Abstract
The enhancement of enzyme activity has garnered significant attention in biotransformation processes and applications. This enhancement is achieved through the use of specific nanomaterials (NMs) with unique physicochemical characteristics responsive to external stimuli. In this study, an enzyme–Fe3O4 nano-biocatalytic system (NBS) was developed to enable real-time activation of enzymatic catalysis under alternating magnetic field (AMF) and near-infrared (NIR) irradiation using dual-functional Fe3O4 magnetic nanoparticles (MNPs). When exposed to an AMF, Fe3O4 MNPs generate molecular vibrations through mechanisms such as Néel or Brown relaxation while acting as a photothermal agent in response to NIR irradiation. The synergistic effect of AMF and NIR irradiation significantly enhanced energy transfer between the enzyme and Fe3O4 MNPs, resulting in a maximum 4.3-fold increase in enzyme activity. Furthermore, the system reduced aldol reaction time by 66% (from 4 h to 1.5 h) while achieving 90% product yield. Additionally, factors such as nanoparticle size and NIR power were found to play a critical role in the efficiency of this real-time regulation strategy. The results also demonstrate that the enzyme–Fe3O4 nanocomposites (NCs) significantly enhanced catalytic efficiency and reduced the reaction time for aldol reactions. This study demonstrates an efficient NBS controlled via the synergistic effects of AMF and NIR irradiation, enabling spatiotemporal control of biochemical reactions. This work also provides a breakthrough strategy for dynamic biocatalysis, with potential applications in industrial biomanufacturing, on-demand drug synthesis, and precision nanomedicine.
1. Introduction
Enzymes are considered to be efficient macromolecular biological catalysts that play an important role in cellular metabolism and chemical reaction. Their essential advantages are high catalytic efficiency, substrate specificity, minimal formation of byproducts, and low energy consumption [1,2]. Regulation of enzyme activity is crucial for making biocatalysts exert preferable properties in many applications. Therefore, significant efforts have been directed toward developing diverse enzyme activity regulation strategies such as enzyme molecule modification or alteration of the reaction environment (e.g., pH, temperature, and ionic strength). However, these traditional regulation strategies may lead to uncontrollable and irreversible inactivity of enzymes and require complex and long-term processes [3,4].
Recently, real-time and remote activation of enzyme activity, containing NIR, AMF, microwave, and ultrasound irradiation [5], has been an emerging strategy (photothermal effect) to achieve biotransformation function due to its high efficiency, deep penetrability, and sustainability [6]. Localized surface plasmon resonance (LSPR) has emerged as a promising strategy to enhance the enzyme-like activity of nanozymes in catalytic reactions [7]. Specifically, NIR activation facilitates the transfer of internal energy (photothermal effect) from plasmonic NMs to immobilized enzymes, thereby enabling remote control of enzyme activity [8]. It is also well known that inorganic nanoparticles (NPs) can be activated as hotspots by an AMF as extrinsic energy sources and thus act as nanoactuators to enhance enzymatic catalysis [9]. Microwave and ultrasound irradiation can regulate some specific enzymes to alter enzymatic structure into favorable and flexible conformation, which increases the stability, selectivity, or kinetics of enzymes [6]. Therefore, these real-time activation strategies can make it possible to achieve the precise and spatiotemporal control of biocatalytic processes, which have been applied in many fields, including chemical synthesis, industrial manufacture, and disease therapy. For example, Zhang et al. [10] constructed oxygen vacancy molybdenum trioxide nanodots (MoO3−xNDs) which can possess triple-therapy synergistic efficiency for bacterial infections based on the single NIR-regulated combination of photodynamic, photothermal, and peroxidase-like enzymatic activities [6]. Wang et al. [11] immobilized glucose oxidase (GOx) onto a ferrimagnetic vortex iron oxide nanoring (Fe3O4 NR) functionalized with poly (ethylene glycol) of different molecular weights to construct a series of Fe3O4 NR@GOx, which can be stimulated by Fe3O4 NR-mediated local heat in response to an AMF. Additionally, many studies have shown interest in promoting enzymatic reactions combined with microwaves or ultrasound to improve chemical synthesis [6,12,13].
NMs with unique energy-absorbing and physical characteristics have become a significant media for real-time and precise activation strategies for enzyme activity. The mechanism of NMs for controlling enzyme activity upon external stimuli relies on their ability to transfer absorbed energy into internal energy, including generating thermal effect and improving molecular diffusion and stability, which can fulfill high efficiency and controllability of real-time activation. For instance, many plasmatic nanoparticles with excellent light absorption and efficient photothermal conversion, such as Au, Pt, CdS, TiO2, and graphene oxide, can convert NIR light into heat or transfer electrons to achieve the activation of enzymes [14,15,16]. In a previous study, a NIR-activated enzyme, Au NBS, was constructed, which demonstrated that the activity of thermophilic enzymes can be stimulated efficiently by the excellent photothermal effect of Au nanorods under NIR irradiation [17]. Furthermore, magnetic nanoparticles (MNPs) that are responsive to an AMF have drawn considerable interest in enzyme immobilization due to their ability to control the activity of enzymes upon AMF irradiation [18]. With proper surface modification, these magnetic nanoparticles can be dispersed into water, forming water-based suspensions, which can interact with an external magnetic field. Amongst these NMs, Fe3O4 magnetic nanoparticles possess a high specific surface area, low toxicity, and good biocompatibility. It is simple to separate them and easy to modify them, and they have been widely used in drug delivery in tumor cells, magnetic resonance imaging (MRI), and magnetothermal therapy [19]. Interestingly, Fe3O4 magnetic nanoparticles not only can produce molecular vibrations by affecting the magnetic moment arrangement but can also generate a photothermal effect upon NIR irradiation [20,21]. Concas et al. reported γ-Fe2O3 nanospheres decorated with gold nanoparticles on the surface to be efficient magnetothermal and photothermal agents, a type of magnetic vortex. These NMs can significantly increase local temperature, induce tumor cell apoptosis, and cause massive denaturation of collagen fibers when exposed to near-infrared laser (808 nm) and alternating magnetic field (AMF) irradiation [22]. Therefore, it is proposed that enzymes can be activated by the synergistic effect of AMF and NIR irradiation with the help of dual-functional Fe3O4 MNPs, which may lead to greater enhancement of enzyme activity. However, the subject of controlling the activity of enzymes immobilized on Fe3O4 nanoparticles under AMF and NIR irradiation remains largely underexplored.
