Enhanced H2 Production Efficiency in Photo-Reforming of PET Waste Plastic Using Dark-Deposited Atom/Nanocomposite Pt/TiO2 Photocatalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts with Transmission Electron Microscopy
2.2. X-Ray Diffraction (XRD) Analysis of the Catalysts
2.3. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) Analysis
2.4. XPS Analysis
2.5. UV-Vis Absorbance of the Catalysts
2.6. Analysis of Hydrogen Production with Dark-Deposited Catalysts
2.7. Comparison of Hydrogen Production with Different Catalysts
| Experimental Conditions | Light Source | Hydrogen Conversion Efficiency (%) | EQY (%) |
|---|---|---|---|
| Direct-deposited 0.7%(w/w) Pt/TiO2 with PET substrate (This study) | UVC LED 70 mW | 6.4 | 1 |
| Dark-deposited 0.4%(w/w) Pt/TiO2 annealed at 500 °C with PET substrate (This study) | UVC LED 70 mW | 33.4 | 7 |
| Dark-deposited 0.04%(w/w) Pt/TiO2 annealed at 500 °C with PET substrate (This study) | UVC LED 70 mW | 25.9 | 4 |
| CdS/CdOx Quantum dots with PET substrate [62] | Simulated solar light (AM 1.5 G, 100 mW/cm2) | 16.6 | 4 |
| NCNCNx|Ni2P with PET substrate [62] | Simulated solar light (AM 1.5 G, 100 mW/cm2) | 17.1 | - |
2.8. Principal Component Analysis (PCA)
3. Methodology
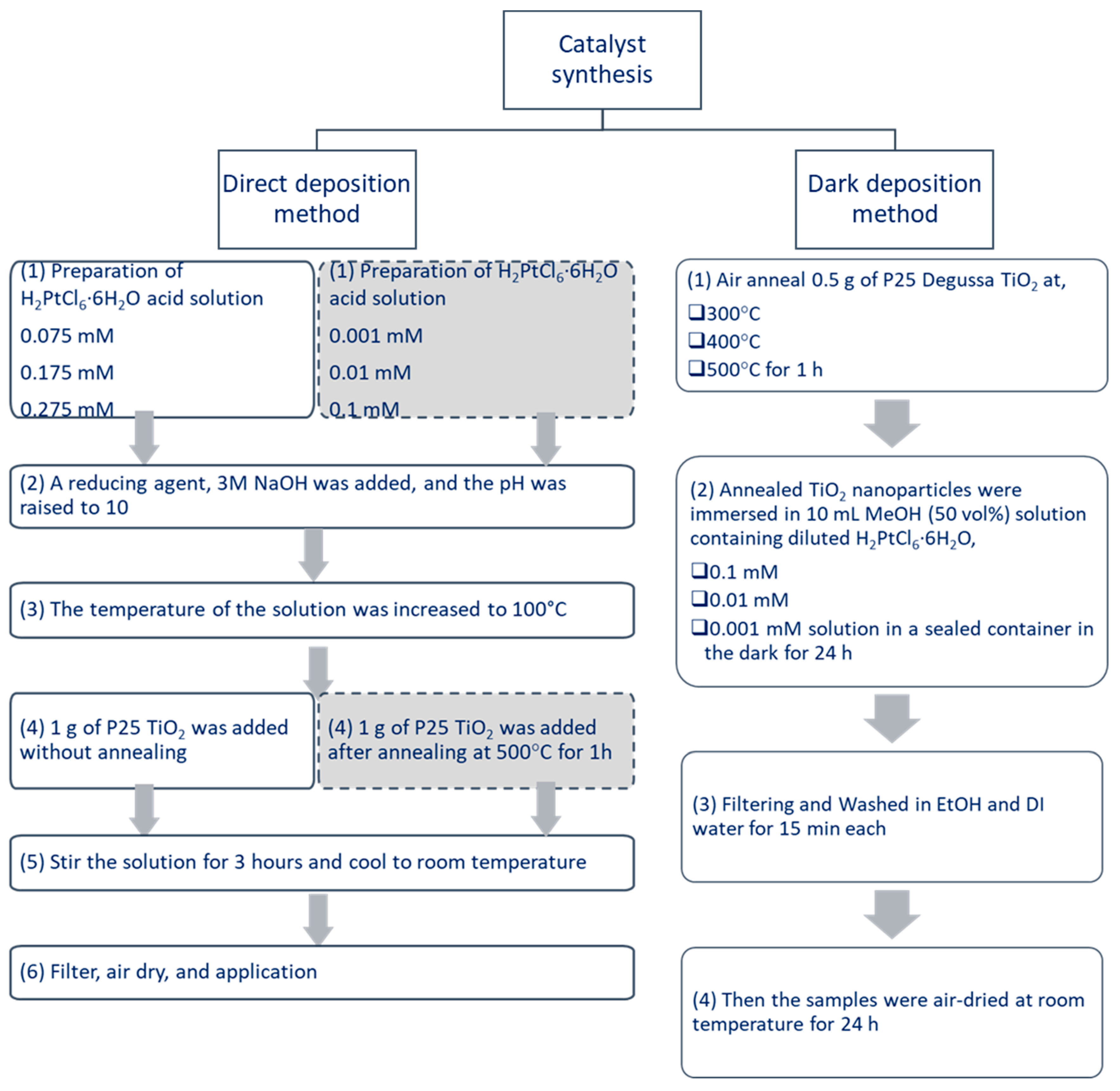
3.1. Dark Deposition Method of Pt/TiO2
3.2. Direct Deposition Method
3.3. Characterization of the Catalysts
3.4. Evaluation of Hydrogen Production
3.5. Method of Principal Component Analysis (PCA)
4. Conclusions and Future Perspectives
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting-An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, S.; Zhang, D.; Li, G.; Leung, M.K.H. Controlling charge transfer in quantum-size titania for photocatalytic applications. Appl. Catal. B Environ. 2017, 215, 85–92. [Google Scholar] [CrossRef]
- Lian, Z.; Wang, W.; Li, G.; Tian, F.; Schanze, K.S.; Li, H. Pt-Enhanced Mesoporous Ti3+/TiO2 with Rapid Bulk to Surface Electron Transfer for Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 16959–16966. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhu, Y.; Wu, Y.; An, X.; Liu, L.-M. Effect of single-atom cocatalysts on the activity of faceted TiO2 photocatalysts. Langmuir 2018, 35, 391–397. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Gihoon, C.; Anca, M.; Imgon, H.; Nikita, D.; Johannes, W.; Tadahiro, Y.; Zdeněk, B.; Giorgio, Z.; Alexander, B.T.; Erdmann, S.; et al. A facile “dark”-deposition approach for Pt single-atom trapping on facetted anatase TiO2 nanoflakes and use in photocatalytic H2 generation. Electrochim. Acta 2022, 412, 140129. [Google Scholar] [CrossRef]
- Yu, X.; Deng, J.; Liu, Y.; Jing, L.; Hou, Z.; Pei, W.; Dai, H. Single-Atom Catalysts: Preparation and Applications in Environmental Catalysis. Catalysts 2022, 12, 1239. [Google Scholar] [CrossRef]
- Li, C.-f.; Pan, W.-g.; Zhang, Z.-r.; Wu, T.; Guo, R.-t. Recent Progress of Single-Atom Photocatalysts Applied in Energy Conversion and Environmental Protection. Small 2023, 19, 2300460. [Google Scholar] [CrossRef]
- Wang, L.; Huang, L.; Liang, F.; Liu, S.; Wang, Y.; Zhang, H. Preparation, characterization and catalytic performance of single-atom catalysts. Chin. J. Catal. 2017, 38, 1528–1539. [Google Scholar]
- Hiragond, C.B.; Powar, N.S.; Lee, J.; In, S.-I. Single-Atom Catalysts (SACs) for Photocatalytic CO2 Reduction with H2O: Activity, Product Selectivity, Stability, and Surface Chemistry. Small 2022, 18, 2201428. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Wu, Y.; Li, Y. Recent advances in the precise control of isolated single-site catalysts by chemical methods. Natl. Sci. Rev. 2018, 5, 673–689. [Google Scholar]
- Saptal, V.B.; Ruta, V.; Bajada, M.A.; Vilé, G. Single-Atom Catalysis in Organic Synthesis. Angew. Chem. Int. Ed. 2023, 62, e202219306. [Google Scholar] [CrossRef]
