Application of Clay-Based Catalysts in Co-Conversion and Co-Pyrolysis of Chlorella and Oil Shale
Abstract
1. Introduction
2. Results and Discussion
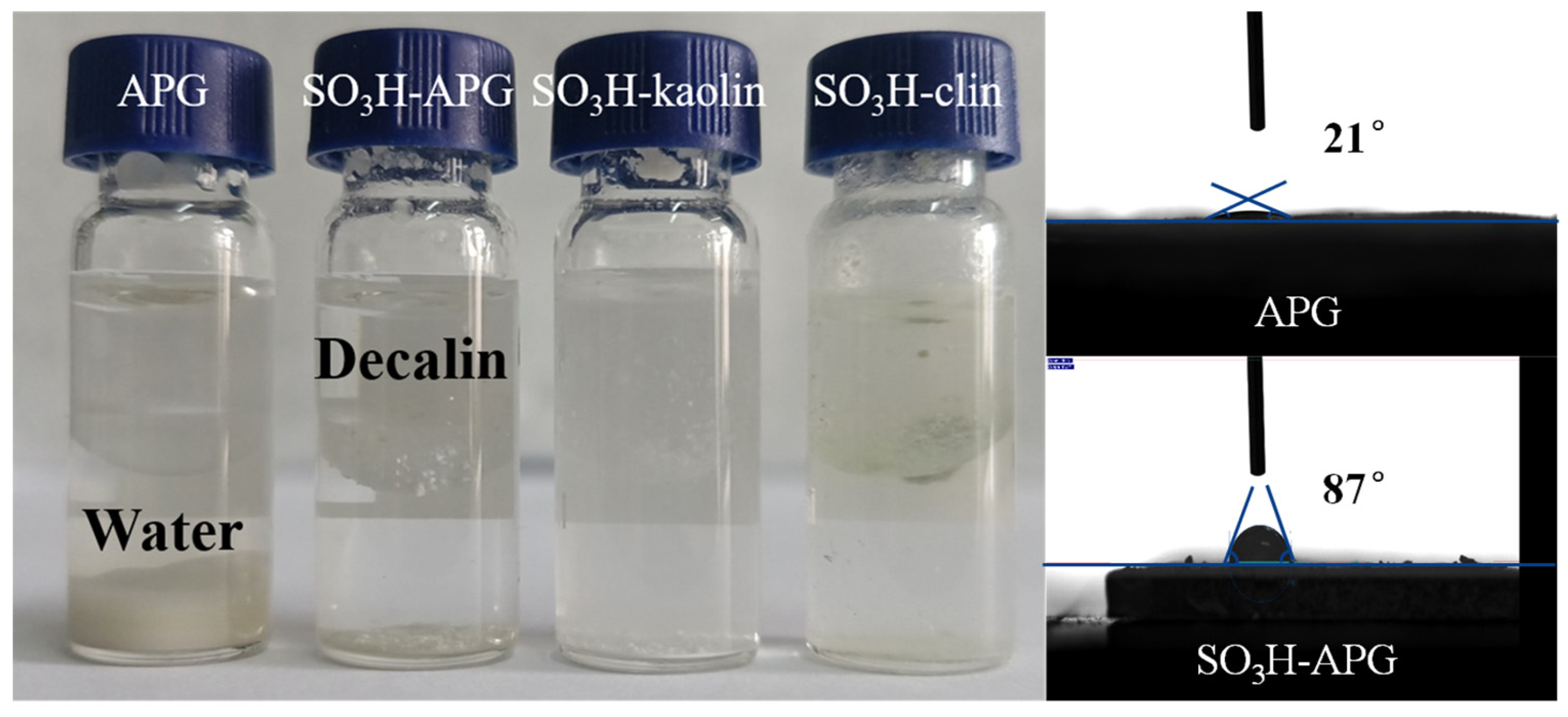
2.1. Hydrophobicity Tests of Clay-Based Catalysts
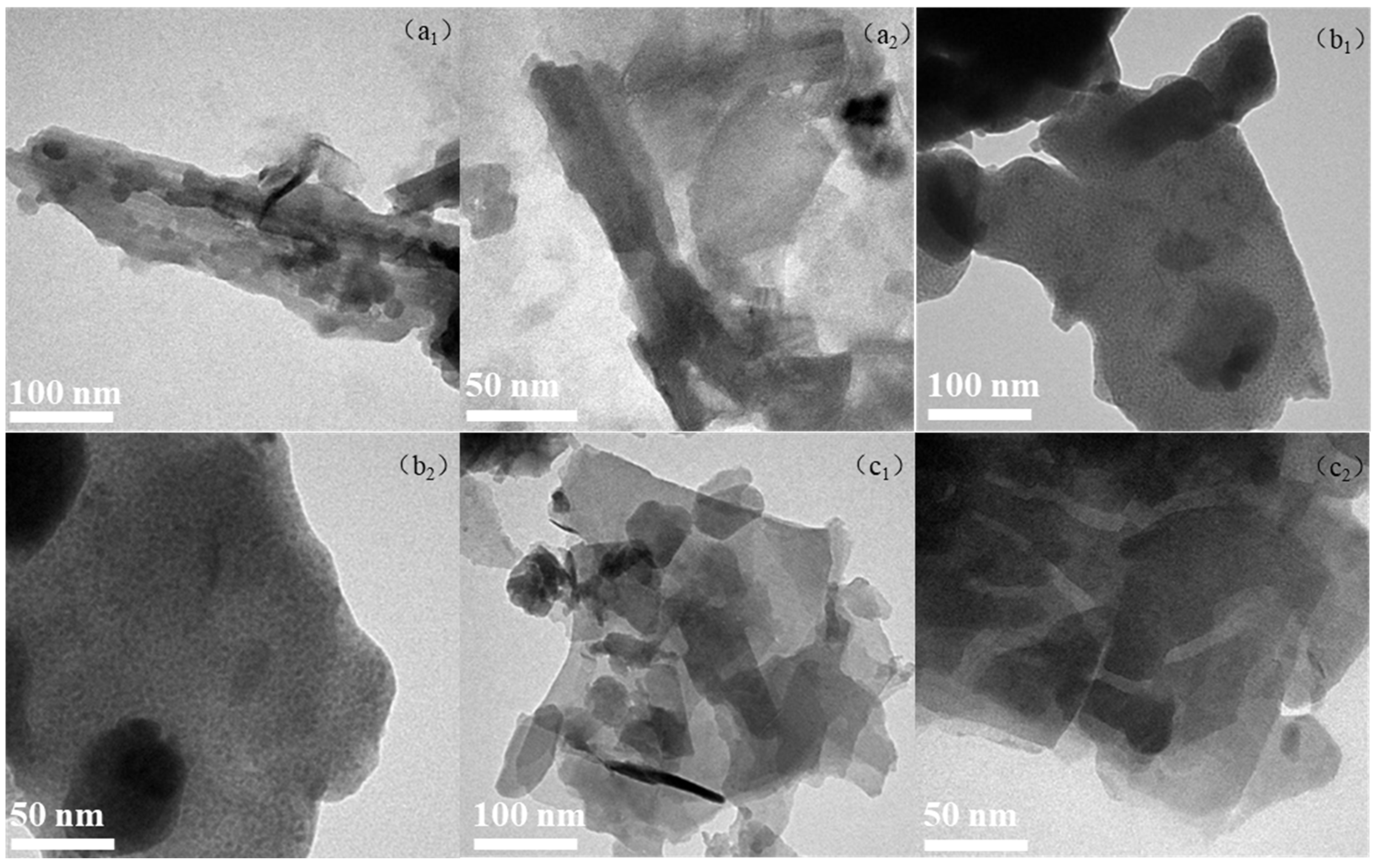
2.2. TEM Images of Clay-Based Catalysts
2.3. BET Analysis of Clay-Based Catalysts
2.4. FTIR Analysis of Clay-Based Catalysts
2.5. Co-Liquefaction of Chlorella and Oil Shale
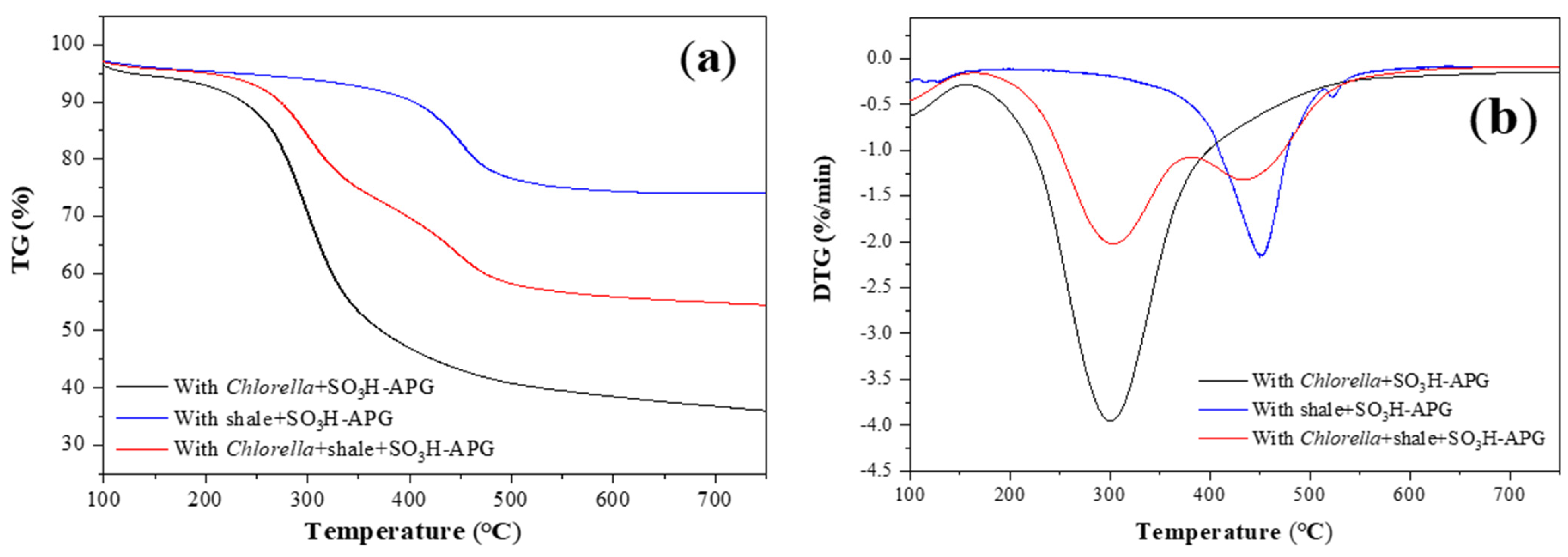
2.6. Co-Pyrolysis of Chlorella and Oil Shale
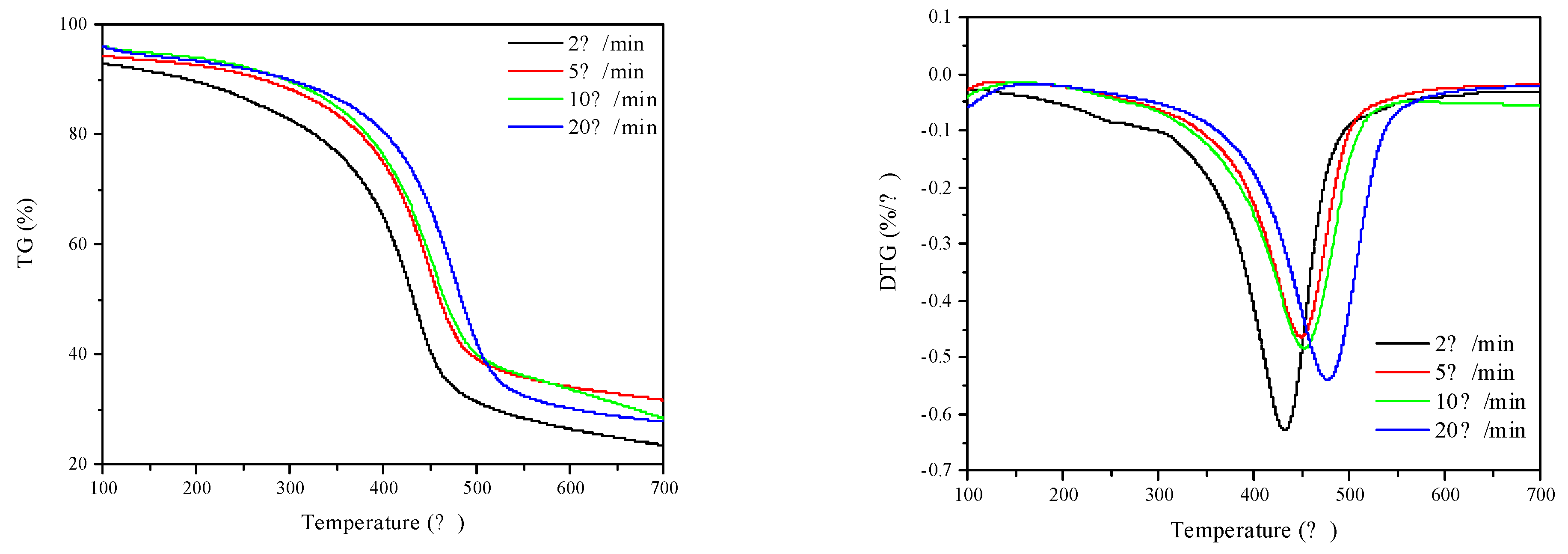
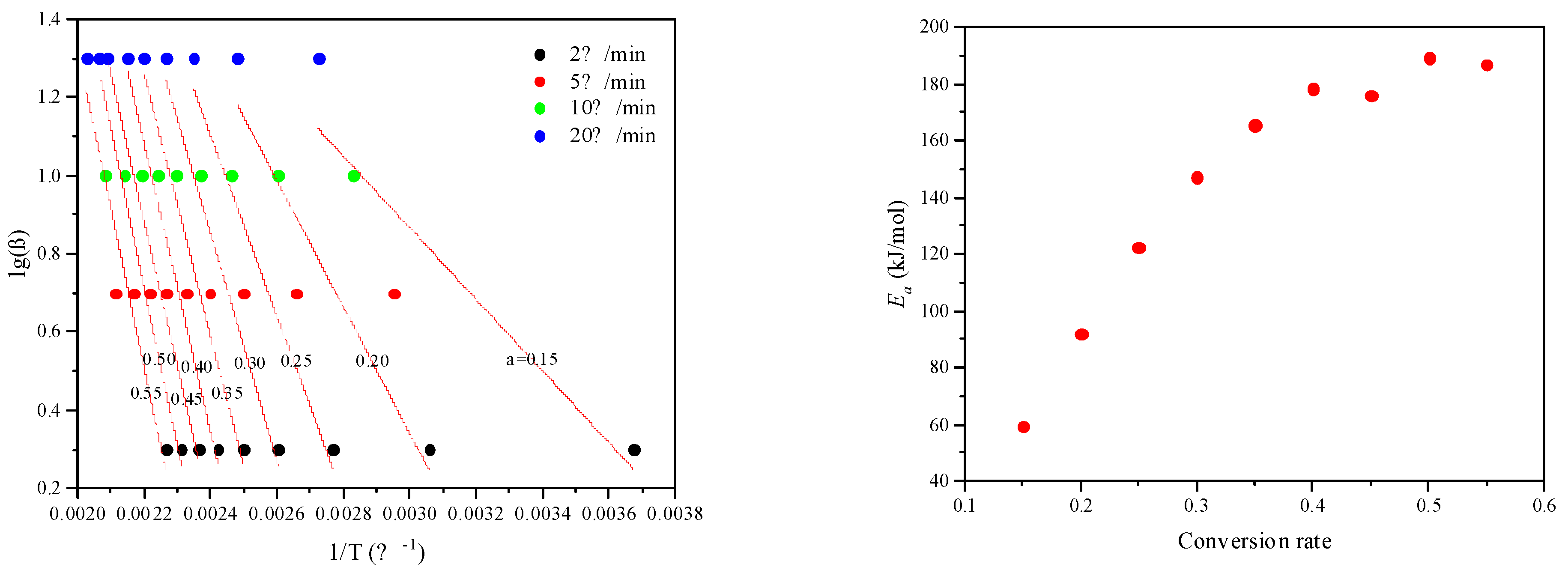
2.7. Kerogen Catalytic Pyrolysis
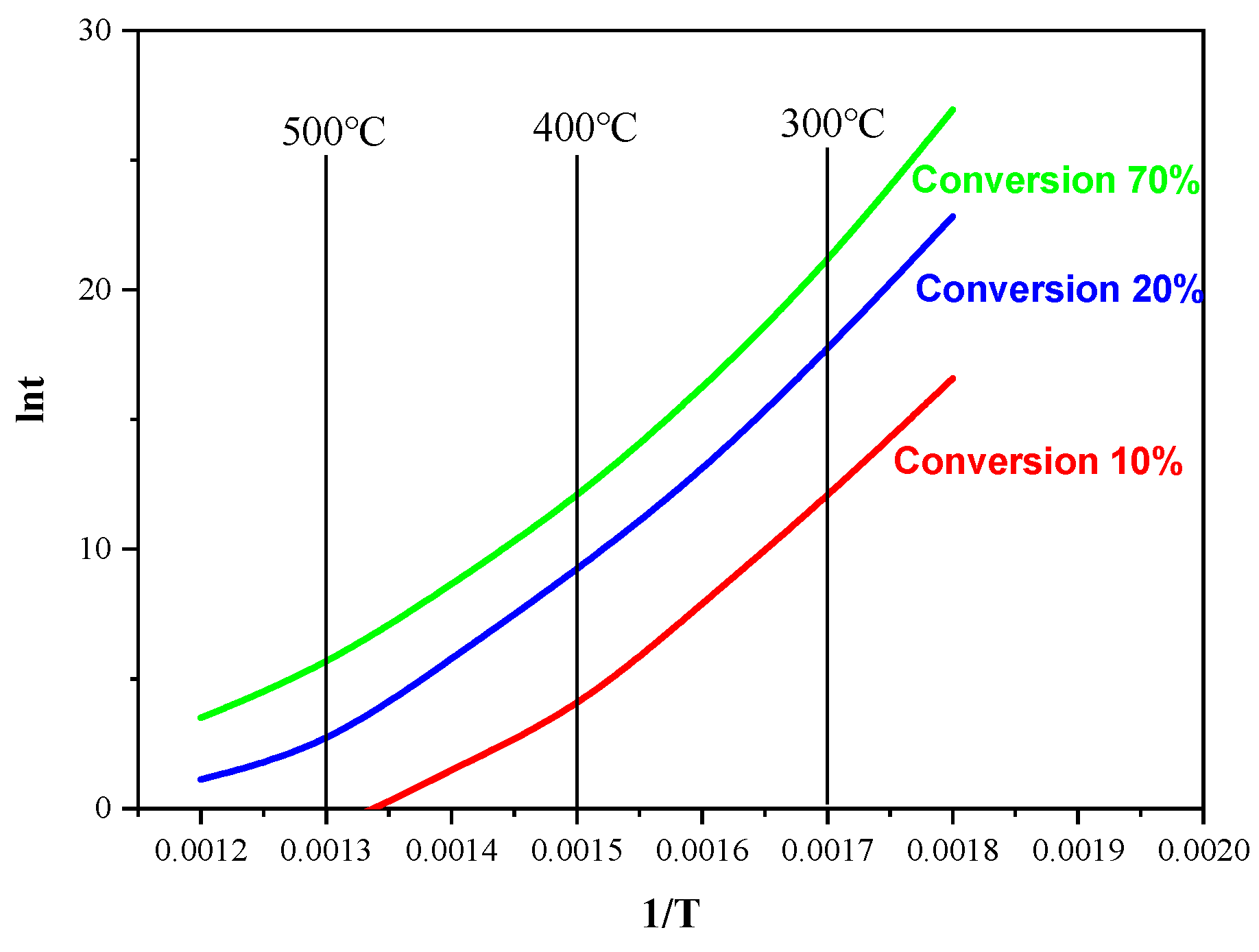
2.8. Temperature–Time-Conversion Diagram
3. Experimental Section
3.1. Reagents and Instruments
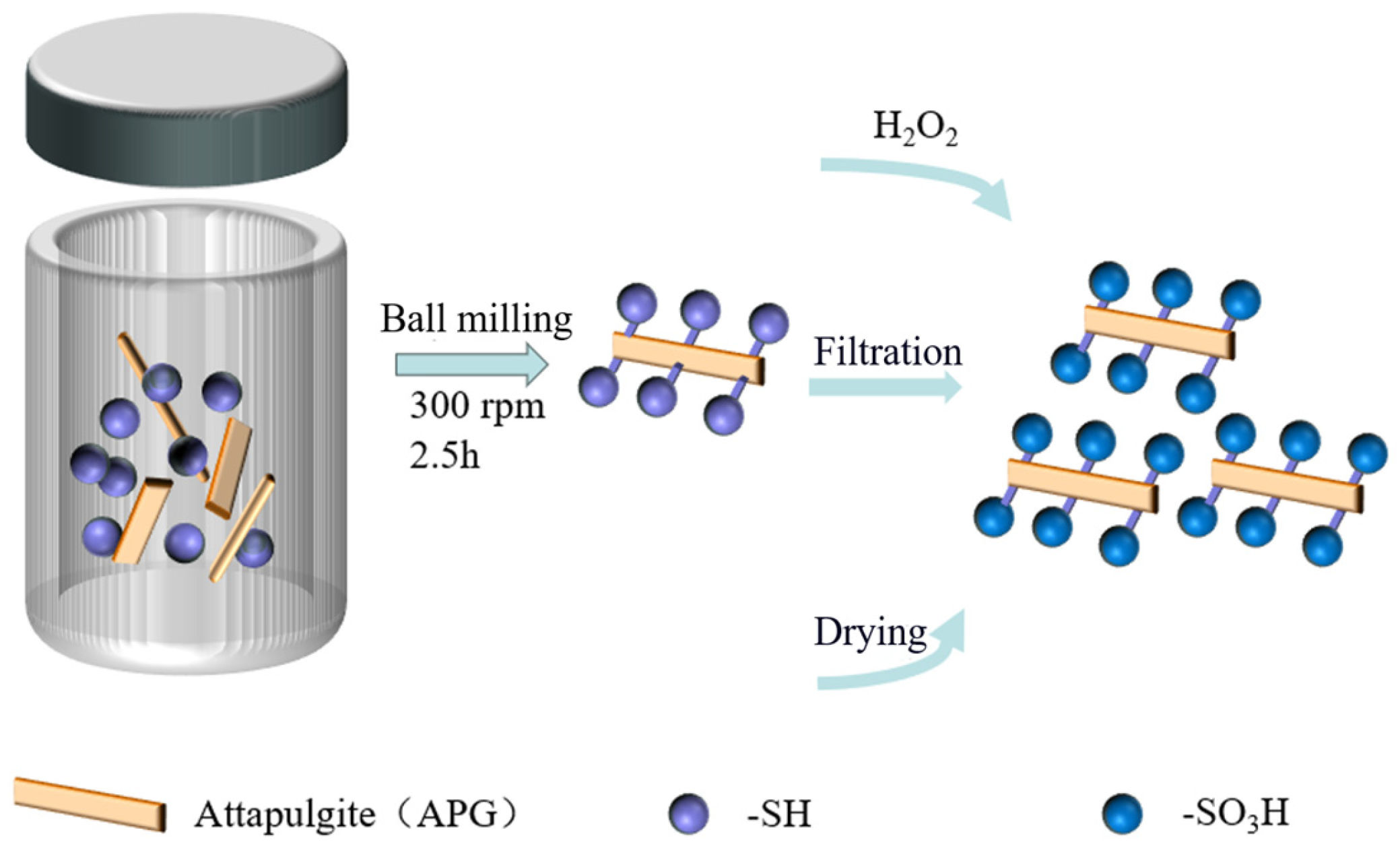
3.2. Catalyst Preparation
3.3. Catalyst Activity Evaluation
3.4. Catalyst Characterization Methods
3.5. Analytical and Computational Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alazaiza, M.Y.D.; Alzghoul, T.M.; Ramu, M.B.; Nassani, D.E. Catalysis in Biofuel Production and Biomass Valorization: Trends, Challenges, and Innovations Through a Bibliometric Analysis. Catalysts 2025, 15, 227. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Samuel, D.; Cedric Karel Fonzeu, M.; Hao, W.; Ahmed, I.O.; Zhen-Yu, T. Catalytic production of aviation jet biofuels from biomass: A review. Environ. Chem. Lett. 2025, 23, 419–461. [Google Scholar] [CrossRef]
- Faizan, M.; Song, H. Critical review on catalytic biomass gasification: State-of-Art progress, technical challenges, and perspectives in future development. J. Clean. Prod. 2023, 408, 137224. [Google Scholar] [CrossRef]
- de Deus Junior, J.O.; Alves, J.L.F.; de Melo e Melo, V.R.; de Oliveira, Â.A.S.; de Oliveira, K.F.S.; de Araújo Melo, D.M.; Braga, R.M. Conversion of microalgae into renewable hydrocarbons through catalytic pyrolysis: A bibliometric analysis. Algal Res. 2024, 81, 103602. [Google Scholar] [CrossRef]
