Abstract
This study focuses on the fabrication and application of heterogeneous acid catalytic filaments for free fatty acid (FFA) reduction in crude palm oil (CPO) via esterification. Amberlyst-15 catalyst was blended with acrylonitrile butadiene styrene (ABS) using a single-screw filament extruder to produce Amberlyst-15/ABS catalytic filaments. A 5 wt.% concentration of fine Amberlyst-15 particles was considered optimal for blending with ABS, making them a suitable acid catalyst for FFA reduction. The mechanical properties, thermal behavior, and morphology of the Amberlyst-15/ABS catalytic filaments were assessed. The esterification process was optimized by varying three independent variables: the methanol-to-oil molar ratio, catalytic filament loading, and reaction time. The results revealed that under the recommended conditions—26.7:1 methanol-to-oil molar ratio, 78.5 wt.% catalytic filament loading, and a reaction time of 20.2 h at 500 rpm and 60 °C—the FFA content in CPO was reduced from 10.05 to 0.83 wt.%. Additionally, the reusability of the catalytic filaments was evaluated under the recommended conditions of the esterification process. The results demonstrated that the filaments remained effective for at least two cycles, achieving FFA levels below 2 wt.%, thereby confirming their stability and catalytic efficiency. The methodology employed in this study for the preparation and characterization of Amberlyst-15/ABS catalytic filaments offers a promising approach for fabricating acid catalytic materials via 3D printing, especially for heterogeneous catalysis in esterification reactions.
1. Introduction
Petroleum fuels are crucial for various sectors, including transportation, manufacturing, power generation, and agriculture. However, their increasing use has resulted in rising costs and environmental concerns, particularly in terms of air pollution [1,2,3]. Biodiesel, a renewable and sustainable biofuel, has received considerable attention as an alternative to petroleum-based fuels. Biodiesel is typically produced from diverse sources, including vegetable oils (such as palm, coconut, rapeseed, and soybean), waste cooking oils, and animal fats [4,5,6,7]. Compared to petroleum diesel, biodiesel offers environmental and economic advantages, including lower emissions of NOx, CO, CO2, HC, and particulate matter, and can be used in diesel engines without modification [4,5,8,9,10]. Consequently, the use of biodiesel can reduce greenhouse gas emissions from transportation and improve energy security [11]. In Thailand, crude palm oil (CPO) is considered a crucial raw material for producing commercial biodiesel, which is blended with petroleum diesel for use in the transportation sector. However, the high free fatty acid (FFA) content in CPO presents a major challenge for its use as a feedstock. If CPO contains more than 1 wt.% of FFAs, the base-catalyzed transesterification process triggers saponification reactions, which hinder biodiesel production. To achieve high yields and purity, the FFA content must be reduced to below 1 wt.% [12,13,14,15,16]. To address this issue, a two-step biodiesel production process is commonly used to convert high-FFA oil into biodiesel [7,16]. In the first step, FFAs are converted into fatty acid methyl esters (FAMEs) via acid-catalyzed esterification, reducing the FFA content to below 1–2% to prevent soap formation during the subsequent reaction [12,16]. The esterified oil from the first step is then subjected to base-catalyzed transesterification, in which triglycerides and other remaining components are converted into esters and glycerol [12,15,17]. Typically, homogeneous acid catalysts such as sulfuric acid, p-toluene sulfonic acid, phosphoric acid, and hydrochloric acid are used to convert the FFAs in oil into esters [18,19]. Although these catalysts provide advantages in terms of cost and availability compared with solid catalysts, they can significantly corrode reactor equipment [7,12,14,17,20]. Heterogeneous acid catalysts, particularly those in the sulfonate ion exchange resin group, have been widely investigated for biodiesel production [12]. Due to their superior efficiency in converting FFAs, the heterogeneous sulfonate ion exchange catalysts, such as Amberlyst-15, are commonly used in esterification processes [7,16,21]. Previous research has explored the use of sulfonate acid catalysts to accelerate the esterification reaction. Ridwan et al. analyzed the effects of co-solvent and methanol on biodiesel production from oleic acid using Amberlyst-15. The findings revealed that the highest FFA conversion of 28.6% was achieved at a methanol-to-oleic acid molar ratio of 25:1, with a catalyst loading of 10%, a co-solvent concentration of 8%, and a reaction temperature of 60 °C for 60 min. After four reuse cycles, the catalytic activity of Amberlyst-15 remained stable, with only a slight decrease observed after the fifth cycle [16]. Boz et al. investigated the esterification and transesterification processes using unmodified and modified Amberlyst-15 to produce biodiesel from waste cooking oils. Moreover, Amberlyst-15 was used to reduce the FFAs in CPO through an esterification reaction for at least three reuse cycles. Additionally, the heat treatment of the catalyst at temperatures above 220 °C for 48 h could enhance its activity. The optimal conditions for biodiesel production were identified as a 1:12 oil-to-methanol ratio, 3 wt.% unmodified catalyst loading, a temperature of 65 °C, and a reaction time of 9 h, resulting in a maximum biodiesel yield of 78% [7].
Three-dimensional (3D) printing, an additive manufacturing technique, is increasingly applied in various industries—including the automotive, robotics, medical, construction, food, electronics, aerospace, renewable energy, biotechnology, and chemical processing industries—due to its ability to produce complex, customized structures [22,23,24,25]. In particular, fused deposition modeling (FDM) is an effective printing method for producing parts from computer-aided design models through a layer-by-layer manufacturing process, which deposits thin layers of material to form a 3D product [22,24,26,27]. FDM has gained widespread adoption due to its speed, adaptability, low cost, and ease of use [22,23,24,26]. Additionally, pure polymer filaments are often combined with additives to enhance their structural, mechanical, thermal, chemical, and electrical properties, meeting the specific requirements of each application [22,28,29,30,31,32]. Botti et al. investigated the use of geopolymers modified with the addition of sodium hydroxide (NaOH), potassium hydroxide (KOH), and NaOH/KOH combinations. These modified geopolymers served as heterogeneous base catalysts for biodiesel production from soybean oil through a transesterification process. These geopolymers were printed using 3D printing and tested for biodiesel production under conditions of 31.62 vol.% methanol, 5 wt.% catalyst, and a 6 h reaction time at 75 °C. The results revealed high FAME yields of 73.5%, 85.3%, and 71.3% for the NaOH, KOH, and NaOH/KOH geopolymers, respectively [33]. Borges et al. explored the application of 3D printing technology to develop heterogeneous catalysts for biodiesel production from waste feedstocks. They loaded 6 wt.% KOH on to aluminum silicate, which served as the support material. The stirred blades of a catalytic stirring reactor were used to shape these materials for the transesterification reaction. The results revealed that a 96% FAME yield was obtained under the conditions of a 24:1 methanol-to-oil molar ratio, a 17% catalyst loading (using three catalyst-stirred blade pieces), and a temperature of 55 °C for 2 h [34]. To produce biodiesel from pretreated sludge palm oil through transesterification, Pongraktham and Somnuk investigated the combination of CaO and ABS plastic. To create the CaO/acrylonitrile butadiene styrene (ABS) catalytic filaments, ABS pellets were mixed with 15% CaO. The findings indicated that a methyl ester (ME) purity of 96.58% could be achieved under the recommended conditions of a 9:1 methanol-to-oil molar ratio, 75 wt.% catalytic filament loading, and a reaction time of 4 h. Additionally, the biodiesel’s purity remained above 95% even after four reuse cycles with these CaO catalytic filaments [35].
