Nickel-Decorated Carbocatalysts for the UV-Driven Photodegradation of Rhodamine B
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Textural and Porosimetry Properties
2.1.2. Morphology and Composition
2.1.3. Structural Characterization
2.2. Adsorption and Photodegradation of RhB
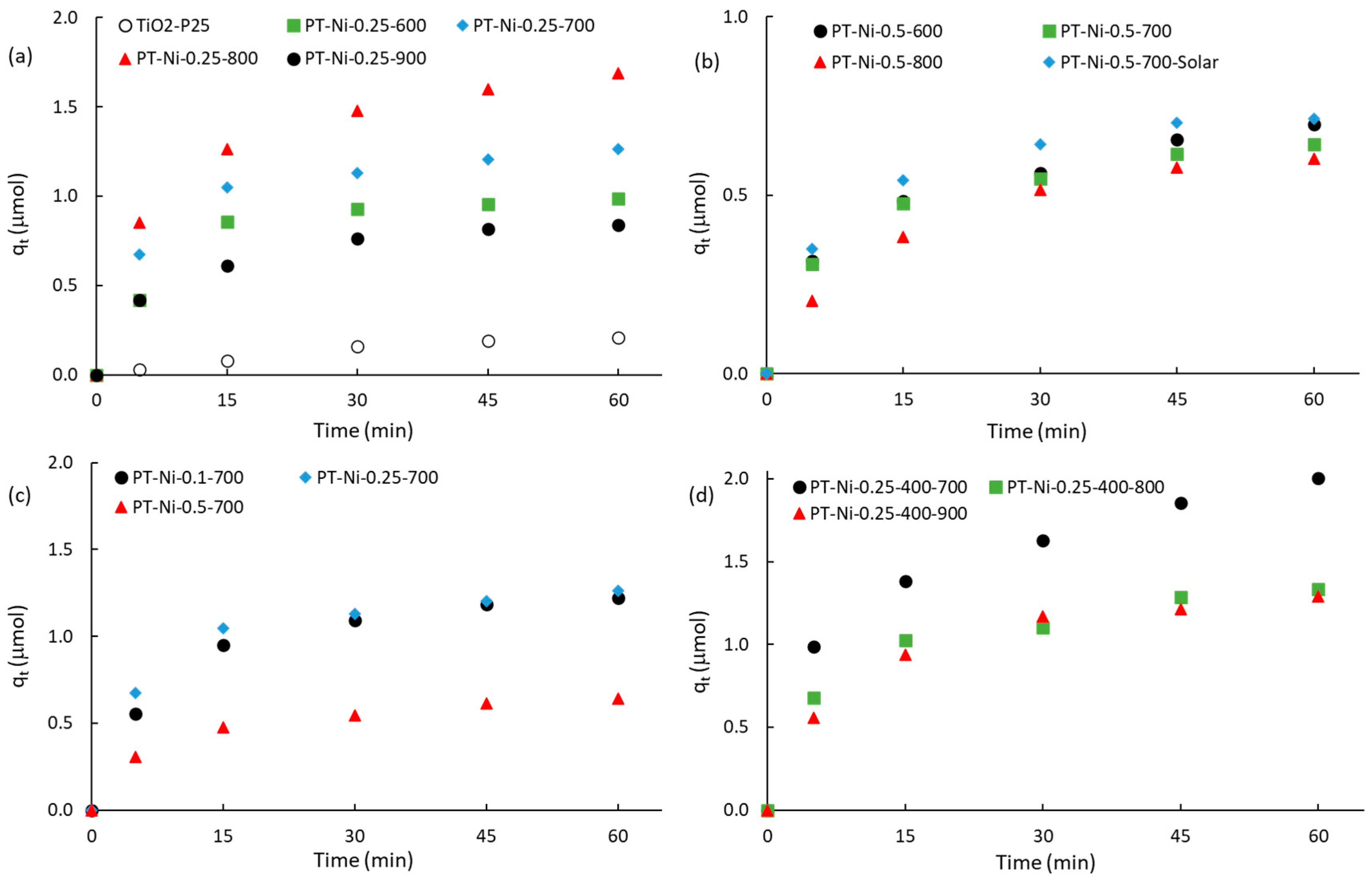
2.2.1. Kinetics of RhB Adsorption in the Dark
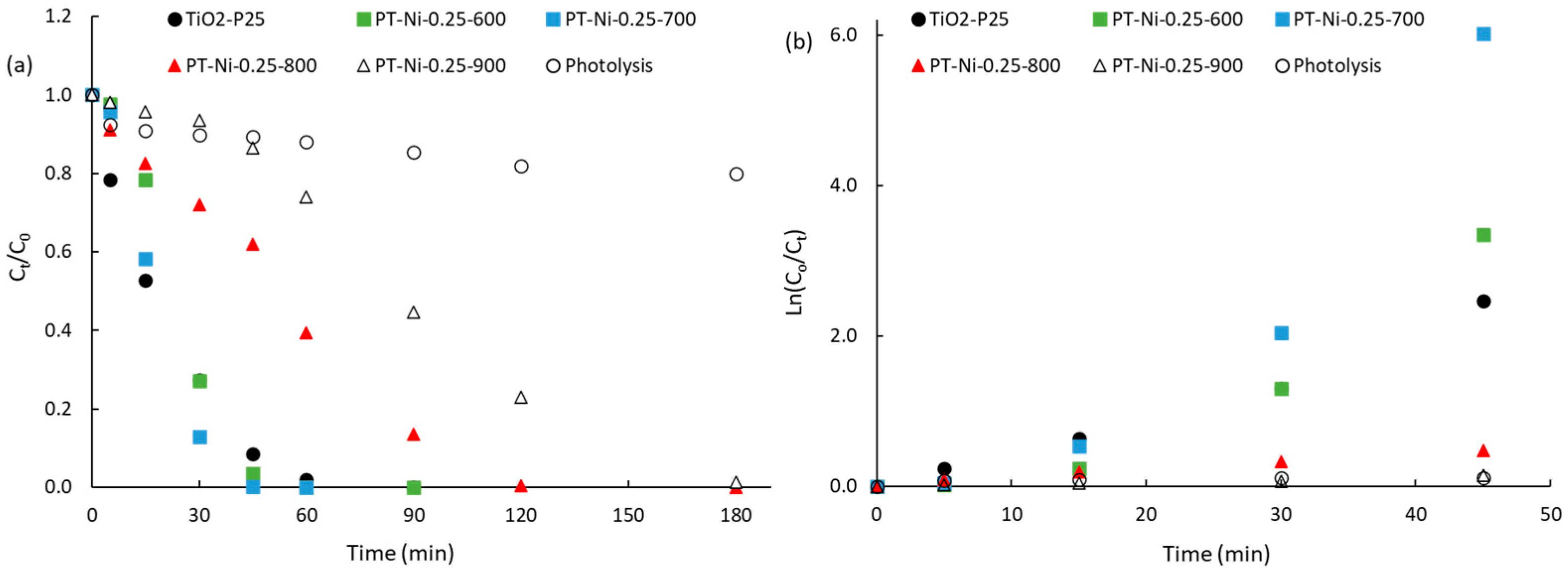
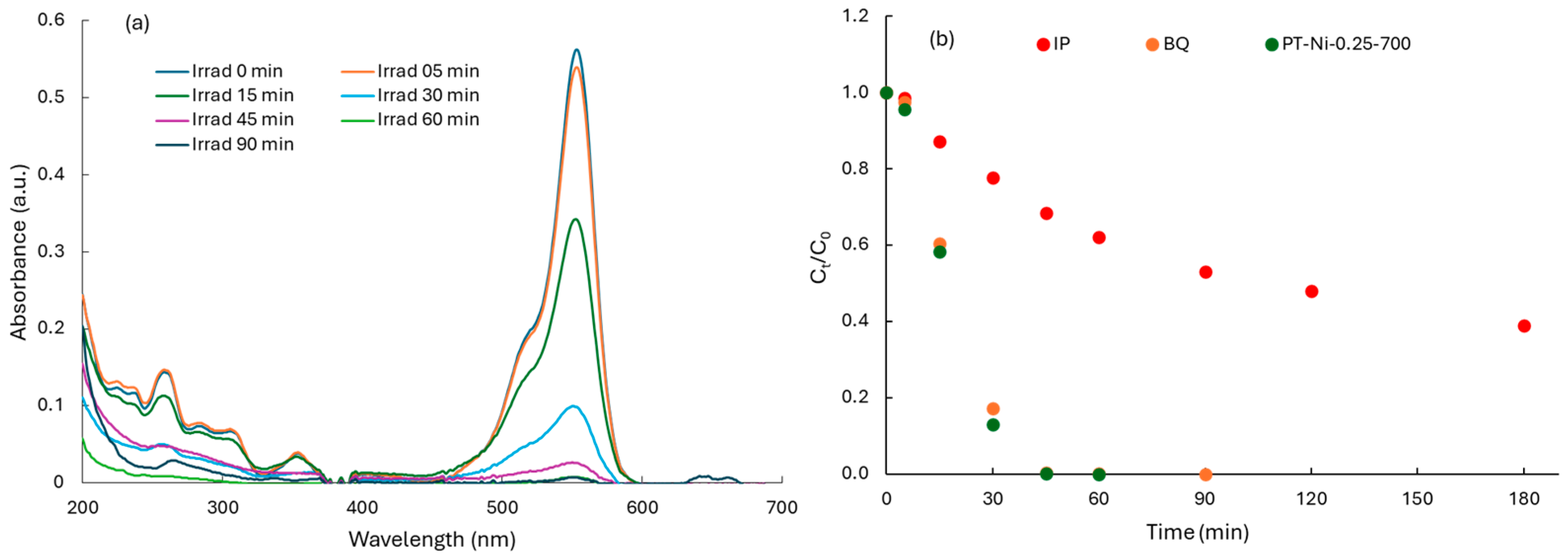
2.2.2. Photocatalytic Degradation of RhB
2.3. General Discussion
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Two-Step Pyrolysis
3.1.2. One-Step Pyrolysis
3.2. Characterization
3.3. Photocatalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Wivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar Singh, C.; Kamesh Misra, S.; Singh, B.P.; Bhardwaj, A.K.; Chandra, K.K. Water biodiversity: Ecosystem services, threats, and conservation. In Biodiversity and Bioeconomy; Singh, K., Ribeiro, M.C., Calicioglu, Ö., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 347–380. [Google Scholar]
- Ojha, A.; Tiwary, D. Organic pollutants in water and its health risk assessment through consumption. In Contamination of Water; Ahamad, A., Siddiqui, S.I., Singh, P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 237–250. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Kumar, P.S.; Mohamed, R.M.S.R.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of rhodamine B from textile wastewater using nanoparticle photocatalysts: A review for sustainable approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye wastewater. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Muñoz-Flores, P.; Poon, P.S.; Ania, C.O.; Matos, J. Performance of a C-containing Cu-based photocatalyst for the degradation of tartrazine: Comparison of performance in a slurry and CPC photoreactor under artificial and natural solar light. J. Colloid. Interface Sci. 2022, 623, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Flores, P.; Poon, P.S.; Sepulveda, C.; Ania, C.O.; Matos, J. Photocatalytic performance of carbon-containing CuMo-Based catalysts under sunlight illumination. Catalysts 2022, 12, 46. [Google Scholar] [CrossRef]