- Raíssa Aparecida da Silveira, R.; Janaína Miranda, B.; Marcos Antonio de Souza, B.; Luiz Gustavo Martins, V. Catalytic solar hydropyrolysis of the Chlamydomonas reinhardtii microalgae. Biomass Bioenergy 2021, 152, 106183. [Google Scholar] [CrossRef]
- Elhassan, M.; Abdullah, R.; Kooh, M.R.R.; Chou Chau, Y.-F. Hydrothermal liquefaction: A technological review on reactor design and operating parameters. Bioresour. Technol. Rep. 2023, 21, 101314. [Google Scholar] [CrossRef]
- Ocampo, E.; Beltrán, V.V.; Gómez, E.A.; Ríos, L.A.; Ocampo, D. Hydrothermal liquefaction process: Review and trends. Curr. Res. Green Sustain. Chem. 2023, 7, 100382. [Google Scholar] [CrossRef]
- Shahbeik, H.; Kazemi Shariat Panahi, H.; Dehhaghi, M.; Guillemin, G.J.; Fallahi, A.; Hosseinzadeh-Bandbafha, H.; Amiri, H.; Rehan, M.; Raikwar, D.; Latine, H.; et al. Biomass to biofuels using hydrothermal liquefaction: A comprehensive review. Renew. Sustain. Energy Rev. 2024, 189, 113976. [Google Scholar] [CrossRef]
- Goswami, G.; Makut, B.B.; Das, D. Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci. Rep. 2019, 9, 15016. [Google Scholar] [CrossRef]
- Piyatilleke, S.; Thevarajah, B.; Nimarshana, P.H.V.; Ariyadasa, T.U. Microalgal biofuels: Challenges and prospective in the framework of circular bioeconomy. Energy Nexus 2025, 17, 100338. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Kantzas, A. A comprehensive review of microwave application on the oil shale: Prospects for shale oil production. Fuel 2021, 305, 121519. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development potential and technical strategy of continental shale oil in China. Pet. Explor. Dev. 2020, 47, 877–887. [Google Scholar] [CrossRef]
