Copper Molybdate-Catalyzed Esterification of Levulinic Acid: A Heterogeneous Approach for Biofuel Synthesis
Abstract
1. Introduction
2. Results and Discussion
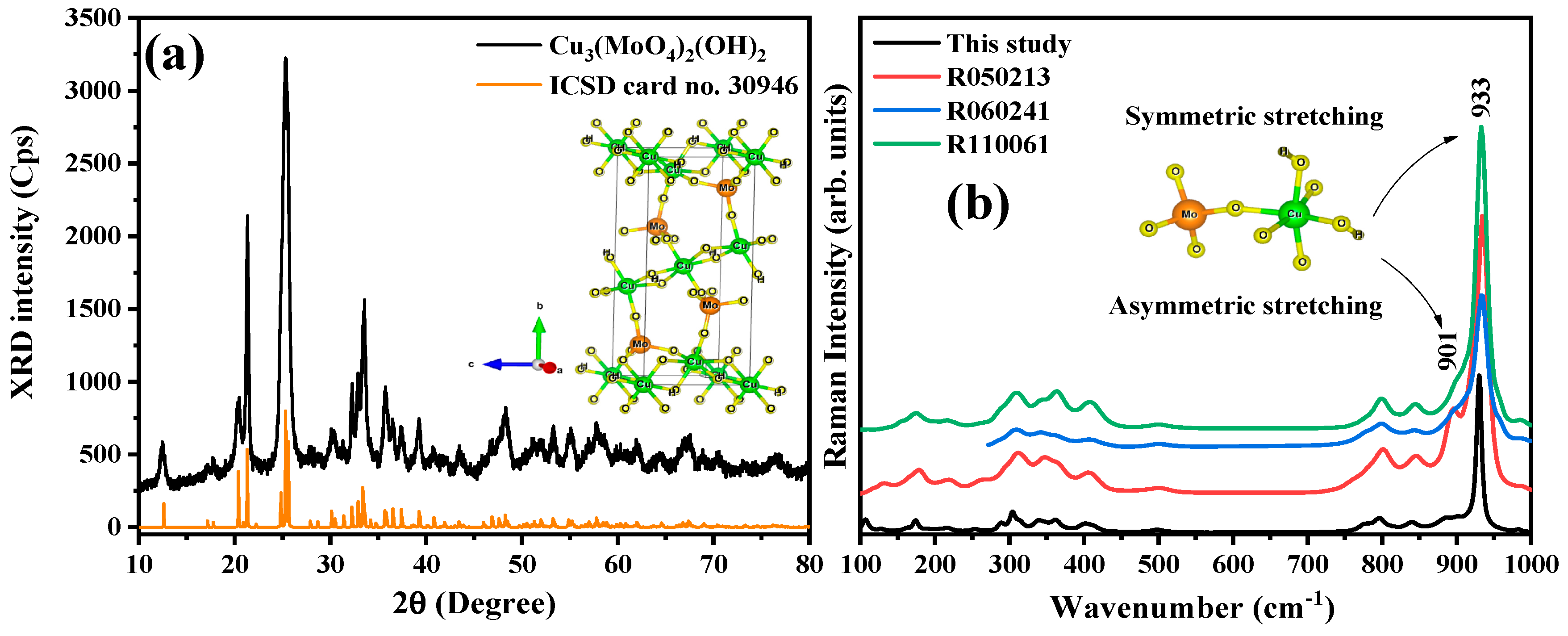
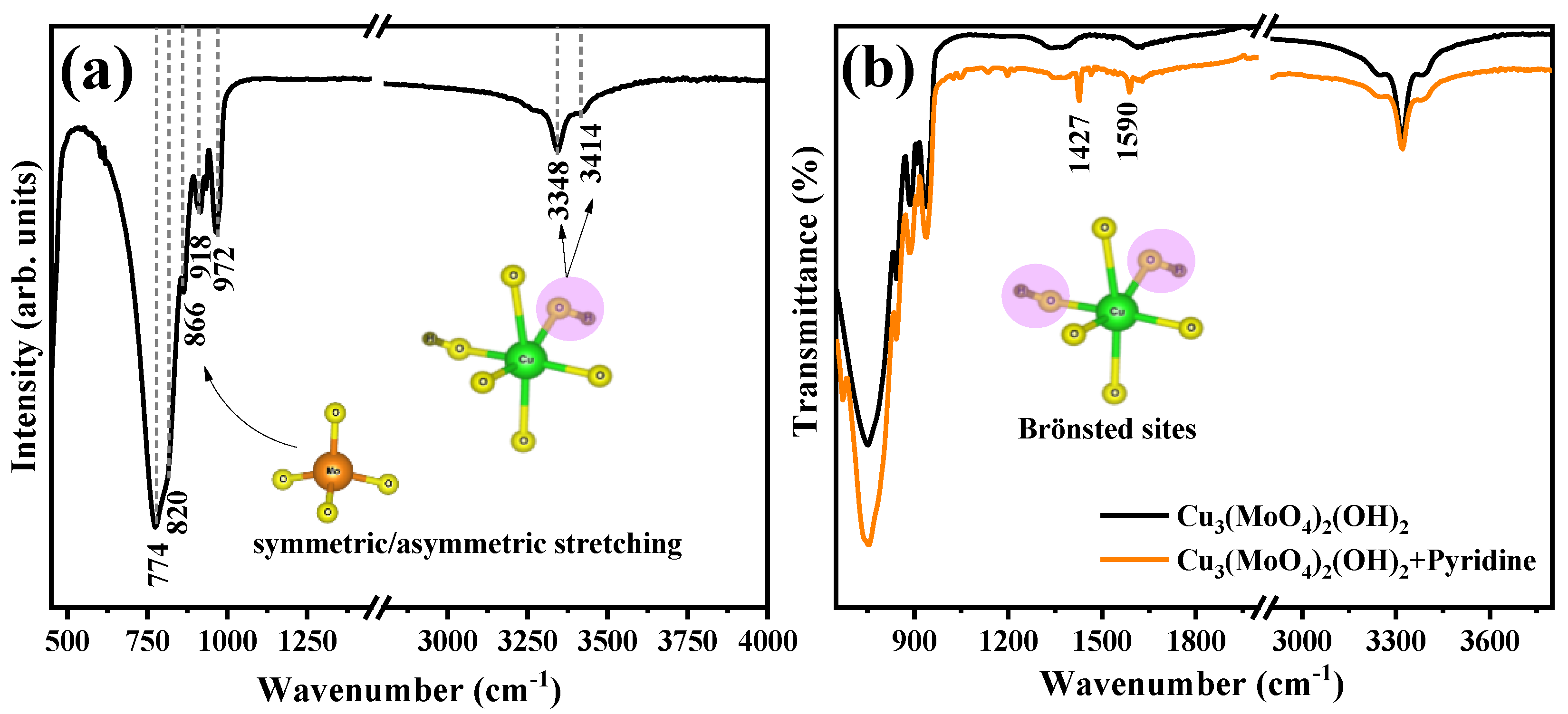
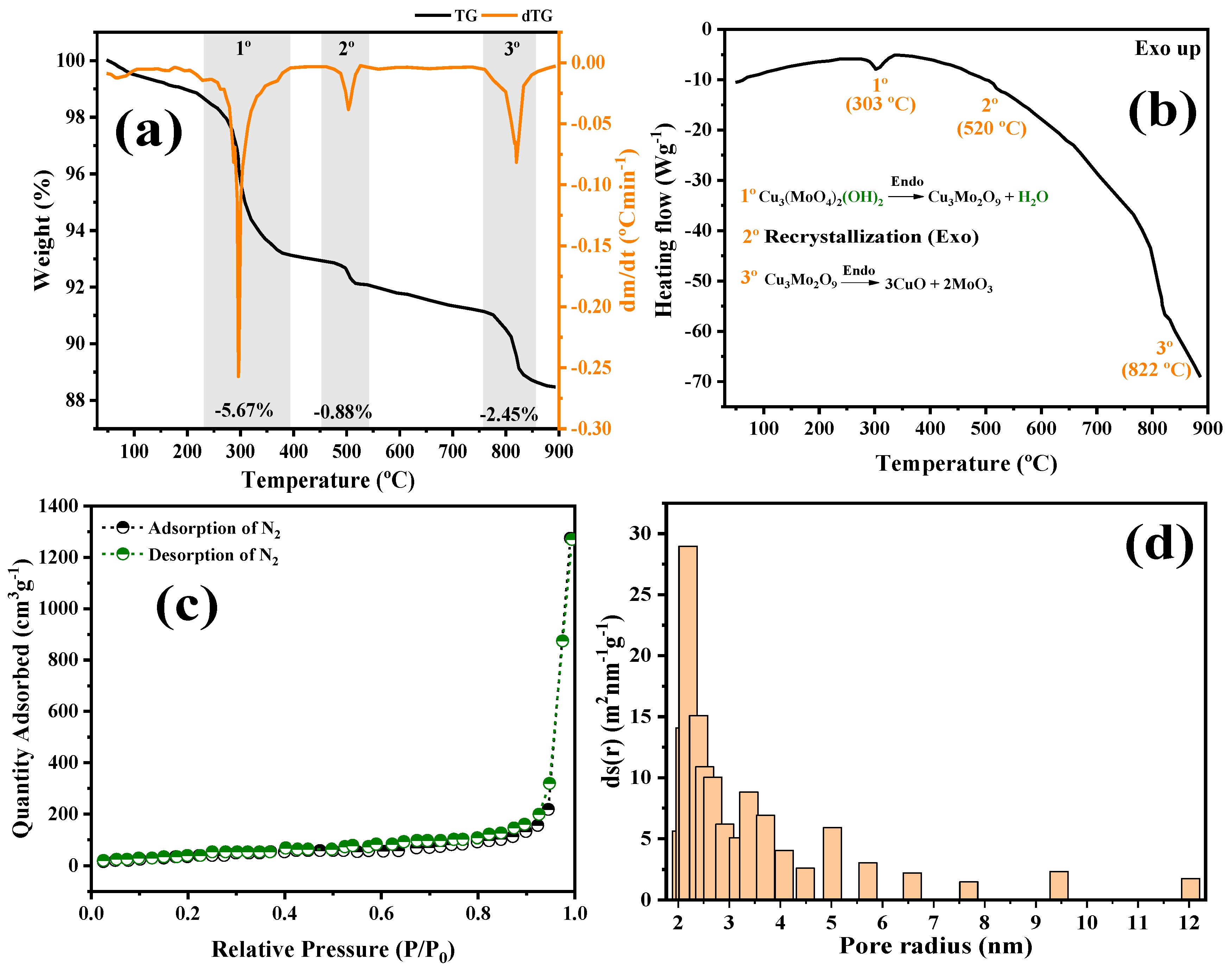
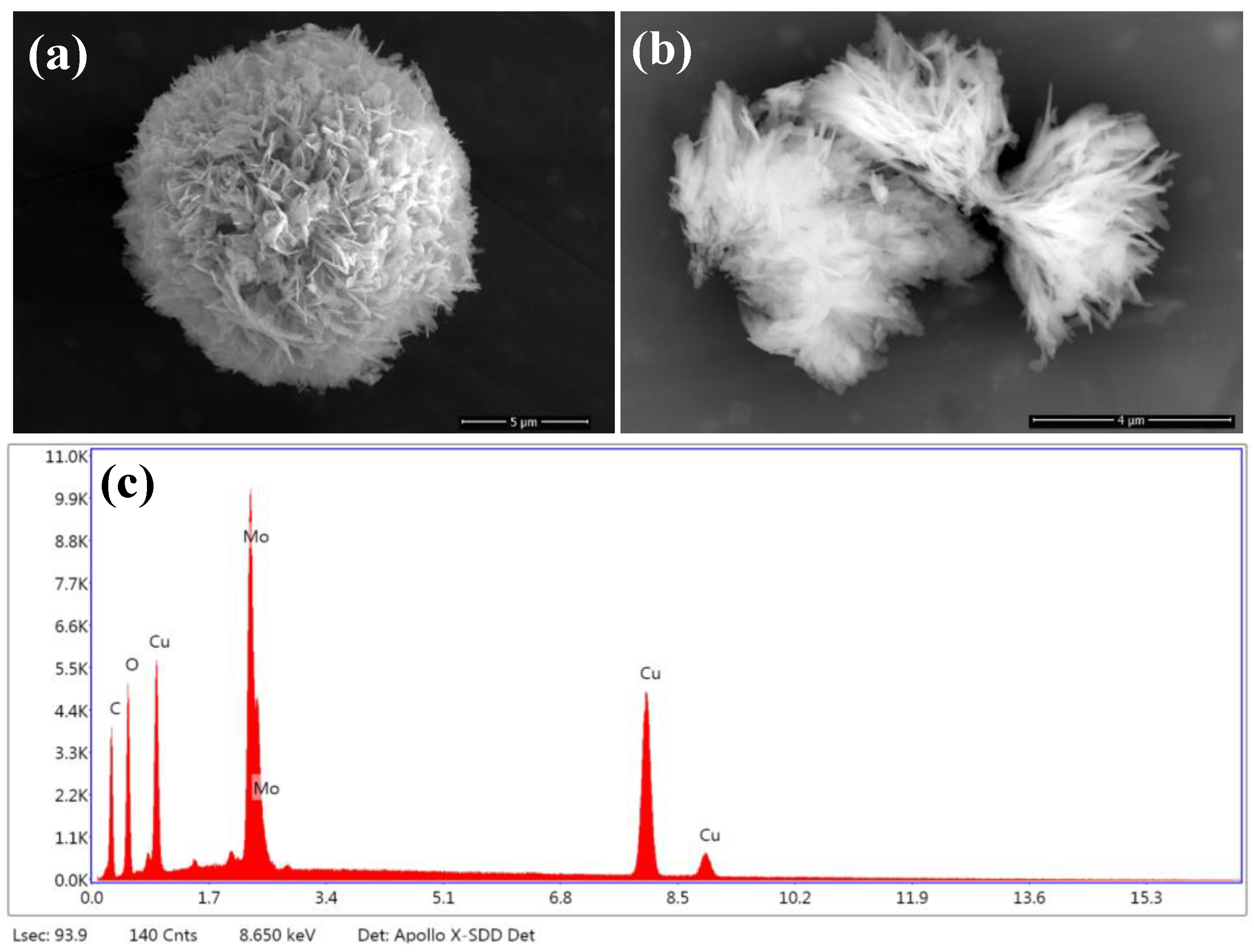
2.1. Structural and Morphological Characterization
2.2. Catalytic Conversion of Levulinic Acid into Methyl Levulinate
2.2.1. Effect of Temperature
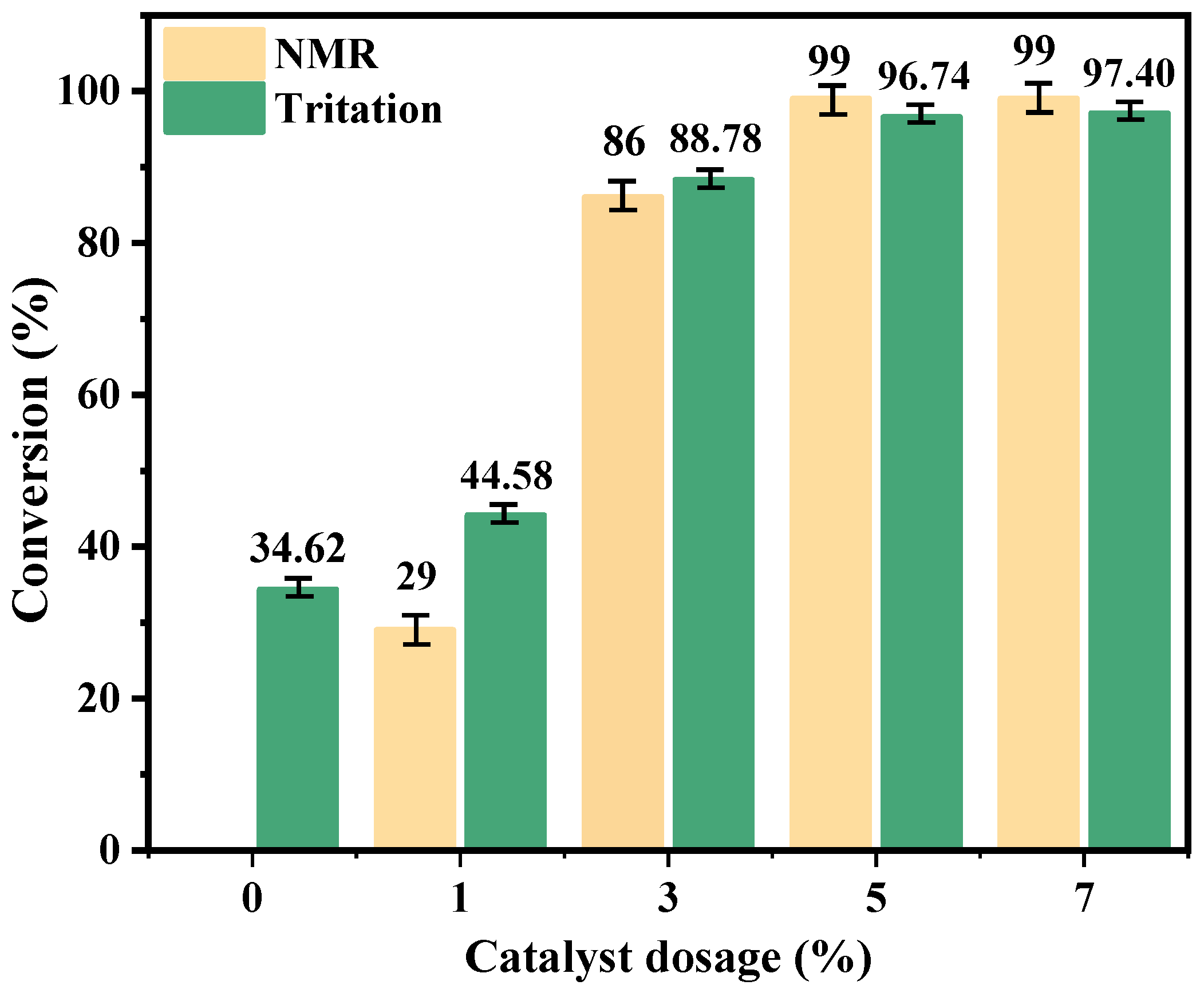
2.2.2. Catalyst Dosage
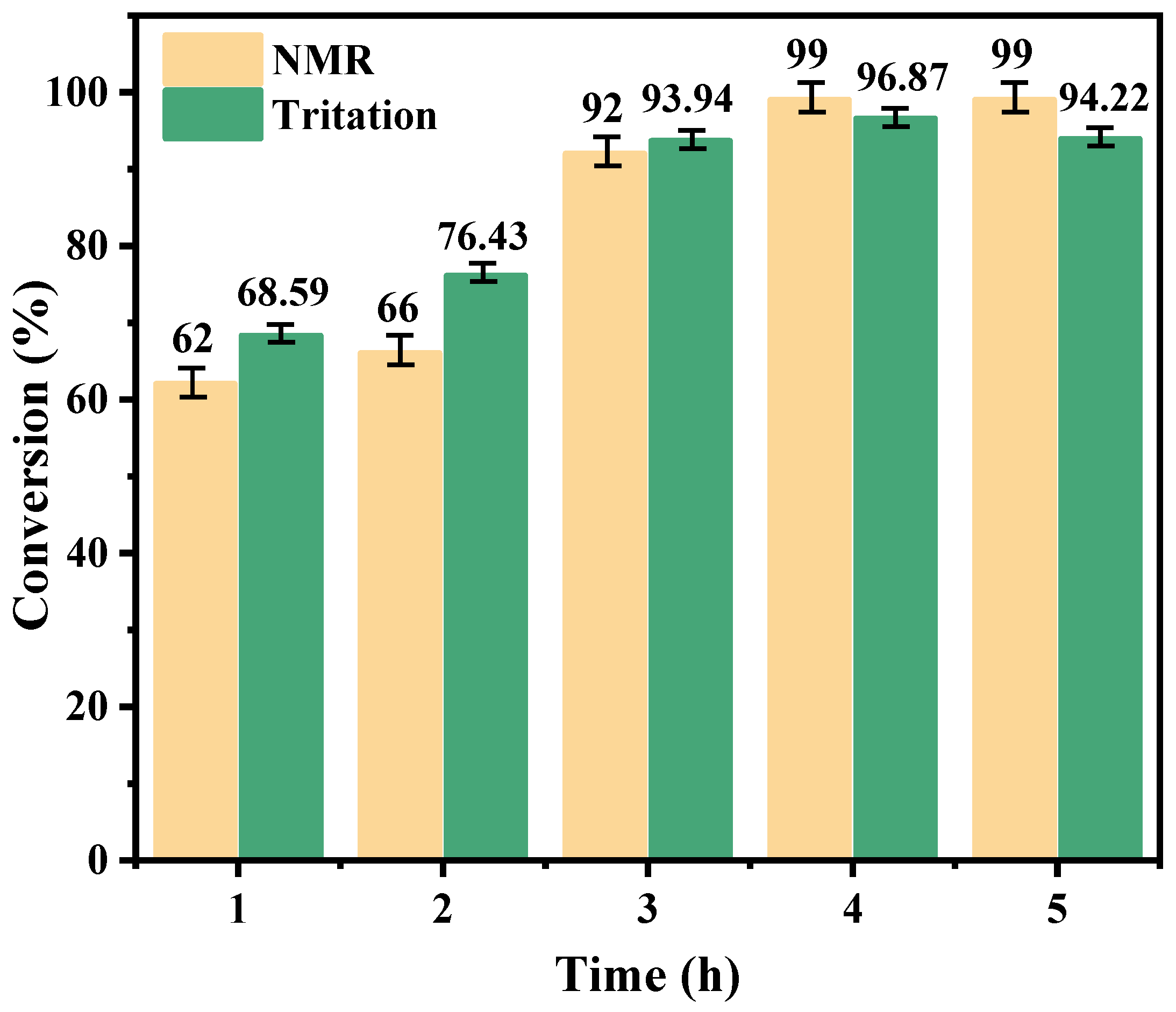
2.2.3. Effect of Time
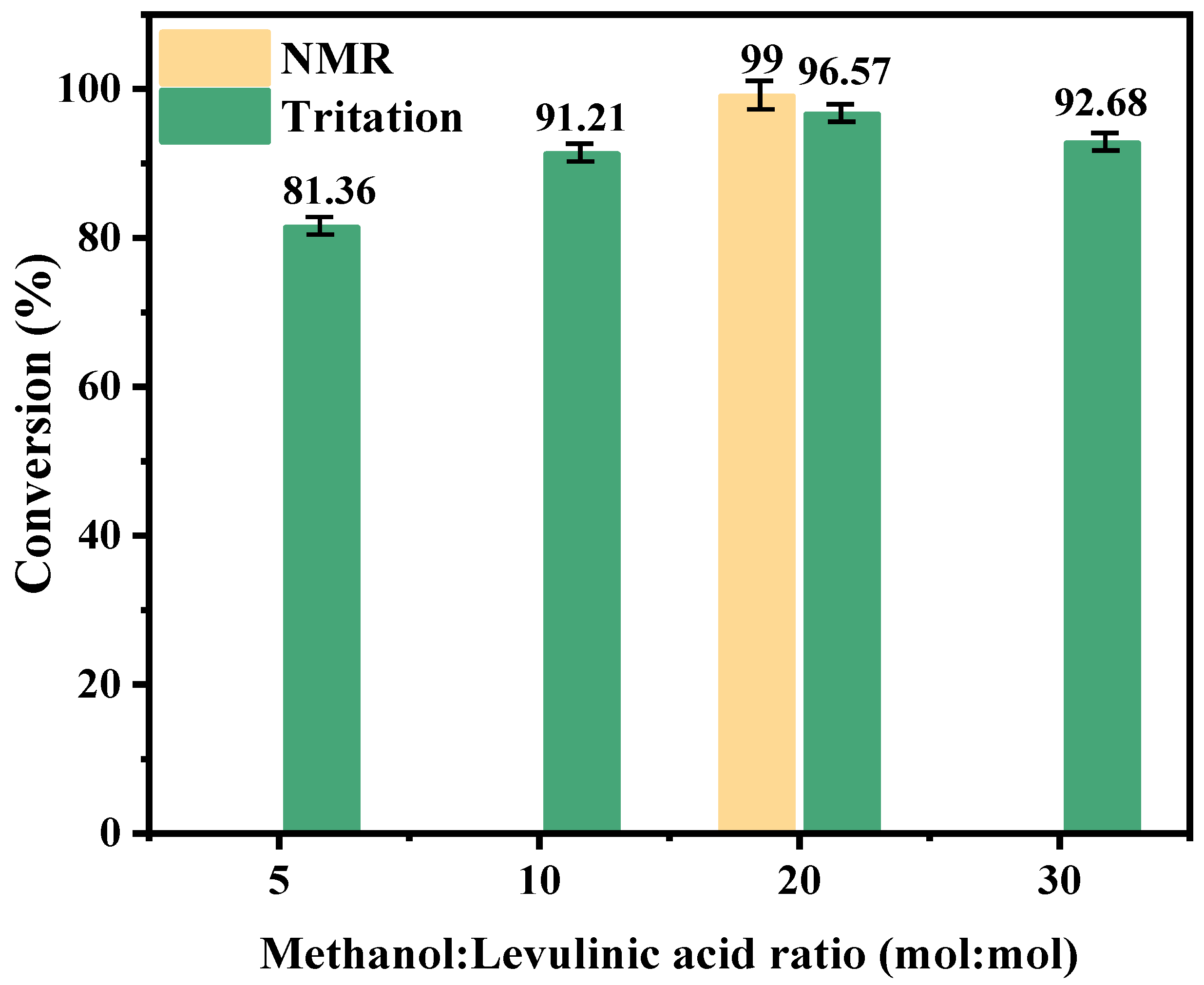
2.2.4. Effect of Molar Ratio of Levulinic Acid to Methanol
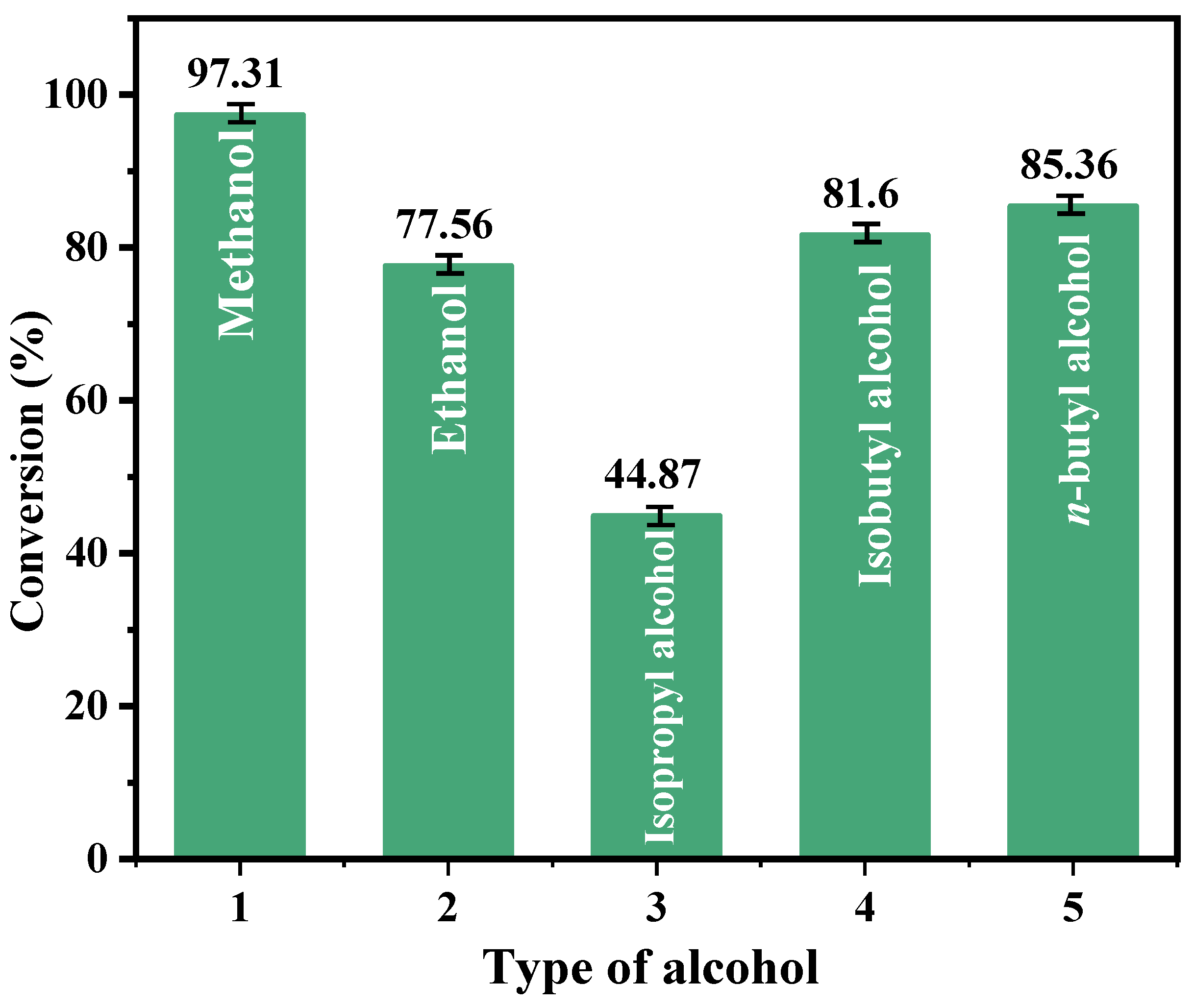
2.2.5. Effects of Different Alcohol
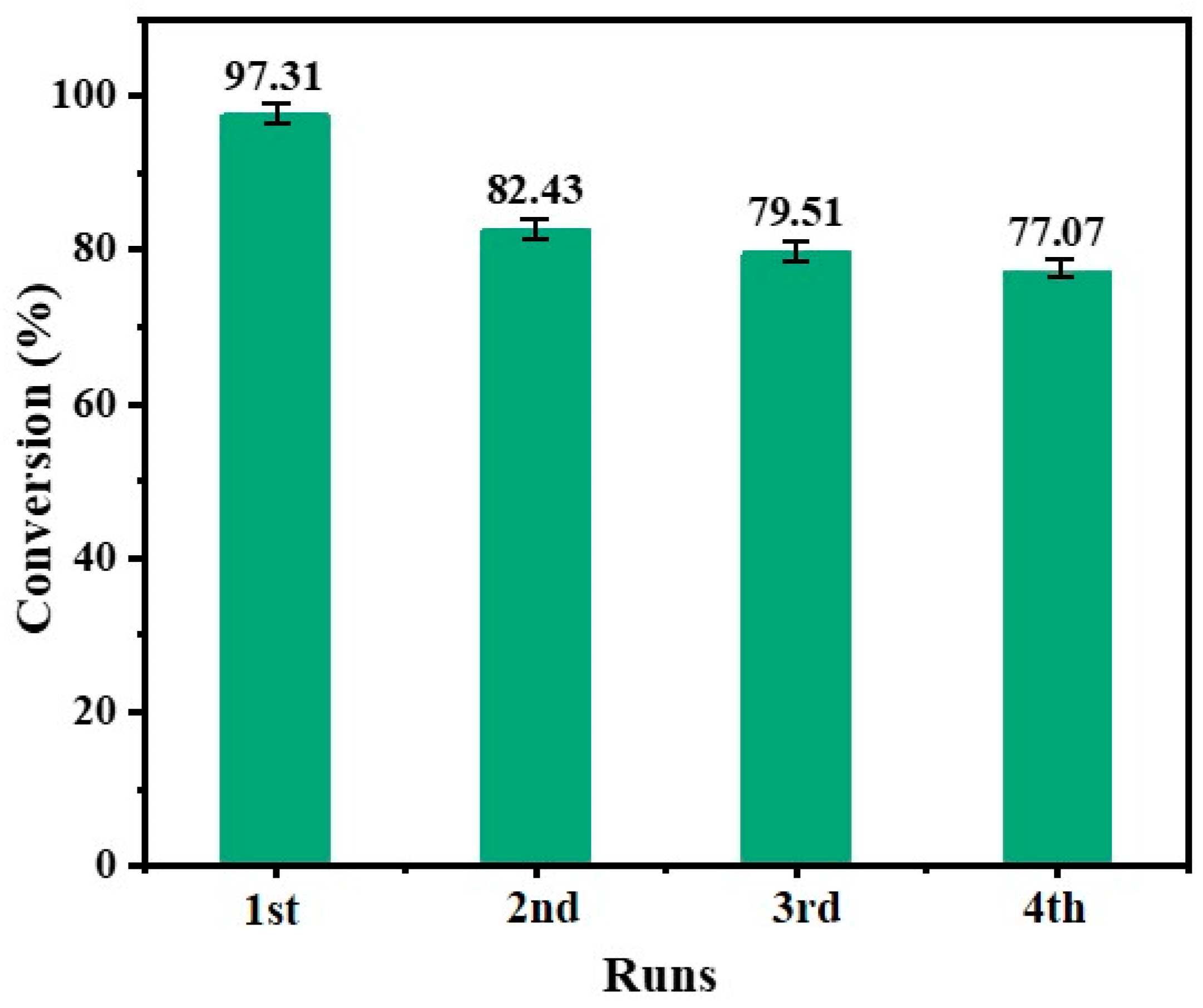
2.2.6. Recycling
3. Materials and Methods
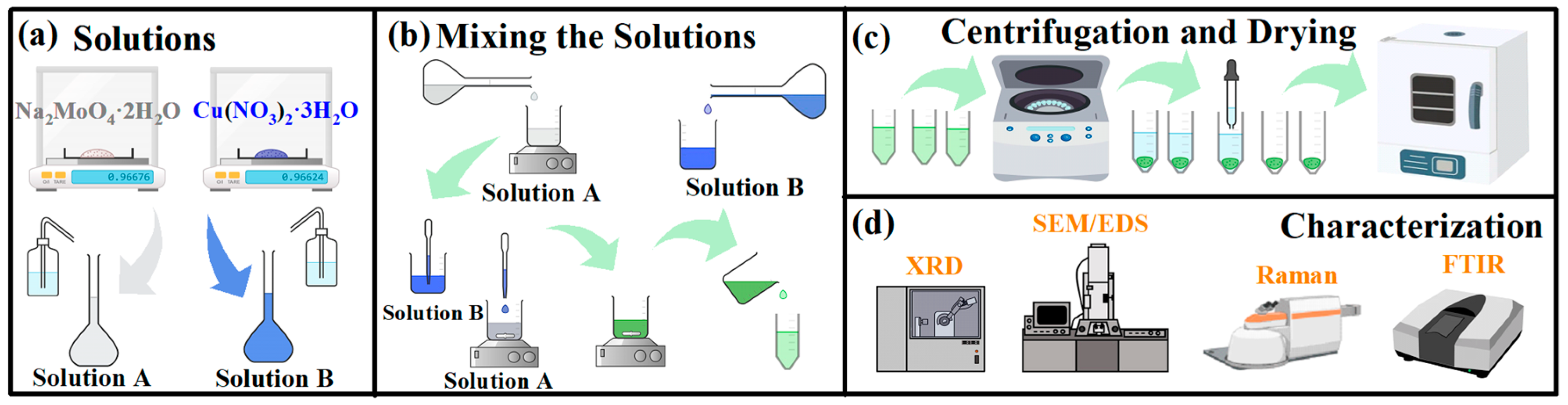
3.1. Materials
3.2. Methods
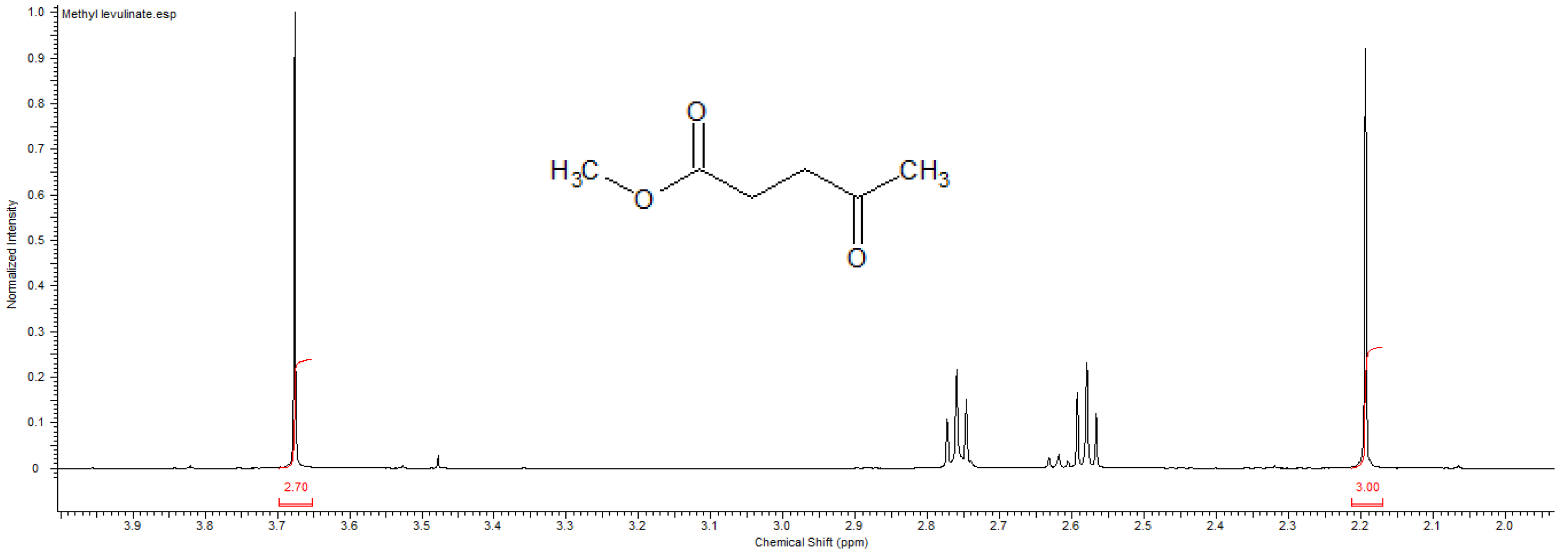
3.3. Characterization
3.4. Catalytic Esterification of Levulinic Acid
3.5. Catalyst Recycling and Leaching Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, S.; Singh, H. Pharmaceutical Waste: Risk to Human Health And Environment. Eur. J. Mol. Clin. Med. 2020, 7, 3512–3518. [Google Scholar]
- Capurro, R.; Fiorentino, R.; Galeotti, R.M.; Garzella, S. The Impact of Digitalization and Sustainability on Governance Structures and Corporate Communication: A Cross-Industry and Cross-Country Approach. Sustainability 2023, 15, 2064. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health—A Review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Zaller, J.G.; Kruse-Plaß, M.; Schlechtriemen, U.; Gruber, E.; Peer, M.; Nadeem, I.; Formayer, H.; Hutter, H.P.; Landler, L. Pesticides in Ambient Air, Influenced by Surrounding Land Use and Weather, Pose a Potential Threat to Biodiversity and Humans. Sci. Total Environ. 2022, 838, 156012. [Google Scholar] [CrossRef]
- O’Sullivan, J.N. Demographic Delusions: World Population Growth Is Exceeding Most Projections and Jeopardising Scenarios for Sustainable Futures. World 2023, 4, 545–568. [Google Scholar] [CrossRef]
- Deus, M.S.; Deus, K.C.O.; Lira, D.S.; Oliveira, J.A.; Padilha, C.E.A.; Souza, D.F.S. Esterification of Oleic Acid for Biodiesel Production Using a Semibatch Atomization Apparatus. Int. J. Chem. Eng. 2023, 2023, 6957812. [Google Scholar] [CrossRef]
- Fallah, Z.; Zare, E.N.; Ghomi, M.; Ahmadijokani, F.; Amini, M.; Tajbakhsh, M.; Arjmand, M.; Sharma, G.; Ali, H.; Ahmad, A.; et al. Toxicity and Remediation of Pharmaceuticals and Pesticides Using Metal Oxides and Carbon Nanomaterials. Chemosphere 2021, 275, 130055. [Google Scholar] [CrossRef]
