Carbon-Coated Magnetic Catalysts for Enhanced Degradation of Nitrophenols: Stability and Efficiency in Catalytic Wet Peroxide Oxidation
Abstract
1. Introduction
2. Results and Discussion
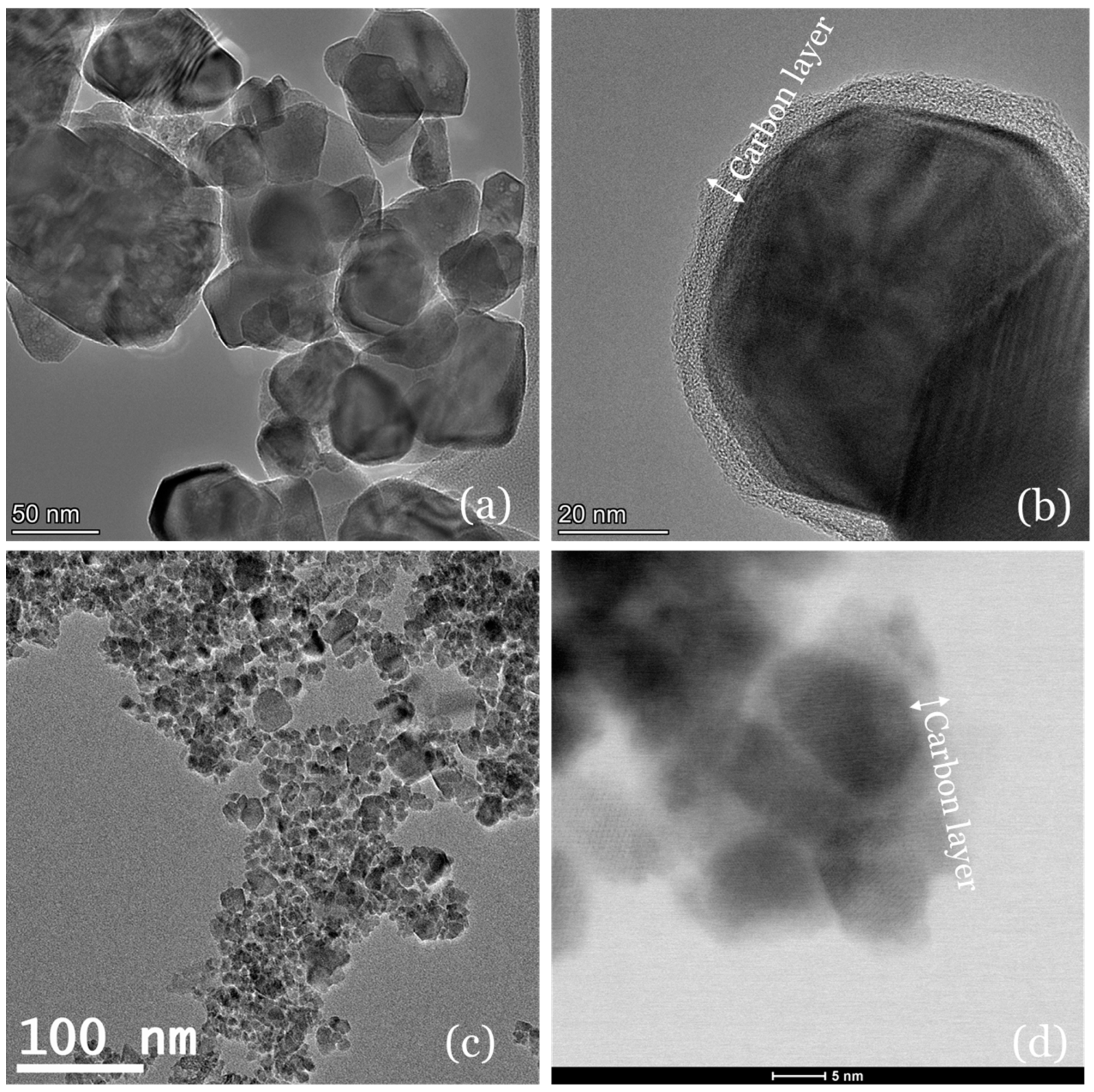
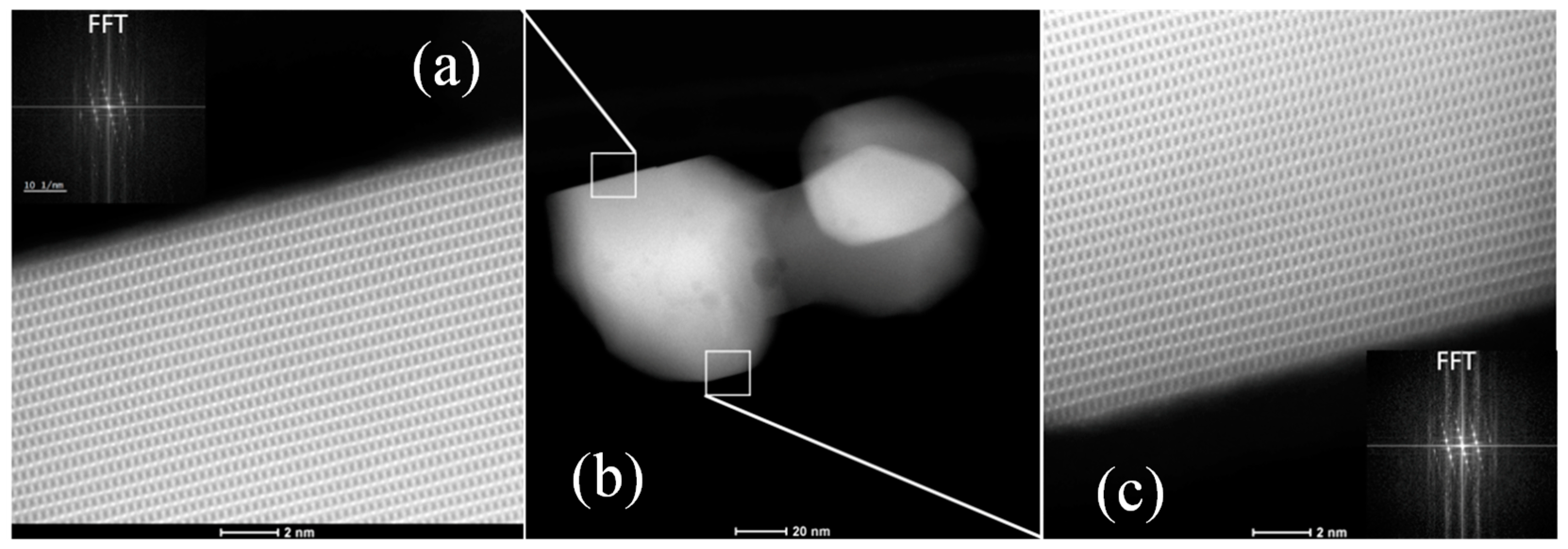
2.1. Morphology and Textural Properties
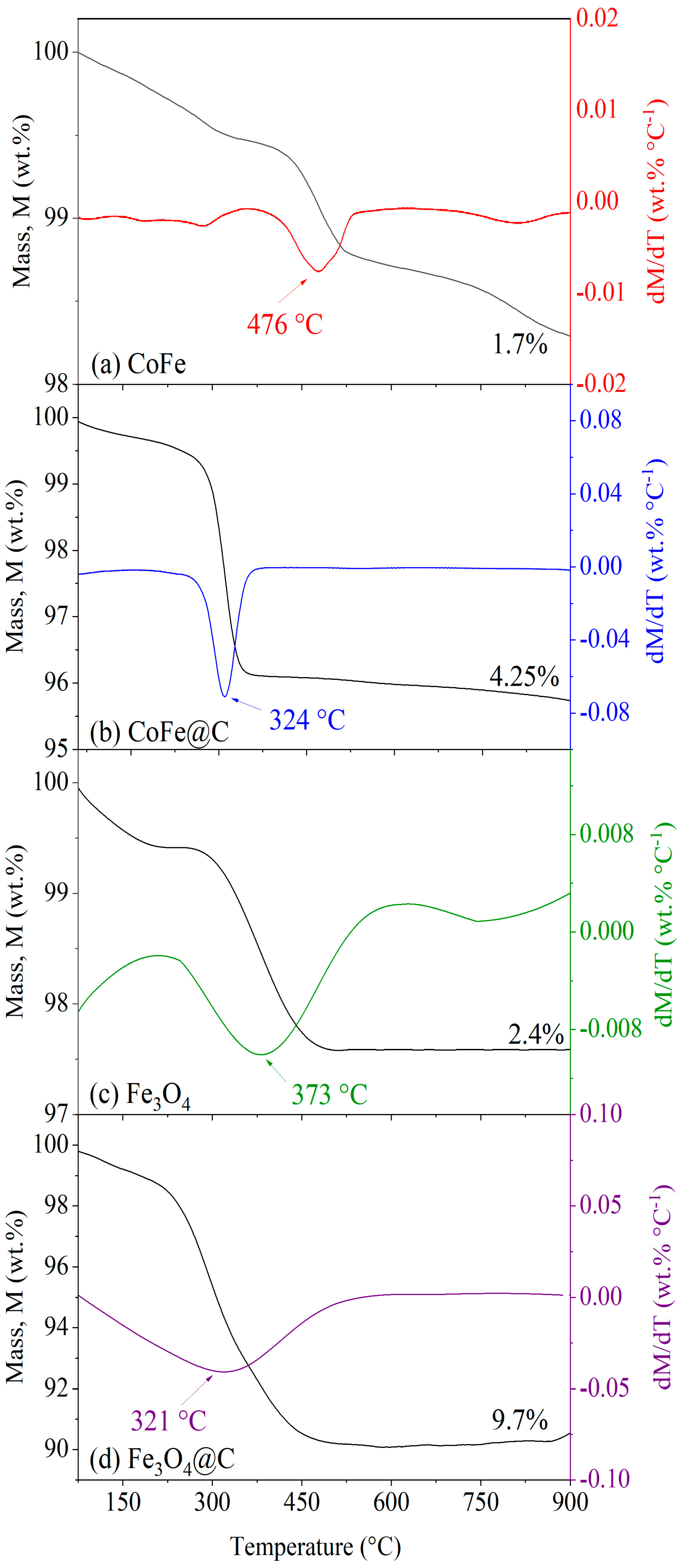
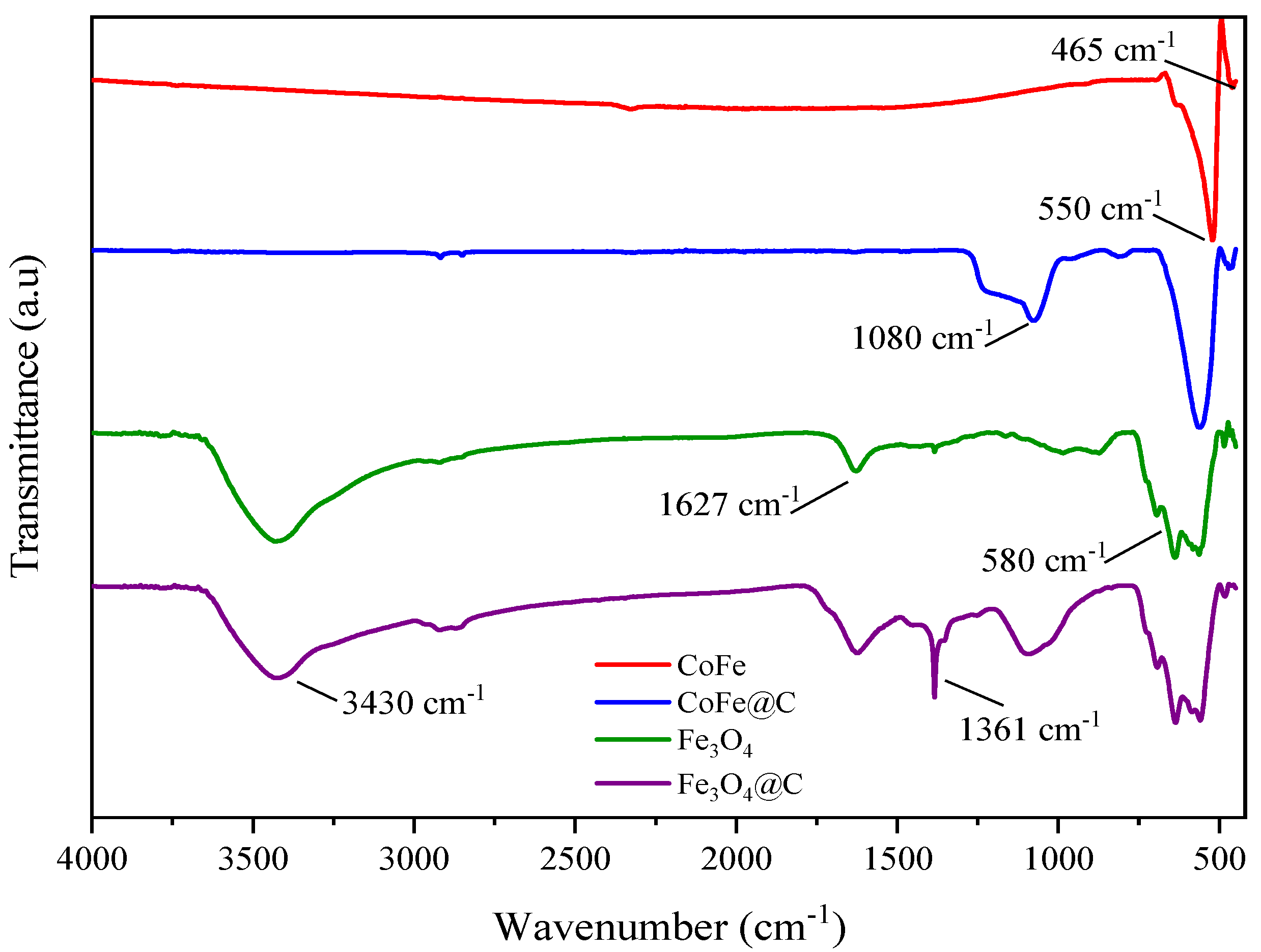
2.2. Thermal and Surface Properties
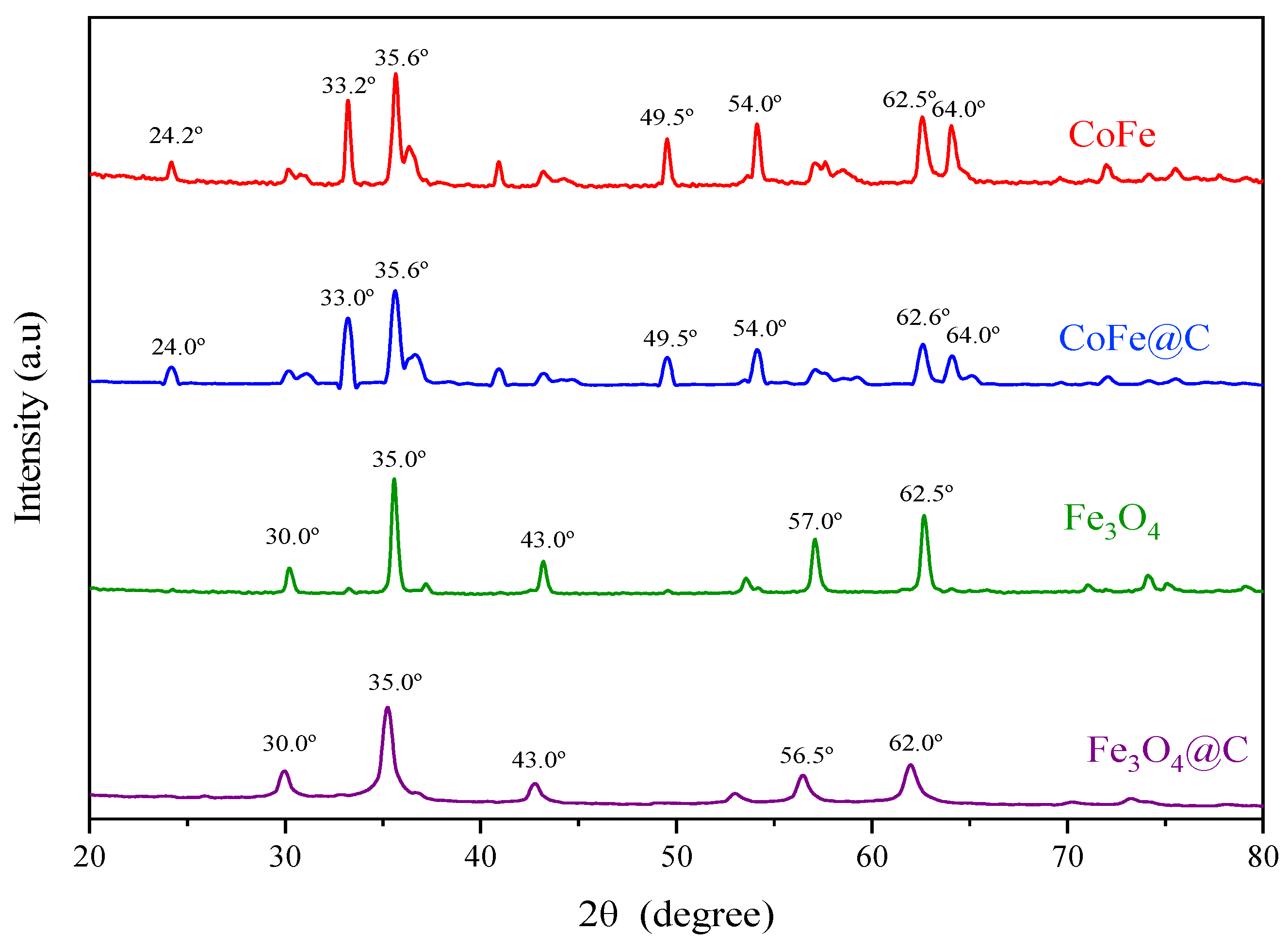
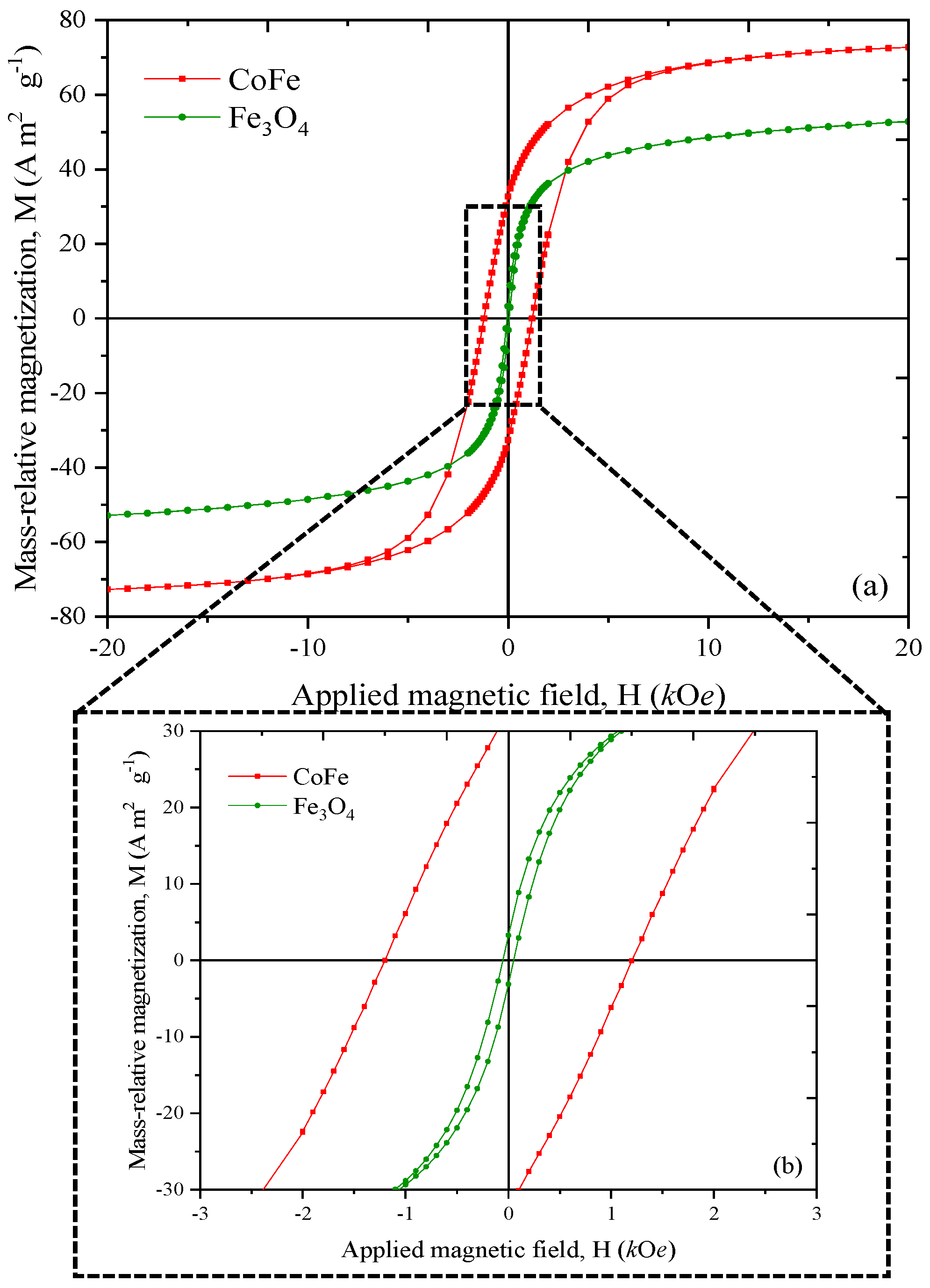
2.3. Crystalline Phase and Magnetic Properties
2.4. Single-Component CWPO Experiments
2.5. Multi-Component CWPO Experiment
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of Magnetic Cores
3.3. Carbon Coating
3.4. Characterization Techniques
3.5. Liquid-Phase Oxidation Runs and Analytical Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future Global Urban Water Scarcity and Potential Solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Filho, P.Z.; Ferreira, A.P.; Roman, F.F.; Baldo, A.P.; Rauhauser, M.; Diaz de Tuesta, J.L.; Pereira, A.I.; Silva, A.M.T.; Pietrobelli, J.M.T.; et al. Occurrence of Micropollutants in Surface Water and Removal by Catalytic Wet Peroxide Oxidation Enhanced Filtration Using Polymeric Membranes Loaded with Carbon Nanotubes. Chem. Eng. J. Adv. 2025, 21, 100707. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, H.; Zhang, W.; Lai, B.; Yao, G. Removal of Nitrophenols and Their Derivatives by Chemical Redox: A Review. Chem. Eng. J. 2019, 359, 13–31. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Yang, Y.; Yang, P.; Wang, L.; Lu, J.; Li, J.; Zhou, L.; Ferronato, C.; Chovelon, J.-M. Rethinking Sulfate Radical-Based Oxidation of Nitrophenols: Formation of Toxic Polynitrophenols, Nitrated Biphenyls and Diphenyl Ethers. J. Hazard. Mater. 2019, 361, 152–161. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. Degradation of Nitrophenols by Fenton and Photo-Fenton Processes. J. Photochem. Photobiol. A Chem. 2005, 170, 83–95. [Google Scholar] [CrossRef]