- Zhan, H.; Yang, Q.; Qin, F.; Meng, Z.; Chen, R.; Miao, X.; Zhao, K.; Yue, W. Comprehensive preparation and multiscale characterization of kerogen in oil shale. Energy 2022, 252, 124005. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, Y.; Wu, W.; Liu, Q.; Liu, Z. The structural characteristics of kerogens in oil shale with different density grades. Fuel 2018, 219, 151–158. [Google Scholar] [CrossRef]
- Gong, M.; Jiang, W.; Wang, S.; Liu, P.; Xu, F.; Wang, W.; Fan, Y. Bio-oil production from hydrothermal liquefaction of algal biomass: Effects of feedstock properties and reaction parameters. J. Environ. Chem. Eng. 2024, 12, 114010. [Google Scholar] [CrossRef]
- Cui, S.; Yang, T.; Zhai, Y.; Zhu, Y.; Yu, Z.; Kai, X. Investigation on the characteristics and interaction of co-pyrolysis of oil shale and peanut shell. Fuel 2023, 340, 127502. [Google Scholar] [CrossRef]
- Xian, S.; Xu, Q.; Feng, Y. Simultaneously remove organic pollutants and improve pyrolysis gas quality during the co-pyrolysis of soybean straw and oil shale. J. Anal. Appl. Pyrolysis 2022, 167, 105665. [Google Scholar] [CrossRef]
- Wu, A.; Wu, J.; Chen, L.; Su, J.; Yu, W. Recent Progress on In Situ Catalytic Conversion Catalysts for Oil Shale. ACS Omega 2024, 9, 36904–36916. [Google Scholar] [CrossRef]
- Li, D.; Pan, H.; Di, X.; Liu, X.; Hu, H. Progress in Catalytic Pyrolysis of Oil Shale. Scanning 2021, 2021, 6759176. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Zhang, L.; Zhang, X.; Sun, G. Effects of shale ash and FCC catalyst on adjusting secondary reaction of volatiles in oil shale pyrolysis. CIESC J. 2017, 68, 3770–3778. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Ma, X.; Tang, X.; Meng, X.; Wang, X. Investigation of catalytic hydrothermal co-liquefaction of oil shale and algal biomass. J. Anal. Appl. Pyrolysis 2025, 186, 106974. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, X.; Hao, C.; Kuang, W.; Wang, C.; Zhou, L.; Chu, M. Non-catalytic and In-situ catalytic co-pyrolysis of Huadian oil shale and microalgae using several additives as catalyst. J. Anal. Appl. Pyrolysis 2023, 172, 106012. [Google Scholar] [CrossRef]
