Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives
Abstract
1. Introduction
2. Discussion
2.1. Industrial Relevance and Market Size
2.2. Evolution in the Ester Production Processes Using Standard Catalysts
2.3. New Trends for Ester Production: Biocatalysis
2.4. Immobilization of Enzymes
2.5. Current Status of Immobilized Lipases on Short-Chain Ester Syntheses
2.6. Performance Indicators on Enzymatic Esterification Studies and Environmental Aspects
2.7. Solvents and Solvent-Free Systems on Enzymatic Esterification Studies
2.8. The Use of Ultrasound and Microwave on Enzymatic Esterification Studies
2.9. Biocatalysts on Enzymatic Esterifications and Reusability Studies
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Sá, A.G.A.; de Meneses, A.C.; de Araújo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Brask, J.; Fjerbaek, L. Enzymatic biodiesel production: Technical and economical considerations. Eur. J. Lipid Sci. Technol. 2008, 110, 692–700. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Manjón, A.; Iborra, J.L.; Arocas, A. Short-chain flavour ester synthesis by immobilized lipase in organic media. Biotechnol. Lett. 1991, 13, 339–344. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Thum, O.; Oxenbøll, K.M. Biocatalysis—A Sustainable Method for the Production of Emollient Esters. SÖFW-Journal 2008, 134, 44–47. [Google Scholar]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Straathof, A.J.J. Transformation of biomass into commodity chemicals using enzymes or cells. Chem. Rev. 2014, 114, 1871–1908. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.R.; Costa, M.; Pinto, C.; Cristina, E.; Aguieiras, G.; Cipolatti, E.P.; Andrade, E.; Ayla, M.; Ana, S.; Pinto, J.C.; et al. Comparative performance and reusability studies of lipases on syntheses of octyl esters with an economic approach. Bioprocess Biosyst. Eng. 2022, 45, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Bélafi-Bakó, K.; Badr, A.K.; Ehrenstein, U.; Gubicza, L. Kinetics of Ethyl Acetate Formation by Lipase in Organic Solvent and Solvent-Free System. Chem. Pap. 2003, 57, 278–281. [Google Scholar]
- Bezbradica, D.; Mijin, D.; Šiler-Marinković, S.; Knežević, Z. The effect of substrate polarity on the lipase-catalyzed synthesis of aroma esters in solvent-free systems. J. Mol. Catal. B Enzym. 2007, 45, 97–101. [Google Scholar] [CrossRef]
- Nordblad, M.; Adlercreutz, P. Effects of acid concentration and solvent choice on enzymatic acrylation by Candida antarctica lipase B. J. Biotechnol. 2008, 133, 127–133. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipases and Lipase-Catalyzed Esterification Reactions in Nonaqueous Media. Catal. Rev. 2002, 44, 499–591. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-free esterifications mediated by immobilized lipases: A review from thermodynamic and kinetics perspective. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- de Sousa, R.R.; da Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Simplified method to optimize enzymatic esters syntheses in solvent-free systems: Validation using literature and experimental data. Catalysts 2021, 11, 1357. [Google Scholar] [CrossRef]
- Gao, P.; Jiang, Q.; Xu, Y.; Xia, W. Biosynthesis of acetate esters by dominate strains, isolated from Chinese traditional fermented fish (Suan yu). Food Chem. 2018, 244, 44–49. [Google Scholar] [CrossRef]
- Gao, W.; Wu, K.; Chen, L.; Fan, H.; Zhao, Z.; Gao, B.; Wang, H.; Wei, D. A novel esterase from a marine mud metagenomic library for biocatalytic synthesis of short-chain flavor esters. Microb. Cell Fact. 2016, 15, 41. [Google Scholar] [CrossRef]
- Lorenzo, B.; Fernández, L.; Ortega, J.; Domínguez, L. Improvements in the Modeling and Kinetics Processes of the Enzymatic Synthesis of Pentyl Acetate. Processes 2023, 11, 1640. [Google Scholar] [CrossRef]
- Brault, G.; Shareck, F.; Hurtubise, Y.; Lépine, F.; Doucet, N. Short-Chain Flavor Ester Synthesis in Organic Media by an E. coli Whole-Cell Biocatalyst Expressing a Newly Characterized Heterologous Lipase. PLoS ONE 2014, 9, e91872. [Google Scholar] [CrossRef]
- Kruis, A.J.; Bohnenkamp, A.C.; Patinios, C.; Van Nuland, Y.M.; Levisson, M.; Mars, A.E.; Van Den Berg, C.; Kengen, S.W.M.; Weusthuis, R.A. Microbial production of short and medium chain esters: Enzymes, pathways, and applications. Biotechnol. Adv. 2019, 37, 107407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, X.; Abdo, A.A.A.; Kaddour, B.; Li, X.; Teng, C.; Wan, C. Ultrasound-assisted lipase-catalyzed synthesis of ethyl acetate: Process optimization and kinetic study. Biotechnol. Biotechnol. Equip. 2021, 35, 255–263. [Google Scholar] [CrossRef]
- Mehta, A.; Grover, C.; Bhardwaj, K.K.; Gupta, R. Application of lipase purified from Aspergillus fumigatus in the syntheses of ethyl acetate and ethyl lactate. J. Oleo Sci. 2020, 69, 23–29. [Google Scholar] [CrossRef]
- Ferone, M.; Raganati, F.; Olivieri, G. Bioreactors for succinic acid production processes. Crit. Rev. Biotechnol. 2019, 39, 571–586. [Google Scholar] [CrossRef]
- Barel, A.O.; Paye, M.; Maibach, H.I. (Eds.) Handbook of Cosmetic Science and Technology, 3rd ed.; Informa Healthcare: London, UK, 2010; Volume 36, ISBN 9781420069631. [Google Scholar]
- Vogel, A.I. 130. Physical properties and chemical constitution. Part XIII. Aliphatic carboxylic esters. J. Chem. Soc. 1948, 624–644. [Google Scholar] [CrossRef]
- Kononenko, O.K.; Herstein, K.M. Nonaqueous Solvents for Sucrose. Ind. Eng. Chem. Chem. Eng. Data Ser. 1956, 1, 87–92. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- International Organization of the Flavor Industry. IOFI Code of Practices; IOFI: Washington, DC, USA, 2012; Volume 1.3. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540699330. [Google Scholar]
- Sun, J.; Liu, S.Q. Ester Synthesis in Aqueous Media by Lipase: Alcoholysis, Esterification and Substrate Hydrophobicity. J. Food Biochem. 2015, 39, 11–18. [Google Scholar] [CrossRef]
- Sun, J.; Yu, B.; Curran, P.; Liu, S.Q. Lipase-catalysed transesterification of coconut oil with fusel alcohols in a solvent-free system. Food Chem. 2012, 134, 89–94. [Google Scholar] [CrossRef]
- Kuperkar, V.V.; Lade, V.G.; Prakash, A.; Rathod, V.K. Synthesis of isobutyl propionate using immobilized lipase in a solvent free system: Optimization and kinetic studies. J. Mol. Catal. B Enzym. 2014, 99, 143–149. [Google Scholar] [CrossRef]
- Chowdary, G.V.; Ramesh, M.N.; Prapulla, S.G. Enzymic synthesis of isoamyl isovalerate using immobilized lipase from Rhizomucor miehei: A multivariate analysis. Process Biochem. 2000, 36, 331–339. [Google Scholar] [CrossRef]
- Ergan, F.; Trani, M.; André, G. Use of lipases in multiphasic systems solely composed of substrates. J. Am. Oil Chem. Soc. 1991, 68, 412–417. [Google Scholar] [CrossRef]
- Fellechner, O.; Blatkiewicz, M.; Smirnova, I. Reactive Separations for In Situ Product Removal of Enzymatic Reactions: A Review. Chemie-Ingenieur-Technik 2019, 91, 1522–1543. [Google Scholar] [CrossRef]
- Lee, D.E.; Park, K.M.; Choi, S.J.; Shim, J.H.; Chang, P.S. Enhancing operational stability and exhibition of enzyme activity by removing water in the immobilized lipase-catalyzed production of erythorbyl laurate. Biotechnol. Prog. 2013, 29, 882–889. [Google Scholar] [CrossRef]
- Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Green synthesis of isopropyl myristate in novel single phase medium Part I: Batch optimization studies. Biotechnol. Rep. 2015, 8, 133–137. [Google Scholar] [CrossRef]
- Sandoval, G.; Condoret, J.S.; Monsan, P.; Marty, A. Esterification by Immobilized Lipase in Solvent-Free Media: Kinetic and Thermodynamic Arguments. Biotechnol. Bioeng. 2002, 78, 313–320. [Google Scholar] [CrossRef]
- Castillo, E.; Dossat, V.; Marty, A.; Stéphane Condoret, J.; Combes, D. The role of silica gel in lipase-catalyzed esterification reactions of high-polar substrates. JAOCS J. Am. Oil Chem. Soc. 1997, 74, 77–85. [Google Scholar] [CrossRef]
- Kobayashi, T.; Adachi, S. Reaction equilibrium for lipase-catalyzed condensation in organic solvent systems. Biotechnol. Lett. 2004, 26, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.R.; Pazutti, L.V.B.; Dalmaso, G.Z.L.; Siqueira, D.F.; Silva, A.S.; Ferreira-Leitão, V.S. A practical approach to obtain high yield lipase-mediated synthesis of octyl caprylate with Novozym 435. Biocatal. Biotransform. 2020, 38, 293–303. [Google Scholar] [CrossRef]
- Sose, M.T.; Bansode, S.R.; Rathod, V.K. Solvent free lipase catalyzed synthesis of butyl caprylate. J. Chem. Sci. 2017, 129, 1755–1760. [Google Scholar] [CrossRef]
- Santos, J.C.; Bueno, T.; Rós, P.C.M.; de Castro, H.F. Lipase-catalyzed Synthesis of Butyl Esters by Direct Esterification in Solvent-Free System. J. Chem. Technol. Biotechnol. 2007, 82, 956–961. [Google Scholar] [CrossRef]
- Gubicza, L.; Kabiri-Badr, A.; Keoves, E.; Belafi-Bako, K. Large-scale enzymatic production of natural flavour esters in organic solvent with continuous water removal. J. Biotechnol. 2000, 84, 193–196. [Google Scholar] [CrossRef]
- Woodcock, L.L.; Wiles, C.; Greenway, G.M.; Watts, P.; Wells, A.; Eyley, S. Enzymatic synthesis of a series of alkyl esters using Novozym 435 in a packed-bed, miniaturized, continuous flow reactor. Biocatal. Biotransform. 2008, 26, 466–472. [Google Scholar] [CrossRef]
- Reis, C.; Yvay, E.; De Sousa, A.; De França, J.; Casemiro, R. Design of immobilzed enzyme biocatalyst: Drawbacks and opportinities. Quim. Nov. 2019, 42, 768–783. [Google Scholar]
- Serrano-Arnaldos, M.; Bastida, J.; Máximo, F.; Ortega-Requena, S.; Montiel, C. One-Step Solvent-Free Production of a Spermaceti Analogue Using Commercial Immobilized Lipases. ChemistrySelect 2018, 3, 748–752. [Google Scholar] [CrossRef]
- Gubicza, L.; Bélafi-Bakó, K.; Fehér, E.; Fráter, T. Waste-free process for continuous flow enzymatic esterification using a double pervaporation system. Green Chem. 2008, 10, 1284–1287. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Verma, H.K.; Pathak, S.; Kaushal, R.K.; Kumar, Y.; Verma, M.L.; Chimni, S.S.; Chauhan, G.S. Enhancement of ethyl propionate synthesis by poly (AAc-co-HPMA-cl-MBAm)-immobilized Pseudomonas aeruginosa MTCC-4713, exposed to Hg2+ and NH4+ ions. Acta Microbiol. Immunol. Hung. 2006, 53, 195–207. [Google Scholar] [CrossRef]
- Foukis, A.; Gkini, O.A.; Stergiou, P.Y.; Papamichael, E.M. New insights and tools for the elucidation of lipase catalyzed esterification reaction mechanism in n-hexane: The synthesis of ethyl butyrate. Mol. Catal. 2018, 455, 159–163. [Google Scholar] [CrossRef]
- Santos, M.; Velez, A.; Abburra, R.; Magario, I. Thermodynamic and kinetic aspects of the solvent-free liquid–liquid synthesis of tailor-made glyceride esters. Fuel 2023, 333, 126461. [Google Scholar] [CrossRef]
- Hartman, L. Miscibility of glycerol with fatty acids and glycerides. J. Am. Oil Chem. Soc. 1963, 40, 142. [Google Scholar] [CrossRef]
- Dhake, K.P.; Thakare, D.D.; Bhanage, B.M. Lipase: A potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragr. J. 2013, 28, 71–83. [Google Scholar] [CrossRef]
- Foresti, M.L.; Pedernera, M.; Bucalá, V.; Ferreira, M.L. Multiple effects of water on solvent-free enzymatic esterifications. Enzym. Microb. Technol. 2007, 41, 62–70. [Google Scholar] [CrossRef]
- Marty, A.; Dossat, V.; Condoret, J.S. Continuous operation of lipase-catalyzed reactions in nonaqueous solvents: Influence of the production of hydrophilic compounds. Biotechnol. Bioeng. 1997, 56, 232–237. [Google Scholar] [CrossRef]
- Halling, P.J.; Finney, J.L.; Ho, M.W.; Franks, F.; Littlechild, J.A. What can we learn by studying enzymes in non-aqueous media? Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1287–1297. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-chaabouni, M.; Bezzine, S.; Miled, N.; Gargouri, Y. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzym. Microb. Technol. 2006, 38, 788–794. [Google Scholar] [CrossRef]
- Foresti, M.L.; Pedernera, M.; Ferreira, M.L.; Bucalá, V. Kinetic modeling of enzymatic ethyl oleate synthesis carried out in biphasic systems. Appl. Catal. A Gen. 2008, 334, 65–72. [Google Scholar] [CrossRef]
- Foresti, M.L.; Ferreira, M.L. Solvent-free ethyl oleate synthesis mediated by lipase from Candida antarctica B adsorbed on polypropylene powder. Catal. Today 2005, 107–108, 23–30. [Google Scholar] [CrossRef]
- Vaysse, L.; Ly, A.; Moulin, G.; Dubreucq, E. Chain-length selectivity of various lipases during hydrolysis, esterification and alcoholysis in biphasic aqueous medium. Enzym. Microb. Technol. 2002, 31, 648–655. [Google Scholar] [CrossRef]
- Goldberg, M.; Thomas, D.; Legoy, M.D. Water activity as a key parameter of synthesis reactions: The example of lipase in biphasic (liquid/solid) media. Enzym. Microb. Technol. 1990, 12, 976–981. [Google Scholar] [CrossRef]
- de Sousa, R.R.; de Castro, R.d.P.V.; Assis, N.M.; da Silva, A.S.; Freire, D.M.G.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Technical–Economic Assessment—The Missing Piece for Increasing the Attractiveness of Applied Biocatalysis in Ester Syntheses? Catalysts 2023, 13, 223. [Google Scholar] [CrossRef]
- Clyburn, F. IFF Proxy Statement and Notice of 2022 Annual Meeting of Sharingholders. Available online: https://ir.iff.com/static-files/d874db63-45ff-4a28-8bf5-88b2d50ff719 (accessed on 12 August 2024).