- Matos, J.; Poon, P.S.; Montaña, R.; Romero, R.; Gonçalves, G.R.; Schettino, A.A.; Passamani, E.C.; Freitas, J.C.C. Photocatalytic activity of P-Fe/activated carbon nanocomposites under artificial solar irradiation. Catal. Today 2020, 356, 226–240. [Google Scholar] [CrossRef]
- Lanfredi, S.; Nobre, M.A.L.; Poon, P.S.; Matos, J. Hybrid material based on an amorphous-carbon matrix and ZnO/Zn for the solar photocatalytic degradation of basic blue 41. Molecules 2020, 25, 96. [Google Scholar] [CrossRef]
- Bello, R.; Rodríguez-Aguado, E.; Smith, V.A.; Grachev, D.; Rodriguez-Castellón, E.; Bashkova, S. Ni-doped ordered nanoporous carbon prepared from chestnut wood tannins for the removal and photocatalytic degradation of methylene blue. Nanomaterials 2022, 12, 1625. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Das, B.; Dhara, H.N.; Dahiya, A.; Patel, B.K. Recent advances in photocatalytic degradation of dyes using heterogeneous catalysts. In Trends and Contemporary Technologies for Photocatalytic Degradation of Dyes. Environmental Science and Engineering; Dave, S., Das, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Konstantinou, I.K.; Albanis, A.T. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A review on transition metal oxides based photocatalysts for degradation of synthetic organic pollutants. J. Environ. Sci. 2024, 139, 389–417. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Wu, H.; Guo, S. Control of the formation of rod-like ZnO mesocrystals and their photocatalytic properties. CrystEngComm 2013, 15, 2608–2615. [Google Scholar] [CrossRef]
- Kozlov, Y.A.; Dorogov, M.V.; Chirkunova, N.V.; Sosnin, I.M.; Vikarchuk, A.A.; Romanov, A.E. CuO Nanowhiskers-based photocatalysts for wastewater treatment. Nano Hyb. Comp. 2017, 13, 183–189. [Google Scholar] [CrossRef]
- Huang, L.; Fang, W.; Yang, Y.; Wu, J.; Yu, H.; Dong, X.; Wang, T.; Liu, Z.; Zhao, B. Three-dimensional MoO3 nanoflowers assembled with nanosheets for rhodamine B degradation under visible light. Mater. Res. Bull. 2018, 108, 38–45. [Google Scholar] [CrossRef]
- Kumar, V.V.; Gayathri, K.; Anthony, S.P. Synthesis of α-MoO3 nanoplates using organic aliphatic acids and investigation of sunlight enhanced photodegradation of organic dyes. Mater. Res. Bull. 2016, 76, 147–154. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M. Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 2016, 155, 34–38. [Google Scholar] [CrossRef]