- Priyanka, M.; Saravanakumar, M.P. New Insights on Aging Mechanism of Microplastics Using PARAFAC Analysis: Impact on 4-Nitrophenol Removal via Statistical Physics Interpretation. Sci. Total Environ. 2022, 807, 150819. [Google Scholar] [CrossRef]
- ToxFAQs TM for Nitrophenols. 1995. Available online: https://wwwn.cdc.gov/TSp/ToxFAQs/ToxFAQsDetails.aspx?faqid=879&toxid=172 (accessed on 9 April 2025).
- Chaara, D.; Pavlovic, I.; Bruna, F.; Ulibarri, M.A.; Draoui, K.; Barriga, C. Removal of Nitrophenol Pesticides from Aqueous Solutions by Layered Double Hydroxides and Their Calcined Products. Appl. Clay Sci. 2010, 50, 292–298. [Google Scholar] [CrossRef]
- Asif, M.; Deng, L.; Mao, Y.; Khan, H.U.; Tang, Q. Efficient Degradation of 2-Nitrophenol during the UV/Chlorine Treatment in the Presence of Fe(III): Kinetics, DFT Calculation and HNMs Formation. J. Environ. Chem. Eng. 2025, 13, 115712. [Google Scholar] [CrossRef]
- Diaz De Tuesta, J.L.; Pantuzza, G.F.; Silva, A.M.T.; Praça, P.; Faria, J.L.; Gomes, H.T. Catalysts Prepared with Matured Compost Derived from Mechanical-Biological Treatment Plants for the Wet Peroxide Oxidation of Pollutants with Different Lipophilicity. Catalysts 2020, 10, 1243. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Removal of 2-Nitrophenol by Catalytic Wet Peroxide Oxidation Using Carbon Materials with Different Morphological and Chemical Properties. Appl. Catal. B 2013, 140–141, 356–362. [Google Scholar] [CrossRef]
- Silva, A.S.; Roman, F.F.; Dias, A.V.; Diaz de Tuesta, J.L.; Narcizo, A.; da Silva, A.P.F.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Ferrari, A.M.C.; et al. Hybrid Multi-Core Shell Magnetic Nanoparticles for Wet Peroxide Oxidation of Paracetamol: Application in Synthetic and Real Matrices. J. Environ. Chem. Eng. 2023, 11, 110806. [Google Scholar] [CrossRef]
- Kalmakhanova, M.S.; Diaz de Tuesta, J.L.; Malakar, A.; Gomes, H.T.; Snow, D.D. Wastewater Treatment in Central Asia: Treatment Alternatives for Safe Water Reuse. Sustainability 2023, 15, 14949. [Google Scholar] [CrossRef]