The aim of this study was to investigate the synergistic effects of combining AMF with NIR irradiation on enhancing the activity of enzyme–Fe3O4 nanocomposites. Three acyl aminopeptidases (EC 3.4.19.1) from different sources were chosen as model enzymes: mesophilic BSU32203 from Bacillus subtilis 168 (optimal temperature: 55 °C), thermophilic ST0779 from Sulfolobus tokodaii (optimal temperature: 70 °C), and hyperthermophile APE1547 from Aeropyrum pernix K1 (optimal temperature: 90 °C) [23,24]. The aldehyde-tagged enzymes were covalently linked to amino-modified Fe3O4 magnetic nanoparticles through site-specific immobilization, as previously reported. A comparison of the impact of different activation strategies on enzyme activity, including exposure to AMF or NIR irradiation exclusively or to both stimuli, was conducted, as shown in Scheme 1B, to optimize the strategy systematically. Moreover, the application of NBS to catalyze aldol reaction was explored; this is one of the most important carbon–carbon bond-forming reactions in industrial applications. This study provides a new method for real-time and synergistic activation of enzyme activity by combining AMF and NIR irradiation.
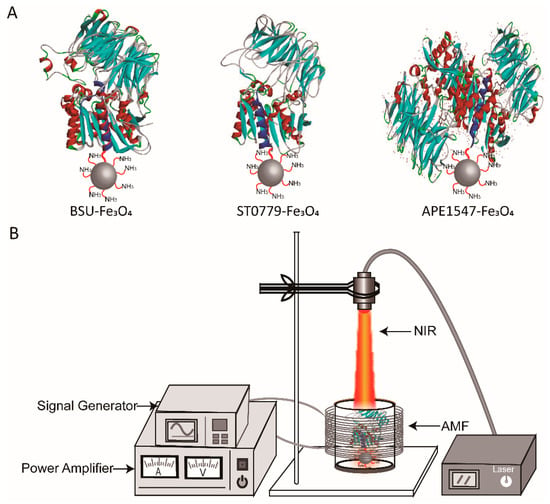
Scheme 1.
Schematic diagram of (A) covalent conjugation of aldehyde-tagged acyl aminopeptidases (BSU32203, ST0779, APE1547) to amino-functionalized Fe3O4 MNPs (200–300 nm) via Schiff base reaction (the structures of APE1547, ST0779, and BSU32203 were all modeled by SWISS-MODEL). (B) The regulation process of enzyme–Fe3O4 NC under AMF and NIR.
2. Results and Discussion
2.1. Activation of Enzyme–Fe3O4 NC Under AMF and NIR
The three kinds of recombinant enzymes with aldehyde tags at the C-terminal have been constructed successfully in previous work [25]. For enzyme immobilization, a Schiff reaction occurs when Fe3O4 magnetic nanoparticles modified with amino group are mixed with aldehyde-tag enzymes. This covalently site-specific immobilization ensures uniform distribution of the proteins and accessibility of the active center [25]. In our previous work, we determined the immobilization efficiency of ST0779 on Fe3O4 nanoparticles to be 84.6% [25]. The thermal conversion efficiency of enzyme–Fe3O4 NC was measured under an AMF of 10 kHz and 16 G or exposure to 808 nm NIR laser irradiation at different power levels (1–2.5 W), as shown in Figure 1A. This reveals that the AMF stimulation cannot influence the temperature of the reaction system, which is probably because the magnetothermal effect of Fe3O4 is generally dependent on the frequency and intensity of the AMF. Many studies have shown that higher magnetic frequency and intensity, such as 345 kHz and 300 G, enabled magnetic particles to generate heat due to Neel or Brown relaxation loss [26], whereas magnetic particles oscillated and behaved like microscopic stirrers at a lower magnetic frequency and intensity, such as 600 Hz and 10 G [27]. However, there is still no definite division of the low- or high-frequency and intensity range. The thermal conversion efficiency results indicate that Fe3O4 MNPs functioned as microscopic stirrers under a magnetic field of 10 kHz and 16 G. Moreover, by adjusting the laser power of the NIR irradiation, the temperature can be maintained within the range of 25–45 °C, which exhibited the good photothermal conversion of Fe3O4 magnetic nanoparticles.