- Yu, Z.; Dai, M.; Huang, M.; Fang, S.; Xu, J.; Lin, Y.; Ma, X. Catalytic characteristics of the fast pyrolysis of microalgae over oil shale: Analytical Py-GC/MS study. Renew. Energy 2018, 125, 465–471. [Google Scholar] [CrossRef]
- Yin, Y.; Qi, Y.; Ma, C.; Li, W.; Luo, S.; Liu, S. Efficient conversion of cellulose to 5-hydroxymethylfurfural using a bifunctional hydrophobic SBA-15 catalyst: The effects of hydrophobicity, morphology and acidity. Fuel Process. Technol. 2023, 245, 107752. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, K.; Jiang, Y.; Chen, L.; Zhang, H.; Li, H.; Yang, S. Biomass-derived hydrophobic metal-organic frameworks solid acid for green efficient catalytic esterification of oleic acid at low temperatures. Fuel Process. Technol. 2023, 239, 107558. [Google Scholar] [CrossRef]
- Zhang, Z.; Volkman, J.K.; Xie, X.; Snowdon, L.R. Stepwise pyrolysis of the kerogen from the Huadian oil shale, NE China: Algaenan-derived hydrocarbons and mid-chain ketones. Org. Geochem. 2016, 91, 89–99. [Google Scholar] [CrossRef]
- Veski, R.; Veski, S. Aliphatic dicarboxylic acids from oil shale organic matter–historic review. Oil Shale 2019, 36, 76–95. [Google Scholar] [CrossRef]
- Gelin, F.; Volkman, J.K.; Largeau, C.; Derenne, S.; Sinninghe Damsté, J.S.; De Leeuw, J.W. Distribution of aliphatic, nonhydrolyzable biopolymers in marine microalgae. Org. Geochem. 1999, 30, 147–159. [Google Scholar] [CrossRef]
- Ariskina, K.A.; Yuan, C.; Abaas, M.; Emelianov, D.A.; Rodionov, N.; Varfolomeev, M.A. Catalytic effect of clay rocks as natural catalysts on the combustion of heavy oil. Appl. Clay Sci. 2020, 193, 105662. [Google Scholar] [CrossRef]