- Roman, F.F.; Silva, A.S.; Diaz de Tuesta, J.L.; Baldo, A.P.; Lopes, J.P.M.; Gonçalves, G.; Pereira, A.I.; Praça, P.; Silva, A.M.T.; Faria, J.L.; et al. Plastic Waste-Derived Carbon Nanotubes: Influence of Growth Catalyst and Catalytic Activity in CWPO. J. Environ. Chem. Eng. 2025, 13, 115206. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jo, S.; Lee, Y.; Kang, S.H.; Kim, J.W.; Lee, S.C.; Kim, J.C. Influence of Ni on Fe and Co-Fe Based Catalysts for High-Calorific Synthetic Natural Gas. Catalysts 2021, 11, 697. [Google Scholar] [CrossRef]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Metal-Free Carbon Materials as Catalysts for Wet Air Oxidation. Catal. Today 2020, 356, 189–196. [Google Scholar] [CrossRef]
- Anjaneyulu, B.; Rana, R.; Versha; Afshari, M.; Carabineiro, S.A.C. The Use of Magnetic Porous Carbon Nanocomposites for the Elimination of Organic Pollutants from Wastewater. Surfaces 2024, 7, 120–142. [Google Scholar] [CrossRef]
- Guari, N.M.C.; Silva, A.S.; Diaz de Tuesta, J.-L.; Pottker, W.E.; Cordeiro, P.Y.; Gomes, H.T. Magnetic CoFe2O4@carbon Yolk-Shell Nanoparticles as Catalysts for the Catalytic Wet Peroxide Oxidation of Paracetamol: Kinetic Insights. Glob. NEST J. 2023, 25, 57–66. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Tavares, P.B.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Hybrid Magnetic Graphitic Nanocomposites for Catalytic Wet Peroxide Oxidation Applications. Catal. Today 2017, 280, 184–191. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, X.; Hou, X.; Xu, H.; Yao, Z.; Jiang, Z. A High-Efficient Carbon-Coated Iron-Based Fenton-Like Catalyst with Enhanced Cycle Stability and Regenerative Performance. Catalysts 2020, 10, 1486. [Google Scholar] [CrossRef]
