Harnessing Alcohol Dehydrogenases in Organic Reaction Cascades: A Strategy for Enhanced Efficiency in Synthetic Organic Chemistry
Abstract
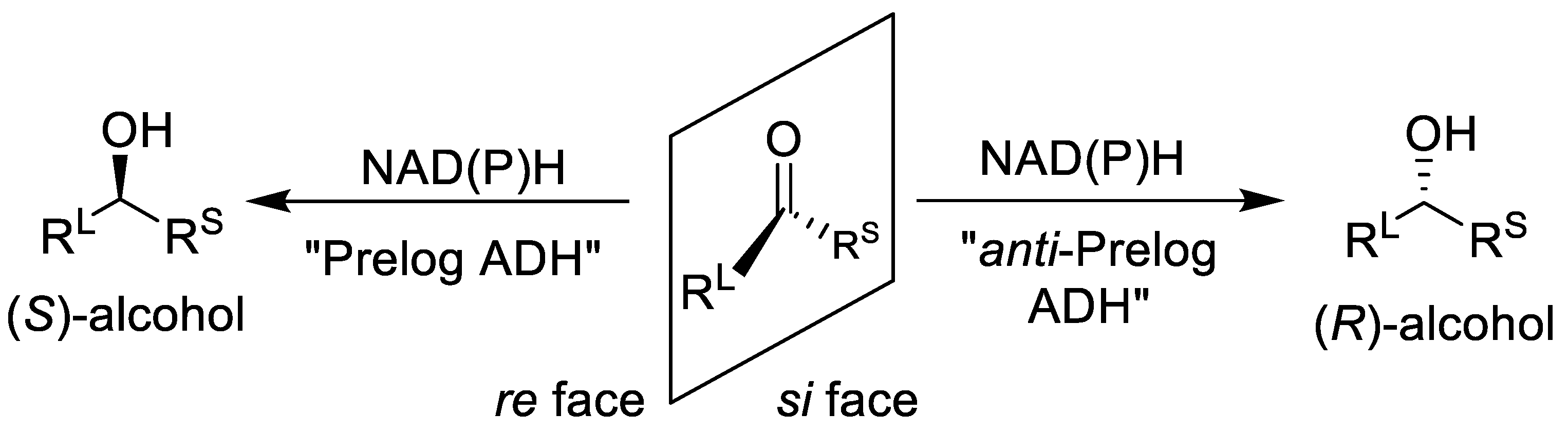
1. Introduction
2. One-Pot, Two-Step Reactions Utilizing Alcohol Dehydrogenases
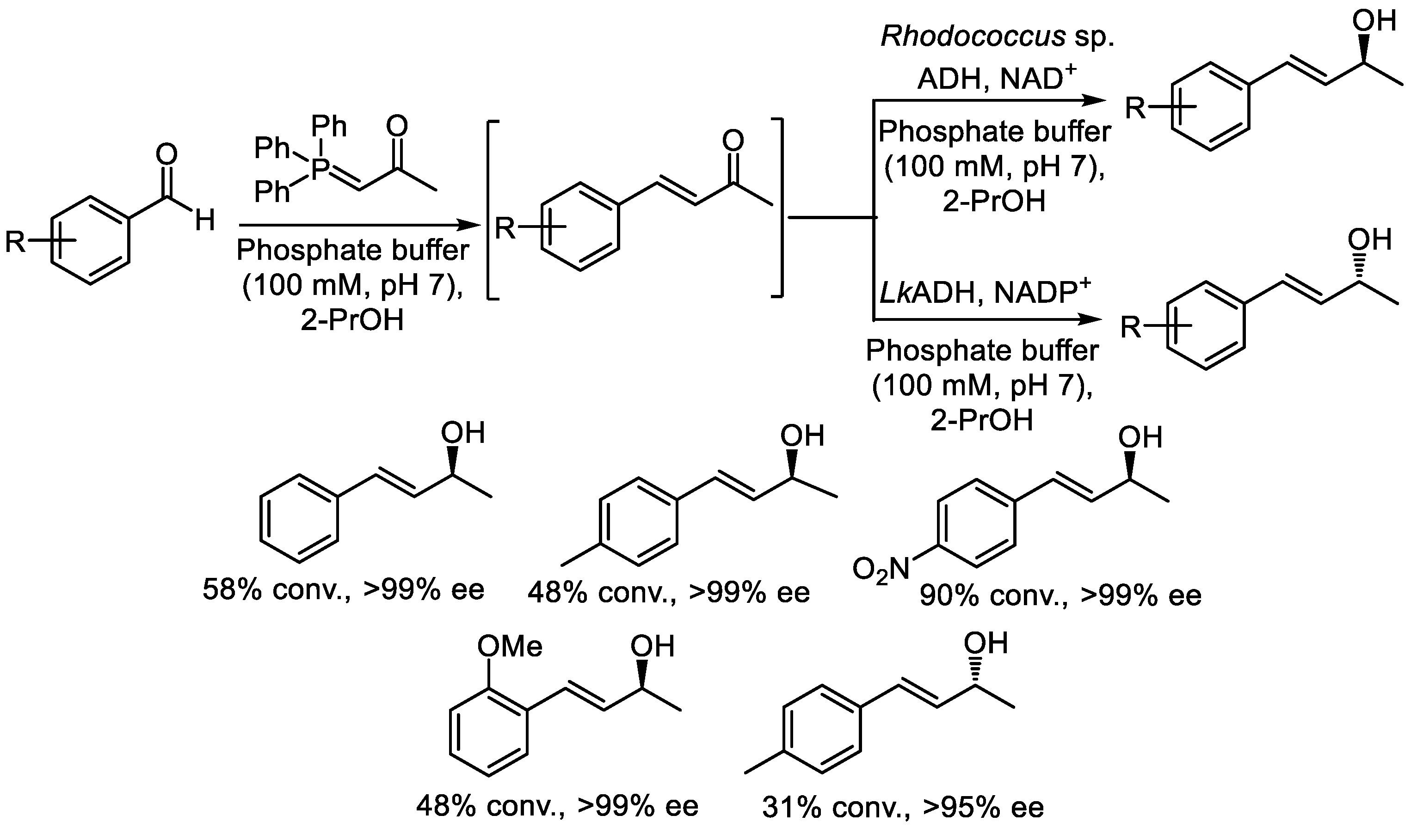
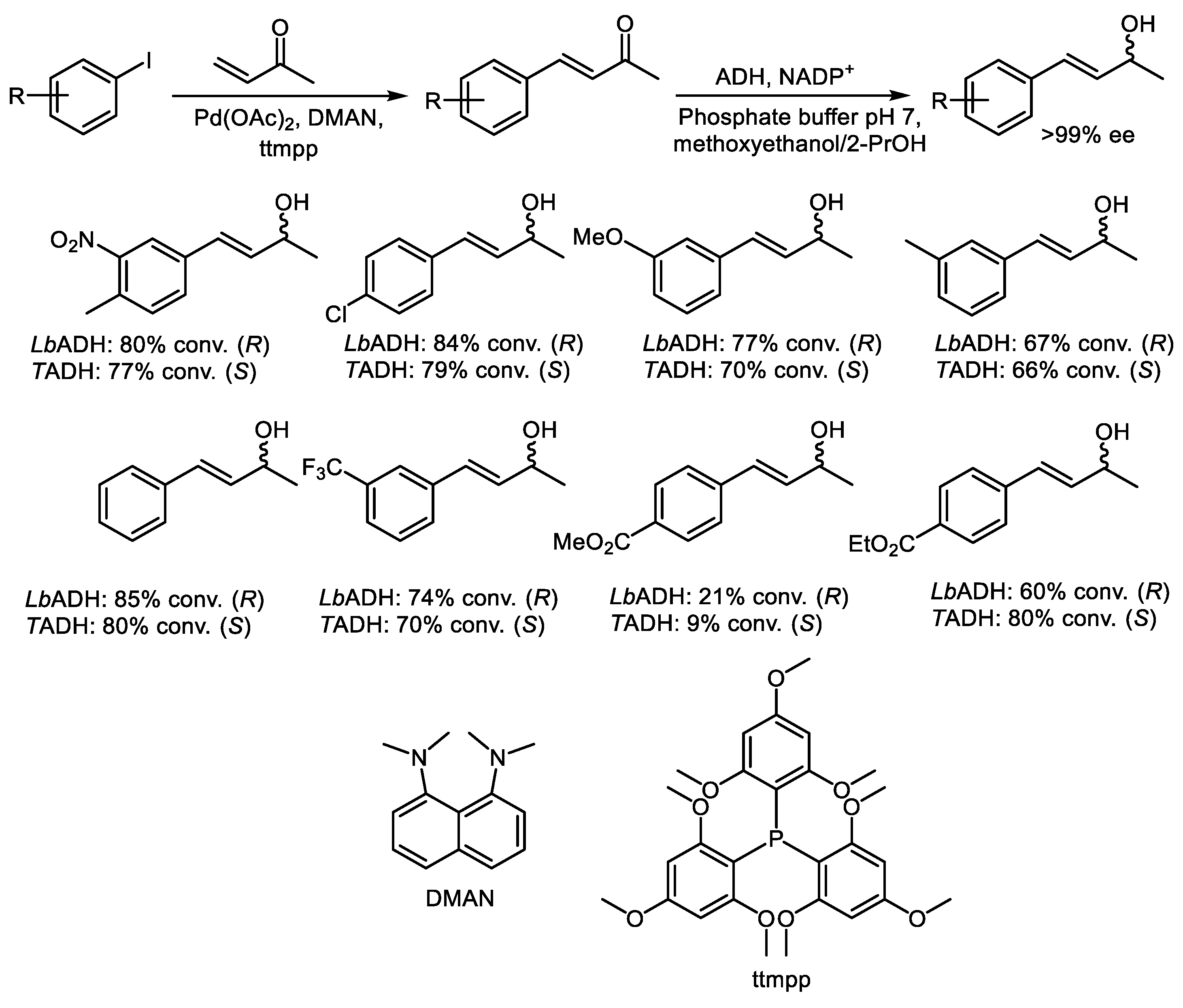
2.1. Two-Step Sequential Reactions in a One-Pot Setup
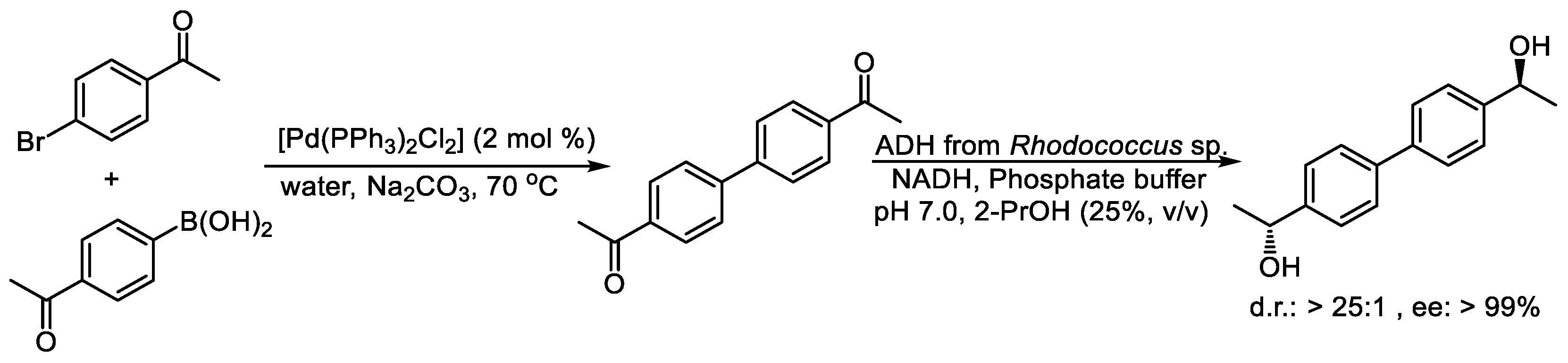
2.2. Concurrent Reaction Cascades Involving Alcohol Dehydrogenases
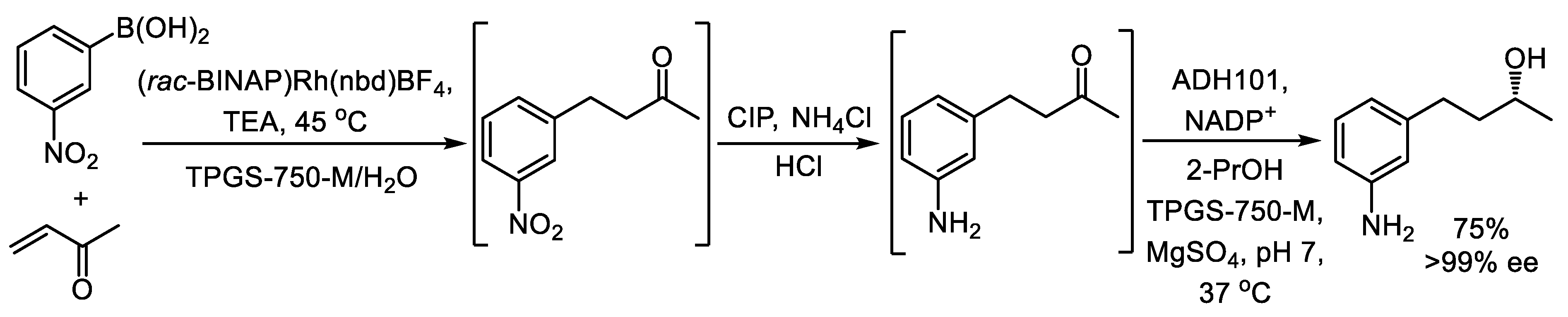
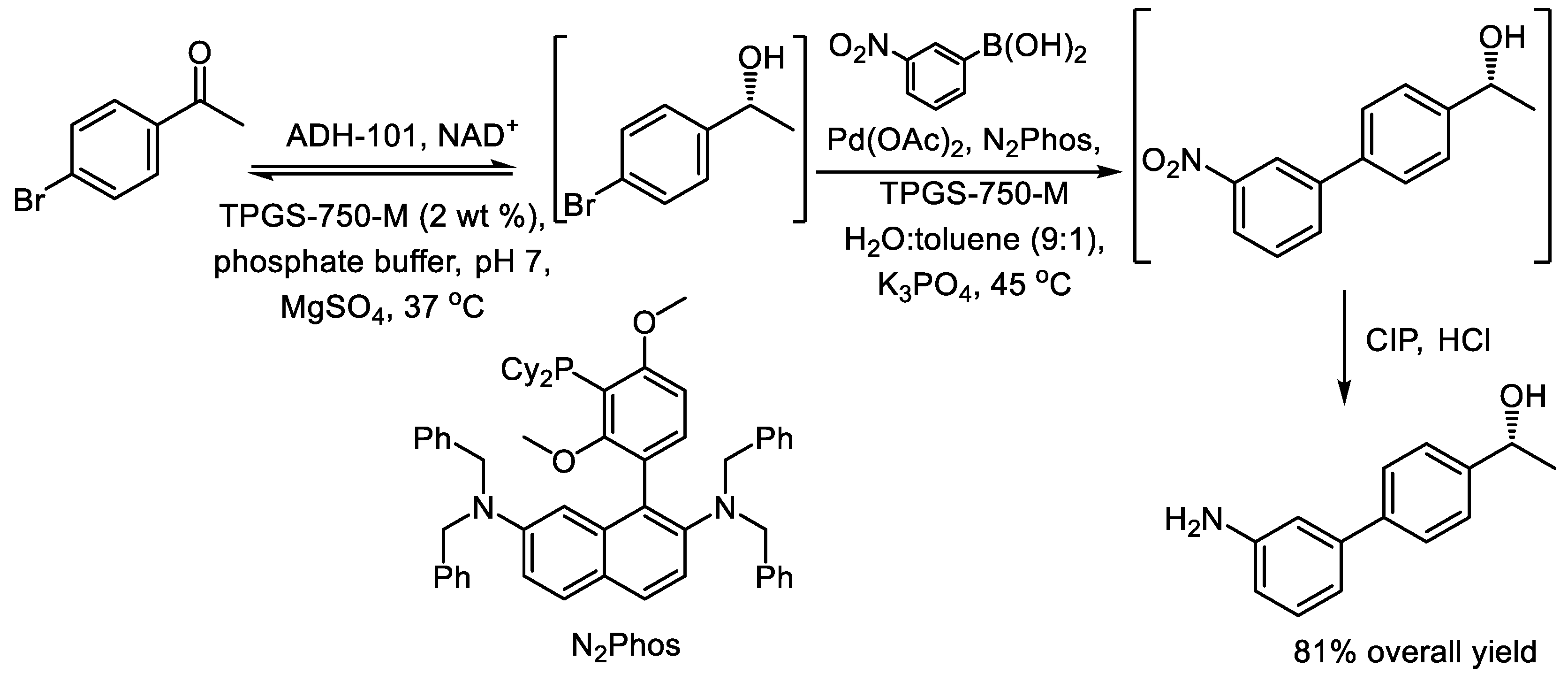
3. One-Pot, Three-Step Reaction Cascades Utilizing Alcohol Dehydrogenases
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Broadwater, S.J.; Roth, S.L.; Price, K.E.; Kobašlija, M.; McQuade, D.T. One-pot multi-step synthesis: A challenge spawning innovation. Org. Biomol. Chem. 2005, 16, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, W. Recent developments in one-pot stepwise synthesis (OPSS) of small molecules. iScience 2022, 25, 105005. [Google Scholar] [CrossRef] [PubMed]
- Kornet, M.M.; Müller, T.J.J. Recent Advances in Sequentially Pd-Catalyzed One-Pot Syntheses of Heterocycles. Molecules 2024, 29, 5265. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C. Green and sustainable chemistry—The case for a systems-based, interdisciplinary approach. iScience 2021, 24, 103489. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 4, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Bommarius, A.S. Enantioenriched compounds via enzyme-catalyzed redox reactions. Chem. Rev. 2011, 111, 4088–4110. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, A.S.; Milagre, C.D.; Hollmann, F. Alcohol Dehydrogenases as Catalysts in Organic Synthesis. Front. Catal. 2022, 2, 900554. [Google Scholar] [CrossRef]