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef] [PubMed]
- Europe, C.D. The In-Cosmetics Global Award Winners 2024. Available online: https://www.cosmeticsdesign-europe.com/Article/2024/04/22/The-In-Cosmetics-Global-award-winners-2024 (accessed on 31 July 2024).
- Cosmetic Business. The Winners of the Pure Beauty Global Awards 2024 Revealed. Available online: https://cosmeticsbusiness.com/pure-beauty-global-awards-winners-2024 (accessed on 3 January 2025).
- Barcelona Perfumery Congress. The Barcelona Perfumery Awards Already Have Their Winners Among 120 International Nominations from Across the Value Chain of the Sector. Available online: https://perfumerycongress.com/meet-the-winners-of-the-2023-barcelona-perfumery-awards/ (accessed on 3 January 2025).
- Future Market Insights Inc. Esters Market Outlook from 2023 to 2033. Available online: https://www.futuremarketinsights.com/reports/esters-market (accessed on 12 August 2024).
- Grand View Research U.S. Esters Market Size, Share & Trends Analysis Report By Product (Fatty Esters, Phosphate Esters, Acrylic Esters, Cellulose Esters, Allyl and Aromatic Esters), By Application, and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/us-esters-market (accessed on 13 August 2024).
- Csanádi, Z.; Kurdi, R.; Bélafi-Bakó, K. Ethyl-Acetate Synthesis in Gas Phase by Immobilised Lipase. Hung. J. Ind. Chem. Veszpr. 2012, 40, 39–44. [Google Scholar]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Mary Bailey. BASF to Expand Production Capacity of Synthetic Ester Base Stocks in China—Chemical Engineering. Available online: https://www.chemengonline.com/basf-to-expand-production-capacity-of-synthetic-ester-base-stocks-in-china/?printmode=1 (accessed on 4 May 2024).
- Wray, J. Evonik Grows Green Emollient Esters Business in China. Available online: https://cosmeticsbusiness.com/news/article_page/Evonik_grows_green_emollient_esters_business_in_China/201432 (accessed on 6 October 2023).
- Business Wire. Investment in Research and Development: OQ Chemicals Launches New Pilot Plant for Customized Esters. Business Wire. Available online: https://www.businesswire.com/news/home/20230504005125/en/Investment-in-Research-and-Development-OQ-Chemicals-Launches-New-Pilot-Plant-for-Customized-Esters (accessed on 5 February 2024).
- IFF. IFF Commissions Fragrance Ingredients Plant in China; Builds Industry Leadership; Sets New Sustainability Standards. International Flavors & Fragrances Inc. Available online: https://ir.iff.com/news-releases/news-release-details/iff-commissions-fragrance-ingredients-plant-china-builds-0 (accessed on 12 August 2024).
- Solvay. Solvay Opens Its Second Sustainable Solvent Plant in Brazil. Solvay. Available online: https://www.solvay.com/en/press-release/new-augeo-plant (accessed on 4 May 2024).
- Villa, C.; Mariani, E.; Loupy, A.; Grippo, C.; Grossi, G.C.; Bargagna, A. Solvent-free reactions as green chemistry procedures for the synthesis of cosmetic fatty esters. Green Chem. 2003, 5, 623–626. [Google Scholar] [CrossRef]
- Ishida, T.; Ogihara, Y.; Ohashi, H.; Akita, T.; Honma, T.; Oji, H.; Haruta, M. Base-free direct oxidation of 1-octanol to octanoic acid and its octyl ester over supported gold catalysts. ChemSusChem 2012, 5, 2243–2248. [Google Scholar] [CrossRef]
- Maruyama, S.A.; Kanda, L.R.S.; Wypych, F. Isopropyl octanoate synthesis catalyzed by layered zinc n-octanoate. J. Braz. Chem. Soc. 2017, 28, 985–994. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J.; Bhoi, S. Homogeneous and Heterogeneous Catalyzed Esterification of Acrylic Acid with Ethanol: Reaction Kinetics and Modeling. Int. J. Chem. Kinet. 2018, 50, 370–380. [Google Scholar] [CrossRef]
- Jiang, H. Reaction-controlled recovery of the copper(II) methanesulfonate catalyst for esterification. React. Kinet. Catal. Lett. 2005, 84, 223–228. [Google Scholar] [CrossRef]
- Zang, Y.; Shi, J.; Zhang, F.; Zhong, Y.; Zhu, W. Sulfonic acid-functionalized MIL-101 as a highly recyclable catalyst for esterification. Catal. Sci. Technol. 2013, 3, 2044–2049. [Google Scholar] [CrossRef]
- Park, Y.-M.; Lee, J.Y.; Chung, S.-H.; Park, I.S.; Lee, S.-Y.; Kim, D.-K.; Lee, J.-S.; Lee, K.-Y. Esterification of used vegetable oils using the heterogeneous WO3/ZrO2 catalyst for production of biodiesel. Bioresour. Technol. 2010, 101, S59–S61. [Google Scholar] [CrossRef]
- Ben Mya, O.; Bita, M.; Louafi, I.; Djouadi, A. Esterification process catalyzed by ZSM-5 zeolite synthesized via modified hydrothermal method. MethodsX 2018, 5, 277–282. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, L.; Lei, Z. Quaternary Ammonium Salt Functionalized Methoxypolyethylene Glycols-Supported Phosphotungstic Acid Catalyst for the Esterification of Carboxylic Acids with Alcohols. Catal. Lett. 2014, 144, 585–589. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Ziarani, G.; Lashgari, N.; Badiei, A. Sulfonic acid-functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catal. A Chem. 2015, 397, 166–191. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Enzymatic reactors for biodiesel synthesis: Present status and future prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Contente, M.L.; Tamborini, L.; Molinari, F.; Paradisi, F. Aromas flow: Eco-friendly, continuous, and scalable preparation of flavour esters. J. Flow Chem. 2020, 10, 235–240. [Google Scholar] [CrossRef]
- Thum, O. Enzymatic production of care specialities based on fatty acid esters. Tenside Surfactants Deterg. 2004, 41, 287–290. [Google Scholar] [CrossRef]
- Baum, S.; Mueller, J.J.; Hilterhaus, L.; Eckstein, M.; Thum, O.; Liese, A. The bubble column reactor: A novel reactor type for cosmetic esters. In Applied Biocatalysis: From Fundamental Science to Industrial Applications; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 343–366. [Google Scholar] [CrossRef]
- Juan, J.C.; Zhang, J.; Yarmo, M.A. Efficient Esterification of Fatty Acids with Alcohols Catalyzed by Zr(SO4)2·4H2O under Solvent-Free Condition. Catal. Lett. 2008, 126, 319–324. [Google Scholar] [CrossRef]
- Devaraj Naik, B.; Udayakumar, M. Optimization studies on esterification of waste cooking oil using sulfated montmorillonite clay acidic catalyst. Mater. Today Proc. 2021, 46, 9855–9861. [Google Scholar] [CrossRef]
- Cao, M.; Peng, L.; Xie, Q.; Xing, K.; Lu, M.; Ji, J. Sulfonated Sargassum horneri carbon as solid acid catalyst to produce biodiesel via esterification. Bioresour. Technol. 2021, 324, 124614. [Google Scholar] [CrossRef]
- Mustafa, A.; Niikura, F. Green synthesis of isopropyl palmitate using immobilized Candida antarctica lipase: Process optimization using response surface methodology. Clean. Eng. Technol. 2022, 8, 100516. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Sukumara, S.; Herrgård, M.J.; Fantke, P. Combining Environmental and Economic Performance for Bioprocess Optimization. Trends Biotechnol. 2020, 38, 1203–1214. [Google Scholar] [CrossRef]
- Delgove, M.A.F.; Laurent, A.B.; Woodley, J.M.; De Wildeman, S.M.A.; Bernaerts, K.V.; van der Meer, Y. A Prospective Life Cycle Assessment (LCA) of Monomer Synthesis: Comparison of Biocatalytic and Oxidative Chemistry. ChemSusChem 2019, 12, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Arnaldos, M.; Ortega-Requena, S.; Montiel, M.C.; Máximo, F.; Bastida, J.; Murcia, M.D. Preliminary economic assessment: A valuable tool to establish biocatalytic process feasibility with an in-lab immobilized lipase. J. Chem. Technol. Biotechnol. 2019, 94, 409–417. [Google Scholar] [CrossRef]
- Burton, S.G.; Cowan, D.A.; Woodley, J.M. The search for the ideal biocatalyst. Nat. Biotechnol. 2002, 20, 37–45. [Google Scholar] [CrossRef]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1689–1703. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Montiel, M.C.; Ortega-Requena, S.; Máximo, F.; Bastida, J. Development and economic evaluation of an eco-friendly biocatalytic synthesis of emollient esters. Bioprocess Biosyst. Eng. 2020, 43, 495–505. [Google Scholar] [CrossRef]
- Martins, A.B.; Da Silva, A.M.; Schein, M.F.; Garcia-Galan, C.; Záchia Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Comparison of the performance of commercial immobilized lipases in the synthesis of different flavor esters. J. Mol. Catal. B Enzym. 2014, 105, 18–25. [Google Scholar] [CrossRef]
- Hills, G. Industrial use of lipases to produce fatty acid esters. Eur. J. Lipid Sci. Technol. 2003, 105, 601–607. [Google Scholar] [CrossRef]
- López-Fernández, J.; Benaiges, M.D.; Sebastian, X.; Bueno, J.M.; Valero, F. Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase. Catalysts 2022, 12, 639. [Google Scholar] [CrossRef]
- Raghavendra, T.; Panchal, N.; Divecha, J.; Shah, A.; Madamwar, D. Biocatalytic synthesis of flavor ester “pentyl valerate” using Candida rugosa lipase immobilized in microemulsion based organogels: Effect of parameters and reusability. BioMed Res. Int. 2014, 2014, 353845. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef]
- Gunukula, S.; Runge, T.; Anex, R. Assessment of Biocatalytic Production Parameters to Determine Economic and Environmental Viability. ACS Sustain. Chem. Eng. 2017, 5, 8119–8126. [Google Scholar] [CrossRef]
- Enzymes REACH Consortium. Welcome to Enzymes REACH Consortium. Available online: http://www.enzymes-reach.org/content/welcome-enzymes-reach-consortium (accessed on 6 January 2025).
