Rotating Photo-Disc Reactor (RPR) Used in the Photo-Degradation of Pyridine Using Zinc Oxide as a Catalyst Composited with Aluminum Nanoparticles and Irradiated with Natural Light
Abstract
1. Introduction
2. Results
2.1. SEM Results
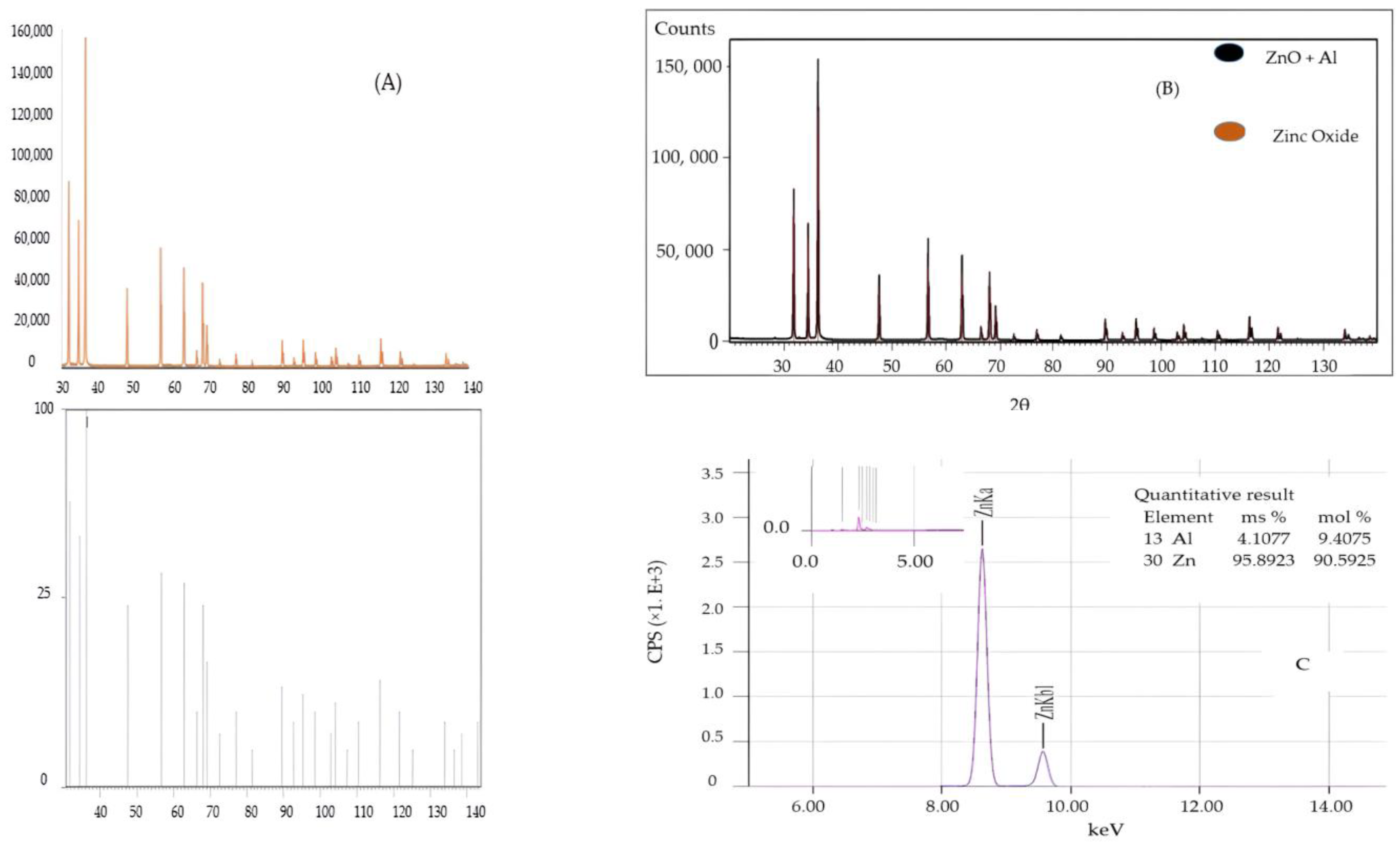
2.2. X-Rays
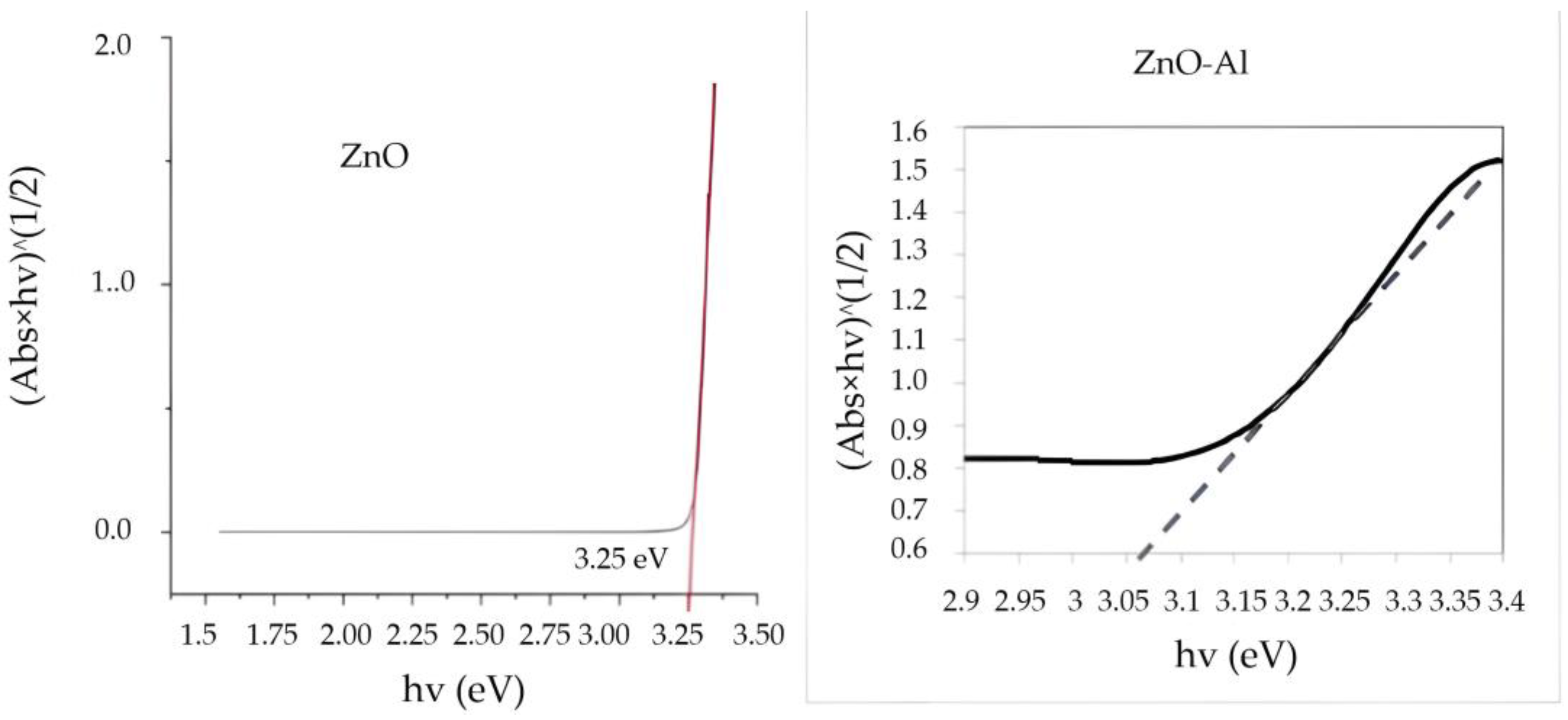