- Mishra, B.; Mohanta, Y.K.; Reddy, C.N.; Reddy, S.D.M.; Mandal, S.K.; Yadavalli, R.; Sarma, H. Valorization of Agro-Industrial Biowaste to Biomaterials: An Innovative Circular Bioeconomy Approach. Circ. Econ. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive Edible Films for Food Applications: Mechanisms of Antimicrobial and Antioxidant Activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef]
- Cesca, R.S.; Fonseca, G.G.; Paz, M.F.D.; Cortez-Vega, W.R. Advances and Perspectives on the Application of Essential Oils in Food Packaging Films, Coatings, and Nanoencapsulated Materials. Bragantia 2024, 83, e20230132. [Google Scholar] [CrossRef]
- Asomadu, R.O.; Ezeorba, T.P.C.; Ezike, T.C.; Uzoechina, J.O. Exploring the Antioxidant Potential of Endophytic Fungi: A Review on Methods for Extraction and Quantification of Total Antioxidant Capacity (TAC). 3 Biotech 2024, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Vaithyanathan, V.K.; Goyette, B.; Rajagopal, R. A Critical Review of the Transformation of Biomass into Commodity Chemicals: Prominence of Pretreatments. Environ. Chall. 2023, 11, 100700. [Google Scholar] [CrossRef]
- Wang, J.; Cui, H.; Wang, Y.; Zhao, R.; Xie, Y.; Wang, M.; Yi, W. Efficient Catalytic Conversion of Cellulose to Levulinic Acid in the Biphasic System of Molten Salt Hydrate and Methyl Isobutyl Ketone. Green Chem. 2020, 22, 4240–4251. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C.; Huber, G.W. Production of Levulinic Acid from Cellulose by Hydrothermal Decomposition Combined with Aqueous Phase Dehydration with a Solid Acid Catalyst. Energy Environ. Sci. 2012, 5, 7559. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Hydrogenolysis of Cellulose to C4–C7 Alcohols over Bi-Functional CuO–MO/Al2O3 (M=Ce, Mg, Mn, Ni, Zn) Catalysts Coupled with Methanol Reforming Reaction. Bioresour. Technol. 2013, 137, 311–317. [Google Scholar] [CrossRef]
- Galadima, A.; Masudi, A.; Muraza, O. Conversion of Cellulose to Glucose and Further Transformation into Fuels over Solid Acid Catalysts: A Mini Review. Microporous Mesoporous Mater. 2022, 336, 111846. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Cellulose Depolymerization over Heterogeneous Catalysts. Acc. Chem. Res. 2018, 51, 761–768. [Google Scholar] [CrossRef]
- Boonyakarn, T.; Wataniyakul, P.; Boonnoun, P.; Quitain, A.T.; Kida, T.; Sasaki, M.; Laosiripojana, N.; Jongsomjit, B.; Shotipruk, A. Enhanced Levulinic Acid Production from Cellulose by Combined Brønsted Hydrothermal Carbon and Lewis Acid Catalysts. Ind. Eng. Chem. Res. 2019, 58, 2697–2703. [Google Scholar] [CrossRef]