However, no studies have been published on the extrusion and 3D printing of acid catalytic filaments from fine Amberlyst-15/ABS particles for FFA reduction through esterification processes. Therefore, this study aims to evaluate the application of catalytic filaments in 3D printing and their potential for reducing FFAs in CPO. The concentration of Amberlyst-15 blended with ABS was investigated to assess its suitability for extrusion and 3D printing, along with an analysis of the resulting filaments’ mechanical, thermal, and structural properties. The catalytic performance of the filaments was evaluated through batch esterification, and optimal conditions for FFA reduction were identified using the response surface methodology (RSM) based on three independent variables: the methanol-to-oil molar ratio, catalyst filament loading, and reaction time. Additionally, the reusability of the catalytic filaments was assessed through multiple cycles of the esterification process. In summary, the current study intends to create Amberlyst-15/ABS catalytic filaments for 3D printing. This acid catalytic filament was used as a catalyst to reduce FFAs in CPO using the esterification process. The material properties of the acid catalytic filaments were tested to investigate their mechanical, thermal, and structural properties. Furthermore, their catalytic performance was evaluated under optimal conditions using the RSM. Finally, the number of reuse cycles for which the catalyst could be used in the batch esterification process was investigated to determine the reusability of the catalytic filaments.
2. Results and Discussion
2.1. Mechanical Properties of Acid Catalytic Filaments
The Amberlyst-15/ABS catalytic filaments were produced using a single-screw filament extruder and a combination of fine ABS and Amberlyst-15 particles. In this study, the acid catalytic filaments were created by mixing Amberlyst-15 particles with ABS particles at concentrations ranging from 3 to 6 wt.%. The mechanical properties and catalytic activity of the Amberlyst-15/ABS catalytic filaments are interrelated through the composition and structural integrity of the composite. In general, increasing the concentration of Amberlyst-15 particles enhances the number of available acidic sites, which can promote catalytic activity. As a result, using ABS plastic with higher acid concentrations will speed up the esterification process. However, the maximum concentration of Amberlyst-15 was limited to 5 wt.% due to constraints in the extrusion process. Higher concentrations of Amberlyst-15 particles tend to reduce the mechanical strength and flexibility of the filaments due to the rigid and brittle nature of the Amberlyst-15 particles, which may interrupt the continuity of the polymer matrix. The extrusion test revealed that blending 6 wt.% Amberlyst-15 particles was unsuccessful, as the resulting filaments were more brittle and continuous filaments could not be produced. Although increasing the concentration of Amberlyst-15 particles could enhance catalytic activity, it also compromises the mechanical performance of the catalytic filaments. Therefore, to successfully produce Amberlyst-15/ABS catalytic filaments using an extruder, the Amberlyst-15 concentration should be limited to 5 wt.%. The tensile strength test results of the 3D-printed objects, the ABS filaments and 5 wt.% Amberlyst-15/ABS catalytic filaments, were compared (Figure S1a). The pure ABS filaments exhibited a tensile strength of 45.6 MPa, while the Amberlyst-15/ABS catalytic filaments featured a tensile strength of 32.7 MPa. Consequently, the tensile strength of the Amberlyst-15/ABS catalytic filaments was 28.3% lower than that of the ABS filaments. The reduced tensile strength of the Amberlyst-15/ABS catalytic filaments may be attributed to the weakened adhesion between the pure ABS plastic structures, likely caused by the inconsistent arrangement of fine Amberlyst-15 particles in the catalytic filament. Moreover, the layer-by-layer 3D printing process may contribute to these filaments’ reduced mechanical properties due to specimen shrinkage during printing [36]. The elongation at break for ABS and the Amberlyst-15/ABS catalytic filament is illustrated in Figure S1a. The elongation at break of the Amberlyst-15/ABS catalytic filament was 88.52% lower than that of the ABS filament, indicating increased brittleness. This result suggests that the Amberlyst-15/ABS catalytic filament loses its flexibility and capacity for elongation before breaking. The addition of fine Amberlyst-15 particles reduced the tensile strength and elongation at break of the composite [37]. Figure S1b compares the compressive strength of the Amberlyst-15/ABS catalytic filaments and ABS filament. The ABS filament had a maximum compressive strength of 41.0 MPa, while the Amberlyst-15/ABS catalytic filament featured a maximum compressive strength of 23.5 MPa. The compressive strength of the Amberlyst-15/ABS catalytic filament was 42.7% lower than that of the pure ABS filament. The addition of fine Amberlyst-15 particles to the catalytic filaments may reduce the density and increase brittleness, resulting in a lower compressive strength. Figure S1c compares the flexural strength and flexural strain at break between the Amberlyst-15/ABS catalytic filament and the ABS filament. The ABS filament had a maximum flexural strength of 31.7 MPa, while the Amberlyst-15/ABS catalytic filament exhibited a maximum flexural strength of 47.2 MPa. The flexural strength of the Amberlyst-15/ABS catalytic filament was 48.9% higher than that of the ABS filaments. These results indicate that the addition of fine Amberlyst-15 enhanced the strength of the catalytic filament during flexural strength testing. The even distribution of Amberlyst-15 particles within the filament improved its resistance to bending forces applied perpendicular to the specimen layers. Additionally, the horizontal arrangement of the layers during the 3D printing process contributed to improved vertical flexural strength, as the vertical force was evenly distributed across all layers. The flexural strain at break of the Amberlyst-15/ABS catalytic filament was 42.0% lower than that of the ABS filament. These results demonstrated that the addition of fine Amberlyst-15 particles made the composites more brittle and reduced their flexural resistance, which is consistent with the observed decrease in elongation at break. The ABS filament exhibited a hardness of 69.8, while the Amberlyst-15/ABS catalytic filament exhibited a slightly lower hardness of 68.4 (Figure S1d). The hardness of the Amberlyst-15/ABS catalytic filament was 2% lower than that of the ABS filament. The addition of fine Amberlyst-15 particles to the ABS plastic did not considerably affect the hardness of the catalytic filament.
2.2. Characterization of Acid Catalytic Filaments
The surface area of the catalytic filaments was analyzed using a surface area and porosity analyzer. The Amberlyst-15 catalytic filament had a BET surface area of 0.9726 m2/g and an average adsorption pore diameter of 18.69 nm. These results suggest that the catalytic filament is suitable for catalyzing heterogeneous mixtures using a stirring method [38]. Additionally, the distribution of chemical elements within the catalytic filaments was examined through scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) analyses to investigate the distribution of Amberlyst-15 particles within the catalytic filaments after extrusion [39]. Figure 1 shows the distribution of chemical elements and the EDS spectra on the surface of a catalytic filament at 100× magnification. The mapping analysis was performed through the scanning of the sample surface with an electron beam to obtain images. These images revealed the distribution characteristics of the chemical elements within the analyzed area. The filament surface contained sulfur, carbon, and oxygen in specific regions corresponding to the chemical composition of the Amberlyst-15 catalyst. Sulfur is a major component of Amberlyst-15 due to the presence of sulfonic acid (–SO3H) functional groups. Figure 1b clearly illustrates the presence of sulfur, with a uniform distribution of sulfur across the filament surface shown. The EDS spectrum (Figure 1e) showed that Amberlyst-15 was added to the ABS matrix. Sulfur, which was not present in pure ABS, was found at a weight percentage of 1.4 wt.%. The detected carbon mainly originated from the ABS polymer, while the oxygen content was attributed to the sulfonic acid functional groups in the Amberlyst-15 structure and minor oxygenated components in the ABS. The presence of carbon (92.4 wt.%), oxygen (6.3 wt.%), and sulfur (1.4 wt.%) was consistent with the expected elemental composition of Amberlyst-15 blended into the ABS matrix. Therefore, the elemental mapping and EDS spectra provided clear evidence of Amberlyst-15 particle incorporation into the ABS matrix.
