MOF-808 as Effective Support for Cu-Based Catalyst for CO2 Hydrogenation to Methanol
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. Inductively Coupled Plasma (ICP) Analysis and N2 Physisorption
2.1.2. XRD Characterization
2.1.3. SEM and TEM Characterization
2.1.4. Temperature-Programmed Reduction (TPR) and N2O Chemisorption
2.1.5. XPS Analysis
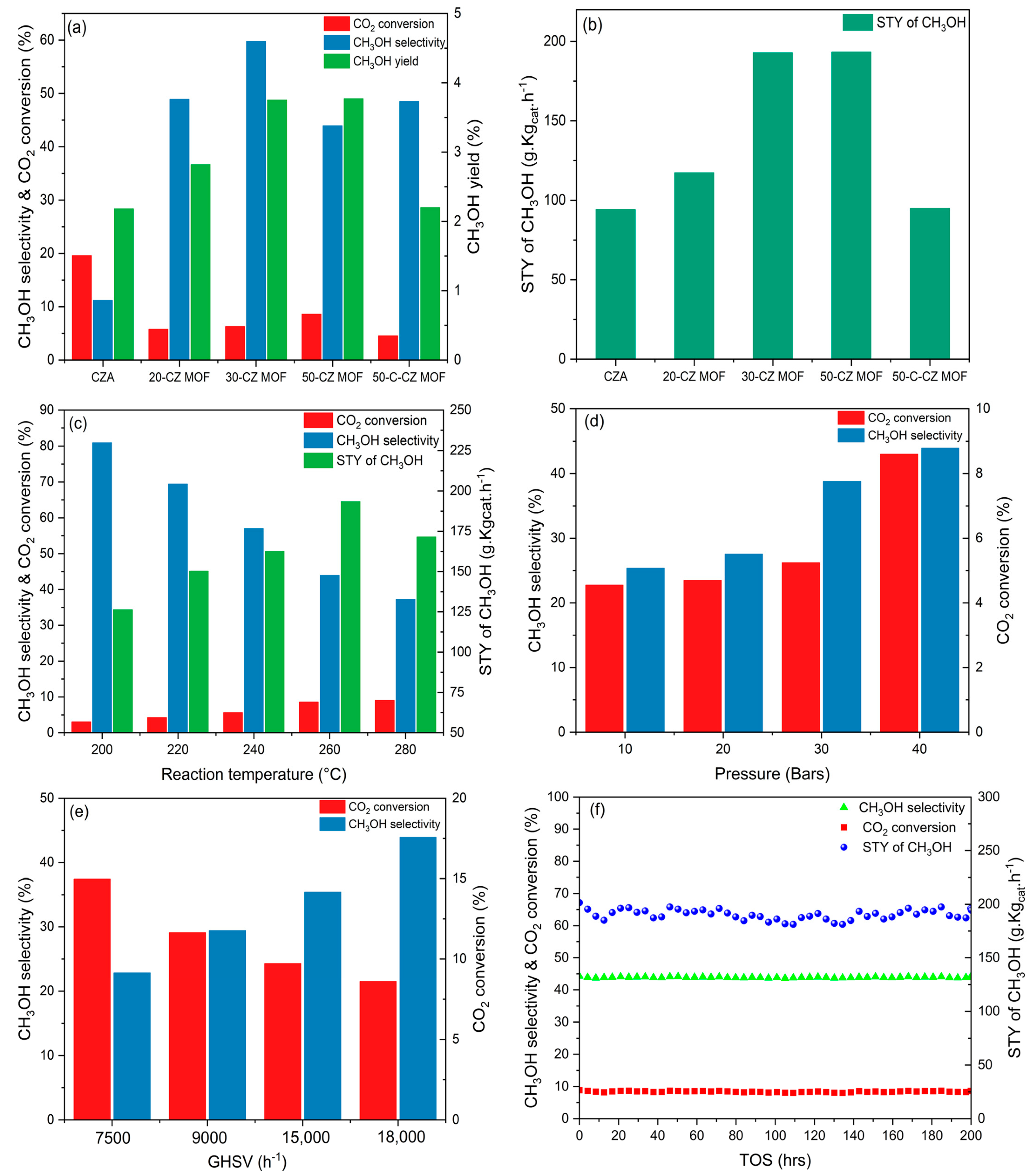
2.2. Activity Tests
3. Materials and Methods
3.1. Catalyst Preparation
- a.
- Synthesis of Cu/ZnO/Al2O3 (CZA) catalyst
- b.