- Majeed, I.; Ali, H.; Idrees, A.; Arif, A.; Ashraf, W.; Rasul, S.; Khan, M.A.; Nadeem, M.A.; Nadeem, M.A. Understanding the role of metal supported on TiO2 in photoreforming of oxygenates. Energy Adv. 2022, 1, 842–867. [Google Scholar] [CrossRef]
- Choi, Y.; Koo, M.S.; Bokare, A.D.; Kim, D.H.; Bahnemann, D.W.; Choi, W. Sequential Process Combination of Photocatalytic Oxidation and Dark Reduction for the Removal of Organic Pollutants and Cr(VI) using Ag/TiO2. Environ. Sci. Technol. 2017, 51, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.W.; Wu, P.; Xie, R.; Shang, J.K. Palladium Oxide Nanoparticles on Nitrogen-Doped Titanium Oxide: Accelerated Photocatalytic Disinfection and Post-Illumination Catalytic “Memory”. Adv. Mater. 2008, 20, 3717–3723. [Google Scholar] [CrossRef]
- Takai, A.; Kamat, P.V. Capture, store, and discharge. Shuttling photogenerated electrons across TiO2-silver interface. ACS Nano 2011, 5, 7369–7376. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Wang, L.; Zhang, S.; Ma, J.; Dong, W.; Zeng, Y.; Yuan, J.; Liu, C.; Luo, S. “Dark Deposition” of Ag Nanoparticles on TiO2: Improvement of Electron Storage Capacity To Boost “Memory Catalysis” Activity. ACS Appl. Mater. Interfaces 2018, 10, 25350–25359. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Disdier, J.M.; Pichat, P. Photoassisted platinum deposition on TiO2 powder using various platinum complexes. J. Phys. Chem. 1986, 90, 6028–6034. [Google Scholar]
- Lai, W.H.; Miao, Z.; Wang, Y.X.; Wang, J.Z.; Chou, S.L. Atomic-local environments of single-atom catalysts: Synthesis, electronic structure, and activity. Adv. Energy Mater. 2019, 9, 1900722. [Google Scholar]
- Porter, J.F.; Li, Y.-G.; Chan, C.K. The effect of calcination on the microstructural characteristics and photoreactivity of Degussa P-25 TiO2. J. Mater. Sci. 1999, 34, 1523–1531. [Google Scholar]
- He, F.; Ma, F.; Li, J.; Li, T.; Li, G. Effect of calcination temperature on the structural properties and photocatalytic activities of solvothermal synthesized TiO2 hollow nanoparticles. Ceram. Int. 2014, 40, 6441–6446. [Google Scholar]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar]
- Wu, N.-L.; Lee, M.-S.; Pon, Z.-J.; Hsu, J.-Z. Effect of calcination atmosphere on TiO2 photocatalysis in hydrogen production from methanol/water solution. J. Photochem. Photobiol. A Chem. 2004, 163, 277–280. [Google Scholar]
- Lu, G.; Linsebigler, A.; Yates, J.T., Jr. Ti3+ defect sites on TiO2 (110): Production and chemical detection of active sites. J. Phys. Chem. 1994, 98, 11733–11738. [Google Scholar]
- Savva, A.I.; Smith, K.A.; Lawson, M.; Croft, S.R.; Weltner, A.E.; Jones, C.D.; Bull, H.; Simmonds, P.J.; Li, L.; Xiong, H. Defect generation in TiO2 nanotube anodes via heat treatment in various atmospheres for lithium-ion batteries. Phys. Chem. Chem. Phys. 2018, 20, 22537–22546. [Google Scholar] [CrossRef]
- Goodman, D. “Catalytically active Au on Titania:” Yet another example of a strong metal support interaction (SMSI)? Catal. Lett. 2005, 99, 1–4. [Google Scholar]
- Dulub, O.; Hebenstreit, W.; Diebold, U. Imaging cluster surfaces with atomic resolution: The strong metal-support interaction state of Pt supported on TiO2(110). Phys. Rev. Lett. 2000, 84, 3646. [Google Scholar]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Wang, H.B.; Talipov, M.R.; Xu, P.; Wang, H. Photo-reforming and degradation of waste plastics under UV and visible light for H2 production using nanocomposite photocatalysts. J. Environ. Chem. Eng. 2023, 11, 109580. [Google Scholar] [CrossRef]
- Muthee, D.K.; Dejene, B.F. Effect of annealing temperature on structural, optical, and photocatalytic properties of titanium dioxide nanoparticles. Heliyon 2021, 7, e07269. [Google Scholar] [CrossRef]
