Abstract
A high-activity, low-cost, and easy-to-prepare monolithic catalyst is crucial for the industrial catalytic combustion of volatile organic compounds (VOCs) in a cost-effective manner. In this study, a highly efficient monolithic catalyst, designated as 4GM/COR, was developed by loading 4% graphene-doped α-MnO2 (4GM) catalyst onto pre-etched cordierite (COR) blocks using a straightforward “ball-milling-assisted impregnation” method. The anchoring force of the cordierite pores, generated through oxalic acid etching, enables the uniform and robust loading of powdered 4GM onto COR, preventing detachment under high temperatures or high gas flow rates. The loading rate, specific surface area, and concentrations of Mn3+ and surface-lattice and absorbed oxygen species in the monolithic catalyst increase with impregnation times from 2 to 4, indicating that catalytic activity is optimized through repeated impregnation. Catalytic performance tests demonstrated that the 4-4GM/COR exhibited the highest activity, achieving 90% degradation of toluene at 200 °C under both dry and humid (relative humidity is 85%) conditions. Furthermore, the 4-4GM/COR maintains high catalytic stability and activity even at a large GHSV of 6000 h−1. To conclude, the 4-4GM/COR monolithic catalyst developed in this study not only represents a promising option for industrial applications but also serves as an important reference for the synthesis of monolithic catalysts.
1. Introduction
The rapid advancement of industrial processes has intensified atmospheric challenges posed by volatile organic compounds (VOCs), which act as critical precursors in photochemical ozone production and secondary fine particulate matter (PM2.5) formation, while concurrently triggering severe public health risks—from respiratory impairments to carcinogenic effects [1,2]. This dual environmental and epidemiological impact has elevated VOCs emission control to a paramount priority in sustainable development agendas worldwide.
Catalytic combustion stands as a pivotal technology for VOCs abatement, yet its industrial scalability hinges critically on the economic viability and catalytic performance of employed materials, which is intrinsically governed by two interdependent factors: (1) the catalyst’s intrinsic properties (activity, durability, cost) and (2) its structural configuration. Developing low-cost catalysts with high intrinsic activity and enhanced durability directly reduces operational expenditures (OPEX) by minimizing both catalyst consumption and energy demands. Concurrently, optimizing catalyst architecture to maximize active site accessibility—through engineered pore structures or surface functionalization—improves both catalyst utilization efficiency and VOCs treatment efficiency by enhancing reactant-catalyst contact and mass transfer dynamics [3,4]. Compared with the powdered catalysts, the strategic deposition of active catalytic coatings onto honeycomb monolithic substrates can achieve this effect by mitigating flow resistance, preventing channel blockage, and maximizing active site accessibility [5]. Therefore, the integration of a monolithic structured substrate and an active component to fabricate monolithic integrated catalyst aligns with industrial requirements for scalable, energy-efficient VOCs treatment systems.
Noble metal catalysts, while renowned for their superior catalytic activity in combustion processes, face limitations in industrial applications due to their high cost and scarcity [6,7]. This inherent vulnerability has driven substantial research interest toward transition metal oxides as alternative catalysts. Among various transition metal oxides, manganese-based catalysts have emerged as particularly promising candidates for VOCs oxidation, owing to their structural versatility and multivalent redox characteristics [8,9,10]. In particular, α-MnO2 has been proven to have higher activity among the various crystal types (i.e., α-, β-, γ-, and δ-) [9,10,11]. The catalytic mechanism of manganese oxides primarily involves oxygen activation and transfer processes mediated by reversible Mn3⁺/Mn4⁺ redox cycles, where surface electron transfer plays a critical role [8]. This fundamental understanding suggests that enhancing electrical conductivity could improve catalytic efficiency by accelerating charge transfer during redox reactions. Recent advancements in catalyst engineering have found the catalytic activity can be further optimized through strategic modifications including heteroatom doping (e.g., (i.e., Cu [12], Co [13])) and graphene hybridization [8]. In particular, graphene hybridization can achieve multifaceted synergistic effects: (1) The two-dimensional carbon matrix prevents nanoparticle aggregation, significantly increasing accessible catalytic surfaces for VOCs adsorption/desorption; (2) Graphene’s superlative conductivity establishes efficient electron transport pathways, facilitating rapid redox cycling; (3) Structural integration promotes oxygen vacancy regeneration through interfacial charge redistribution [14]. These combined advantages position graphene-incorporated α-MnO2 (GM) composites as ideal active components for developing monolithic integrated catalyst systems with industrial-scale applicability.
Cordierite honeycomb ceramic, distinguished by its exceptional thermal stability, mechanical robustness, and cost-effectiveness, has become the substrate of choice for monolithic catalysts in various domains such as industrial flue gas treatment, vehicle exhaust purification, chemical synthesis, and biochemistry [15,16,17]. Since the catalytic performance is directly related to the active component, how to firmly fix the active component onto the cordierite substrate without affecting its catalytic activity is of great importance [18]. In the last decades, various kinds of loading methods have been developed, including impregnation [19], coating [20], hydrothermal synthesis [21], and in-situ synthesis [16,22], among others. Despite the widespread research and utilization of the cordierite monolithic integrated catalyst, challenges persist, including the non-uniform distribution of active components (notably in the coating method), the propensity of coatings to peel under the influence of flue gas flow and thermal shocks, and the complexity and stringency of preparation processes (as observed in in-situ/hydrothermal synthesis) that hinder scalability [23]. Therefore, despite a low-temperature active component, a facile, efficient, and easy-to-operate loading method also plays an important role in fabricating monolithic integrated catalyst.
