CrossRef]
- Ban, C.; Wang, Y.; Feng, Y.; Zhu, Z.; Duan, Y.; Ma, J.; Zhang, X.; Liu, X.; Zhou, K.; Zou, H.; et al. Photochromic single atom Ag/TiO2 catalysts for selective CO2 reduction to CH4. Energy Environ. Sci. 2024, 17, 518–530. [Google Scholar] [CrossRef]
- Cheng, T.; Ma, Q.; Gao, H.; Meng, S.; Lu, Z.; Wang, S.; Yi, Z.; Wu, X.; Liu, G.; Wang, X.; et al. Enhanced photocatalytic activity, mechanism and potential application of I doped-Bi4Ti3O12 photocatalysts. Mater. Today Chem. 2022, 23, 100750. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr(VI) ions. Colloid Surf. A 2022, 633, 127918. [Google Scholar] [CrossRef]
- Li, L.; Yan, Y.; Du, J.; Fu, S.; Liu, H.; Zhao, F.; Zhou, J. Glucose-assisted hydrothermal synthesis of plasmonic Bi deposited nested Bi2O2-xCO3 photocatalysts with enhanced photocatalytic activity. Colloid Surf. A 2019, 583, 123946. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Synthesis of homogeneous sphere-like Bi2WO6 nanostructure by silica protected calcination with high visible-light-driven photocatalytic activity under direct sunlight. Cryst. Eng. Comm. 2015, 17, 1037–1049. [Google Scholar] [CrossRef]
- Di, L.; Sun, X.; Xian, T.; Li, H.; Gao, Y.; Yang, H. Preparation of Z-scheme Au-Ag2S/Bi2O3 composite by selective deposition method and its improved photocatalytic degradation and reduction activity. Adv. Powder Technol. 2021, 32, 3672–3688. [Google Scholar] [CrossRef]
- Xian, T.; Sun, X.; Di, L.; Zhou, Y.; Li, H.; Yang, H. Carbon quantum dots (CQDs) decorated Bi2O3-x hybrid photocatalysts with promising NIR-light-driven photodegradation activity for AO7. Catalysts 2019, 9, 1031. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Huang, C.; Liu, M.; Qiu, X. Enhanced photocatalytic activity of Bi2O3 under visible light-irradiation by Cu(II) clusters modification. Appl. Catal. B 2013, 142–143, 598–603. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Zhang, W. Facile preparation of heterostructured Bi2O3/Bi2MoO6 hollow microspheres with enhanced visible-light-driven photocatalytic and antimicrobial activity. Mater. Res. Bull. 2013, 48, 1420–1427. [Google Scholar] [CrossRef]
- Xian, T.; Sun, X.; Di, L.; Li, H.; Yang, H. Improved photocatalytic degradation and reduction performance of Bi2O3 by the decoration of AuPt alloy nanoparticles. Opt. Mater. 2021, 111, 110614. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Sun, G.; Gao, H.; Yu, X.; Tang, S.; Zhao, X.; Yi, Z.; Wang, Y.; Wei, Y. Facile preparation of MgAl2O4/CeO2/Mn3O4 heterojunction photocatalyst and enhanced photocatalytic activity. Mater. Today Chem. 2021, 19, 100390. [Google Scholar] [CrossRef]
- Gao, H.; Yu, C.; Wang, Y.; Wang, S.; Yang, H.; Wang, F.; Tang, S.; Yi, Z.; Li, D. A novel photoluminescence phenomenon in a SrMoO4/SrWO4 micro/nano heterojunction phosphors obtained by the polyacrylamide gel method combined with low temperature calcination technology. J. Lumin. 2022, 243, 118660. [Google Scholar] [CrossRef]