- Monalisha, G.; Nirab, C.A.; Suparna, B. Effect of annealing temperatures on the structural and optical properties of zinc oxide nanoparticles prepared by chemical precipitation method. Optik 2018, 158, 1006–1015. [Google Scholar] [CrossRef]
- Zaki, M.Y.; Sava, F.; Simandan, I.D.; Buruiana, A.T.; Mihai, C.; Velea, A.; Galca, A.C. Effect of the stacking order, annealing temperature and atmosphere on crystal phase and optical properties of Cu2SnS3. Sci. Rep. 2022, 12, 7958. [Google Scholar] [CrossRef]
- Zarattini, M.; Dun, C.; Isherwood, L.H.; Felten, A.; Filippi, J.; Gordon, M.P.; Zhang, L.; Kassem, O.; Song, X.; Zhang, W.; et al. Synthesis of 2D anatase TiO2 with highly reactive facets by fluorine-free topochemical conversion of 1T-TiS2 nanosheets. J. Mater. Chem. A Mater. 2022, 10, 13884–13894. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Single-atom catalysis: A new field that learns from tradition. Natl. Sci. Rev. 2018, 5, 690–693. [Google Scholar] [CrossRef]
- He, L.; Guan, C.; Bulushev, D.A.; Xiang, Q. Regulation of Metal-Support Interaction in Single-Atom Catalysis. Small 2024, 940, 2410976. [Google Scholar] [CrossRef]
- Al-Hilfi, S.H.; Jiang, X.; Heuer, J.; Akula, S.; Tammeveski, K.; Hu, G.; Yang, J.; Wang, H.I.; Bonn, M.; Landfester, K.; et al. Single-Atom Catalysts through Pressure-Controlled Metal Diffusion. J. Am. Chem. Soc. 2024, 146, 19886–19895. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Li, B.; Li, Z. Single-Atom Long-Range Interaction: Basic Principles and Applications. Adv. Sustain. Syst. 2024, 8, 2300564. [Google Scholar] [CrossRef]
- Bhosale, R.; Hyam, R.; Dhanya, P.; Ogale, S. Chlorate ion mediated rutile to anatase reverse phase transformation in the TiO2 nanosystem. Dalton Trans. 2011, 40, 11374–11377. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A.; Wei, Z.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-Controlled Synthesis of Pt Particles on TiO2 Surface: Physicochemical Characteristic and Photocatalytic Activity. Catalysts 2019, 9, 940. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Ostwald ripening in a Pt/SiO2 model catalyst studied by in situ TEM. J. Catal. 2011, 281, 147–155. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Jovic, V.; Sun-Waterhouse, D.; Murai, K.-i.; Idriss, H.; Waterhouse, G.I.N. Effect of the TiO2 Crystallite Size, TiO2 Polymorph and Test Conditions on the Photo-Oxidation Rate of Aqueous Methylene Blue. Top. Catal. 2015, 58, 85–102. [Google Scholar] [CrossRef]
- Zhou, Y.; Muhich, C.L.; Neltner, B.T.; Weimer, A.W.; Musgrave, C.B. Growth of Pt Particles on the Anatase TiO2 (101) Surface. J. Phys. Chem. C 2012, 116, 12114–12123. [Google Scholar] [CrossRef]
- Prucnal, S.; Gago, R.; Calatayud, D.G.; Rebohle, L.; Liedke, M.O.; Butterling, M.; Wagner, A.; Helm, M.; Zhou, S. TiO2 Phase Engineering by Millisecond Range Annealing for Highly Efficient Photocatalysis. J. Phys. Chem. C 2023, 127, 12686–12694. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2 Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef] [PubMed]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.-R.; Xiao, Y.-Y.; Yin, Y.-C.; Huang, W.-M.; Huang, Z.-C.; Zheng, Y.-Z.; Mu, F.-Y.; Huang, R.; Shi, G.-Y.; et al. Electronic metal–support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Wu, Z.; Hwang, I.; Cha, G.; Qin, S.; Tomanec, O.; Badura, Z.; Kment, S.; Zboril, R.; Schmuki, P. Optimized Pt single atom harvesting on TiO2 nanotubes—Towards a most efficient photocatalyst. Small 2022, 18, 2104892. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Vidya, R.; Anja Olafsen, S. Band gap engineering of anatase TiO2 by ambipolar doping: A first principles study. Mater. Chem. Phys. 2023, 299, 127467. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Meynen, V.; Awan, S.U.; Khan, A.R.; Hussain, S.; Zain-ul-Abdin; Din, S.U.; Hafeez, M.; et al. Effect of Annealing Temperature on Structural Phase Transformations and Band Gap Reduction for Photocatalytic Activity of Mesopores TiO2 Nanocatalysts. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1312–1322. [Google Scholar] [CrossRef]