- MOF-808 and CZ MOF catalyst synthesis
3.2. Catalyst Characterization
3.3. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Rosado, P.; Roser, M. Emissions by Sector; Our World in Data: Oxford, UK, 2023. [Google Scholar]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar]
- Alshalif, A.F.; Irwan, J.M.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S. A systematic review on bio-sequestration of carbon dioxide in bio-concrete systems: A future direction. Eur. J. Environ. Civ. Eng. 2022, 26, 1209–1228. [Google Scholar]
- Deng, L.; Wang, Z.; Jiang, X.; Xu, J.; Zhou, Z.; Li, X.; You, Z.; Ding, M.; Shishido, T.; Liu, X.; et al. Catalytic aqueous CO2 reduction to formaldehyde at Ru surface on hydroxyl-groups-rich LDH under mild conditions. Appl. Catal. B Environ. 2023, 322, 122124. [Google Scholar]
- Kalck, P.; Le Berre, C.; Serp, P. Recent advances in the methanol carbonylation reaction into acetic acid. Coord. Chem. Rev. 2020, 402, 213078. [Google Scholar] [CrossRef]
- Kubas, D.; Gierse, M.; Salem, O.; Krossing, I. Is Direct DME Synthesis Superior to Methanol Production in Carbon Dioxide Valorization? From Thermodynamic Predictions to Experimental Confirmation. ACS Catal. 2023, 13, 3960–3970. [Google Scholar]
- Ye, Y.; Abou-Hamad, E.; Gong, X.; Shoinkhorova, T.B.; Dokania, A.; Gascon, J.; Chowdhury, A.D. Mapping the Methanol-to-Gasoline Process Over Zeolite Beta. Angew. Chem. Int. Ed. 2023, 62, e202303124. [Google Scholar]
- Wang, X.; Zeng, C.; Gong, N.; Zhang, T.; Wu, Y.; Zhang, J.; Song, F.; Yang, G.; Tan, Y. Effective Suppression of CO Selectivity for CO2 Hydrogenation to High-Quality Gasoline. ACS Catal. 2021, 11, 1528–1547. [Google Scholar]
- Cui, X.; Liu, Y.; Yan, W.; Xue, Y.; Mei, Y.; Li, J.; Gao, X.; Zhang, H.; Zhu, S.; Niu, Y.; et al. Enhancing methanol selectivity of commercial Cu/ZnO/Al2O3 catalyst in CO2 hydrogenation by surface silylation. Appl. Catal. B Environ. 2023, 339, 123099. [Google Scholar]
- Chen, F.; Zhang, P.; Zeng, Y.; Kosol, R.; Xiao, L.; Feng, X.; Li, J.; Liu, G.; Wu, J.; Yang, G.; et al. Vapor-phase low-temperature methanol synthesis from CO2-containing syngas via self-catalysis of methanol and Cu/ZnO catalysts prepared by solid-state method. Appl. Catal. B Environ. 2020, 279, 119382. [Google Scholar]