- Li, T.; Quan, S.; Shi, X.; Liu, C.; Yang, L. Photocatalytic Activity of Bi2O3 Enhanced by the Addition of Ce3+/Ce4+ Synthesized by Ethylene Glycol-assisted Solvothermal Method. ChemistrySelect 2020, 5, 5799–5808. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Wang, Z.; Jiao, S.; Zhu, H. Bi2O3 quantum dots decorated anatase TiO2 nanocrystals with exposed {0 0 1} facets on graphene sheets for enhanced visible-light photocatalytic performance. Appl. Catal. B 2013, 129, 333–341. [Google Scholar] [CrossRef]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Teodoro, V.; Gouveia, A.; Machado, T.; Trench, A.; Jacomaci, N.; Assis, M.; Marques, G.; Teodoro, M.; San-Miguel, M.; Andrés, J.; et al. Connecting morphology and photoluminescence emissions in β-Ag2MoO4 microcrystals. Ceram. Int. 2022, 48, 3740–3750. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Hao, H.; Zhu, F.; Zhang, G.; Bi, J.; Yan, S.; Hou, H. Construction of an S-Scheme Ag2MoO4/ZnFe2O4 nanofiber heterojunction for enhanced photoelectrocatalytic activity under visible light irradiation. Langmuir 2022, 38, 13437–13447. [Google Scholar] [CrossRef] [PubMed]
- Kokilavani, S.; Syed, A.; Kumar, B.H.; Elgorban, A.; Bahkali, A.; Ahmed, B.; Das, A.; Khan, S. Facile synthesis of MgS/Ag2MoO4 nanohybrid heterojunction: Outstanding visible light harvesting for boosted photocatalytic degradation of MB and its antimicrobial applications. Colloids Surf. A 2021, 627, 127097. [Google Scholar] [CrossRef]
- Lian, Y.; Wang, Y.; Zhang, D.; Xu, L. Visible light-driven photocatalytic and enzyme-like properties of novel AgBr/Ag2MoO4 for degradation of pollutants and improved antibacterial application. Colloids Surf. A 2022, 639, 128348. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Sun, X.; Yi, Z.; Xian, T.; Wu, X.; Liu, G.; Wang, X.; Yang, H. Construction of Z-scheme Ag2MoO4/ZnWO4 heterojunctions for photocatalytically removing pollutants. Langmuir 2023, 39, 1159–1172. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, Y.; Zheng, J.; Ge, S.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. In-situ constructing visible light CdS/Cd-MOF photocatalyst with enhanced photodegradation of methylene blue. Particuology 2022, 69, 111–122. [Google Scholar] [CrossRef]
- Rani, M.; Meenu; Shanker, U. Efficient cleanup of emerging contaminants by green biosynthesized Z-scheme-type Bi2O3@CdS nanocomposite with improved photoactivity. Nanotechnol. Environ. Eng. 2023, 8, 197–218. [Google Scholar] [CrossRef]
- Chen, P.; Liu, F.; Ding, H.; Chen, S.; Chen, L.; Li, Y.; Au, C.T.; Yin, S. Porous double-shell CdS@C3N4 octahedron derived by in situ supramolecular self-assembly for enhanced photocatalytic activity. Appl. Catal. B Environ. 2019, 252, 33–40. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, X.; Zhang, H.; Chen, J.; Wang, S.; Yang, H. Construction of 2D/0D/2D face-to-face contact g-C3N4@Au@Bi4Ti3O12 heterojunction photocatalysts for degradation of rhodamine B. J. Electron. Mater. 2020, 49, 5248–5259. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Wang, S.; Yi, Z.; Liu, G.; Pu, Z.; Wang, X.; Yang, H. Surface doping of Bi4Ti3O12 with S: Enhanced photocatalytic activity, mechanism and potential photodegradation application. Mater. Res. Bull. 2022, 149, 111711. [Google Scholar] [CrossRef]