- Wu, B.; Du, F.; Wang, H.; Wu, C.; Chu, J.; Wang, X.; Xiong, S. Effects of annealing temperature of PtCu/MWCNT catalysts on their electrocatalytic performance of electrooxidation of methanol. Ionics 2022, 28, 369–382. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Lei, Y.; Wang, Q.; Li, A.; Chen, W.; Zhou, G.; Zhang, Z.; Wang, Y.; et al. Engineering the Atomic Interface with Single Platinum Atoms for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2020, 59, 1295–1301. [Google Scholar] [CrossRef]
- Li, X.; Su, Z.; Jiang, H.; Liu, J.; Zheng, L.; Zheng, H.; Wu, S.; Shi, X. Band Structure Tuning via Pt Single Atom Induced Rapid Hydroxyl Radical Generation toward Efficient Photocatalytic Reforming of Lignocellulose into H2. Small 2024, 20, 2400617. [Google Scholar] [CrossRef]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Talipov, M.R.; Wang, H. Optimization of green hydrogen evolution from low-density plastics using TiO2-based nano-photocatalysts with techno-economic and carbon footprint assessment. Nanotechnol. Environ. Eng. 2024, 9, 817–832. [Google Scholar] [CrossRef]
- Han, X.; Jiang, M.; Li, H.; Li, R.; Sulaiman, N.H.M.; Zhang, T.; Li, H.; Zheng, L.; Wei, J.; He, L.; et al. Upcycle polyethylene terephthalate waste by photoreforming: Bifunction of Pt cocatalyst. J. Colloid Interface Sci. 2024, 665, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Carozo, A.M.; López-Tenllado, F.J.; Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; Estevez, R.; Ariza-Pérez, A.; Urbano, F.J.; Marinas, A. Pt-TiO2 Systems for Enhanced Hydrogen Production from Glycerol: Direct vs Sequential Incorporation Through Photodeposition. Materials 2024, 17, 5109. [Google Scholar] [CrossRef]
- Pichler, C.M.; Bhattacharjee, S.; Rahaman, M.; Uekert, T.; Reisner, E. Conversion of Polyethylene Waste into Gaseous Hydrocarbons via Integrated Tandem Chemical–Photo/Electrocatalytic Processes. ACS Catal. 2021, 11, 9159–9167. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from water by the decomposition of poly-vinylchloride, protein, algae, dead insects, and excrement. Chem. Lett. 2006, 10, 81–84. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Zhang, C.; Fan, W.; Li, G.; Fang, J.; Lu, L. Atomically engineering the metal-support interaction of single-atom Cu/TiO2 for efficient polyethylene terephthalate plastic photoreforming. Chem Catal. 2024, 4, 100902. [Google Scholar] [CrossRef]
- Zi, B.; Zheng, H.; Zhou, T.; Lu, Q.; Chen, M.; Xiao, B.; Zhang, Y.; Qiu, Z.; Sun, H.; Zhao, J.; et al. Changeable Active Sites by Pr Doping CuSA-TiO2 Photocatalyst for Excellent Hydrogen Production. Small 2024, 20, 2305779. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chen, X.; Sisay Wolde, G.; Kuo, D.-H. Photocatalytic hydrogen production over highly oxygen deficient Cu-doped TiO2 and its composites: Insights of kinetic reaction micromechanisms. Sep. Purif. Technol. 2024, 339, 126671. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Dong, Q.; Gao, T.; Cao, M.; Zhao, K.; Lichtfouse, E.; Patrocinio, A.O.T.; Wang, C. Photo- and electrochemical processes to convert plastic waste into fuels and high-value chemicals. Chem. Eng. J. 2024, 482, 148827. [Google Scholar] [CrossRef]
- Uekert, T. Plastic and Mixed Waste as Feedstocks for Solar-Driven H2 Production. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2021. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar]
- Origin(Pro), version 2024; OriginLab Corporation: Northampton, MA, USA, 2024.