- Abeyta, K.P.; Silva, M.L.A.d.; Silva, C.L.S.; Pontes, L.A.M.; Teixeira, L.S.G. Clay-based catalysts applied to glycerol valorization: A review. Sustain. Chem. Pharm. 2024, 40, 101641. [Google Scholar] [CrossRef]
- Khadijeh, R. Recent Application of Clay-Based Heterogeneous Catalyst in Organic Reactions. J. Inorg. Organomet. Polym. Mater. 2025, 1–22. [Google Scholar] [CrossRef]
- Luo, C.; Liu, H.; Zhou, S.; Li, J.; Li, X.; Huang, Y. Catalytic role of various clay minerals during the thermal reaction process with oxidized and pyrolyzed oils. J. Therm. Anal. Calorim. 2024, 149, 8681–8691. [Google Scholar] [CrossRef]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, M.A.; Cadús, L.E.; Morales, M.R. Tailoring materials by high-energy ball milling: TiO2 mixtures for catalyst support application. Mater. Today Chem. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Huang, F.; Wang, H.; Chen, J.-S.; Yang, B. Dry ball milling and wet ball milling for fabricating copper–yttria composites. Rare Met. 2018, 37, 859–867. [Google Scholar] [CrossRef]
- Mañosa, J.; Alvarez-Coscojuela, A.; Maldonado-Alameda, A.; Chimenos, J.M. A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments. Materials 2024, 17, 4651. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, J.; Wang, L.; Lyu, C.; Chen, H.; Lyu, Y.; Nie, B. Preparation and performance of superhydrophobic surfaces with low surface energy modified attapulgite. J. Mol. Struct. 2024, 1295, 136586. [Google Scholar] [CrossRef]
- Xue, S.; Li, B.; Mu, P.; Li, J. Designing attapulgite-based self-healing superhydrophobic coatings for efficient corrosion protection of magnesium alloys. Prog. Org. Coat. 2022, 170, 106966. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Wang, D.; Li, G. Study on surface modification of attapulgite. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Qingdao, China, 12–14 June 2020. [Google Scholar] [CrossRef]
