Ni-Doped Co-Based Metal–Organic Framework with Its Derived Material as an Efficient Electrocatalyst for Overall Water Splitting
Abstract
1. Introduction
2. Results and Discussion
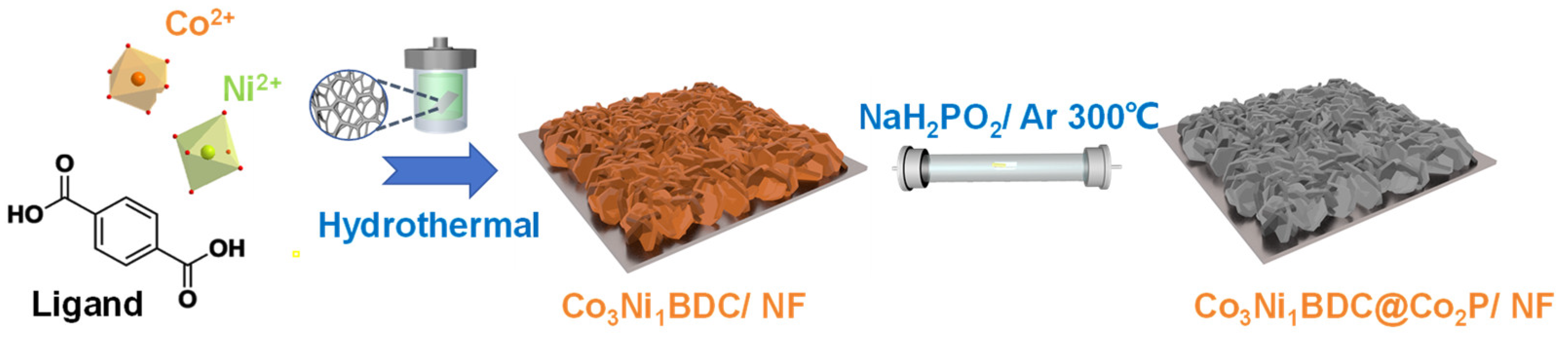
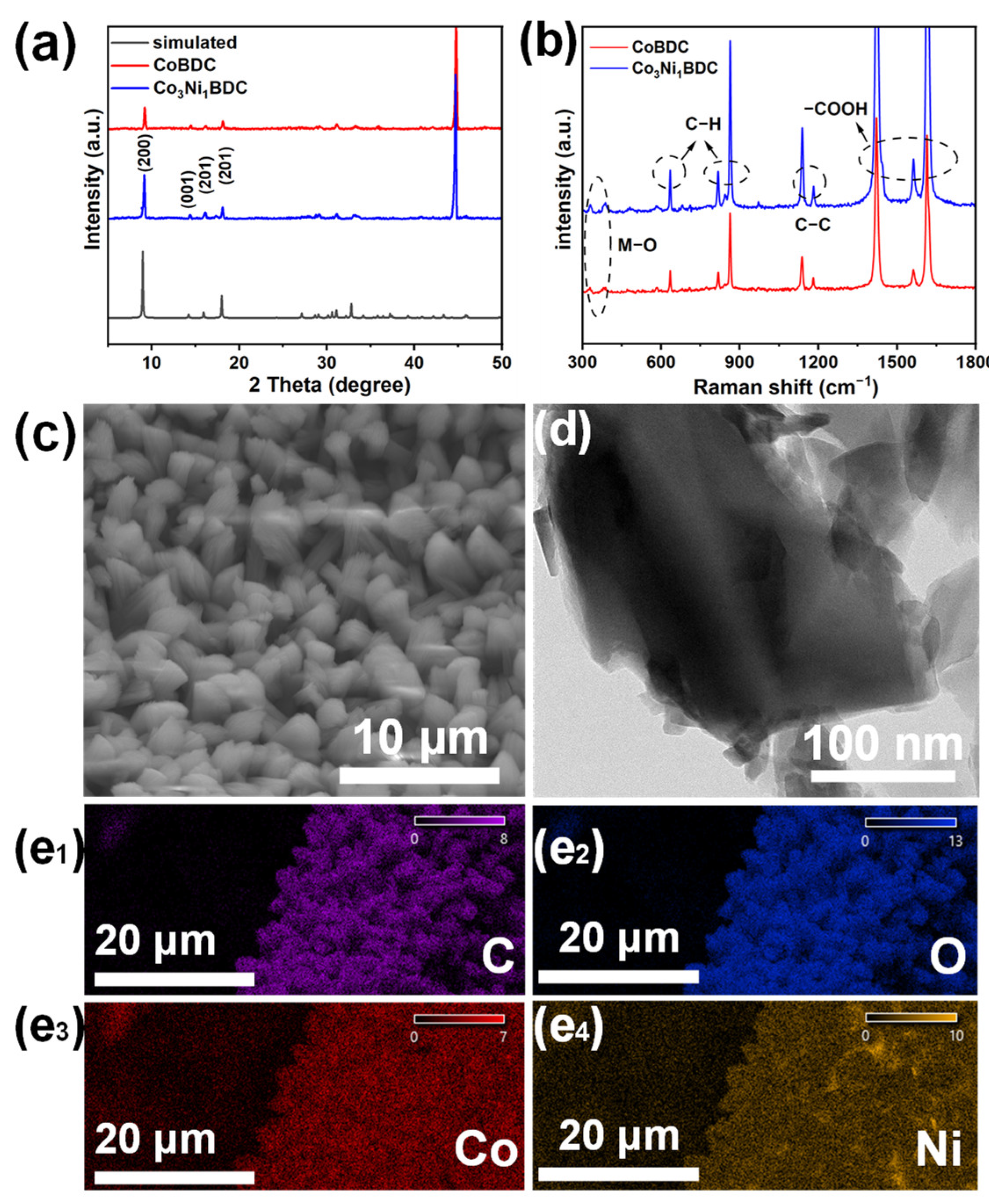
2.1. Catalyst Synthesis and Characterization
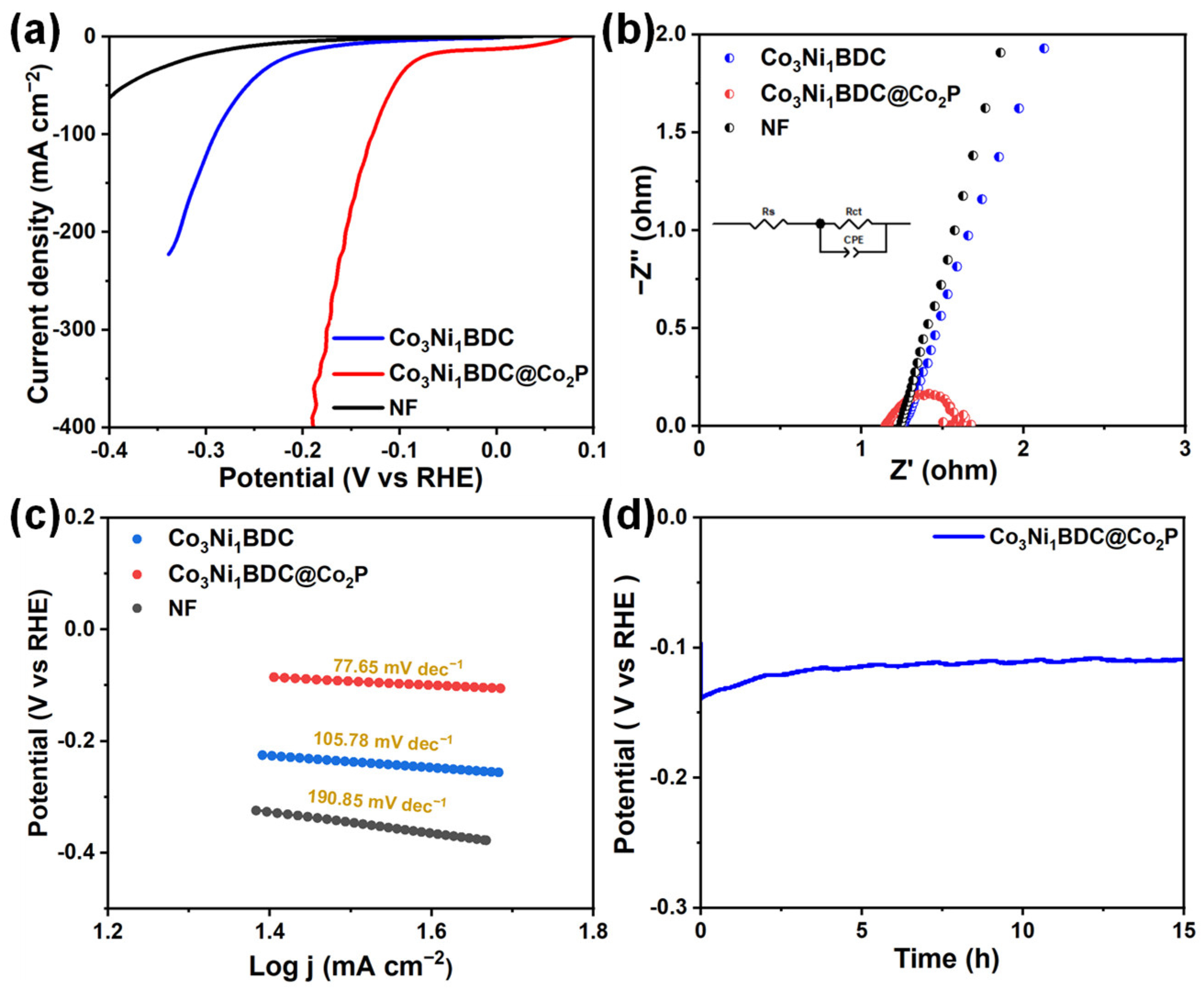
2.2. Oxygen Evolution Reaction Performance
2.3. Hydrogen Evolution Reaction Performance
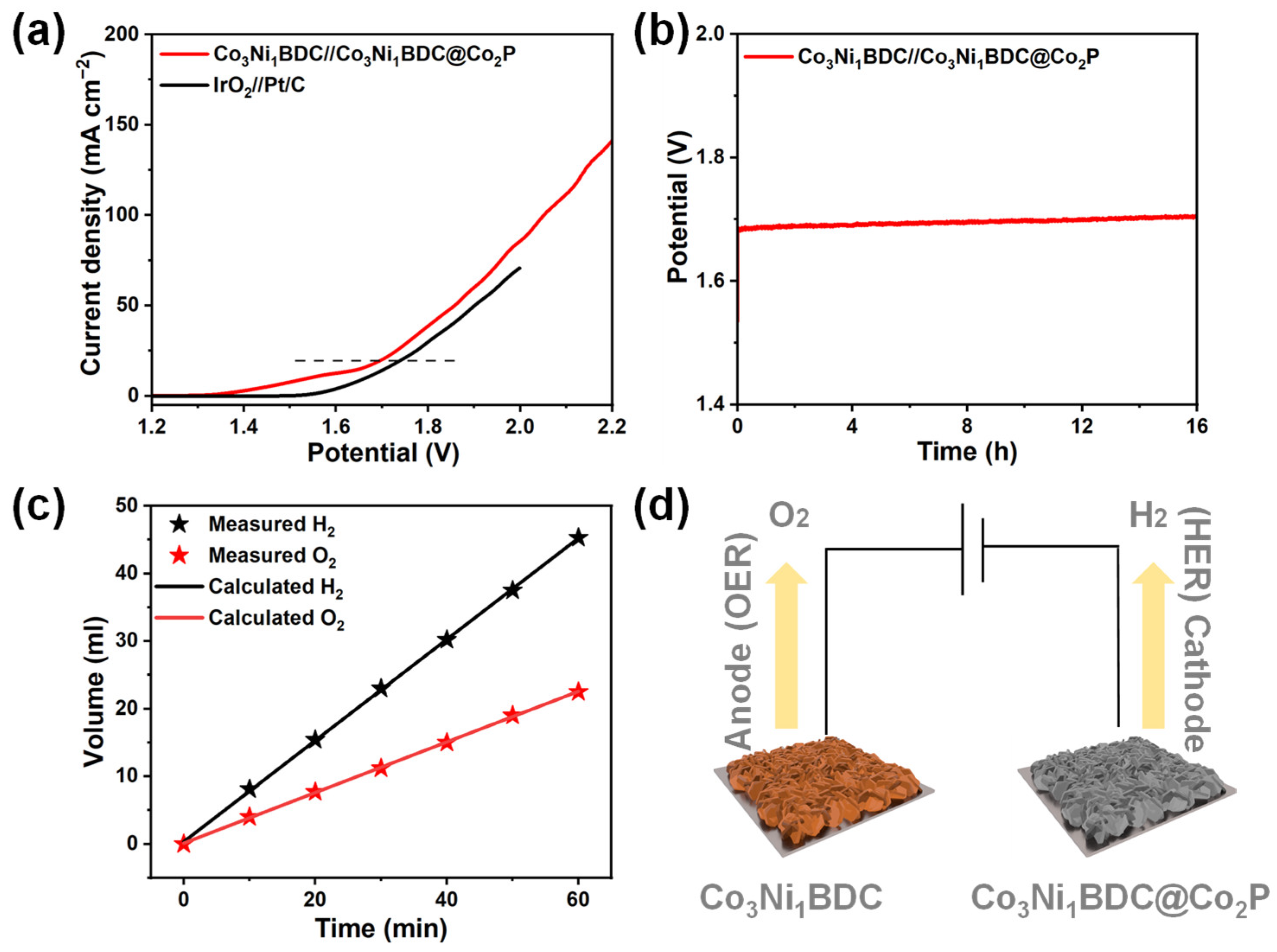
2.4. Overall Water Splitting Performance
3. Experimental Section
3.1. Materials
3.2. Preparation of Co3Ni1BDC and Co3Ni1BDC@Co2P
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdechafik, E.H.; Ousaleh, H.A.; Mehmood, S.; Baba, Y.F.; Bürger, I.; Linder, M.; Faik, A. An analytical review of recent advancements on solid-state hydrogen storage. Int. J. Hydrog. Energy 2024, 52, 1182–1193. [Google Scholar] [CrossRef]
- Hu, C.; Yue, K.; Han, J.; Liu, X.; Liu, L.; Liu, Q.; Kong, Q.; Pao, C.W.; Hu, Z.; Suenaga, K.; et al. Misoriented high-entropy iridium ruthenium oxide foracidic water splitting. Sci. Adv. 2023, 9, adf9144. [Google Scholar] [CrossRef]
- Chen, D.; Yu, R.; Yu, K.; Lu, R.; Zhao, H.; Jiao, J.; Yao, Y.; Zhu, J.; Wu, J.; Mu, S. Bicontinuous RuO2 nanoreactors for acidic water oxidation. Nat. Commun. 2024, 15, 3928. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, R.; Zhang, Y.; Zhang, Q.Q.; He, Y.; Li, Z.B.; Liu, W.H.; Liu, X.J.; Lu, Z.P. Durable Acidic Oxygen Evolution Via Self-Construction of Iridium Oxide/Iridium-Tantalum Oxide Bi-Layer Nanostructure with Dynamic Replenishment of Active Sites. Nano-Micro. Lett. 2025, 17, 165. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, M.Y.; Ren, B.Y.; Yi, Q.; Yu, H.; Zhang, B.Y.; Li, A.; Hu, X.Y.; Li, Z.; Chen, G.Y.; et al. Interfacial built-in electric field in 2D Ni(OH)2 heterojunction with the sodium organic compound for enhanced oxygen evolution catalysis. Chem. Eng. J. 2025, 503, 158565. [Google Scholar] [CrossRef]
- Yu, Y.T.; Zhu, Z.J.; Huang, H.W. Surface Engineered Single-atom Systems for Energy Conversion. Adv. Mater. 2024, 36, 2311148. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yang, L.; Sun, Y.; Lin, G.Y.; Manners, I.; Qiu, H.B. Surface-Initiated Living Self-Assembly of Polythiophene-Based Conjugated Block Copolymer into Erect Micellar Brushes. Angew. Chem. Int. Ed. 2024, 63, e202315740. [Google Scholar] [CrossRef]
- Du, N.; Wang, C.; Wang, X.; Lin, Y.; Jiang, J.; Xiong, Y. Trimetallic TriStar Nanostructures: Tuning Electronic and Surface Structures for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2016, 28, 2077–2084. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the Electrochemistry of the Hydrogen-Evolution Reaction through Combining Experiment and Theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Declerck, K.; Savić, N.D.; Moussawi, M.A.; Seno, C.; Pokratath, R.; De Roo, J.; Parac-Vogt, T.N. Molecular Insights into Sequence-Specific Protein Hydrolysis by a Soluble Zirconium-Oxo Cluster Catalyst. J. Am. Chem. Soc. 2024, 146, 11400–11410. [Google Scholar] [CrossRef]