- Edirisooriya, E.M.N.T.; Punhasa, S.S.; Pei, X.; Huiyao, W. Hydrogen production and value-added chemical recovery from the photo-reforming process using waste plastics. J. Environ. Chem. Eng. 2023, 11, 111429. [Google Scholar] [CrossRef]
- Wang, H.; Edirisooriya, E.M.N.T.; Xu, P. Method of Hydrogen Manufacture. U.S. Patent Application 18/378586, 6 February 2025. [Google Scholar]
- Qin, J.; Dou, Y.; Wu, F.; Yao, Y.; Andersen, H.R.; Hélix-Nielsen, C.; Lim, S.Y.; Zhang, W. In-situ formation of Ag2O in metal-organic framework for light-driven upcycling of microplastics coupled with hydrogen production. Appl. Catal. B Environ. 2022, 319, 121940. [Google Scholar] [CrossRef]

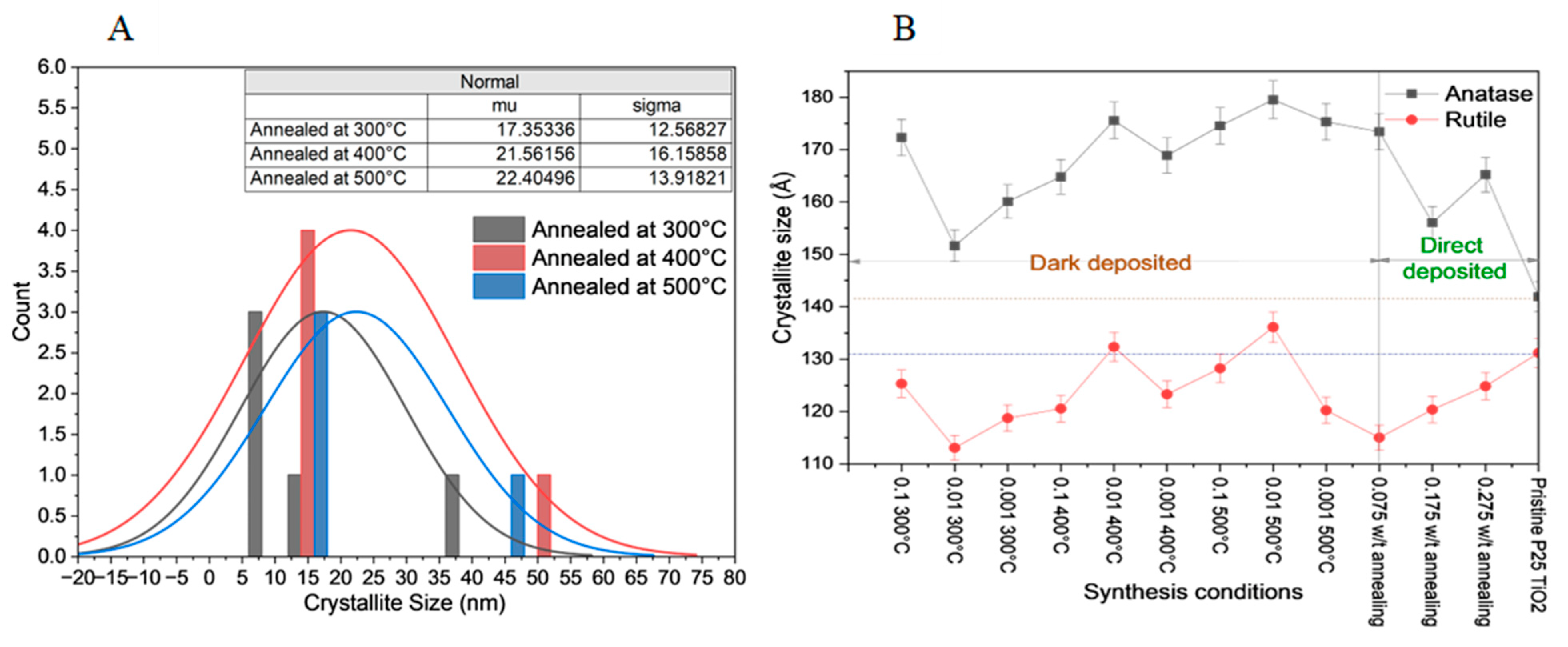
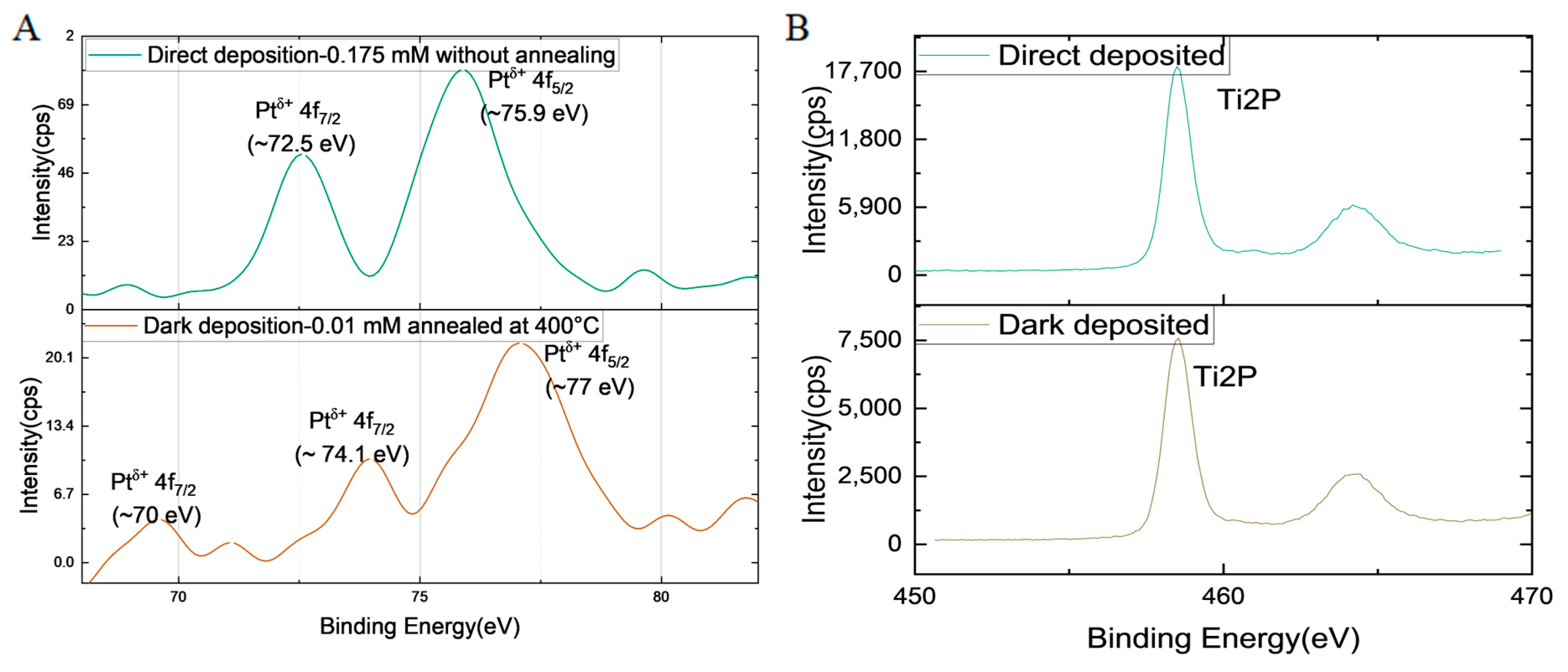


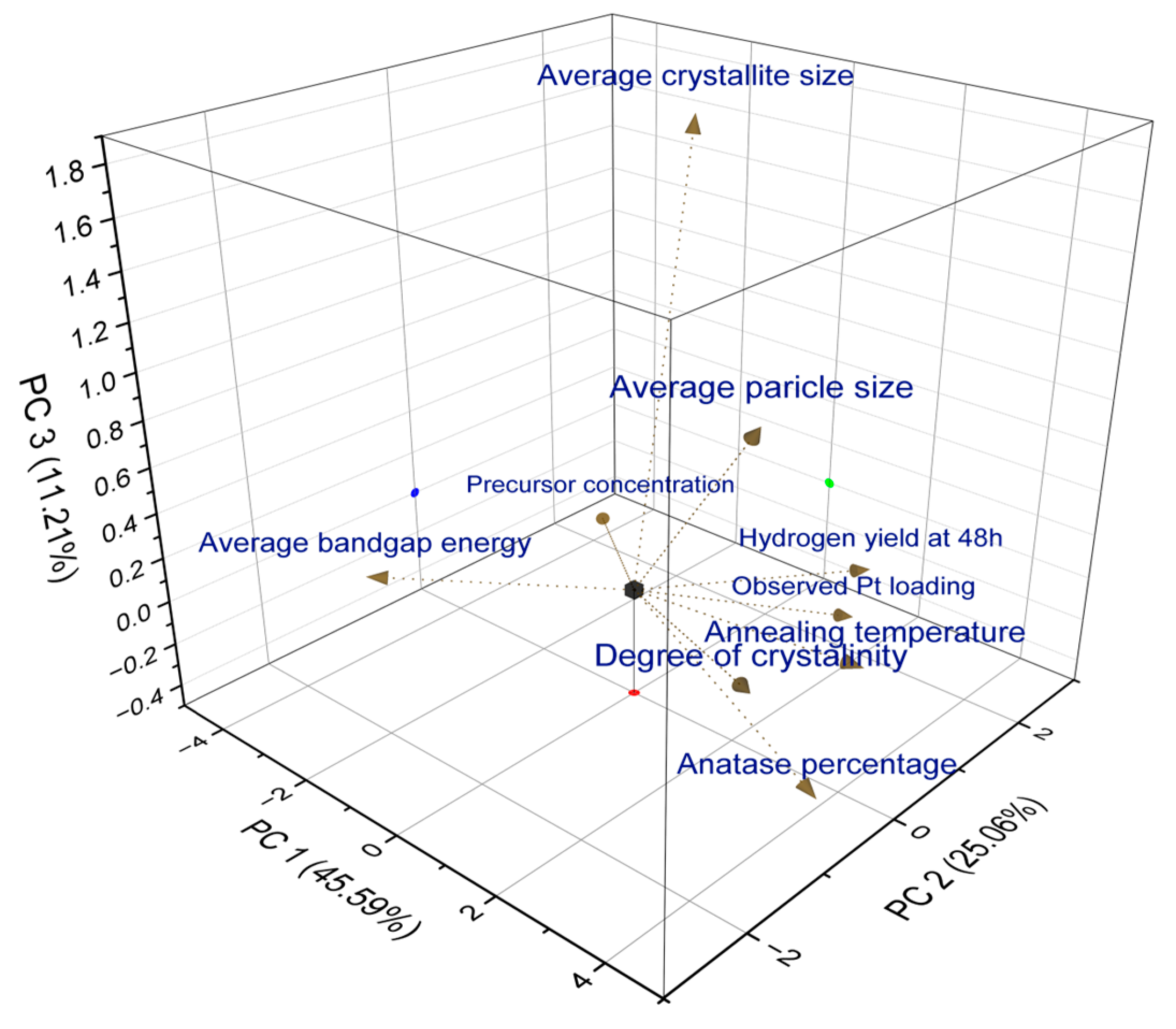
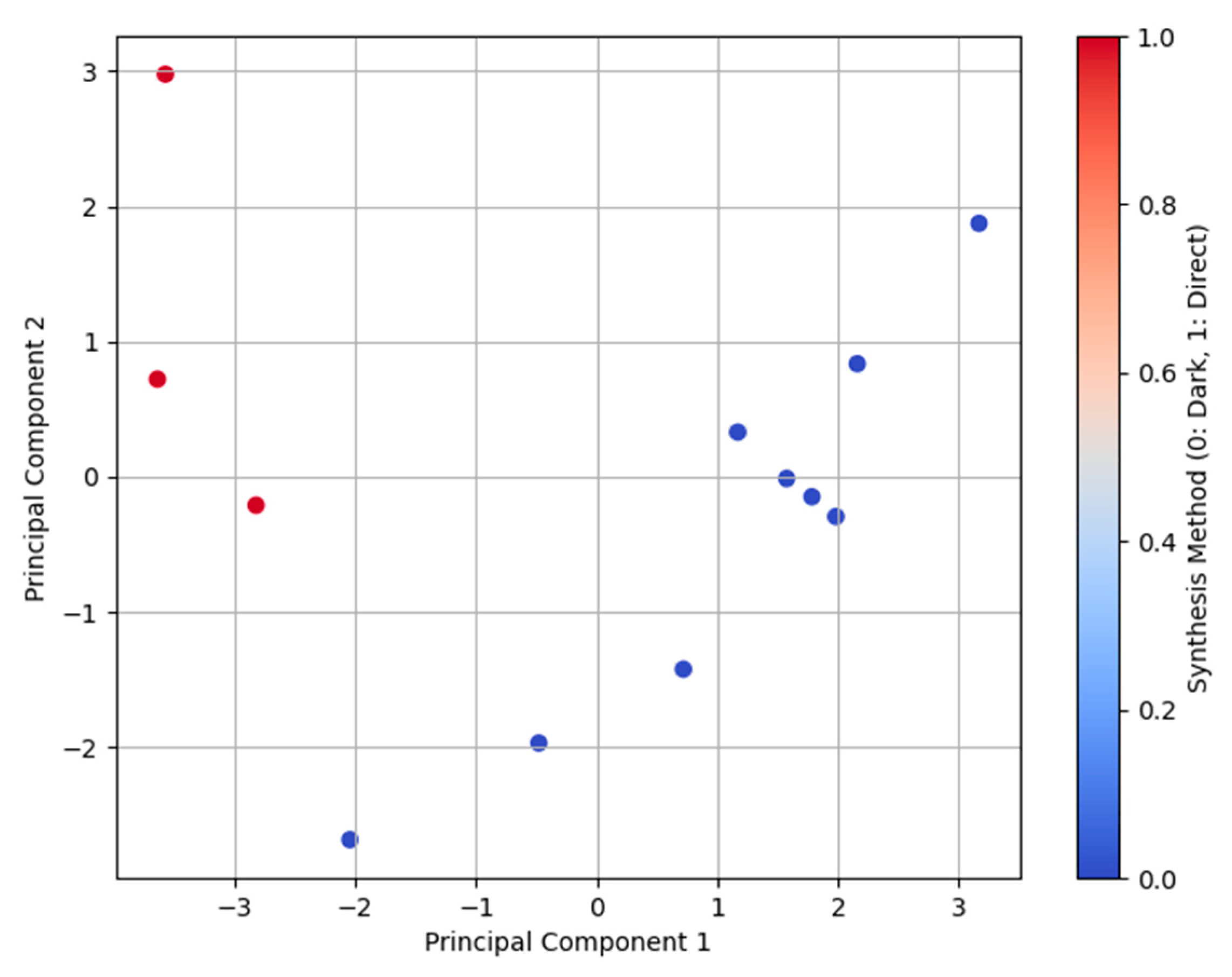
| Synthesis Condition | Degree of Crystallinity (%) | Average Crystallite Size (nm) | Anatase Percentage (%) |
|---|---|---|---|
| 0.1 mM annealed at 300 °C | 83.96 | 20.20 | 92 |
| 0.01 mM annealed at 300 °C | 94.03 | 17.35 | 93 |