- Saedy, S.; Newton, M.A.; Zabilskiy, M.; Lee, J.H.; Krumeich, F.; Ranocchiari, M.; van Bokhoven, J.A. Copper–zinc oxide interface as a methanol-selective structure in Cu–ZnO catalyst during catalytic hydrogenation of carbon dioxide to methanol. Catal. Sci. Technol. 2022, 12, 2703–2716. [Google Scholar]
- Niu, J.; Liu, H.; Jin, Y.; Fan, B.; Qi, W.; Ran, J. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis. Int. J. Hydrogen Energy 2022, 47, 9183–9200. [Google Scholar]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Nagababu, P.; Prabhu, Y.T.; Kularkar, A.; Subbalakshmi, M.S.; Nagarkar, J.; Rayalu, S. Manifestation of Cu-MOF-templated TiO2 nanocomposite for synergistic photoreduction of CO2 to methanol production. Emergent Mater. 2021, 4, 503–514. [Google Scholar] [CrossRef]
- Zou, N.; Qiu, T.; Zheng, Y. Synthesis of MOFs catalyst containing Cu(I) sites for CO2 hydrogenation: Room-temperature controlled reduction via plasma reducing treatment strategy. Catal. Today 2023, 421, 114176. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, X.; Qiu, Z.; Feng, B.; Liu, Y.; Xing, A.; Fan, M. Improved methanol synthesis performance of Cu/ZnO/Al2O3 catalyst by controlling its precursor structure. Green Energy Environ. 2022, 7, 772–781. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.L.; et al. The Active Site of Methanol Synthesis over Cu/ZnO/Al2O3 Industrial Catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Qaderi, J. A brief review on the reaction mechanisms of CO2 hydrogenation into methanol. Int. J. Innov. Res. Sci. Stud. 2020, 3, 33–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Kang, J.; Yu, Z.; Tian, J.; Gong, Z.; Jia, A.; You, R.; Qian, K.; He, S.; et al. The active sites of Cu–ZnO catalysts for water gas shift and CO hydrogenation reactions. Nat. Commun. 2021, 12, 4331. [Google Scholar] [CrossRef]