- Tulaphol, S.; Hossain, M.A.; Rahaman, M.S.; Liu, L.Y.; Phung, T.K.; Renneckar, S.; Grisdanurak, N.; Sathitsuksanoh, N. Direct Production of Levulinic Acid in One Pot from Hemp Hurd by Dilute Acid in Ionic Liquids. Energy Fuels 2020, 34, 1764–1772. [Google Scholar] [CrossRef]
- Santos, L.; Fraga, G.; Pontes, D.; Campos, L.; Pontes, L.; Teixeira, L. Alternatives for the Production of Levulinic Acid Obtained from Biomass. Quim. Nova 2021, 44, 1300–1310. [Google Scholar] [CrossRef]
- Lopes, E.S.; Silva, J.F.L.; do Nascimento, L.A.; Bohórquez, J.F.C.; Lopes, M.S.; Tovar, L.P.; Filho, R.M. Feasibility of the Conversion of Sugarcane Molasses to Levulinic Acid: Reaction Optimization and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2021, 60, 15646–15657. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Liu, Q.; Liang, M.; Yang, J.; Lu, S.; Li, G.; Lu, L.; Si, C. Valorization of Corn Stover into Furfural and Levulinic Acid over SAPO-18 Zeolites: Effect of Brønsted to Lewis Acid Sites Ratios. Ind. Crops Prod. 2019, 141, 111759. [Google Scholar] [CrossRef]
- Perveen, F.; Farooq, M.; Ramli, A.; Naeem, A.; Khan, I.W.; Saeed, T.; Khan, J. Levulinic Acid Production from Waste Corncob Biomass Using an Environmentally Benign WO3-Grafted ZnCo2O4@CeO2 Bifunctional Heterogeneous Catalyst. ACS Omega 2022, 8, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, K.; Vogeler, N.; Kärkkäinen, J.; Dong, Y.; Niemelä, M.; Rusanen, A.; Ruotsalainen, A.L.; Wäli, P.; Markkola, A.; Lassi, U. Microwave-Assisted Conversion of Novel Biomass Materials into Levulinic Acid. Biomass Convers. Biorefin. 2018, 8, 965–970. [Google Scholar] [CrossRef]
- Santana Junior, C.C.; Rambo, M.C.D.; Teófilo, R.F.; Cardoso, W.J.; Bertuol, D.A.; Rambo, M.K.D. Production of Levulinic Acid from Coconut Residues (Cocos Nucifera) Using Differents Approaches. Waste Biomass Valorization 2021, 12, 6875–6886. [Google Scholar] [CrossRef]
- Freitas, F.A.; Licursi, D.; Lachter, E.R.; Galletti, A.M.R.; Antonetti, C.; Brito, T.C.; Nascimento, R.S.V. Heterogeneous Catalysis for the Ketalisation of Ethyl Levulinate with 1,2-Dodecanediol: Opening the Way to a New Class of Bio-Degradable Surfactants. Catal. Commun. 2016, 73, 84–87. [Google Scholar] [CrossRef]
- Ravutsov, M.A.; Marinova, M.M.; Kurutos, A.; Simeonov, S.P. Sources, Sustainability and Directions in the Chemical Synthesis of δ-Aminolevulinic Acid. Sustain. Chem. Pharm. 2024, 38, 101491. [Google Scholar] [CrossRef]
- Wu, H.; Song, J.; Xie, C.; Hu, Y.; Zhang, P.; Yang, G.; Han, B. Surface Engineering in PbS: Via Partial Oxidation: Towards an Advanced Electrocatalyst for Reduction of Levulinic Acid to γ-Valerolactone. Chem. Sci. 2019, 10, 1754–1759. [Google Scholar] [CrossRef]