| 0.001 mM annealed at 300 °C | 92.01 | 18.11 | 92 |
| 0.1 mM annealed at 400 °C | 89.47 | 20.66 | 91 |
| 0.01 mM annealed at 400 °C | 95.29 | 21.56 | 92 |
| 0.001 mM annealed at 400 °C | 88.45 | 22.12 | 92 |
| 0.1 mM annealed at 500 °C | 88.89 | 20.56 | 94 |
| 0.01 mM annealed at 500 °C | 93.19 | 22.40 | 94 |
| 0.001 mM annealed at 500 °C | 93.89 | 18.51 | 94 |
| 0.075 mM without annealing | 83.16 | 20.52 | 86 |
| 0.175 mM without annealing | 82.74 | 21.80 | 89 |
| 0.275 mM without annealing | 63.99 | 20.54 | 83 |
| Pristine P25 TiO2 | 86.65 | 18.32 | 85 |
| Synthesis Method | Annealing Temperature | Precursor Concentration | Expected Maximum Pt Loading %(w/w) | Measured Pt Loading by ICP-OES %(w/w) |
|---|---|---|---|---|
| Dark deposition method | 300 °C | 0.001 mM | 0.004 | 0.011 |
| 0.010 mM | 0.040 | 0.038 | ||
| 0.100 mM | 0.400 | 0.346 | ||
| 400 °C | 0.001 mM | 0.004 | 0.013 | |
| 0.010 mM | 0.040 | 0.039 | ||
| 0.100 mM | 0.400 | 0.380 | ||
| 500 °C | 0.001 mM | 0.004 | 0.004 | |
| 0.010 mM | 0.040 | 0.035 | ||
| 0.100 mM | 0.400 | 0.362 | ||