- AMFEP. Major Regulatory Aspects on Technical Enzymes. Available online: https://amfep.org/about-enzymes/regulatory/technical-enzymes-regulations/ (accessed on 6 January 2025).
- Enzymes REACH Consortium. Safety Evaluation of Technical Enzyme Products with Regards to the REACH Legislation. 2021. Available online: http://www.enzymes-reach.org/content/documents (accessed on 6 January 2025).
- Melo, A.D.Q.; Silva, F.F.M.; Dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef]
- Jawale, P.V.; Bhanage, B.M. Synthesis of propyl benzoate by solvent-free immobilized lipase-catalyzed transesterification: Optimization and kinetic modeling. Bioprocess Biosyst. Eng. 2021, 44, 369–378. [Google Scholar] [CrossRef]
- Yadav, G.D.; Devendran, S. Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochem. 2012, 47, 496–502. [Google Scholar] [CrossRef]
- Sun, J.; Yu, B.; Curran, P.; Liu, S.Q. Lipase-catalysed ester synthesis in solvent-free oil system: Is it esterification or transesterification? Food Chem. 2013, 141, 2828–2832. [Google Scholar] [CrossRef]
- Verma, M.L.; Azmi, W.; Kanwar, S.S. Synthesis of ethyl acetate employing celite-immobilized lipase of Bacillus cereus MTCC 8372. Acta Microbiol. Immunol. Hung. 2009, 56, 229–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.; Park, C. Enzymatic synthesis of phenethyl ester from phenethyl alcohol with acyl donors. Enzym. Microb. Technol. 2017, 100, 37–44. [Google Scholar] [CrossRef]
- Gómez, J.L.; Gómez, M.; Murcia, M.D.; Gómez, E.; Hidalgo, A.M.; Montiel, C.; Martínez, R. Biosynthesis of benzyl acetate: Optimization of experimental conditions, kinetic modelling and application of alternative methods for parameters determination. Bioresour. Technol. Rep. 2020, 11, 100519. [Google Scholar] [CrossRef]
- Chen, M.L.; Vali, S.R.; Lin, J.Y.; Ju, Y.H. Synthesis of the structured lipid 1,3-dioleoyl-2-palmitoylglycerol from palm oil. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 525–532. [Google Scholar] [CrossRef]
- Palla, C.A.; Carrín, M.E. Kinetics modeling of the acidolysis with immobilized Rhizomucor miehei lipases for production of structured lipids from sunflower oil. Biochem. Eng. J. 2014, 90, 184–194. [Google Scholar] [CrossRef]
- Hita, E.; Robles, A.; Camacho, B.; González, P.A.; Esteban, L.; Jiménez, M.J.; Muñío, M.M.; Molina, E. Production of structured triacylglycerols by acidolysis catalyzed by lipases immobilized in a packed bed reactor. Biochem. Eng. J. 2009, 46, 257–264. [Google Scholar] [CrossRef]
- Jiménez, M.J.; Esteban, L.; Robles, A.; Hita, E.; González, P.A.; Muñío, M.M.; Molina, E. Production of triacylglycerols rich in palmitic acid at sn-2 position by lipase-catalyzed acidolysis. Biochem. Eng. J. 2010, 51, 172–179. [Google Scholar] [CrossRef]
- Gandhi, N.N.; Mukherjee, K.D. Reactivity of medium-chain substrates in the interesterification of tripalmitin catalyzed by papaya lipase. JAOCS J. Am. Oil Chem. Soc. 2001, 78, 965–968. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Ultrasound assisted intensification of biodiesel production using enzymatic interesterification. Ultrason. Sonochem. 2016, 29, 67–75. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Immobilized lipase on core-shell structured Fe3O4-MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard. Food Chem. 2016, 194, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Fu, G.-N.; Du, L.-H.; Lin, H.; Zhang, A.-Y.; Xie, H.-J.; Sheng, Z.-K.; Xue, M.-M.; Yan, B.-L.; Liu, Y.; et al. Continuous flow biocatalysis: Synthesis of purine nucleoside esters catalyzed by lipase TL IM from Thermomyces lanuginosus. RSC Adv. 2024, 14, 10953–10961. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Wang, T. An effective method for reducing free fatty acid content of high-acid rice bran oil by enzymatic amidation. J. Ind. Eng. Chem. 2017, 48, 119–124. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Wang, C.; Zheng, L.; Wang, Z.; Zhao, R.; Wang, L. Lipase-mediated amidation of anilines with 1,3-diketones via C–C bond cleavage. Catalysts 2017, 7, 115. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Jin, Q.; Wang, X.; Hu, J.; Wang, X. Preparation of arachidonoyl ethanolamide by enzymatic amidation of arachidonic acid purified from microbial oil. Process Biochem. 2018, 66, 120–125. [Google Scholar] [CrossRef]
- Du, L.; Zheng, L.; Pan, Y.; Sheng, Z.; Zhang, S.; Lin, H.; Zhang, A.; Xie, H.; Luo, X. Highly Efficient Synthesis of Cinnamamides from Methyl Cinnamates and Phenylethylamines Catalyzed by Lipozyme® TL IM under Continuous-Flow Microreactors. Catalysts 2022, 12, 1265. [Google Scholar] [CrossRef]
- Zhang, A.-Y.; Huang, Z.-H.; Du, L.-H.; Lin, H.; Xie, H.-J.; Yan, B.-L.; Xue, M.M.; Wang, L.; Shao, W.-X.; Fu, G.-N.; et al. Pyrazine derivative synthesis in a continuous-flow system: A green synthesis of pyrazinamide from pyrazine esters and amines catalyzed by Lipozyme® TL IM from Thermomyces lanuginosus. RSC Adv. 2024, 14, 39560–39568. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Verger, R. “Interfacial activation” of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Mancheño, J.M.; Pernas, M.A.; Martínez, M.J.; Ochoa, B.; Rúa, M.L.; Hermoso, J.A. Structural insights into the lipase/esterase behavior in the Candida rugosa lipases family: Crystal structure of the lipase 2 isoenzyme at 1.97A resolution. J. Mol. Biol. 2003, 332, 1059–1069. [Google Scholar] [CrossRef]
- Uppenberg, J.; Hansen, M.T.; Patkar, S.; Jones, T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 1994, 2, 293–308. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modification. J. Mol. Catal. B Enzym. 2010, 66, 15–32. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Yu, H.; Qin, L.; Zhou, J. Effect of Oil Polarity on the Protein Adsorption at Oil-Water Interfaces. Langmuir 2023, 39, 10701–10710. [Google Scholar] [CrossRef]
- Stahmann, K.P.; Böddecker, T.; Sahm, H. Regulation and properties of a fungal lipase showing interfacial inactivation by gas bubbles, or droplets of lipid or fatty acid. Eur. J. Biochem. 1997, 244, 220–225. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, Z.; Wang, P.; Zheng, S.-P.; Han, S.-Y.; Lin, Y. Improving the catalytic characteristics of lipase-displaying yeast cells by hydrophobic modification. Bioprocess Biosyst. Eng. 2017, 40, 1689–1699. [Google Scholar] [CrossRef]
- Wang, P.; He, J.; Sun, Y.; Reynolds, M.; Zhang, L.; Han, S.; Liang, S.; Sui, H.; Lin, Y. Display of fungal hydrophobin on the Pichia pastoris cell surface and its influence on Candida antarctica lipase B. Appl. Microbiol. Biotechnol. 2016, 100, 5883–5895. [Google Scholar] [CrossRef]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Ortiz, C.; Fuentes, M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Use of immobilized lipases for lipase purification via specific lipase-lipase interactions. J. Chromatogr. A 2004, 1038, 267–273. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General trend of lipase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Palomo, J.M.; Fernández-Lorente, G.; Illanes, A.; Guisán, J.M.; Fernández-Lafuente, R. Effect of lipase–lipase interactions in the activity, stability and specificity of a lipase from Alcaligenes sp. Enzym. Microb. Technol. 2006, 39, 259–264. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. Lipase Liquid. Available online: https://www.sigmaaldrich.com/BR/pt/search/lipase-liquid?focus=products&page=1&perpage=30&sort=relevance&term=lipaseliquid&type=product (accessed on 6 January 2025).
