Mechanochemically Modified TiO2 Photocatalysts: Combination of Visible-Light Excitability and Antibacterial Effect
Abstract
1. Introduction
2. Results and Discussion
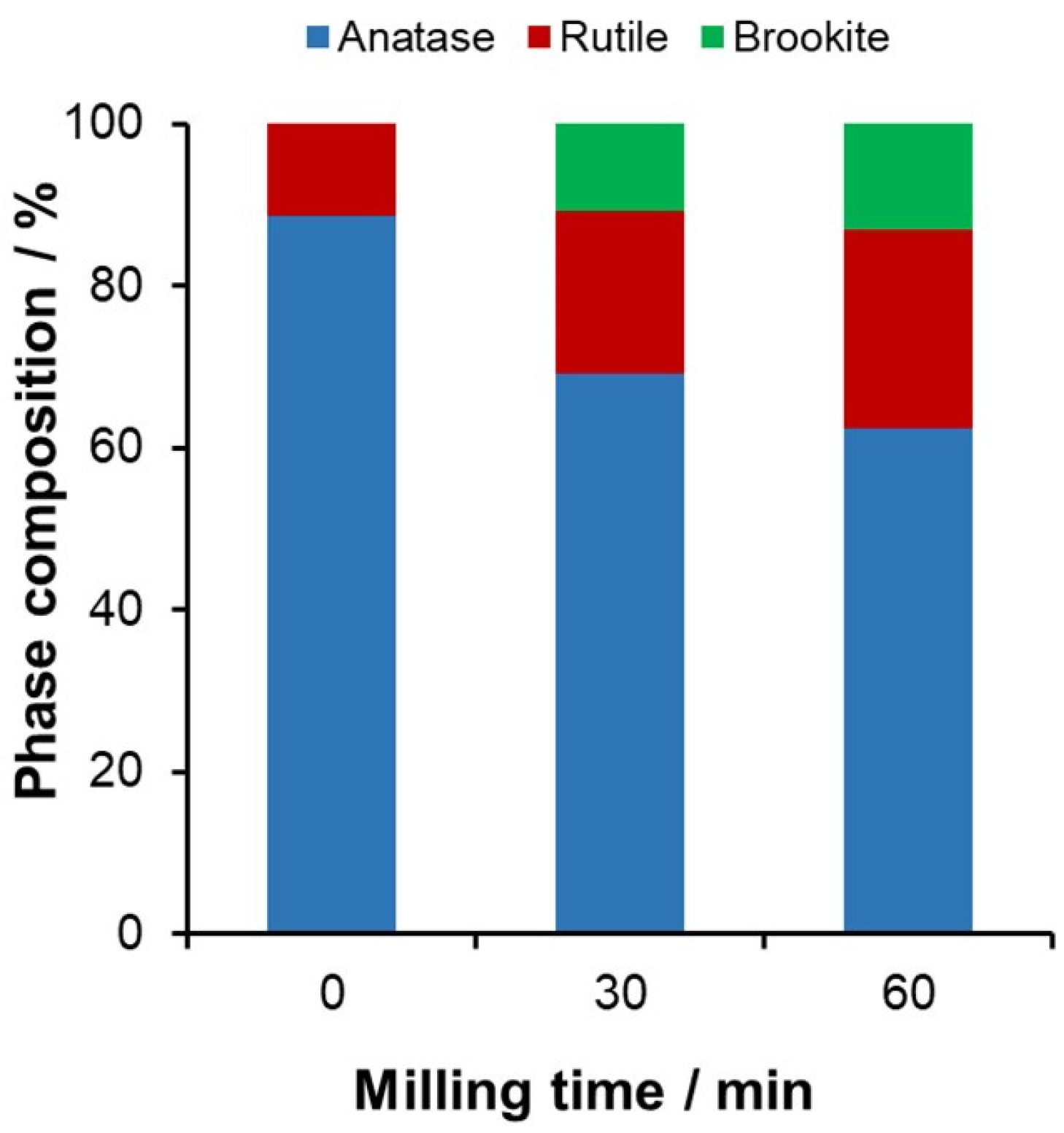
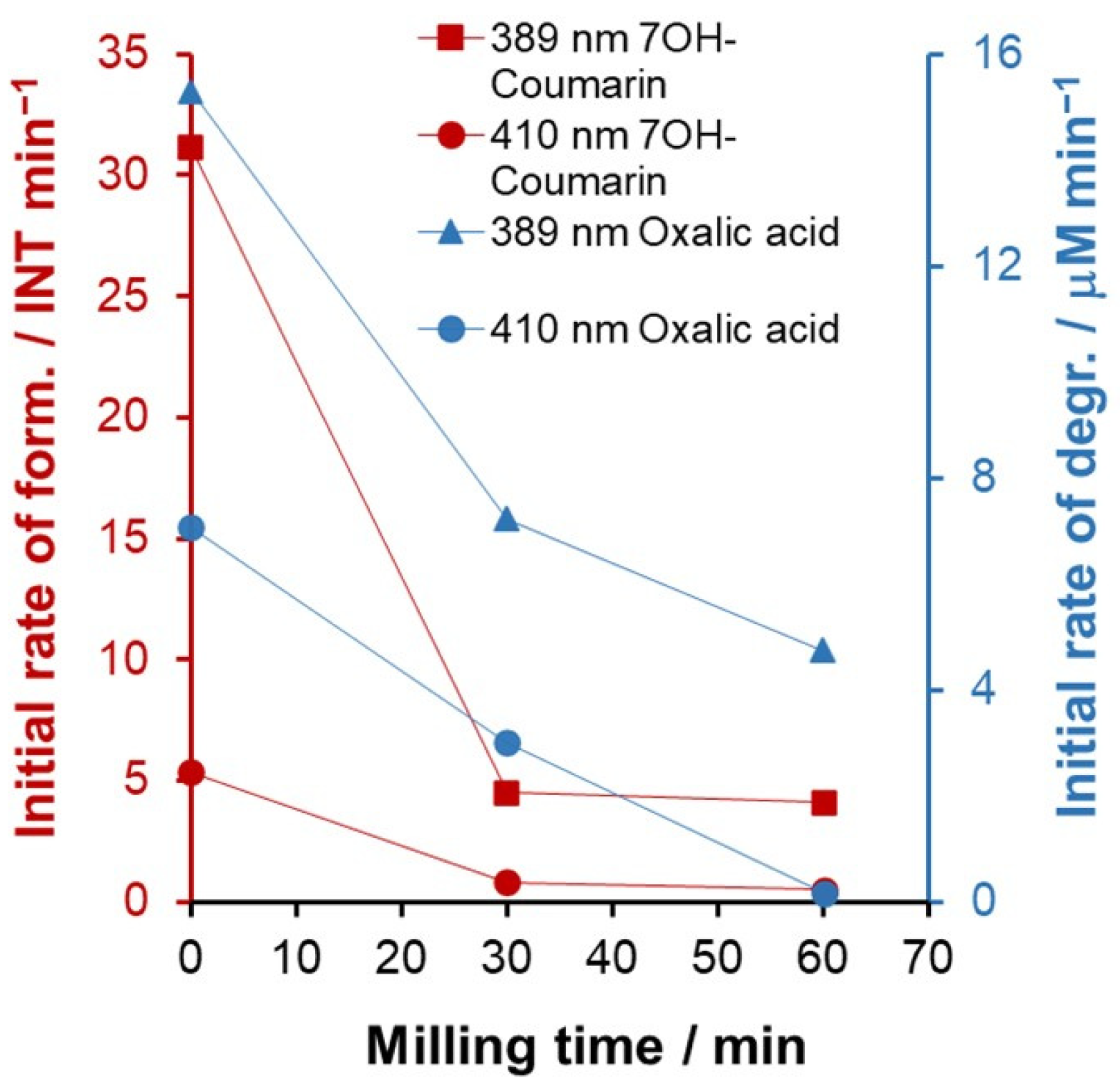
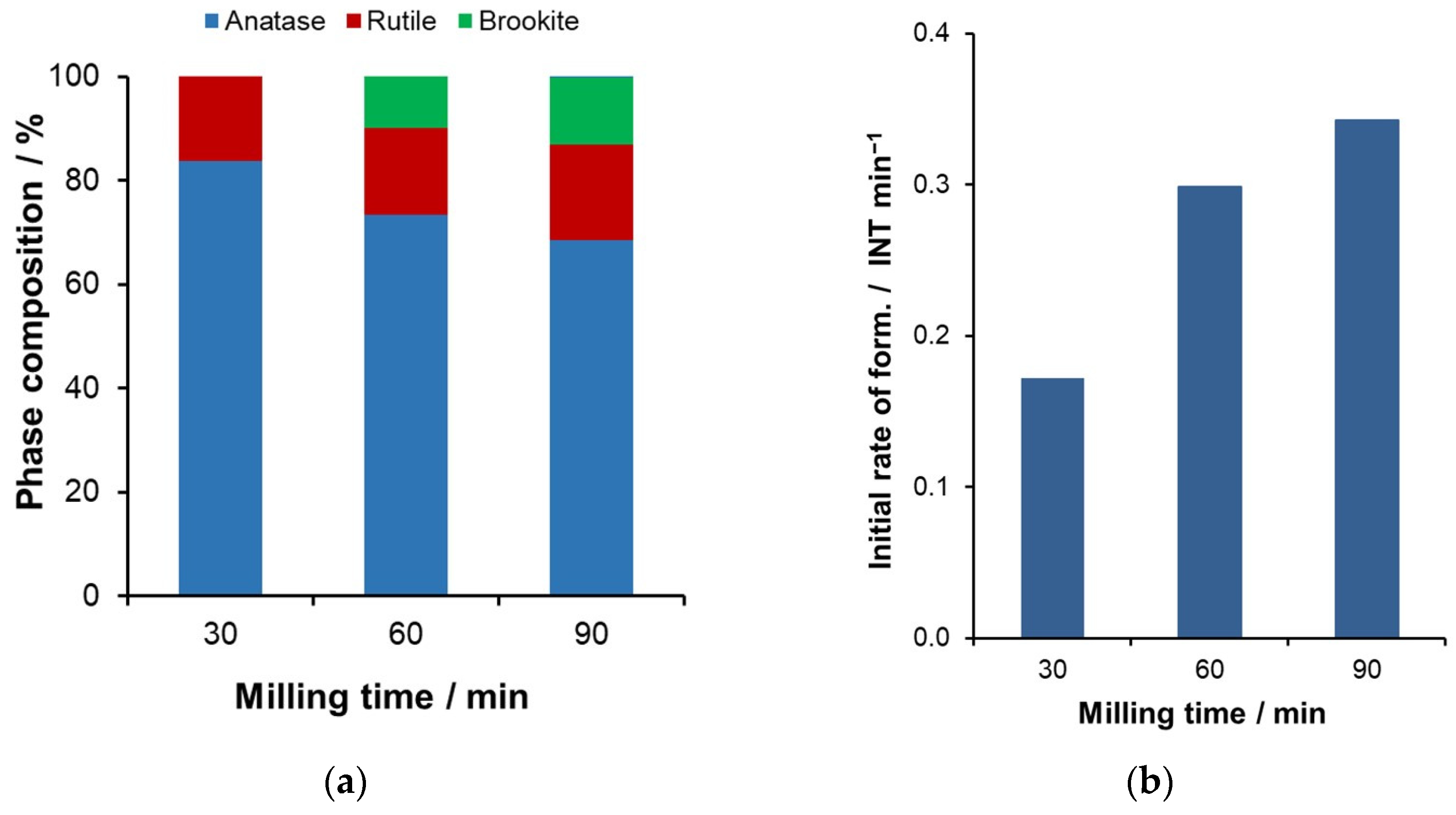
2.1. Mechanochemical Treatment of TiO2
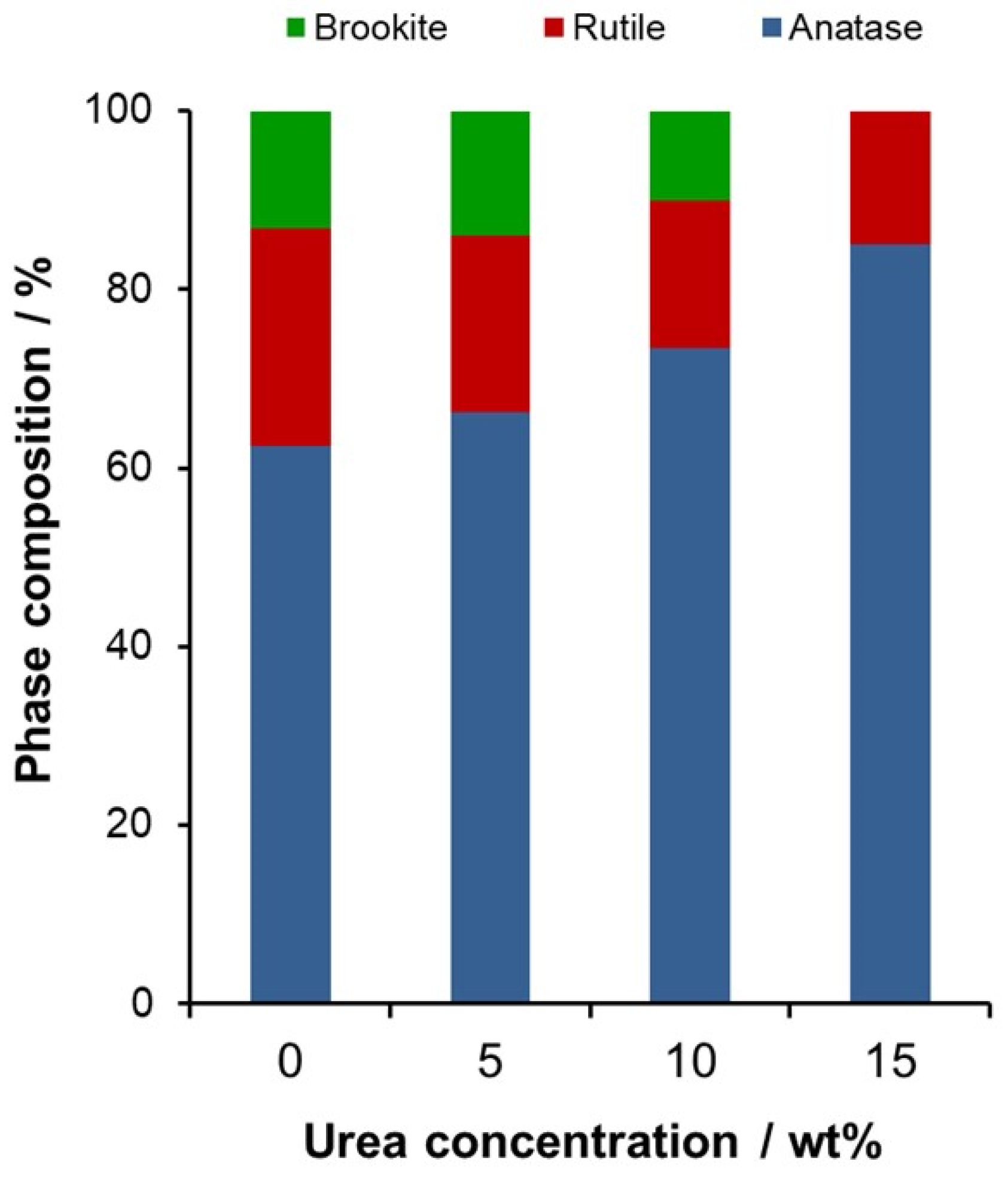
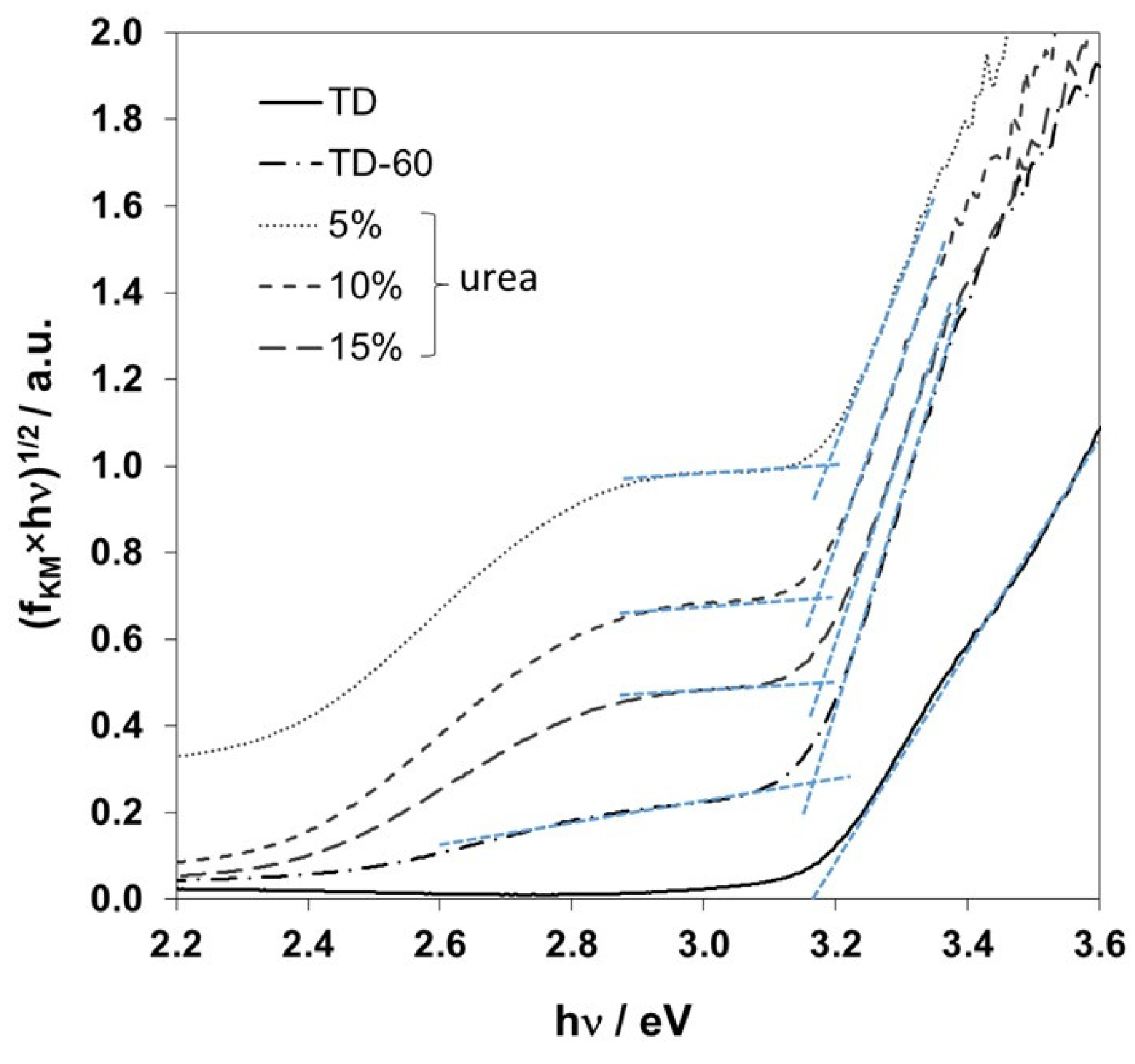
2.2. Modification with Nitrogen
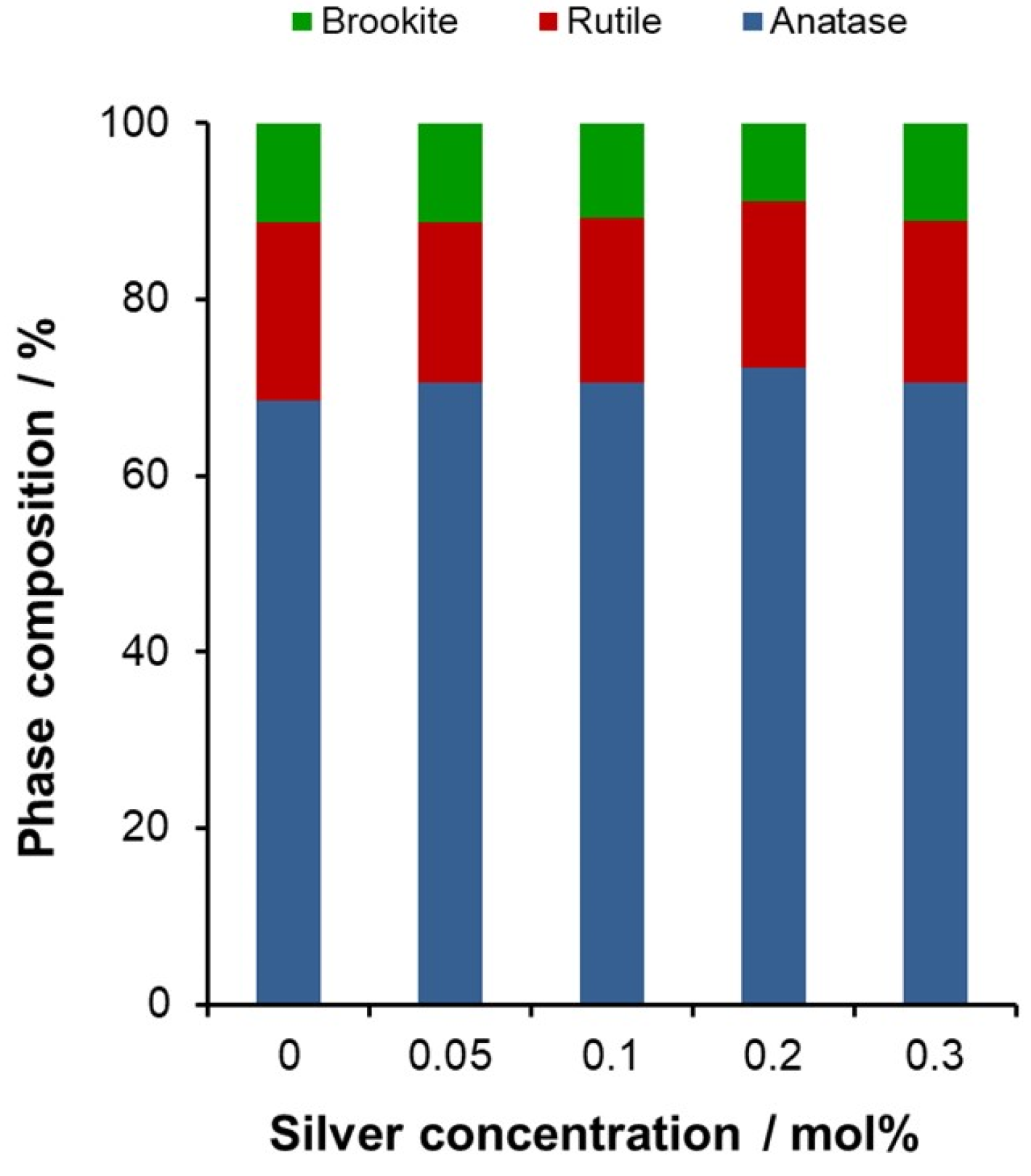
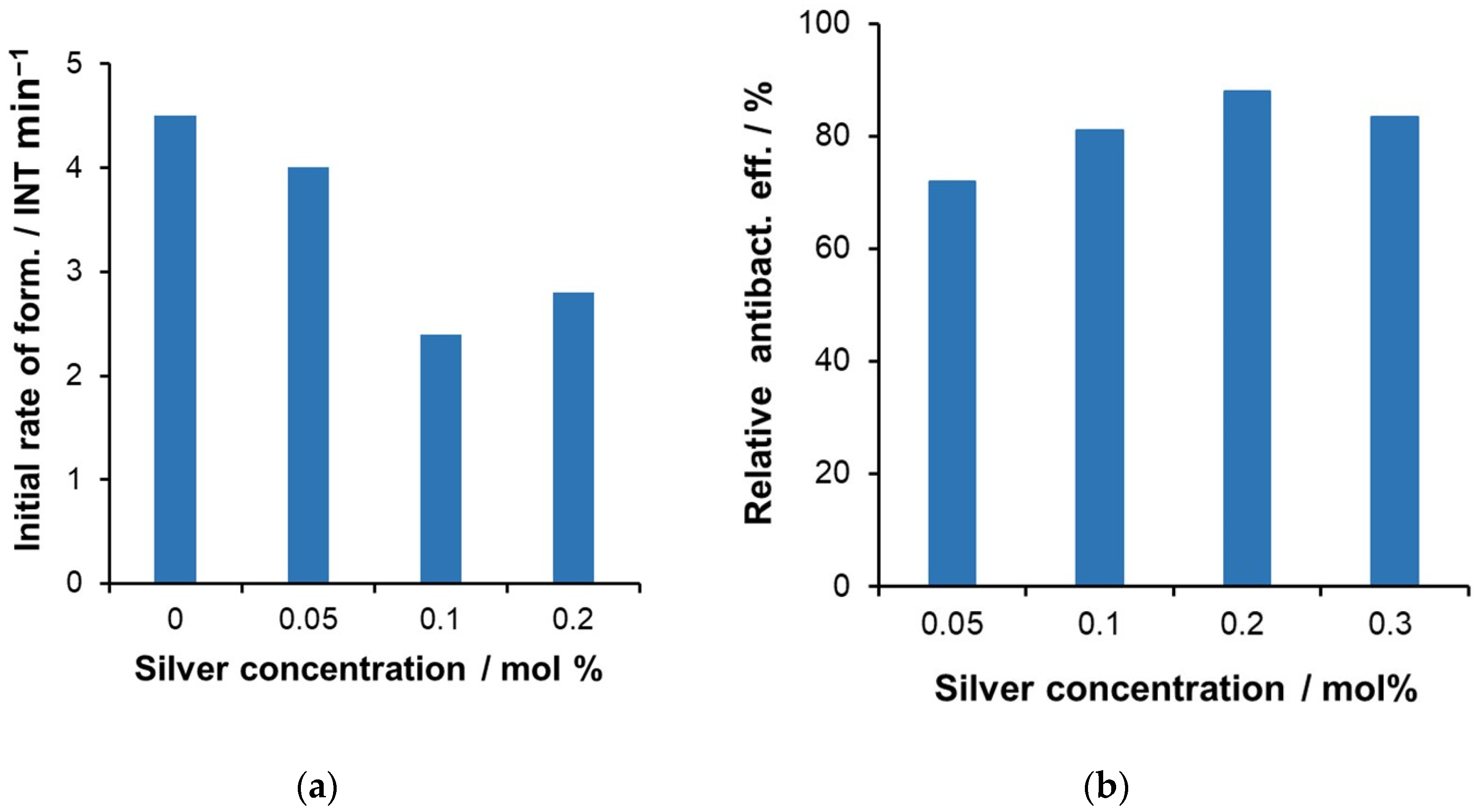
2.3. Modification with Silver
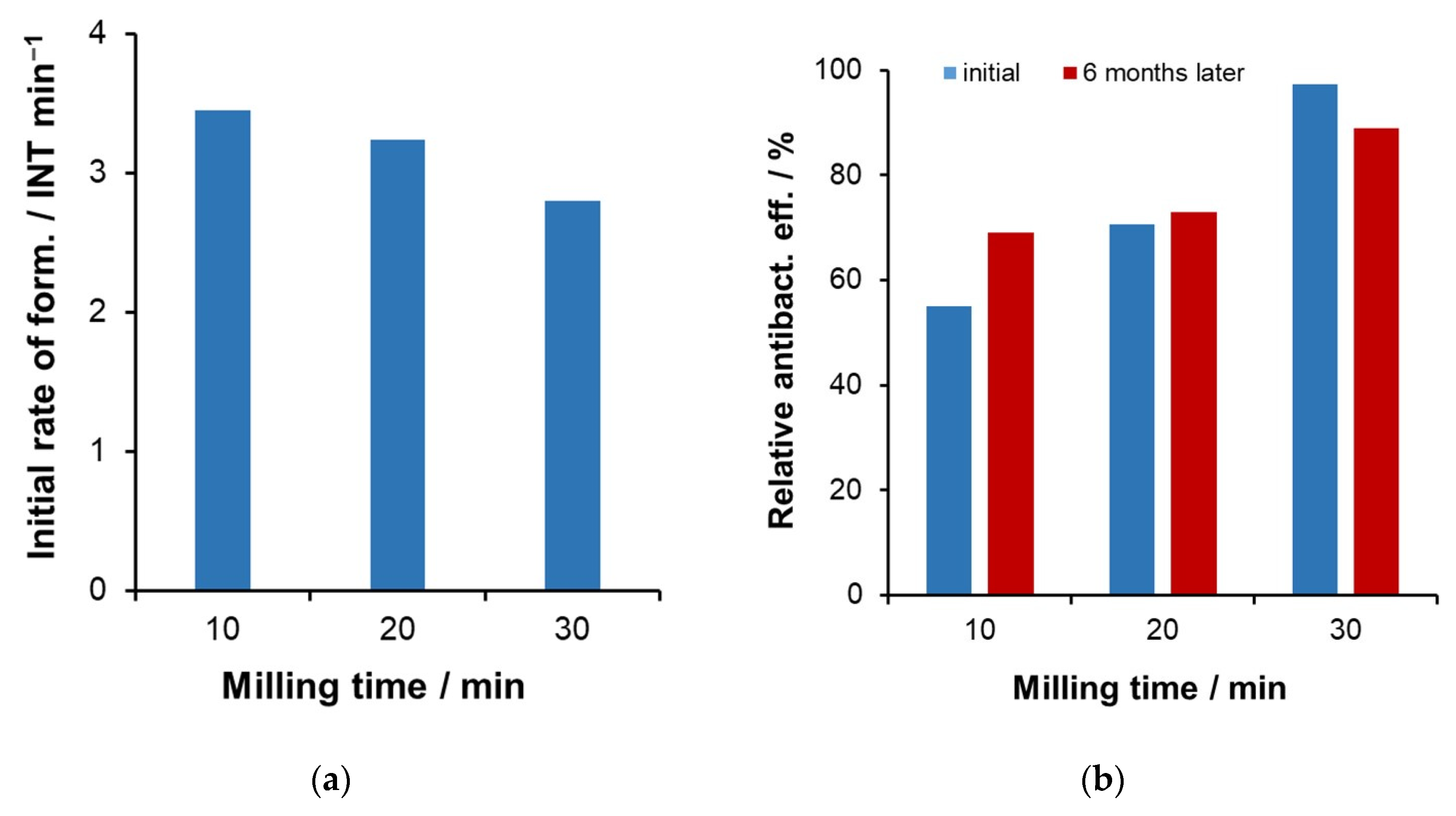
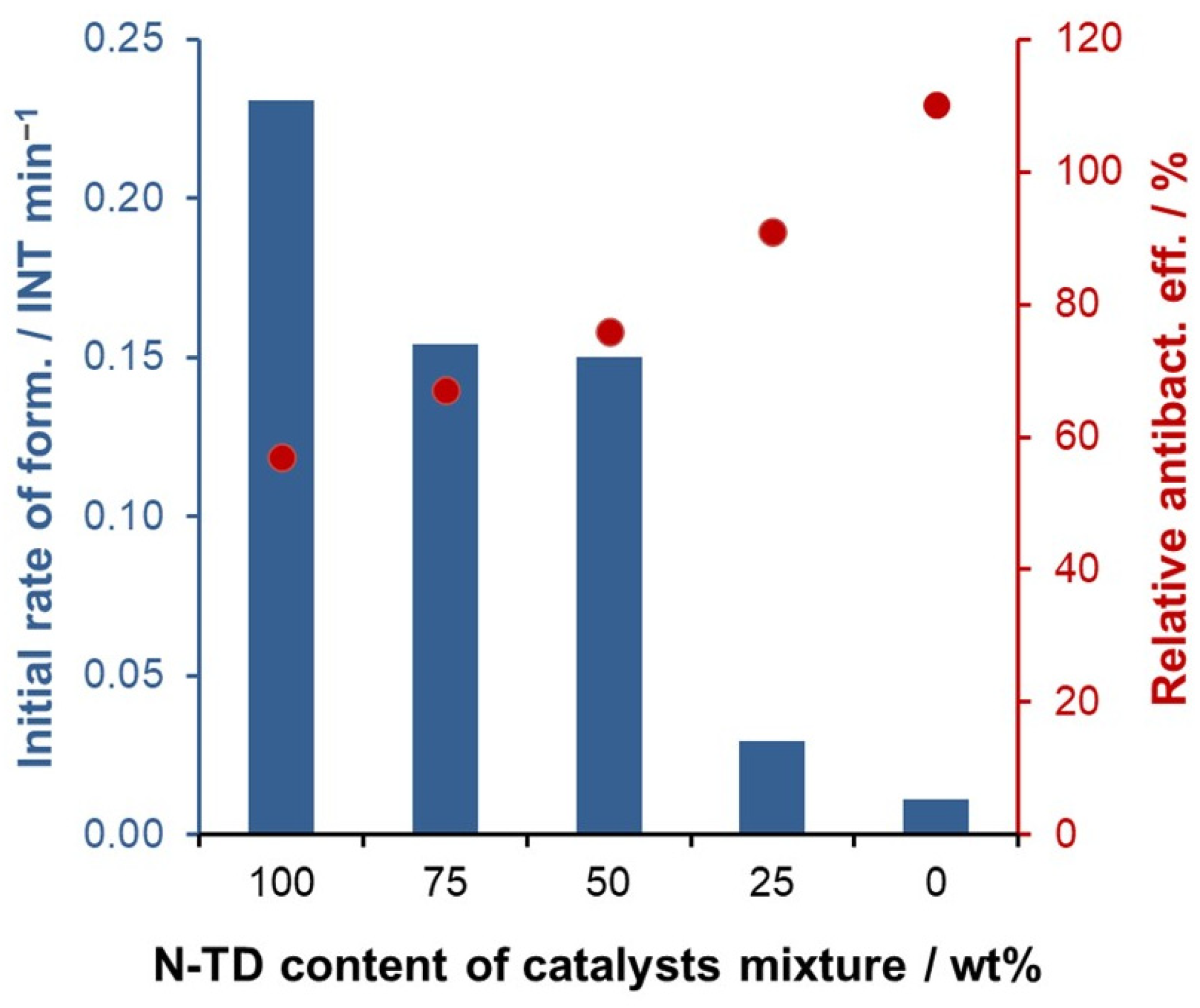
2.4. Combination of Photocatalytic and Antibacterial Activities
3. Materials and Methods
3.1. Materials
3.2. Mechanochemical Modification of the Catalysts
3.3. Photocatalytic Experiments
3.4. Analytical Procedures
3.4.1. Characterization of the Catalysts
3.4.2. Measurements of Photocatalytic Activity
3.4.3. Determination of Antibacterial Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bregnocchi, A.; Jafari, R.; Momen, G. Design Strategies for Antiviral Coatings and Surfaces: A Review. Appl. Surf. Sci. Adv. 2022, 8, 100224. [Google Scholar] [CrossRef]
- Lee, A.-C.; Lin, R.-H.; Yang, C.-Y.; Lin, M.-H.; Wang, W.-Y. Preparations and Characterization of Novel Photocatalysts with Mesoporous Titanium Dioxide (TiO2) via a Sol–Gel Method. Mater. Chem. Phys. 2008, 109, 275–280. [Google Scholar] [CrossRef]
- Beh Gin-Colin, S.; Girot, T.; Le Caek, G.; Mocellin, A. Kinetics and Mechanisms of Phase Transformations Induced by Ball-Milling in Anatase TiO2. J. Solid State Chem. 2000, 149, 41–48. [Google Scholar]
- Huberty, J.; Xu, H. Kinetics Study on Phase Transformation from Titania Polymorph Brookite to Rutile. J. Solid State Chem. 2008, 181, 508–514. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-Specific Photocatalytic Activities of TiO2 and Multiactivity Test for Water Treatment Application. Environ. Sci. Technol. 2008, 42, 294–300. [Google Scholar] [CrossRef]