- Huang, C.; Wen, J.; Sun, Y.; Zhang, M.; Bao, Y.; Zhang, Y.; Liang, L.; Fu, M.; Wu, J.; Ye, D.; et al. CO2 hydrogenation to methanol over Cu/ZnO plate model catalyst: Effects of reducing gas induced Cu nanoparticle morphology. Chem. Eng. J. 2019, 374, 221–230. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Lv, Y.; Liu, P.; Hao, F.; Xiong, W.; Luo, H. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy. Fuel 2022, 314, 123035. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Liu, J.; Zhang, J.; Zhang, F.; Zhou, T.; Rui, N.; Yao, S.; Deng, Y.; Yang, F.; et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 2020, 11, 5767. [Google Scholar] [CrossRef]
- Yang, M.; Yu, J.; Tong, X.; Sun, X.; Xu, H.; Sun, J. Flame-made Cu/ZrO2catalysts with metastable phase and strengthened interactions for CO2 hydrogenation to methanol. Chem. Commun. 2021, 57, 7509–7512. [Google Scholar]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy Chem. 2020, 40, 22–30. [Google Scholar]
- Tan, Q.; Shi, Z.; Wu, D. CO2 Hydrogenation to Methanol over a Highly Active Cu–Ni/CeO2–Nanotube Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar]
- Yan, Y.; Wong, R.J.; Ma, Z.; Donat, F.; Xi, S.; Saqline, S.; Fan, Q.; Du, Y.; Borgna, A.; He, Q.; et al. CO2 hydrogenation to methanol on tungsten-doped Cu/CeO2 catalysts. Appl. Catal. B Environ. 2022, 306, 121098. [Google Scholar] [CrossRef]
- Qi, T.; Zhao, Y.; Chen, S.; Li, W.; Guo, X.; Zhang, Y.; Song, C. Bimetallic metal organic framework-templated synthesis of a Cu-ZnO/Al2O3 catalyst with superior methanol selectivity for CO2 hydrogenation. Mol. Catal. 2021, 514, 111870. [Google Scholar]
- Lam, E.; Corral-Pérez, J.J.; Larmier, K.; Noh, G.; Wolf, P.; Comas-Vives, A.; Urakawa, A.; Copéret, C. CO2 Hydrogenation on Cu/Al2O3: Role of the Metal/Support Interface in Driving Activity and Selectivity of a Bifunctional Catalyst. Angew. Chem. Int. Ed. 2019, 58, 13989–13996. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Li, J.; Hu, Y.; Duanmu, C. Enhanced CO2 Hydrogenation to Methanol on the Mesostructured Cu–ZnO/Al2O3–ZrO2 Catalyst. ACS Appl. Energy Mater. 2021, 4, 8311–8321. [Google Scholar]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal–Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar]
- Liu, T.; Hong, X.; Liu, G. In Situ Generation of the Cu@3D-ZrOx Framework Catalyst for Selective Methanol Synthesis from CO2/H2. ACS Catal. 2020, 10, 93–102. [Google Scholar]