- Sun, X.; Xian, T.; Li, R.; Yang, H. Heterojunction interface field and piezoelectric polarization field cooperatively facilitating photocarrier separation in heterojunction piezo-photocatalysts: Experimental and theoretical characterization. Colloids Surf. A 2023, 677, 132430. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Ma, J.; Yi, Z.; Xian, T.; Wang, S.; Liu, G.; Wang, X.; Yang, H. Development of highly-efficient 0D/1D/0D dual Z-scheme CdS/ZnWO4/ZnS heterojunction photocatalysts in pollutant removal and involved mechanism. Appl. Surf. Sci. 2023, 611, 155681. [Google Scholar] [CrossRef]
- Madhavi, J.; Prasad, V.; Reddy, K.R.; Reddy, C.V.; Raghu, A.V. Facile synthesis of Ni-doped ZnS-CdS composite and their magnetic and photoluminescence properties. J. Environ. Chem. Eng. 2021, 9, 106335. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Sun, X.; Xian, T.; Wang, S.; Yi, Z.; Liu, G.; Wang, X.; Yang, H. An excellent Z-scheme Ag2MoO4/Bi4Ti3O12 heterojunction photocatalyst: Construction strategy and application in environmental purification. Adv. Powder Technol. 2021, 32, 951–962. [Google Scholar] [CrossRef]
- Balasurya, S.; Das, A.; Alyousef, A.A.; Alqasim, A.; Almutairi, N.; Khan, S.S. Facile synthesis of Bi2MoO6-Ag2MoO4 nanocomposite for the enhanced visible light photocatalytic removal of methylene blue and its antimicrobial application. J. Mol. Liq. 2021, 337, 116350. [Google Scholar] [CrossRef]
- Moinuddin, A.A.; Kotkondawar, A.V.; Hippargi, G.; Anshul, A.; Rayalu, S. Morphologically and hierarchically controlled Ag/Ag2MoO4 microspheres for photocatalytic hydrogen generation. Appl. Surf. Sci. 2022, 597, 153554. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Yi, Z.; Wang, S.; Wu, X.; Li, R.; Yang, H. Comparative investigation on synthesis, morphological tailoring and photocatalytic activities of Bi2O2CO3 nanostructures. Colloids Surf. A 2022, 644, 128758. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, Z.; Ma, J.; Xian, T.; Liu, G.; Yang, H. Development of ternary Pt/BaTiO3/Bi2O3 heterostructured piezo-photocatalysts for antibiotic degradation. Appl. Surf. Sci. 2024, 653, 159421. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, X.; Tu, S.; Zuo, X.; Nan, J. Synthesis of flower-like heterostructured β-Bi2O3/Bi2O2CO3 microspheres using Bi2O2CO3 self-sacrifice precursor and its visible-light-induced photocatalytic degradation of o-phenylphenol. Appl. Catal. B Environ. 2015, 163, 510–519. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Volanti, D.P.; Nogueira, A.E.; Zamperini, C.A.; Vergani, C.E.; Longo, E. Well-designed β-Ag2MoO4 crystals with photocatalytic and antibacterial activity. Mater. Design 2017, 115, 73–81. [Google Scholar] [CrossRef]
- Sun, X.; Xu, T.; Xian, T.; Yi, Z.; Liu, G.; Dai, J.; Yang, H. Insight on the enhanced piezo-photocatalytic mechanism of In2O3/BiFeO3 heterojunctions for degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2023, 640, 158408. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Xian, T.; Liu, G.; Yang, H. Photocatalytic purification of simulated dye wastewater in different pH environments by using BaTiO3/Bi2WO6 heterojunction photocatalysts. Opt. Mater. 2021, 113, 110853. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Guo, Y.; Wei, Z.; Han, M.; He, H.; Yang, S.; Sun, C. Facile synthesis and high activity of novel BiVO4/FeVO4 heterojunction photocatalyst for degradation of metronidazole. Appl. Surf. Sci. 2015, 351, 270–279. [Google Scholar] [CrossRef]