- Di Paola, A.; Cufalo, G.; Addamo, M.; Bellardita, M.; Campostrini, R.; Ischia, M.; Ceccato, R.; Palmisano, L. Photocatalytic Activity of Nanocrystalline TiO2 (Brookite, Rutile and Brookite-Based) Powders Prepared by Thermohydrolysis of TiCl4 in Aqueous Chloride Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 366–376. [Google Scholar] [CrossRef]
- Luo, W.; Taleb, A. Large-Scale Synthesis Route of TiO2 Nanomaterials with Controlled Morphologies Using Hydrothermal Method and TiO2 Aggregates as Precursor. Nanomaterials 2021, 11, 365. [Google Scholar] [CrossRef]
- Vajedi, F.S.; Dehghani, H. Synthesis of Titanium Dioxide Nanostructures by Solvothermal Method and Their Application in Preparation of Nanocomposite Based on Graphene. J. Mater. Sci. 2016, 51, 1845–1854. [Google Scholar] [CrossRef]
- Domingues, L.A.C.S.; Carriello, G.M.; Pegoraro, G.M.; Mambrini, G.P. Synthesis of TiO2 Nanoparticles by the Solvothermal Method and Application in the Catalysis of Esterification Reactions. An. Acad. Bras. Cienc. 2024, 96, e20240096. [Google Scholar] [CrossRef]
- Buraso, W.; Lachom, V.; Siriya, P.; Laokul, P. Synthesis of TiO2 Nanoparticles via a Simple Precipitation Method and Photocatalytic Performance. Mater. Res. Express 2018, 5, 115003. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cao, X.; Wu, S.; Liu, C.; Li, G.; Jiang, W.; Wang, H.; Wang, N.; Ding, W. Preparation and Characterization of TiO2 Nanoparticles by Two Different Precipitation Methods. Ceram. Int. 2020, 46, 15333–15341. [Google Scholar] [CrossRef]
- Szabó-Bárdos, E.; Czili, H.; Horváth, A. Photocatalytic Oxidation of Oxalic Acid Enhanced by Silver Deposition on a TiO2 Surface. J. Photochem. Photobiol. A Chem. 2003, 154, 195–201. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.B.; Safshekan, S. Photoinduced Deposition of Palladium Nanoparticles on TiO2 Nanotube Electrode and Investigation of Its Capability for Formaldehyde Oxidation. Electrochim. Acta 2014, 138, 468–475. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase. Materials 2018, 12, 40. [Google Scholar] [CrossRef]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non-Metal-Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Murzin, D.Y. Effect of Catalyst Synthesis Parameters on the Metal Particle Size. Appl. Catal. A Gen. 2013, 451, 251–281. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-Doped Titanium Dioxide: An Overview of Material Design and Dimensionality Effect over Modern Applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A Review on Metal Ions Modified TiO2 for Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Durango-Giraldo, G.; Cardona, A.; Zapata, J.F.; Santa, J.F.; Buitrago-Sierra, R. Titanium Dioxide Modified with Silver by Two Methods for Bactericidal Applications. Heliyon 2019, 5, e01608. [Google Scholar] [CrossRef]
- Xiong, Z.; Ma, J.; Ng, W.J.; Waite, T.D.; Zhao, X.S. Silver-Modified Mesoporous TiO2 Photocatalyst for Water Purification. Water Res. 2011, 45, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, X.; Liu, T.; Duan, C.; Zhu, Z. In-Situ Fabrication of Silver-Modified TiO2 Microspheres with Enhanced Visible Photocatalytic Activity. Mater. Lett. 2013, 110, 111–113. [Google Scholar] [CrossRef]
- Liu, H.; Dong, X.; Liu, T.; Zhu, Z. In-Situ Fabrication of Silver-Modified TiO2 Microspheres for Enhanced Visible Light Driven Photocatalytic Activities. Sol. Energy Mater. Sol. Cells 2015, 132, 86–93. [Google Scholar] [CrossRef]
- Liu, H.; Dong, X.; Duan, C.; Su, X.; Zhu, Z. Silver-Modified TiO2 Nanorods with Enhanced Photocatalytic Activity in Visible Light Region. Ceram Int. 2013, 39, 8789–8795. [Google Scholar] [CrossRef]
- Vamathevan, V.; Amal, R.; Beydoun, D.; Low, G.; McEvoy, S. Photocatalytic Oxidation of Organics in Water Using Pure and Silver-Modified Titanium Dioxide Particles. J. Photochem. Photobiol. A Chem. 2002, 148, 233–245. [Google Scholar] [CrossRef]
- Chaker, H.; Chérif-Aouali, L.; Khaoulani, S.; Bengueddach, A.; Fourmentin, S. Photocatalytic Degradation of Methyl Orange and Real Wastewater by Silver Doped Mesoporous TiO2 Catalysts. J. Photochem. Photobiol. A Chem. 2016, 318, 142–149. [Google Scholar] [CrossRef]
- He, P.; Tao, J.; Huang, X.; Xue, J. Preparation and Photocatalytic Antibacterial Property of Nitrogen Doped TiO2 Nanoparticles. J. Solgel. Sci. Technol. 2013, 68, 213–218. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Sun, Y.; Hu, J.; Li, C.; Xue, G.; Ognier, S. A Comparison of N-doped TiO2 Photocatalysts Preparation Methods and Studies on Their Catalytic Activity. J. Chem. Technol. Biotechnol. 2013, 88, 1815–1821. [Google Scholar] [CrossRef]