| Direct deposition method | 500 °C | 0.001 mM | 0.004 | 0.009 |
| 0.010 mM | 0.040 | 0.018 | ||
| 0.100 mM | 0.400 | 0.012 | ||
| 0.075 mM | 0.300 | 0.024 | ||
| 0.175 mM | 0.700 | 0.022 | ||
| Without annealing | 0.075 mM | 0.300 | 0.004 | |
| 0.175 mM | 0.700 | 0.029 | ||
| 0.275 mM | 1.500 | 0.040 |
| Synthesis Method | Synthesis Conditions | Bandgap (eV) | Maximum Wavelength (nm) | ||
|---|---|---|---|---|---|
| Direct | Indirect | Average | |||
| Dark deposition | 0.1 mM 300 °C | 2.28 | 2.50 | 2.39 | 520 |
| 0.1 mM 400 °C | 2.19 | 2.18 | 2.19 | 567 | |
| 0.1 mM 500 °C | 2.28 | 2.50 | 2.39 | 520 | |
| 0.01 mM 300 °C | 2.48 | 2.19 | 2.34 | 531 | |
| 0.01 mM 400 °C | 2.48 | 2.19 | 2.34 | 531 | |
| 0.01 mM 500 °C | 2.48 | 2.19 | 2.34 | 531 | |
| 0.001 mM 300 °C | 2.97 | 3.00 | 2.99 | 415 | |
| 0.001 mM 400 °C | 2.64 | 2.86 | 2.75 | 451 | |
| 0.001 mM 500 °C | 2.51 | 2.62 | 2.57 | 483 | |
| Direct deposition | 0.075 mM 25 °C | 2.66 | 2.98 | 2.82 | 440 |
| 0.175 mM 25 °C | 2.78 | 2.95 | 2.86 | 434 | |