- Zhang, Y.; Kokculer, I.Y.; de Azambuja, F.; Parac-Vogt, T.N. Dynamic Environment at the Zr6 Oxo Cluster Surface Is Key for the Catalytic Formation of Amide Bonds. Catal. Sci. Technol. 2023, 13, 100–110. [Google Scholar] [CrossRef]
- Zhang, Y.; de Azambuja, F.; Parac-Vogt, T.N. Zirconium oxo clusters as discrete molecular catalysts for the direct amide bond formation. Catal. Sci. Technol. 2022, 12, 3190–3201. [Google Scholar] [CrossRef]
- Van den Eynden, D.; Pokratath, R.; Mathew, J.P.; Goossens, E.; De Buysser, K.; De Roo, J. Fatty acid capped, metal oxo clusters as the smallest conceivable nanocrystal prototypes. Chem. Sci. 2023, 14, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, J.Y.; Hu, Y.T.; Wang, L.Q.; Zhang, X.F.; Zhao, B. Ni Doped Co-MOF-74 Synergized with 2D Ti3C2Tx MXene as an Efficient Electrocatalyst for Overall Water-Splitting. Catalysts 2024, 14, 184. [Google Scholar] [CrossRef]
- Wei, K.L.; Wang, X.; Jiao, X.L.; Li, C.; Chen, D.R. Self-supported 2D Fe-doped Ni-MOF nanosheets as highly efficient and stable electrocatalysts for benzylamine oxidation. Appl. Surf. Sci. 2022, 578, 152065. [Google Scholar] [CrossRef]
- Li, Y.; He, N.N.; Chen, X.H.; Fang, B.; Liu, X.J.; Li, H.B.; Gong, Z.W.; Lu, T.; Pan, L.K. Interface regulation of Zr-MOF/Ni2P@nickel foam as high-efficient electrocatalyst for pH-universal hydrogen evolution reaction. J. Colloid Interface Sci. 2024, 656, 289–296. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Ferey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Yang, J.X.; Loh, K.P. Structure-Directing Role of Graphene in the Synthesis of Metal−Organic Framework Nanowire. J. Am. Chem. Soc. 2010, 132, 14487–14495. [Google Scholar] [CrossRef]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like NiCoP for Highly Efficient Overall Water Splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.M.; Xing, Y.Y.; Li, D.; Jiang, D.L.; Chen, M.; Shi, W.D.; Yuan, S.Q. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B-Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Li, S.; Zhou, L.; Li, Z.; Li, J.; Shao, M. Interface engineering of (Ni, Fe)S2@MoS2 heterostructures for synergetic electrochemical water splitting. Appl. Catal. B-Environ. 2019, 247, 107–114. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, T.; Fu, G.; Gao, T.; Han, J.; Yao, T.; Zhang, Y.; Zhong, W.; Wang, X.; Song, B. Skutterudite-Type Ternary Co1–xNixP3 Nanoneedle Array Electrocatalysts for Enhanced Hydrogen and Oxygen Evolution. ACS Energy Lett. 2018, 3, 1744–1752. [Google Scholar] [CrossRef]
- Song, J. Are Metal Chalcogenides, Nitrides, and Phosphides Oxygen Evolution Catalysts or Bifunctional Catalysts? ACS Energy Lett. 2017, 2, 1937–1938. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel–Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Tang, B.; Yu, Z.G.; Zhang, Y.; Tang, C.; Seng, H.L.; Seh, Z.W.; Zhang, Y.W.; Pennycook, S.J.; Gong, H.; Yang, W. Metal–organic framework-derived hierarchical MoS2/CoS2 nanotube arrays as pH-universal electrocatalysts for efficient hydrogen evolution. J. Mater. Chem. A 2019, 7, 13339–13346. [Google Scholar] [CrossRef]