- Wang, X.; Lim, T.-T. Effect of Hexamethylenetetramine on the Visible-Light Photocatalytic Activity of C–N Codoped TiO2 for Bisphenol A Degradation: Evaluation of Photocatalytic Mechanism and Solution Toxicity. Appl. Catal. A Gen. 2011, 399, 233–241. [Google Scholar] [CrossRef]
- Devi, L.G.; Nagaraj, B.; Rajashekhar, K.E. Synergistic Effect of Ag Deposition and Nitrogen Doping in TiO2 for the Degradation of Phenol under Solar Irradiation in Presence of Electron Acceptor. Chem. Eng. J. 2012, 181–182, 259–266. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Pósfai, M.; Makó, É.; Juzsakova, T.; Fónagy, O. The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect. Nanomaterials 2020, 10, 2261. [Google Scholar] [CrossRef]
- Kocsis, G.; Szabó-Bárdos, E.; Fónagy, O.; Farsang, E.; Juzsakova, T.; Jakab, M.; Pekker, P.; Kovács, M.; Horváth, O. Characterization of Various Titanium-Dioxide-Based Catalysts Regarding Photocatalytic Mineralization of Carbamazepine Also Combined with Ozonation. Molecules 2022, 27, 8041. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Mondal, M.; Mandal, S.; Roy, A.; Chakrabarty, S.; Chakrabarti, G.; Pradhan, S.K. Enhanced Photocatalytic and Antibacterial Activities of Mechanosynthesized TiO2–Ag Nanocomposite in Wastewater Treatment. J. Mol. Struct. 2020, 1211, 128076. [Google Scholar] [CrossRef]
- Ellouzi, I.; Bouddouch, A.; Bakiz, B.; Benlhachemi, A.; Abou Oualid, H. Glucose-Assisted Ball Milling Preparation of Silver-Doped Biphasic TiO2 for Efficient Photodegradation of Rhodamine B: Effect of Silver-Dopant Loading. Chem. Phys. Lett. 2021, 770, 138456. [Google Scholar] [CrossRef]
- Šubrt, J.; Criado, J.M.; Szatmáry, L.; Diánez-Millán, M.J.; Murafa, N.; Pérez-Maqueda, L.A.; Brezová, V. Mechanochemical Synthesis of Visible Light Sensitive Titanium Dioxide Photocatalyst. Int. J. Photoenergy 2011, 2011, 156941. [Google Scholar] [CrossRef][Green Version]