2.3. Diffuse Reflectance
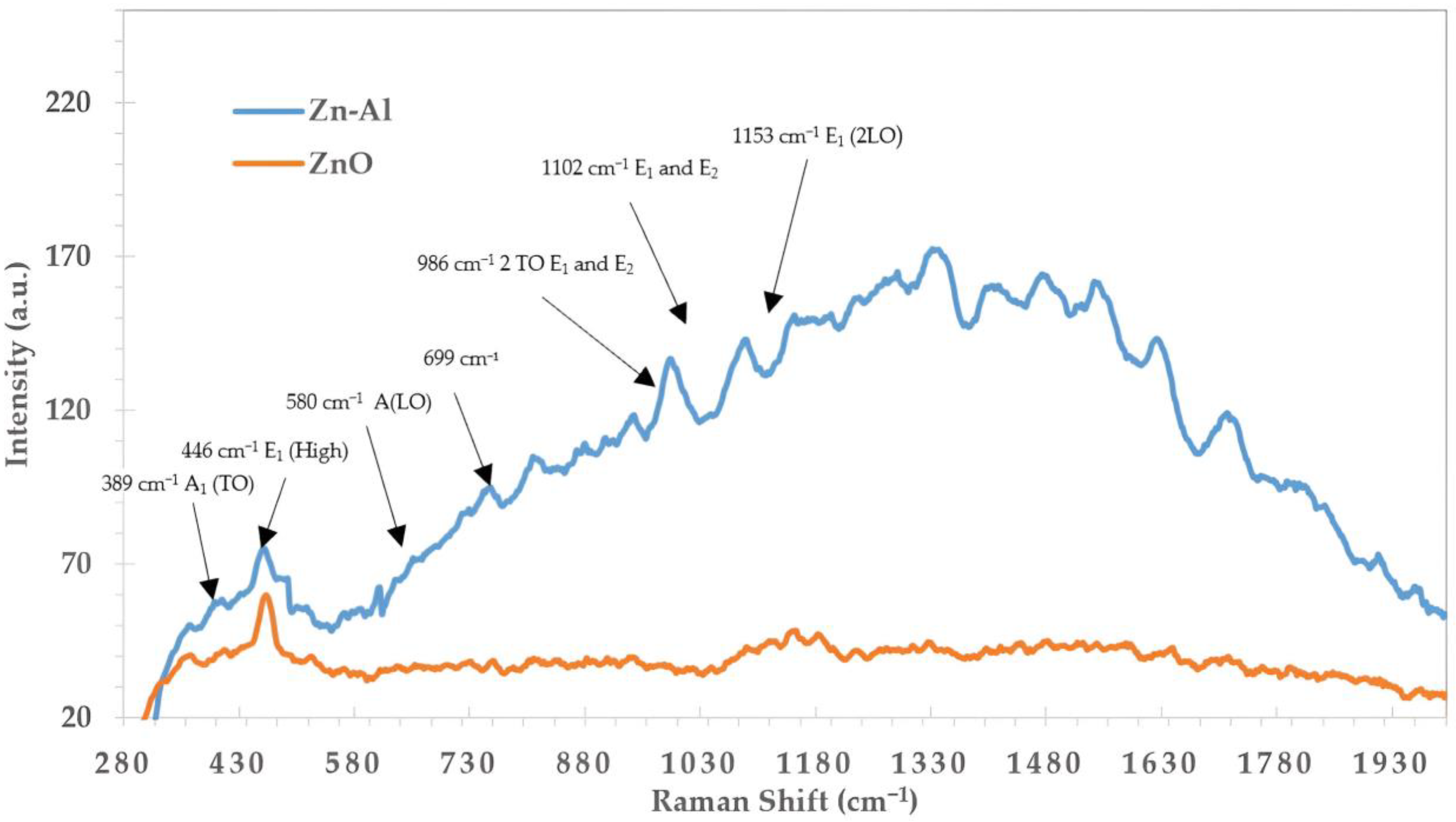
2.4. Raman Spectroscopy
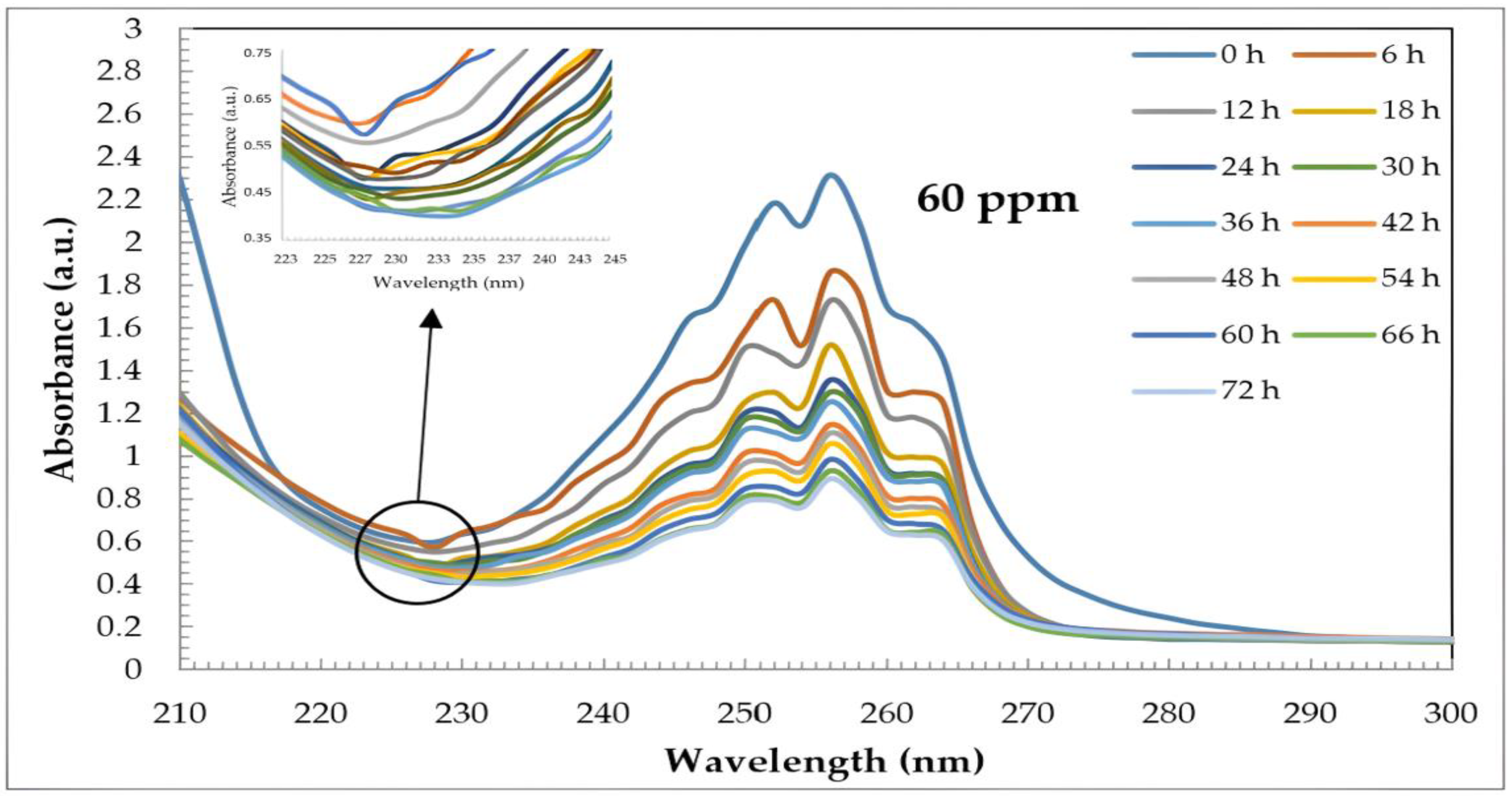
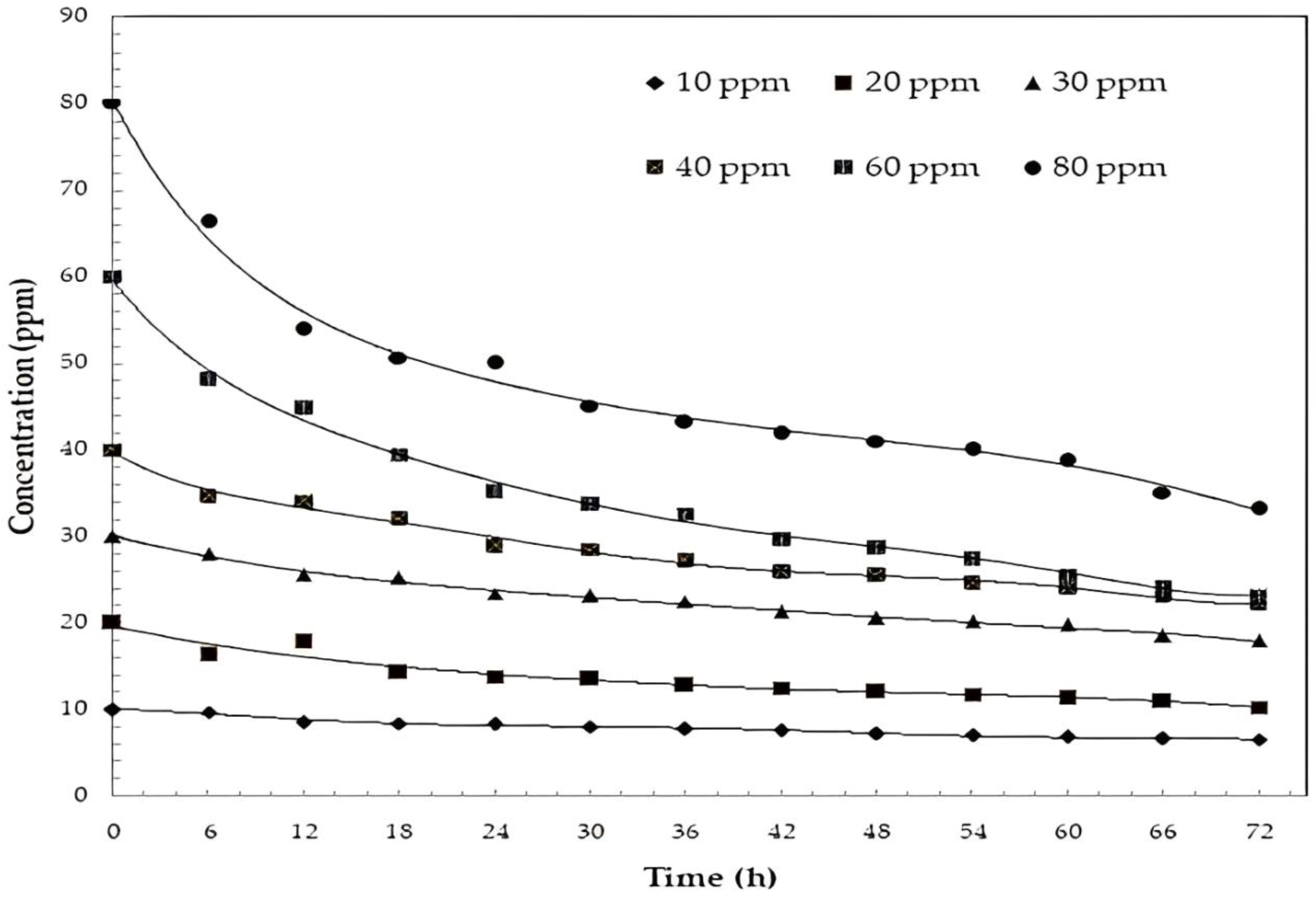
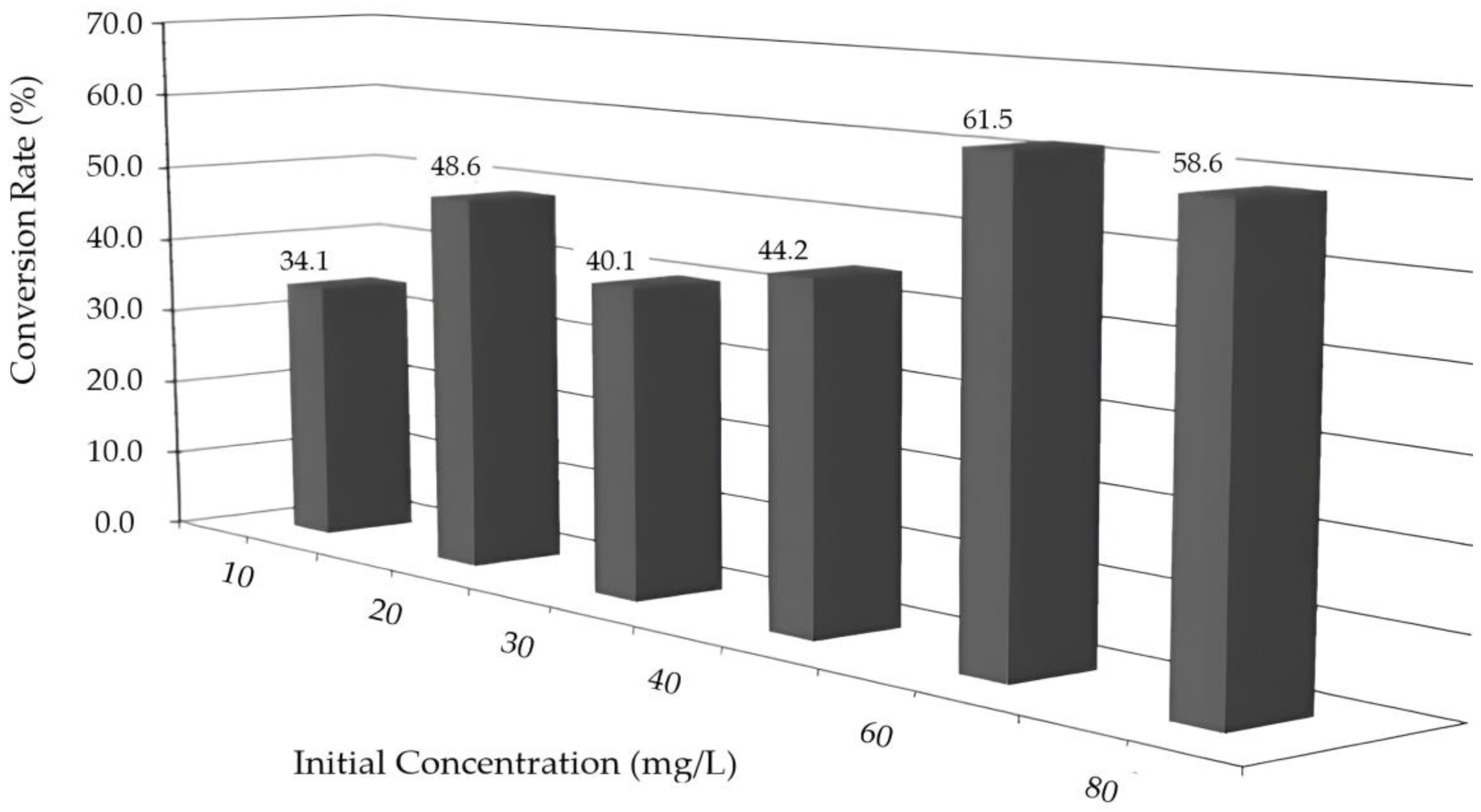
2.5. Uv–Vis and HPLC Results
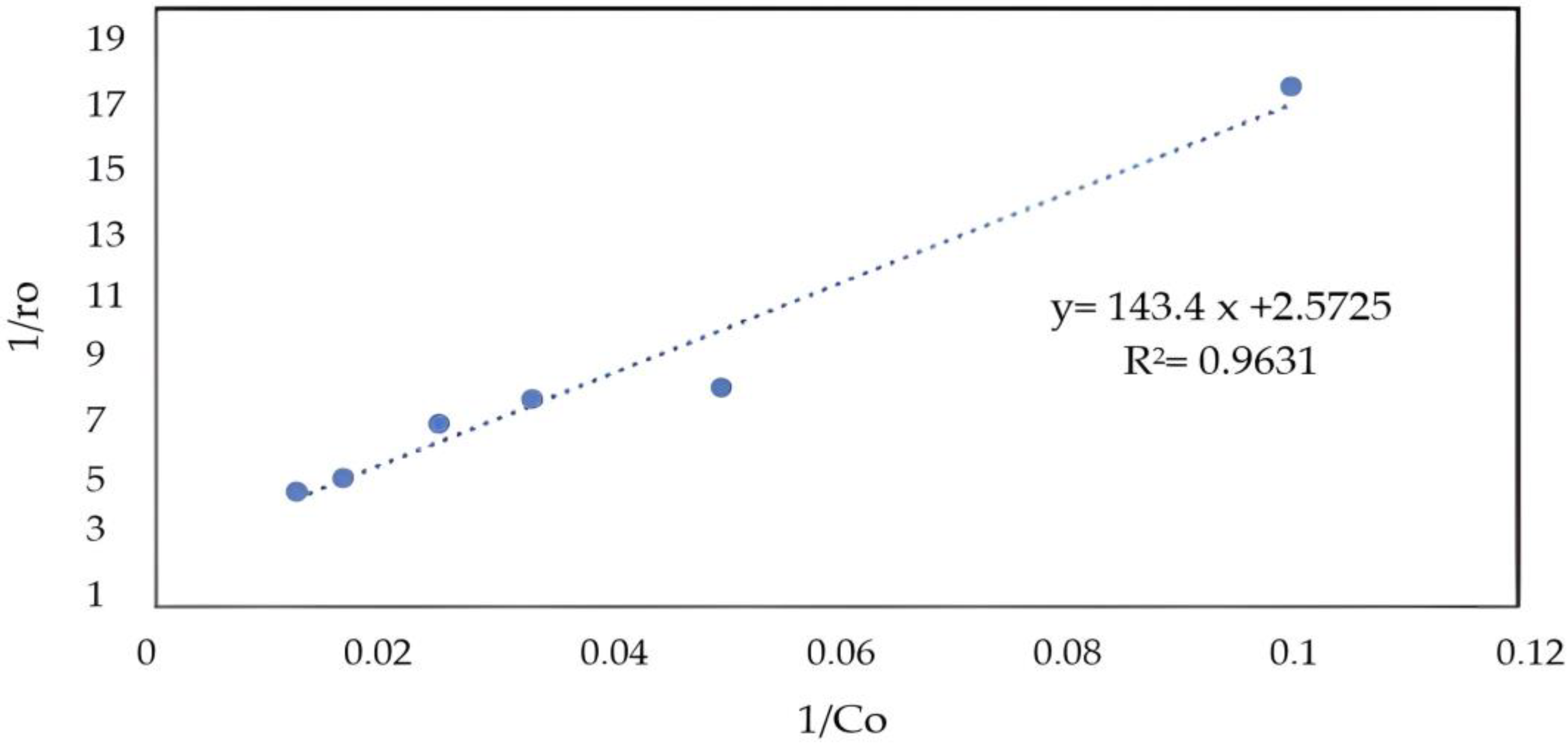
2.6. Kinetic Analysis
2.7. Proposed Reaction Mechanism
3. Methodology
3.1. Reagents and Chemicals
3.2. Analytical Methods
3.2.1. Rotating Photo-Disk Reactor (RPR)
3.2.2. Doping Process
3.3. Catalyst Characterization Tests
3.3.1. SEM Tests
3.3.2. Diffuse Reflectance
3.3.3. X-Rays
3.3.4. Raman
3.4. Preparation of Pyridine Solutions
3.5. Degradation Tests
3.6. Gas Masses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef]
- Santhi, K.; Manikandan, P.; Rani, C.; Karuppuchamy, S. Synthesis of nanocrystalline titanium dioxide for photodegradation treatment of remazol brown dye. Appl. Nanosci. 2015, 5, 373–378. [Google Scholar] [CrossRef][Green Version]
- Bello, M.M.; Raman, A. Adsorption and Oxidation Techniques to Remove Organic Pollutants from Water. In Green Adsorbents for Pollutant Removal; Springer: Cham, Switzerland, 2018; pp. 249–300. Available online: https://www.researchgate.net/publication/326012580_Adsorption_and_Oxidation_Techniques_to_Remove_Organic_Polltants_from_Water (accessed on 20 March 2025).