- Chadha, A.; Padhi, S.K.; Stella, S.; Venkataraman, S.; Saravanan, T. Microbial alcohol dehydrogenases: Recent developments and applications in asymmetric synthesis. Org. Biomol. Chem. 2024, 22, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, J. Biocatalytic ketone reduction: A green and efficient access to enantiopure alcohols. Biotechnol. Adv. 2012, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.M.; Vieille, C.; Phillips, R.S. Secondary Alcohol Dehydrogenases from Thermoanaerobacter pseudoethanolicus and Thermoanaerobacter brockii as Robust Catalysts. ChemBioChem 2021, 22, 1884–1893. [Google Scholar] [CrossRef]
- Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Recent advances in the biocatalytic reduction of ketones and oxidation of sec -alcohols. Curr. Opin. Chem. Biol. 2004, 8, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Prelog, V. Specification of the stereospecificity of some oxido-reductases by diamond lattice sections. Pure Appl. Chem. 1964, 9, 119–130. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Liu, J.; Huang, Z.; Chen, F. Application of Ketoreductase in Asymmetric Synthesis of Pharmaceuticals and Bioactive Molecules: An Update (2018–2020). Chem. Rec. 2021, 7, 1611–1630. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Xu, Y. Structure-based mechanisms: On the way to apply alcohol dehydrogenases/reductases to organic-aqueous systems. Int. J. Biol. Macromol. 2021, 7, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Nealon, C.M.; Musa, M.M.; Patel, J.M.; Phillips, R.S. Controlling Substrate Specificity and Stereospecificity of Alcohol Dehydrogenases. ACS Catal. 2015, 5, 2100–2114. [Google Scholar] [CrossRef]
