Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate
Abstract
1. Introduction
2. Results and Discussion
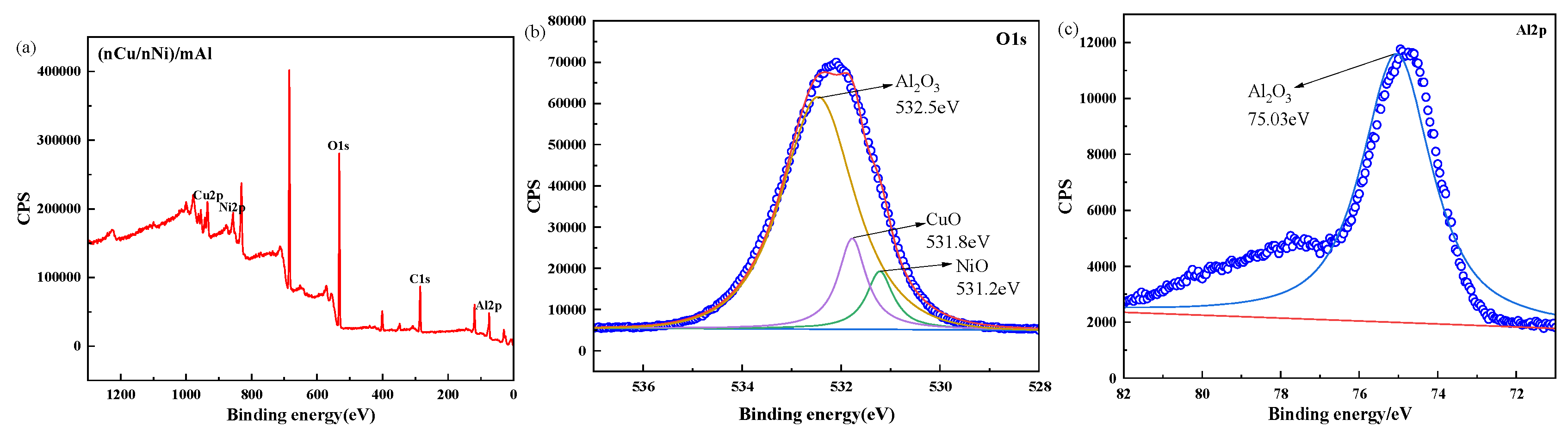
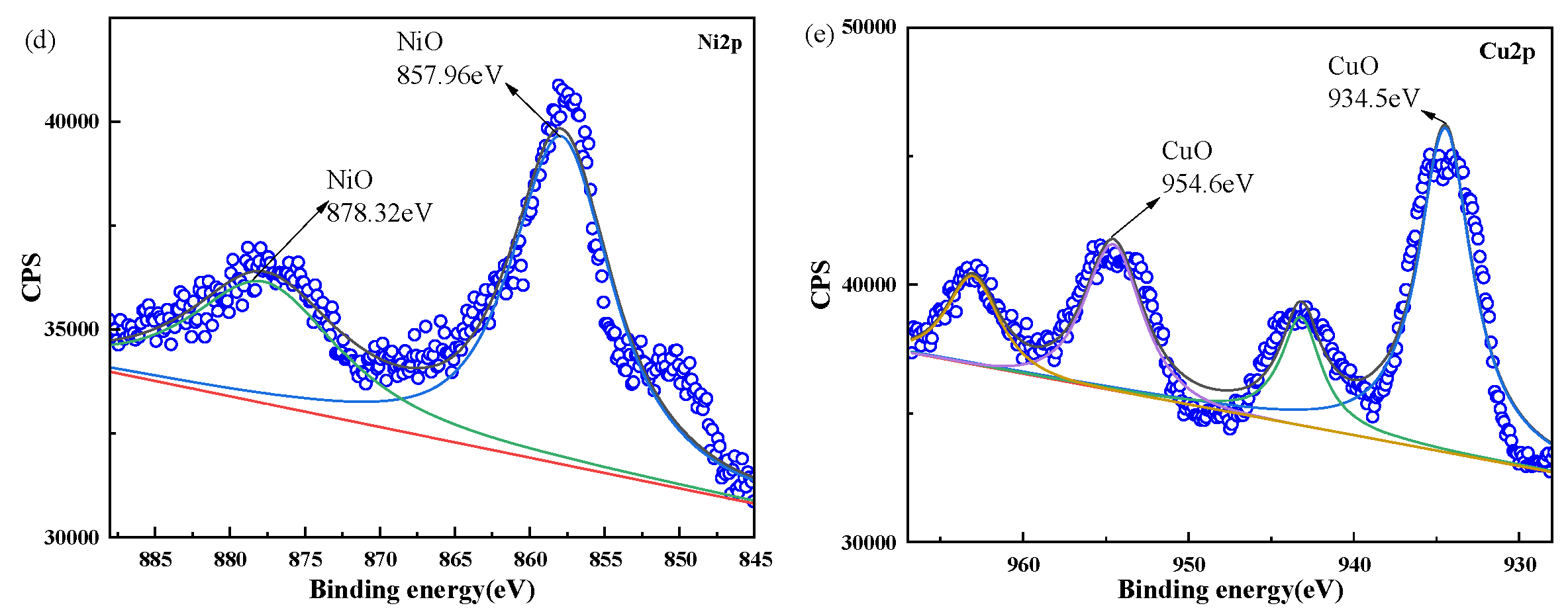
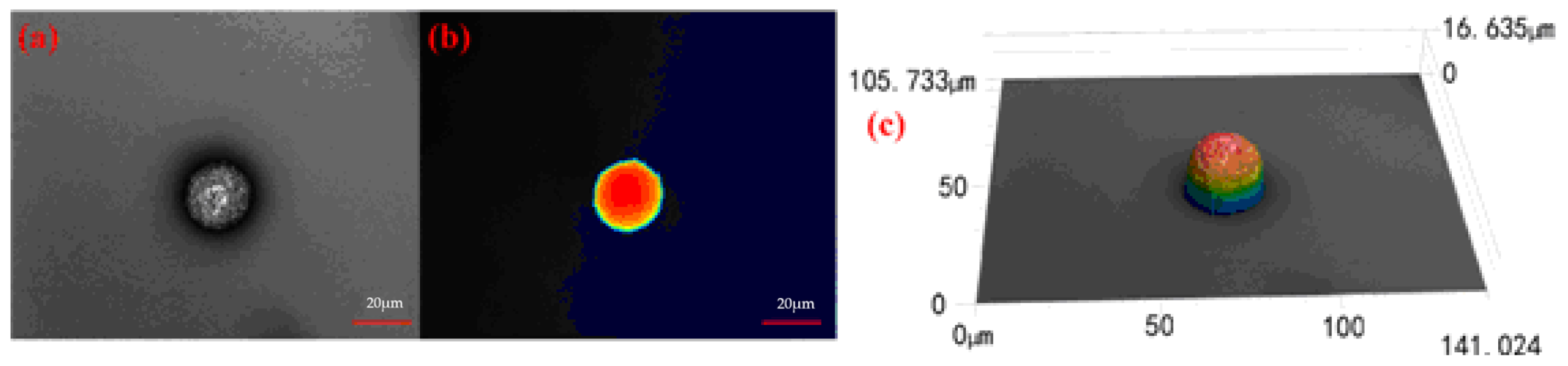
2.1. Characterization of [nCu+nNi]/μAl
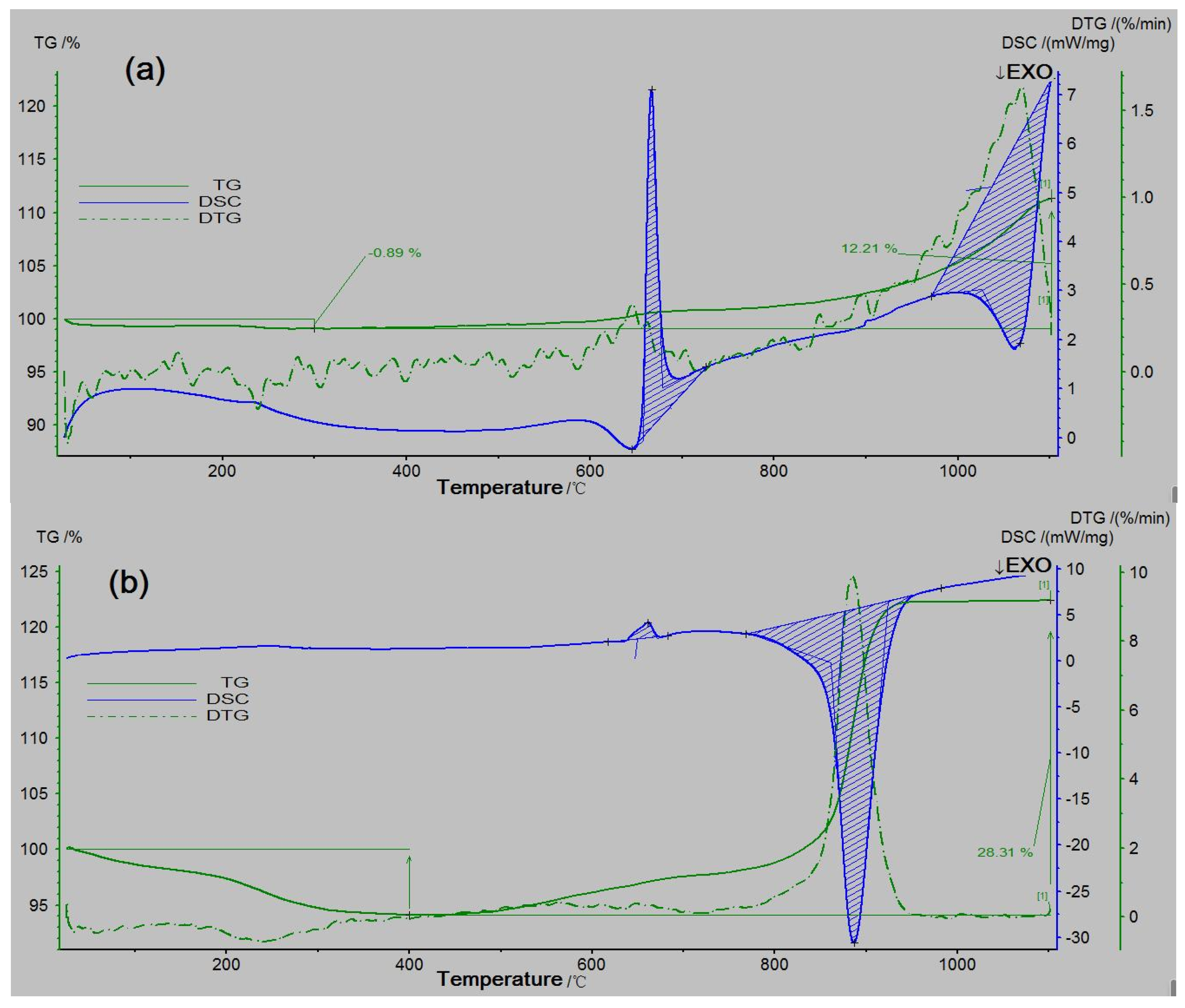
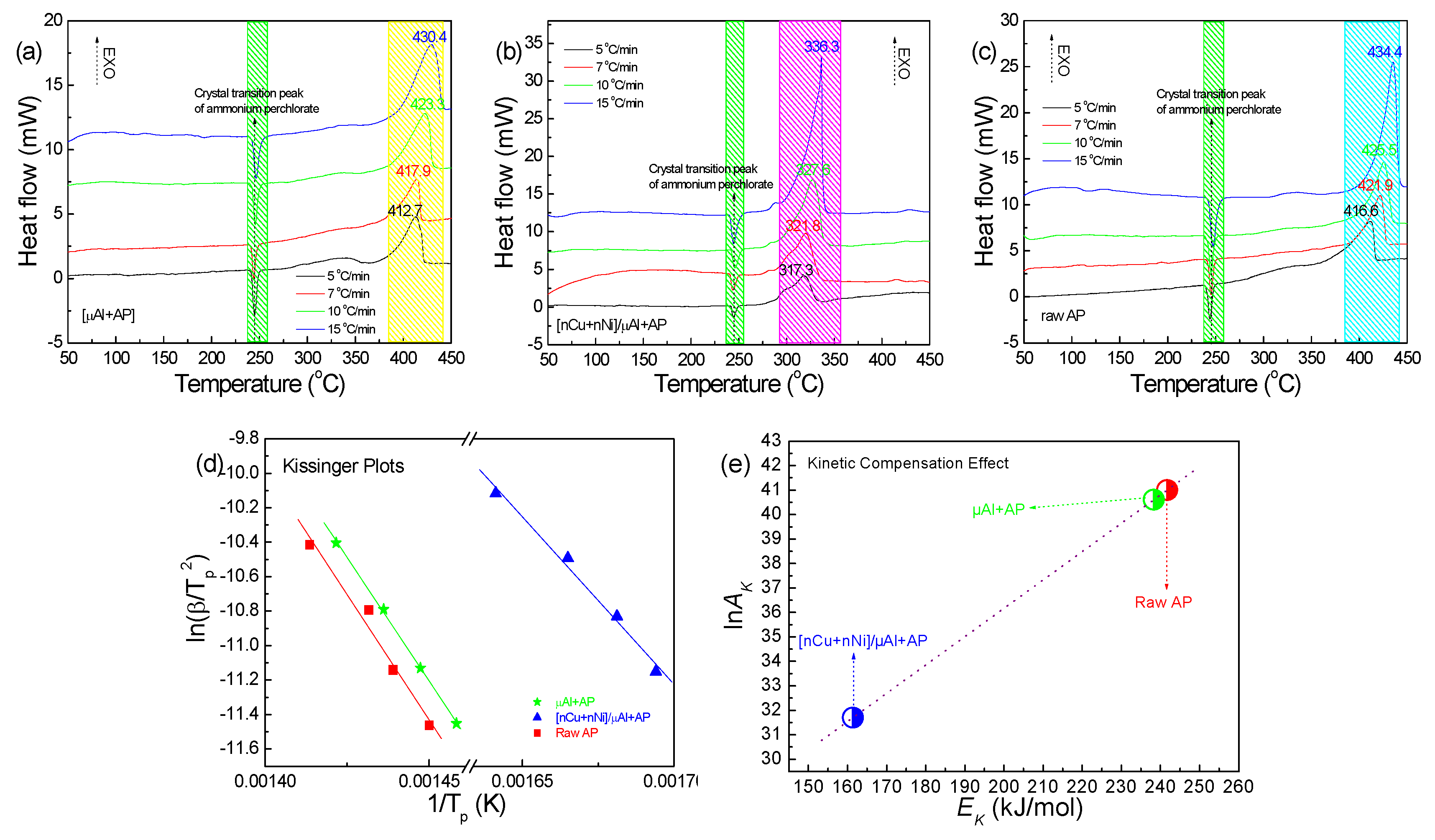
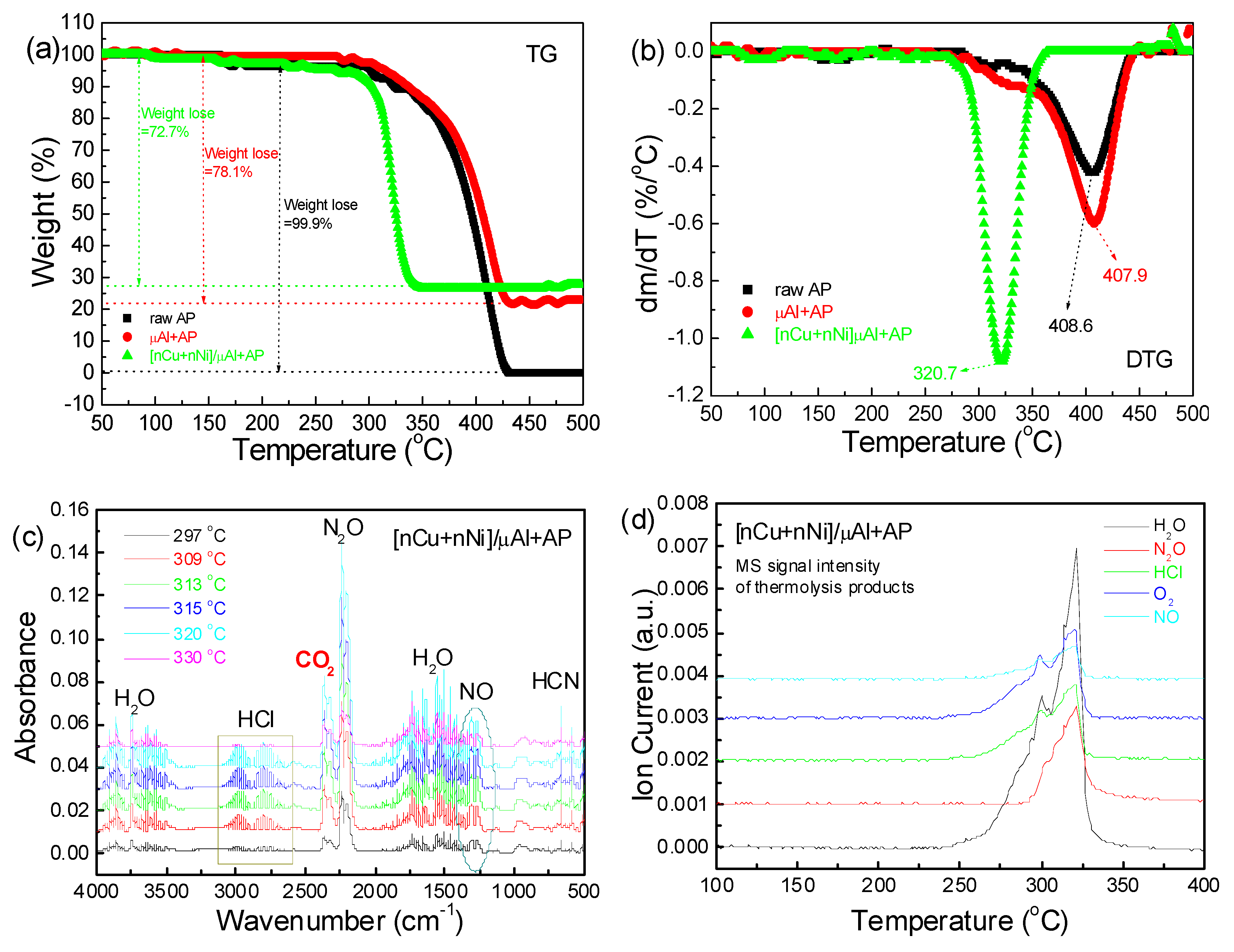
2.2. Thermal Analyses
2.3. Combustion Performance
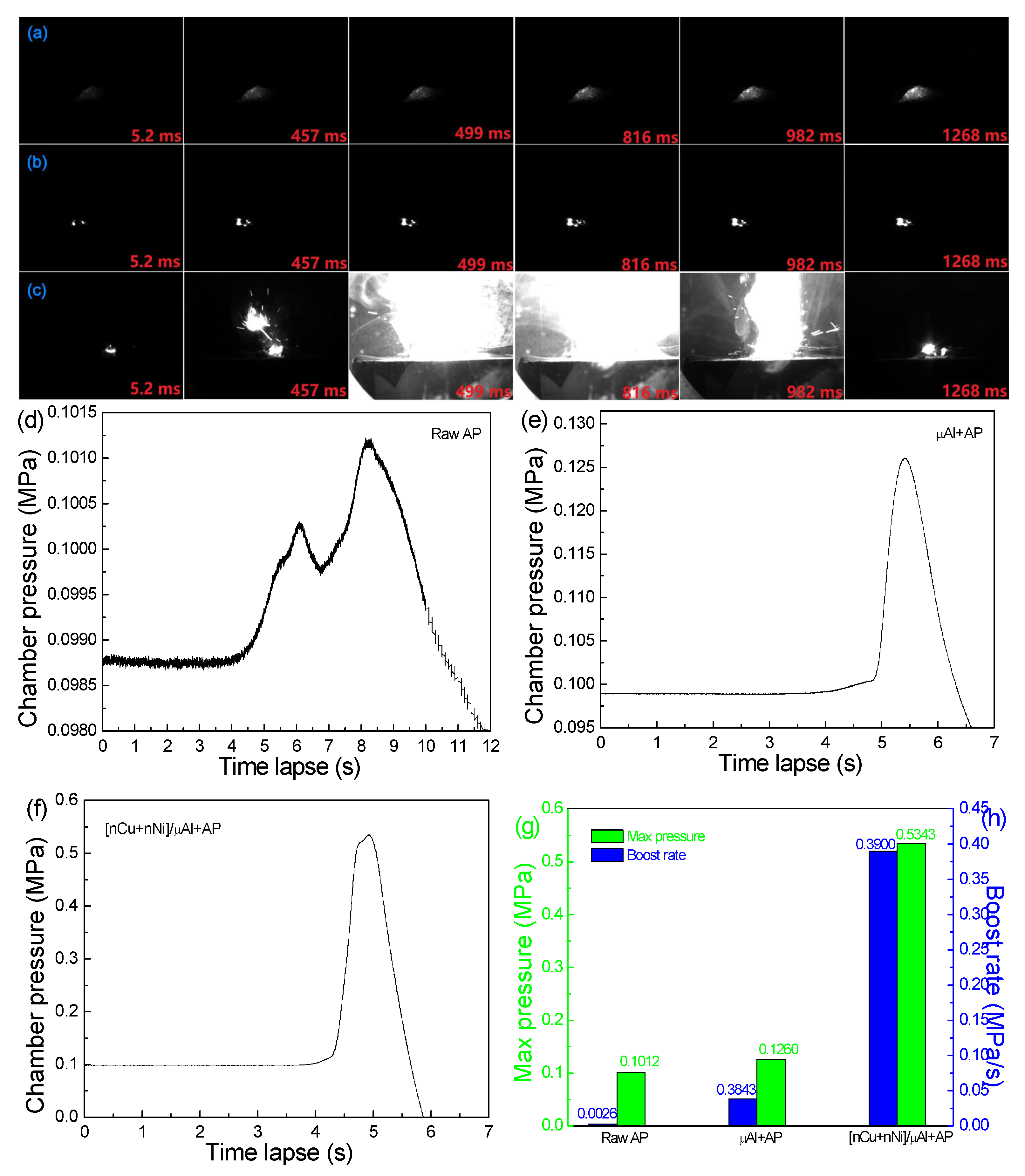
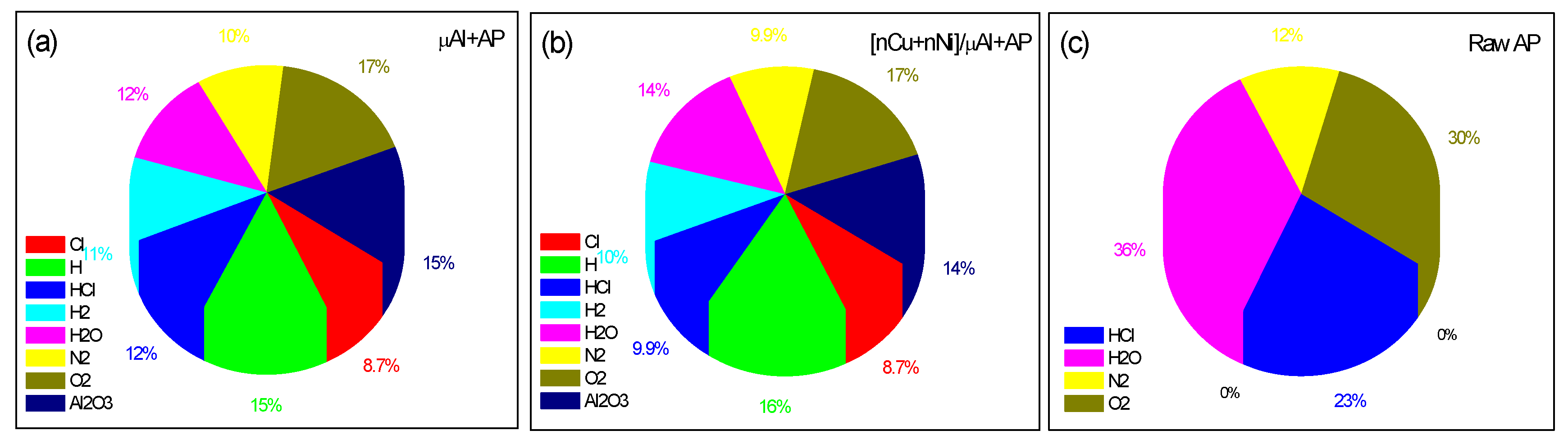
2.3.1. Combustion in Closed Bomb
2.3.2. Combustion in the Air
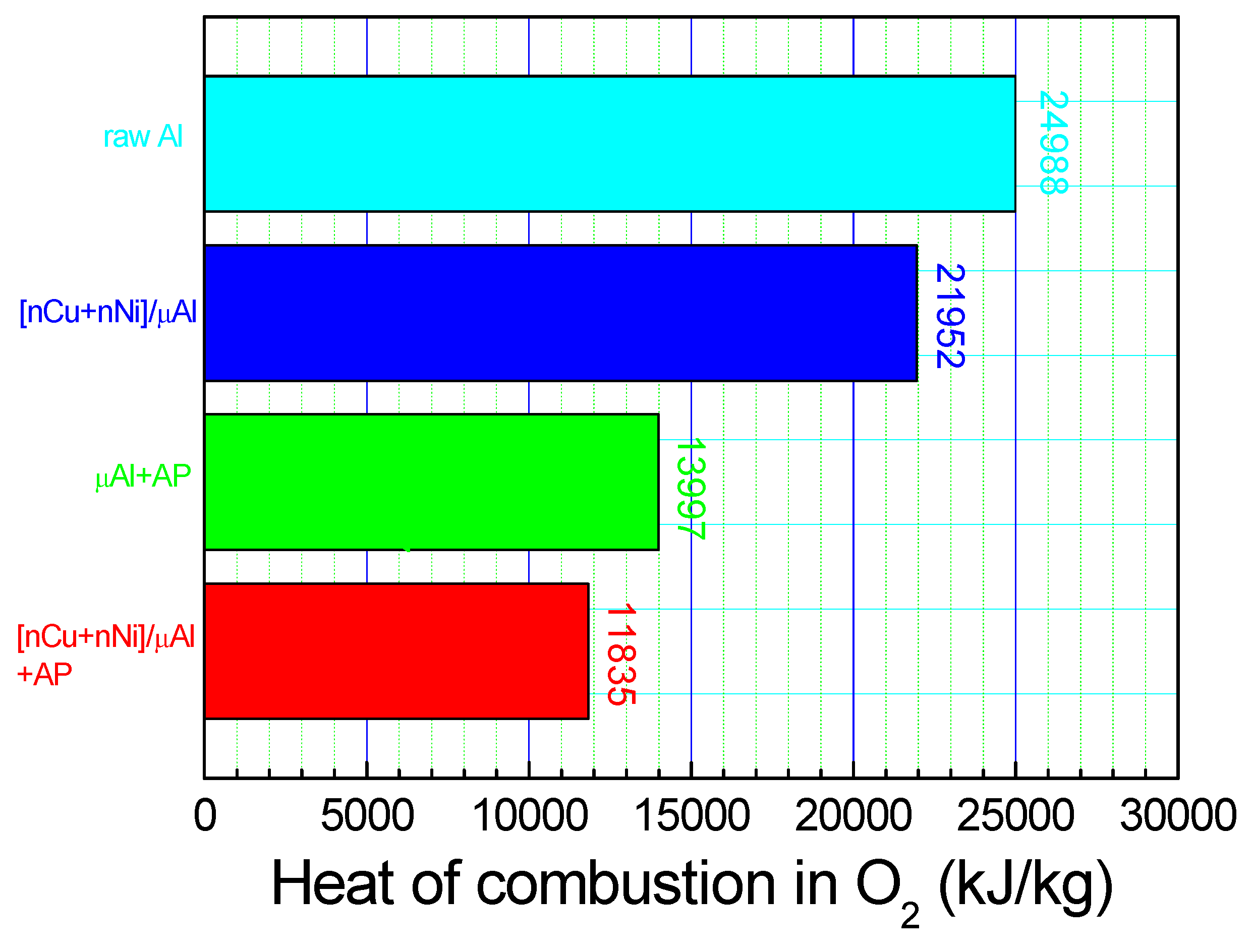
2.3.3. Heats of Combustion in Oxygen Bomb
3. Experimental Sections
3.1. Materials
3.2. Preparation of [nCu+nNi]/μAl
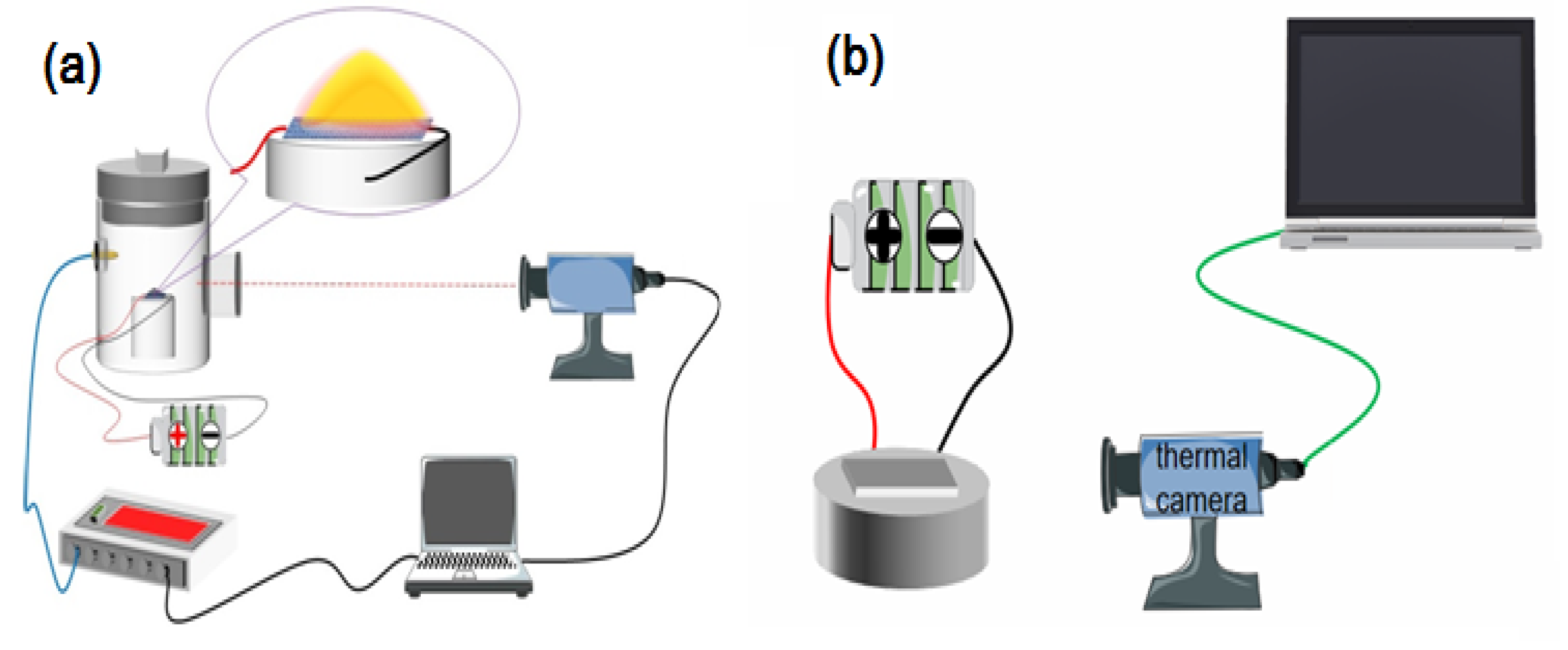
3.3. Characterization and Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, T.L.; Buckmaster, J.; Wang, X. Modeling of propellants containing ultrafine aluminum. J. Propuls. Power 2007, 23, 158–165. [Google Scholar]
- Yuan, J.F.; Liu, J.Z.; Zhou, Y.N.; Wang, J.; Xv, T. Aluminum agglomeration of AP/HTPB composite propellant. Acta Astronaut. 2019, 156, 14–22. [Google Scholar]
- Gautham, M.G.; Ramakrishna, P.A. Combustion characteristics of aluminum-water gelled composite propellant. J. Propuls. Power 2018, 34, 1345–1353. [Google Scholar]
- Gottfried, J.L.; Smith, D.K.; Wu, C.C.; Pantoya, M.L. Improving the explosive performance of aluminum nanoparticleswith aluminum iodate hexahydrate (AIH). Sci. Rep. 2018, 8, 8036. [Google Scholar]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S.; Kohsari, I.; Fathollahi, M.; Hosseini, S. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel 2008, 87, 244–251. [Google Scholar]