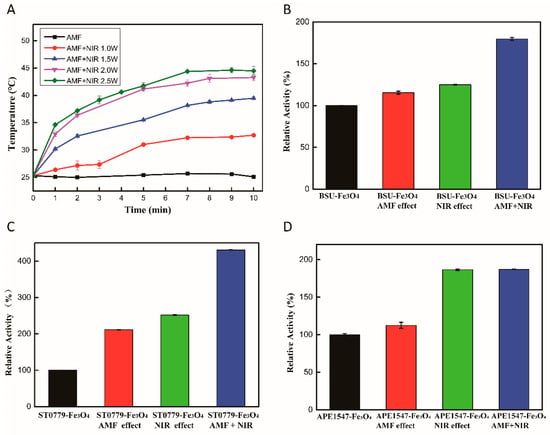
Figure 1.
Thermal conversion and enzymatic activity enhancement of Fe3O4 nanocomposites under AMF and NIR. (A) Heat generation of Fe3O4 MNPs at different NIR powers (808 nm, 1–2.5 W) or AMF (10 kHz, 16 G). Evaluation of enzyme activity under different regulation strategies, including AMF, NIR, or both. (B) BSU-Fe3O4 nanocomposite, (C) ST0779-Fe3O4 nanocomposite, and (D) APE1547-Fe3O4 nanocomposite.
To explore the effect of different activation strategies on enzyme activity at room temperature, the activity of enzyme–Fe3O4 nanocomposites was measured using the substrate p-nitrophenyl butyrate (pNPC4). When exposed to external stimuli, the activities of three immobilization enzymes were higher than those without any activation in different degrees, as shown in Figure 1B–D. As for AMF activation, enzyme activity can be increased slightly at 1.1–2-fold, compared to NIR activation, which increased enzyme activity by 1.3–2.8-fold. This result demonstrates that the photothermal effect was the main reason for the improvement in enzyme activity, which also confirmed the previous assumption that Fe3O4 performed like microscopic stirrers under AMF irradiation to accelerate collisions between a substrate and enzyme rather than producing a magnetothermal effect. Importantly, the maximum activity was observed when AMF and NIR irradiation were used simultaneously; in this case, the catalytic activity increased by 1.8–4.3-fold compared with the control group without any treatment. The results demonstrate that enzyme catalysis was greatly improved by the synergistic effect between AMF and NIR irradiation. This synergistic effect allows a rapid transfer from external stimuli to enzyme molecules, thereby increasing the activity of the enzyme. Notably, the activity of the thermophilic ST0779-Fe3O4 NC was more significantly enhanced under AMF, NIR, or combined stimulation compared to the other two nanocomposites. This indicated that the effect of this strategy on enzymes from different sources is different even for the same type of enzymes, while the energy generated by photothermal effect and microscopic vibration cannot efficiently activate BSU-Fe3O4 nanocomposite and APE1547-Fe3O4 nanocomposite, which might be because the different enzymatic conformations affected the energy transfer efficiency between Fe3O4 MNPs and enzymes. Based on its enhanced enzyme activity, the ST0779-Fe3O4 nanocomposite was chosen as a model enzyme for further investigation. To observe the effect of NIR power on enzyme activity directly, the experiment of measuring the activity of ST0779-Fe3O4 nanocomposite under AMF and NIR irradiation with different powers of the laser was carried out. The result indicates that the change of activity was power-dependent or temperature-dependent, as shown in Figure 2. NIR irradiation performed like a heating switch which could control the temperature of the reaction system in real time and activate the reaction rapidly. This result confirms that better photothermal conversion of Fe3O4 MNPs can lead to efficient energy transfer to enzymes. Instead, once the NIR irradiation was removed, the reaction process catalyzed by thermophilic enzymes was terminated due to the reduced temperature, achieving the controllable and real-time activation of enzyme activity.
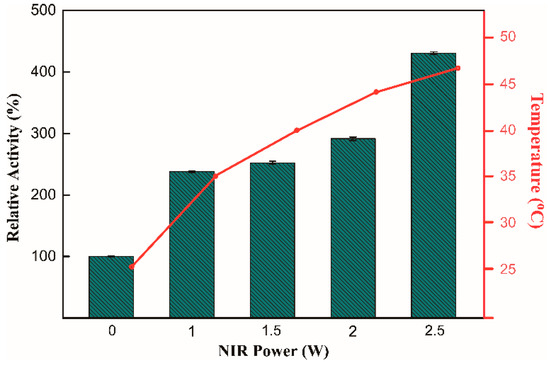
Figure 2.
Detection of enzymatic activity of ST0779-Fe3O4 nanocomposite upon different NIR powers. The fitting lines figure indicates the temperature change with power.