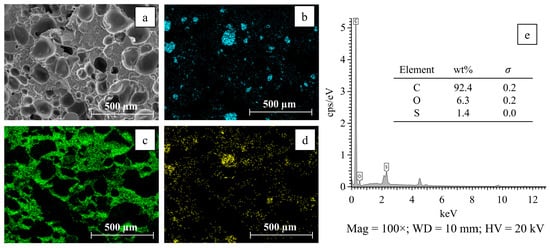
Figure 1.
Characterization of 5 wt.% Amberlyst-15/ABS catalytic filaments: (a) surface morphology, (b) sulfur distribution, (c) carbon distribution, (d) oxygen distribution, and (e) EDS spectra.
2.3. Thermal Stability of Acid Catalytic Filaments
Figure 2 presents the thermogravimetric (TGA) and derivative thermogravimetric (DTG) analysis profiles of the ABS and Amberlyst-15/ABS catalytic filaments, providing insight into their thermal decomposition behavior. The TGA analysis of the pristine ABS filament revealed a two-stage thermal degradation behavior [40]. The initial decomposition occurred in the temperature range of approximately 255–420 °C and was attributed to the thermal degradation of the polybutadiene phase and the partial decomposition of the styrene–acrylonitrile copolymer [41]. The second stage, observed between approximately 420 and 485 °C, corresponds to the decomposition of the remaining styrene–acrylonitrile copolymer matrix [41]. However, the Amberlyst-15/ABS catalytic filament exhibited a more complex thermal degradation behavior consisting of five stages. The first stage occurred between approximately 255 and 405 °C, resulting in the decomposition of the ABS matrix and the sulfonic acid groups in Amberlyst-15 [42]. The second stage, the decomposition of the crosslinked sulfonate polymer backbone in the Amberlyst-15, occurred at approximately 410–445 °C [42]. The third and fourth stages occurred within the temperature ranges of approximately 445–480 °C and 480–540 °C, respectively, indicating the continued breakdown of the composite components. Finally, a continuous mass loss was observed at temperatures above 540 °C. To assess the suitability of the catalytic filament for 3D printing, the initial temperature at which 1% weight loss occurred was determined through TGA analysis to identify the initial degradation temperature of the material [43]. We observed a 1% weight loss (Td, 1%) from the ABS at approximately 274 °C and from the catalytic filament at 264 °C. Consequently, the blending of Amberlyst-15 particles resulted in a slight reduction in initial heat stability. This means that the minor reduction in degradation temperature may be attributed to the presence of Amberlyst-15, which could potentially accelerate the thermal degradation of the polymer matrix. However, the catalytic filaments retained more than 90% of their weight up to 380 °C, which confirmed that the catalytic filaments remained thermally stable within the operating temperature range for extrusion and 3D printing. These results suggest that the catalytic filament can withstand temperatures exceeding 260 °C during extrusion and 3D printing without significant weight loss or a degradation in quality [44]. The glass transition temperature of the pure ABS filament was measured to be 107 °C, consistent with the findings reported by Hart et al. [45]. The Amberlyst-15/ABS catalytic filament exhibited a slightly lower glass transition temperature of 105 °C compared to the other filaments, likely due to the dispersion of fine Amberlyst-15 particles in the ABS plastic matrix. The reduction in the glass transition temperature and heat resistance of ABS may result from the Amberlyst-15’s dispersion, which weakened the intermolecular adhesion of the plastic matrix [46]. This glass transition temperature result was used to set the platform temperature to above 105 °C, thereby ensuring proper adhesion of the printed materials and minimizing warping during the 3D printing process. Therefore, a platform temperature of 110 °C was selected to achieve an optimal 3D printing performance [47].
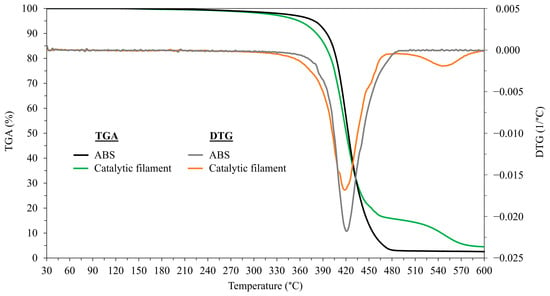
Figure 2.
Thermogravimetric (TGA) and derivative thermogravimetric (DTG) analysis profiles of ABS and Amberlyst-15/ABS catalytic filaments.
2.4. Experimental Results of FFA Reduction in CPO Using Acid Catalytic Filaments
2.4.1. Experimental Results and Statistical Analysis
The results of the reduction in the FFAs in CPO when using Amberlyst-15/ABS catalytic filaments in the esterification process are shown in Table 1. According to the 18 experimental trials presented in Table 1, the residual FFA content in CPO decreased from an initial FFA content of 10.05 wt.% to a range of 0.25–4.34 wt.%. A multiple regression analysis was conducted to examine the relationship between the independent and dependent variables, with a 95% confidence level used. Moreover, a predictive model was developed to accurately forecast FFA reduction conditions through a comprehensive analysis of the statistical data. Table 2 presents the analysis of variance for the models predicting the influence of factors on FFA reduction in CPO. The statistical significance of each coefficient (β) in the prediction model was evaluated using the probability of error (p-value). A p-value below 0.05 indicated that the variable considerably influences the model’s prediction, with smaller p-values reflecting a stronger impact on FFA reduction. Conversely, variables with p-values greater than 0.05 were considered insignificant and excluded from the model. The final relationship between FFAs and the independent variables is expressed in Equation (1). In the relationship prediction analysis, the coefficients β1M and β6M2 exhibited the lowest p-values, indicating that the methanol-to-oil molar ratio had the greatest influence on FFA reduction in CPO during the esterification process. The catalytic filament loading, represented by β2C and β7C2, exhibited the third and fourth strongest impacts on FFA reduction, followed by the reaction time, indicated by β3T. The predictive accuracy of the model was assessed using statistical indicators. The coefficient of multiple determination (R2) and the adjusted R2 were 0.998 and 0.997, respectively, confirming the model’s high predictive accuracy. An F-test was conducted to assess the hypothesis and the significance of the variability among the samples. The F-test yielded an F0 value of 707.38, which exceeded the critical F value of 3.23 (F0.05,8,9). Therefore, the model is statistically significantly able to predict the reduction in the FFAs in CPO during the esterification process. Figure S2 shows the relationship between the predicted and actual experimental FFA values for Amberlyst-15/ABS catalytic filaments in the esterification process. The graphs and statistical analysis confirm that the model is highly accurate and effective at predicting FFA values and determining optimal conditions.
where FFA represents the free fatty acid content in esterified oil from CPO (ECPO, wt.%), M denotes the methanol-to-oil molar ratio, C indicates the Amberlyst-15/ABS catalytic filament loading (wt.%), and T signifies the reaction time (h).

Table 1.
Design of experiment, coded factor levels, and results of the esterification process using catalytic filaments.

Table 2.
Coefficients and statistical analysis of the variance in the predictive model.