- Juárez, P.; López-Aguado, C.; Paniagua, M.; Melero, J.A.; Mariscal, R.; Morales, G. Self-Condensation of Levulinic Acid into Bio-Jet Fuel Precursors over Acid Zeolites: Elucidating the Role of Nature, Strength and Density of Acid Sites. Appl. Catal. A Gen. 2022, 631, 118480. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, H.; Zhu, J.; Liu, X.; Xu, Q.; Yin, D. Esterification of Levulinic Acid into n-Butyl Levulinate Catalyzed by Sulfonic Acid-Functionalized Lignin-Montmorillonite Complex. J. Bioresour. Bioprod. 2020, 5, 291–299. [Google Scholar] [CrossRef]
- Chaffey, D.R.; Bere, T.; Davies, T.E.; Apperley, D.C.; Taylor, S.H.; Graham, A.E. Conversion of Levulinic Acid to Levulinate Ester Biofuels by Heterogeneous Catalysts in the Presence of Acetals and Ketals. Appl. Catal. B Environ. 2021, 293, 120219. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, K.; Liu, Y.; Wu, S. Esterification of Levulinic Acid into Ethyl Levulinate Catalyzed by Sulfonated Bagasse-Carbonized Solid Acid. BioResources 2019, 14, 2186–2196. [Google Scholar] [CrossRef]
- Gallego-Villada, L.A.; Alarcón, E.A.; Cerrutti, C.; Blustein, G.; Sathicq, Á.G.; Romanelli, G.P. Levulinic Acid Esterification with n-Butanol over a Preyssler Catalyst in a Microwave-Assisted Batch Reactor: A Kinetic Study. Ind. Eng. Chem. Res. 2023, 62, 10915–10929. [Google Scholar] [CrossRef]
- Subalakshmi, A.; Kavitha, B.; Srinivasan, N.; Rajarajan, M.; Suganthi, A. An Affordable Efficient SrWO4 Decorated Bi2O3 Nanocomposite: Photocatalytic Activity for the Degradation of Methylene Blue under Visible Light Irradiation. Mater. Today Proc. 2022, 48, 409–419. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Mei, Y.; Chen, L.; Guo, H.; Liu, B. The Antibacterial Properties of Nano ZnMoO4 Powder. J. Mater. Sci. 2022, 57, 16820–16829. [Google Scholar] [CrossRef]
- Saraj, C.S.; Singh, S.C.; Verma, G.; Alquliah, A.; Li, W.; Guo, C. Rapid Fabrication of CuMoO4 Nanocomposites via Electric Field Assisted Pulsed-Laser Ablation in Liquids for Electrochemical Hydrogen Generation. Appl. Surf. Sci. Adv. 2023, 13, 100358. [Google Scholar] [CrossRef]
- Murugan, E.; Govindaraju, S.; Santhoshkumar, S. Hydrothermal Synthesis, Characterization and Electrochemical Behavior of NiMoO4 Nanoflower and NiMoO4/RGO Nanocomposite for High-Performance Supercapacitors. Electrochim. Acta 2021, 392, 138973. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, T.; Sun, Y.; Wang, X.; Wu, X. Co3O4@NiMoO4 Composite Electrode Materials for Flexible Hybrid Capacitors. Front. Optoelectron. 2022, 15, 25. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Khajuria, H.; Sharma, S.; Sheikh, H.N. Studies on Hydrothermal Synthesis of Photolumniscent Rare Earth (Eu3+ & Tb3+) Doped NG@FeMoO4 for Enhanced Visible Light Photodegradation of Methylene Blue Dye. Solid State Sci. 2018, 76, 38–47. [Google Scholar] [CrossRef]