- Mitsuka, Y.; Ogiwara, N.; Mukoyoshi, M.; Kitagawa, H.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Haneda, M.; Kawaguchi, S.; Kubota, Y.; et al. Fabrication of Integrated Copper-Based Nanoparticles/Amorphous Metal–Organic Framework by a Facile Spray-Drying Method: Highly Enhanced CO2 Hydrogenation Activity for Methanol Synthesis. Angew. Chem. Int. Ed. 2021, 60, 22283–22288. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, J.; Ye, J.; Cui, Y.; Koh, K.; Kovarik, L.; Camaioni, D.M.; Fulton, J.L.; Truhlar, D.G.; Neurock, M.; et al. Copper-zirconia interfaces in UiO-66 enable selective catalytic hydrogenation of CO2 to methanol. Nat. Commun. 2020, 11, 5849. [Google Scholar] [PubMed]
- Stawowy, M.; Ciesielski, R.; Maniecki, T.; Matus, K.; Łużny, R.; Trawczynski, J.; Silvestre-Albero, J.; Łamacz, A. CO2 Hydrogenation to Methanol over Ce and Zr Containing UiO-66 and Cu/UiO-66. Catalysts 2020, 10, 39. [Google Scholar]
- Candian Firmino Marcos, F.; Costa, M.J.F.; Catuzo, G.L.; de Moraes, D.A.; Junior, M.d.O.; Mastelaro, V.R.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Supported Cu catalysts on UiO-66 toward enhanced methanol selectivity by CO2 hydrogenation: Effect of Cu loading. J. Catal. 2023, 427, 115104. [Google Scholar]
- Song, M.; Liu, T.; Hong, X.; Liu, G. Coordination Environment Dependent Surface Cu State for CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2023, 11, 12135–12144. [Google Scholar]
- Wang, C.; Kosari, M.; Xi, S.; Zeng, H.C. Uniform Si-Infused UiO-66 as a Robust Catalyst Host for Efficient CO2 Hydrogenation to Methanol. Adv. Funct. Mater. 2023, 33, 2212478. [Google Scholar]
- Yu, J.; Chen, G.; Guo, Q.; Guo, X.; Da Costa, P.; Mao, D. Ultrasmall bimetallic Cu/ZnOx nanoparticles encapsulated in UiO-66 by deposition–precipitation method for CO2 hydrogenation to methanol. Fuel 2022, 324, 124694. [Google Scholar]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar]
- Aunan, E.; Affolter, C.W.; Olsbye, U.; Lillerud, K.P. Modulation of the Thermochemical Stability and Adsorptive Properties of MOF-808 by the Selection of Non-Structural Ligands. Chem. Mater. 2021, 33, 1471–1476. [Google Scholar]
- Han, C.; Zhang, H.; Li, C.; Huang, H.; Wang, S.; Wang, P.; Li, J. The regulation of Cu-ZnO interface by Cu-Zn bimetallic metal organic framework-templated strategy for enhanced CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2022, 643, 118805. [Google Scholar]