- Li, C.D.; Pi, Z.L.; Wang, F.; Wu, Y. Pore structure regulation of aluminum powder as a filler for high energy solid propellants. Mater. Chem. Phys. 2024, 314, 128911. [Google Scholar]
- Makhov, M.N. Effect of aluminum and boron additives on the heat of explosion and acceleration ability of high explosives. Russ. J. Phys. Chem. B 2015, 9, 50–55. [Google Scholar]
- Cudzilo, S.; Trzcinski, W.A.; Paszula, J.; Szala, M.; Chyłek, Z. Performance of magnesium, Mg-Al alloy and silicon in thermobaric explosives—A comparison to aluminium. Propellant Explos. Pyrotech. 2020, 45, 1691–1697. [Google Scholar]
- Azhagurajan, A.; Selvakumar, N.; Thanulingam, T.L. Thermal and sensitivity analysis of nano aluminium powder for firework application. J. Therm. Anal. Calorim. 2011, 105, 259–267. [Google Scholar]
- Han, Z.Y.; Jiang, Q.; Du, Z.M.; Hoon, H.H.; Yu, Y.; Zhang, Y.; Li, G.; Sun, Y. A novel environmental-friendly and safe unpacking powder without magnesium, aluminum and sulphur for fireworks. J. Hazard. Mater. 2019, 373, 835–843. [Google Scholar]
- Pantoya, M.L.; Granier, J.J. The effect of slow heating rates on the reaction mechanisms of nano and micron composite thermite reactions. J. Therm. Anal. Calorim. 2006, 85, 37–43. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Zhu, X.Y.; Dreizin, E.L. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust. Flame 2005, 140, 310–318. [Google Scholar]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E. Composition and chemical state of the ions of aluminium-oxide films formed by thermal oxidation of aluminum. Surf. Sci. 2002, 506, 313–332. [Google Scholar]
- Sánchez-López, J.C.; Fernández, A.; Conde, C.F.; Conde, A.; Morant, C.; Sanz, J. The melting behavior of passivated nanocrystalline aluminum. Nanostruct. Mater. 1996, 7, 813–822. [Google Scholar]