- Collaço, A.C.A.; Aguieiras, E.C.G.; Santos, J.G.; de Oliveira, R.A.; de Paula Vieira de Castro, R.; Freire, D.M.G. Experimental study and preliminary economic evaluation of enzymatic biodiesel production by an integrated process using co-products from palm (Elaeais guineensis Jaquim) industry. Ind. Crops Prod. 2020, 157, 112904. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-industrial wastes as potential carriers for enzyme immobilization: A review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- Collaço, A.C.A.; Aguieiras, E.C.G.; Cavalcanti, E.D.C.; Freire, D.M.G. Development of an integrated process involving palm industry co-products for monoglyceride / diglyceride emulsifier synthesis: Use of palm cake and fiber for lipase production and palm fatty-acid distillate as raw material. LWT 2021, 135, 110039. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Ribeiro, D.S.; Couteiro, P.P.; Bastos, C.M.B.; de Queiroz, D.S.; Parreira, J.M.; Langone, M.A.P. Investigation of the Reuse of Immobilized Lipases in Biodiesel Synthesis: Influence of Different Solvents in Lipase Activity. Appl. Biochem. Biotechnol. 2016, 179, 485–496. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Delavault, A.; Grüninger, J.; Kapp, D.; Hollenbach, R.; Rudat, J.; Ochsenreither, K.; Syldatk, C. Enzymatic Synthesis of Alkyl Glucosides by β-Glucosidases in a 2-in-1 Deep Eutectic Solvent System. Chemie-Ingenieur-Technik 2022, 94, 417–426. [Google Scholar] [CrossRef]
- Guajardo, N.; de María, P.D. Lipases in Green Chemistry: Deep Eutectic Solvents (DES) as New Green Solvents. In Lipases and Phospholipases: Methods and Protocols, Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1835, pp. 288–297. ISBN 9781493986729. [Google Scholar]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Miranda, L.P.; Fernandez-Lafuente, R.; Tardioli, P.W. Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil. Molecules 2021, 26, 193. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Mahapatra, P.; Kumar, G.V.; Banerjee, R. Comparative study of thermostabilty and ester synthesis ability of free and immobilized lipases on cross linked silica gel. Bioprocess Biosyst. Eng. 2008, 31, 291–298. [Google Scholar] [CrossRef]
- Junior, J.M.; Mattos, F.R.; Costa, G.R.; Zurlo, A.B.R.; Fernandez-Lafuente, R.; Mendes, A.A. Improved Catalytic Performance of Lipase Eversa® Transform 2.0 via Immobilization for the Sustainable Production of Flavor Esters—Adsorption Process and Environmental Assessment Studies. Catalysts 2022, 12, 1412. [Google Scholar] [CrossRef]
- Kaur, P.; Jana, A.K.; Jana, M.M. Immobilization of Candida rugosa lipase on optimized polyamidoamine dendrimer functionalized magnetic multiwalled carbon nanotubes for green manufacture of butyl butyrate ester. Mol. Catal. 2024, 553, 113779. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. The combine use of ultrasound and lipase immobilized on co-polymer matrix for efficient biocatalytic application studies. J. Mol. Catal. B Enzym. 2015, 122, 255–264. [Google Scholar] [CrossRef]
- Da Silva, V.C.F.; Contesini, F.J.; Carvalho, P.D.O. Characterization and catalytic activity of free and immobilized lipase from Aspergillus niger: A comparative study. J. Braz. Chem. Soc. 2008, 19, 1468–1474. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Yang, J.; Ma, X.; Zhang, Z.; Chen, B.; Li, S.; Wang, G. Lipase immobilized by modification-coupled and adsorption-cross-linking methods: A comparative study. Biotechnol. Adv. 2010, 28, 644–650. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized multi-wall carbon nanotubes for lipase immobilization. Adv. Eng. Mater. 2010, 12, 179–183. [Google Scholar] [CrossRef]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.G.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads improves butyl acetate synthesis. Biotechnol. Prog. 2012, 28, 406–412. [Google Scholar] [CrossRef]
- Friedrich, J.L.R.; Peña, F.P.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Effect of immobilization protocol on optimal conditions of ethyl butyrate synthesis catalyzed by lipase B from Candida antarctica. J. Chem. Technol. Biotechnol. 2012, 88, 1089–1095. [Google Scholar] [CrossRef]
- Basri, M.; Wong, C.C.; Ahmad, M.B.; Razak, C.N.A.; Salleh, A.B. Immobilization of lipase on poly(N-vinyl-2-pyrrolidone-co-2-hydroxyethyl methacrylate) hydrogel for the synthesis of butyl oleate. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 571–577. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Entrapment of surfactant modified lipase within zeolitic imidazolate framework (ZIF)-8. Int. J. Biol. Macromol. 2020, 146, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.A.; Lopes, F.M.; Yamashita, F.; Fernandes, K.F. Lipase entrapment in PVA/Chitosan biodegradable film for reactor coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1696–1701. [Google Scholar] [CrossRef]
- Kheadr, E.E.; Vuillemard, J.-C.; El-Deeb, S.A. Acceleration of Cheddar Cheese Lipolysis by Using Liposome-entrapped Lipases. J. Food Sci. 2002, 67, 485–492. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Simpelkamp, J. Efficient immobilization of lipases by entrapment in hydrophobic sol-gel materials. Biotechnol. Bioeng. 1996, 49, 527–534. [Google Scholar] [CrossRef]
- Sittko, I.; Kremser, K.; Roth, M.; Kuehne, S.; Stuhr, S.; Tiller, J.C. Amphiphilic polymer conetworks with defined nanostructure and tailored swelling behavior for exploring the activation of an entrapped lipase in organic solvents. Polymer 2015, 64, 122–129. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Du, Y.; Xiao, H.; McClements, D.J. Control of lipase digestibility of emulsified lipids by encapsulation within calcium alginate beads. Food Hydrocoll. 2011, 25, 122–130. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef]
- Reetz, M.T.; Tielmann, P.; Wiesenhöfer, W.; Könen, W.; Zonta, A. Second Generation Sol-Gel Encapsulated Lipases: Robust Heterogeneous Biocatalysts. Adv. Synth. Catal. 2003, 345, 717–728. [Google Scholar] [CrossRef]
- Ozturk, T.K.; Kilinc, A. Immobilization of lipase in organic solvent in the presence of fatty acid additives. J. Mol. Catal. B Enzym. 2010, 67, 214–218. [Google Scholar] [CrossRef]
- Bayramoǧlu, G.; Hazer, B.; Altintaş, B.; Arica, M.Y. Covalent immobilization of lipase onto amine functionalized polypropylene membrane and its application in green apple flavor (ethyl valerate) synthesis. Process Biochem. 2011, 46, 372–378. [Google Scholar] [CrossRef]
- Mendes, A.A.; de Castro, H.F.; Giordano, R.L.C. Covalent attachment of lipases on glyoxyl-agarose beads: Application in fruit flavor and biodiesel synthesis. Int. J. Biol. Macromol. 2014, 70, 78–85. [Google Scholar] [CrossRef]
- Kartal, F. Enhanced esterification activity through interfacial activation and cross-linked immobilization mechanism of Rhizopus oryzae lipase in a nonaqueous medium. Biotechnol. Prog. 2016, 32, 899–904. [Google Scholar] [CrossRef]
- Sampaio, C.S.; Angelotti, J.A.F.; Fernandez-Lafuente, R.; Hirata, D.B. Lipase immobilization via cross-linked enzyme aggregates: Problems and prospects—A review. Int. J. Biol. Macromol. 2022, 215, 434–449. [Google Scholar] [CrossRef]
- Kanwar, S.S. Synthesis of Ethyl Propionate Catalyzed by Poly(NAEAAm-co-AAc)-cl-MBAm Hydrogel-Immobilized Lipase of Bacillus coagulans MTCC-6375. J. Appl. Polym. Sci. 2007, 116, 2658–2667. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Pinto, M.C.C.; Robert, J.d.M.; da Silva, T.P.; Beralto, T.d.C.; Santos, J.G.F.; de Castro, R.d.P.V.; Fernandez-Lafuente, R.; Manoel, E.A.; Pinto, J.C.; et al. Pilot-scale development of core–shell polymer supports for the immobilization of recombinant lipase B from Candida antarctica and their application in the production of ethyl esters from residual fatty acids. J. Appl. Polym. Sci. 2018, 135, 46727. [Google Scholar] [CrossRef]
- Silva, M.V.C.; Rosa, C.M.R.; Aguiar, L.G.; Oliveira, P.C.; de Castro, H.F.; Freitas, L. Synthesis of Isopropyl Palmitate by Lipase Immobilized on a Magnetized Polymer Matrix. Chem. Eng. Technol. 2020, 43, 1741–1748. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Cerqueira Pinto, M.C.; Lorente, G.F.; Manoel, E.A.; Guisán, J.M.; Ninow, J.L.; de Oliveira, D.; Pessela, B.C. Production of new nanobiocatalysts via immobilization of lipase B from C. antarctica on polyurethane nanosupports for application on food and pharmaceutical industries. Int. J. Biol. Macromol. 2020, 165, 2957–2963. [Google Scholar] [CrossRef]
- Gawlitza, K.; Wu, C.; Georgieva, R.; Wang, D. Immobilization of lipase B within micron-sized poly- N -isopropylacrylamide hydrogel particles by solvent exchange. Phys. Chem. Chem. Phys. 2012, 14, 9594–9600. [Google Scholar] [CrossRef]
- Poojari, Y.; Beemat, J.S.; Clarson, S.J. Enzymatic synthesis of poly(ε-caprolactone): Thermal properties, recovery, and reuse of lipase B from Candida antarctica immobilized on macroporous acrylic resin particles. Polym. Bull. 2013, 70, 1543–1552. [Google Scholar] [CrossRef]
- Ben Salah, R.; Ghamghui, H.; Miled, N.; Mejdoub, H.; Gargouri, Y. Production of butyl acetate ester by lipase from novel strain of Rhizopus oryzae. J. Biosci. Bioeng. 2007, 103, 368–372. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Effect of the immobilization protocol on the properties of lipase B from Candida antarctica in organic media: Enantiospecifc production of atenolol acetate. J. Mol. Catal. B Enzym. 2011, 71, 124–132. [Google Scholar] [CrossRef]
- Dosanjh, N.S.; Kaur, J. Immobilization, stability and esterification studies of a lipase from a Bacillus sp. Biotechnol. Appl. Biochem. 2002, 36, 7. [Google Scholar] [CrossRef] [PubMed]
- Ghamgui, H.; Karra-Chaabouni, M.; Gargouri, Y. 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: A comparative study between n-hexane and solvent-free system. Enzym. Microb. Technol. 2004, 35, 355–363. [Google Scholar] [CrossRef]
- Shu, C.; Cai, J.; Huang, L.; Zhu, X.; Xu, Z. Biocatalytic production of ethyl butyrate from butyric acid with immobilized Candida rugosa lipase on cotton cloth. J. Mol. Catal. B Enzym. 2011, 72, 139–144. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Pinheiro, Á.D.T.; Ferreira, A.L.O.; Gonçalves, L.R.B. Immobilization of Candida antarctica lipase B by adsorption to green coconut fiber. Appl. Biochem. Biotechnol. 2008, 146, 173–187. [Google Scholar] [CrossRef]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of agriculture waste as a support for lipase immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Bonet-Ragel, K.; López-Pou, L.; Tutusaus, G.; Benaiges, M.D.; Valero, F. Rice husk ash as a potential carrier for the immobilization of lipases applied in the enzymatic production of biodiesel. Biocatal. Biotransform. 2018, 36, 151–158. [Google Scholar] [CrossRef]