- Mo, Y.; Che, C.; Han, W.; Chen, F.; Guan, J.; Zhang, F.; Yang, B.; Ren, X.; Li, H.; Ahmed, S.; et al. Fabrication of Carbon-Coated Iron-Based Porous Heterogeneous Fenton Catalyst and Enhanced Degradation Performance towards Ciprofloxacin. Chem. Eng. J. 2024, 497, 154680. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, B.; Yang, Y.; Ming, H.; Liu, G.; Zhang, J.; Hou, Y. Carbon-Coated ZnFe2O4 Nanoparticles as an Efficient, Robust and Recyclable Catalyst for Photocatalytic Ozonation of Organic Pollutants. J. Environ. Chem. Eng. 2022, 10, 107419. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Gallo, J.; Bañobre-López, M.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Enhanced Performance of Cobalt Ferrite Encapsulated in Graphitic Shell by Means of AC Magnetically Activated Catalytic Wet Peroxide Oxidation of 4-Nitrophenol. Chem. Eng. J. 2018, 376, 120012. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and Magnetic Properties of Cobalt Ferrite (CoFe2O4) Nanoparticles Prepared by Wet Chemical Route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Saragi, T.; Nurjannah, S.; Novia, R.; Syakir, N.; Simanjuntak, E.; Safriani, L.; Risdiana; Bahtiar, A. Synthesis of Cobalt Ferrite Particles by Utilized Sol-Gel Method. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2015; Volume 827, pp. 219–222. [Google Scholar]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Abbas, M.; Parvatheeswara Rao, B.; Nazrul Islam, M.; Kim, K.W.; Naga, S.M.; Takahashi, M.; Kim, C. Size-Controlled High Magnetization CoFe2O4 Nanospheres and Nanocubes Using Rapid One-Pot Sonochemical Technique. Ceram. Int. 2014, 40, 3269–3276. [Google Scholar] [CrossRef]
- Akter, S.; Khan, M.N.I.; Ferdous, F.; Das, H.N.; Alam, M.K.; Rahman, M.A.; Hasan, T.; Syed, I.M. Unveiling the Role of Sintering Temperatures in the Physical Properties of Cu-Mg Ferrite Nanoparticles for Photocatalytic Application. Heliyon 2024, 10, e40771. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Lendzion-Bielun, Z. Synthesis and Characterization of Magnetic Nanomaterials with Adsorptive Properties of Arsenic Ions. Molecules 2020, 25, 4117. [Google Scholar] [CrossRef]