- Wang, S.X.; Liang, K.M.; Zhang, X.H.; Wang, G.-L. Influence of heating-rate on DTA curve of the aluminothermic reaction. J. Mater. Sci. Lett. 2003, 22, 855–856. [Google Scholar]
- Schoenitz, M.; Ward, T.S.; Dreizin, E.L. Fully dense nano-composite energetic powders prepared by arrested reactive milling. Proc. Combust. Inst. 2005, 30, 2071–2078. [Google Scholar]
- Kim, K.T.; Kim, D.; Kim, C.K.; Choi, Y.J. A facile synthesis and efficient thermaloxidation of polytetrafluoroethylene-coated aluminum powders. Mater. Lett. 2016, 167, 262–265. [Google Scholar]
- Guo, L.G.; Song, W.L.; Xie, C.S.; Zhang, X.; Hu, M. Characterization and thermalproperties of carbon-coated aluminum nanopowders prepared by laser-induction complex heating in methane. Mater. Lett. 2007, 61, 3211–3214. [Google Scholar]
- Lyu, J.Y.; Chen, S.W.; He, W.; Zhang, X.X.; Tang, D.Y.; Liu, P.J.; Yan, Q.L. Fabrication of high-performancegraphene axidle doped PVDF/CuO/Al nanocomposites via electrospinning. Chem. Eng. 2019, 368, 129–137. [Google Scholar]
- Zhou, H.; Lv, B.L.; Wu, D.; Xu, Y. Synthesis of polycrystalline Co3O4 nanowires with excellent ammonium perchlorate catalytic decompositionproperty. Mater. Res. Bull. 2014, 60, 492–497. [Google Scholar]
- Cheng, Z.P.; Chu, X.Z.; Yin, J.Z.; Dai, B.; Zhao, W.; Jiang, Y.; Xu, J.; Zhong, H.; Zhao, P.; Zhang, L. Formation of compositefuels by coating aluminum powder with a cobalt nanocalalyst: Enhanced heat release and catalytic performance. Chem. Eng. 2020, 385, 123859. [Google Scholar]
- Cheng, Z.P.; Chu, X.Z.; Zhao, W.; Yin, J.; Dai, B.; Zhong, H.; Xu, J.; Jiang, Y. Controllable synthesis of Cu/Al energetic nanocomposites with excellent heat release and combustion performance. Appl. Surf. Sci. 2020, 513, 145704. [Google Scholar]