- Rajput, R.B.; Jamble, S.N.; Kale, R.B. A review on TiO2/SnO2 heterostructures as a photocatalyst for the degradation of dyes and organic pollutants. J. Environ. Manag. 2022, 307, 114533. [Google Scholar] [CrossRef]
- Vilé, G.; Sharma, P.; Nachtegaal, M.; Tollini, F.; Moscatelli, D.; Sroka-Bartnicka, A.; Tomanec, O.; Petr, M.; Filip, J.; Pieta, I.S.; et al. An Earth-abundant Ni-based single-atom catalyst for selective photodegradation of pollutants. Solar RRL 2021, 5, 2100176. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Akbari, A.; Sabouri, Z.; Hosseini, H.A.; Hashemzadeh, A.; Khatami, M.; Darroudi, M. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Inorg. Chem. Commun. 2020, 115, 107867. [Google Scholar] [CrossRef]
- Negash, A.; Mohammed, S.; Weldekirstos, H.D.; Ambaye, A.D.; Gashu, M. Enhanced photocatalytic degradation of methylene blue dye using eco-friendly synthesized rGO@ZnO nanocomposites. Sci. Rep. 2023, 13, 22234. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Xiong, Z.; Cheng, L.; Du, M.; Liu, Z.; Li, J.; Li, D. TiO2/carbon composites from waste sawdust for methylene blue photodegradation. Diam. Relat. Mater. 2023, 136, 109918. [Google Scholar] [CrossRef]
- Matos, J.; Ocares-Riquelme, J.; Poon, P.S.; Montaña, R.; García, X.; Campos, K.; Hernández-Garrido, J.C.; Titirici, M.M. C-doped anatase TiO2: Adsorption kinetics and photocatalytic degradation of methylene blue and phenol, and correlations with DFT estimations. J. Colloid. Inter. Sci. 2019, 547, 14–29. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- European Scientist. Available online: https://www.europeanscientist.com/fr/sante/la-france-va-interdire-le-dioxyde-de-titane-a-compter-de-2020/ (accessed on 2 April 2025).
- Fernández de Cordoba, M.C.; Matos, J.; Montaña, R.; Poon, P.S.; Lanfredi, S.; Praxedes, F.R.; Hernández-Garrido, J.C.; Calvino, J.J.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E.; et al. Sunlight photoactivity of rice husks-derived biogenic silica. Catal. Today 2019, 328, 125–135. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Libbrecht, W.; Verberckmoes, A.; Thybaut, J.W.; Van der Voort, P.; Clercq, J.D. Soft templated mesoporous carbons: Tuning the porosity for the adsorption of large organic pollutants. Carbon 2017, 116, 528–546. [Google Scholar] [CrossRef]