- Che, C.; Wu, J.; Shen, Z.; Ning, H.; Tang, R.; Liang, S.; Zhang, J.; Jiang, H.; Yuan, S. Catalytic Conversion of Oil Shale over Fe or Ni Catalysts under Sub-Critical Water. Processes 2024, 12, 949. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S. Catalytic hydrothermal liquefaction of lactuca scariola with a heterogeneous catalyst: The investigation of temperature, reaction time and synergistic effect of catalysts. Bioresour. Technol. 2020, 309, 123375. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Zhang, L.; Liu, Z.; Wu, Y.; Yang, M. Catalytic hydrothermal liquefaction of microalgae over mesoporous silica-based materials with site-separated acids and bases. Fuel 2020, 279, 118529. [Google Scholar] [CrossRef]
- Yu, C.; Qi, Z.; Bian, J.; Song, R.; Wang, W.; Li, C. Insight into acid-base bifunctional catalysts for microalgae liquefaction and bio-oil pyrolysis: Product characteristics, energy recovery and kinetics. J. Anal. Appl. Pyrolysis 2021, 155, 105086. [Google Scholar] [CrossRef]
- Cui, Z.; Lu, Y.; Cao, R.; Huang, D.; Zhang, G.; Zhang, Y. Synergistic effect and hydrogen migration during co-pyrolysis of oil shale and cherry pit. Fuel 2024, 365, 131275. [Google Scholar] [CrossRef]
- Baruah, B.; Tiwari, P. Investigation of co-pyrolysis mechanism of oil shale and rubber seed shell, product yield and optimization of pyrolysis parameter using response surface methodology. Fuel 2022, 317, 123441. [Google Scholar] [CrossRef]
- SY-T 5779-2008. Available online: https://codeofchina.com/standard/SYT5779-2008.html (accessed on 22 March 2025).
- GB/T 19143-2017. Available online: https://codeofchina.com/standard/GBT19143-2017.html (accessed on 22 March 2025).
- GB/T 37160-2019. Available online: https://www.chinesestandard.net/PDF.aspx/GBT37160-2019 (accessed on 22 March 2025).