In this work, a simple ball-milling-assisted impregnation method for loading active graphene-doped α-MnO2 (GM) catalyst onto cordierite blocks was developed for preparation of GM/cordierite monolithic catalyst. First, the powdered 4GM (α-MnO2 doped with 4 wt.% graphene) catalyst was prepared through a hydrothermal synthesis, followed by being ground into nanosized particles using a planetary ball mill. Second, the honeycomb cordierite blocks were etched with oxalic acid to create more micropore structures on the surface. Finally, the treated cordierite blocks were impregnated in the powdered GM suspension to complete the active component loading. This methodology demonstrates three distinctive technical merits: (1) Mechanical anchoring enhancement: oxalic acid etching induces porous surface topographies on cordierite, providing physical interlocking sites for GM nanoparticle immobilization; (2) Interfacial bonding reinforcement: π-π interactions between graphene and the silicate framework generate covalent-like interfacial adhesion, significantly improving peel resistance; (3) Nanostructural precision: ball milling enables controlled particle size reduction, while the graphene matrix suppresses nanoparticle aggregation during impregnation, collectively optimizing active surface accessibility and uniformity. Finally, the catalytic performance of the GM/cordierite monolithic catalyst for toluene oxidation under both dry and humid atmospheres were comprehensively evaluated by temperature-programmed testing, catalytic cycling testing, and stability testing. Meanwhile, structural and electronic characterizations (XRD, BET, SEM, XPS, H2-TPR) were conducted to reveal the underpinning mechanisms of catalytic performance. Furthermore, the catalytic oxidation performance of toluene at varied Gas Hourly Space Velocity (GHSV) from 750 h−1 to 6000 h−1, as well as the catalytic oxidation performance of different kinds of VOCs (i.e., toluene and acetone), were investigated to demonstrate its application potentials.
2. Results and Discussions
2.1. Catalyst Characterization
The active component 4GM was loaded onto cordierite (COR) supports to fabricate monolithic catalysts through different methodologies. As evidenced by the macroscopic observations of prepared catalysts (Figure S1), the ball-milling-assisted impregnation method yielded x-4GM/COR samples (Figure S1a–c) with superior catalyst distribution uniformity. Systematic optimization revealed a positive correlation between impregnation cycles and loading efficiency, achieving progressively enhanced loading contents of 4.7 wt.%, 6.0 wt.%, and 6.8 wt.% for 2-4GM/COR, 3-4GM/COR, and 4-4GM/COR, respectively. In contrast, the 4GM/COR prepared by both the coating method (Figure S1d,e) and the hydrothermal treatment (Figure S1f) not only presents uneven distribution of catalyst particles on the surface of cordierite block with partial channel blockage, but also poor adhesion stability, as evidenced by visible catalyst detachment from the cordierite substrates. This comparative analysis confirms the ball-milling-assisted impregnation method as an effective strategy for achieving both homogeneous catalyst distribution and stable immobilization on cordierite supports, addressing the critical challenges of particle aggregation and interfacial adhesion encountered in conventional preparation approaches.
XRD analysis was performed to discern the crystalline structural differences between COR and x-4GM/COR, as depicted in Figure S2. The diffractograms for both COR and x-4GM/COR displayed characteristic cordierite diffraction peaks (PDF#84-1897), with no notable phase alterations in the cordierite structure after loading the 4GM catalyst. Moreover, the XRD patterns for x-4GM/COR did not reveal any distinct peaks ascribable to MnO2 species, which was mainly attributed to the low loading quantity and/or the dispersion of 4GM particles across the cordierite surface. Importantly, as the number of impregnation cycles increased, a gradual diminishment in the intensity of primary diffraction peaks corresponding to crystal planes such as (200) and (312) was observed. This trend likely results from the increased loading rate and dispersion of 4GM catalyst particles on the cordierite substrate.
To investigate the morphological evolution of cordierite during pretreatment and catalyst loading, SEM analysis was conducted on pristine cordierite, 4GM particles, and impregnated monolithic catalysts (Figure 1a–f). The untreated cordierite substrate (Figure 1a) exhibits a continuous, smooth surface with inherent structural integrity. Acid etching pretreatment (Figure 1b) generates a porous architecture characterized by uniformly distributed submicron-scale cavities (3–5 μm in length), creating an enhanced surface area for catalyst anchoring. Ball-milled 4GM precursor particles (Figure 1c) demonstrate a well-defined coral-like morphology with hierarchical nano-features. Progressive catalyst loading through multiple impregnation cycles is evidenced by SEM characterization. The 2-4GM/COR sample (Figure 1d) shows partial pore occupation with isolated 4GM clusters, confirming initial stage deposition. Subsequent impregnation cycles (3-4GM/COR, Figure 1e) yield increased surface coverage but the 4GM were unevenly distributed on the cordierite surface with obvious particle agglomeration. Optimal dispersion is achieved in 4-4GM/COR (Figure 1f), where the 4GM particles were present in reduced size and uniformly distributed on the subsurface pore networks. Complementary EDS mapping (Figure 1g,h) verifies the homogeneous spatial distribution of MnO2 and graphene components within the composite architecture. This systematic investigation demonstrates that iterative impregnation cycles not only enhance catalyst loading capacity (from 4.7 wt.% to 6.8 wt.%) but critically improve deposition uniformity. Quadruple impregnation achieves optimal catalyst dispersion, balancing pore accessibility with maximal active site exposure while preventing particle over-accumulation.
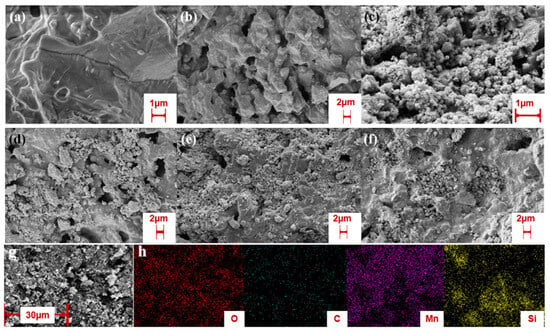
Figure 1.
SEM image of cordierite monolithic catalysts: (a) cordierite; (b) oxalic acid treated cordierite; (c) 4GM micron particles; (d) 2-4GM/COR; (e) 3-4GM/COR; (f) 4-4GM/COR; (g,h) EDS of 4-4GM/COR.
The textural properties of 4GM, pre-etched cordierite (COR), and x-4GM/COR composites were systematically analyzed using nitrogen adsorption/desorption isotherms, with key parameters summarized in Table 1 and the isotherm profiles shown in Figure S3. The 4GM catalyst exhibited the highest specific surface area among the tested materials, displaying characteristic type IV adsorption isotherms indicative of its well-defined mesoporous structure (Figure S3a,b). In contrast, the pre-etched cordierite substrate demonstrated a significantly lower surface area, primarily due to its macroporous architecture featuring submicron-scale cavities (3–5 μm in length), as revealed by SEM. Notably, while macropores are easily visible, structural analysis reveals the coexistence of residual mesopores (Figure S3c). The integration of 4GM particles into the cordierite matrix induced distinct textural modifications: the hysteresis loop magnitude decreased progressively, accompanied by reductions in both surface area and pore volume. These observations suggest partial infiltration of 4GM particles into the cordierite’s pore network, coupled with potential pore-blocking effects that render some mesopores inaccessible (“dead pores”). Intriguingly, the composite materials exhibited progressive increases in specific surface area with successive impregnation cycles. This phenomenon is mainly attributable to two synergistic factors: (1) the inherently superior surface area of 4GM relative to cordierite, and (2) enhanced loading rate and dispersion uniformity of 4GM particles across the COR substrate with repeated loading, maximizing the exposure of 4GM’s high-surface-area architecture. This trend underscores the critical role of multi-cycle impregnation in achieving both optimal particle distribution and maximized exposure of active sites. The 4-4GM/COR catalyst, characterized by an optimal loading capacity, surface area, and pore volume, is anticipated to exhibit enhanced catalytic performance in VOCs oxidation.