- Gang, D.; Ahmad, Z.U.; Lian, Q.; Yao, L.; Zappi, M.E. A review of adsorptive remediation of environmental pollutants from aqueous phase by ordered mesoporous carbon. Chem. Eng. J. 2021, 403, 1385–8947. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakatani, N.; Doi, A.; Miyake, Y. Preparation of ordered mesoporous carbon membranes by a soft-templating method. Carbon 2011, 49, 3184–3189. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, M.; Yang, J.; Shen, F.; Wang, X.; Qi, X. One-pot self-assembly synthesis of Ni-doped ordered mesoporous carbon for quantitative hydrogenation of furfural to furfuryl alcohol. Green Chem. 2021, 23, 1861–1870. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef]
- Matos, J.; Samudio-González, D.; Blanco, E.; Poon, P.S.; Escalona, N. Alkali-driven selectivity of products on carbon-supported Ni-based catalysts during the HDO of guaiacol. Fuel 2024, 374, 132442. [Google Scholar] [CrossRef]
- Yung, T.Y.; Huang, L.Y.; Chan, T.Y.; Wang, K.S.; Liu, T.Y.; Chen, P.T.; Chao, C.Y.; Liu, L.K. Synthesis and characterizations of Ni-NiO nanoparticles on PDDA-modified graphene for oxygen reduction reaction. Nanoscale Res. Lett. 2014, 9, 444. [Google Scholar] [CrossRef]
- Ding, K.; Chen, J.; Liu, Y.; Zhou, L.; Zhang, D.; Tian, W.; Sun, Y.; Pan, J.; Wang, H. Peony-shaped micron-sized NiO particles: Their excellent electrochemical performances as anode materials of lithium-ion batteries (LIBs). J. Solid State Electrochem. 2022, 26, 985–996. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Cao, Y.; Li, D.; Zhang, Z.; Wang, K.; Ding, F.; Wang, X.; Meng, D.; Fan, L.; et al. Size and morphology-controlled synthesis of Ni3C nanoparticles in a TEG solution and their magnetic properties. RSC Adv. 2016, 6, 81989–81994. [Google Scholar] [CrossRef]
- Matos, J.; Díaz, K.; García, V.; Cordero, T.C.; Brito, J.L. Methane transformation in presence of carbon dioxide on activated carbon supported nickel-calcium catalysts. Catal. Lett. 2006, 109, 163–169. [Google Scholar] [CrossRef]