- Musa, M.M. Alcohol Dehydrogenases with anti-Prelog Stereopreference in Synthesis of Enantiopure Alcohols. ChemistryOpen 2022, 11, e202100251. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Bering, L.; Thompson, J.; Micklefield, J. New reaction pathways by integrating chemo- and biocatalysis. Trends Chem. 2022, 4, 392–408. [Google Scholar] [CrossRef]
- Musa, M.M.; Hollmann, F.; Mutti, F.G. Synthesis of enantiomerically pure alcohols and amines via biocatalytic deracemisation methods. Catal. Sci. Technol. 2019, 9, 5487–5503. [Google Scholar] [CrossRef] [PubMed]
- Rachwalski, M.; Vermue, N.; Rutjes, F.P.J.T. Recent advances in enzymatic and chemical deracemisation of racemic compounds. Chem. Soc. Rev. 2013, 42, 9268–9282. [Google Scholar] [CrossRef] [PubMed]
- Kraußer, M.; Hummel, W.; Gröger, H. Enantioselective One-Pot Two-Step Synthesis of Hydrophobic Allylic Alcohols in Aqueous Medium through the Combination of a Wittig Reaction and an Enzymatic Ketone Reduction. Eur. J. Org. Chem. 2007, 31, 5175–5179. [Google Scholar] [CrossRef]
- Dambacher, J.; Zhao, W.; El-Batta, A.; Anness, R.; Jiang, C.; Bergdahl, M. Water is an efficient medium for Wittig reactions employing stabilized ylides and aldehydes. Tetrahedron Lett. 2005, 46, 4473–4477. [Google Scholar] [CrossRef]
- Sgalla, S.; Fabrizi, G.; Cirilli, R.; Macone, A.; Bonamore, A.; Boffi, A.; Cacchi, S. Chiral (R)- and (S)-allylic alcohols via a one-pot chemoenzymatic synthesis. Tetrahedron Asymmetry 2007, 18, 2791–2796. [Google Scholar] [CrossRef]
- Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Combined Suzuki Coupling—Wittig Olefination Reaction in Aqueous Medium. Z. Für Naturforsch B 2005, 60, 1299–1307. [Google Scholar] [CrossRef]
- Franzén, R.; Xu, Y. Review on green chemistry; Suzuki cross coupling in aqueous media. Can. J. Chem. 2005, 83, 266–272. [Google Scholar] [CrossRef]
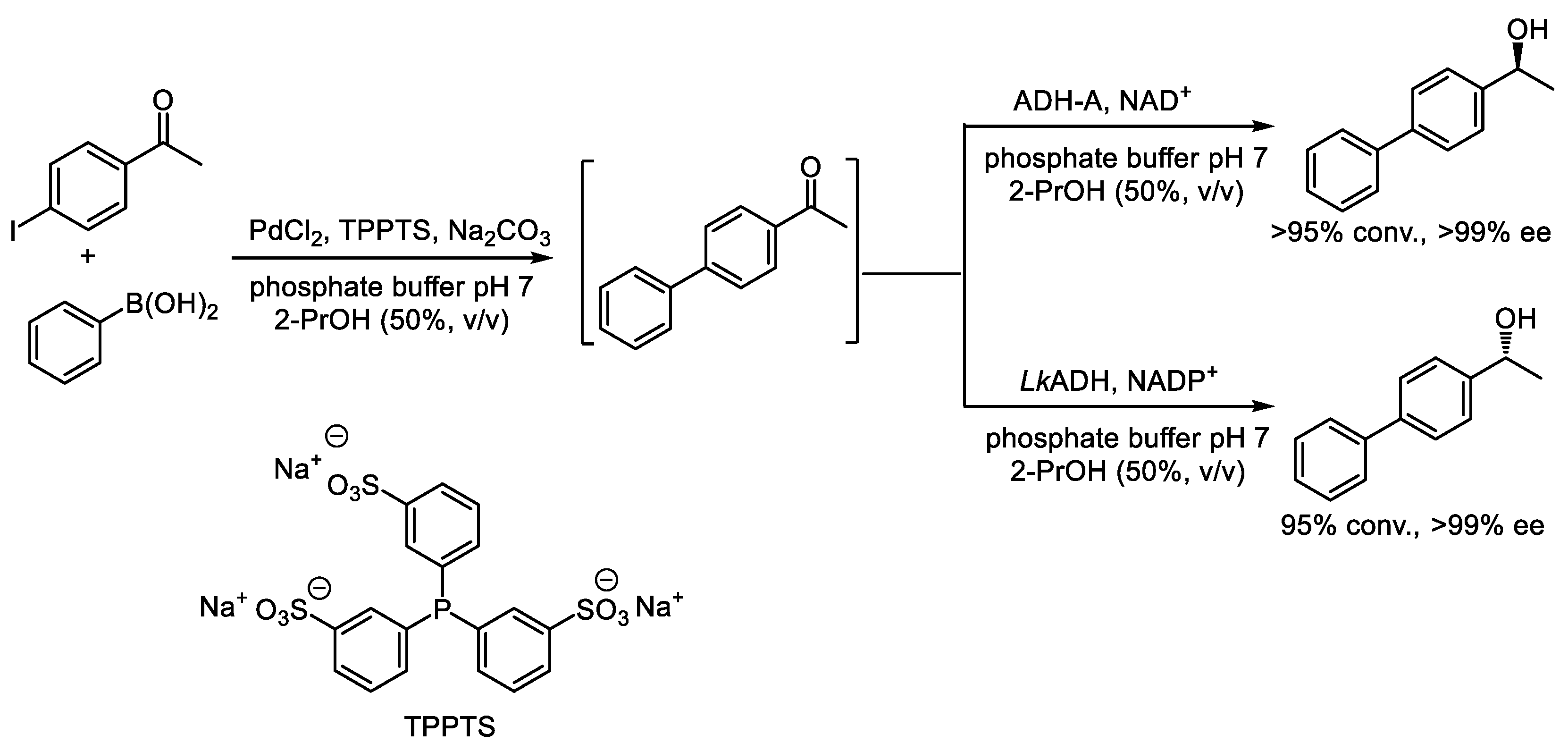
- Burda, E.; Hummel, W.; Gröger, H. Modular chemoenzymatic one-pot syntheses in aqueous media: Combination of a palladium-catalyzed cross-coupling with an asymmetric biotransformation. Angew. Chem. Int. Ed. 2008, 47, 9551–9554. [Google Scholar] [CrossRef] [PubMed]
- Burda, E.; Bauer, W.; Hummel, W.; Gröger, H. Enantio- and Diastereoselective Chemoenzymatic Synthesis of C2-Symmetric Biaryl-Containing Diols. ChemCatChem 2010, 2, 67–72. [Google Scholar] [CrossRef]
- Borchert, S.; Burda, E.; Schatz, J.; Hummel, W.; Gröger, H. Combination of a Suzuki cross-coupling reaction using a water-soluble palladium catalyst with an asymmetric enzymatic reduction towards a one-pot process in aqueous medium at room temperature. J. Mol. Catal. B Enzym. 2012, 84, 89–93. [Google Scholar] [CrossRef]
- González-Martínez, D.; Gotor, V.; Gotor-Fernández, V. Chemoenzymatic Synthesis of an Odanacatib Precursor through a Suzuki-Miyaura Cross-Coupling and Bioreduction Sequence. ChemCatChem 2019, 23, 5800–5807. [Google Scholar] [CrossRef]
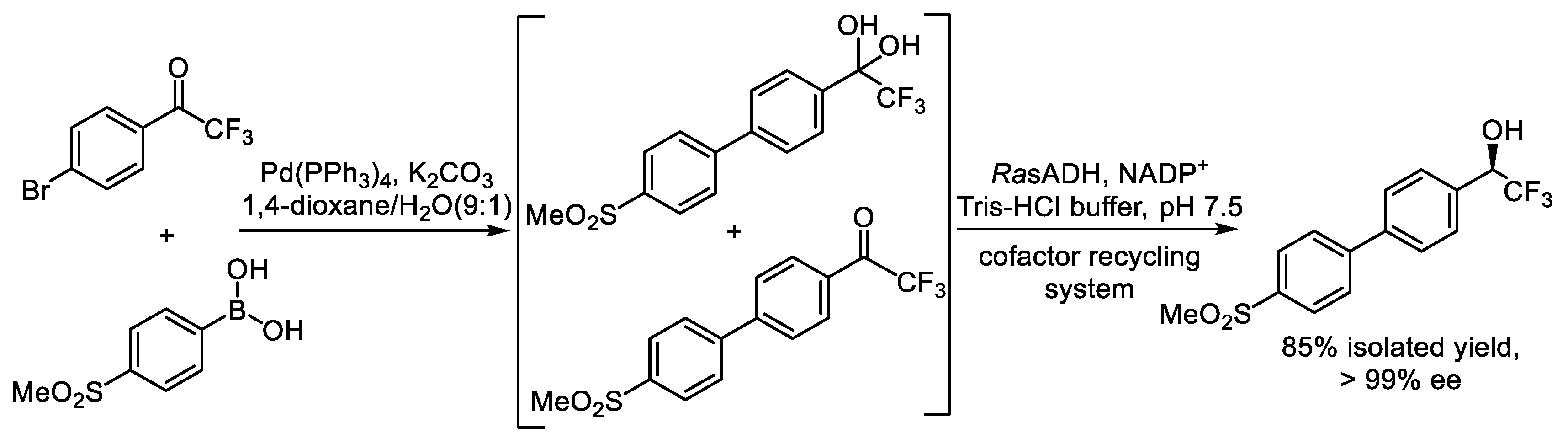