- Denisova, K.; Ilyin, A.A.; Rumyantsev, R.; Sakharova, J.; Ilyin, A.P.; Gordina, N. Low-Temperature Synthesis and Catalytic Activity of Cobalt Ferrite in Nitrous Oxide (N2O) Decomposition Reaction. Catalysts 2021, 11, 889. [Google Scholar] [CrossRef]
- Raut, S.D.; Dahotre, S.G.; Singh, L.N.; Jadhav, S.N. Synthesis and Characterization of Magnetite and Cobalt Ferrite Nanoparticles by Sol-Gel Auto Combustion Technique. Int. J. 2021, 6, 17–22. [Google Scholar] [CrossRef]
- Kaidar, B.; Lesbayev, A.; Imash, A.; Baskanbayeva, D.; Akalim, D.; Keneshbekova, A.; Yensep, E.; Ilyanov, A.; Smagulova, G. Magnetite Nanoparticles Obtained by Solution Combustion Synthesis. Гoрение и Плазмoхимия 2023, 21, 147–157. [Google Scholar] [CrossRef]
- Gao, J.; Ma, Z.; Liu, F.; Chen, C. Synthesis of Carbon-Coated Cobalt Ferrite Core–Shell Structure Composite: A Method for Enhancing Electromagnetic Wave Absorption Properties by Adjusting Impedance Matching. Chin. J. Chem. Eng. 2022, 47, 206–217. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Curr. Nanosci. 2015, 12, 455–468. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Liu, Q.; Liu, Q.; Li, Z.; Yan, C. Field Test on the Biodegradation of Poly(Butylene Adipate-Co-Terephthalate) Based Mulch Films in Soil. Polym. Test. 2021, 93, 107009. [Google Scholar] [CrossRef]
- Ansari, S.M.; Ghosh, K.C.; Devan, R.S.; Sen, D.; Sastry, P.U.; Kolekar, Y.D.; Ramana, C.V. Eco-Friendly Synthesis, Crystal Chemistry, and Magnetic Properties of Manganese-Substituted CoFe2O4 Nanoparticles. ACS Omega 2020, 5, 19315–19330. [Google Scholar] [CrossRef]
- Li, Z.; Ren, X.; Zheng, Y.; Tian, W.; An, L.; Sun, J.; Guo, J.; Wen, L.; Wang, L.; Liang, G. Double-Layer Carbon-Coating Method for Simultaneous Improvement of Conductivity and Tap Density of LiMn0.65Fe0.35PO4/C/KB Cathode Materials. ACS Appl. Energy Mater. 2020, 3, 8573–8582. [Google Scholar] [CrossRef]
- Robinson, I. Nanoparticle Structure by Coherent X-ray Diffraction. J. Phys. Soc. Jpn. 2013, 82, 021012. [Google Scholar] [CrossRef]