- de Castro, H.F.; de Lima, R.; Roberto, I.C. Rice Straw as a Support for Immobilization of Microbial Lipase. Biotechnol. Prog. 2001, 17, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, R.; Sanny, S.A.; Derman, E. Stability studies of immobilized lipase on rice husk and eggshell membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012032. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzym. Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Torres, R.; Barbosa, O.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Evaluation of divinylsulfone activated agarose to immobilize lipases and to tune their catalytic properties. Process Biochem. 2015, 50, 918–927. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase from Pseudomonas fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Biotechnol. 2020, 8, 36. [Google Scholar] [CrossRef]
- Lokha, Y.; Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Gonçalves, L.R.B.; Lopez-Gallego, F.; Fernandez-Lafuente, R. Modulating the properties of the lipase from Thermomyces lanuginosus immobilized on octyl agarose beads by altering the immobilization conditions. Enzym. Microb. Technol. 2020, 133, 109461. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Immobilization of lipases via interfacial activation on hydrophobic supports: Production of biocatalysts libraries by altering the immobilization conditions. Catal. Today 2021, 362, 130–140. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzym. Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Virgen-Ortíz, J.J.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers: Avoiding enzyme release from the support. Process Biochem. 2017, 54, 81–88. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Virgen-OrtÍz, J.J.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Optimization of the coating of octyl-CALB with ionic polymers to improve stability and decrease enzyme leakage. Biocatal. Biotransform. 2018, 36, 47–56. [Google Scholar] [CrossRef]
- Pizarro, C.; Brañes, M.C.; Markovits, A.; Fernández-Lorente, G.; Guisán, J.M.; Chamy, R.; Wilson, L. Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis. J. Mol. Catal. B Enzym. 2012, 78, 111–118. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzym. Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Yu, M.; Zhou, H.; Zhou, H.; Wei, P. Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa Lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J. Mol. Catal. B Enzym. 2016, 133, 1–5. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Filice, M.; Lopez-Vela, D.; Pizarro, C.; Wilson, L.; Betancor, L.; Avila, Y.; Guisan, J.M. Cross-Linking of Lipases Adsorbed on Hydrophobic Supports: Highly Selective Hydrolysis of Fish Oil Catalyzed by RML. J. Am. Oil Chem. Soc. 2011, 88, 801–807. [Google Scholar] [CrossRef]
- De Almeida, D.G.; Soares Da Silva, R.d.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef]
- Ren, L.; Jia, H.; Yu, M.; Shen, W.; Zhou, H.; Wei, P. Enhanced catalytic ability of Candida rugosa lipase immobilized on pore-enlarged hollow silica microspheres and cross-linked by modified dextran in both aqueous and non-aqueous phases. Biotechnol. Bioprocess Eng. 2013, 18, 888–896. [Google Scholar] [CrossRef]
- Bernal, C.; Poveda-Jaramillo, J.C.; Mesa, M. Raising the enzymatic performance of lipase and protease in the synthesis of sugar fatty acid esters, by combined ionic exchange-hydrophobic immobilization process on aminopropyl silica support. Chem. Eng. J. 2018, 334, 760–767. [Google Scholar] [CrossRef]
- Vescovi, V.; Kopp, W.; Guisán, J.M.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino-glutaraldehyde spacer arms. Process Biochem. 2016, 51, 2055–2066. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Selectivity of R-α-monobenzoate glycerol synthesis catalyzed by Candida antarctica lipase B immobilized on heterofunctional supports. Process Biochem. 2015, 50, 1870–1877. [Google Scholar] [CrossRef]
- Hirata, D.B.; Albuquerque, T.L.; Rueda, N.; Sánchez-Montero, J.M.; Garcia-Verdugo, E.; Porcar, R.; Fernandez-Lafuente, R. Advantages of Heterofunctional Octyl Supports: Production of 1,2-Dibutyrin by Specific and Selective Hydrolysis of Tributyrin Catalyzed by Immobilized Lipases. ChemistrySelect 2016, 1, 3259–3270. [Google Scholar] [CrossRef]
- Souza, P.M.P.; Carballares, D.; Lopez-Carrobles, N.; Gonçalves, L.R.B.; Lopez-Gallego, F.; Rodrigues, S.; Fernandez-Lafuente, R. Enzyme-support interactions and inactivation conditions determine Thermomyces lanuginosus lipase inactivation pathways: Functional and florescence studies. Int. J. Biol. Macromol. 2021, 191, 79–91. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Carballares, D.; Rocha-Martin, J.; Alcántara, A.R.; Tardioli, P.W.; Fernandez-Lafuente, R. Heterofunctional Methacrylate Beads Bearing Octadecyl and Vinyl Sulfone Groups: Tricks to Obtain an Interfacially Activated Lipase from Thermomyces lanuginosus and Covalently Attached to the Support. Catalysts 2023, 13, 108. [Google Scholar] [CrossRef]
- Hirata, D.B.; Albuquerque, T.L.; Rueda, N.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Fernandez-Lafuente, R. Evaluation of different immobilized lipases in transesterification reactions using tributyrin: Advantages of the heterofunctional octyl agarose beads. J. Mol. Catal. B Enzym. 2016, 133, 117–123. [Google Scholar] [CrossRef]
- Souza, P.M.P.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R.; Rodrigues, S. Immobilization of Lipase B from Candida antarctica in Octyl-Vinyl Sulfone Agarose: Effect of the Enzyme-Support Interactions on Enzyme Activity, Specificity, Structure and Inactivation Pathway. Int. J. Mol. Sci. 2022, 23, 14268. [Google Scholar] [CrossRef]
- Li, K.; Fan, Y.; He, Y.; Zeng, L.; Han, X.; Yan, Y. Burkholderia cepacia lipase immobilized on heterofunctional magnetic nanoparticles and its application in biodiesel synthesis. Sci. Rep. 2017, 7, 16473. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional Hydrophilic–Hydrophobic Porous Silica as Support for Multipoint Covalent Immobilization of Lipases: Application to Lactulose Palmitate Synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.S.; Mendez-Sanchez, C.; Arana-Peña, S.; Rueda, N.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Immobilization of lipase from Pseudomonas fluorescens on glyoxyl-octyl-agarose beads: Improved stability and reusability. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Suescun, A.; Rueda, N.; dos Santos, J.C.S.; Castillo, J.J.; Ortiz, C.; Torres, R.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of lipases on glyoxyl–octyl supports: Improved stability and reactivation strategies. Process Biochem. 2015, 50, 1211–1217. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Rueda, N.; Albuquerque, T.L.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; Dos Santos, J.C.S.; Barbosa, O.; Fernandez-Lafuente, R. Reversible Immobilization of Lipases on Heterofunctional Octyl-Amino Agarose Beads Prevents Enzyme Desorption. Molecules 2016, 21, 646. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Rueda, N.; dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Rios, N.S.; Arana-Peña, S.; Mendez-Sanchez, C.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Reuse of Lipase from Pseudomonas fluorescens via Its Step-by-Step Coimmobilization on Glyoxyl-Octyl Agarose Beads with Least Stable Lipases. Catalysts 2019, 9, 487. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. Coimmobilization of lipases exhibiting three very different stability ranges. Reuse of the active enzymes and selective discarding of the inactivated ones. Int. J. Biol. Macromol. 2022, 206, 580–590. [Google Scholar] [CrossRef]
- Dossat, V.; Combes, D.; Marty, A. Continuous enzymatic transesterification of high oleic sunflower oil in a packed bed reactor: Influence of the glycerol production. Enzym. Microb. Technol. 1999, 25, 194–200. [Google Scholar] [CrossRef]
- Séverac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.S.; Monsan, P.; Marty, A. Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzym. Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef]
- Lopresto, G.C.; Calabrò, V.; Woodley, J.M.; Tufvesson, P. Enzymatic kinetic study on the enzymatic esterification of octanoic acid and hexanol by immobilized Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2014, 110, 64–71. [Google Scholar] [CrossRef]
- Varma, M.N.; Madras, G. Effect of chain length of alcohol on the lipase-catalyzed esterification of propionic acid in supercritical carbon dioxide. Appl. Biochem. Biotechnol. 2010, 160, 2342–2354. [Google Scholar] [CrossRef]
- Alves, J.S.; Garcia-Galan, C.; Schein, M.F.; Silva, A.M.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules 2014, 19, 9562–9576. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.B.; Friedrich, J.L.R.; Cavalheiro, J.C.; Garcia-Galan, C.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads. Bioresour. Technol. 2013, 134, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J.K.; Garcia-Galan, C.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J. Mol. Catal. B Enzym. 2013, 94, 51–56. [Google Scholar] [CrossRef]
- Fallavena, L.P.; Antunes, F.H.F.; Alves, J.S.; Paludo, N.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei. RSC Adv. 2014, 4, 8675–8681. [Google Scholar] [CrossRef]
- Martins, A.B.; Schein, M.F.; Friedrich, J.L.R.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Ultrasound-assisted butyl acetate synthesis catalyzed by Novozym 435: Enhanced activity and operational stability. Ultrason. Sonochem. 2013, 20, 1155–1160. [Google Scholar] [CrossRef]
- Paludo, N.; Alves, J.S.; Altmann, C.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason. Sonochem. 2014, 22, 89–94. [Google Scholar] [CrossRef]
- Alves, J.S.; Garcia-Galan, C.; Danelli, D.; Paludo, N.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Use of Lecitase-Ultra immobilized on styrene-divinylbenzene beads as catalyst of esterification reactions: Effects of ultrasounds. Catal. Today 2015, 255, 27–32. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Transesterification of Waste Frying Oil and Soybean Oil by Combi-lipases Under Ultrasound-Assisted Reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef] [PubMed]
- José, C.; Bonetto, R.D.; Gambaro, L.A.; Torres, M.P.G.; Foresti, M.L.; Ferreira, M.L.; Briand, L.E. Enzymatic Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol–aqueous media. J. Mol. Catal. B Enzym. 2011, 71, 95–107. [Google Scholar] [CrossRef]
- Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.M.; Shin, W.H.; Oh, J.M.; Park, C. Enzymatic synthesis of formate ester through immobilized lipase and its reuse. Polymers 2020, 12, 1802. [Google Scholar] [CrossRef]
- Seo, J.; Shin, M.; Lee, J.; Lee, T.; Oh, J.M.; Park, C. Novel and highly efficient lipase-catalyzed esterification of formic acid with hexanol for waste gas reutilization. J. Ind. Eng. Chem. 2021, 93, 430–435. [Google Scholar] [CrossRef]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: Reaction optimization by response surface methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Divakar, S.; Prapulla, S.G.; Karanth, N.G. Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J. Biotechnol. 2001, 87, 193–201. [Google Scholar] [CrossRef]
- Güvenç, A.; Kapucu, N.; Kapucu, H.; Aydoǧan, Ö.; Mehmetoǧlu, Ü. Enzymatic esterification of isoamyl alcohol obtained from fusel oil: Optimization by response surface methodolgy. Enzym. Microb. Technol. 2007, 40, 778–785. [Google Scholar] [CrossRef]
- Nyari, N.; Paulazzi, A.; Zamadei, R.; Steffens, C.; Zabot, G.L.; Tres, M.V.; Zeni, J.; Venquiaruto, L.; Dallago, R.M. Synthesis of isoamyl acetate by ultrasonic system using Candida antarctica lipase B immobilized in polyurethane. J. Food Process Eng. 2018, 41, e12812. [Google Scholar] [CrossRef]
- Narwal, S.K.; Saun, N.K.; Dogra, P.; Gupta, R. Green synthesis of isoamyl acetate via silica immobilized novel thermophilic lipase from Bacillus aerius. Russ. J. Bioorg. Chem. 2016, 42, 69–73. [Google Scholar] [CrossRef]
- De Oliveira, T.P.; Pereira, M.; Santos, F. Incorporation of metallic particles in activated carbon used in lipase immobilization for production of isoamyl acetate. J. Chem. Technol. Biotechnol. 2022, 97, 1736–1746. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, T.; Ni, X.; Xia, J.; Suo, H.; Yan, L.; Zou, B. Immobilized lipase based on SBA-15 adsorption and gel embedding for catalytic synthesis of isoamyl acetate. Food Biosci. 2024, 60, 104427. [Google Scholar] [CrossRef]
- Diaz, M.D.R.; Gómez, J.M.; Díaz-Suelto, B.; García-Sanz, A. Enzymatic synthesis of short-chain esters in n-hexane and supercritical carbon dioxide: Effect of the acid chain length. Eng. Life Sci. 2010, 10, 171–176. [Google Scholar] [CrossRef]
- Bourg-Garros, S.; Razafindramboa, N.; Pavia, A.A. Optimization of lipase-catalyzed synthesis of (z)-3-hexen-1-yl acetate by direct esterification in hexane and a solvent-free medium. Enzym. Microb. Technol. 1998, 22, 240–245. [Google Scholar] [CrossRef]
- Dai, W.C.; Chiu, S.J.; Huang, D.Y.; Juan, H.Y.; Chen, C.Y.; Chen, S.S.; Su, C.H.; Li, S.Y. Lipase-catalyzed synthesis of butyl propionate in solvent-free system: Optimization by response surface methodology. J. Taiwan Inst. Chem. Eng. 2014, 45, 2233–2237. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. Microwave assisted solvent-free synthesis of n-butyl propionate by immobilized lipase as catalyst. Biocatal. Agric. Biotechnol. 2018, 14, 264–269. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Acoustic cavitation promoted lipase catalysed synthesis of isobutyl propionate in solvent free system: Optimization and kinetic studies. Ultrason. Sonochem. 2018, 40, 727–735. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; da Fonseca, M.M.R.; Ferreira-Dias, S. Esterification activity and operational stability of Candida rugosa lipase immobilized in polyurethane foams in the production of ethyl butyrate. Biochem. Eng. J. 2010, 48, 246–252. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, J.M.; Roura, E.; Contreras, E. Biosynthesis of ethyl butyrate using immobilized lipase: A statistical approach. Process Biochem. 2005, 40, 63–68. [Google Scholar] [CrossRef]
- Rahman, I.N.A.; Manan, F.M.A.; Marzuki, N.H.C.; Mahat, N.A.; Attan, N.; Keyon, A.S.A.; Jamalis, J.; Aboul-Enein, H.Y.; Wahab, R.A. A statistical approach for optimizing the high yield green production of the flavor ester butyl butyrate. J. Teknol. 2017, 79, 141–151. [Google Scholar] [CrossRef][Green Version]
- Sjöblom, M.; Risberg, P.; Filippova, A.; Öhrman, O.G.W.; Rova, U.; Christakopoulos, P. In Situ Biocatalytic Synthesis of Butyl Butyrate in Diesel and Engine Evaluations. ChemCatChem 2017, 9, 4529–4537. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Bachmann, L.; Said, S.; Oliveira, W.P. General Enhancement lipase activity via immobilization onto chitosan beads used as seed particles during fluidized bed drying: Application in butyl butyrate production. Appl. Catal. A Gen. 2021, 622, 118217. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Sahney, R.; Dahiya, P. Green synthesis of iron-alginate nanoparticle for Taguchi-assisted immobilization of Candida rugosa lipase and its application in the synthesis of butyl butyrate ester. Process Biochem. 2024, 139, 81–92. [Google Scholar] [CrossRef]
- Gonçalves, G.R.F.; Reinert, O.; Gandolfi, R.; Junqueira, M.; Brito, P.; Cristina, R.; Bonomo, F.; Ilh, C.; Veloso, C.M. Immobilization of porcine pancreatic lipase on activated carbon by adsorption and covalent bonding and its application in the synthesis of butyl butyrate. Process Biochem. 2021, 111, 114–123. [Google Scholar] [CrossRef]
- Kaur, P.; Kumar, A. Amino functionalization of magnetic multiwalled carbon nanotubes with flexible hydrophobic spacer for immobilization of Candida rugosa lipase and application in biocatalytic production of fruit flavour esters ethyl butyrate and butyl butyrate. Appl. Nanosci. 2023, 13, 4291–4311. [Google Scholar] [CrossRef]
- Anschau, A.; da Mota Huerta, K.; Rêgo, T.V.; de Oliveira, J.M.G.; Borba, C.M.; Kalil, S.J.; Burkert, C.A.V.; de Medeiros Burkert, J.F. Enzymatic synthesis optimization of isoamyl butyrate from fusel oil. Acta Sci. Biol. Sci. 2021, 43, e54966. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Sattur, A.P.; Karanth, N.G. Lipase-catalyzed synthesis of isoamyl isobutyrate—Optimization using a central composite rotatable design. Process Biochem. 2001, 37, 9–16. [Google Scholar] [CrossRef]
- Santos, M.M.O.; Menezes, L.H.S.; Santo, E.L.E.; Carvalho, M.S.; Gonçalves, M.S.; Tavares, I.M.C.; Mendes, A.A.; Ruiz, H.A.; Salay, L.C.; Franco, M.; et al. Synthesis of hexyl butyrate (apple and citrus aroma) by Candida rugosa lipase immobilized on Diaion HP-20 using the Box-Behnken design. Food Sci. Biotechnol. 2023, 32, 689–696. [Google Scholar] [CrossRef]
- Raghavendra, T.; Sayania, D.; Madamwar, D. Synthesis of the ‘green apple ester’ ethyl valerate in organic solvents by Candida rugosa lipase immobilized in MBGs in organic solvents: Effects of immobilization and reaction parameters. J. Mol. Catal. B Enzym. 2010, 63, 31–38. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. Process intensification by microwave irradiation in immobilized-lipase catalysis in solvent-free synthesis of ethyl valerate. Mol. Catal. 2018, 461, 34–39. [Google Scholar] [CrossRef]
- Cebrián-García, S.; Balu, A.M.; García, A.; Luque, R. Sol-gel immobilisation of lipases: Towards active and stable biocatalysts for the esterification of valeric acid. Molecules 2018, 23, 2283. [Google Scholar] [CrossRef] [PubMed]
- Hussin, N.H.; Wahab, R.A.; Elias, N.; Jacob, A.G.; Zainal-Abidin, M.H.; Abdullah, F.; Sulaiman, N.J.; Misson, M. Electrospun magnetic nanocellulose–polyethersulfone-conjugated Aspergillus oryzae lipase for synthesis of ethyl valerate. Membranes 2021, 11, 972. [Google Scholar] [CrossRef]
- Padilha, G.S.; De Barros, M.; Alegre, R.M.; Tambourgi, E.B. Production of ethyl valerate from Burkholderia cepacia lipase immobilized in alginate. Chem. Eng. Trans. 2013, 32, 1063–1068. [Google Scholar] [CrossRef]
- Jacob, A.G.; Wahab, R.A.; Chandren, S.; Jumbri, K.; Mahmood, W.M.A. Physicochemical properties and operational stability of Taguchi design-optimized Candida rugosa lipase supported on biogenic silica/magnetite/graphene oxide for ethyl valerate synthesis. Adv. Powder Technol. 2022, 33, 103374. [Google Scholar] [CrossRef]
- Elias, N.; Wahab, R.A.; Chandren, S.; Jamalis, J.; Mahat, N.A.; Jye, L.W. Structure and properties of lipase activated by cellulose-silica polyethersulfone membrane for production of pentyl valerate. Carbohydr. Polym. 2020, 245, 116549. [Google Scholar] [CrossRef]
- Musa, N.; Latip, W.; Abd Rahman, R.N.Z.; Salleh, A.B.; Ali, M.S.M. Immobilization of an antarctic Pseudomonas AMS8 lipase for low temperature ethyl hexanoate synthesis. Catalysts 2018, 8, 234. [Google Scholar] [CrossRef]
- Shin, M.; Seo, J.; Baek, Y.; Lee, T.; Jang, M.; Park, C. Novel and efficient synthesis of phenethyl formate via enzymatic esterification of formic acid. Biomolecules 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; de Meneses, A.C.; Lerin, L.A.; de Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Biocatalysis of aromatic benzyl-propionate ester by different immobilized lipases. Bioprocess Biosyst. Eng. 2018, 41, 585–591. [Google Scholar] [CrossRef]
- de Meneses, A.C.; Lerin, L.A.; Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Benzyl propionate synthesis by fed-batch esterification using commercial immobilized and lyophilized CALB lipase. Bioprocess Biosyst. Eng. 2019, 42, 1625–1634. [Google Scholar] [CrossRef]
- Jeromin, G.E.; Zoor, A. A new irreversible enzyme-aided esterification method in organic solvents. Biotechnol. Lett. 2008, 30, 925–928. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhong, H.; Zhu, H.; Wang, H.; Goh, K.L.; Zhang, J.; Zheng, M. Efficient and sustainable preparation of cinnamic acid flavor esters by immobilized lipase microarray. LWT 2023, 173, 114322. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Enzymatic catalysis in organic media at 100 °C. Science 1984, 224, 1249–1251. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef]
- Valivety, R.H.; Johnston, G.A.; Suckling, C.J.; Halling, P.J. Solvent effects on biocatalysis in organic systems: Equilibrium position and rates of lipase catalyzed esterification. Biotechnol. Bioeng. 1991, 38, 1137–1143. [Google Scholar] [CrossRef]
- García-Alles, L.F.; Gotor, V. Alcohol Inhibition and Specificity Studies of Lipase B from Candida antarctica in Organic Solvents. Biotechnol. Bioeng. 1998, 59, 163–170. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Levashov, A.V.; Klyachko, N.L.; Martinek, K. Engineering biocatalytic systems in organic media with low water content. Enzym. Microb. Technol. 1988, 10, 710–724. [Google Scholar] [CrossRef]
- Branco, R.J.F.; Graber, M.; Denis, V.; Pleiss, J. Molecular mechanism of the hydration of Candida antarctica lipase B in the gas phase: Water adsorption isotherms and molecular dynamics simulations. ChemBioChem 2009, 10, 2913–2919. [Google Scholar] [CrossRef]