2.2. The Effect of Different Sizes of Fe3O4 on Enzyme Activity
The dimension and morphology of nanoparticles are also very important, accounting for their physicochemical characteristics and biomedical and biological applications [28,29,30]. Like other nanoparticles, the size of MNPs affects their magnetic and biological properties, such as biodistribution, transportation, and toxicity, which are closely associated with the synthesis size, methodology, and surface characteristics [31]. To investigate the effect of different dimensions of MNPs on enzyme activity, ST0779 was immobilized on different sizes of Fe3O4 MNPs (100–200 nm, 200–300 nm, 300–400 nm), and their activity under different regulation strategies was also measured (Figure 3). The immobilization enzyme without regulation at each size range was chosen as the control, and the relative activity was defined as 100%. The result shows that enzymes immobilized on 200–300 nm Fe3O4 MNPs exerted a higher activity upon the AMF, NIR, or synergistic effect than those of 100–200 nm and 300–400 nm when compared to their respective control groups. This indicated that Fe3O4 MNPs with a size range of 200–300 nm exhibited a better capacity to respond to external stimuli, especially an AMF that could accelerate the molecular motion of Fe3O4 MNPs to promote contact between enzymes and the substrate. Therefore, the exhibited unique size-dependent property of Fe3O4 MNPs was important for physicochemical characteristics, affecting the activity of immobilized enzymes under AMF and NIR irradiation.
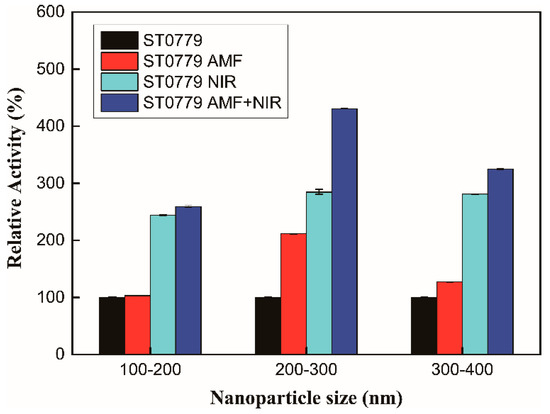
Figure 3.
Relative enzymatic activity of ST0779-Fe3O4 nanocomposites immobilized on MNPs of different sizes: 100–200 nm, 200–300 nm, and 300–400 nm. Activity was measured under AMF (10 kHz, 16 G), NIR (2.5 W), or both.
2.3. Evaluation of Storage Stability and Reusability of ST0779-Fe3O4 Nanocomposites
The storage stability of enzymes is an important factor for many applications, including industrial processing and chemical synthesis. We evaluated the storage stability of immobilized enzymes at 4 °C over seven days. Notably, the enzymatic activity remained at a higher level after 7 days, which exhibited excellent stability of immobilized enzymes, as shown in Figure 4A. The recycling of immobilized enzymes is one of the advantages of immobilized enzymes. Thus, we evaluated the recycling numbers of the enzyme–Fe3O4 nanosystems after being exposed to AMF (10 kHz, 13 G) and NIR (2.5 W) irradiation. After each reaction cycle, the immobilized enzymes in the solution were separated through a magnet and reused for repeated catalysis reactions. It was found that the activity of the immobilized enzyme could maintain higher activity after four recycles, which exhibited potential value for industrial application, as seen in Figure 4B. Furthermore, the main reason why enzyme activity decreased after five cycles is due to the physical adsorption of MNPs during the recycling processes. Therefore, an appropriate immobilization method can improve stability and ensure the long-term utilization of enzymes.
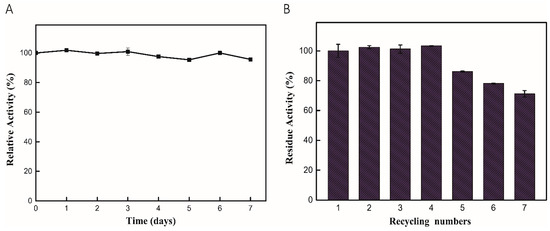
Figure 4.
Stability and reusability of ST0779-Fe3O4 nanocomposites. (A) Evaluation of long-term stability of ST0779-Fe3O4 nanocomposite after 7 days of incubation at 4 °C. (B) The number of recycled immobilized enzymes.
2.4. Catalysis of ST0779-Fe3O4 Nanocomposites for Aldol Reaction
Aldol addition is one of the most useful methods for the construction of new carbon–carbon bond formation in organic synthesis, attracting considerable interest in various applications over the years, including the synthesis of pharmaceuticals, fine chemicals, and natural products [32,33,34]. ST0779 has been identified as promiscuously catalyzing the addition reaction of acetone to p-nitrobenzaldehyde in our previous study [23]. Here, we used this real-time activation strategy to estimate the promiscuous catalysis of enzyme–Fe3O4 NC, as observed in Scheme 2. The productivity of the aldol reaction was detected by HPLC with isopropanol/n-hexane as the mobile phase. The results show that the yield of the aldol reaction catalyzed by ST0779-Fe3O4 nanocomposites reached 90% in 4 h under conventional heating, whereas when exposed to AMF and NIR irradiation, ST0779-Fe3O4 nanocomposites, remarkably, achieved higher productivity in 1.5 h, which improved catalytic efficiency in a short time, as shown in Figure 5. The enhanced reaction rate under AMF/NIR irradiation stems from rapid energy transfer between Fe3O4 nanoparticles and enzymes, enabling real-time catalytic activation. Unlike traditional heating, which elevates bulk temperature and risks side reactions, AMF/NIR selectively heats the Fe3O4 microenvironment, minimizing thermal degradation and improving specificity while accelerating catalysis.
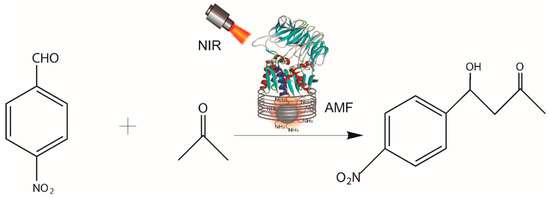
Scheme 2.