- Karthikeyan, M. Production of Biodiesel from Cordiamyxa Bio-Oil Using BaMoO4-Ce2O3 Nanoparticles as an Alternative Fuel for Diesel Engine. Mater. Lett. 2019, 243, 199–201. [Google Scholar] [CrossRef]
- Feitosa de Carvalho, T.A.; Nobre, F.X.; de Lima Barros, A.; Ghosh, A.; de Almeida Lima e Silva, A.; Oliveira dos Santos Fontenelle, R.; Rita de Morais Chaves Santos, M.; Elias de Matos, J.M. Investigation of Optical, Structural, and Antifungal Properties of Lindgrenite Obtained by Conventional Coprecipitation and Ultrasound-Assisted Coprecipitation Methods. J. Solid State Chem. 2021, 295, 121957. [Google Scholar] [CrossRef]
- Silva Junior, J.L.; Nobre, F.X.; de Freitas, F.A.; de Carvalho, T.A.F.; de Barros, S.S.; Nascimento, M.C.; Manzato, L.; Matos, J.M.E.; Brito, W.R.; Leyet, Y.; et al. Copper Molybdate Synthesized by Sonochemistry Route at Room Temperature as an Efficient Solid Catalyst for Esterification of Oleic Acid. Ultrason. Sonochem. 2021, 73, 105541. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jenkins, R.A.; Thompson, R.M.; Downs, R.T.; Evans, S.H.; Bloch, E.M. Markascherite, Cu3(MoO4)(OH)4, a New Mineral Species Polymorphic with Szenicsite, from Copper Creek, Pinal County, Arizona, U.S.A. Am. Mineral. 2012, 97, 197–202. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, X.; Zhang, H.; Wang, J.; Liu, G.; Yu, W.; Dong, X. Hydrothermal Synthesis of Rod-like CoMoO4 and Its Excellent Properties for the Anode of Lithium-ion Batteries. J. Chin. Chem. Soc. 2020, 67, 2012–2018. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, C.; Ma, Z.; Sun, C.; Chen, L.; Chen, Y. MoO3 Nanorods/Fe2(MoO4)3 Nanoparticles Composite Anode for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2014, 39, 14411–14415. [Google Scholar] [CrossRef]
- Carvalho, T.A.F.D.; Brenord, G.; Pinto, B.F.; Mendes, M.K.D.A.; Silva, D.S.N.; Silva, A.D.A.L.E.; de Moura, E.M.; Vieira, E.C.; Ghosh, A.; Nobre, F.X.; et al. Lindgrenite as an Efficient Heterogeneous Catalyst to Obtain Biodiesel. J. Environ. Chem. Eng. 2024, 12, 111672. [Google Scholar] [CrossRef]
- Dummer, N.F.; Sodiq-Ajala, Z.; Morgan, D.J.; Davies, T.E. Investigating the Preparation of Cu3Mo2O9 as a Photocatalyst. Catal. Commun. 2022, 163, 106414. [Google Scholar] [CrossRef]
- Wen, P.; He, Q.; Hu, D.; Ren, L.; Zhao, W.; Wei, F. Preparation and Characterization of Cu3(MoO4)2(OH)2 Nanosheets with Solar Photocatalytic Activity. ChemistrySelect 2022, 7, e202200087. [Google Scholar] [CrossRef]