2.4.2. Influence of Independent Variables on FFA Reduction
The relationship between the dependent variable (the FFA content in CPO) and the independent variables (methanol-to-oil molar ratio, catalytic filament loading, and reaction time) is illustrated in the 3D response surface plots in Figure 3. Statistical analysis revealed that the methanol content and catalytic filament loading were the most important factors influencing the esterification process. Figure 3a shows the response surface plot showing the interaction between the methanol-to-oil molar ratio and catalytic filament loading on the FFA content in CPO. An increase in methanol content and catalytic filament loading accelerated the esterification reaction, leading to a significant reduction in the FFA content. Additionally, a higher methanol content reduced the oil viscosity and increased the contact surface area, thereby enhancing mass transfer during the blending process [48]. At a methanol-to-oil molar ratio of 21.5:1–42.5:1 and with a catalytic filament loading range of 66.0–130.5 wt.%, the FFA content in CPO decreased to less than 1 wt.%. However, lower methanol concentrations may reduce FFA conversion. Conversely, an excessively high methanol concentration diluted the mixture of methanol, oil, and filaments, leading to a slower reaction rate [49,50]. Binnal et al. reported similar results, which indicated a decrease in the esterification reaction rate with higher methanol concentrations [51]. An increase in methanol concentration in the mixture can lead to a decrease in FFA conversion due to a reverse reaction occurring during the esterification process. Moreover, the loading of catalytic filaments must be carefully balanced with the methanol content. A high density of catalytic filaments in the mixture can hinder agitation, thereby reducing catalytic activity. This interplay highlights the need to optimize both parameters for efficient FFA reduction [52]. Figure 3b shows the response surface plot illustrating the influence of the reaction time and catalytic filament loading on the FFA content in CPO. The experimental results reveal that a reaction time of 12.5–37.5 h and a catalytic filament loading of 52.0–130.5 wt.% effectively reduced the FFA content in CPO to below 1 wt.%. The significant decrease in FFA content with increasing catalytic filament loading can be attributed to the additional active surface area provided by the filaments, which enhances reaction activity [16]. Furthermore, the sulfonic acid groups on the Amberlyst-15 within the catalytic filaments facilitated ion exchange, thereby aiding the esterification process [53]. Although the reaction time is less significant compared with the methanol content and catalytic filament loading, the statistical analysis still indicated its importance in the esterification process. The 3D response surface plots confirmed that reaction time is a critical parameter for achieving FFA reduction. Figure 3c illustrates the combined influence of the reaction time and the methanol-to-oil molar ratio on the FFA content. The experimental results revealed that the FFA level could be reduced to below 1% as the reaction time ranged from 18.0 to 37.5 h and the methanol-to-oil molar ratio varied between 21.0:1 and 42.5:1. Achieving an optimal balance between the methanol concentration and reaction time enhanced mass transfer within the mixture, thereby accelerating FFA conversion during the esterification process [48,54]. A longer reaction time can improve the mixing of CPO, methanol, and catalytic filaments, thereby enhancing the esterification process [54]. However, prolonged reaction times increase energy consumption. The recommended conditions identified in this experiment optimized the batch esterification process through balancing reaction efficiency and resource usage, thereby effectively reducing chemical and energy consumption.
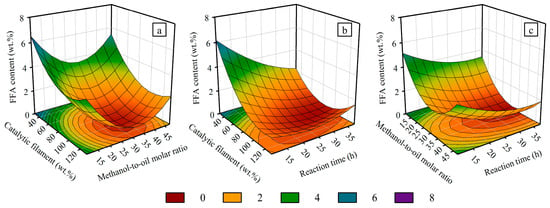
Figure 3.
Three-dimensional response surface and contour plots illustrating the influence of three variables on the FFA content in ECPO: (a) methanol-to-oil molar ratio and catalytic filament loading, (b) reaction time and catalytic filament loading, and (c) reaction time and methanol-to-oil molar ratio.
2.4.3. Optimal Conditions for the Reduction of the FFAs in CPO
The optimal conditions for reducing the FFA in CPO using Amberlyst-15/ABS catalytic filaments were determined based on the model in Equation (1). The predicted model was optimized using Excel Solver. Under the conditions of a 31.9:1 methanol-to-oil molar ratio, 93.0 wt.% catalytic filament loading, and a 30.7 h reaction time with the stirrer at 500 rpm and a 60 °C reaction temperature, the lowest achievable FFA content was 0.07 wt.%. However, achieving the lowest FFA content required considerable chemical usage and an extended reaction time, leading to higher operating costs. Consequently, the operating time and chemical costs increased under these optimal conditions. Therefore, targeting an FFA level of 1 wt.% was recommended, as this level was suitable for the subsequent second step of the transesterification process [12,16]. To determine the new optimal conditions for the 1 wt.% FFA level, this value was substituted into Equation (1). The three parameters in the model equation were recalculated using Excel Solver. The recommended conditions for achieving the target FFA level were a 26.7:1 methanol-to-oil molar ratio, 78.5 wt.% catalytic filament loading, and a 20.2 h reaction time. Under these recommended conditions, the consumption of methanol, catalytic filament loading, and reaction time decreased by 16.3%, 15.6%, and 34.2%, respectively. These conditions were validated through actual experiments to confirm the accuracy and reliability of the FFA content predictions from the model. The results revealed that the FFA content decreased to 0.09 wt.% under the optimal conditions and 0.83 wt.% under the recommended conditions. The properties and composition of ECPO from the optimal and recommended conditions are summarized in Table 3.

Table 3.
Properties and compositions of CPO and ECPO.
2.4.4. Reusability of Acid Catalytic Filaments
The reusability of the Amberlyst-15/ABS catalytic filaments for FFA reduction in CPO was evaluated by conducting the esterification process under the recommended conditions. After each reaction cycle, the catalytic filaments were recovered by washing them with methanol to remove any residual oil from the catalyst surface before proceeding to the next cycle. To assess the catalytic activity of the recovered filaments, the FFA content in the ECPO was measured during each reaction cycle (Figure S3). The results revealed an increase in FFA content remaining with each reuse of the catalytic filaments, with FFA contents of 0.86, 1.75, and 3.58 wt.% achieved during the first, second, and third cycles, respectively. The detachment of Amberlyst-15 particles from the catalytic filaments was evaluated through the measurement of the catalyst’s weight loss after each esterification cycle. In this study, the catalyst particles were separated from the esterified oil using a centrifuge, and then dried and weighed. The recovered weight was then compared with the initial loading of the fresh filaments. The observed weight losses were 3.66%, 5.19%, and 3.57% for the first, second, and third cycles, respectively. These results indicate the physical detachment of the Amberlyst-15 particles embedded within the ABS matrix of the catalytic filaments. This detachment likely led to a reduction in the number of available active sites, which corresponds with the observed decline in catalytic activity during repeated use. Therefore, the weight loss measurements of the catalyst provide supporting evidence of the occurrence of particle separation during the reusability tests. However, the reused catalytic filaments could reduce the FFA content in the oil. After the second cycle, the FFA content in the ECPO was 1.75 wt.%. Because the FFA level in the raw material was below 2 wt.%, the conversion of FFA to ester was more efficient. However, at an FFA level below 2 wt.%, the product exhibited a higher level of soap formation than the raw material [12,16]. These results indicate that Amberlyst-15/ABS catalytic filaments can be reused for at least two cycles in the batch esterification process, thereby effectively reducing the FFA content in ECPO to below 2 wt.% and ensuring the cost-effective use of chemicals. To create Amberlyst-15/ABS catalytic filaments with over 5 wt.% of Amberlyst-15 particles, this study recommends optimizing the filament extrusion process to enhance particle dispersion, melting, and material flow. These factors influence the efficiency and consistency of catalytic filament production. Optimizing these parameters could increase the concentration of Amberlyst-15 particles in the catalytic filaments to over 5 wt.%, enabling the filaments’ reuse in additional reaction cycles.