- Hobday, C.L.; Krause, S.; Rogge, S.M.J.; Evans, J.D.; Bunzen, H. Perspectives on the Influence of Crystal Size and Morphology on the Properties of Porous Framework Materials. Front. Chem. 2021, 9, 772059. [Google Scholar] [CrossRef]
- Sanpo, N.; Wang, J.; Berndt, C.C. Sol-Gel Synthesized Copper-Substituted Cobalt Ferrite Nanoparticles for Biomedical Applications. J. Nano Res. 2013, 22, 95–106. [Google Scholar] [CrossRef]
- Ma, J.; Chen, K. Discovery of Superparamagnetism in Sub-Millimeter-Sized Magnetite Porous Single Crystals. Phys. Lett. A 2016, 380, 3313–3318. [Google Scholar] [CrossRef]
- Baldini, A.; Petrecca, M.; Sangregorio, C.; Anselmi-Tamburini, U. Magnetic Properties of Bulk Nanocrystalline Cobalt Ferrite Obtained by High-Pressure Field Assisted Sintering. J. Phys. D Appl. Phys. 2021, 54, 194006. [Google Scholar] [CrossRef]
- Wirecka, R.; Maćkosz, K.; Żywczak, A.; Marzec, M.M.; Zapotoczny, S.; Bernasik, A. Magnetoresistive Properties of Nanocomposites Based on Ferrite Nanoparticles and Polythiophene. Nanomaterials 2023, 13, 879. [Google Scholar] [CrossRef]
- Milutinović, A.; Lazarević, Z.; Šuljagić, M.; Andjelković, L. Synthesis-Dependent Structural and Magnetic Properties of Monodomain Cobalt Ferrite Nanoparticles. Metals 2024, 14, 833. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Dai, M.; Niu, Q.; Wu, S.; Lin, Y.; Biswas, J.K.; Yang, C. Hydroxyl Radicals in Ozone-Based Advanced Oxidation of Organic Contaminants: A Review. Environ. Chem. Lett. 2024, 22, 3059–3106. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; Wei, Y.; You, L.; Cai, C.; Wei, Q.; Zhou, K. Designing Hierarchically Porous Zero-Valent Iron via 3D Printing to Degrade Organic Pollutants by Activating Peroxymonosulfate Using High-Valent Iron-Oxo Species. Chem. Eng. J. 2023, 476, 146523. [Google Scholar] [CrossRef]
- Hao, T.; Rao, X.; Li, Z.; Niu, C.; Wang, J.; Su, X. Synthesis of Magnetic Separable Iron Oxide/Carbon Nanocomposites for Efficient Adsorptive Removal of Congo Red. J. Alloys Compd. 2014, 617, 76–80. [Google Scholar] [CrossRef]