- Ávila-López, M.A.; Luévano-Hipólito, E.; Torres-Martínez, L.M. In-Situ Fabrication of Cu3(MoO4)2(OH)2 Films Decorated with MO (M = Zn, Cu, and Ni) for CO2 Photoconversion into Value-Added Compounds. J. Alloys Compd. 2021, 873, 159846. [Google Scholar] [CrossRef]
- Bomio, M.R.D.; Tranquilin, R.L.; Motta, F.V.; Paskocimas, C.A.; Nascimento, R.M.; Gracia, L.; Andres, J.; Longo, E. Toward Understanding the Photocatalytic Activity of PbMoO4 Powders with Predominant (111), (100), (011), and (110) Facets. A Combined Experimental and Theoretical Study. J. Phys. Chem. C 2013, 117, 21382–21395. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Zhang, Y.; Li, Z.; Li, J.; Fu, X. Solvothermal Preparation, Electronic Structure and Photocatalytic Properties of PbMoO4 and SrMoO4. Appl. Catal. B Environ. 2009, 91, 135–143. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Martínez-de la Cruz, A.; Torres-Martínez, L.M. Photocatalytic Degradation of Organic Compounds by PbMoO4 Synthesized by a Microwave-Assisted Solvothermal Method. Ceram. Int. 2016, 42, 3096–3103. [Google Scholar] [CrossRef]
- Ismail, A.A.; Alsheheri, S.Z.; Albukhari, S.M.; Mahmoud, M.H.H. Facile Synthesis of Visible-Light-Induced Mesoporous Ag2O/Fe2(MoO4)3 Photocatalysts for Degradation of Tetracycline. Opt. Mater. 2021, 121, 111505. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, L.; Liu, Y.; Mei, Y.; Chen, L.; Zheng, A.; Yin, K.; Xiao, X.; Li, S.; Sun, X.; et al. Highly Stable and Antifungal Properties on the Oilseed Rape of Cu3(MoO4)2(OH)2 Nanoflakes Prepared by Simple Aqueous Precipitation. Sci. Rep. 2024, 14, 5235. [Google Scholar] [CrossRef]
- Hassani, H.O.; Akouibaa, M.; Rakass, S.; Abboudi, M.; El Bali, B.; Lachkar, M.; Al Wadaani, F. A Simple and Cost-Effective New Synthesis Method of Copper Molybdate CuMoO4 Nanoparticles and Their Catalytic Performance. J. Sci. Adv. Mater. Devices 2021, 6, 501–507. [Google Scholar] [CrossRef]
- Medeiros Leão, G.S.; Silva Ribeiro, M.D.; Filho, R.L.D.F.; Saraiva, L.B.; Peña-Garcia, R.R.; Teixeira, A.P.D.C.; Lago, R.M.; Freitas, F.A.; de Sá Barros, S.; Junior, S.D.; et al. The Synergic Effect of h -MoO3, α-MoO3, and β-MoO3 Phase Mixture as a Solid Catalyst to Obtain Methyl Oleate. ACS Appl. Mater. Interfaces 2024, 16, 60103–60121. [Google Scholar] [CrossRef]
- Rahmani, A.; Farsi, H. Nanostructured Copper Molybdates as Promising Bifunctional Electrocatalysts for Overall Water Splitting and CO2 reduction. RSC Adv. 2020, 10, 39037–39048. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, P.; Kuo, D.H.; Wu, B.; Abdeta, A.B.; Su, Z.; Chen, L.; Zelekew, O.A.; Lin, J.; Chen, X. Activation of Cu3(MoO4)2(OH)2 with the Hydrazine-Driven Cation Reduction into a Highly Efficient Catalyst for the Reduction of Organic Dyes and Heavy Metals Ion. J. Environ. Chem. Eng. 2023, 11, 109974. [Google Scholar] [CrossRef]