- Liu, L.L.; Li, F.S.; Tan, L.; Ming, L.; Yi, Y. Effects of nanometer Ni, Cu, Al and NiCu powders on the thermal decomposition of ammonium perchlorate. Propellants Explos. Pyrotech. 2004, 29, 34–38. [Google Scholar]
- Song, M.R.; Chen, M.; Zhang, Z.J. Effect of Zn powders on the thermal decomposition of ammonium perchlorate. Propellants Explos. Pyrotech. 2008, 33, 261–265. [Google Scholar]
- Yan, J.; Wang, H.; Jin, B.; Zeng, M.; Peng, R. Cu-MOF derived Cu/Cu2O/C nanocomposites for the efficient thermal decomposition of ammonium perchlorate. J. Solid State Chem. 2021, 297, 122060. [Google Scholar]
- Duan, H.Z.; Lin, X.Y.; Liu, G.P.; Li, F.S. Synthesis of Co nanoparticles and their catalytic effect on the decomposition of ammonium perchlorate. Chin. J. Chem. Eng. 2008, 16, 325–328. [Google Scholar]
- Duan, H.Z.; Lin, X.Y.; Liu, G.P.; Xu, L.; Li, F.S. Synthesis of Ni nanoparticles and their catalytic effect on the decomposition of ammonium perchlorate. J. Mater. Process. Technol. 2008, 208, 494–498. [Google Scholar]
- Kou, Y.; Wang, Y.; Zhang, J.; Guo, K.G.; Song, X.L. Iron/aluminum nanocomposites prepared by one-step reduction method and their effects on thermal decomposition of AP and AN. Def. Technol. 2023, 22, 74–87. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Cheng, Z.P.; Chen, W.F.; An, C.; Song, X.; Li, F. Thermite reaction of Al/Cu core-shell nanocomposites with WO3. Thermochim. Acta 2007, 463, 69–76. [Google Scholar]
- Wang, Y.; Song, X.L.; Jiang, W.; Deng, G.D.; Guo, X.D.; Liu, H.Y.; Li, F.S. Synthesis of nano-nickel-coated micro-aluminum and thermal reactivity of aluminum/nickel stannic-oxide thermite. Int. J. Energetic Mater. Chem. Propuls. 2011, 10, 231–243. [Google Scholar]