Schematic illustration of enzyme-catalyzed aldol reaction under AMF and NIR.
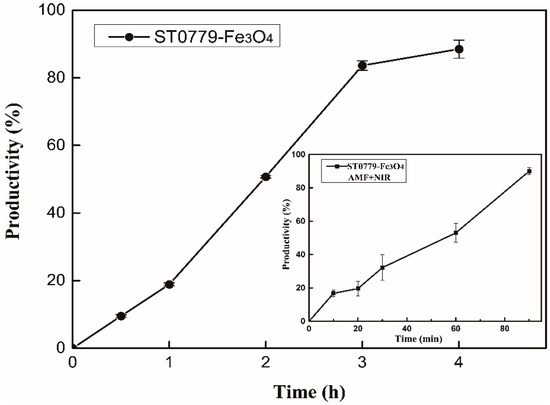
Figure 5.
The comparison of productivity of aldol reaction catalyzed by ST0779-Fe3O4 nanocomposites under AMF and NIR or under conventional heating at 55 °C.
Moreover, many studies have reported that several enzyme–Fe3O4 nanocomposites activated by an AMF individually can be applied in many fields. For instance, Xia et al. constructed a fixed-bed reactor by immobilizing laccase on polyethyleneimine (PEI)-modified amine-functionalized Fe3O4 nanoparticles, which achieved the continuous degradation of phenolic compounds under a high-gradient magnetic field [5,35]. Zhou et al. studied natural glucose oxidase (GOx) and superparamagnetic Fe3O4 nanoparticles that were integrated into poly(lactic-co-glycolic acid) (PLGA) to fabricate a sequential nanocatalyst (designated as GOx@PLGAFe3O4), which can be modulated in activity by an AMF [36]. This work confirmed that the synergistic effect of AMF and NIR irradiation can activate the activity of enzymes in real time effectively and can probably be used in extensive applications such as chemical synthesis, biodegradation, and tumor therapies.
3. Materials and Methods
3.1. Materials and Chemicals
The acyl aminopeptidase ST0779 was cloned from thermophilic archaea Sulfolobus tokodaii strain T7 [23], APE1547 was cloned from Aeropyrum pernix strain K1, and BSU32203 was cloned from Bacillus subtilis 168 (all cells of strain 7 T JCM 10545 were obtained from Biological Resource Center, National Institute of Technology and Evaluation, Tokyo, Japan) [24]. The Fe3O4 magnetite nanoparticles of different sizes (100–200 nm, 200–300 nm, 300–400 nm) modified with amino groups were purchased from Beisile (Tianjin, China). Isopropyl β-D-thiogalactopyranoside (IPTG) and sodium borohydride (NaBH4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-arabinose was obtained from the Aladdin Company (Shanghai, China). Ampicillin and kanamycin were purchased from Takara Biotechnology (Kusatsu, Shiga, Japan,). The pET-28a-derived plasmids with target proteins were constructed in the laboratory, and the pBAD-Mtb-FGE plasmid was obtained from a nonprofit plasmid repository (AddGene, Cambridge, MA, USA) [37].
3.2. Expression of Recombinant Proteins
The recombinant proteins with aldehyde tags were all expressed in E. coli BL21 (DE3) cells with the pBAD–Mtb–FGE plasmid and incubated in LB media with ampicillin and kanamycin. Then, they were shaken at the speed of 170 rpm and the temperature of 37 °C up to an optical density at 600 nm (OD600) up to 0.8. Then, 0.2% L-arabinose was added to induce the expression of formylglycine-generating enzyme (FGE), which can specifically oxidize cysteine or serine residues in proteins, generating formylglycine (FGly) that contains an aldehyde group [37]. After incubation at 37 °C for 30 min, 0.5 mM of isopropyl β-D-thiogalactoside (IPTG) was added to induce the expression of recombinant enzymes. Then, the cells were incubated at 25 °C for 12 h.
The induced bacteria were harvested via centrifugation at 4000 rpm for 30 min at 4 °C. Afterward, the cells of ST0779, BSU32203, and APE1547 were resuspended with 10 volumes of 50 mM Tris-HCl buffer (pH 8.0) [37]. The cells were treated by ultrasound fragmentation until the solution was no longer viscous, and the solution was centrifuged at 10,000 rpm at 4 °C for 20 min to obtain the supernatants with target enzymes. Then, the supernatants with ST0779, as well as APE1547, were purified by thermal treatment to remove the contaminated protein [37]. The discarded, deformed protein was precipitated by centrifugation at 10,000 rpm for 20 min at 4 °C, retaining the supernatants.
3.3. Immobilization of Enzyme
First, the Fe3O4 nanoparticle suspension was washed five times with deionized water and then resuspended by Tris-HCl buffer (pH 8.0). Before immobilization, nanoparticles were well dispersed by ultrasonication. Then, 3 mg of Fe3O4 nanoparticles were mixed with an appropriate amount of enzyme solution, shaken at 10 °C for 6 h, and reduced with 1% (w/w) NaBH3 for 1 h. The immobilized enzymes were washed twice with NaCl solution (1 mol/L) and separately with deionized water [25].