- Grigor’eva Nellya, G.; Filippova Nadezhda, A.; Tselyutina Marina, I.; Kutepov Boris, I. Synthesis of pyridine and methylpyridines over zeolite catalysts. Appl. Petrochem. Res. 2015, 5, 99–104. [Google Scholar] [CrossRef][Green Version]
- Ashok, B.; Hariram, N.; Siengchin, S.; Rajulu, A.V. Modification of tamarind fruit shell powder with in situ generated copper nanoparticles by single step hydrothermal method. J. Bioresour. Bioprod. 2020, 5, 180–185. [Google Scholar] [CrossRef]
- Frolov, N.A.; Vereshchagin, A.N. Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 2937. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.; Popa, C.-V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef]
- Moctezuma, E.; López, M.; Zermeño, B. Reaction pathways for the photocatalytic degradation of phenol under different experimental conditions. Rev. Mex. Ing. Química 2016, 15, 129–137. [Google Scholar]
- Elsayed, M. Successive advanced oxidation of pyridine by ultrasonic irradiation: Effect of additives and kinetic study. Desalination Water Treat. 2015, 53, 57–65. [Google Scholar] [CrossRef]
- Aguilar, U.C.; Anguebes, F.F.; Cerón, B.J.; Cerón, B.R.; Córdova-Quiroz, A.V.; Montalvo-Romero, C.; Rangel-Marrón, M.; Robles-Heredia, J.C.; y Zavala-Loría, J.C. Tópicos Selectos de Ingeniería Química; UNACAR: Campeche, México, 2012. [Google Scholar]
- Song, K.K.; Chen, J.; Song, Y. The degradation of pyridine wastewater by ozonation catalyzed by calcined zinc-magnesium-aluminum hydrotalcites. J. Beijing Univ. Chem. Technol. 2021, 8, 8–15. [Google Scholar] [CrossRef]
- Swarnakar, P.; Kanel, S.R.; Nepal, D.; Jiang, Y.; Jia, H.; Kerr, L.; Goltz, M.N.; Levy, J.; Rakovan, J. Silver deposited titanium dioxide thin film for photocatalysis of organic compounds using natural light. Sol. Energy 2013, 88, 242–249. [Google Scholar] [CrossRef]
- Czech, B.; Rubinowska, K. TiO2-assisted photocatalytic degradation of diclofenac, metoprolol, estrone and chloramphenicol as endocrine disruptors in water. Adsorption 2013, 19, 619–630. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, G.; Ibhadon, A.O.; Salunke, D.B.; Sinha, A.; Kansal, S.K. A Facile synthesis of silver modified ZnO nanoplates for efficient removal of ofloxacin drug in aqueous phase under solar irradiation. J. Environ. Chem. Eng. 2017, 6, 3621–3630. [Google Scholar] [CrossRef]
- El Nemr, A.; Helmy, E.T.; Gomaa, E.A.; Eldafrawy, S.; Mousa, M. Photocatalytic and Biological Activities of Undoped and Doped TiO2 Prepared by Green Method for Water Treatment. J. Environ. Chem. Eng. 2019, 7, 103385. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Asiri, A.M.; Alamry, K.A.; Khan, A.A.P.; Khan, A.; Rub, M.A.; Azum, N. Acetone sensor based on solvothermally prepared ZnO doped with Co3O4 nanorods. Microchim. Acta 2013, 180, 675–685. [Google Scholar] [CrossRef]
- Islam, M.T.; Jing, H.; Yang, T.; Zubia, E.; Goos, A.G.; Bernal, R.A.; Botez, C.E.; Narayan, M.; Chan, C.K.; Noveron, J.C. Fullerene Stabilized Gold Nanoparticles Supported on Titanium Dioxide for Enhanced Photocatalytic Degradation of Methyl Orange and Catalytic Reduction of 4-Nitrophenol. J. Environ. Chem. Eng. 2018, 6, 3827–3836. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- He, R.; Hocking, R.K.; Tsuzuki, T. Local structure and photocatalytic property of sol–gel synthesized ZnO doped with transition metal oxides. J. Mater. Sci. 2012, 47, 3150–3158. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Mosleh, S.; Ghaedi, M. Chapter 13—Photocatalytic reactors: Technological status, opportunities, and challenges for development and industrial upscaling. Interface Sci. Technol. 2021, 32, 761–790. [Google Scholar] [CrossRef]
- Molinari, R.; Severino, A.; Lavorato, C.; Argurio, P. Which Configuration of Photocatalytic Membrane Reactors Has a Major Potential to Be Used at an Industrial Level in Tertiary Sewage Wastewater Treatment? Catalysts 2023, 13, 1204. [Google Scholar] [CrossRef]
- Sannino, D.; Morante, N.; Sacco, O.; Mancuso, A.; De Guglielmo, L.; Di Capua, G.; Femia, N.; Vaiano, V. Visible light-driven degradation of Acid Orange 7 by light modulation techniques. Photochem. Photobiol. Sci. 2023, 22, 185–193. [Google Scholar] [CrossRef]
- Chakravorty, A.; Roy, S. A review of photocatalysis, basic principles, processes, and materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Hassard, F.; Biddle, J.; Cartmell, E.; Jefferson, B.; Tyrrel, S.; Stephenson, T. Rotating biological contactors for wastewater treatment—A review. Process. Saf. Environ. Prot. 2015, 94, 285–306. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Rotating biological contactors: A review on main factors affecting performance. Rev. Environ. Sci. Bio/Technol. 2008, 7, 155–172. [Google Scholar] [CrossRef]
- Boiarkina, I.; Norris, S.; Patterson, D.A. Investigation into the effect of flow structure on the photocatalytic degradation of methylene blue and dehydroabietic acid in a spinning disc reactor. Chem. Eng. J. 2013, 222, 159–171. [Google Scholar] [CrossRef]
- Imayama, S. Studies of the Rotating-Disk Boundary-Layer Flow. Technical Reports from Royal Institute of Technology KTH Mechanics. December 2014, Stockholm, Sweden. Available online: https://www.diva-portal.org/smash/get/diva2:781517/SUMMARY01.pdf (accessed on 6 March 2025).