- Li, Y.; Liu, G.; Zhou, L.; Ma, L.; He, Y.; Gao, J.; Jiang, Y.; Ren, L.; Liu, Y. Resin-Immobilized Palladium Acetate and Alcohol Dehydrogenase for Chemoenzymatic Enantioselective Synthesis of Chiral Diarylmethanols. J. Org. Chem. 2024, 7, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Schnapperelle, I.; Hummel, W.; Gröger, H. Formal Asymmetric Hydration of Non-Activated Alkenes in Aqueous Medium through a “Chemoenzymatic Catalytic System. Chem. Eur. J. 2012, 18, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, P.; Gojic, V.; Bayer, T.; Rudroff, F.; Schnürch, M.; Mihovilovic, M.D. Easy Access to Enantiopure (S)- and (R)-Aryl Alkyl Alcohols by a Combination of Gold(III)-Catalyzed Alkyne Hydration and Enzymatic Reduction. ChemCatChem 2018, 10, 920–924. [Google Scholar] [CrossRef]
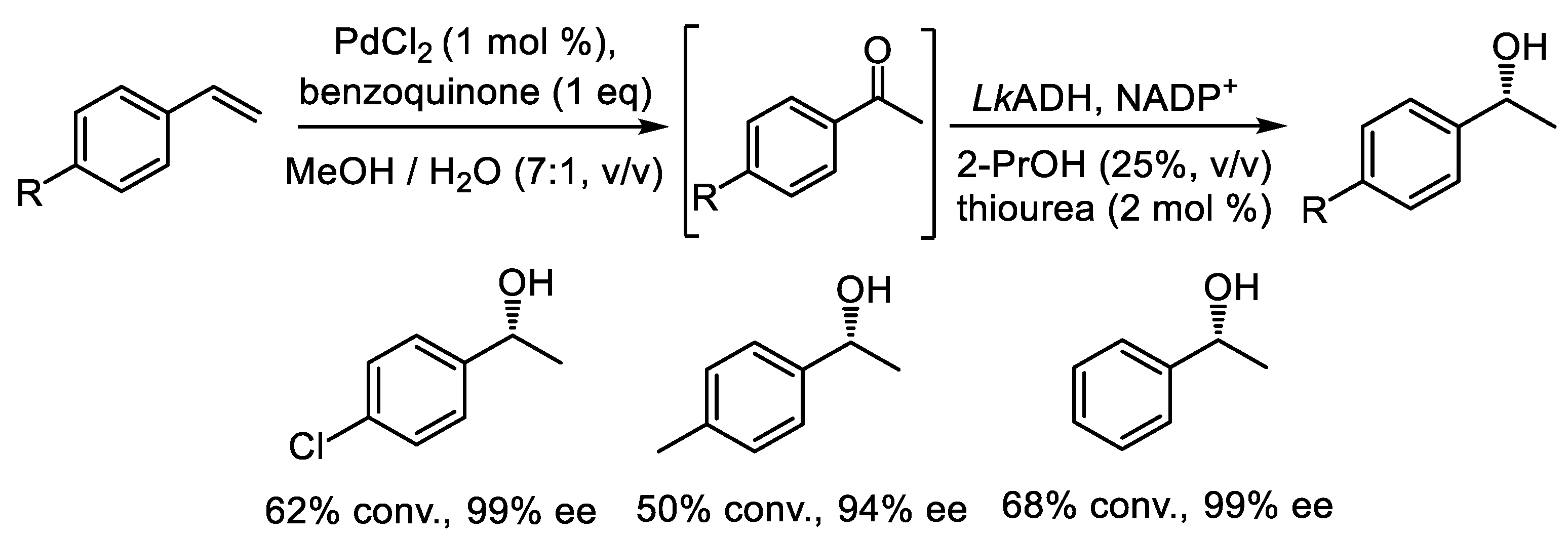
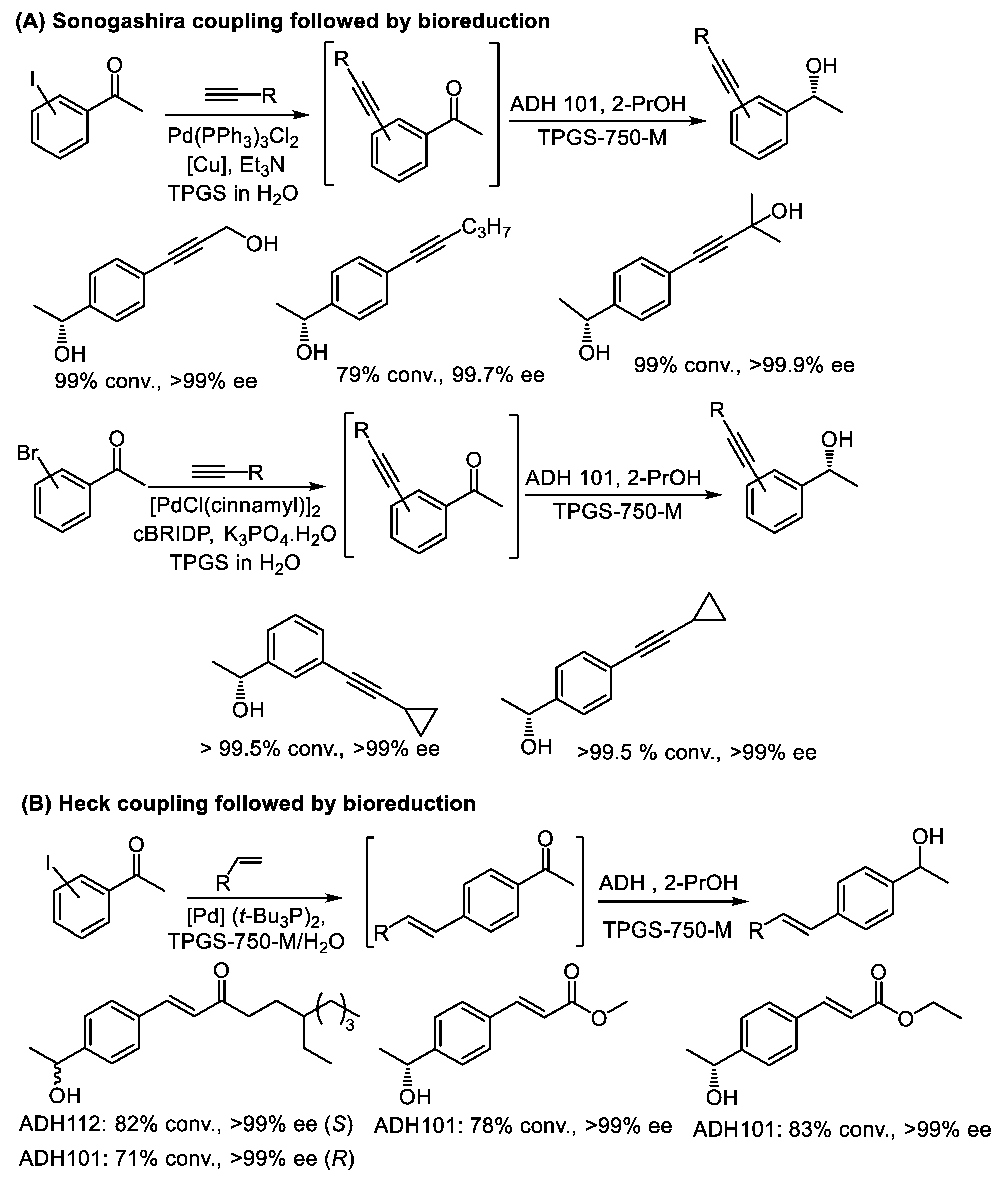
- Cortes-Clerget, M.; Akporji, N.; Zhou, J.; Gao, F.; Guo, P.; Parmentier, M.; Gallou, F.; Berthon, J.; Lipshutz, B.H. Bridging the gap between transition metal- and bio-catalysis via aqueous micellar catalysis. Nat. Commun. 2019, 10, 2169. [Google Scholar] [CrossRef]
- López-Agudo, M.; Ríos-Lombardía, N.; González-Sabín, J.; Lavandera, I.; Gotor-Fernández, V. Chemoenzymatic Oxosulfonylation-Bioreduction Sequence for the Stereoselective Synthesis of β-Hydroxy Sulfones. ChemSusChem 2022, 15, e202101313. [Google Scholar] [CrossRef]
- Raj, M.; Vishnumaya, G.; Sandeep, K.; Singh, V.K. Highly Enantioselective Direct Aldol Reaction Catalyzed by Organic Molecules. Org. Lett. 2006, 8, 4097–4099. [Google Scholar] [CrossRef]