- Dulian, P.; Buras, M.; Żukowski, W. Modyfication of Photocatalytic Properties of Titanium Dioxide by Mechanochemical Method. Pol. J. Chem. Technol. 2016, 18, 68–71. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Sushnikova, A.A.; Rempel, A.A. Phase Composition Tuning by High-Energy Ball Milling of Titanium Dioxide Powders. Inorg Chem. Commun. 2024, 159, 111727. [Google Scholar] [CrossRef]
- Jalalah, M.; Faisal, M.; Bouzid, H.; Ismail, A.A.; Al-Sayari, S.A. Dielectric and Photocatalytic Properties of Sulfur Doped TiO2 Nanoparticles Prepared by Ball Milling. Mater. Res. Bull. 2013, 48, 3351–3356. [Google Scholar] [CrossRef]
- Yin, S.; Komatsu, M.; Zhang, Q.; Saito, F.; Sato, T. Synthesis of Visible-Light Responsive Nitrogen/Carbon Doped Titania Photocatalyst by Mechanochemical Doping. J. Mater. Sci. 2007, 42, 2399–2404. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Komatsu, M.; Zhang, Q.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Preparation of Nitrogen-Doped Titania with High Visible Light Induced Photocatalytic Activity by Mechanochemical Reaction of Titania and Hexamethylenetetramine. J. Mater. Chem. 2003, 13, 2996. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Komatsu, M.; Zhang, Q.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Synthesis of Visible-Light Reactive TiO2−N Photocatalyst by Mechanochemical Doping. Solid State Sci. 2005, 7, 1479–1485. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A. Nitrogen-Doped Titanium Dioxide Photocatalysts for Visible Response Prepared by Using Organic Compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Zhang, Q.; Komatsu, M.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Mechanochemical Synthesis of Nitrogen-Doped Titania and Its Visible Light Induced NO Destruction Ability. Solid State Ion. 2004, 172, 205–209. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Huang, X.-H.; Yu, H.-Q.; Tang, L.-H. Nitrogen-Doped Photocatalyst Prepared by Mechanochemical Method: Doping Mechanisms and Visible Photoactivity of Pollutant Degradation. Int. J. Photoenergy 2012, 2012, 960726. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Chen, M.; Yan, L.; He, H.; Chang, Q.; Yu, Y.; Qu, J. Catalytic Sterilization of Escherichia Coli K 12 on Ag/Al2O3 Surface. J. Inorg. Biochem. 2007, 101, 817–823. [Google Scholar] [CrossRef]