- Matos, J.; Arcibar-Orozco, J.; Poon, P.S.; Pecchi, G.; Rangel-Mendez, J.R. Influence of phosphorous upon the formation of DMPO-•OH and POBN-O2•¯ spin-trapping adducts in carbon-supported P-promoted Fe-based photocatalysts. J. Photochem. Photobiol. A. Chem. 2020, 391, 112362. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. The removal of color from effluent using various adsorbents—III. Silica: Rate processes. Water Res. 1980, 14, 15–20. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G.A. Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Perez-Rodriguez, S.; Pinto, O.; Izquierdo, M.T.; Segura, C.; Poon, P.S.; Celzard, A.; Matos, J.; Fierro, V. Upgrading of pine tannin biochars as electrochemical capacitor electrodes. J. Coll. Interface Sci. 2021, 601, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Burgos, O.; Castro-Gutiérrez, J.; Poon, P.S.; Izquierdo, M.T.; Celzard, A.; Fierro, V.; Matos, J. Enhancing electrochemical capacitor performance of N-doped tannin-derived carbons by hydrothermal treatment in ammonia. J. Power Sources 2024, 602, 234332. [Google Scholar] [CrossRef]
- Rangel-Mendez, J.R.; Matos, J.; Cházaro-Ruiz, L.F.; González-Castillo, A.C.; Barrios-Yáñez, G. Microwave-assisted synthesis of C-doped TiO2 and ZnO hybrid nanostructured materials as quantum-dots sensitized solar cells. Appl. Surf. Sci. 2018, 434, 744–755. [Google Scholar] [CrossRef]
- Gonzalez-Vera, A.S.; Pineda-Arellano, C.A.; Matos, J.; Chazaro-Ruiz, L.F.; Ramírez-Monroy, A.; Rangel-Mendez, J.R.; Ania, C.O. Influence of the sequential purification of biomass-derived carbon dots on their colloidal and optical properties. Carbon Trends 2025, 19, 100460. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Abdelkader-Fernandeza, V.K.; Fayad, A.; Ania, C.O.; Praxedes, F.R.; Lanfredi, S.; Rodríguez-Castellón, E.; Rodríguez-Aguado, E.; Hernández-Garrido, J.C.; Quintana-González, J.J.; et al. Ni and Co-based bifunctional electrocatalysts supported on TiO2@C for oxygen evolution and reduction reactions. Electrochim. Acta 2024, 506, 145030. [Google Scholar] [CrossRef]
- Merka, O.; Yarovyi, V.; Bahnemann, D.W.; Wark, M. pH-Control of the photocatalytic degradation mechanism of Rhodamine B over Pb3Nb4O13. J. Phys. Chem. C 2011, 115, 8014–8023. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of Rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]