- Lobo, W.V.; Paes, O.A.R.L.; Sousa, A.D.S.; Pinheiro, W.; Sousa, C.A.S.; de Araújo Bezerra, J.; Novita, L.; Cheng, I.F.; de Souza, L.K.C.; de Freitas, F.A. Application of Sulfonated Passion Fruit Seeds as a Heterogeneous Catalyst in the Esterification of Oleic and Levulinic Acids: Optimization of Reaction Parameters. Renew. Energy 2025, 241, 122355. [Google Scholar] [CrossRef]
- Patel, A.; Dave, R. Phosphotungstic Acid Supported on Waste Sugarcane Bagasse: A Sustainable and Recyclable Catalyst for the Synthesis of Biofuel Additives from Levulinic Acid. J. Indian Chem. Soc. 2025, 102, 101574. [Google Scholar] [CrossRef]
- Alsalim, M.; Najafi Chermahini, A.; Dinari, M.; Luque, R.; Pineda, A.; Vargas Fernández, C. Synthesis of Carrageenan/DFNS Composite and Its Application in the Esterification of Levulinic Acid with Alcohols. Inorg. Chem. Commun. 2024, 169, 113054. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, C.; Chen, S.; Liu, P. Controlled Synthesis of Defective MOF-808 and Its Application in Levulinic Acid Esterification. Appl. Catal. A Gen. 2024, 681, 119773. [Google Scholar] [CrossRef]
- Qin, L.; Wang, J.; Mao, H.; Ge, L.; Yao, C. Efficient Cascade Reactions Involving Esterification, Hydrogen Transfer, and Lactonization for Biomass Conversion Using MOF/Metal Oxide Composites as Heterogeneous Catalysts. Mol. Catal. 2024, 568, 114525. [Google Scholar] [CrossRef]
- Desai, D.S.; Yadav, G.D. Synthesis of Energy Rich Fuel Additive from Biomass Derived Levulinic Acid and Furfuryl Alcohol Using Novel Tin-Exchanged Heteropoly Acid Supported on Titania Nanotubes as Catalyst. Fuel 2023, 331, 125700. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.R.L.; Maia, P.J.S.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S.; de Freitas, F.A. New Heterogeneous Catalyst for Biodiesel Production from Waste Tucumã Peels (Astrocaryum Aculeatum Meyer): Parameters Optimization Study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, L.; Bai, J.; Lei, M.; Long, M.; Huang, K. Catalytic Esterification of Levulinic Acid into the Biofuel n-Butyl Levulinate over Nanosized TiO2 Particles. Nanomaterials 2022, 12, 3870. [Google Scholar] [CrossRef]
- Suminar, D.R.; Pribadi, C.Z.; Fitriana, Q.R.; Andrijanto, E.; Permana, M.D.; Eddy, D.R.; Rahayu, I. Corncob Waste Derived Carbon with Sulfonic Acid Group: An Efficient Heterogeneous Catalyst for Production of Ethyl Levulinate as Biodiesel Additives. Heliyon 2024, 10, e37687. [Google Scholar] [CrossRef]