Table 1.
Table of pore structure parameters of 4GM/COR monolithic catalyst.
X-Ray Photoelectron Spectroscopy (XPS) enables systematic investigation of surface elemental composition and chemical states in monolithic catalysts. Figure S4 presents the Mn 2p and O 1s spectra for 4GM and three monolithic catalysts, with corresponding quantitative data summarized in Table 2. Analysis of the Mn 2p spectra (Figure S4a) reveals three characteristic peaks through deconvolution: surface Mn3+ at 639.89 eV, Mn4+ at 641.70 eV, and a satellite peak at 651.54 eV. Notably, the 2-4GM/COR catalyst exhibits pronounced oscillations in its Mn 2p spectrum, indicative of heterogeneous distribution and relatively low concentration of active components. In contrast, the 4-4GM/COR catalyst demonstrates minimal spectral fluctuations, suggesting improved homogeneity in the dispersion of active components across the cordierite substrate. The proportion of Mn3+ is observed to increase progressively with more maceration cycles, approaching the Mn3+ level of the 4GM particles (refer to Figure S4a and Table 2). Since the weaker bond energy of Mn3+-O compared to Mn4+-O bonds facilitates the dissociation and activation of oxygen molecules to participate in oxidation reaction, a higher proportion of Mn3+ is pivotal for the catalytic oxidation of VOCs at lower temperatures, which is extensively confirmed in previous literature [10]. Moreover, the large existence of surface low valence Mn would promote dissociation and activation of circumambient oxygen atoms since Mn3+ ions induce localized charge imbalance (Mn4+ → Mn3+ reduction), compensated by the formation of surface oxygen vacancies, which act as active sites for O2 adsorption and dissociation, lowering the activation energy for molecular oxygen activation [9].

Table 2.
XPS analyses of catalysts.
The deconvoluted O 1s XPS spectra in Figure S4b resolve into three oxygen species: surface lattice oxygen (Olatt) at 527.33 eV, surface adsorbed oxygen species (Oabs) at 529.41 eV, and oxygen containing functional groups (Ofunc) at 531.37 eV. Quantitative analysis (Table 2) reveals a significant redistribution of oxygen species upon loading 4GM onto the cordierite (COR) support. Specifically, the Olatt content undergoes a sharp reduction from 43.85% in pristine 4GM to 4.74–5.54% in supported catalysts, while Ofunc increases dramatically from 3.32% to 25.44–37.91%. This inversion is attributed to the dominance of oxygen-rich surface bonds inherent to the cordierite substrate (e.g., Al–O, Si–O, and Ca–O bonds), which weaken the lattice oxygen signal from the active phase. Notably, despite this overall suppression of surface lattice oxygen, its relative proportion systematically increases with successive impregnation cycles. This trend suggests progressive reconstruction of the active phase’s oxygen framework, likely due to improved dispersion and stronger interfacial interactions between 4GM nanoparticles and the COR substrate, indicating the 4-4GM/COR possesses an optimized ability to storage and release oxygen species. Concurrently, the Oads fraction generally increases with impregnation cycles (e.g., 4-4GM/COR vs. 2-4GM/COR), directly correlating with oxygen vacancies that arise from charge compensation (Mn4+ → Mn3+ reduction) and serve as critical adsorption/activation sites for molecular O2, and thereby play a pivotal role in catalytic oxidation of VOCs.
The synergistic interplay of these factors in 4-4GM/COR—elevated Mn3+ content, abundant Olatt, Oads, and oxygen vacancies—creates an optimized micro-environment for oxygen activation. Lattice oxygen facilitates bulk redox cycles, while adsorbed oxygen and vacancies lower the activation energy for O2 dissociation, collectively enhancing VOCs oxidation efficiency at low temperatures.
2.2. Catalyst Performance Analysis
2.2.1. Evaluation of Catalytic Activity
The catalytic activity of cordierite, x-4GM/COR, and monolithic catalyst prepared by the in-situ hydrothermal method were evaluated by using 300 ppm toluene/air as simulated VOC gas at a GHSV of 750 h−1. The T90 is an important parameter to evaluate catalytic performance, which corresponds to toluene degradation at 90%. As depicted in Figure S5, the bare cordierite support demonstrated limited catalytic activity within the temperature range of 150 to 330 °C, indicating that 4GM catalyst particles are the predominant active components. As expected, catalytic activity of x-4GM/COR is better than that of monolithic catalyst prepared by the in-situ hydrothermal method.
According to the analyses above, the x-4GM/COR monolithic catalysts prepared by the ball-milling assisted impregnation technique exhibited superior catalytic performance, and the effect of impregnation times on catalytic activity was further investigated in both dry and humid environments (RH = 85%), as shown in Figure 2. In the dry environment (Figure 2a), increasing the impregnation cycles is beneficial for the monolithic catalyst to improve catalytic activity. The 2-4GM/COR monolithic catalyst has poor performance with T90 of 287 °C, while the 3-4GM/COR presents similar catalytic performance as the 4-4GM/COR, arriving T90 at 200 °C. The loading amounts of 4GM catalysts on 3-4GM/COR and 4-4GM/COR are higher than that of 2-4GM/COR, leading to the enhanced catalytic performances. Furthermore, the excellent catalytic performances of 3-4GM/COR and 4-4GM/COR may be attributed to the synergy effect of abundant Olatt, Oads, and Mn3+ species (Table 2), which implies abundant oxygen vacancies and excellent mobility of oxygen species [11,24,25], playing a key role in the catalytic processes.