3. Materials and Methods
3.1. Materials
Amberlyst-15, a cation-exchange resin with sulfonic acid functionality, is used as a heterogeneous catalyst in chemical reactions. Amberlyst-15 (Thermo Scientific Chemicals, Fair Lawn, NJ, USA) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and reduced to a smaller particle size. The solid acid catalyst was then combined with ABS pellets (LG ABS HP171, LG Chem, Huizhou, China) to produce acid catalytic filaments for 3D printing. The CPO used in the study, characterized by a high FFA content, was obtained from a small-scale palm oil mill in southern Thailand. The CPO comprised 10.05 wt.% FFA, 81.78 wt.% triglycerides (TGs), 7.92 wt.% diglycerides (DGs), and 0.25 wt.% monoglycerides (MGs). The esterification reaction was conducted using commercial methanol with a 99.7% purity. The properties of the CPO and the products obtained after the esterification process are summarized in Table 3.
3.2. Preparation of Acid Catalytic Filaments
Figure S4 illustrates the preparation process for acid catalytic filaments. Amberlyst-15 was first ground using a grinder (GM-800S1, OrmiSmart, Shenzhen, China) and then passed through a 100-mesh sieve (0.1 mm) to obtain fine Amberlyst-15 particles. These particles were dried at 80 °C for 8 h to remove any residual moisture. Similarly, the ABS plastic pellets were ground and passed through an 18-mesh sieve (1.0 mm) to produce fine ABS particles, which were then combined with the fine Amberlyst-15 particles. The fine ABS particles were dried at 80 °C for 8 h to ensure moisture removal. The acid catalytic filaments were formed through the combination of Amberlyst-15 particles with ABS particles at concentrations ranging from 3 to 6 wt.%. The Amberlyst-15/ABS particles were passed through a single-screw filament extruder (Desktop extruder SJ20, RobotDigg, Shanghai, China) to produce acid catalytic filaments. To ensure optimal extrusion conditions, the outlet water temperature was maintained below 45 °C. The extruder was configured with a 1.75 mm nozzle diameter and a 12.5 rpm screw speed, enabling the production of Amberlyst-15/ABS catalytic filaments at a rate of 0.18 kg/h. A digital temperature controller (AK6, WINPARK, Guangzhou, China) was used to keep the extruder jacket zone at a constant temperature of 185 °C. The diameter of the acid catalytic filaments was measured in real time during the extrusion process using a digital vernier caliper (TDG50 Digital Depth Gauge, Bartec USA, Spartanburg, SC, USA). The acid catalytic filaments were stored in a zip-lock plastic bag to prevent moisture adsorption, ensuring their suitability for further use in the esterification process after their extrusion. The FDM method was used to 3D print the acid catalytic filaments layer by layer and fabricate test specimens. The 3D printing conditions included a 100% infill density, a 0.4 mm layer height, a 0.8 mm nozzle diameter, a 245 °C nozzle temperature, a 110 °C platform temperature, and a base printing speed of 14 mm/s.
3.3. Analysis of the Characteristics of the Acid Catalytic Filaments
The stability and thermal behavior of the composite materials were analyzed using a thermogravimetric analyzer (TGA/DSC3+, Mettler Toledo, Greifensee, Switzerland). Thermogravimetric analysis (TGA) and differential scanning calorimetry were performed on the catalytic filaments [43]. The analysis was conducted over a temperature range of 30–600 °C, with a heating rate of 10 °C/min and a N2 flow rate of 50 mL/min. The distribution of Amberlyst-15 in the catalytic filaments was examined via scanning electron microscopy (SEM, SU3900, Hitachi, Tokyo, Japan). The chemical composition of the catalytic filaments was analyzed via energy-dispersive X-ray spectroscopy (EDS) coupled with SEM. Additionally, the surface area and porosity of the acid catalytic filaments were investigated through the Brunauer–Emmett–Teller (BET) method using a surface area and porosity analyzer (ASAP2460, Micromeritics, Norcross, GA, USA). The BET analysis was conducted at a constant temperature of −196.85 °C using static volumetric N2 gas adsorption.
3.4. Analysis of the Mechanical Properties of the Acid Catalytic Filaments
This study focuses on the development of 3D-printed Amberlyst-15/ABS catalytic filaments, in which their mechanical performance is essential for successful extrusion and printing. Mechanical testing was conducted to identify the optimal balance between catalytic activity and filament processability, ensuring that the filaments possessed adequate tensile, compressive, flexural, and hardness properties for extrusion and 3D printing. The specimens used for the mechanical property analysis were created through the 3D printing of the acid catalytic filaments layer by layer using the FDM method. Various mechanical tests, including tensile, flexural, compressive, and hardness tests, were performed to evaluate the properties of the acid catalytic filaments. The specimen used for tensile testing was designed and printed according to ASTM D638 Type V standards (Figure 4a) [55]. The dimensions of the specimens included a total length of 63.5 mm, a gauge length of 9.53 mm, a gauge width of 3.18 mm, an end width of 9.53 mm, a thickness of 3.4 mm, and a fillet radius of 12.7 mm between the gauge section and the wider ends. Tensile tests were conducted using a universal testing machine (UTM, ZwickRoell 2005, Ulm, Germany) with a 1000 N load cell at a pulling speed of 1 mm/min. Flexural tests were conducted using the UTM but with a 2500 N load cell, according to ASTM D790 standards [56]. A rectangular specimen 50.8 mm in length, 12.7 mm in width, and 1.6 mm in thickness was used for the flexural test (Figure 4b) [57]. The test was performed at a speed of 5 mm/min with a support span of 25.4 mm. Compression tests were performed according to ASTM D695 standards [58], using an UTM (Z010, ZwickRoell, Ulm, Germany) equipped with a 10 kN load cell at a test speed of 1.3 mm/min. A cylindrical specimen with a diameter of 12.7 mm and a length of 25.4 mm was used for this evaluation (Figure 4c) [36]. The hardness of the specimens was measured using a hardness tester (Digitest II, Bareiss, Oberdischingen, Germany), according to the ASTM D2240 standard [59], at a temperature of 23 ± 2 °C and a relative humidity of 50 ± 5%. A square specimen 50 mm in length, 50 mm in width, and 6.4 mm in thickness was used for the hardness test (Figure 4d) [60]. Five repetitions of each mechanical property test were conducted, and the average values and standard deviations were calculated to ensure accuracy.
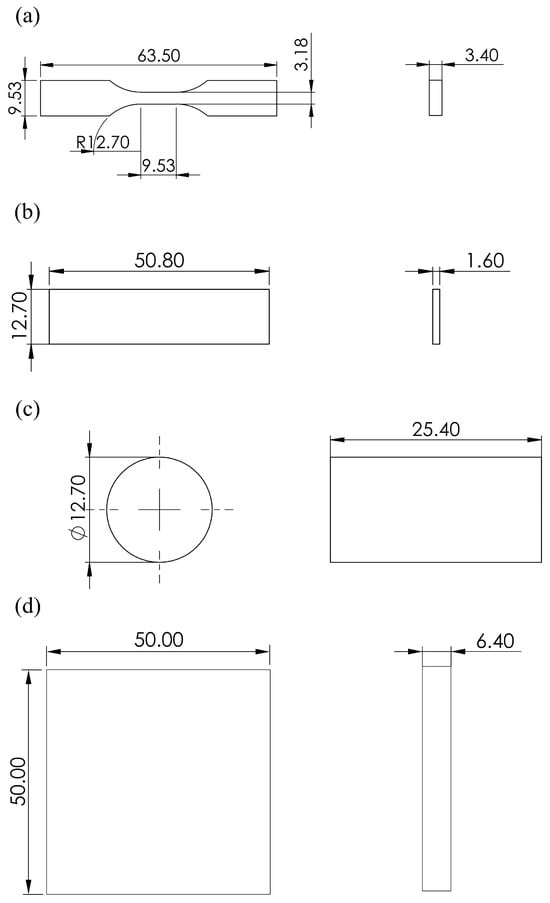
Figure 4.