- Zi, R.C.; Han, Z.Y.; Yu, Y.; Wang, C.; Zhang, X.; Guo, X.; Chen, J.; Zhang, X.; Yang, J. Properties of mixed crystal coprecipitation substances of ammonium nitrate and potassium perchlorate prepared by the evaporative solvent method. ACS Omega 2023, 9, 1573–1590. [Google Scholar] [PubMed]
- Wang, Y.; Song, X.L.; Li, F.S. Thermal behavior and decomposition mechanism of ammonium perchlorate and ammonium nitrate in the presence of nanometer triaminoguanidine nitrate. ACS Omega 2019, 4, 214–225. [Google Scholar] [PubMed]
- Lee, D.G.; Lee, C.J. Fuel-Rich combustion with ammonium perchlorate addition in a staged hybrid rocket engine. J. Propuls. Power 2017, 33, 1581–1588. [Google Scholar]
- Zhou, L.Y.; Cao, S.B.; Zhang, L.L.; Xiang, G.; Wang, J.; Zeng, X.; Chen, J. Facet effect of Co3O4 nanocatalysts on the catalytic decomposition of ammonium perchlorate. J. Hazard. Mater. 2020, 392, 122358. [Google Scholar]
- Vara, J.A.; Dave, P.N.; Chaturvedi, S. Investigating catalytic properties of nanoferrites for both AP and nano-AP based composite solid propellant. Combust. Sci. Technol. 2021, 193, 2290–2304. [Google Scholar]
- Song, X.L.; Wang, Y.; Song, D.; An, C.; Wang, J. Catalysis of a nanometer solid super acid of SO42−/TiO2 on the thermal decomposition of ammonium nitrate. Nanomater. Nanotechnol. 2016, 6, 23. [Google Scholar] [CrossRef]
- Song, X.L.; Wang, Y.; Song, D.; An, C.; Wang, J. Thermal decomposition of ammonium perchlorate and ammonium nitrate doped with nanometer SO42−/Fe2O3 solid strong acid as catalyst. Sci. Technol. Energetic Mater. 2016, 77, 65–71. [Google Scholar]
- Wang, Y.; Song, X.L.; Li, F.S. Nanometer ammonium perchlorate and ammonium nitrate with 2D network structure prepared via rapid freezing technology. Nanomaterials 2019, 9, 1605. [Google Scholar]