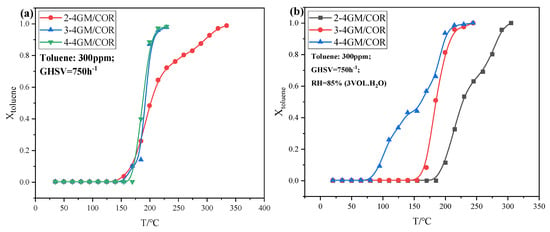
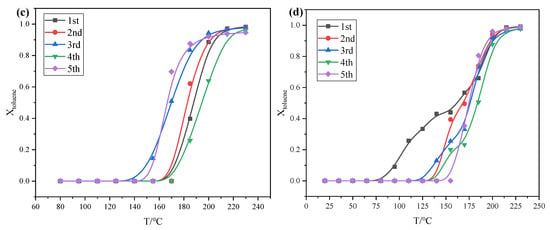
Figure 2.
Catalytic activity test of 4GM/COR monolithic catalyst at dry (a) and humid atmosphere (b), and cycling performance of 4-4GM/COR monolithic catalyst at dry (c) and humid atmosphere (d).
The catalytic activities of x-4GM/COR in the humid environment were shown in Figure 2b. The catalytic activities of the x-4GM/COR catalysts at an RH of 85% increased with increasing impregnation cycles. As shown in Figure S6, the 4GM catalyst particles exhibited excellent water resistance, with a T90 of approximately 155 °C (RH = 85%) [14]. As expected, the activity of the 2-4GM/COR catalyst was significantly reduced with increasing humidity, which may be directly related to the low loading amount of active components. In contrast, both the 3-4GM/COR and 4-4GM/COR monolithic catalysts exhibited excellent catalytic activity in the humid atmosphere (Figure 2b), achieving 95% toluene degradation at around 210 °C. It is worth noting that the 4-4GM/COR monolithic catalyst exhibited superior catalytic activity in the low-temperature region (<200 °C) in the humid (RH = 85%) environment, with T50 and T90 values of 160 °C and 198 °C, respectively. This phenomenon may be attributed to the reaction between partial Oads and water molecules, resulting in the generation of OH* species, which can participate in the process of toluene degradation. In summary, it is believed that the high loading amount of the ball-milled 4GM particles retains the characteristic of high moisture resistance, contributing to the excellent catalytic performance of the 4GM/COR monolithic catalyst in a humid atmosphere.
The catalytic performances of the 4-4GM@COR monolithic catalyst were investigated over five cycles to ensure the repeatability of the catalytic reaction and the stability of the catalyst, as shown in Figure 2c,d. Whether in dry or humid (RH = 85%) environments, the 4-4GM/COR monolithic catalyst maintained a T90 of approximately 200 °C, confirming its high cycling stability. It can be concluded that the uniformly dispersed active components and large specific surface area are conducive to the adsorption of VOCs and the dissociation of products, thereby accelerating the mass transfer efficiency of the catalytic process and improving the catalytic cycling performance. In addition, the high concentration of surface-adsorbed species (Table 2) ensures the excellent cycling performance, as it represents abundant oxygen vacancies. Overall, the high cycling stability of the 4-4GM/COR monolithic catalyst indicates that catalyst deactivation caused by the repeated start and stop of equipment can be effectively avoided in industrial scenarios.
2.2.2. Evaluation of Catalyst Applicability
After exploring the cycling performance of 4-4GM/COR on toluene degradation, the catalytic stability of the 4-4GM/COR monolithic catalyst was further investigated at 270 °C, as depicted in Figure 3. It is found that the catalyst was able to maintain almost 100% toluene degradation with CO2 selectivity, amounting to 75–80% in the dry atmosphere during the 10 h test (Figure 3a), demonstrating that most of the toluene is effectively degraded into the final product CO2 with only a small amount of by-products generated. Similarly, in a humid (RH = 85%) environment, the 4-4GM/COR monolithic catalyst maintained almost 100% toluene degradation, but there is a slight decrease in CO2 selectivity (65~75%). The presence of water vapor introduces hydroxyl radicals (OH*), which seem to participate in the oxidation process, leading to the formation of small amounts of by-products like benzyl alcohol and formic acid [26]. The reduction in CO2 selectivity in the presence of humidity is likely due to these alternate reaction pathways facilitated by the hydroxyl radicals. Overall, the high CO2 selectivity demonstrates the high proportion of surface adsorbed oxygen species provides sufficient active species for the sustained catalytic reactions to achieve efficient mineralization of toluene. The efficient mineralization of toluene to CO2 also indicates that the catalyst could be an excellent candidate for applications in air purification or industrial exhaust treatment, where VOCs like toluene need to be removed to mitigate environmental and health impacts.
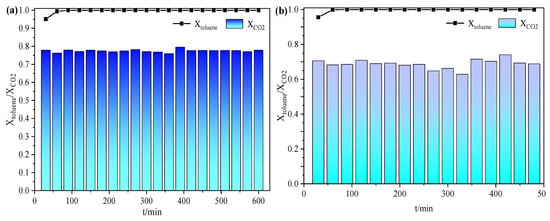
Figure 3.
Lifetime test of 4-4GM/COR monolithic catalyst on toluene conversion: (a) dry atmosphere; (b) humid atmosphere (toluene: 300 ppm; WHSV: 750 h−1).
In-situ DRIFTS was further conducted to elucidate the catalytic mechanism of toluene oxidation and product generation pathway. As shown in Figure 4, the obvious main characteristic peak detected at 2348 cm−1 can be attributed to CO2 [27]. The characteristic peak of CO2 gradually increases with the increase of temperature, suggesting that the continuous increase in temperature is conducive to accelerate the catalytic oxidation of toluene. The peak at 1695 cm−1 can be attributed to the tensile vibration of the benzene ring skeleton ν(C=C), which confirmed that the benzene intermediate was attached to the catalyst surface during the catalytic process. The characteristic peaks of the in-plane and out-plane bending vibration of methyl ν(C-H) detected at 821, 3035, and 3139 cm−1 [28] indicates that toluene gas molecules first adsorb on the catalyst surface and then undergo oxidation reaction. Additional peaks at 1172 and 1236 cm−1, assigned to ν(-OH), and those at 2800 and 2913 cm−1, linked to benzyl species [29], emerge with increasing temperature, signifying benzyl alcohol as an intermediate in the toluene oxidation pathway. The characteristic peak of ν(-COO) at 1409 and 1535 cm−1 is attributable to carboxylate species [29]. The characteristic peak of ν(-C-O) at 920, 1041 cm−1 is ascribed to the generation of alkoxide species [30,31]. Combined with the XPS results, toluene molecules that adsorbed on the catalyst surface were oxidized by adsorbed oxygen species to generate CO2 as major product with minor intermediate products (such as benzyl alcohol and benzoic acid).