- Baer, K.; Kraußer, M.; Burda, E.; Hummel, W.; Berkessel, A.; Gröger, H. Sequential and modular synthesis of chiral 1,3-diols with two stereogenic centers: Access to all four stereoisomers by combination of organo- and biocatalysis. Angew. Chem. Int. Ed. 2009, 48, 9355–9358. [Google Scholar] [CrossRef] [PubMed]
- Rulli, G.; Duangdee, N.; Baer, K.; Hummel, W.; Berkessel, A.; Gröger, H. Direction of Kinetically versus Thermodynamically Controlled Organocatalysis and Its Application in Chemoenzymatic Synthesis. Angew. Chem. Int. Ed. 2011, 50, 7944–7947. [Google Scholar] [CrossRef] [PubMed]
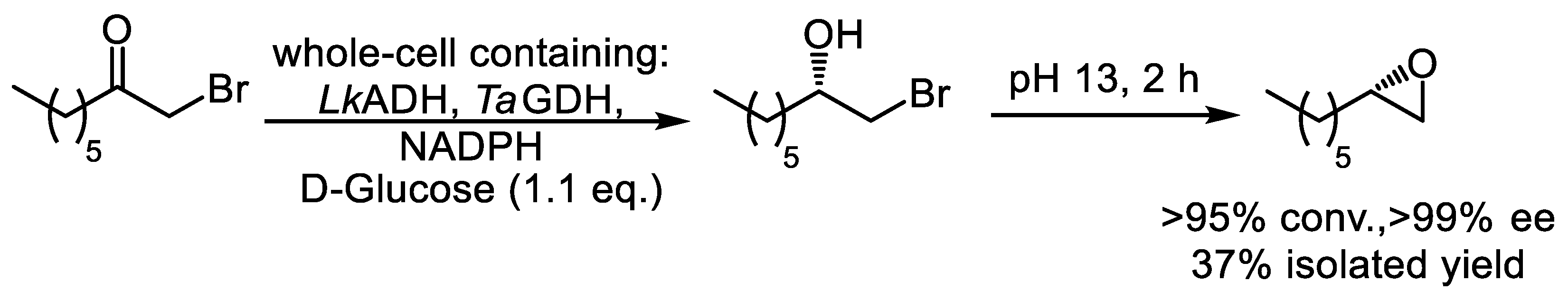
- Berkessel, A.; Rollmann, C.; Chamouleau, F.; Labs, S.; May, O.; Gröger, H. Practical two-step synthesis of an enantiopure aliphatic terminal (S)-epoxide based on reduction of haloalkanones with ‘designer cells. Adv. Synth. Catal. 2007, 349, 2697–2704. [Google Scholar] [CrossRef]
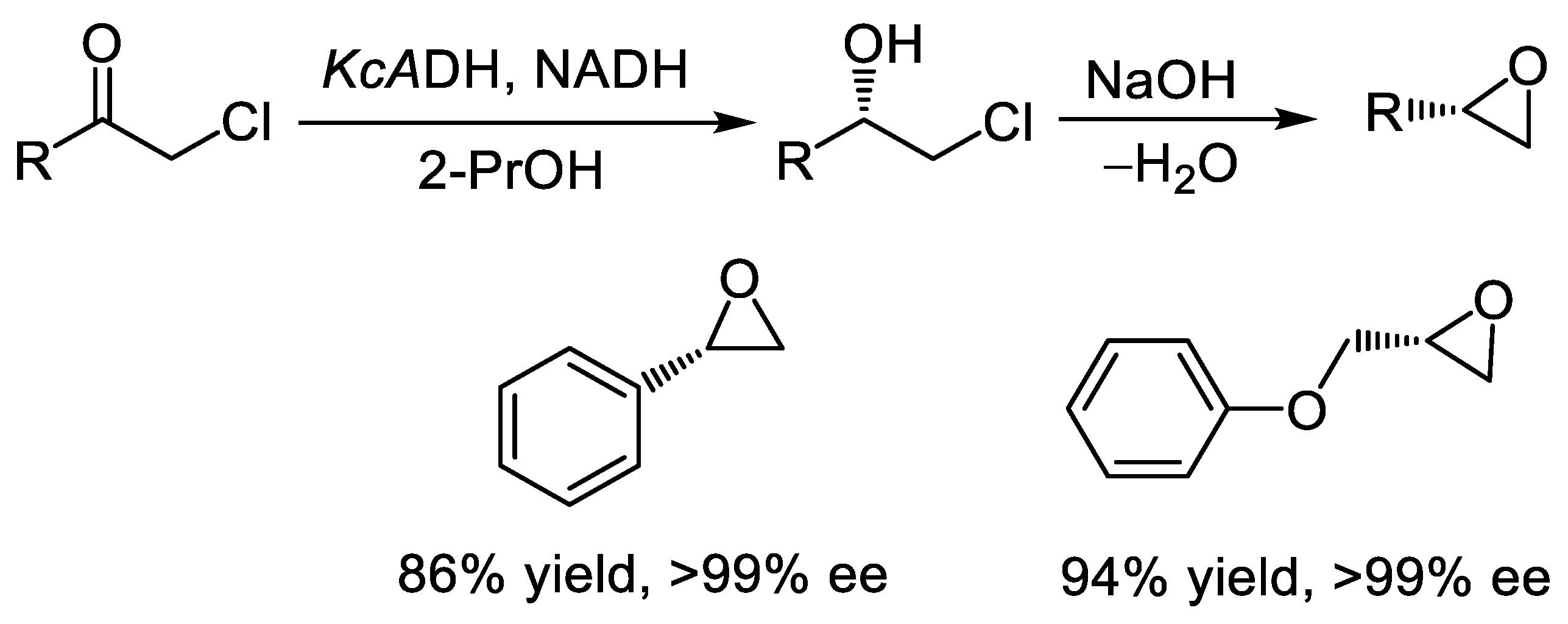
- Wu, K.; Chen, L.; Fan, H.; Zhao, Z.; Wang, H.; Wei, D. Synthesis of enantiopure epoxide by ‘one pot’ chemoenzymatic approach using a highly enantioselective dehydrogenase. Tetrahedron Lett. 2016, 57, 899–904. [Google Scholar] [CrossRef]
- Schaaf, P.; Gojic, V.; Bayer, T.; Rudroff, F.; Schnürch, M.; Mihovilovic, M.D. Biocompatible metal-assisted C-C cross-coupling combined with biocatalytic chiral reductions in a concurrent tandem cascade. Chem. Commun. 2018, 54, 12978–12981. [Google Scholar] [CrossRef] [PubMed]
- Liardo, E.; González-Fernández, R.; Ríos-Lombardía, N.; Morís, F.; García-Álvarez, J.; Cadierno, V.; Crochet, P.; Rebolledo, F.; González-Sabín, J. Strengthening the Combination between Enzymes and Metals in Aqueous Medium: Concurrent Ruthenium-Catalyzed Nitrile Hydration—Asymmetric Ketone Bioreduction. ChemCatChem 2018, 10, 4676–4682. [Google Scholar] [CrossRef]