- Mu, W.; Xu, M.; Sun, X.; Liu, G.; Yang, H. Oxygen-vacancy-tunable mesocrystalline ZnO twin “cakes” heterostructured with CdS and Cu nanoparticles for efficiently photodegrading sulfamethoxazole. J. Environ. Chem. Eng. 2024, 12, 112367. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Z.; Liu, D.; Tan, Z.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. S-Scheme Bi2S3/CdS Nanorod Heterojunction Photocatalysts with Improved Carrier Separation and Redox Capacity for Pollutant Removal. ACS Appl. Nano Mater. 2022, 5, 5448–5458. [Google Scholar] [CrossRef]
- He, Z.; Siddique, M.S.; Yang, H.; Xia, Y.; Su, J.; Tang, B.; Wang, L.; Kang, L.; Huang, Z. Novel Z-scheme In2S3/Bi2WO6 core-shell hetero junctions with synergistic enhanced photocatalytic degradation of tetracycline hydrochloride. J. Clean. Prod. 2022, 339, 130634. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Sun, X.; Wang, S.; Yi, Z.; Liu, G.; Li, R.; Yang, H. Enhanced piezophotocatalytic activity of Bi2MoO6 nanosheets: Theory and experimental studies. Ceram. Int. 2023, 49, 36545–36559. [Google Scholar] [CrossRef]

| Photocatalyst | Degradation Efficiency | Reference |
|---|---|---|
| 25C10AB | 60 min, 99% | This work |
| C3N4/BiVO4 | 90 min, 94% | [6] |
| CeO2/MgAl2O4 | 180 min, 95.5% | [9] |
| Cu2O/RGO/In2O3 | 120 min, 95.1 | [12] |
| CdS/Bi4Ti3O12 | 50 min, 96.3% | [22] |
| MgAl2O4/CeO2/Mn3O4 | 180 min, 94.6% | [31] |
| SrMoO4/SrWO4 | 180 min, ~90% | [32] |
| MgS/Ag2MoO4 | 200 min, 90% | [38] |
| CdS/Cd-MOF | 100 min, 91.9% | [41] |
| CdS/ZnWO4/ZnS | 50 min, 95.6% | [47] |
| BaTiO3/Bi2WO6 | 120 min, 88.4% | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Xing, Y.; Sun, X.; Ma, Q.; Gu, Y.; Zhou, H.; Liu, G.; Ma, J.; Yang, H. Rational Synthesis of a Dual Z-Scheme CdS/Ag2MoO4/β-Bi2O3 Heterojunction for the Deep Photodegradation of Methylene Blue and Analysis of Its Mechanisms. Catalysts 2025, 15, 438. https://doi.org/10.3390/catal15050438
Ma W, Xing Y, Sun X, Ma Q, Gu Y, Zhou H, Liu G, Ma J, Yang H. Rational Synthesis of a Dual Z-Scheme CdS/Ag2MoO4/β-Bi2O3 Heterojunction for the Deep Photodegradation of Methylene Blue and Analysis of Its Mechanisms. Catalysts. 2025; 15(5):438. https://doi.org/10.3390/catal15050438
Chicago/Turabian StyleMa, Weiyi, Yelin Xing, Xiaofeng Sun, Qianfei Ma, Yufen Gu, Hui Zhou, Guorong Liu, Jinyuan Ma, and Hua Yang. 2025. "Rational Synthesis of a Dual Z-Scheme CdS/Ag2MoO4/β-Bi2O3 Heterojunction for the Deep Photodegradation of Methylene Blue and Analysis of Its Mechanisms" Catalysts 15, no. 5: 438. https://doi.org/10.3390/catal15050438
APA StyleMa, W., Xing, Y., Sun, X., Ma, Q., Gu, Y., Zhou, H., Liu, G., Ma, J., & Yang, H. (2025). Rational Synthesis of a Dual Z-Scheme CdS/Ag2MoO4/β-Bi2O3 Heterojunction for the Deep Photodegradation of Methylene Blue and Analysis of Its Mechanisms. Catalysts, 15(5), 438. https://doi.org/10.3390/catal15050438