- Trodler, P.; Pleiss, J. Modeling structure and flexibility of Candida antarctica lipase B in organic solvents. BMC Struct. Biol. 2008, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Rehm, S.; Trodler, P.; Pleiss, J. Solvent-induced lid opening in lipases: A molecular dynamics study. Protein Sci. 2010, 19, 2122–2130. [Google Scholar] [CrossRef]
- Cebrián-García, S.; Balu, A.M.; Luque, R. Ultrasound-assisted esterification of valeric acid to alkyl valerates promoted by biosilicified lipases. Front. Chem. 2018, 6, 197. [Google Scholar] [CrossRef]
- Ewing, T.A.; Nouse, N.; van Lint, M.; van Haveren, J.; Hugenholtz, J.; van Es, D.S. Fermentation for the production of biobased chemicals in a circular economy: A perspective for the period 2022–2050. Green Chem. 2022, 24, 6373–6405. [Google Scholar] [CrossRef]
- Kovalenko, G.; Perminova, L.; Pykhtina, M.; Beklemishev, A. Lipase-active heterogeneous biocatalysts for enzymatic synthesis of short-chain aroma esters. Biocatal. Agric. Biotechnol. 2021, 36, 102124. [Google Scholar] [CrossRef]
- Kütt, A.; Selberg, S.; Kaljurand, I.; Tshepelevitsh, S.; Heering, A.; Darnell, A.; Kaupmees, K.; Piirsalu, M.; Leito, I. pKa values in organic chemistry—Making maximum use of the available data. Tetrahedron Lett. 2018, 59, 3738–3748. [Google Scholar] [CrossRef]
- Rosell, C.M.; Terreni, M.; Fernandez-Lafuente, R.; Guisan, J.M. A criterion for the selection of monophasic solvents for enzymatic synthesis. Enzym. Microb. Technol. 1998, 23, 64–69. [Google Scholar] [CrossRef]
- van Schie, M.M.C.H.; Spöring, J.-D.; Bocola, M.; Domínguez de María, P.; Rother, D. Applied biocatalysis beyond just buffers—From aqueous to unconventional media. Options and guidelines. Green Chem. 2021, 23, 3191–3206. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Van Spronsen, J.; Witkamp, G. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Bode, M.L.; Mathebula, N. Green and sustainable solvents for biocatalytic oxidations. Curr. Opin. Green Sustain. Chem. 2023, 39, 100741. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipase-catalyzed synthesis of isoamyl butyrate: A kinetic study. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 2001, 1547, 262–267. [Google Scholar] [CrossRef]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing: A Tutorial; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–393. [Google Scholar] [CrossRef]
- Santos, D.; da Rocha, E.C.L.; Santos, R.L.M.; Cancelas, A.J.; Franceschi, E.; Santos, A.F.; Fortuny, M.; Dariva, C. Demulsification of water-in-crude oil emulsions using single mode and multimode microwave irradiation. Sep. Purif. Technol. 2017, 189, 347–356. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave material processing—A review. AIChE J. 2012, 58, 330–363. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Stencel, L.M. Fast, Easy Preparation of Biodiesel Using Microwave Heating. Energy Fuels 2006, 20, 2281–2283. [Google Scholar] [CrossRef]
- Choedkiatsakul, I.; Ngaosuwan, K.; Assabumrungrat, S.; Mantegna, S.; Cravotto, G. Biodiesel production in a novel continuous flow microwave reactor. Renew. Energy 2015, 83, 25–29. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Ma, D.; Wu, H.; Wang, Z.; Wang, L.; Fang, X. Microwave-assisted fatty acid methyl ester production from soybean oil by Novozym 435. Green Chem. 2010, 12, 844–850. [Google Scholar] [CrossRef]
- Khan, N.R.; Rathod, V.K. Microwave assisted enzymatic synthesis of speciality esters: A mini-review. Process Biochem. 2018, 75, 89–98. [Google Scholar] [CrossRef]
- Hasegawa, S.; Azuma, M.; Takahashi, K. Enzymatic esterification of lactic acid, utilizing the basicity of particular polar organic solvents to suppress the acidity. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 1503–1510. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Jackson, M.; Blanco, R.M. Active-site titration analysis of surface influences on immobilized Candida antarctica lipase B activity. J. Mol. Catal. B Enzym. 2011, 69, 60–65. [Google Scholar] [CrossRef]
- Chen, B.; Hu, J.; Miller, E.M.; Xie, W.; Cai, M.; Gross, R.A. Candida antarctica Lipase B Chemically Immobilized on Epoxy-Activated Micro- and Nanobeads: Catalysts for Polyester Synthesis. Biomacromolecules 2008, 9, 463–471. [Google Scholar] [CrossRef]
- Chen, B.; Miller, E.M.; Miller, L.; Maikner, J.J.; Gross, R.A. Effects of Macroporous Resin Size on Candida antarctica Lipase B Adsorption, Fraction of Active Molecules, and Catalytic Activity for Polyester Synthesis. Langmuir 2007, 23, 1381–1387. [Google Scholar] [CrossRef]
- Tambe, A.; Vyasarayani, R.; Datla, A.; Ponrathnam, S.; Demnerova, K. Macroporous Poly (Vinyl Acetate-Co-Divinyl Benzene) Copolymer Beads as Macroporous Poly (Vinyl Acetate-Co-Divinyl Benzene) Copolymer Beads as Adsorptive Support for the Direct Immobilization of Candida antarctica Lipase B. Enzym. Eng. 2015, 4, 1000130. [Google Scholar]
- Nicolás, P.; Lassalle, V.L.; Ferreira, M.L. About the role of typical spacer/crosslinker on the design of efficient magnetic biocatalysts based on nanosized magnetite. J. Mol. Catal. B Enzym. 2015, 122, 296–304. [Google Scholar] [CrossRef]
- Weinberg, S.; Pellis, A.; Comerford, J.W.; Farmer, T.J.; Guebitz, G.M. Efficient Physisorption of Candida Antarctica Lipase B on Polypropylene Beads and Application for Polyester Synthesis. Catalysts 2018, 8, 369. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Z. Migration of reactive trace compounds from Novozym® 435 into organic solvents and ionic liquids. Biochem. Eng. J. 2010, 49, 113–118. [Google Scholar] [CrossRef]
- Pires-cabral, P.; Dubreucq, E.; Fonseca, M.M.R.; Ferreira-dias, S. Partitioning of water in organic systems with lipase immobilized in polyurethane foams. Biochem. Eng. J. 2005, 26, 29–37. [Google Scholar] [CrossRef]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: Experimental and molecular modeling aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar] [CrossRef]
- Toledo, M.V.; José, C.; Collins, S.E.; Bonetto, R.D.; Ferreira, M.L.; Briand, L.E. Enzymatic Esterification of R/S-ketoprofen with 2-propanol as reactant and solvent catalyzed by Novozym® 435 at selected conditions. J. Mol. Catal. B Enzym. 2012, 83, 108–119. [Google Scholar] [CrossRef]
- Ru, M.T.; Dordick, J.S.; Reimer, J.A.; Clark, D.S. Optimizing the salt-induced activation of enzymes in organic solvents: Effects of lyophilization time and water content. Biotechnol. Bioeng. 1999, 63, 233–241. [Google Scholar] [CrossRef]
- Leszczak, J.-P.; Tran-Minh, C. Optimized enzymatic synthesis of methyl benzoate in organic medium. Operating conditions and impact of different factors on kinetics. Biotechnol. Bioeng. 1998, 60, 356–361. [Google Scholar] [CrossRef]
- Sun, S.Y.; Xu, Y.; Wang, D. Novel minor lipase from Rhizopus chinensis during solid-state fermentation: Biochemical characterization and its esterification potential for ester synthesis. Bioresour. Technol. 2009, 100, 2607–2612. [Google Scholar] [CrossRef]
- Cybulski, A.; Stankiewicz, A.; Edvinsson Albers, R.K.; Moulijn, J.A. Monolithic reactors for fine chemicals industries: A comparative analysis of a monolithic reactor and a mechanically agitated slurry reactor. Chem. Eng. Sci. 1999, 54, 2351–2358. [Google Scholar] [CrossRef]
- Hagen, L.H.; Vivekanand, V.; Linjordet, R.; Pope, P.B.; Eijsink, V.G.H.; Horn, S.J. Microbial community structure and dynamics during co-digestion of whey permeate and cow manure in continuous stirred tank reactor systems. Bioresour. Technol. 2014, 171, 350–359. [Google Scholar] [CrossRef] [PubMed]
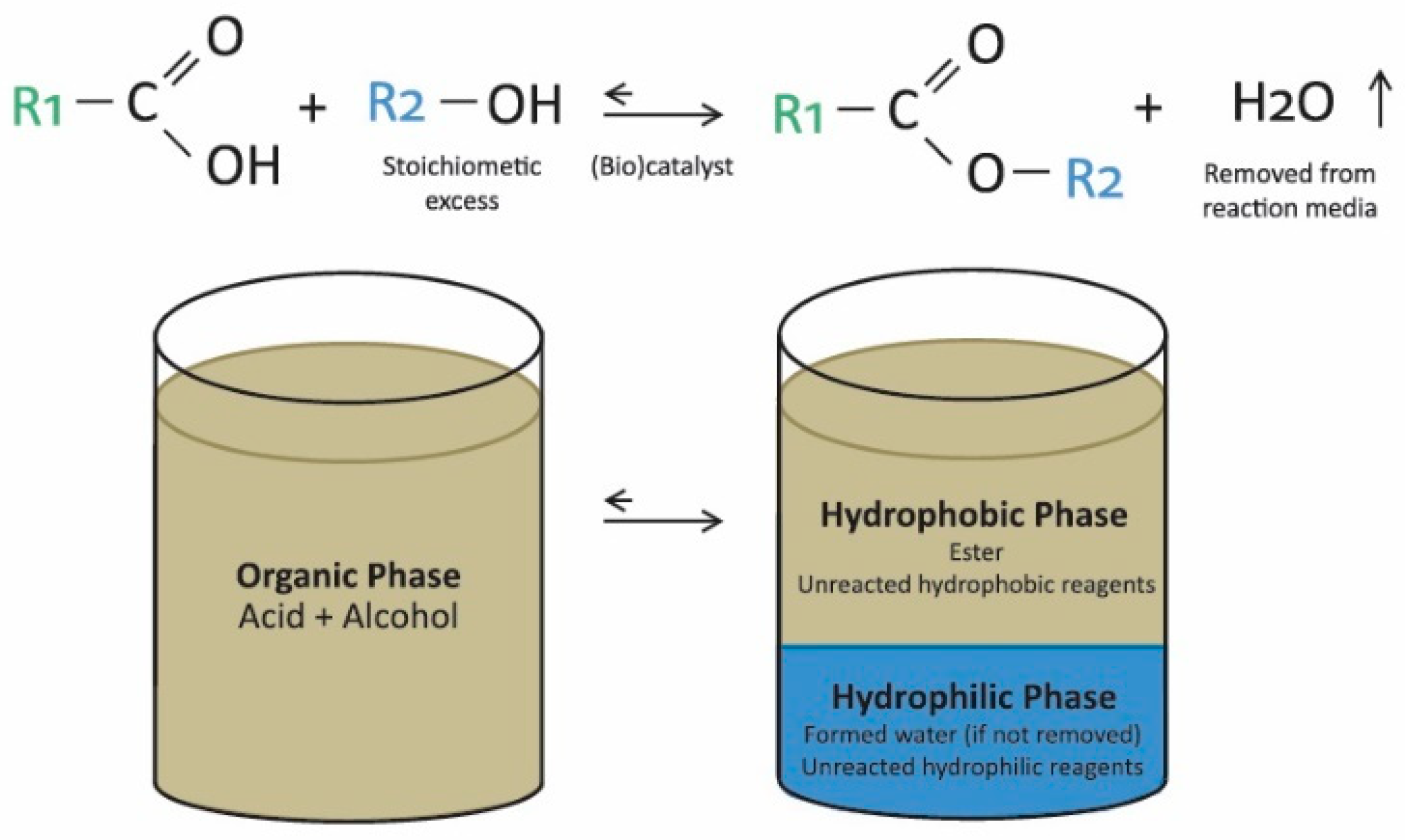
| Ester | Biocatalyst Biocat. Loading | Reaction Conditions | Acid: Alcohol Molar Ratio | Solvent | Conversion (%) | Concentration (g L−1) | Productivity (g L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Octyl Formate | Novozym® 435 15 g L−1 | Orbital shaker, 150 rpm, 1 h, 40 °C | 1:7 | 1,2-dichloro- ethane | 96.5 | 7.6 | 7.6 | [264] |
| Hexyl Formate | Novozym® 435 15 g L−1 | Orbital shaker, 150 rpm, 1.5 h, 40 °C | 1:5 | 1,2-dichloro- ethane | 98.3 | 12.8 | 8.5 | [265] |
| Ethyl Acetate | Novozym® 435 8 g L−1 | Orbital shaker, 150 rpm, 40 °C | 1:4.4 | n-heptane solvent-free | Not ment- ioned (only reaction rate) | - | - | [13] |
| Ethyl Acetate | Novozym® 435 6% (w/v) | Ultrasound (150 W/28 kHz), ~3 h | 1:4 | n-hexane | ~82.0 | - | - | [24] |
| Butyl Acetate | Lipase B from Candida antarctica immobilized onto porous styrene-divinylben-zene beads 10.0% (w/w) Novozym® 435 10.0% (w/w) | Orbital shaker, 200 rpm, 2 h Orbital shaker, 200 rpm, 2 h | 1:5 1:3 | n-hexane n-hexane | ~90.0 ~90.0 | 31.4 31.4 | 15.7 15.7 | [182] |
| Butyl Acetate | Candida antarctica immobilized onto porous styrene-divinylben-zene beads 7.5% (w/w) | Ultrasound (220 W/40 kHz), ~49 °C, 1.5 h | 1:3.46 | n-hexane | 90.0 | 31.4 | 20.9 | [255] |
| Butyl Acetate | Rhizopus oryzae lipase immobilized onto Celite 545 500 U in 5 mL | Orbital shaker, 200 rpm, 37 °C, ~12 h [a] | 1:1 | solvent-free n-heptane n-hexane | 60.0 80.0 76.7 | 334.0 18.6 17.6 | 27.8 1.6 1.5 | [203] |
| Butyl Acetate | Novozym® 435 7.5% (w/w) | Orbital shaker, 200 rpm, 2.5 h, 40 °C | 1:3 | n-hexane | ~90.0 | 31.4 | 15.7 | [266] |
| Butyl Acetate | Novozym® 435 7.0% (w/w) | Ultrasound, 2.5 h, 46 °C | 1:3.6 | n-hexane | 94.0 | 32.8 | 13.1 | [260] |
| Isopentyl Acetate | Lipozyme® RM IM 9.7% (w/w) | Orbital shaker, 150 rpm, 72 h, 40 °C | 1:4 | n-heptane | 95.3 | 150 | 2.1 | [267] |
| Pentyl Acetate | Lipozyme® 435 10.0% (w/w acid) | Shaken glass reactor, 100 rpm, 8 h, 40 °C | 1:2 | solvent-free | 89.0 | 398.3 | 49.8 | [21] |
| Isopentyl Acetate | Novozym® 435 5.0% (w/w) | Orbital shaker, 150 rpm, 6 h, 30 °C | 1:2 | solvent-free | 80.0 | 0.57 g g−1 | - | [268] |
| Isopentyl Acetate | Staphylococcus simulans lipase immobilized onto CaCO3 60 U | Screw-capped tubes, 200 rpm, 8 h, 37 °C [a] | 1:2 | solvent-free | 64.0 | 495.7 | 61.2 | [61] |
| Isopentyl Acetate | Candida antarctica immobilized onto polyurethane 10% (w/w) | Mechanical agitation, 160 rpm, 6 h, 64 °C Ultrasonic power, 105 W, 1 h, 65 °C | 1:7 1:9 | solvent-free | 94.4 95.4 | 158.2 125.7 | 26.3 125.7 | [269] |
| Isopentyl Acetate | Bacillus aerus lipase immobilized onto silica gel cross-linked with glutaraldehyde 1% (w/w) | Constant shaking, 10 h, 55 °C | 1:1 | solvent-free | 68.0 | 65.1 | 0.7 | [270] |
| Isopentyl Acetate | Porcine pancreas lipase immobilized onto activated carbon 0.5 g | Orbital shaker, 200 rpm, 4 h, 40 °C | 1:1 | n-hexane | 93.0 | 29.1 | 7.28 | [271] |
| Isopentyl Acetate | Candida rugosa lipase immobilized onto silica support/sodium alginate/calcium chloride 4 mg protein | Orbital shaker, 200 rpm, 8 h, 50 °C | 1:2.6 | n-hexane | 85.2 | 374.0 | 46.7 | [272] |