- González-Granda, S.; Lavandera, I.; Gotor-Fernández, V. Alcohol Dehydrogenases and N-Heterocyclic Carbene Gold(I) Catalysts: Design of a Chemoenzymatic Cascade towards Optically Active β,β-Disubstituted Allylic Alcohols. Angew. Chem. Int. Ed. 2021, 60, 13945–13951. [Google Scholar] [CrossRef] [PubMed]
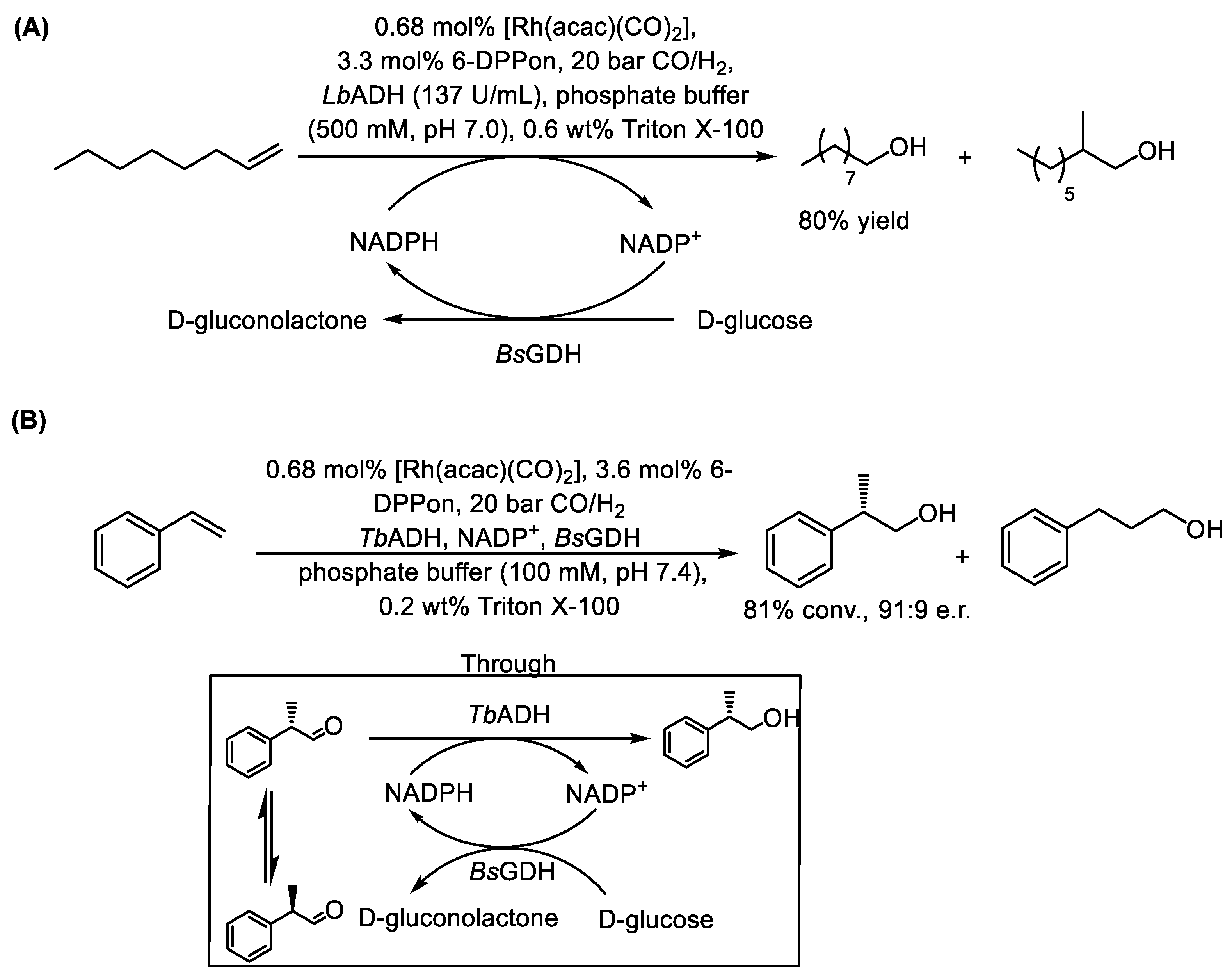
- Bork, H.; Naße, K.; Vorholt, A.J.; Gröger, H. Merging High-Pressure Syngas Metal Catalysis and Biocatalysis in Tandem One-Pot Processes for the Synthesis of Nonchiral and Chiral Alcohols from Alkenes in Water. Angew. Chem. Int. Ed. 2024, 63, e202401989. [Google Scholar] [CrossRef]
- Poessl, T.M.; Kosjek, B.; Ellmer, U.; Gruber, C.C.; Edegger, K.; Faber, K.; Hildebrandt, P.; Bornscheuer, U.T.; Kroutil, W. Non-Racemic Halohydrins via Biocatalytic Hydrogen-Transfer Reduction of Halo-Ketones and One-Pot Cascade Reaction to Enantiopure Epoxides. Adv. Synth. Catal. 2005, 347, 1827–1834. [Google Scholar] [CrossRef]
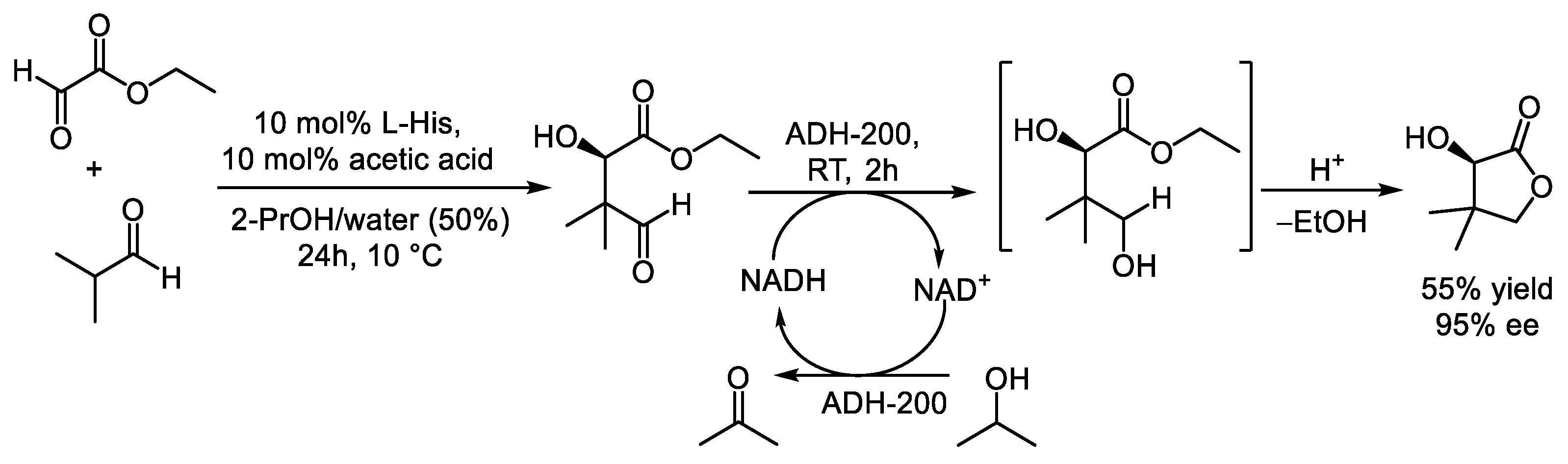
- Heidlindemann, M.; Hammel, M.; Scheffler, U.; Mahrwald, R.; Hummel, W.; Berkessel, A.; Gröger, H. Chemoenzymatic Synthesis of Vitamin B5-Intermediate (R)-Pantolactone via Combined Asymmetric Organo- and Biocatalysis. J. Org. Chem. 2015, 80, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Gallou, F.; Isley, N.A.; Ganic, A.; Onken, U.; Parmentier, M. Surfactant technology applied toward an active pharmaceutical ingredient: More than a simple green chemistry advance. Green Chem. 2016, 18, 14–19. [Google Scholar] [CrossRef]