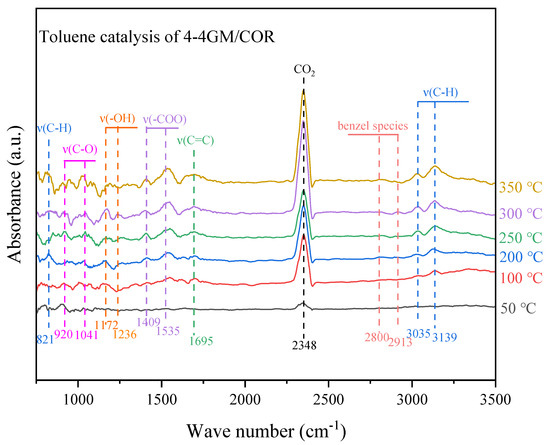
Figure 4.
In-situ DRIFTS of the toluene catalytic process of 4−4GM/COR.
After exploring the catalytic stability, the effect of gas hourly space velocity (GHSV) on catalytic performance was further investigated to evaluate the adaptability of 4-4GM/COR in industrial processes (Figure 5a). As the GHSV increased from 750 h−1 to 6000 h−1 (equivalent to an airspeed of approximately 1 to 8 m/s and a retention time of approximately 0.2 to 0.025s), the 4-4GM/COR monolithic catalyst maintained a stable T90 at 200 °C, signifying the monolithic catalyst exhibited superior adaptability to high wind speeds. This can be attributed to the parallel arrangement of honeycomb pores in the cordierite, which is beneficial for catalytic processes via providing a high surface area with a low pressure drop. Furthermore, by loading 4GM onto the cordierite, the effective contact area between the catalyst and the VOCs was increased and the reaction path length within the same volume was extended, contributing to a more thorough interaction between VOCs and the catalyst, which leads to efficient degradation. In summary, the 4-4GM/COR monolithic catalyst demonstrated great potentials for industrial VOC degradation applications due to its stability and adaptability to different GHSV.
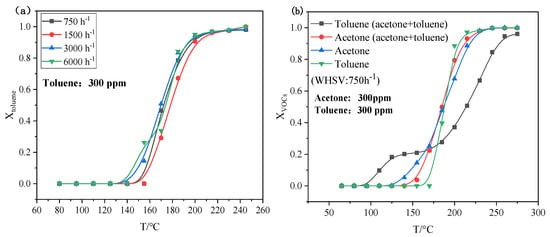
Figure 5.
Catalytic performance of 4−4GM/COR monolithic catalyst on toluene oxidation at different GHSV (a) and different VOCs (b).
In order to comprehensively evaluate the applicability of 4-4GM/COR, the catalytic performance for different kinds of VOCs degradation was investigated by introducing acetone as another kind of VOCs. The performances for catalytic oxidation of toluene (alone), acetone (alone), and a mixture of toluene and acetone were investigated by setting the concentration of each kind of VOC at 300 ppm and the total GHSV at 750 h−1, as illustrated in Figure 5b. With regard to single-type VOC, the catalytic performance of acetone over 4-4GM/COR is similar to that of toluene, achieving 98% conversion at 230 °C. When toluene and acetone were catalyzed together, it is interesting to find the toluene conversion was greatly promoted while the acetone conversion was slightly inhibited at the low-temperature region (<165 °C), which is speculated to be attributed to the preferential adsorption of toluene owing to the π-π accumulation effect between benzene rings of graphene and toluene. With the increase of temperature, the toluene conversion curve shifted to a higher temperature, mainly because of competition between toluene and acetone on the active sites. Even though the presence of acetone raises the T90 for toluene to 250 °C, this performance is deemed equivalent to some noble metal catalysts, highlighting the potential of 4-4GM/COR as a cost-effective alternative to expensive noble metal catalysts for VOC degradation.
2.2.3. Discussion on Application Potentials
Whether a catalyst can be used in industrial processes depends on many factors, including activity, stability, cost, scalability and availability, toxicity and environmental impact, and so on. Table 3 compares the 4-4GM/COR monolithic catalyst extensively with other representative monolithic catalysts used for toluene oxidation in the literature. First, the 4-4GM/COR catalyst developed in this study demonstrates an attractive option for industrial applications since it has superior catalytic activity, which can reduce energy consumption and costs. Additionally, the 4-4GM/COR catalyst exhibits excellent water resistance, durability, and stability, rendering it suitable for a wider range of applications. Second, compared with commercial noble metal catalysts, the 4-4GM/COR catalyst has the advantage of low cost, as the raw materials (MnOx and graphite) required for the monolithic catalyst are readily available and inexpensive. Furthermore, the ball-milling assisted impregnation method employed for the preparation of the monolithic catalysts not only ensures strong adherence of the catalyst particles to the support material but also eliminates the need for additional binders or high-temperature/high-pressure synthesis processes, thereby further reducing preparation costs. Last, the 4-4GM/COR monolithic catalyst features easy scalability and excellent adaptability to high gas hourly space velocities (GHSV) due to its robust mechanical strength and low pressure drop. Clearly, the 4-4GM/COR monolithic catalyst proposed in this study exhibits outstanding overall performance in terms of activity, cost-effectiveness, availability, and scalability.

Table 3.
Performance comparison of 4-4GM/COR with other related monolithic catalysts.
3. Materials and Methodologies
3.1. Materials
Cordierite, purchased from Haichuan Chemical Co., Ltd., Shanghai, China, is a cylindrical honeycomb block with a diameter of 34 mm, a height of 8 mm, and an internal square hole side length of 1mm. Oxalic acid dihydrate (H2C2O4·2H2O, AR, ≥99.5%) and absolute ethanol (C2H5OH, AR, ≥99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
3.2. Monolithic Catalyst Preparation
- (1)
- Cordierite pretreatment
Cordierite blocks were repeatedly cleaned using deionized water and anhydrous ethanol to remove surface impurities, then dried in an oven at 110 °C for 12 h. Subsequently, cordierite blocks were placed in the 200 mL oxalic acid solution (10 wt.%) for 12 h to etch the surface of the support. After soaking, the cordierite block was picked out and washed with deionized water, and then dried for subsequent treatment.