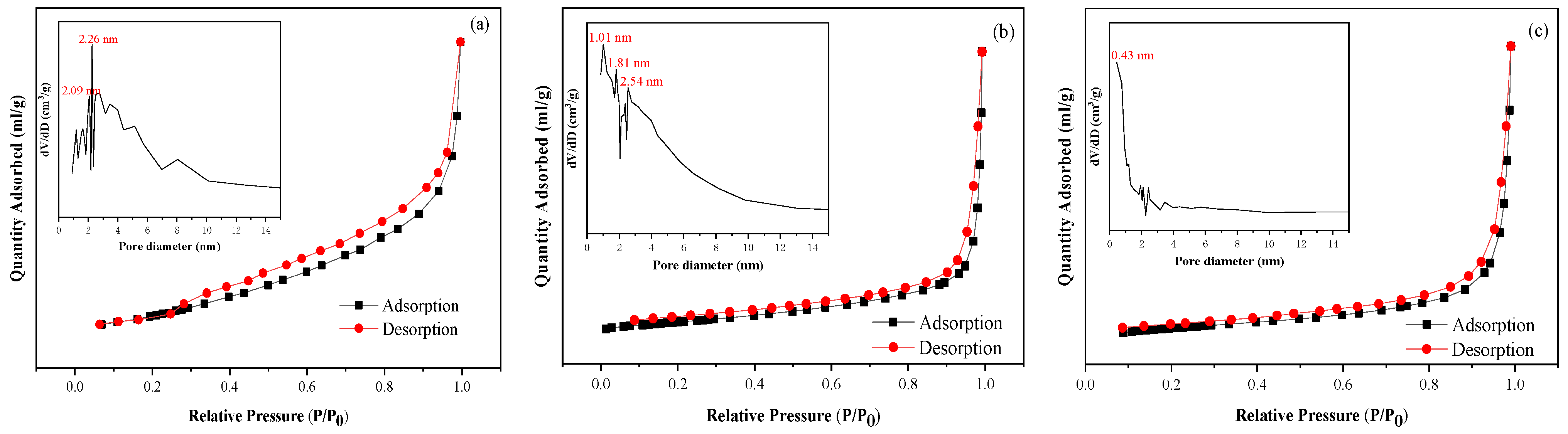


| Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter DFT (nm) | ||
|---|---|---|---|---|
| Dmicro | Dmeso | |||
| APG | 1152 | 0.22 | - | - |
| SO3H-APG | 127 | 0.03 | 1.64 | 2.26 |
| Kaolin | 62 | 0.30 | - | - |
| SO3H-kaolin | 117 | 0.14 | 1.01 | 2.54 |
| clin | 260 | 0.76 | - | - |
| SO3H-clin | 111 | 0.26 | 0.43 | 2.09 |
| Hydrocarbons | N-Containing Compounds | Alcohols | Ketones | Phenols | Organic Acids | Esters | ||
|---|---|---|---|---|---|---|---|---|
| Without catalyst | 31.28 | 29.74 | 4.73 | 15.37 | 4.86 | 10.34 | 3.68 | |
| With SO3H-APG | 53.21 | 21.14 | 3.17 | 10.61 | 2.40 | 4.84 | 4.63 | |
| With SO3H-kaolin | 49.68 | 23.55 | 3.07 | 11.49 | 3.92 | 4.97 | 3.32 | |
| With SO3H-clin | 45.83 | 26.23 | 2.86 | 12.58 | 2.66 | 5.46 | 4.38 | |
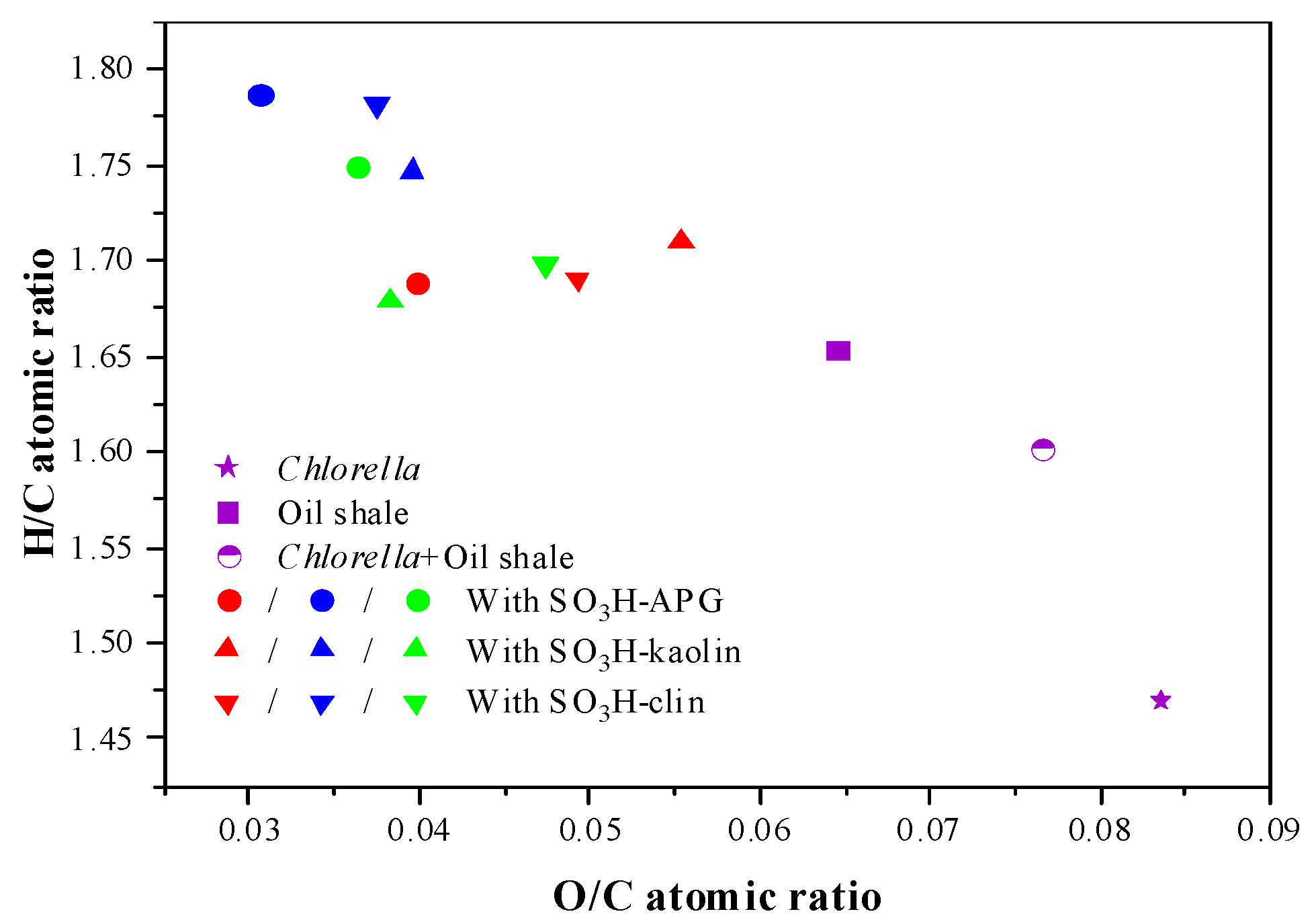
| Element/wt% | Atomic Ratio | HHV (MJ/kg) | DOD (%) | |||||
| C | H | O | N | H/C | O/C | |||
| Without catalyst | 76.34 | 10.19 | 7.75 | 5.72 | 1.6018 | 0.0765 | 38.93 | -- |
| With SO3H-APG | 80.43 | 11.73 | 3.89 | 3.95 | 1.7501 | 0.0363 | 43.20 | 49.81 |
| With SO3H-kaolin | 79.53 | 11.13 | 4.06 | 5.28 | 1.6794 | 0.0383 | 42.01 | 47.61 |
| With SO3H-clin | 78.32 | 11.08 | 4.95 | 5.65 | 1.6977 | 0.0474 | 41.37 | 36.13 |
| Feed + Catalyst | Maximum Weight Loss TM (°C) | Active Energy Ea (kJ·mol−1) | Correlation Coefficient R2 | Pre-Exponential Factor A (h−1) | Temperature Range (°C) |
|---|---|---|---|---|---|
| Chlorella + SO3H-APG | 299.99 | 76.89 | 0.98 | 5.45 × 106 | 150–500 |
| shale + SO3H-APG | 449.17 | 107.74 | 0.99 | 2.79 × 109 | 350–500 |
| Chlorella + shale + SO3H-APG | 300.08 | 68.05 | 0.98 | 2.92 × 105 | 180–380 |
| 435.08 | 100.43 | 0.99 | 1.72 × 109 | 380–500 |
| Conversion Rate | β (°C/min) | Ea (kJ/mol) | r | |||
|---|---|---|---|---|---|---|
| 2 | 5 | 10 | 20 | |||
| 0.05 | 58.1 | 85.1 | 138.17 | 123.64 | - | <0.92 |
| 0.10 | 191.54 | 271.07 | 295.77 | 298.89 | - | <0.92 |
| 0.15 | 272.08 | 338.57 | 353.35 | 367.24 | 59.49 | 0.9338 |
| 0.20 | 326.92 | 375.99 | 384.04 | 403.10 | 92.05 | 0.9546 |
| 0.25 | 361.10 | 400.01 | 405.81 | 425.61 | 122.43 | 0.9653 |