- Liu, H.T.; Guan, J.Y.; Yang, S.X.; Yu, Y.H.; Shao, R.; Zhang, Z.P.; Dou, M.L.; Wang, F.; Xu, Q. Metal–Organic-Framework-Derived Co2P Nanoparticle/Multi-Doped Porous Carbon as a Trifunctional Electrocatalyst. Adv. Mater. 2020, 32, 2003649. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.Y.; Zha, J.L.; Huo, J.J.; Qu, J.T.; Yang, J.; Li, Y.; Zhu, M.Y.; Cairney, J.M.; Zheng, R.K.; Li, S.; et al. Multi-interfacial Ni/Mo2C ultrafine hybrids anchored on nitrogen-doped carbon nanosheets as a highly efficient electrocatalyst for water splitting. Mater. Today Nano 2022, 20, 100248. [Google Scholar] [CrossRef]
- Huang, H.W.; Zhou, S.; Yu, C.; Huang, H.L.; Zhao, J.J.; Dai, L.M.; Qiu, J.S. Rapid and energy-efficient microwave pyrolysis for high-yield production of highly-active bifunctional electrocatalysts for water splitting. Energ. Environ. Sci. 2020, 13, 545–553. [Google Scholar] [CrossRef]
- Shi, J.H.; Qiu, F.; Yuan, W.B.; Guo, M.M.; Lu, Z.H. Nitrogen-doped carbon-decorated yolk-shell CoP@FeCoP micro-polyhedra derived from MOF for efficient overall water splitting. Chem. Eng. J. 2021, 403, 126312. [Google Scholar] [CrossRef]
- Chen, J.S.; Guo, Z.Z.; Luo, Y.X.; Cai, M.D.; Gong, Y.X.; Sun, S.; Li, Z.X.; Mao, C.J. Engineering Amorphous Nickel Iron Oxyphosphide as a Highly Efficient Electrocatalyst toward Overall Water Splitting. ACS Sustain. Chem. Eng. 2021, 9, 9436–9443. [Google Scholar] [CrossRef]
- Ma, G.Y.; Ye, J.T.; Qin, M.Y.; Sun, T.Y.; Tan, W.X.; Fan, Z.H.; Huang, L.F.; Xin, X. Mn-doped NiCoP nanopin arrays as high-performance bifunctional electrocatalysts for sustainable hydrogen production via overall water splitting. Nano Energy 2023, 115, 108679. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Zhu, J.L.; Li, Y.Y.; Tsiakarasc, P.; Shen, P.K. Electronic modulation of cobalt phosphide nanosheet arrays via copper doping for highly efficient neutral-pH overall water splitting. Appl. Catal. B-Environ. 2020, 265, 118555. [Google Scholar] [CrossRef]
- Choi, H.; Surendran, S.; Sim, Y.; Je, M.; Janani, G.; Choi, H.; Kim, J.K.; Sim, U. Enhanced electrocatalytic full water-splitting reaction by interfacial electric field in 2D/2D heterojunction. Chem. Eur. J. 2022, 450, 137789. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ni, H.; Yu, J.; Zhao, B. Ni-Doped Co-Based Metal–Organic Framework with Its Derived Material as an Efficient Electrocatalyst for Overall Water Splitting. Catalysts 2025, 15, 355. https://doi.org/10.3390/catal15040355
Zhang J, Ni H, Yu J, Zhao B. Ni-Doped Co-Based Metal–Organic Framework with Its Derived Material as an Efficient Electrocatalyst for Overall Water Splitting. Catalysts. 2025; 15(4):355. https://doi.org/10.3390/catal15040355
Chicago/Turabian StyleZhang, Jingyuan, Hui Ni, Jianing Yu, and Bin Zhao. 2025. "Ni-Doped Co-Based Metal–Organic Framework with Its Derived Material as an Efficient Electrocatalyst for Overall Water Splitting" Catalysts 15, no. 4: 355. https://doi.org/10.3390/catal15040355
APA StyleZhang, J., Ni, H., Yu, J., & Zhao, B. (2025). Ni-Doped Co-Based Metal–Organic Framework with Its Derived Material as an Efficient Electrocatalyst for Overall Water Splitting. Catalysts, 15(4), 355. https://doi.org/10.3390/catal15040355