Drawings and dimensions (in millimeters) of the 3D-printed specimens used for (a) tensile testing, (b) compressive testing, (c) flexural testing, and (d) hardness testing.
3.5. FFA Reduction in CPO Using Acid Catalytic Filaments
3.5.1. Experimental Design
The optimal conditions for FFA reduction in high-FFA CPO were determined using the RSM with the multiple regression tool in Excel. The experimental design followed a circumscribed central composite design (CCD), where each factor was assigned rotatable levels encoded as –α, –1, 0, +1, and +α. In this setup, –α and +α represent the star points at the minimum and maximum values of each parameter, respectively. The star points (αx) of the rotated CCD were positioned at a distance α from the center, calculated based on the number of factors (k) in the experimental design. This study examined the effects of three independent variables—the methanol-to-oil molar ratio, catalytic filament loading, and reaction time—on FFA reduction in CPO. The star point (αx) was calculated as 1.682 through the substitution of the number of factors (k = 3) into Equation (2). Consequently, five levels were established for each variable, with coded factor levels of –1.682, –1, 0, +1, and +1.682. This setup resulted in a total of 18 experimental conditions (Table 1). The parameter ranges investigated in the esterification process included a methanol-to-oil molar ratio of 10.5–47.5 g/mol, catalytic filament loading of 29.5–130.5 wt.%, and reaction time of 10.5–37.5 h. After the experiments, the FFA content in CPO under these various conditions was modeled through multiple regression analysis. A second-degree polynomial (Equation (3)) was developed to identify the optimal conditions for FFA reduction.
where Y represents the response variable, xi and xj denote the factors, β0 indicates the constant coefficient, βi represents the linear terms, βii denotes the quadratic term, βij signifies the interaction term, k denotes the number of factors, and ε signifies the prediction error.
3.5.2. Experimental Procedures
The influence of key parameters—the methanol-to-oil molar ratio, acid catalytic filament loading, and reaction time—was investigated to evaluate the reduction in the FFAs in CPO through the esterification process. The loading potential of the Amberlyst-15/ABS catalytic filaments as acid catalysts in the batch esterification reaction was assessed. The acid catalyst concentrations were adjusted through the segmentation of the long catalytic filaments into shorter segments with an average length of 5.0 mm, a diameter of 1.8 mm, and a weight of 5.53 mg. For the FFA reduction experiments, 40 g of CPO was placed in a 400 mL reactor, heated to 60 °C, and stirred at 500 rpm using a magnetic stirrer (RCT basic, IKA, Staufen, Germany). The oil temperature was continuously monitored using a digital thermometer (Fluke 51 II, Fluke Corporation, Everett, WA, USA). The methanol concentration and the catalytic filament loading (Table 1) were the key chemical parameters studied. Moreover, the reaction time was varied to examine its effect on FFA reduction in CPO. The timing of the experiment was initiated upon the addition of the catalytic filament to the reactor. Table 1 provides the variation in these three parameters over the different experiments conducted. To minimize methanol evaporation, the reactor was covered with aluminum foil after the addition of methanol and catalytic filament. Upon completion of the esterification process, the samples were filtered through a mesh to remove the catalytic filaments, yielding ECPO. The crude ECPO in the sample bottle was rapidly cooled through immersion in cold water to prevent any potential forward or backward reactions. The sample was then washed with water to remove residual methanol and impurities until the wastewater pH reached a neutral value of 7. After washing, the ECPO was dried at 105 °C to eliminate any remaining moisture. For the analysis of the oil composition, the weight percentages of ME, TGs, FFAs, DGs, and MGs in the ECPO were determined via thin-layer chromatography coupled with flame ionization detection.
3.5.3. Reusability of Catalytic Filaments
Heterogeneous catalysts provide considerable benefits, including reusability, which lowers production costs, minimizes treatment requirements, reduces waste disposal, and limits the use of toxic chemicals. This study evaluated the reusability of catalytic filaments in reducing FFA levels in CPO during the esterification process. Fresh catalytic filaments were used in the first cycle under the recommended conditions. After esterification, the catalytic filaments were separated from the esterified oil using a mesh filter. To prepare the filaments for reuse, they were thoroughly cleaned with methanol to remove any residual oil. During the washing procedure, the filaments were agitated with methanol at 300 rpm for 5 min, and the process was repeated twice to ensure thorough cleaning. The filaments were then dried at 80 °C for 6 h to remove any remaining solvent. To evaluate the reusability of the catalytic filaments, the FFA content in the esterified oil after each esterification cycle was measured. To determine the percentage of catalyst weight loss after each esterification cycle, the esterified oil was subjected to centrifugation to separate catalytic filament particles from the oil sample. The recovered catalytic filaments were then dried and weighed. The remaining weight was compared with the initial weight of the fresh catalytic filaments. The percentage of residual catalyst (wt.%) lost to each cycle was calculated by the weight loss of the catalytic filament particles in the esterified oil (g) with respect to the weight of the initial catalytic filament loading (g) and given in terms of 100 wt.% of the initial catalytic filament loading.
4. Conclusions
This study demonstrates the successful fabrication and application of Amberlyst-15/ABS catalytic filaments in reducing FFA in CPO through the esterification process. The catalytic filaments, composed of 5 wt.% Amberlyst-15, were produced using a single-screw filament extruder and exhibited sufficient thermal stability (up to 260 °C) for 3D printing applications. Regarding their mechanical properties, the Amberlyst-15/ABS catalytic filaments exhibited decreases of 28.3%, 88.5%, and 42.7% in tensile strength, elongation at break, and compressive strength, respectively, compared to pure ABS. In contrast, the flexural strength of the catalytic filaments was 48.9% higher than that of ABS, while their flexural strain at break was 42.0% lower. For the batch esterification process, the RSM was employed to determine the optimal conditions for the reduction of the FFAs in CPO using Amberlyst-15/ABS catalytic filaments. Under the recommended conditions—a 26.7:1 methanol-to-oil molar ratio, 78.5 wt.% catalytic filament loading, 20.2 h reaction time, 500 rpm stirring speed, and 60 °C—the FFA content decreased significantly, from 10.05 to 0.83 wt.%. These results confirm the effectiveness of the Amberlyst-15/ABS catalytic filaments in treating high-FFA oils. A future study will use these acid catalytic filaments to fabricate catalytic 3D-printed reactors such as static mixers, packed beds, and catalytic tubular reactors that can be used in the esterification process to reduce FFA levels in oil. Furthermore, the stability and reusability of Amberlyst-15/ABS catalytic reactors should be investigated to determine their efficiency in the esterification process.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15040356/s1; Figure S1. Mechanical properties of ABS and Amberlyst-15/ABS catalytic filaments: (a) tensile strength and elongation at break, (b) compressive strength, (c) flexural strength and flexural strain at break, and (d) hardness; Figure S2. Relationship between predicted and actual experimental FFA values; Figure S3. FFA content in ECPO during the esterification process using fresh and recycled Amberlyst-15/ABS catalytic filaments after the second and third reaction cycles; Figure S4. Preparation of Amberlyst-15/ABS catalytic filaments for 3D printing.