- (2)
- Preparation of monolithic catalysts
Graphene-doped α-MnO2 was synthesized by the in-situ hydrothermal method, which is described in detail in the Supplementary Materials. To prepare the monolithic catalysts, 3 g of the 4GM powder were initially ground for 2 h at a 200 r/min rate in a planetary mill. Subsequent to milling, the 3 g 4GM catalyst was dispersed in 200 mL of deionized water to form a nano-sized particle suspension. The cordierite was then immersed in this suspension for 6 h, after which it was removed, and the excess solution was expelled from the honeycomb structure using a blower. The sample was subsequently dried at 110 °C for 12 h. The resulting monolithic catalyst was designated as 1-4GM/COR. The impregnation process described above was repeated to synthesize x-4GM/COR catalysts (where x = 2, 3, or 4, indicating the number of impregnation cycles). The weights of the x-4GM/COR catalysts were recorded before and after impregnation to calculate loading rate. In comparison, in-situ hydrothermal synthesis and a coating method using binders were also investigated for the preparation of the monolithic catalyst, with comprehensive details provided in the Supplementary Materials.
3.3. Catalyst Characterization Method
The crystal phase of the samples was characterized by an X-Ray diffractometer (XRD, PANalyticalAeris, Almelo, Holland), in which the Cu-Kα radiation voltage was 40 kV, the current was 15 mA, the scanning range was 8~65°, and the step size was 0.02°. N2 adsorption-desorption isotherms were performed on Autosorb-iQ (Quantachrome Instruments Co., Ltd., Boynton Beach, FL, USA) using N2 as the analytical gas at -196 °C. Before the measurement, the catalyst was degassed at 200 °C for 6 h to remove physically adsorbed water and impurities. The specific surface area was determined by the Brunauer Emmett Teller (BET) equation, and the pore size distribution was analyzed by the quenched solid density functional theory (QSDFT) method. Thermal field emission scanning electron microscopy (SEM, TESCAN MIRA LMS, Beijing, China) was used to characterize the surface morphology of the samples. The surface chemistry was investigated by X-Ray photoelectron spectroscopy (XPS) using a 150 W Al Kα probe beam on a KRATOS AXIS Ultra DLD instrument (XPS, SHIMADZU, Kyoto, Japan). H2 temperature-programmed reduction (H2-TPR) was performed on ChemBET Pulsar TPR/TPD (Quantachrome Instruments Co., Ltd., Boynton Beach, FL, USA) to characterize the redox ability of the catalysts. In the TPR experiment, the sample mass was uniformly set to 5 mg, He was used as the carrier gas, 5% H2/He was used as the reaction gas, and the flow rate of the reaction gas was controlled at 110–120 mL/min. The in-situ DRIFTS experiment was performed on a Fourier transform infrared spectrometer (DRIFTS, Thermo Nicolet Co., Ltd., Waltham, MA, USA) to investigate the catalytic mechanism of toluene.
3.4. Catalyst Performance Testing
In this study, a VOC generator (FD-PG02, Flender Testing Equipment Co., Ltd., Suzhou, China) was used to simulate the VOC gas. Synthetic air was used as the carrier gas, and the flow rate was stabilized at 250 mL/min, 500 mL/min, or 1000 mL/min by a mass flow meter (FL-802, Flowmethod Co., Ltd., Shenzhen, China). Liquid toluene (acetone when needed) was injected into the carrier gas using a micro-syringe pump (SPLab 01, Shenzhen Pump Industry Co., Ltd., Shenzhen, China.) and vaporized at 110 °C to generate a VOC gas stream.
The catalytic reaction was carried out in a quartz reactor (530 mm in length, 40 mm in outer diameter, 35 mm in inner diameter), and the temperature could be adjusted from room temperature to 1200 °C. For each experiment, three pieces of cordierite catalyst blocks were filled into the reactor with the inlet toluene concentration set to 300 ppm. The outlet of the reaction device was connected to a GC/MS gas chromatograph (SP-3420A, BFRL Co., Ltd., Beijing, China) to analyze the concentrations of toluene (acetone when acetone was used as simulated VOC gas), CO2, and CO. In order to study the effect of humidity on the catalytic performance, deionized water was injected into the VOC generator through another micro-syringe pump, and a humidity detector was equipped to measure the atmospheric humidity in real-time. The experimental setup was illustrated in Figure S7 in the Supplementary Materials.
The main parameters to evaluate the catalytic performance of catalysts for toluene oxidation are the toluene degradation efficiency (Xtoluene) and the CO2 selectivity (SCO2). The calculation formulas are as follows:
where [Toluene]in and [Toluene]out are the concentrations of toluene at the reactor inlet and outlet, respectively, and [CO2] is the concentration of CO2 at the reactor outlet. The number 7 in Formula (2) represents that 1 mole of toluene can be completely degraded into 7 moles of CO2.
4. Conclusions
The monolithic catalyst was fabricated via a facile ball-milling-assisted impregnation strategy. A cordierite honeycomb substrate, pre-etched with oxalic acid to enhance surface roughness and area for catalyst anchoring, served as the structured support. Nano-sized 4GM particles, obtained by mechanical ball milling, were uniformly and firmly anchored onto the cordierite’s submicron-scale cavities (3–5 μm in length) without blocking the pristine COR channels. Structural analyses revealed that iterative impregnation cycles (up to four cycles) progressively enhanced the loading rate and distribution uniformity of the active components, specific surface area, and pore volume. Concurrently, the optimized surface chemistry—characterized by elevated Mn3⁺ content, abundant lattice oxygen species, and oxygen vacancy density—synergistically facilitated the activation and mobility of reactive oxygen species, thereby boosting catalytic performance.