| Hexyl Acetate | Novozym® 435 13.8 g mol−1 | Orbital shaker, 200 rpm, 3 h, 40 °C | 1:1 | n-hexane | 93.0 | 67.1 | 22.3 | [273] |
| 3-Hexen-1-yl Acetate | Novozym® 435 2% (w/w) 9% (w/w) | Round bottom flask, 250 rpm, ~8 h, 40 °C Round bottom flask, 250 rpm, 24 h, 40 °C | 1:1 | n-hexane solvent-free | 94.0 70.0 | 100.2 566.5 | 12.5 23.6 | [274] |
| Ethyl Propionate | Bacillus coagulants (MTCC-6375) lipase immobilized onto polyhydrogel 31.4% (w/w) | Water-batch-incub- ator shaker and molecular sieves, 160 rpm, 9 h, 65 °C | 1:3 | n-heptane | 89.3 | 9.1 | 1.0 | [197] |
| Ethyl Propionate | Pseudomonas aeruginosa (BTS-2) lipase immobilized onto polyhydrogel network 23.6% (w/w) | Orbital shaker, 160 rpm, 3 h, 65 °C | 1:3 | n-nonane | 94.0 | 9.6 | 3.2 | [53] |
| Butyl Propionate | Novozym® 435 2.35% (w/w acid) | Orbital shaker, 130 rpm, ~25 h, ~43 °C | 1:9 | solvent-free | 93.7 | 132.5 | 5.3 | [275] |
| Butyl Propionate | Novozym® 435 Lipozyme® RM IM Lipozyme® TL-IM 0.98% (w/w) each | Microwave, 50 W, 400 rpm, 8 h, 60 °C | 1:12 | solvent-free | 92.0 14.0 9.0 | 102.2 15.6 10.0 | 12.8 1.9 1.1 | [276] |
| Isobutyl Propionate | Fermase® CALB 1000 4% (w/w) | Ultrasound, 40 W, 25 kHz, 150 rpm, 3 h, 60 °C | 1:3 | solvent-free | 95.1 | 354.5 | 118.1 | [277] |
| Isobutyl Propionate | Novozym® 435 5% (w/w) | Orbital shaker, 300 rpm, 10 h, 40 °C | 1:3 | solvent-free | 92.5 | 345.0 | 34.5 | [37] |
| Hexyl Propionate | Novozym® 435 13.8 g mol−1 | Orbital shaker, 200 rpm, 3 h, 40 °C | 1:1 | n-hexane | 94.0 | 74.3 | 24.7 | [273] |
| Ethyl Butyrate | Candida rugosa lipase immobilized onto cotton cloth 25.4% (w/w) | Orbital shaker, 220 rpm, 24 h, 25 °C | 2.4:1 | Cyclohexane | 91.2 | 26.5 | 1.1 | [207] |
| Ethyl Butyrate | Candida rugosa lipase immobilized into polyurethane foams 1560 mg | Continuous packed-bed reactor, 260 min, residence time, 36 h, 30 °C | 1:1.5 | n-hexane | 80.7 | 32.7 | 0.7 | [278] |
| Ethyl Butyrate | Novozym® 435 7% (w/w) | Orbital shaker, 150 rpm, 96 h, 34 °C | 1:3 | n-heptane | 72.9 | 33.9 | 0.4 | [279] |
| Ethyl Butyrate | Candida antarctica lipase B immobilized onto styrene beads 10% (w/w) Novozym® 435 7.5% (w/w) | Orbital shaker, 200 rpm, 1 h, 37.5 °C [a] Orbital shaker, 200 rpm, 1 h, 44 °C [a] | 1:4 | n-hexane n-hexane | 86.2 87.7 | 69.9 70.7 | 69.9 70.7 | [183] |
| Isobutyl Butyrate | Lipozyme® RM-IM 14% (w/w) | Ultrasound, 2.5 h, 45 °C | 1:1 | n-hexane | 86.0 | - | - | [259] |
| Butyl Butyrate | Novozym® 435 - | Shaken round bottom flask, ~10 h, 56 °C | 1:3.93 | solvent-free | 99.6 | - | - | [280] |
| Butyl Butyrate | Thermomyces lanuginosus lipase immobilized onto glyoxyl-agarose beads 10% (w/w) | Orbital shaker, 200 rpm, 24 h, 37 °C | 1:1 | heptane | 85.0 | - | - | [194] |
| Butyl Butyrate | Novozym® 435 12% (w/w) | Round-bottom flasks, 250 rpm, 0.5 h, 57 °C | 1:1 | diesel oil | 90.0 | 129.8 | 259.6 | [281] |
| Butyl Butyrate | Cercospora kikuchii lipase immobilized onto chitosan beads 42.0% (w/w) | 45 °C | 1:1.5 | n-heptane | 95.0 | 18.0 | 3.0 | [282] |
| Butyl Butyrate | Candida rugosa lipase immobilized onto iron-alginate nanoparticles - | Orbital shaker, 100 rpm, 6 h, 50 °C | 1:4 | solvent-free | 90.0 | - | - | [283] |
| Butyl Butyrate | Porcine pancreas lipase immobilized onto activated carbon 0.5 g | Orbital shaker, 200 rpm, 4 h, 40 °C | 1:2 | n-hexane | 82.0 | 14.2 | 3.5 | [284] |
| Butyl Butyrate | Candida rugosa lipase immobilized onto multiwalled carbon nanotubes 11.6% (w/w) | Orbital shaker, 200 rpm, 4 h, 37 °C | 1:3 | n-hexane | 91.5 | 13.2 | 3.3 | [285] |
| Butyl Butyrate | Candida rugosa lipase immobilized onto multiwalled carbon nanotubes 4.2% (w/w) | Orbital shaker, 200 rpm, 4 h, 30 °C | 1:1 | n-hexane | 92.7 | 13.4 | 3.3 | [175] |
| Isopentyl Butyrate | Lipozyme® TL IM 14.7% (w/w) | Orbital shaker, 180 rpm, 6 h, 40 °C, | 1:2 | n-hexane | 96.1 | 259.5 | 43.3 | [286] |
| Isopentyl Butyrate | Lipozyme® IM-20 1.9% (w/w) | Orbital shaker, 150 rpm, 24 h, 40 °C | 1:1 | n-hexane | ~92.0 | 36.3 | 1.51 | [287] |
| Isopentyl Butyrate | Rhizopus oryzae lipase immobilized onto Purolite (PMMA with epoxide and octadecyl groups) 35 U | Orbital shaker, 1200 rpm, 12 h, 30 °C | 1:2 | cyclohexane | 91.0 | 59.0 | 4.9 | [108] |
| Hexyl Butyrate | Novozym® 435 13.8 g mol−1 | Orbital shaker, 200 rpm, 3 h, 40 °C | 1:1 | n-hexane | 93.0 | 80.1 | 26.7 | [273] |
| Hexyl Butyrate | Candida rugosa lipase immobilized onto Diaion HP20 10.8% (w/w) | Orbital shaker, 200 rpm, 3 h, 40 °C | 1:2 | n-heptane | 94.5 | 81.8 | 27.6 | [288] |
| Ethyl Valerate | Candida rugosa lipase immobilized onto microemulsion-based organogels - | Orbital shaker, 150 rpm, 144 h, 40 °C | 1:1.6 | cyclohexane | 99.9 | 13.0 | 0.1 | [289] |
| Ethyl Valerate | Candida rugosa lipase immobilized onto app membrane 696.6 U in 20 mL | Screw-capped flasks, 150 rpm, 24 h, 40 °C [a] | 2:1 1:1 | solvent-free n-hexane | 34.8 67.2 | 164.2 583.2 | 6.8 24.3 | [178] |
| Ethyl Valerate | Novozym® 435 10.06 g L−1 | Microwave reactor, 0.67 h, 50 °C | 1:1 | solvent-free | 69.2 | 8.0 | 12.0 | [290] |
| Ethyl Valerate | Candida antarctica lipase B 12% (w/v) | Orbital shaker, 1000 rpm, 2 h, 40 °C Microwave, 100 W, 1 h, 40 °C | 1:2 1:2 | solvent-free | 90.0 82.0 | 518.1 472.1 | 259.1 472.1 | [291] |
| Ethyl Valerate | Aspergillus oryzae immobilized onto an electrospun nanofibrous membrane 1.10 g L−1 | Magnetical stirring in oil bath, 250 rpm, 24 h, 50 °C [a] | 1:1 | solvent-free | 72.1 | 23.3 | 1.0 | [292] |
| Ethyl Valerate | Burkholderia cepacian lipase immobilized onto sodium alginate 20% (w/v) | Orbital shaker, 150 rpm, 48 h, 37 °C | 1:2 | n-heptane | 87.1 | 58.6 | 1.2 | [293] |
| Ethyl Valerate | Candida rugosa lipase immobilized onto biogenic sílica/magnetite/ graphene oxide 2.1% (w/w) | Orbital shaker, 200 rpm, 3 h, 40 °C | 1:2 | n-heptane | 90.4 | 87.5 | 29.1 | [294] |
| Pentyl Valerate | Candida rugosa lipase immobilized onto nanocrystalline cellulose, silica, and polyethersulfone 3 mg mL−1 | Orbital shaker, 200 rpm, 3 h, 50 °C | 1:2 | cyclohexane | 91.3 | - | - | [295] |
| Pentyl Valerate | Candida rugosa lipase immobilized onto microemulsion-based organogels 70 g L−1 | Orbital shaker, 150 rpm, 199 h, 37 °C | 2.9:2 | cyclohexane/AOT | 99.9 | 17.0 | 0.1 | [109] |
| Ethyl Hexanoate | Antarctic pseudomonas (AMS8) immobillized onto chitosan 5% (w/w) | Orbital shaker, 200 rpm, 2 h, 20 °C | 1:1 | toluene solvent-free | 69.0 47.0 | 2.5 372.8 | 1.3 93.2 | [296] |
| Ester | Biocatalyst Biocat. Loading | Reaction Conditions | Acid: Alcohol Molar Ratio | Solvent | Conversion (%) | Concentration (g L−1) | Productivity (g L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Phenethyl Formate | Novozym® 435 15 g L−1 | Orbital shaker, 150 rpm, 4 h, 40 °C | 1:5 | 1,2-dichloroethane | 95.9 | 14.4 | 3.6 | [297] |
| Phenethyl Formate | Novozym® 435 15 g L−1 | Orbital shaker, 150 rpm, 30 min, 30 °C | 1:1 | n-hexane | 69.2 | - | - | [121] |
| Benzyl Propionate | Novozym® 435 10% (w/w) | Thermal bath with mechanical agitation, 24 h, 65 °C | 1:5 | solvent-free | 44.0 | 127.6 | 5.3 | [298] |
| Benzyl Propionate | Novozym® 435 10% (w/w) | Fed-batch system with molecular sieves, 150 rpm, 6 h, 50 °C | 1:5 | solvent-free | 99.0 | 270.7 | 45.1 | [299] |
| Benzyl Butyrate | Pseudomonas cepacian lipase immobilized onto polyvinyl alcohol and chitosan 8.6% (w/w) | Ultrasound, 100 W, 3 h, 52 °C | 1:3 | isooctane | 40.0 | - | - | [176] |
| Benzyl Butyrate | Novozym® 435 2% (w/w) | Round bottom flask with fish-clip spinner, 20 h, 52 °C; irreversible esterification reaction (chemical drying) | 1:1 | methyl tert-butyl ether | 82.0 | 160.4 | 8.0 | [300] |
| Methyl Cinnamate | Candida sp. lipase immobilized onto ordered mesoporous silicon C8 10 mg | Orbital shaker, 220 rpm, 2 h, 70 °C | 1:3 | isooctane | 90.4 | 1.5 | 0.8 | [301] |
| Ester | Biocatalyst | Number of Sequential Reactions | Ref. |
|---|---|---|---|
| Butyl Propionate | Novozym® 435 | 20 | [275] |
| Hexyl Formate | Novozym® 435 | 20 | [265] |
| Ethyl Butyrate | Candida rugosa lipase immobilized into polyurethane foams | 20 | [278] |
| Butyl Acetate | Novozym® 435 | 14 | [259] |
| Octyl Formate | Novozym® 435 | 10 | [264] |
| Pentyl Acetate | Lipozyme® 435 | 10 | [21] |
| Pentyl Valerate | Candida rugosa lipase immobilized onto microemulsion-based organogels | 10 | [109] |
| Ethyl Butyrate | Candida antarctica lipase B immobilized onto styrene beads | 8 | [183] |
| Isobutyl Propionate | Fermase® CALB 10000 | 7 | [277] |
| Isobutyl Propionate | Novozym® 435 | 7 | [37] |
| Butyl Acetate | Candida antarctica lipase B immobilized onto porous styrene-divinylbenzene beads | 6 | [182] |
| Butyl Butyrate | Thermomyces lanuginosus lipase immobilized onto glyoxyl-agarose beads | 5 | [194] |
| Butyl Acetate | Novozym® 435 | 5 | [256] |
| Ethyl Valerate | Candida rugosa lipase immobilized onto amine polypropylene membrane | 5 | [178] |
| Ethyl Valerate | Aspergillus oryzae immobilized onto an electrospun nanofibrous membrane | 5 | [292] |
| Isopentyl Butyrate | Rhizopus oryzae lipase immobilized onto Purolite (PMMA with epoxide and octadecyl groups) | 5 | [108] |
| Isobutyl Butyrate | Lipozyme® RM-IM | 4 | [259] |
| Isopentyl Acetate | Staphylococcus simulans lipase immobilized onto CaCO3 | 4 | [61] |
| Isopentyl Propionate | Novozym® 435 | 4 | [254] |
| Butyl Propionate | Novozym® 435 | 3 | [276] |
| Butyl Acetate | Rhyzopus oryzae lipase immobilized onto Celite 545 | 3 | [210] |
| Ethyl Valerate | Novozym® 435 | 2 | [290] |
| Isopentyl Acetate | Lipozyme® IM-20 | 2 | [266] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, R.R.d.; dos Santos, M.M.; Medeiros, M.W.R.; Manoel, E.A.; Berenguer-Murcia, Á.; Freire, D.M.G.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives. Catalysts 2025, 15, 375. https://doi.org/10.3390/catal15040375
Sousa RRd, dos Santos MM, Medeiros MWR, Manoel EA, Berenguer-Murcia Á, Freire DMG, Fernandez-Lafuente R, Ferreira-Leitão VS. Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives. Catalysts. 2025; 15(4):375. https://doi.org/10.3390/catal15040375
Chicago/Turabian StyleSousa, Ronaldo Rodrigues de, Michelle M. dos Santos, Matheus W. R. Medeiros, Evelin A. Manoel, Ángel Berenguer-Murcia, Denise Maria Guimarães Freire, Roberto Fernandez-Lafuente, and Viridiana Santana Ferreira-Leitão. 2025. "Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives" Catalysts 15, no. 4: 375. https://doi.org/10.3390/catal15040375
APA StyleSousa, R. R. d., dos Santos, M. M., Medeiros, M. W. R., Manoel, E. A., Berenguer-Murcia, Á., Freire, D. M. G., Fernandez-Lafuente, R., & Ferreira-Leitão, V. S. (2025). Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives. Catalysts, 15(4), 375. https://doi.org/10.3390/catal15040375