3.4. Enzyme Assay
The activity of each kind of immobilized enzyme was tested by the hydrolysis of p-Nitrophenyl butyrate at room temperature [37]. The reaction system used 1 mL, consisting of 1 mM of substrate and 3 mg of immobilized enzyme in the Tris-HCl buffer (50 mM, pH 8.0). After reacting for 1 min, the p-nitrophenol product was quantified spectrophotometrically at 420 nm.
As for different regulation strategies, a custom-built setup consisting of a signal generator and a power amplifier was used to generate alternating magnetic field and NIR laser irradiation. The magnetic frequency and intensity were fixed at 10 kHz and 6.5 G, and NIR power was controlled at 1–2.5 W at 808 nm for regulation. The thermal response of the enzyme-Fe3O4 NC was measured as follows: aqueous dispersions of the NC were irradiated under the respective stimuli, and the temperature of the system was monitored using a non-contact infrared thermometer until thermal equilibrium was achieved. This protocol ensured real-time tracking of localized heating effects mediated by Fe3O4 nanoparticles. The substrate concentration and measurement methods were the same as those used for immobilized enzymes. After pretreating the mixture with AMF and NIR irradiation for 10 min to stabilize the temperature and reaction conditions, the substrates were added to start the reaction, in which a continuous regulating function was applied to the whole process. The reaction vessel was placed in the central core of a Faraday coil to accept the AMF, while the laser emitter was immobilized on an iron support stand to illuminate the reaction vessel vertically [17]. The distance between the NIR emitter and the reaction vessel was about 10 cm.
3.5. Examination of Different Sizes of Fe3O4
The size of Fe3O4 magnetic particles was divided into three gradients ranging from 100 to 200 nm, 200 to 300 nm, and 300 to 400 nm. The same amount of acyl aminopeptidase ST0779 was separately immobilized on 3 mg of Fe3O4 nanoparticles with different sizes using the same method mentioned before. Meanwhile, the activity of immobilized enzymes under AMF and NIR irradiation was detected as mentioned above.
3.6. Evaluation of Long-Term Stability and Recycling
To detect the stability of the immobilized enzyme, the prepared sample solution was incubated at 4 °C for 7 days. A certain amount of immobilized enzyme was removed for daily activity measurement.
To determine the recycling of Fe3O4-ST0779, the immobilized enzymes were harvested after each reaction and washed twice with Tris-HCl buffer (pH 8.0) before being utilized in the next cycle. The reusability of the Fe3O4-ST0779 enzyme was evaluated by measuring the relative hydrolyzing activity. The initial activity of the immobilized enzyme was defined as 100%.
3.7. Catalysis of Aldol Reaction
The aldol reaction was conducted by using 4-nitrobenzaldehyde and acetone as substrates in a mixture containing 4 mL of acetone and 0.03 mM of 4-nitrobenzaldehyde with 1 mL of deionized water. For the control group without regulation, the reaction was initiated by addition of the enzyme at a concentration of 0.032 μM at 55 °C for 4 h, as previously reported. For the immobilized enzyme, the mixture solution was preheated under AMF and NIR irradiation for 10 min before adding 4-nitrobenzaldehyde. Pooled extracts were dried using anhydrous Na2SO4 to remove the solvent under reduced pressure. The quantality of products was analyzed by high-performance liquid chromatography (HPLC) with isopropanol/n-hexane, as previously reported [37].
3.8. Statistical Analysis
All experiments were performed in triplicate, and data are presented as means. Statistical significance between experimental groups (e.g., enzyme activity under AMF/NIR irradiation vs. control or effects of nanoparticle sizes) was evaluated using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. For pairwise comparisons (e.g., activity enhancement before and after regulation), a two-tailed Student’s t-test was applied. A p-value of <0.05 was considered statistically significant. Data normality and homogeneity of variances were verified using Shapiro–Wilk testing and Levene’s test, respectively. All statistical analyses were conducted using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
4. Conclusions
In summary, we constructed a real-time activatable enzyme–Fe3O4 nanosystem leveraging the dual functionality of Fe3O4 MNPs under AMF and NIR irradiation. Key findings include the following: (1) the synergistic AMF/NIR stimulation enhanced enzymatic activity by up to 4.3-fold through rapid energy transfer, where Fe3O4 MNPs served triply as an immobilization matrix, a microscale stirrer to accelerate mass transfer, and a photothermal nanoheater. (2) Thermophilic ST0779 exhibited exceptional activation, enabling efficient catalysis at ambient temperatures, while the 200–300 nm MNPs demonstrated optimal responsiveness due to enhanced enzyme–substrate collision dynamics. (3) This strategy reduced aldol reaction time by 66% (1.5 h) with 90% yield, highlighting its potential in chemical synthesis and precision biocatalysis. These results underscore the promise of AMF/NIR-responsive nanosystems for applications spanning industrial biomanufacturing, on-demand drug synthesis, and therapeutic nanocarriers, though scalability and long-term stability under operational conditions require further validation. Future studies should expand to diverse enzyme classes, optimize nanoparticle design for broader stimuli responsiveness, and integrate multi-modal regulation strategies (e.g., pH, redox) to advance real-time biocatalysis. Furthermore, we will aim to further investigate and compare the effects of different fixation methods to optimize this system. These efforts will bridge the gap between laboratory-scale innovation and practical applications in on-demand biomanufacturing, precision nanomedicine, and sustainable chemical synthesis.