- Zhao, L.; Zhang, L.; Wu, Z.; Huang, C.; Chen, K.; Wang, H.; Yang, F. Size Effect of Cu Particles on Interface Formation in Cu/ZnO Catalysts for Methanol Synthesis. Catalysts 2023, 13, 1190. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Moafor, S.N.; Tsobnang, P.K.; Oyedotun, K.O.; Fomekong, R.L.; Kabongo, G.L.; Lebohang, M.; Lambi, J.N.; Jewell, L.L. Effect of SiO2/Al2O3 ratio on the electrochemical performance of amorphous zeolite loaded with cobalt oxide grown via steam-assisted crystallization method. RSC Adv. 2023, 13, 21393–21402. [Google Scholar] [CrossRef]
- Golunski, S.; Burch, R. CO2 Hydrogenation to Methanol over Copper Catalysts: Learning from Syngas Conversion. Top. Catal. 2021, 64, 974–983. [Google Scholar] [CrossRef]
- Ahouari, H.; Soualah, A.; Le Valant, A.; Pinard, L.; Magnoux, P.; Pouilloux, Y. Methanol synthesis from CO2 hydrogenation over copper based catalysts. React. Kinet. Mech. Catal. 2013, 110, 131–145. [Google Scholar] [CrossRef]
- Wang, Y.; Kattel, S.; Gao, W.; Li, K.; Liu, P.; Chen, J.G.; Wang, H. Exploring the ternary interactions in Cu–ZnO–ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Barberis, L.; Hakimioun, A.H.; Plessow, P.N.; Visser, N.L.; Stewart, J.A.; Vandegehuchte, B.D.; Studt, F.; de Jongh, P.E. Competition between reverse water gas shift reaction and methanol synthesis from CO2: Influence of copper particle size. Nanoscale 2022, 14, 13551–13560. [Google Scholar] [CrossRef]
- Guo, S.-J.; Wang, H.; Qin, Z.-F.; Li, Z.-K.; Wang, G.-F.; Dong, M.; Fan, W.-B.; Wang, J.-G. Conversion of the CO and CO2 mixture to alcohols and hydrocarbons by hydrogenation under the influence of the water-gas shift reaction, a thermodynamic consideration. J. Fuel Chem. Technol. 2023, 51, 482–491. [Google Scholar]
- Behrens, M.; Brennecke, D.; Girgsdies, F.; Kißner, S.; Trunschke, A.; Nasrudin, N.; Zakaria, S.; Idris, N.F.; Hamid, S.B.A.; Kniep, B.; et al. Understanding the complexity of a catalyst synthesis: Co-precipitation of mixed Cu,Zn,Al hydroxycarbonate precursors for Cu/ZnO/Al2O3 catalysts investigated by titration experiments. Appl. Catal. A Gen. 2011, 392, 93–102. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, G.; Yang, L.; Li, F. Tuning surface-interface structures of ZrO2 supported copper catalysts by in situ introduction of indium to promote CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2020, 605, 117805. [Google Scholar] [CrossRef]