- Chang, Q.; He, H.; Ma, Z. Efficient Disinfection of Escherichia Coli in Water by Silver Loaded Alumina. J. Inorg. Biochem. 2008, 102, 1736–1742. [Google Scholar] [CrossRef]
- Pham, T.-D.; Lee, B.-K. Feasibility of Silver Doped TiO2/Glass Fiber Photocatalyst under Visible Irradiation as an Indoor Air Germicide. Int. J. Environ. Res. Public Health 2014, 11, 3271–3288. [Google Scholar] [CrossRef]
- Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Colón, G.; Navío, J.A.; Macías, M.; Pérez Peña, J. Effect of Deposition of Silver on Structural Characteristics and Photoactivity of TiO2-Based Photocatalysts. Appl. Catal. B 2012, 127, 112–120. [Google Scholar] [CrossRef]
- Madadi, M.; Ghorbanpour, M.; Feizi, A. Antibacterial and Photocatalytic Activity of Anatase Phase Ag-doped TiO2 Nanoparticles. Micro. Nano Lett. 2018, 13, 1590–1593. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced Photocatalytic and Antibacterial Activities of Ag-Doped TiO2 Nanoparticles under Visible Light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Backhaus, T.; Grimme, L.H. The Toxicity of Antibiotic Agents to the Luminescent Bacterium Vibrio Fischeri. Chemosphere 1999, 38, 3291–3301. [Google Scholar] [CrossRef]
- Hernando, M.D.; De Vettori, S.; Martínez Bueno, M.J.; Fernández-Alba, A.R. Toxicity Evaluation with Vibrio Fischeri Test of Organic Chemicals Used in Aquaculture. Chemosphere 2007, 68, 724–730. [Google Scholar] [CrossRef]
- Vihodceva, S.; Šutka, A.; Sihtmäe, M.; Rosenberg, M.; Otsus, M.; Kurvet, I.; Smits, K.; Bikse, L.; Kahru, A.; Kasemets, K. Antibacterial Activity of Positively and Negatively Charged Hematite (α-Fe2O3) Nanoparticles to Escherichia Coli, Staphylococcus Aureus and Vibrio Fischeri. Nanomaterials 2021, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Gmurek, M.; Horn, H.; Majewsky, M. Phototransformation of Sulfamethoxazole under Simulated Sunlight: Transformation Products and Their Antibacterial Activity toward Vibrio Fischeri. Sci. Total Environ. 