| 0.275 mM 25 °C | 2.88 | 2.48 | 2.68 | 463 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Wang, H. Enhanced H2 Production Efficiency in Photo-Reforming of PET Waste Plastic Using Dark-Deposited Atom/Nanocomposite Pt/TiO2 Photocatalysts. Catalysts 2025, 15, 334. https://doi.org/10.3390/catal15040334
Edirisooriya EMNT, Senanayake PS, Xu P, Wang H. Enhanced H2 Production Efficiency in Photo-Reforming of PET Waste Plastic Using Dark-Deposited Atom/Nanocomposite Pt/TiO2 Photocatalysts. Catalysts. 2025; 15(4):334. https://doi.org/10.3390/catal15040334
Chicago/Turabian StyleEdirisooriya, E. M. N. Thiloka, Punhasa S. Senanayake, Pei Xu, and Huiyao Wang. 2025. "Enhanced H2 Production Efficiency in Photo-Reforming of PET Waste Plastic Using Dark-Deposited Atom/Nanocomposite Pt/TiO2 Photocatalysts" Catalysts 15, no. 4: 334. https://doi.org/10.3390/catal15040334
APA StyleEdirisooriya, E. M. N. T., Senanayake, P. S., Xu, P., & Wang, H. (2025). Enhanced H2 Production Efficiency in Photo-Reforming of PET Waste Plastic Using Dark-Deposited Atom/Nanocomposite Pt/TiO2 Photocatalysts. Catalysts, 15(4), 334. https://doi.org/10.3390/catal15040334