Author Contributions
Conceptualization, R.G. and L.X.; methodology and investigation, F.W., Y.L., Q.D., Z.L., S.L. and T.Z.; writing—original draft preparation, F.W., Y.L. and Q.D.; writing—review and editing, R.G.; project administration, R.G. and L.X.; supervision, R.G.; funding acquisition, R.G. and L.X. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was supported by the National Natural Science Foundation of China (grant no. 32471471).
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Liangtao Xu is employed by the Technology Center of China Tobacco Jiangsu Industrial Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NMs | Nanomaterials |
| NBS | Nano-biocatalytic system |
| NC | Nanocomposites |
| AMF | Alternating magnetic field |
| NIR | Near-infrared |
| MNPs | Magnetic nanoparticles |
References
- Scheibel, D.M.; Gitsov, I.P.I.; Gitsov, I. Enzymes in “Green” Synthetic Chemistry: Laccase and Lipase. Molecules 2024, 29, 989. [Google Scholar] [CrossRef] [PubMed]
- Intasian, P.; Prakinee, K.; Phintha, A.; Trisrivirat, D.; Weeranoppanant, N.; Wongnate, T.; Chaiyen, P. Enzymes, In Vivo Biocatalysis, and Metabolic Engineering for Enabling a Circular Economy and Sustainability. Chem. Rev. 2021, 121, 10367–10451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.; Liu, Y. Advanced Strategies of Enzyme Activity Regulation for Biomedical Applications. ChemBioChem 2022, 23, e202200358. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of Lipases on Heterofunctional Octyl–Glyoxyl Agarose Supports. Methods Enzymol. 2016, 571, 73–85. [Google Scholar] [PubMed]
- Khan, A.; Beg, M.R.; Waghmare, P. Intensification of Biokinetics of Enzymes Using Ultrasound-Assisted Methods: A Critical Review. Biophys. Rev. 2021, 13, 417–423. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Du, C.; Gao, R. Current Strategies for Real-Time Enzyme Activation. Biomolecules 2022, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Y.; Niu, R.; Tang, Y.; Chen, Y.; Su, H.; Yang, Z.; Jing, X.; Guan, H.; Gao, R.; et al. NIR Plasmonic Nanozymes: Synergistic Enhancement Mechanism and Multi-Modal Anti-Infection Applications of MXene/MOFs. Adv. Mater. 2024, 36, 2307839. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yuan, J.; Gao, F.; Weng, B.; Hu, W.; Lei, Y.; Huang, X.; Yang, L.; Shen, J.; Xu, D.; et al. Piezopotential-Driven Simulated Electrocatalytic Nanosystem of Ultrasmall MoC Quantum Dots Encapsulated in Ultrathin N-Doped Graphene Vesicles for Superhigh H2 Production from Pure Water. Nano Energy 2020, 75, 104990. [Google Scholar] [CrossRef]
- Torres-Herrero, B.; Armenia, I.; Alleva, M.; Asín, L.; Correa, S.; Ortiz, C.; Fernández-Afonso, Y.; Gutiérrez, L.; De La Fuente, J.M.; Betancor, L.; et al. Remote Activation of Enzyme Nanohybrids for Cancer Prodrug Therapy Controlled by Magnetic Heating. ACS Nano 2023, 17, 12358–12373. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Tan, J.; Chang, Z.; Liu, X.; Ma, W.; Xu, Y. Near-Infrared Regulated Nanozymatic/Photothermal/Photodynamic Triple-Therapy for Combating Multidrug-Resistant Bacterial Infections via Oxygen-Vacancy Molybdenum Trioxide Nanodots. Small 2021, 17, 2005739. [Google Scholar] [CrossRef]
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zou, Y.; Li, X.; Yang, L.; Zhang, B.; Wu, X. Waxberry-Shaped Biomimetic Nanoparticles for Efficient Photothermal Conversion. Int. J. Therm. Sci. 2024, 195, 108612. [Google Scholar] [CrossRef]
- Guimaraes, M.; Mateus, N.; de Freitas, V.; Branco, L.C.; Cruz, L. Microwave-Assisted Synthesis and lonic Liquids: Green and Sustainable Alternatives toward Enzymatic Lipophilization of Anthocyanin Monoglucosides. J. Agric. Food Chem. 2020, 68, 7387–7392. [Google Scholar] [CrossRef]
- Elabbadi, M.; Boukouvala, C.; Ringe, E. Controllable Synthesis of Pd and Pt Shells on Au Nanoparticles with Electrodeposition. Sci. Rep. 2025, 15, 1292. [Google Scholar] [CrossRef] [PubMed]
- Kazaz, O.; Karimi, N.; Paul, M.C. Radiation and Nanoparticle Interaction for Enhanced Light Absorption and Heat Conversion. J. Mol. Liq. 2024, 411, 125702. [Google Scholar] [CrossRef]