- Meng, S.H.; Liu, J.F.; Kong, X.T.; Du, S.G. Preparation of TiO2/CNTs nanocomposite and its catalytic performance on the thermal decomposition of ammonium perchlorate. Transit. Met. Chem. 2020, 45, 545–551. [Google Scholar]
- Hu, Y.H.; Tao, B.; Shang, F.; Zhou, M.X.; Hao, D.; Fan, R.; Xia, D.; Yang, Y.; Pang, A.; Lin, K. Thermal decomposition of ammonium perchlorate over perovskite catalysts: Catalytic decomposition behavior, mechanism and application. Appl. Surf. Sci. 2020, 513, 145849. [Google Scholar]
- Carignan, L.P.; Lacroix, C.; Ouimet, A.; Ciureanu, M.; Yelon, A.; Ménard, D. Magnetic anisotropy in arrays of Ni, CoFeB, and Ni/Cu nanowires. J. Appl. Phys. 2007, 102, 023905. [Google Scholar] [CrossRef]
- Rani, B.J.; Saravanakumar, B.; Ravi, G.; Ganesh, V.; Sakunthala, A.; Yuvakkumar, R. Structural, optical and magnetic properties of NiO nanopowders. J. Nanosci. Nanotechnol. 2018, 18, 4658–4666. [Google Scholar] [PubMed]
- Liu, X.S.; Hu, F.; Zhu, D.R.; Jia, D.N.; Wang, P.-P.; Ruan, Z.; Cheng, C.-H. One-step synthesis of carbon nanotubes with Ni nanoparticles as a catalyst by the microwave-assisted polyol method. J. Alloys Compd. 2011, 509, 2829–2832. [Google Scholar]
- Chen, L.J.; Li, G.S.; Qi, P.; Li, L.O. Thermal decomposition of ammonium perchlorate activated via addition of NiO nanocrystals. J. Therm. Anal. Calorim. 2008, 92, 765–769. [Google Scholar]
- Chen, L.J.; Li, G.S.; Li, L.O. CuO nanocrystals in thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2008, 91, 581–587. [Google Scholar]
- Li, J.W.; Li, J.F.; Zhang, D.X.; Wu, B.D. Preparation of Micron-scale DAP-4-based Energetic Composite Microspheres by Microfluidic Technology and its Characterization. Chin. J. Energ. Mater. 2024, 32, 249–255. [Google Scholar]
- Shi, J.H.; Wu, B.D.; Zhou, J.Q.; Ren, D.W. One-step rapid preparation of CL-20/TNT co-crystal assembly and spheroidized coating based on droplet microfluidic technology. Def. Technol. 2023, 27, 251–262. [Google Scholar]