Author Contributions
Conceptualization, methodology, validation, formal analysis, writing—original draft, visualization, J.T.; methodology, investigation, data curation, K.P.; supervision, writing—review and editing, conceptualization, project administration, funding acquisition, K.S. All authors contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Prince of Songkla University.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research was supported by Prince of Songkla University under the Postdoctoral Fellowship Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Line Today. Factors Affecting Oil Demand and Prices. Available online: https://today.line.me/th/v2/article/QwGjj2r (accessed on 10 January 2025).
- U.S. Energy Information Administration. Short-Term Energy Outlook (STEO). Available online: https://www.eia.gov/outlooks/steo/?utm.com (accessed on 10 January 2025).
- Afifa; Arshad, K.; Hussain, N.; Ashraf, M.H.; Saleem, M.Z. Air Pollution and Climate Change as Grand Challenges to Sustainability. Sci. Total Environ. 2024, 928, 172370. [Google Scholar] [CrossRef] [PubMed]
- Monika; Banga, S.; Pathak, V.V. Biodiesel Production from Waste Cooking Oil: A Comprehensive Review on The Application of Heterogenous Catalysts. Energy Nexus. 2023, 10, 100209. [Google Scholar] [CrossRef]
- Ebadian, M.; van Dyk, S.; McMillan, J.D.; Saddler, J. Biofuels Policies that have Encouraged their Production and Use: An International Perspective. Energy Policy 2020, 147, 111906. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A Comprehensive Review on Biodiesel as an Alternative Energy Resource and its Characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Esterification and Transesterification of Waste Cooking Oil over Amberlyst 15 and modified Amberlyst 15 Catalysts. Appl. Catal. B 2015, 165, 723–730. [Google Scholar] [CrossRef]
- O’Malley, J.; Searle, S. Air Quality Impacts of Biodiesel in The United States; ICCT: Washington, DC, USA, 2021; pp. 1–36. [Google Scholar]
- Ogunkunle, O.; Ahmed, N.A. Overview of Biodiesel Combustion in Mitigating the Adverse Impacts of Engine Emissions on The Sustainable Human–Environment Scenario. Sustainability 2021, 13, 5465. [Google Scholar] [CrossRef]
- Reddy, S.N.K.; Wani, M.M. A Comprehensive Review on Effects of Nanoparticles–Antioxidant Additives–Biodiesel Blends on Performance and Emissions of Diesel Engine. Appl. Sci. Eng. Prog. 2020, 13, 285–298. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Fattah, I.M.R.; Sanjid, A. Feasibility of Diesel–Biodiesel–Ethanol/Bioethanol Blend as Existing CI Engine Fuel: An Assessment of Properties, Material Compatibility, Safety and Combustion. Renew. Sustain. Energy Rev. 2014, 32, 379–395. [Google Scholar] [CrossRef]
- Banchero, M.; Gozzelino, G. A Simple Pseudo-Homogeneous Reversible Kinetic Model for the Esterification of Different Fatty Acids with Methanol in the Presence of Amberlyst-15. Energies 2018, 11, 1843. [Google Scholar] [CrossRef]
- Gaurav, A.; Dumas, S.; Mai, C.T.Q.; Ng, F.T.T. A Kinetic Model for a Single Step Biodiesel Production from a High Free Fatty Acid (FFA) Biodiesel Feedstock over a Solid Heteropolyacid Catalyst. Green Energy Environ. 2019, 4, 328–341. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. BioEnergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Litinas, A.; Geivanidis, S.; Faliakis, A.; Courouclis, Y.; Samaras, Z.; Keder, A.; Krasnoholovets, A.; Gandzha, I.; Zabulonov, Y.; Puhach, O.; et al. Biodiesel Production from High FFA Feedstocks with A Novel Chemical Multifunctional Process Intensifier. Biofuel Res. J. 2020, 7, 1170–1177. [Google Scholar] [CrossRef]
- Ridwan, I.; Ghazali, M.; Kusmayadi, A.; Putra, R.D.; Marlina, N.; Andrijanto, E. The Effect of Co-solvent on Esterification of Oleic Acid Using Amberlyst 15 as Solid Acid Catalyst in Biodiesel Production. MATEC Web Conf. 2018, 156, 03002. [Google Scholar] [CrossRef]
- Kasirajan, R. Biodiesel Production by Two Step Process from an Energy Source of Chrysophyllum Albidum Oil Using Homogeneous Catalyst. S. Afr. J. Chem. Eng. 2021, 37, 161–166. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current Developments in Esterification Reaction: A Review on Process and Parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Vitiello, R.; Taddeo, F.; Russo, V.; Turco, R.; Buonerba, A.; Grassi, A.; Di Serio, M.; Tesser, R. Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. ChemEngineering 2021, 5, 46. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Gea, S.; Widati, A.A.; Syukri, S.; Eddiyanto; Wardana, D. Esterification of Palm Fatty Acid Distillate to Methyl Ester Using Amberlyst Catalyst in a Semi-Continuous Reactor. AIP Conf. Proc. 2024, 3026, 030008. [Google Scholar] [CrossRef]
- Makki, T.; Vattathurvalappil, S.H.; Theravalappil, R.; Nazir, A.; Alhajeri, A.; Azeem, M.A.; Mahdi, E.; Ummer, A.C.; Ali, U. 3D and 4D printing: A review of Virgin Polymers Used in Fused Deposition Modeling. Compos. Part C Open Access 2024, 14, 100472. [Google Scholar] [CrossRef]
- Fafenrot, S.; Grimmelsmann, N.; Wortmann, M.; Ehrmann, A. Three-Dimensional (3D) Printing of Polymer-Metal Hybrid Materials by Fused Deposition Modeling. Materials 2017, 10, 1199. [Google Scholar] [CrossRef]
- Iqbal, H.; Fernandes, Q.; Idoudi, S.; Basineni, R.; Billa, N. Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry. Polymers 2024, 16, 386. [Google Scholar] [CrossRef]
- Chadha, U.; Abrol, A.; Vora, N.P.; Tiwari, A.; Shanker, S.K.; Selvaraj, S.K. Performance Evaluation of 3D Printing Technologies: A Review, Recent Advances, Current Challenges, and Future Directions. Prog. Addit. Manuf. 2022, 7, 853–886. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused Deposition Modelling: Current Status, Methodology, Applications and Future Prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Puttegowda, M.; Rangappa, S.M.; Alexey, K.; Gorbatyuk, S.; Khan, A.; Doddamani, M.; Siengchin, S. A Comprehensive Review on 3D Printing Advancements in Polymer Composites: Technologies, Materials, and Applications. Int. J. Adv. Manuf. Technol. 2022, 121, 127–169. [Google Scholar] [CrossRef]
- Kichloo, A.F.; Raina, A.; Haq, M.I.U.; Wani, M.S. Impact of Carbon Fiber Reinforcement on Mechanical and Tribological Behavior of 3D-Printed Polyethylene Terephthalate Glycol Polymer Composites—An Experimental Investigation. J. Mater. Eng. Perform. 2022, 31, 1021–1038. [Google Scholar] [CrossRef]