| 0.30 | 383.87 | 416.95 | 421.62 | 441.66 | 147.29 | 0.9706 |
| 0.35 | 400.22 | 430.05 | 435.45 | 454.24 | 165.49 | 0.9762 |
| 0.40 | 412.82 | 440.94 | 446.30 | 464.88 | 178.26 | 0.9782 |
| 0.45 | 423.2 | 450.82 | 456.15 | 478.23 | 175.97 | 0.9803 |
| 0.50 | 432.41 | 460.70 | 467.01 | 483.85 | 189.32 | 0.9792 |
| 0.55 | 441.55 | 472.62 | 479.81 | 493.70 | 186.94 | 0.9714 |
| 0.60 | 452.14 | 493.92 | 501.45 | 505.82 | - | <0.92 |
| 0.65 | 469.59 | 572.31 | 575.24 | 525.98 | - | <0.92 |
| 0.70 | 520.33 | 780.10 | 672.69 | 606.95 | - | <0.92 |
| Temperature | Conversion (%) | ||
|---|---|---|---|
| 10 | 60 | 70 | |
| 280 °C | 7.4 months | 280 years | 11,500 years |
| 300 °C | 2.3 days | 1.5 years | 40.12 years |
| 350 °C | 0.98 h | 7 days | 4 months |
| 400 °C | 0.029 h | 3.2 h | 2.4 days |
| 450 °C | 0.0017 h | 0.15 h | 1.87 h |
| 500 °C | 0.00014 h | 0.008 h | 0.089 h |
| Temperature | Conversion (%) | ||
|---|---|---|---|
| 10 | 60 | 70 | |
| 280 °C | 6.2 months | 260 years | 11,350 years |
| 300 °C | 1.9 days | 1.5 years | 42.12 years |
| 350 °C | 0.76 h | 5 days | 4 months |
| 400 °C | 0.012 h | 2.7 h | 1.8 days |
| 450 °C | 0.0013 h | 0.09 h | 1.56 h |
| 500 °C | 0.0001 h | 0.003 h | 0.068 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Song, R.; Bian, J. Application of Clay-Based Catalysts in Co-Conversion and Co-Pyrolysis of Chlorella and Oil Shale. Catalysts 2025, 15, 322. https://doi.org/10.3390/catal15040322
Meng X, Song R, Bian J. Application of Clay-Based Catalysts in Co-Conversion and Co-Pyrolysis of Chlorella and Oil Shale. Catalysts. 2025; 15(4):322. https://doi.org/10.3390/catal15040322
Chicago/Turabian StyleMeng, Xianglong, Ranran Song, and Junjie Bian. 2025. "Application of Clay-Based Catalysts in Co-Conversion and Co-Pyrolysis of Chlorella and Oil Shale" Catalysts 15, no. 4: 322. https://doi.org/10.3390/catal15040322
APA StyleMeng, X., Song, R., & Bian, J. (2025). Application of Clay-Based Catalysts in Co-Conversion and Co-Pyrolysis of Chlorella and Oil Shale. Catalysts, 15(4), 322. https://doi.org/10.3390/catal15040322