The 4-4GM/COR catalyst demonstrated exceptional toluene oxidation activity, achieving 90% conversion (T90) at 200 °C under dry conditions and 198 °C in humid atmospheres (RH = 85%), outperforming most reported monolithic catalysts. Remarkably, it maintained a stable T90 of 200 °C across five consecutive cycles and under varying gas hourly space velocities (GHSV: 750–6000 h−1), confirming its robustness in practical applications. Additionally, the 4-4GM/COR monolithic catalyst demonstrated excellent catalysis stability with 100% toluene conversion at 270 °C in both dry and humid environments. In summary, the monolithic catalyst prepared using the “ball-milling assisted impregnation method” exhibits high activity, stability, and adaptability to varying GHSV. The preparation method is simple, easy-to-operate, and holds great potential for industrial applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15040321/s1, Figure S1: Appearance of different cordierite monolithic catalysts (a) 2-4GM/COR, (b) 3-4GM/COR, (c) 4-4GM/COR, (d) and (e) 4GM/COR prepared by the coating method, and (f) 4GM/COR prepared by the hydrothermal method; Figure S2: XRD test diagram of 4GM/COR monolithic catalyst; Figure S3: BET test diagram of 4GM/COR monolithic catalyst: (a,c) N2 adsorption-desorption curve (b,d) pore size distribution; Figure S4: XPS test spectrum of 4GM/COR monolithic catalyst (a) Mn2p (b) O1s; Figure S5:Comparison of catalytic activity of 4GM/COR monolithic catalyst prepared by ball-milling assisted impregnation with COR and 4GM/COR monolithic catalyst prepared by in-situ hydrothermal method (test condition: toluene concentration =300 ppm, GHVS = 750 h−1); Figure S6: Catalytic activity of 4GM at different atmosphere; Figure S7: Experimental setup for the catalyst performance evaluation (1. synthetic air, 2. mass flow meter, 3. micro-syringe pump, 4. VOC generator, 5. gas-mixing tanks, 6. catalytic reactor, 7.GC, 8.data acquisition.).
Author Contributions
Conceptualization, Y.D.; investigation, Y.D., Y.Z. and Y.S.; resources, X.Z., W.W., Z.S. and Y.M.; writing—original draft preparation, Y.D. and Y.S.; writing—review and editing, J.S. and Y.Z.; supervision, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was generously funded by the Key Research and Development Project of Shandong province (2020CXGC011402).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to appreciate the support from the 2024 Zhenjiang Jinshan Talent Program for Recruiting Leading Talents for Industrial Strengthening Urban Development and the 2023 Yangzhong “Jinshan Talent, Jiangyan Plan” for Recruiting Leading Talents for Industrial Strengthening Urban Development.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Q.; Zheng, Y.; Song, C.; Liu, Q.; Ji, N.; Ma, D.; Lu, X. Novel monolithic catalysts derived from in-situ decoration of Co3O4 and hierarchical Co3O4@MnOx on Ni foam for VOC oxidation. Appl. Catal. B Environ. 2020, 265, 118552. [Google Scholar]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar]
- Fu, K.X.; Su, Y.; Yang, L.Z.; Ji, N.; Song, C.F.; Ma, D.G.; Lv, X.B.; Han, R.; Liu, Q.L. Pt loaded manganese oxide nanoarray-based monolithic catalysts for catalytic oxidation of acetone. Chem. Eng. J. 2022, 432, 134397. [Google Scholar]
- Guo, Y.; Ren, Z.; Xiao, W.; Liu, C.; Sharma, H.; Gao, H.; Mhadeshwar, A.; Gao, P.X. Robust 3-D configurated metal oxide nano-array based monolithic catalysts with ultrahigh materials usage efficiency and catalytic performance tunability. Nano Energy 2013, 2, 873–881. [Google Scholar]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Yang, P.; Cheng, Z.; Li, J.; Zuo, S. Ce-modified mesoporous γ-Al2O3 supported Pd-Pt nanoparticle catalysts and their structure-function relationship in complete benzene oxidation. Chem. Eng. J. 2019, 356, 255–261. [Google Scholar]
- Ge, Y.; Fu, K.; Zhao, Q.; Ji, N.; Song, C.; Ma, D.; Liu, Q. Performance study of modified Pt catalysts for the complete oxidation of acetone. Chem. Eng. Sci. 2019, 206, 499–506. [Google Scholar]
- Lu, L.; Tian, H.; He, J.H.; Yang, Q.W. Graphene-MnO2 Hybrid Nanostructure as a New Catalyst for Formaldehyde Oxidation. J. Phys. Chem. C 2016, 120, 23660–23668. [Google Scholar]
- Fan, J.; Ren, Q.; Mo, S.; Sun, Y.; Fu, M.; Wu, J.; Chen, L.; Chen, P.; Ye, D. Transient in-situ DRIFTS Investigation of Catalytic Oxidation of Toluene over α-, γ- and β-MnO2. ChemCatChem 2020, 12, 1046–1054. [Google Scholar]
- Yang, W.; Su, Z.a.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B Environ. 2020, 260, 118150. [Google Scholar]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Min, X.; Guo, M.; Li, K.; Gu, J.-n.; Hu, X.; Jia, J.; Sun, T. Boosting the VOCs purification over high-performance α-MnO2 separated from spent lithium-ion battery: Synergistic effect of metal doping and acid treatment. Sep. Purif. Technol. 2022, 295, 121316. [Google Scholar]
- Liu, W.; Xiang, W.; Guan, N.; Cui, R.; Cheng, H.; Chen, X.; Song, Z.; Zhang, X.; Zhang, Y. Enhanced catalytic performance for toluene purification over Co3O4/MnO2 catalyst through the construction of different Co3O4-MnO2 interface. Sep. Purif. Technol. 2021, 278, 119590. [Google Scholar]
- Dong, Y.; Sun, J.; Shen, Y.; Wang, Z.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. 3D snowflake graphene modified α-MnO2 Catalyst: Enhancing the Low-Temperature catalytic activity for VOCs degradation. Chem. Eng. J. 2023, 473, 145130. [Google Scholar]