- Wissamitanan, T.; Dechwayukul, C.; Kalkornsurapranee, E.; Thongruang, W. Proper Blends of Biodegradable Polycaprolactone and Natural Rubber for 3D Printing. Polymers 2020, 12, 2416. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.; Zhang, L.; Gao, Y.; Xuan, F. Strain Sensing Behavior of FDM 3D Printed Carbon Black Filled TPU with Periodic Configurations and Flexible Substrates. J. Manuf. Process. 2022, 74, 283–295. [Google Scholar] [CrossRef]
- Osman, M.A.; Atia, M.R.A. Investigation of ABS-Rice Straw Composite Feedstock Filament for FDM. Rapid Prototyp. J. 2018, 24, 1067–1075. [Google Scholar] [CrossRef]
- Kottasamy, A.; Samykano, M.; Kadirgama, K.; Rahman, M.; Noor, M.M. Experimental Investigation and Prediction Model for Mechanical Properties of Copper-Reinforced Polylactic Acid Composites (Cu-PLA) Using FDM-Based 3D Printing Technique. Int. J. Adv. Manuf. Technol. 2022, 119, 5211–5232. [Google Scholar] [CrossRef]
- Botti, R.F.; Innocentini, M.D.M.; Faleiros, T.A.; Mello, M.F.; Flumignan, D.L.; Santos, L.K.; Franchin, G.; Colombo, P. Additively Manufactured Geopolymer Structured Heterogeneous Catalysts for Biodiesel Production. Appl. Mater. Today 2021, 23, 101022. [Google Scholar] [CrossRef]
- Borges, M.E.; Hernández, L.; Ruiz-Morales, J.C.; Martín-Zarza, P.F.; Fierro, J.L.G.; Esparza, P. Use of 3D Printing for Biofuel Production: Efficient Catalyst for Sustainable Biodiesel Production from Wastes. Clean Technol. Environ. Policy 2017, 19, 2113–2127. [Google Scholar] [CrossRef]
- Pongraktham, K.; Somnuk, K. Heterogeneous Calcium Oxide Catalytic Filaments for Three-Dimensional Printing: Preparation, Characterization, and Use in Methyl Ester Production. ACS Omega 2024, 9, 27578–27591. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, O.; Cong, W.; Bediako, E.; Aladesanmi, V. Additive Manufacturing of Carbon Fiber Reinforced Plastic Composites: The Effect of Fiber Content on Compressive Properties. J. Compos. Sci. 2021, 5, 325. [Google Scholar] [CrossRef]
- Revert, A.; Reig, M.; Seguí, V.J.; Boronat, T.; Fombuena, V.; Balart, R. Upgrading Brewer’s Spent Grain as Functional Filler in Polypropylene Matrix. Polym. Compos. 2017, 38, 40–47. [Google Scholar] [CrossRef]
- Das, S.; Anal, J.M.H.; Kalita, P.; Saikia, L.; Rokhum, S.L. Process Optimization of Biodiesel Production Using Waste Snail Shell as A Highly Active Nanocatalyst. Int. J. Energy Res. 2023, 2023, 6676844. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Gong, Z.; Han, K.; Wang, Y.; Lu, C. Wollastonite Decorated with Calcium Oxide as Heterogeneous Transesterification Catalyst for Biodiesel Production: Optimized by Response Surface Methodology. Renew. Energy 2020, 159, 873–884. [Google Scholar] [CrossRef]
- Dul, S.; Fambri, L.; Pegoretti, A. Filaments Production and Fused Deposition Modelling of ABS/Carbon Nanotubes Composites. Nanomaterials 2018, 8, 49. [Google Scholar] [CrossRef]
- Dhandapani, A.; Krishnasamy, S.; Rajini, N.; Siengchin, S.; Senthil Muthu Kumar, T.; Chandrasekar, M.; Yorseng, K. Thermal and Tensile Properties of 3D Printed ABS–Glass Fibre, ABS–Glass Fibre–Carbon Fibre Hybrid Composites Made by Novel Hybrid Manufacturing Technique. J. Thermoplast. Compos. Mater. 2023, 37, 206–225. [Google Scholar] [CrossRef]
- Fan, G.; Liao, C.; Fang, T.; Luo, S.; Song, G. Amberlyst 15 as a New and Reusable Catalyst for the Conversion of Cellulose into Cellulose Acetate. Carbohydr. Polym. 2014, 112, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Narlıoğlu, N.; Salan, T.; Alma, M.H. Properties of 3D-Printed Wood Sawdust-Reinforced PLA Composites. BioResources 2021, 16, 5467–5480. [Google Scholar]
- Kumar, S.; Ramesh, M.R.; Doddamani, M.S.; Rangappa, M.; Siengchin, S. Mechanical Characterization of 3D Printed MWCNTs/HDPE Nanocomposites. Polym. Test 2022, 114, 107703. [Google Scholar] [CrossRef]
- Hart, K.R.; Dunn, R.M.; Sietins, J.M.; Hofmeister Mock, C.M.; Mackay, M.E.; Wetzel, E.D. Increased Fracture Toughness of Additively Manufactured Amorphous Thermoplastics via Thermal Annealing. Polymer 2018, 144, 192–204. [Google Scholar] [CrossRef]
- Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L.; Balart, R.; Garcia-Garcia, D.; Dominici, F.; Puglia, D.; Torre, L. Development of Biodegradable PLA Composites and Tangerine Peel Flour with Improve Toughness Containing a Natural-Based Terpenoid. Express Polym. Lett. 2023, 17, 789–805. [Google Scholar] [CrossRef]
- Çantı, E.; Aydin, M. Effects of Micro Particle Reinforcement on Mechanical Properties of 3D Printed Parts. Rapid Prototyp. J. 2018, 24, 171–176. [Google Scholar] [CrossRef]
- Chueluecha, N.; Kaewchada, A.; Jaree, A. Biodiesel Synthesis Using Heterogeneous Catalyst in A Packed-Microchannel. Energy Conv. Manag. 2017, 141, 145–154. [Google Scholar] [CrossRef]
- Hykkerud, A.; Marchetti, J.M. Esterification of Oleic Acid with Ethanol in The Presence of Amberlyst-15. Biomass Bioenergy 2016, 95, 340–343. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ma, X.; Guo, M.; Li, Y.; Li, G.; Cui, P.; Zhou, S.; Yu, M. Synthesis of CaO/ZrO2 Based Catalyst by Using UiO–66(Zr) and Calcium Acetate for Biodiesel Production. Renew. Energy 2022, 185, 970–977. [Google Scholar] [CrossRef]
- Binnal, P.; Amruth, A.; Basawaraj, M.P.; Chethan, T.S.; Murthy, K.R.S.; Rajashekhara, S. Microwave-Assisted Esterification and Transesterification of Dairy Scum Oil for Biodiesel Production: Kinetics and Optimisation Studies. Indian Chem. Eng. 2021, 63, 374–386. [Google Scholar] [CrossRef]
- Aisien, F.A.; Aisien, E.T. Modeling and Optimization of Transesterification of Rubber Seed Oil Using Sulfonated CaO Derived from Giant African Land Snail (Achatina fulica) Catalyst by Response Surface Methodology. Renew. Energy 2023, 207, 137–146. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in Solid Acid Catalysts for Biodiesel Production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of Waste Cooking Oil for Biodiesel Production Catalyzed by Zn Substituted Waste Egg Shell Derived CaO Nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- ASTM Standard D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM Standard D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Panneerselvam, T.; Raghuraman, S.; Vamsi Krishnan, N. Investigating Mechanical Properties of 3D-Printed Polyethylene Terephthalate Glycol Material Under Fused Deposition Modeling. J. Inst. Eng. India Ser. C 2021, 102, 375–387. [Google Scholar] [CrossRef]
- ASTM Standard D695-15; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM Standard D2240-15; Standard Test Method for Rubber Property-Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2016.
- Yeh, C.H.; Chou, C.M.; Lin, C.P. Design of Experiment for Optimization of 3D Printing Parameters of Base Plate Structures in Colostomy Bag for Newborns. J. Ind. Prod. Eng. 2021, 38, 523–535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).