- Qin, X.; Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. Highly Efficient Hydroxyl Radicals Production Boosted by the Atomically Dispersed Fe and Co Sites for Heterogeneous Electro-Fenton Oxidation. Environ. Sci. Technol. 2023, 57, 2907–2917. [Google Scholar] [CrossRef]
- Xu, G.; Sun, L.; Tu, Y.; Teng, X.; Qi, Y.; Wang, Y.; Li, A.; Xie, X.; Gu, X. Highly Stable Carbon-Coated NZVI Composite Fe0@RF-C for Efficient Degradation of Emerging Contaminants. Environ. Sci. Ecotechnol. 2024, 22, 100457. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, S.; Alzahrani, K.A.; Khan, S.B. Development and Detailed Investigation of Metal Nanoparticles Decorated Carbon Black/Sodium Alginate Composite Beads for Catalytic Reduction of Environmental Toxicants and Hydrogen Production. Int. J. Biol. Macromol. 2024, 283, 137300. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules 2022, 27, 132. [Google Scholar] [CrossRef]
- Gupta, V.K.; Atar, N.; Yola, M.L.; Üstündaǧ, Z.; Uzun, L. A Novel Magnetic Fe@Au Core–Shell Nanoparticles Anchored Graphene Oxide Recyclable Nanocatalyst for the Reduction of Nitrophenol Compounds. Water Res. 2014, 48, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, T.; Kompotiatis, L.; Kontogeorgakos, A.; Kordas, G. Microwave Behavior of Ferrites Prepared via Sol–Gel Method. J. Magn. Magn. Mater. 2002, 246, 360–365. [Google Scholar] [CrossRef]
- Silva, A.S.; Diaz de Tuesta, J.L.; Sayuri Berberich, T.; Delezuk Inglez, S.; Bertão, A.R.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Gomes, H.T. Doxorubicin Delivery Performance of Superparamagnetic Carbon Multi-Core Shell Nanoparticles: PH Dependence, Stability and Kinetic Insight. Nanoscale 2022, 14, 7220–7232. [Google Scholar] [CrossRef]