- Imayama, S.; Alfredsson, P.H.; Lingwood, R.J. On the laminar–turbulent transition of the rotating-disk flow: The role of absolute instability. J. Fluid Mech. 2014, 745, 132–163. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Wang, Z.; Zhou, Y.; Qin, Y.; Wang, Z.L. Piezotronic Effect Enhanced Photocatalysis in Strained Anisotropic ZnO/TiO2 Nanoplatelets via Thermal Stress. ACS Nano 2016, 10, 2636–2643. [Google Scholar] [CrossRef]
- Porrawatkul, P.; Pimsen, R.; Kuyyogsuy, A.; Rattanaburi, P.; Nuengmatcha, P. Morphology-dependent photocatalytic performance of ZnO nanostructures in organic dye and antibiotic degradation. Int. J. Environ. Sci. Technol. 2024, 21, 7397–7414. [Google Scholar] [CrossRef]
- Salah, N.; Hameed, A.; Aslam, M.; Babkair, S.S.; Bahabri, F. Photocatalytic activity of V doped ZnO nanoparticles thin films for the removal of 2- chlorophenol from the aquatic environment under natural sunlight exposure. J. Environ. Manag. 2016, 177, 53–64. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Medellin, C.N.; Ocampo, R.; Leyva, R.R.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Méndez-Díaz, J.D. Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H2O2, O3/H2O2 and O3/activated carbon). Sci. Total Environ. 2013, 1, 26–35. [Google Scholar] [CrossRef]
- Montalvo, C.; Aguilar, C.; Alcoser, R.; Ramirez-Elias, M.; Cordova-Quiroz, V. Semi-Pilot Photocatalytic Rotating Reactor (RFR) with Supported TiO2/Ag Catalysts for Water Treatment. Molecules 2018, 23, 224. [Google Scholar] [CrossRef]
- Aguilar, C.A.; Montalvo, C.; Ceron, J.G.; Moctezuma, E. Photocatalytic Degradation of Acetaminophen. Int. J. Environ. Res. 2011, 5, 1071–1078. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 3rd ed.; Pearson Education Limited: Hongkong, China, 1999; ISBN 9780135317167. [Google Scholar]
- Matthews, R.W.; McEvoy, S.R. Photocatalytic degradation of phenol in the presence of near-UV illuminated titanium dioxide. J. Photochem. Photobiol. A Chem. 1992, 64, 231–246. [Google Scholar] [CrossRef]
- Elisa, L.; Carlos, M.; Edgar, M.; Socorro, L. Photocatalytic degradation of pyridine in water solution using ZnO as an alternative catalyst to TiO2. J. Ceram. Process. Res. 2008, 9, 455–462. [Google Scholar]
- He, B.; Zhao, Q.; Zeng, Z.; Wang, X.; Han, S. Effect of hydrothermal reaction time and calcination temperature on properties of Au@CeO2 core–shell catalyst for CO oxidation at low temperature. J. Mater. Sci. 2015, 50, 6339–6348. [Google Scholar] [CrossRef]