- Zainol, M.M.; Amin, N.A.S.; Asmadi, M. Kinetics and Thermodynamic Analysis of Levulinic Acid Esterification Using Lignin-Furfural Carbon Cryogel Catalyst. Renew. Energy 2019, 130, 547–557. [Google Scholar] [CrossRef]
- Ntshibongo, S.; Maumela, M.; Bingwa, N. Synthesis, Characterization and Catalytic Evaluation of Supported Catalysts (PdNPs/TiO2, PdNPs/Al2O3, PdNPs/SiO2) for Esterification of Levulinic Acid to Levulinate Esters. Inorg. Chem. Commun. 2022, 146, 110101. [Google Scholar] [CrossRef]
- Pithadia, D.; Patel, A. Anchored Silicotungstic Acid: Study of the Synthesis of Biofuel Additive from Levulinic Acid & Effect of Supports. Catal. Today 2024, 433, 114666. [Google Scholar] [CrossRef]
- Goga, G.; Chauhan, B.S.; Mahla, S.K.; Cho, H.M. Performance and Emission Characteristics of Diesel Engine Fueled with Rice Bran Biodiesel and N-Butanol. Energy Rep. 2019, 5, 78–83. [Google Scholar] [CrossRef]
- Maggi, R.; Shiju, N.R.; Santacroce, V.; Maestri, G.; Bigi, F.; Rothenberg, G. Silica-Supported Sulfonic Acids as Recyclable Catalyst for Esterification of Levulinic Acid with Stoichiometric Amounts of Alcohols. Beilstein J. Org. Chem. 2016, 12, 2173–2180. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.P.d.O.; Lobo, W.V.; de Carvalho, T.A.F.; de Matos, J.M.E.; de Freitas, F.A.; Ruiz, Y.L.; Matos, R.S.; Ţălu, Ş.; da Fonseca Filho, H.D.; Domínguez, L.A.; et al. Copper Molybdate-Catalyzed Esterification of Levulinic Acid: A Heterogeneous Approach for Biofuel Synthesis. Catalysts 2025, 15, 357. https://doi.org/10.3390/catal15040357
Ribeiro APdO, Lobo WV, de Carvalho TAF, de Matos JME, de Freitas FA, Ruiz YL, Matos RS, Ţălu Ş, da Fonseca Filho HD, Domínguez LA, et al. Copper Molybdate-Catalyzed Esterification of Levulinic Acid: A Heterogeneous Approach for Biofuel Synthesis. Catalysts. 2025; 15(4):357. https://doi.org/10.3390/catal15040357
Chicago/Turabian StyleRibeiro, Alyne Pereira de Oliveira, Wyvirlany Valente Lobo, Talles André Feitosa de Carvalho, José Milton Elias de Matos, Flávio Augusto de Freitas, Yurimiler Leyet Ruiz, Robert S. Matos, Ştefan Ţălu, Henrique Duarte da Fonseca Filho, Lianet Aguilera Domínguez, and et al. 2025. "Copper Molybdate-Catalyzed Esterification of Levulinic Acid: A Heterogeneous Approach for Biofuel Synthesis" Catalysts 15, no. 4: 357. https://doi.org/10.3390/catal15040357
APA StyleRibeiro, A. P. d. O., Lobo, W. V., de Carvalho, T. A. F., de Matos, J. M. E., de Freitas, F. A., Ruiz, Y. L., Matos, R. S., Ţălu, Ş., da Fonseca Filho, H. D., Domínguez, L. A., Brito, W. R., & Nobre, F. X. (2025). Copper Molybdate-Catalyzed Esterification of Levulinic Acid: A Heterogeneous Approach for Biofuel Synthesis. Catalysts, 15(4), 357. https://doi.org/10.3390/catal15040357