- Dunphy, D.R.; Sheth, P.H.; Garcia, F.L.; Brinker, C.J. Enlarged Pore Size in Mesoporous Silica Films Templated by Pluronic F127: Use of Poloxamer Mixtures and Increased Template/SiO2 Ratios in Materials Synthesized by Evaporation-Induced Self-Assembly. Chem. Mater. 2014, 27, 75–84. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Pasc, A.; Celzard, A. Easy and Eco-Friendly Synthesis of Ordered Mesoporous Carbons by Self-Assembly of Tannin with a Block Copolymer. Green Chem. 2016, 18, 3265–3271. [Google Scholar] [CrossRef]



| Material | SBET (m2 g−1) | Slangmuir (m2 g−1) | VT (cm3 g−1) | BET (R2) | DBJH (nm) |
|---|---|---|---|---|---|
| CoFe | 9 | 8 | 0.024 | 0.9989 | 1 |
| CoFe@C | 22 | 18 | 0.043 | 0.9971 | 1.1 |
| Fe3O4 | 83 | 97 | 0.022 | 0.9996 | 2.3 |
| Fe3O4@C | 120 | 136 | 0.340 | 0.9998 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldo, A.P.; Bezerra, A.J.B.; Silva, A.S.; Ferreira, A.P.; Roman, F.F.; Çaha, I.; Bañobre-López, M.; Deepak, F.L.; Gomes, H.T. Carbon-Coated Magnetic Catalysts for Enhanced Degradation of Nitrophenols: Stability and Efficiency in Catalytic Wet Peroxide Oxidation. Catalysts 2025, 15, 376. https://doi.org/10.3390/catal15040376
Baldo AP, Bezerra AJB, Silva AS, Ferreira AP, Roman FF, Çaha I, Bañobre-López M, Deepak FL, Gomes HT. Carbon-Coated Magnetic Catalysts for Enhanced Degradation of Nitrophenols: Stability and Efficiency in Catalytic Wet Peroxide Oxidation. Catalysts. 2025; 15(4):376. https://doi.org/10.3390/catal15040376
Chicago/Turabian StyleBaldo, Arthur P., Ana Júlia B. Bezerra, Adriano S. Silva, Ana Paula Ferreira, Fernanda F. Roman, Ihsan Çaha, Manuel Bañobre-López, Francis Leonard Deepak, and Helder T. Gomes. 2025. "Carbon-Coated Magnetic Catalysts for Enhanced Degradation of Nitrophenols: Stability and Efficiency in Catalytic Wet Peroxide Oxidation" Catalysts 15, no. 4: 376. https://doi.org/10.3390/catal15040376
APA StyleBaldo, A. P., Bezerra, A. J. B., Silva, A. S., Ferreira, A. P., Roman, F. F., Çaha, I., Bañobre-López, M., Deepak, F. L., & Gomes, H. T. (2025). Carbon-Coated Magnetic Catalysts for Enhanced Degradation of Nitrophenols: Stability and Efficiency in Catalytic Wet Peroxide Oxidation. Catalysts, 15(4), 376. https://doi.org/10.3390/catal15040376