- Duma, Z.G.; Dyosiba, X.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M.; Duma, Z.G.; Dyosiba, X.; Moma, J.; et al. Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks. Catalysts 2022, 12, 401. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, X.; Boreriboon, N.; Wang, Z.; Song, C.; Cen, K. Effects of supports on bimetallic Pd-Cu catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 585, 117210. [Google Scholar] [CrossRef]
- Zhang, J.; An, B.; Li, Z.; Cao, Y.; Dai, Y.; Wang, W.; Zeng, L.; Lin, W.; Wang, C. Neighboring Zn–Zr Sites in a Metal–Organic Framework for CO2 Hydrogenation. J. Am. Chem. Soc. 2021, 143, 8829–8837. [Google Scholar] [CrossRef]
- Zhang, J.; An, B.; Cao, Y.; Li, Z.; Chen, J.; He, X.; Wang, C. ZnO Supported on a Zr-Based Metal–Organic Framework for Selective CO2 Hydrogenation to Methanol. ACS Appl. Energy Mater. 2021, 4, 13567–13574. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Yang, B.; Guo, L. A highly active and selective mesostructured Cu/AlCeO catalyst for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 571, 51–60. [Google Scholar] [CrossRef]
- Yao, L.; Shen, X.; Pan, Y.; Peng, Z. Synergy between active sites of Cu-In-Zr-O catalyst in CO2 hydrogenation to methanol. J. Catal. 2019, 372, 74–85. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Mu, X.; Yang, G.; Luo, X.; Lester, E.; Wu, T. Cu-ZrO2 catalysts with highly dispersed Cu nanoclusters derived from ZrO2@ HKUST-1 composites for the enhanced CO2 hydrogenation to methanol. Chem. Eng. J. 2021, 419, 129656. [Google Scholar] [CrossRef]
- Han, X.; Li, M.; Chang, X.; Hao, Z.; Chen, J.; Pan, Y.; Kawi, S.; Ma, X. Hollow structured Cu@ZrO2 derived from Zr-MOF for selective hydrogenation of CO2 to methanol. J. Energy Chem. 2022, 71, 277–287. [Google Scholar] [CrossRef]
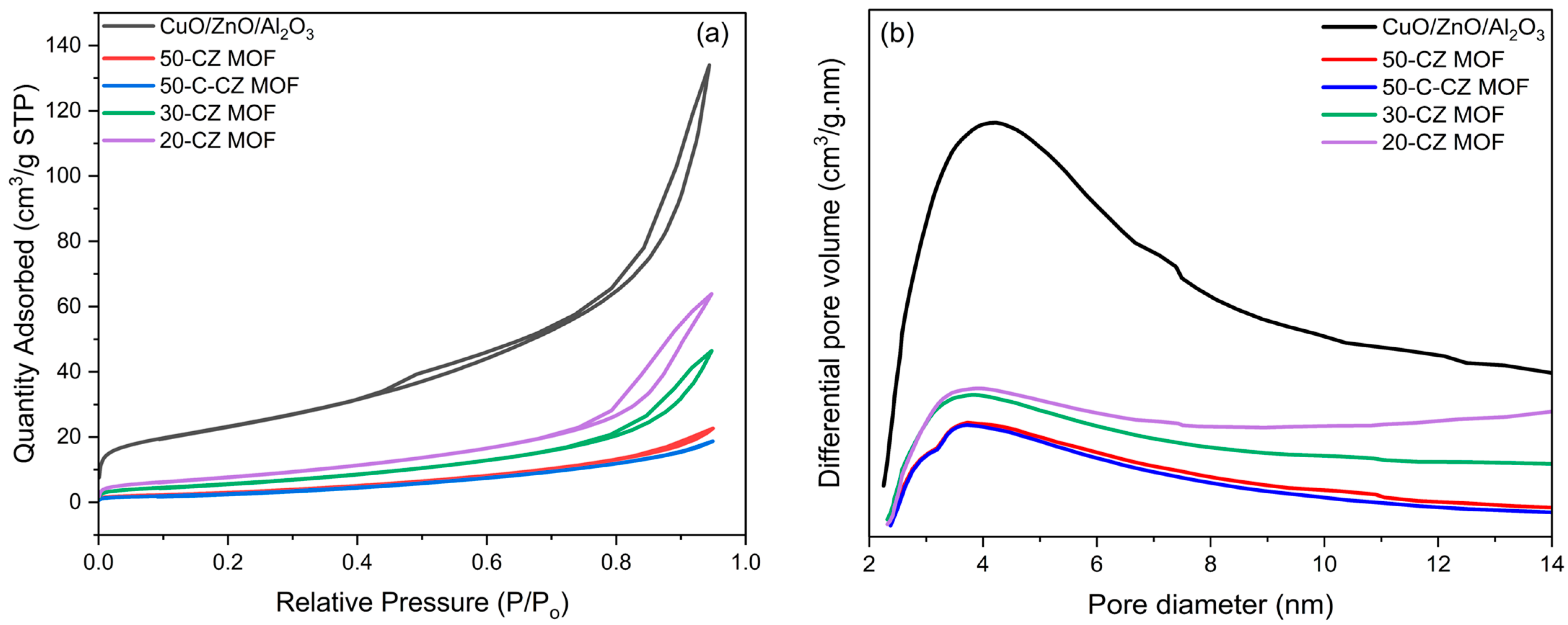
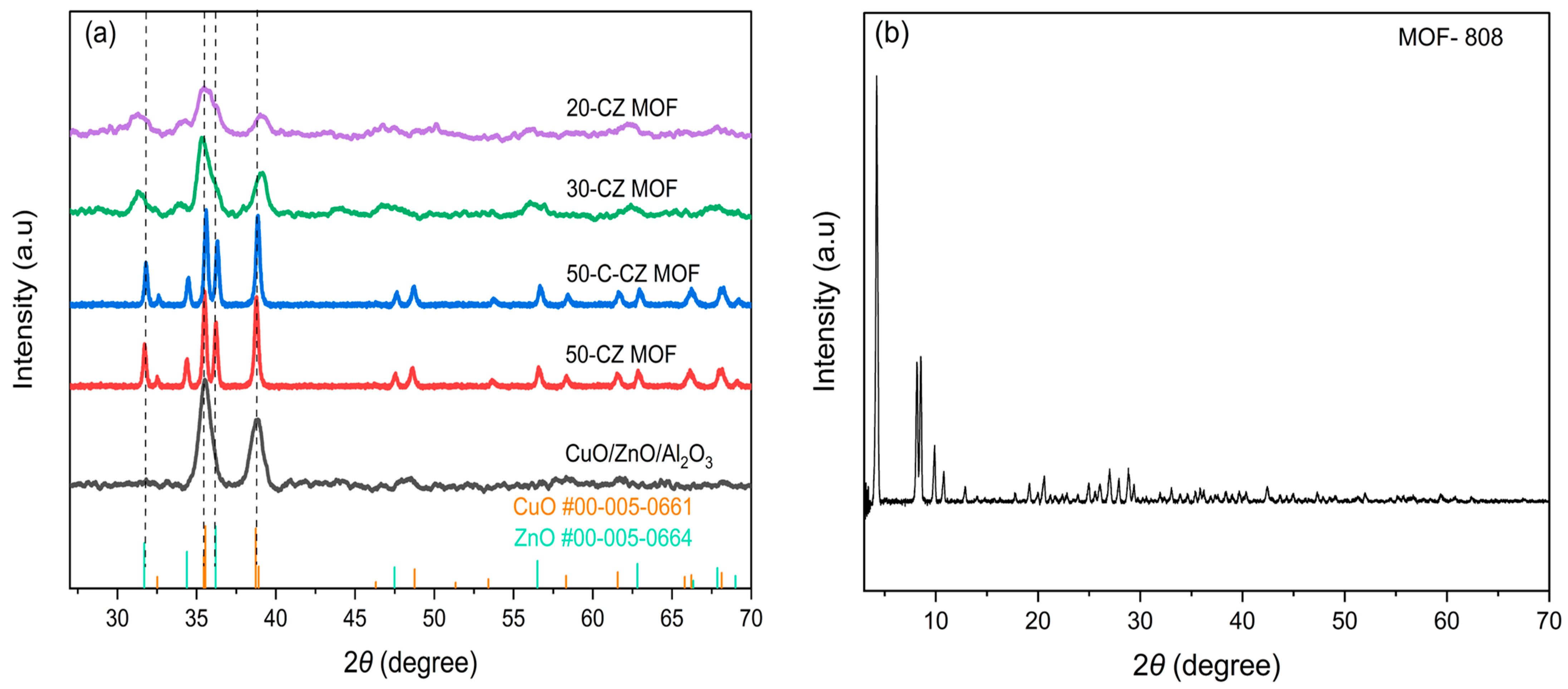

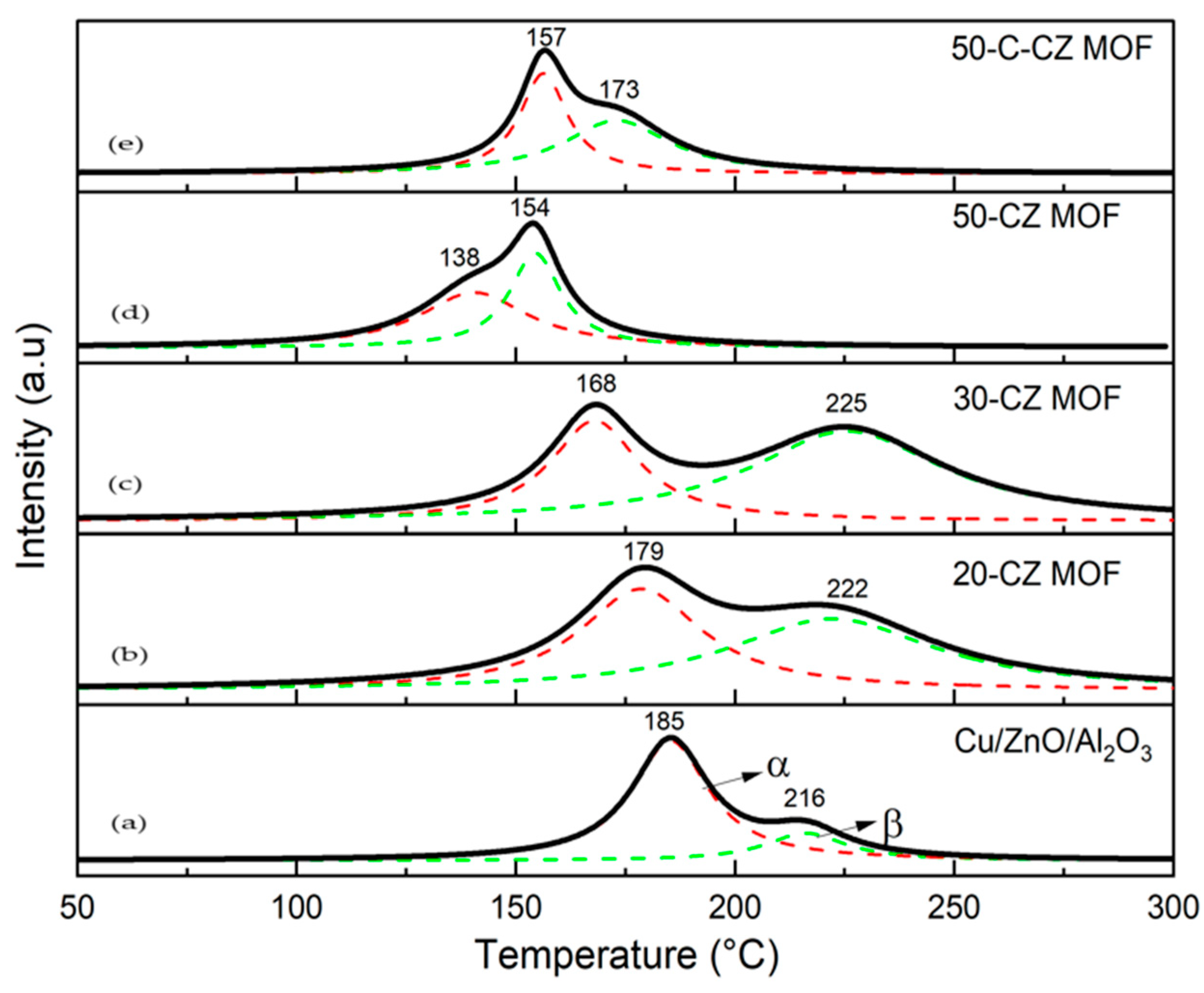
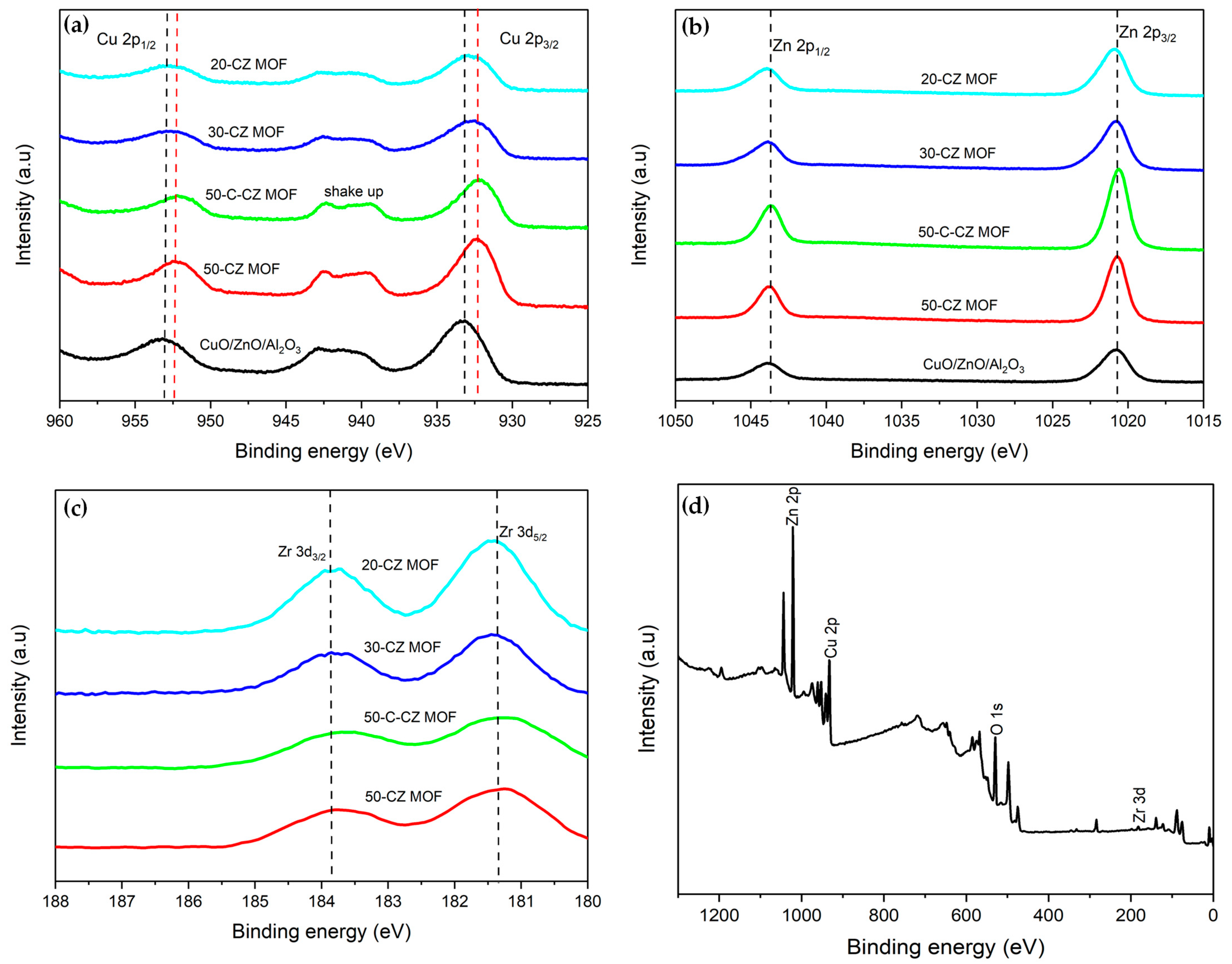