- Guo, Y.Y.; Liu, Y.; Guan, Q. Preparation of micron-sized ammonium perchlorate with narrow particle size distribution based on microfluidic technology. Initiat. Pyrotech. 2024, 33–39. [Google Scholar]
- Zhang, T.T.; Jiang, Y.Q.; Huo, W.L. Preparation and combustion properties of AP@Al composites. Solid Rocket. Technol. 2024, 47, 1–13. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar]
- Han, Z.W.; Han, Y.C.; Xu, S. Preparation of nano-cerium dioxide and its effect on the thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2014, 116, 273–278. [Google Scholar] [CrossRef]
- Fujimura, K.; Miyake, A. The effect of specific surface area of TiO2 on the thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2010, 99, 27–31. [Google Scholar] [CrossRef]
- Shim, H.M.; Lee, E.A.; Kim, J.K. Formation of tungsten/ammonium perchlorate composites and their reaction kinetics. Cent. Eur. J. Energ. Mater. 2015, 12, 703–722. [Google Scholar]
- Song, Y.; Song, X.L.; Song, D.; Liang, L.; An, C.; Wang, J. Synthesis, thermolysis, and sensitivities of HMX/NC energetic nanocomposites. J. Hazard. Mater. 2016, 312, 73–83. [Google Scholar] [CrossRef]
- Yu, Z.H.; Song, X.L.; Kou, Y.; Wang, Y.; An, C. Characterization and testing for lowest eutectic mixture of TNBA/DNTF. Propellants Explos. Pyrotech. 2023, 48, e202200201. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Jia, K.H.; Song, D.; Song, X.; An, C. Preparation and characterization of high-reactivity explosive-based nano-boron microspheres. Particuology 2024, 93, 125–136. [Google Scholar] [CrossRef]







| Sample | Modification Method | Original Peak Temperature/°C | Modified Peak Temperature/°C | Advance Temperature/°C |