- Akporji, N.; Thakore, R.; Cortes-Clerget, M.; Andersen, J.; Landstrom, E.; Aue, D.H.; Gallou, F.; Lipshutz, B.H. N2Phos—An easily made, highly effective ligand designed for ppm level Pd-catalyzed Suzuki-Miyaura cross couplings in water. Chem. Sci. 2020, 11, 5205–5212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed Evolution: Methodologies and Applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with Alpha Fold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzenah, H.; Abdulrasheed, M.; Sardauna, A.E.; Al-Qataisheh, B.; Musa, M.M. Harnessing Alcohol Dehydrogenases in Organic Reaction Cascades: A Strategy for Enhanced Efficiency in Synthetic Organic Chemistry. Catalysts 2025, 15, 223. https://doi.org/10.3390/catal15030223
Abuzenah H, Abdulrasheed M, Sardauna AE, Al-Qataisheh B, Musa MM. Harnessing Alcohol Dehydrogenases in Organic Reaction Cascades: A Strategy for Enhanced Efficiency in Synthetic Organic Chemistry. Catalysts. 2025; 15(3):223. https://doi.org/10.3390/catal15030223
Chicago/Turabian StyleAbuzenah, Heba, Muhammad Abdulrasheed, Auwal Eshi Sardauna, Bayan Al-Qataisheh, and Musa M. Musa. 2025. "Harnessing Alcohol Dehydrogenases in Organic Reaction Cascades: A Strategy for Enhanced Efficiency in Synthetic Organic Chemistry" Catalysts 15, no. 3: 223. https://doi.org/10.3390/catal15030223
APA StyleAbuzenah, H., Abdulrasheed, M., Sardauna, A. E., Al-Qataisheh, B., & Musa, M. M. (2025). Harnessing Alcohol Dehydrogenases in Organic Reaction Cascades: A Strategy for Enhanced Efficiency in Synthetic Organic Chemistry. Catalysts, 15(3), 223. https://doi.org/10.3390/catal15030223