- Praxedes, F.R.; Nobre, M.A.L.; Lanfredi, S.; Poon, P.S.; Matos, J. Influence of the structural properties and W/Nb ratio upon the photocatalytic activity of tungsten-doped potassium sodium niobate-based perovskites. Mater. Res. Bull. 2025, 184, 113256. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J.; Herrmann, J.-M.; Uzcategui, D.; Brito, J.L. Influence of activated carbon upon Titania on aqueous photocatalytic consecutive runs of phenol photomineralization. Appl. Catal. B Environ. 2007, 70, 461–469. [Google Scholar] [CrossRef]
- Velasco, L.F.; Carmona, R.J.; Matos, J.; Ania, C.O. Performance of activated carbons in consecutive phenol photooxidation cycles. Carbon 2014, 73, 206–215. [Google Scholar] [CrossRef]
- Praxedes, F.R.; Nobre, M.A.L.; Olean-Oliveira, A.; Teixeira, M.F.S.; Poon, P.S.; Lanfredi, S.; Matos, J. W-doped niobate-based perovskites: Synthesis by ultrasonic spray pyrolysis and structural characterization. Ceram. Int. 2023, 49, 24004–24017. [Google Scholar] [CrossRef]
- Chu, C.Y.; Huang, M.H. Facet-dependent photocatalytic properties of Cu2O crystals probed by using electron, hole and radical scavengers. J. Mater. Chem. A 2017, 5, 15116–15123. [Google Scholar] [CrossRef]
- Praxedes, F.R.; Nobre, M.A.L.; Poon, P.S.; Matos, J.; Lanfredi, S. Nanostructured KxNa1-xNbO3 hollow spheres as potential materials for the photocatalytic treatment of polluted water. Appl. Catal. B Environ. 2021, 298, 120502. [Google Scholar] [CrossRef]
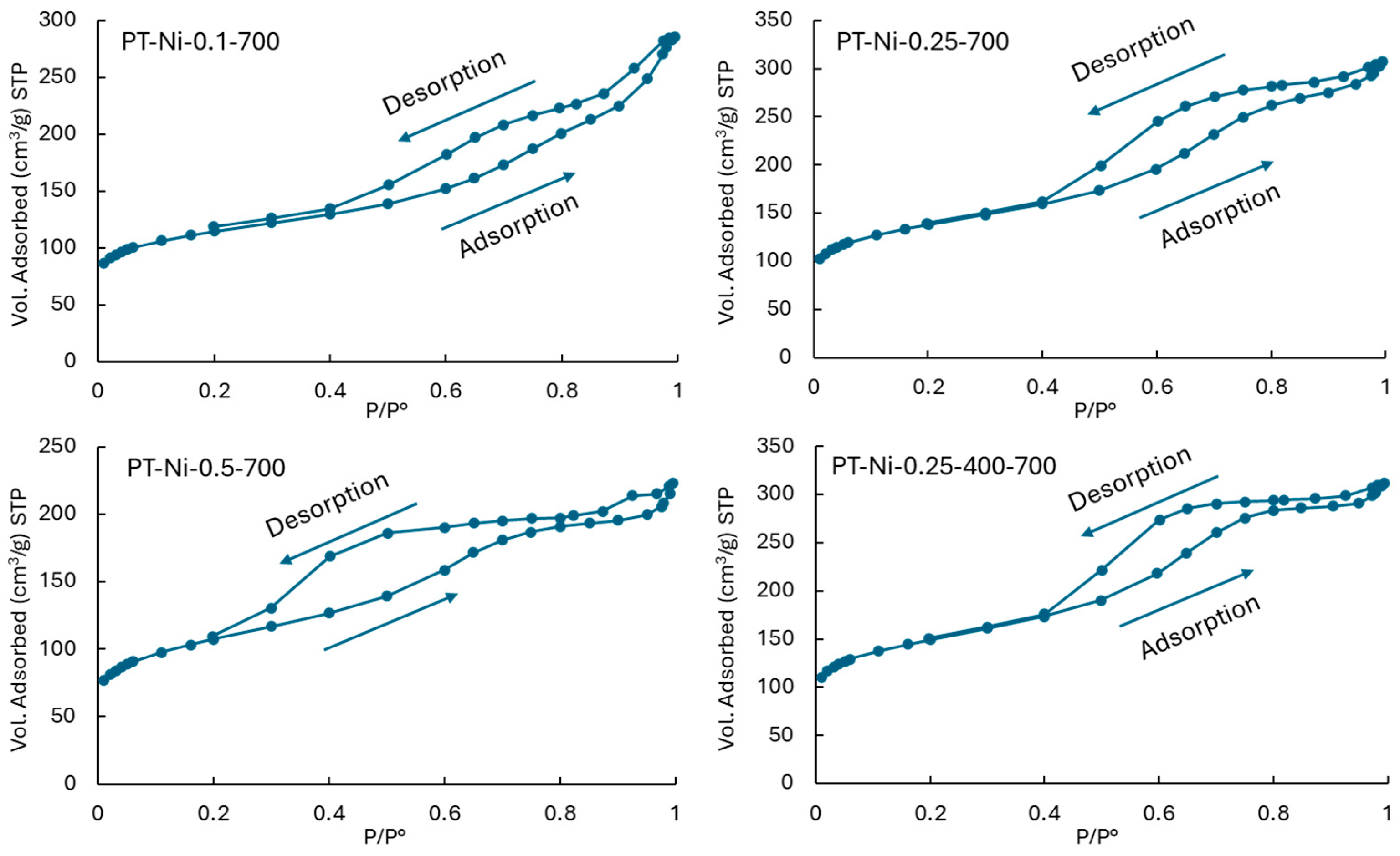
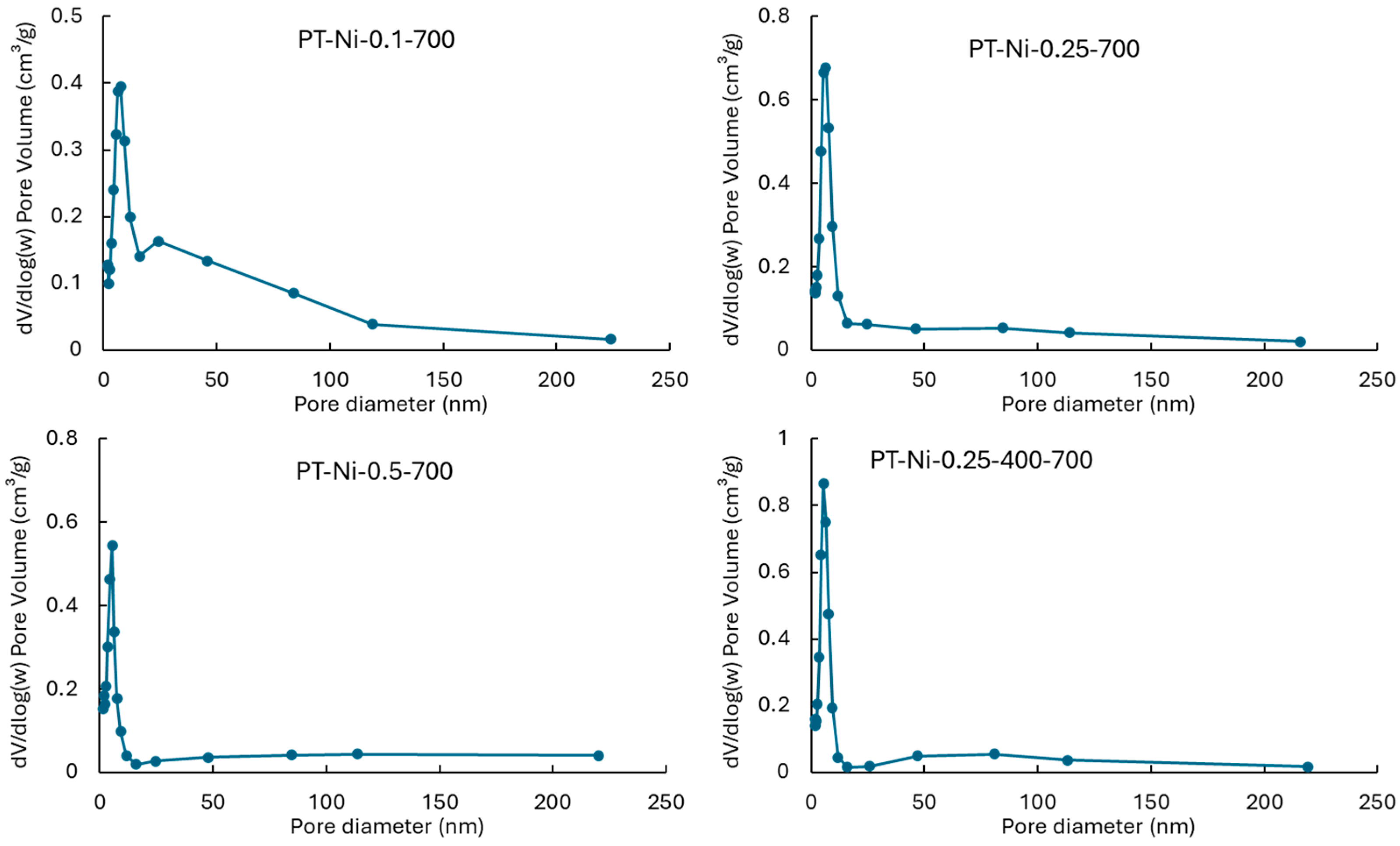
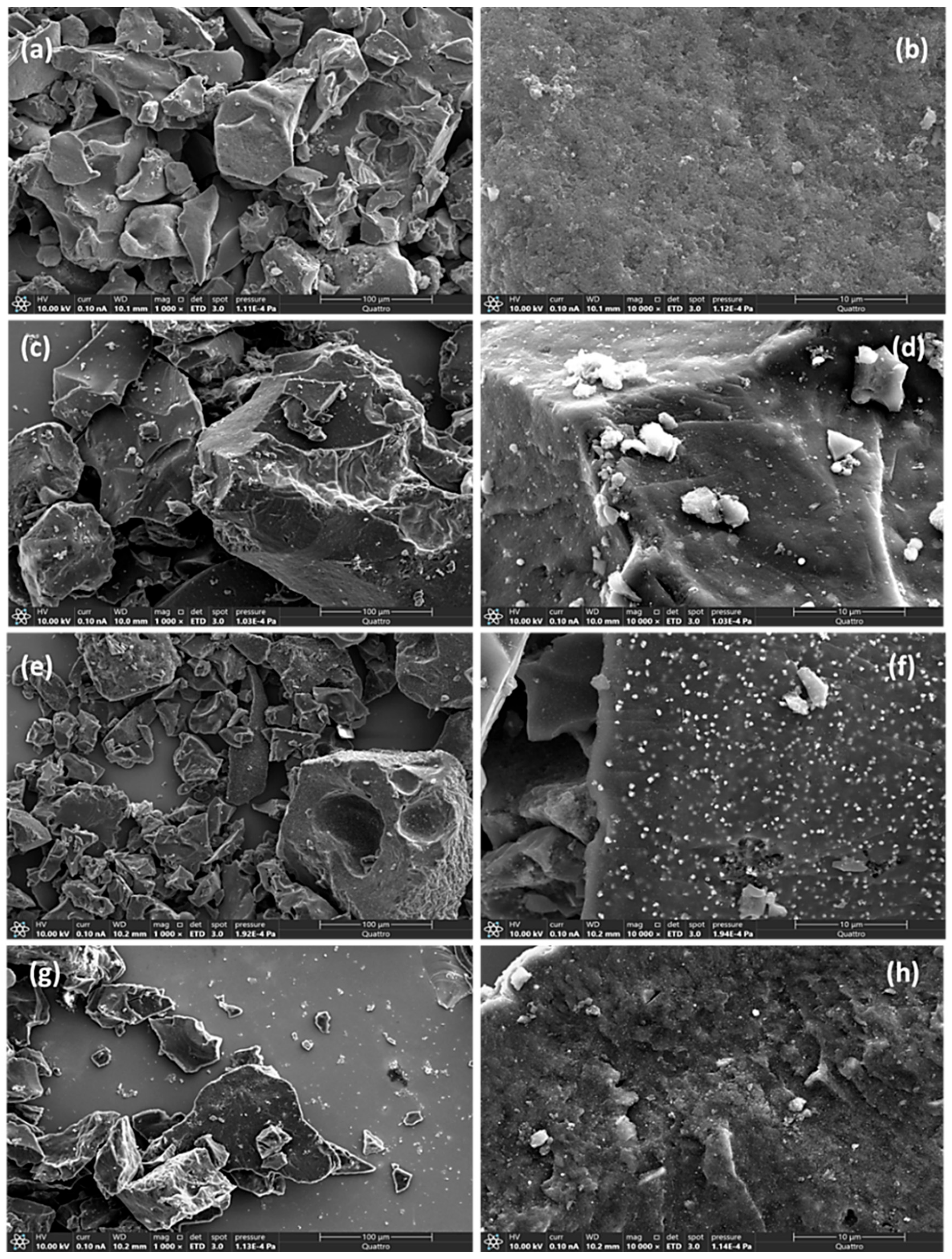
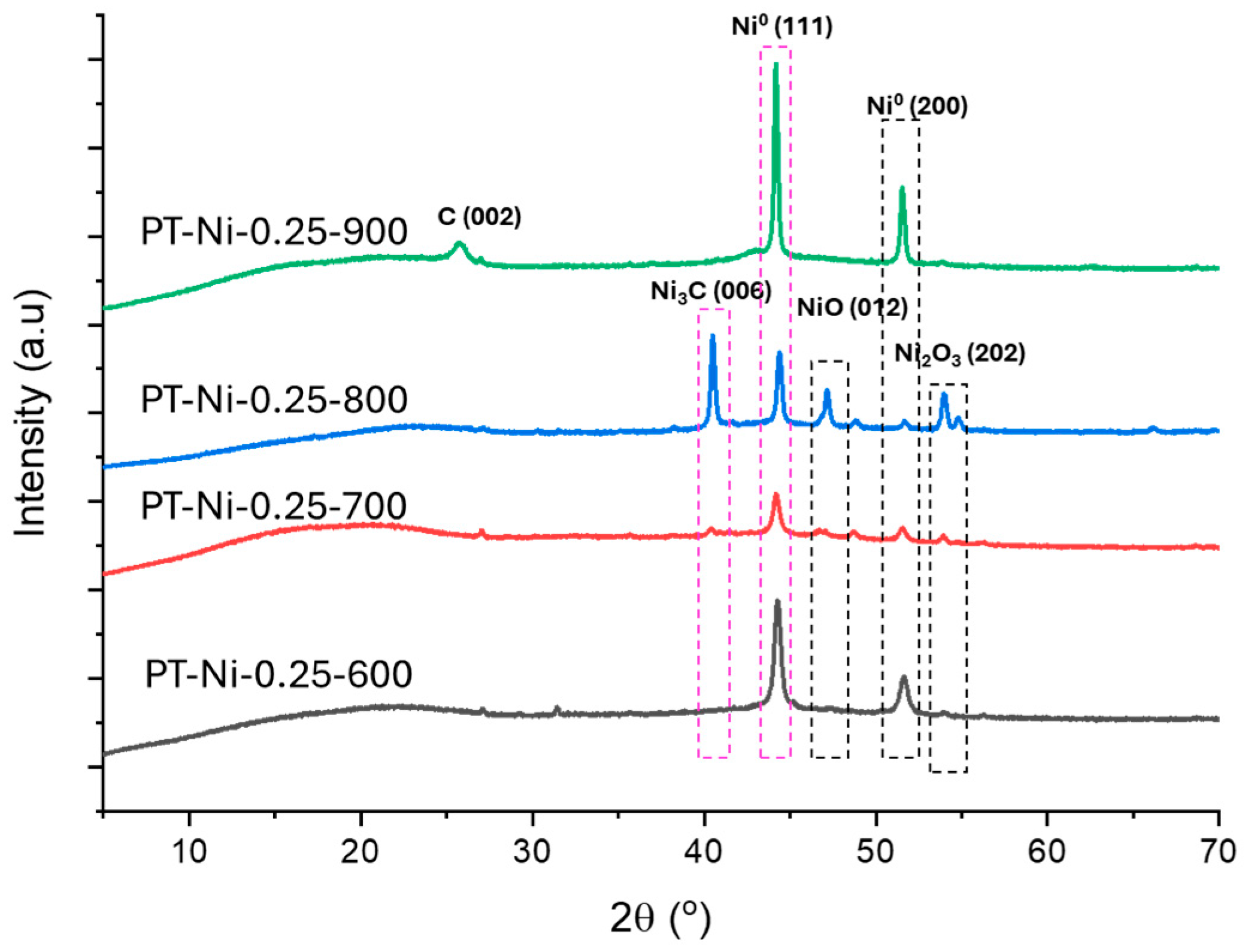
| Catalysts | SBET (m2·g−1) | Vtot (cm3·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) | dpore (nm) |
|---|---|---|---|---|---|
| PT-Ni-0.1-700 | 425 | 0.441 | 0.014 | 0.427 | 7.6 |
| PT-Ni-0.25-600 | 493 | 0.425 | 0.018 | 0.407 | 5.5 |
| PT-Ni-0.25-700 | 504 | 0.474 | 0.015 | 0.459 | 6.4 |
| PT-Ni-0.25-800 | 345 | 0.377 | 0.011 | 0.366 | 5.5 |
| PT-Ni-0.25-900 | 377 | 0.444 | 0.012 | 0.432 | 6.4 |
| PT-Ni-0.5-600 | 415 | 0.317 | 0.020 | 0.297 | 5.5 |
| PT-Ni-0.5-700 | 387 | 0.343 | 0.018 | 0.325 | 5.5 |
| PT-Ni-0.5-800 | 294 | 0.240 | 0.014 | 0.226 | 4.4 |
| PT-Ni-0.25-400-700 | 540 | 0.481 | 0.016 | 0.465 | 5.5 |
| PT-Ni-0.25-400-800 | 437 | 0.417 | 0.014 | 0.403 | 5.5 |
| PT-Ni-0.25-400-900 | 470 | 0.482 | 0.013 | 0.469 | 5.5 |
| Catalysts | C (At.%) | N (At.%) | O (At.%) | Ni (At.%) |
|---|---|---|---|---|