|---|---|---|---|---|
| Ultra-fine AP [48] | Microfluidics | 390.2 | 377.94 | 12.26 |
| Cu+AP [23] | Directly doping | 477.2 | 367 | 110.2 |
| Ni+AP [23] | Directly doping | 477.2 | 388 | 89.2 |
| AP@Al [49] | Surface coating | 399.44 | 387.31 | 12.13 |
| μAl+AP | Directly doping | 416.6 | 412.7 | 3.9 |
| [nCu+nNi]/μAl+AP | Directly doping | 416.6 | 317.3 | 99.3 |
| Samples | Tp (°C) (10 °C/min) | Thermodynamic | Kinetics | Burst Points | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔH≠ (kJ/mol) | ΔG≠ (kJ/mol) | ΔS≠ (J/mol·K) | EK (kJ/mol) | lnAK | k (s−1) | T5s (°C) | E (kJ/mol) | ||
| Raw AP | 425.5 | 235.8 | 179.6 | 0.08 | 241.7 | 41.0 | 0.54 | 288 | 28.4 |
| μAl+AP | 423.3 | 232.5 | 178.6 | 0.08 | 238.3 | 40.6 | 0.59 | 284 | 44.2 |
| [nCu+nNi]/μAl+AP | 327.6 | 156.4 | 153.9 | 0.04 | 161.4 | 31.7 | 0.52 | 302 | 35.4 |
| Samples | TTOP,exp (°C) | Tad,cal (°C) | HR,cal (kJ/kg) | HP,cal (kJ/kg) | ΔHC,cal (kJ/kg) |
|---|---|---|---|---|---|
| Raw AP | - | 1376 | −2520 | −4852 | −2332 |
| μAl+AP | - | 3420 | −1822 | −11,968 | −10,146 |
| [nCu+nNi]/μAl+AP | 1851 | 3389 | −1823 | −11,647 | −9824 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Song, X. Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate. Catalysts 2025, 15, 354. https://doi.org/10.3390/catal15040354
Wang Y, Song X. Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate. Catalysts. 2025; 15(4):354. https://doi.org/10.3390/catal15040354
Chicago/Turabian StyleWang, Yi, and Xiaolan Song. 2025. "Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate" Catalysts 15, no. 4: 354. https://doi.org/10.3390/catal15040354
APA StyleWang, Y., & Song, X. (2025). Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate. Catalysts, 15(4), 354. https://doi.org/10.3390/catal15040354