| Catalysts | Theoretical Loading (wt.%) | Actual Loading a (wt.%) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Al | Cu a | Zn a | Al a | ||||
| CZA | 50 | 30 | 10 | 46 | 21 | 7 | 84.52 | 0.177 | 8.4 |
| 20-CZ MOF | 20 | 10 | 22 | 11 | - | 31.15 | 0.092 | 7.0 | |
| 30-CZ MOF | 30 | 15 | 29 | 14 | - | 24.23 | 0.063 | 6.2 | |
| 50-CZ MOF | 50 | 25 | 43 | 20 | - | 15.64 | 0.026 | 6.0 | |
| 50-C-CZ MOF * | 50 | 25 | 49 | 24 | - | 15.18 | 0.033 | 8.4 | |
| MOF-808 | - | - | - | - | - | - | 2143.20 | 0.911 | 1.5 |
| Catalysts | Scu (m2/g) a | TOF (h−1) | DCu (%) a | D (nm) b |
|---|---|---|---|---|
| CZA | 26.07 | 4.61 | 8.42 | 9.39 |
| 20-CZ MOF | 6.14 | 24.43 | 4.14 | 7.71 |
| 30-CZ MOF | 9.40 | 26.22 | 4.70 | 8.14 |
| 50-CZ MOF | 15.10 | 47.44 | 5.21 | 9.91 |
| 50-C-CZ MOF | 10.37 | 38.64 | 3.14 | 9.96 |
| Catalyst | STY (gMeOH·Kgcat−1 h−1) | CO2 Conversion (%) | CH3OH Selectivity (%) | CO Selectivity (%) | CH3OH Yield (%) |
|---|---|---|---|---|---|
| CZA | 94.11 | 19.57 | 11.17 | 88.83 | 2.23 |
| 20-CZ MOF | 117.32 | 5.78 | 48.90 | 51.10 | 2.83 |
| 30-CZ MOF | 192.78 | 6.29 | 59.76 | 40.24 | 3.75 |
| 50-CZ MOF | 193.32 | 8.60 | 43.93 | 56.07 | 3.78 |
| 50-C-CZ MOF | 94.91 | 4.54 | 48.49 | 51.51 | 2.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramakrishnan, A.; Rathod, S.; Tucho, W.M.; Chavan, S.M.; Yu, Z. MOF-808 as Effective Support for Cu-Based Catalyst for CO2 Hydrogenation to Methanol. Catalysts 2025, 15, 324. https://doi.org/10.3390/catal15040324
Ramakrishnan A, Rathod S, Tucho WM, Chavan SM, Yu Z. MOF-808 as Effective Support for Cu-Based Catalyst for CO2 Hydrogenation to Methanol. Catalysts. 2025; 15(4):324. https://doi.org/10.3390/catal15040324
Chicago/Turabian StyleRamakrishnan, Abinavnataraj, Simmy Rathod, Wakshum Mekonnen Tucho, Sachin M. Chavan, and Zhixin Yu. 2025. "MOF-808 as Effective Support for Cu-Based Catalyst for CO2 Hydrogenation to Methanol" Catalysts 15, no. 4: 324. https://doi.org/10.3390/catal15040324
APA StyleRamakrishnan, A., Rathod, S., Tucho, W. M., Chavan, S. M., & Yu, Z. (2025). MOF-808 as Effective Support for Cu-Based Catalyst for CO2 Hydrogenation to Methanol. Catalysts, 15(4), 324. https://doi.org/10.3390/catal15040324