| PT-Ni-0.1-700 | 72.7 ± 0.4 | 14.4 ± 1.4 | 9.4 ± 0.4 | 3.6 ± 0.2 |
| PT-Ni-0.25-600 | 59.0 ± 0.3 | 15.1 ± 1.5 | 23.4 ± 0.5 | 2.5 ± 0.2 |
| PT-Ni-0.25-700 | 72.1 ± 0.4 | 13.1 ± 0.4 | 9.9 ± 0.4 | 4.9 ± 0.2 |
| PT-Ni-0.25-800 | 63.1 ± 0.4 | 12.5 ± 1.8 | 16.7 ± 0.8 | 7.6 ± 0.3 |
| PT-Ni-0.25-900 | 63.5 ± 1.4 | 18.9 ± 5.8 | 9.7 ± 1.7 | 8.0 ± 0.4 |
| PT-Ni-0.5-600 | 69.2 ± 0.6 | 14.4 ± 2.5 | 10.4 ± 0.8 | 6.0 ± 0.4 |
| PT-Ni-0.5-700 | 69.9 ± 0.2 | 15.2 ± 0.7 | 8.6 ± 0.3 | 6.3 ± 0.1 |
| PT-Ni-0.5-800 | 68.2 ± 0.4 | 14.5 ± 1.2 | 8.9 ± 0.4 | 8.4 ± 0.3 |
| PT-Ni-0.25-400-700 | 65.7 ± 0.3 | 17.4 ± 1.5 | 14.2 ± 0.7 | 2.7 ± 0.2 |
| PT-Ni-0.25-400-800 | 72.2 ± 0.4 | 14.1 ± 1.6 | 9.9 ± 0.5 | 3.8 ± 0.2 |
| PT-Ni-0.25-400-900 | 76.1 ± 0.4 | 13.6 ± 1.6 | 5.9 ± 0.5 | 4.4 ± 0.2 |
| Samples | qeq a (μmol) | k1 b (min−1) | R2k1 c | k2 d (μmol−1·min−1) | R2k2 e | kIPD f (μmol·min−1/2) | R2IPD g | CIPD h (μmol) |
|---|---|---|---|---|---|---|---|---|
| TiO2-P25 | 0.208 | 0.056 | 0.982 | 1.082 | 0.862 | 0.029 | 0.964 | −0.017 |
| PT-Ni-0.1-700 | 1.222 | 0.074 | 0.987 | 0.550 | 0.832 | 0.157 | 0.919 | 0.152 |
| PT-Ni-0.25-600 | 0.986 | 0.078 | 0.937 | 0.723 | 0.970 | 0.129 | 0.876 | 0.133 |
| PT-Ni-0.25-700 | 1.264 | 0.063 | 0.936 | 0.337 | 0.932 | 0.156 | 0.877 | 0.212 |
| PT-Ni-0.25-800 | 1.690 | 0.061 | 0.974 | 0.219 | 0.922 | 0.211 | 0.919 | 0.234 |
| PT-Ni-0.25-900 | 0.841 | 0.074 | 0.993 | 0.779 | 0.855 | 0.171 | 0.926 | 0.109 |
| PT-Ni-0.5-600 | 0.699 | 0.057 | 0.970 | 0.441 | 0.838 | 0.088 | 0.955 | 0.072 |
| PT-Ni-0.5-700 | 0.642 | 0.065 | 0.974 | 0.714 | 0.823 | 0.081 | 0.931 | 0.081 |
| PT-Ni-0.5-800 | 0.602 | 0.069 | 0.994 | 0.810 | 0.816 | 0.081 | 0.974 | 0.029 |
| PT-Ni-0.5-700-Solar | 0.713 | 0.086 | 0.980 | 1.758 | 0.736 | 0.091 | 0.919 | 0.096 |
| PT-Ni-0.25-400-700 | 2.006 | 0.052 | 0.968 | 0.127 | 0.892 | 0.247 | 0.943 | 0.239 |
| PT-Ni-0.25-400-800 | 1.335 | 0.065 | 0.944 | 0.377 | 0.774 | 0.165 | 0.915 | 0.187 |
| PT-Ni-0.25-400-900 | 1.290 | 0.063 | 0.967 | 0.286 | 0.976 | 0.167 | 0.935 | 0.139 |
| Samples | qeq a (μmol) | kapp b (min−1) | R2k1 c | kapp-i/kapp-TiO2 d | kapp-i/kapp-Lysis e |
|---|---|---|---|---|---|
| TiO2-P25 | 0.208 | 0.0528 | 0.978 | 1.0 | 27.8 |
| Photolysis | 0 | 0.0019 | 0.689 | 0.04 | 1.00 |
| PT-Ni-0.1-700 | 1.222 | 0.0339 | 0.959 | 0.64 | 17.8 |
| PT-Ni-0.25-600 | 0.986 | 0.0731 | 0.899 | 1.4 | 38.5 |