2015, 538, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of Nanosized and Bulk ZnO, CuO and TiO2 to Bacteria Vibrio Fischeri and Crustaceans Daphnia Magna and Thamnocephalus Platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Aysin, B.; Ozturk, A.; Park, J. Silver-Loaded TiO2 Powders Prepared through Mechanical Ball Milling. Ceram. Int. 2013, 39, 7119–7126. [Google Scholar] [CrossRef]
- Czili, H.; Horváth, A. Applicability of Coumarin for Detecting and Measuring Hydroxyl Radicals Generated by Photoexcitation of TiO2 Nanoparticles. Appl. Catal. B 2008, 81, 295–302. [Google Scholar] [CrossRef]
- Uzunova-Bujnova, M.; Dimitrov, D.; Radev, D.; Bojinova, A.; Todorovsky, D. Effect of the Mechanoactivation on the Structure, Sorption and Photocatalytic Properties of Titanium Dioxide. Mater. Chem. Phys. 2008, 110, 291–298. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Szabó-Bárdos, E.; Cafuta, A.; Hegedűs, P.; Fónagy, O.; Kiss, G.; Babić, S.; Škorić, I.; Horváth, O. Photolytic and Photocatalytic Degradation of Nitrofurantoin and Its Photohydrolytic Products. J. Photochem. Photobiol. A Chem. 2020, 386, 112093. [Google Scholar] [CrossRef]
- Beranek, R.; Kisch, H. Tuning the Optical and Photoelectrochemical Properties of Surface-Modified TiO2. Photochem. Photobiol. Sci. 2008, 7, 40–48. [Google Scholar] [CrossRef]
- Leandri, V.; Gardner, J.M.; Jonsson, M. Coumarin as a Quantitative Probe for Hydroxyl Radical Formation in Heterogeneous Photocatalysis. J. Phys. Chem. C 2019, 123, 6667–6674. [Google Scholar] [CrossRef]
- Náfrádi, M.; Farkas, L.; Alapi, T.; Hernádi, K.; Kovács, K.; Wojnárovits, L.; Takács, E. Application of Coumarin and Coumarin-3-Carboxylic Acid for the Determination of Hydroxyl Radicals during Different Advanced Oxidation Processes. Radiat. Phys. Chem. 2020, 170, 108610. [Google Scholar] [CrossRef]
- Fernández-Castro, P.; Vallejo, M.; San Román, M.F.; Ortiz, I. Insight on the Fundamentals of Advanced Oxidation Processes. Role and Review of the Determination Methods of Reactive Oxygen Species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Wan, Q.; Cao, S.; Huang, T.; Lu, J.; Ma, J.; Wen, G. Kinetics and Mechanism of Sulfate Radical-and Hydroxyl Radical-Induced Disinfection of Bacteria and Fungal Spores by Transition Metal Ions-Activated Peroxymonosulfate. Water Res. 2023, 243, 120378. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Kanno, T.; Örtengren, U.; Lingström, P.; Niwano, Y. Antimicrobial Activity of Hydroxyl Radicals Generated by Hydrogen Peroxide Photolysis against Streptococcus Mutans Biofilm. Int. J. Antimicrob. Agents 2016, 48, 373–380. [Google Scholar] [CrossRef]
- Sheng, H.; Nakamura, K.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal Effect of Photolysis of H2O2 in Combination with Sonolysis of Water via Hydroxyl Radical Generation. PLoS ONE 2015, 10, e0132445. [Google Scholar] [CrossRef]
- Tenkumo, T.; Ishiyama, K.; Prymak, O.; Nakamura, K.; Shirato, M.; Ogawa, T.; Miyashita, M.; Takahashi, M.; Epple, M.; Kanno, T.; et al. Bactericidal Activity and Recovery Effect of Hydroxyl Radicals Generated by Ultraviolet Irradiation and Silver Ion Application on an Infected Titanium Surface. Sci. Rep. 2020, 10, 8553. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fónagy, O.; Kovács, M.; Szabó-Bárdos, E.; Csicsor-Kulcsár, P.; Fodor, L.; Horváth, O. Mechanochemically Modified TiO2 Photocatalysts: Combination of Visible-Light Excitability and Antibacterial Effect. Catalysts 2025, 15, 316. https://doi.org/10.3390/catal15040316
Fónagy O, Kovács M, Szabó-Bárdos E, Csicsor-Kulcsár P, Fodor L, Horváth O. Mechanochemically Modified TiO2 Photocatalysts: Combination of Visible-Light Excitability and Antibacterial Effect. Catalysts. 2025; 15(4):316. https://doi.org/10.3390/catal15040316
Chicago/Turabian StyleFónagy, Orsolya, Margit Kovács, Erzsébet Szabó-Bárdos, Petra Csicsor-Kulcsár, Lajos Fodor, and Ottó Horváth. 2025. "Mechanochemically Modified TiO2 Photocatalysts: Combination of Visible-Light Excitability and Antibacterial Effect" Catalysts 15, no. 4: 316. https://doi.org/10.3390/catal15040316
APA StyleFónagy, O., Kovács, M., Szabó-Bárdos, E., Csicsor-Kulcsár, P., Fodor, L., & Horváth, O. (2025). Mechanochemically Modified TiO2 Photocatalysts: Combination of Visible-Light Excitability and Antibacterial Effect. Catalysts, 15(4), 316. https://doi.org/10.3390/catal15040316