- Cao, G.; Sun, D.; Gu, T.; Dong, Y.; Wang, G.-L. Photoswitching Enzymatic Activity of Horseradish Peroxidase by Graphene Oxide for Colorimetric Immunoassay. Biosens. Bioelectron. 2019, 145, 111707. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Geng, X.; Li, Z.; Gao, R. Real-Time Regulation of Catalysis by Remote-Controlled Enzyme-Conjugated Gold Nanorod Composites for Aldol Reaction-Based Applications. Catal. Sci. Technol. 2019, 9, 2221–2230. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Zhang, Y.; Wang, Y.; Cui, M.; Li, G.; Liu, X.; Fan, H. Magnetoresponsive Nanozyme: Magnetic Stimulation on the Nanozyme Activity of Iron Oxide Nanoparticles. Sci. China Life Sci. 2022, 65, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Pîrvulescu, D.-C.; Bîrcă, A.C.; Andronescu, E.; Grumezescu, A.M. Magnetite Microspheres for the Controlled Release of Rosmarinic Acid. Pharmaceutics 2022, 14, 2292. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, L.; Chen, M.; Wang, W.; Li, X.; Yang, H.; Yang, S.; Zhou, Z. Self-Amplified Apoptosis Targeting Nanoplatform for Synergistic Magnetic–Thermal/Chemo Therapy In Vivo. Adv. Healthc. Mater. 2020, 9, 2000202. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Allia, P.; Tiberto, P. Temperature-Dependent Heating Efficiency of Magnetic Nanoparticles for Applications in Precision Nanomedicine. Nanoscale 2020, 12, 6360–6377. [Google Scholar] [CrossRef]
- Concas, G.C.; Jalil, W.B.F.; Caraballo-Vivas, R.J.; Gomes, V.L.S.; Camarena, M.A.; Fontes, M.B.; Sharma, S.K.; Almeida, T.P.; Santos, E.C.S.; Garcia, F. Magnetic Vortex γ-Fe2O3 Nanospheres Decorated with Gold Nanoparticles for Dual Hyperthermia Treatment. J. Alloys Compd. 2025, 1010, 177022. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Cao, S.G.; Xie, G.Q.; Gao, R.J. Expression and Characterization of a Thermostable Acyl-peptide Releasing Enzyme ST0779 from Sulfolobus tokodaii. Chem. Res. Chin. Univ. 2012, 28, 851–855. [Google Scholar]
- Bartlam, M.; Wang, G.; Yang, H.; Gao, R.; Zhao, X.; Xie, G.; Cao, S.; Feng, Y.; Rao, Z. Crystal Structure of an Acylpeptide Hydrolase/Esterase from Aeropyrum Pernix K1. Structure 2004, 12, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Ainiwaer, A.; Li, A.; Zhao, X.; Xu, Y.; Han, S.; Gao, R. Site-Specific Covalent Immobilization of Methylobacterium extorquens Non-Blue Laccse Melac13220 on Fe3O4 Nanoparticles by Aldehyde Tag. Catalysts 2022, 12, 1379. [Google Scholar] [CrossRef]
- Li, R.; Perez, B.; Jian, H.; Jensen, M.M.; Gao, R.; Dong, M.; Glasius, M.; Guo, Z. Characterization and mechanism insight of accelerated catalytic promiscuity of Sulfolobus tokodaii (ST0779) peptidase for aldol addition reaction. Appl. Microbiol. Biotechnol. 2015, 99, 9625–9634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhou, Q.; Chen, X.; Jiao, W.; Li, G.; Peng, M.; Liu, X.; He, Y.; Fan, H. Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS Nanoparticles by in Situ Anchoring on Conductive Polymer Nanofibers. ACS Appl. Mater. Inter. 2021, 13, 52395–52405. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, C.Z. Insight into the role of citrate in the citrate cycle in Aspergillus niger. Chem. Eng. J. 2015, 280, 36–40. [Google Scholar] [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Seibt, S.; Pearson, J.; Nixon-Luke, R.; Zhang, H.; Lang, P.R.; Bryant, G.; Cölfen, H.; Mulvaney, P. Size, Diffusion, and Sedimentation of Gold Nanorods. J. Phys. Chem. C 2023, 127, 22336–22346. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.; Zhang, J.; Xi, J.; Du, G.; Ji, Z. MoS2 Nanosheet/ZnO Nanowire Hybrid Nanostructures for Photoelectrochemical Water Splitting. J. Am. Ceram. Soc. 2018, 101, 3989–3996. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size Dependent Biodistribution and Toxicokinetics of Iron Oxide Magnetic Nanoparticles in Mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Kováč, O.; Rode, A.; Atzl, D.; Magauer, T. Total Synthesis of Ganoapplanin Enabled by a Radical Addition/Aldol Reaction Cascade. J. Am. Chem. Soc. 2024, 146, 22937–22942. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ni, Y.; Bučar, D.-K.; Dalby, P.A.; Ward, J.M.; Jeffries, J.W.E.; Hailes, H.C. A Transaminase-Mediated Aldol Reaction and Applications in Cascades to Styryl Pyridines. Catal. Sci. Technol. 2024, 14, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Feng, M.; Liu, C.; Liu, C.; Guo, C. Efficient Phenol Degradation by Laccase Immobilized on Functional Magnetic Nanoparticles in Fixed-Bed Reactor Under High-Gradient Magnetic Field. Eng. Life Sci. 2021, 21, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tang, X.; Huang, J.; Wang, J.; Zhao, J.; Zhang, L.; Wang, Z.; Li, P.; Li, R. Dual-Imaging Magnetic Nanocatalysis Based on Fenton-like Reaction for Tumor Therapy. J. Mater. Chem. B 2022, 10, 3462–3473. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Jian, H.; Huang, Z.; Wang, Y.; Guo, Z.; Gao, R. Design and Construction of an Effective Expression System with Aldehyde Tag for Site-Specific Enzyme Immobilization. Catalysts 2020, 10, 410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).