| PT-Ni-0.25-700 | 1.264 | 0.1295 | 0.879 | 2.5 | 68.2 |
| PT-Ni-0.25-800 | 1.690 | 0.0102 | 0.992 | 0.19 | 5.37 |
| PT-Ni-0.25-900 | 0.841 | 0.0031 | 0.947 | 0.06 | 1.63 |
| PT-Ni-0.5-600 | 0.699 | 0.0187 | 0.871 | 0.35 | 9.84 |
| PT-Ni-0.5-700 | 0.642 | 0.0262 | 0.969 | 0.50 | 13.8 |
| PT-Ni-0.5-800 | 0.602 | 0.018 | 0.963 | 0.33 | 9.26 |
| PT-Ni-0.5-700-Solar | 0.713 | 0.0014 | 0.996 | 0.03 | 0.74 |
| PT-Ni-0.25-400-700 | 2.006 | 0.0128 | 0.989 | 0.24 | 6.74 |
| PT-Ni-0.25-400-800 | 1.335 | 0.0106 | 0.914 | 0.20 | 5.58 |
| PT-Ni-0.25-400-900 | 1.290 | 0.0092 | 0.975 | 0.17 | 4.84 |
| Samples | mcat a (mg) | vsur-cat b (μmol.mg−1.min−1) | φi c |
|---|---|---|---|
| TiO2-P25 | 62.5 | 0.00018 | 1.0 |
| PT-Ni-0.1-700 | 1.6 | 0.0184 | 104.6 |
| PT-Ni-0.25-600 | 3.1 | 0.0461 | 262.1 |
| PT-Ni-0.25-700 | 4.8 | 0.0530 | 301.4 |
| PT-Ni-0.25-800 | 5.0 | 0.0036 | 20.6 |
| PT-Ni-0.25-900 | 3.8 | 0.0005 | 3.0 |
| PT-Ni-0.5-600 | 3.9 | 0.0035 | 19.8 |
| PT-Ni-0.5-700 | 5.3 | 0.0043 | 24.3 |
| PT-Ni-0.5-800 | 3.9 | 0.0020 | 11.5 |
| PT-Ni-0.5-700-Solar | 2.3 | 0.0003 | 1.4 |
| PT-Ni-0.25-400-700 | 1.7 | 0.0152 | 86.4 |
| PT-Ni-0.25-400-800 | 2.4 | 0.0060 | 33.9 |
| PT-Ni-0.25-400-900 | 2.8 | 0.0043 | 24.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.; Smith, R.A.; Bello, R.; Poon, P.S.; Segura-del-Río, R.; Escalona, N.; Bashkova, S. Nickel-Decorated Carbocatalysts for the UV-Driven Photodegradation of Rhodamine B. Catalysts 2025, 15, 385. https://doi.org/10.3390/catal15040385
Matos J, Smith RA, Bello R, Poon PS, Segura-del-Río R, Escalona N, Bashkova S. Nickel-Decorated Carbocatalysts for the UV-Driven Photodegradation of Rhodamine B. Catalysts. 2025; 15(4):385. https://doi.org/10.3390/catal15040385
Chicago/Turabian StyleMatos, Juan, Rory A. Smith, Ruby Bello, Po S. Poon, Rodrigo Segura-del-Río, Néstor Escalona, and Svetlana Bashkova. 2025. "Nickel-Decorated Carbocatalysts for the UV-Driven Photodegradation of Rhodamine B" Catalysts 15, no. 4: 385. https://doi.org/10.3390/catal15040385
APA StyleMatos, J., Smith, R. A., Bello, R., Poon, P. S., Segura-del-Río, R., Escalona, N., & Bashkova, S. (2025). Nickel-Decorated Carbocatalysts for the UV-Driven Photodegradation of Rhodamine B. Catalysts, 15(4), 385. https://doi.org/10.3390/catal15040385