- Tang, L.; Zhao, Z.; Li, K.X.; Yu, X.H.; Wei, Y.C.; Liu, J.; Peng, Y.; Li, Y.Z.; Chen, Y.S. Highly Active Monolith Catalysts of LaKCoO3 Perovskite-type Complex Oxide on Alumina-washcoated Diesel Particulate Filter and the Catalytic Performances for the Combustion of Soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- Tang, W.X.; Lu, X.X.; Liu, F.Y.; Du, S.C.; Weng, J.F.; Hoang, S.; Wang, S.B.; Nam, C.Y.; Gao, P.X. Ceria-based nanoflake arrays integrated on 3D cordierite honeycombs for efficient low-temperature diesel oxidation catalyst. Appl. Catal. B Environ. 2019, 245, 623–634. [Google Scholar]
- Bao, L.; Wu, D.F. Effect of Acid Treatment on the Catalytic Activity and Mechanical Stability of SmMnO3/Cordierite Monolithic Catalysts. Chemistryselect 2021, 6, 7845–7854. [Google Scholar]
- Wu, D.; Zhang, Q.; Gao, R. Mechanical stability of ZSM-5 zeolite washcoated cordierite monoliths. Chem. Eng. Res. Des. 2021, 168, 426–434. [Google Scholar]
- Diaz, C.C.; Yeste, M.P.; Vidal, H.; Gatica, J.M.; Cadus, L.E.; Morales, M.R. In situ generation of Mn1-xCex system on cordierite monolithic supports for combustion of n-hexane. Effects on activity and stability. Fuel 2020, 262, 116564. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z.; Zhou, Y.; Zhu, Q.; Pan, Z.; Lu, H. A facile route for spraying preparation of Pt/TiO2 monolithic catalysts toward VOCs combustion. Appl. Catal. A Gen. 2018, 566, 190–199. [Google Scholar] [CrossRef]
- Xue, T.; Li, R.; Gao, Y.; Wang, Q. Iron mesh-supported vertically aligned Co-Fe layered double oxide as a novel monolithic catalyst for catalytic oxidation of toluene. Chem. Eng. J. 2020, 384, 123284. [Google Scholar]
- Soltan, W.B.; Sun, J.; Wang, W.; Peng, J.; Zhang, Y.; Wang, T.; Chang, Y.; Ding, L.; Cao, Z.; Wang, W.; et al. Modiffcation of Fe on hydrophobic ZSM-5 zeolite: Optimization of adsorption and catalytic performance for decomposition of VOCs at low-temperature. Sep. Purif. Technol. 2024, 333, 125908. [Google Scholar]
- Li, Y.F.; Liao, Q.Y.; Ling, W.Z.; Ye, F.; Liu, F.F.; Zhang, X.P.; He, J.J.; Cheng, G. Pd/d-MnO2 nanoflower arrays cordierite monolithic catalyst toward toluene and o-xylene combustion. Front. Chem. 2022, 10, 978428. [Google Scholar]
- Wu, P.; Jin, X.; Qiu, Y.; Ye, D. Recent Progress of Thermocatalytic and Photo/Thermocatalytic Oxidation for VOCs Purification over Manganese-based Oxide Catalysts. Environ. Sci. Technol. 2021, 55, 4268–4286. [Google Scholar] [PubMed]
- Dong, Y.; Sun, J.; Ma, X.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Li, W. Study on the synergy effect of MnOx and support on catalytic ozonation of toluene. Chemosphere 2022, 303, 134991. [Google Scholar]
- Soltan, W.B.; Sun, J.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Zhang, Z. Discovering the key role of MnO2 and CeO2 particles in the Fe2O3 catalysts for enhancing the catalytic oxidation of VOC: Synergistic effect of the lattice oxygen species and surface-adsorbed oxygen. Sci. Total Environ. 2022, 819, 152844. [Google Scholar] [CrossRef]
- Yang, S.; Qi, Z.; Wen, Y.; Wang, X.; Zhang, S.; Li, W.; Li, S. Generation of abundant oxygen vacancies in Fe doped δ-MnO2 by a facile interfacial synthesis strategy for highly efficient catalysis of VOCs oxidation. Chem. Eng. J. 2023, 452, 139657. [Google Scholar]
- Yang, R.; Han, P.; Fan, Y.; Guo, Z.; Zhao, Q.; Wang, Y.; Che, S.; Lin, S.; Zhu, R. The performance and reaction pathway of δ-MnO2/USY for catalytic oxidation of toluene in the presence of ozone at room temperature. Chemosphere 2020, 247, 125864. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, Y.; Leng, X.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Synthesis of CeaMnOx hollow microsphere with hierarchical structure and its excellent catalytic performance for toluene combustion. Appl. Catal. B Environ. 2019, 245, 502–512. [Google Scholar]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar]
- Bi, F.; Zhang, X.; Chen, J.; Yang, Y.; Wang, Y. Excellent catalytic activity and water resistance of UiO-66-supported highly dispersed Pd nanoparticles for toluene catalytic oxidation. Appl. Catal. B Environ. 2020, 269, 118767. [Google Scholar]
- Wang, H.; Sun, S.; Nie, L.; Zhang, Z.; Li, W.; Hao, Z. A review of whole-process control of industrial volatile organic compounds in China. J. Environ. Sci. 2023, 123, 127–139. [Google Scholar]
- Zhu, J.; Zhang, W.; Qi, Q.; Zhang, H.; Zhang, Y.; Sun, D.; Liang, P. Catalytic oxidation of toluene, ethyl acetate and chlorobenzene over Ag/MnO2-cordierite molded catalyst. Sci. Rep. 2019, 9, 12162. [Google Scholar]
- Zhao, H.; Wang, H.; Qu, Z. Synergistic effects in Mn-Co mixed oxide supported on cordierite honeycomb for catalytic deep oxidation of VOCs. J. Environ. Sci. 2022, 112, 231–243. [Google Scholar]
- Lv, X.; Zhang, T.; Cao, J.; Wang, Y.; Liu, F.; Yin, L. Effect of different coatings on the structure and performance of three-dimensional ordered macropores La0.7Ce0.3CoO3 catalysts. Environ. Sci. Pollut. Res. 2022, 29, 85202–85210. [Google Scholar]
- Lv, C.; Chen, H.; Hu, M.; Ai, T.; Fu, H. Nano-oxides washcoat for enhanced catalytic oxidation activity toward the perovskite-based monolithic catalyst. Environ. Sci. Pollut. Res. 2021, 28, 37142–37157. [Google Scholar]
- Zang, M.; Zhao, C.; Wang, Y.; Liu, X.; Cheng, Y.; Chen, S. Low temperature catalytic combustion of toluene over three-dimensionally ordered La0.8Ce0.2MnO3/cordierite catalysts. Appl. Surf. Sci. 2019, 483, 355–362. [Google Scholar]
- Zhu, A.; Zhou, Y.; Wang, Y.; Zhu, Q.; Liu, H.; Zhang, Z.; Lu, H. Catalytic combustion of VOCs on Pt/CuMnCe and Pt/CeY honeycomb monolithic catalysts. J. Rare Earths 2018, 36, 1272–1277. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).