- Thaha, S.K.S.M.; Nalandhiran, P.; Kaliyamoorthy, S.; Mizota, I.; Mangalaraja, R.V.; Sathishkumar, P. Critical Review of Photocatalytic Reactor Designs for Environmental Applications. In Photocatalysis for Energy and Environmental Applications. Green Energy and Technology; Sathishkumar, P., Ed.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, C.; Zhang, Y.; Gong, Y.; Wu, J.; Fu, Q.; Pan, C. Construction of hierarchical TiO2 nanorod array/graphene/ZnO nanocomposites for high-performance photocatalysis. J. Mater. Sci. 2018, 53, 15376–15389. [Google Scholar] [CrossRef]
- Zyoud, A.; Zu’bi, A.; Helal, M.H.S.; Park, D.; Campet, G.; Hilal, H.S. Optimizing photo-mineralization of aqueous methyl orange by nano-ZnO catalyst under simulated natural conditions. J. Environ. Health Sci. Eng. 2015, 13, 46. [Google Scholar] [CrossRef]
- Gupta, N.; O’Loughlin, E.J.; Sims, G. Microbial degradation of pyridine and pyridine Derivatives. In Microbial Metabolism of Xenobiotic Compounds; Microorganisms for, sustainability, Arora, P., Eds.; Springer: Singapore, 2019. [Google Scholar]



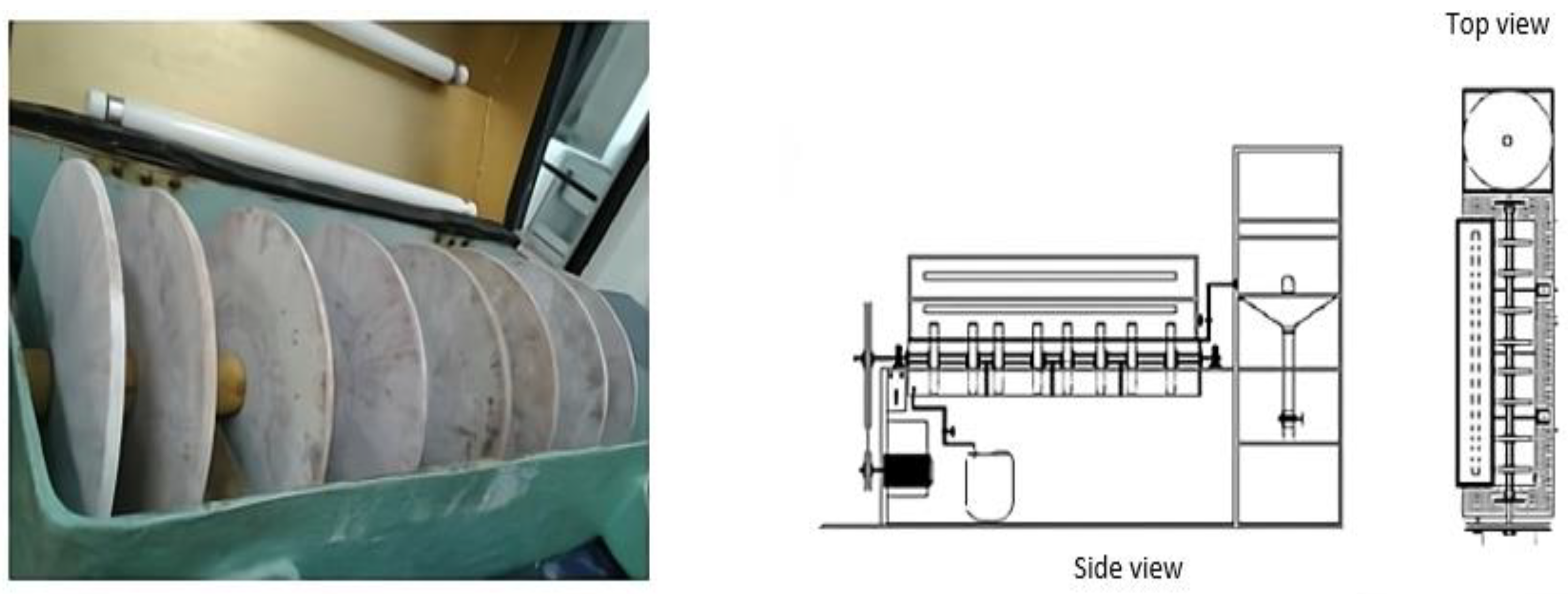
| Reactor Characteristic | Value |
|---|---|
| Number of stages | 4 |
| Number of disks per stage | 2 |
| Disk diameter | 0.23 m |
| Disk thickness | 0.008 m |
| Total area of undoped disks | 0.66476 m2 |
| Total area of doped disks | 329.7209 m2 |
| Area per stage | 41.21512 m2 |
| Total reactor volume | 14.8 L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo, C.; Lemus, E.; Aguilar, C.A.; Cerón, R.M.; Cerón, J.G.; Robles, J.C.; Ruiz, A. Rotating Photo-Disc Reactor (RPR) Used in the Photo-Degradation of Pyridine Using Zinc Oxide as a Catalyst Composited with Aluminum Nanoparticles and Irradiated with Natural Light. Catalysts 2025, 15, 437. https://doi.org/10.3390/catal15050437
Montalvo C, Lemus E, Aguilar CA, Cerón RM, Cerón JG, Robles JC, Ruiz A. Rotating Photo-Disc Reactor (RPR) Used in the Photo-Degradation of Pyridine Using Zinc Oxide as a Catalyst Composited with Aluminum Nanoparticles and Irradiated with Natural Light. Catalysts. 2025; 15(5):437. https://doi.org/10.3390/catal15050437
Chicago/Turabian StyleMontalvo, Carlos, Edith Lemus, Claudia A. Aguilar, Rosa M. Cerón, Julia G. Cerón, Juan C. Robles, and Alejandro Ruiz. 2025. "Rotating Photo-Disc Reactor (RPR) Used in the Photo-Degradation of Pyridine Using Zinc Oxide as a Catalyst Composited with Aluminum Nanoparticles and Irradiated with Natural Light" Catalysts 15, no. 5: 437. https://doi.org/10.3390/catal15050437
APA StyleMontalvo, C., Lemus, E., Aguilar, C. A., Cerón, R. M., Cerón, J. G., Robles, J. C., & Ruiz, A. (2025). Rotating Photo-Disc Reactor (RPR) Used in the Photo-Degradation of Pyridine Using Zinc Oxide as a Catalyst Composited with Aluminum Nanoparticles and Irradiated with Natural Light. Catalysts, 15(5), 437. https://doi.org/10.3390/catal15050437








